Abstract
Older adults often exhibit high levels of lower extremity muscle co-contraction, which may be the cause or effect of age-related impairments in gait and associated falls. Normal gait requires intact executive function and thus can be slowed by challenging executive resources available to the neuromuscular system through the performance of a dual task. We therefore investigated associations between lower limb co-contraction and gait characteristics under normal and dual task conditions in healthy older adults (85.4±5.9 years). We hypothesized that greater co-contraction is associated with slower gait speed during dual task conditions that stress executive and attentional abilities. Co-contraction was quantified during different phases of the gait cycle using surface electromyography (EMG) signals obtained from the anterior tibialis and lateral gastrocnemius while walking at preferred speed during normal and dual task conditions. Variables included the time difference to complete the Trail Making Test A and B (ΔTMT) and gait measures during normal or dual task walking. Higher co-contraction levels during the swing phase of both normal and dual task walking were associated with longer ΔTMT (normal: R2=0.25, p=0.02; dual task: R2=0.27, p=0.01). Co-contraction was associated with gait measures during dual task walking only; greater co-contraction levels during stride and stance were associated with slower gait speed (stride: R2=0.38, p=0.04; stance: R2=0.38, p=0.04), and greater co-contraction during stride was associated with longer stride time (R2=0.16, p=0.03). Our results suggest that relatively high lower limb co-contraction may explain some of the mobility impairments associated with the conduct of executive tasks in older adults.
Keywords: muscle co-contraction, gait, aging, dual task
INTRODUCTION
Aging is associated with a decline in mobility and balance, which may lead to falls and a loss of independent function [1]. One possible mechanism of these impairments in older adults is an increase in lower extremity muscle co-contraction. Muscle co-contraction is the simultaneous activation of agonist and antagonist muscle groups [2]. Older adults, compared to their younger counterparts, exhibit higher levels of lower extremity muscle co-contraction during walking [3] and standing balance [4]. In addition, older adults may unconsciously utilize co-contraction to stiffen joints in order to compensate for deteriorations in postural control and sensory processing [4]. However, high levels of co-contraction also appear to have negative consequences. Co-contraction increases energy expenditure and impedes movement [5], leading to a high cost of walking [6, 7], fatigue, reduced physical performance, and falls [3, 5, 8]. Other potential negative effects include increased forces around joints causing cartilage and joint degeneration [9]. Therefore, it may be important to reduce lower extremity co-contraction in older adults in order to improve gait biomechanics and balance, and thereby reduce their risk of mobility disability and falls.
Recent studies have demonstrated that normal gait requires intact executive function [10] and lower extremity muscle activity can be altered by challenging the executive and attentional resources available to the neuromuscular system through the performance of a dual task [11]. The role of muscle co-contraction in this cognitive-motor interaction remains unknown, especially in older adults who rely heavily on co-contraction to maintain balance. Therefore, we examined the relationships between gait co-contraction and cognitive and physical performances in older adults and how cognitive stress can affect these relationships.
Previous studies have associated co-contraction with postural control during standing, revealing that higher levels of muscle co-contraction have a “deleterious” effect on the regulation of body sway [12]. However, there is a lack of information on lower limb muscle co-contraction during walking. Furthermore, balance control during walking is dynamic and phase dependent; different gait phases such as stance and swing appear to require different levels of cognitive and motor activation [13]. We therefore examined the patterns of muscle co-contraction not only during an overall gait cycle, but also during the different phases of gait.
We designed this study to determine the associations between lower limb co-contraction, functional performance, and gait under normal and dual task conditions in a group of very elderly adults living within supportive housing facilities – a rapidly enlarging, but often overlooked population that accounts for a large proportion of health care spending due to mobility disability. We hypothesized that greater lower limb co-contraction in this cohort is associated with poorer balance and slower gait speed, particularly during dual task conditions that stress executive control and attentional abilities.
METHODS
A secondary analysis was performed on baseline data from a randomized controlled study [NCT01126723] of Tai Chi in elderly residents of senior housing facilities in the Boston area. Participants ranged from 71 to 95 years old. Exclusion criteria included the inability to stand or ambulate unassisted, the presence of symptomatic cardiovascular or respiratory disease, a history of myocardial infarction or stroke, self-reported painful arthritis, spinal stenosis, amputation, painful foot lesions or neuropathy, systolic blood pressure >160 or diastolic blood pressure >100mmHg, known abnormal cardiac rhythm or presence of a cardiac pacemaker, Parkinson’s disease, metastatic cancer, or immunosuppressive therapy [14]. The study was approved by the Institutional Review Board of Hebrew SeniorLife and all participants provided written informed consent.
Protocol
All participants were assessed in their facility by trained research staff. The participants were interviewed to collect demographic, clinical, functional, and medication data. Physical and cognitive assessments included the Short Physical Performance Battery (SPPB), Berg Balance Scale (BBS), Activities-specific Balance Confidence (ABC) scale, and Trail Making Test (TMT). All participants then performed two randomized 90 second walks along an empty indoor hallway at: 1) normal walking speed (NW) and 2) normal walking speed while verbally performing serial subtractions of five from 500 (also known as a dual task walking condition (DT)).
Before the walking trials, surface electromyography (EMG) electrodes (Noraxon, Scottsdale, USA) were placed on the anterior tibialis and lateral gastrocnemius muscles. EMG signals were sampled at a frequency of 1500Hz during the walking trials. The Noraxon equipment contained an EMG Sensor Data Acquisition system with a sample rate of 1500Hz and a selectable low pass filter of 500Hz. The EMG sensors had a 1st order high-pass filter set to 10Hz +/− 10% cutoff. Wireless footswitches were placed on the heel and toe to record heel and toe contact and lift from the ground.
EMG signals were processed with MATLAB (Mathworks, Natick, USA) using a Butterworth bandpass fourth order filter of 20 – 400Hz, full-wave rectification, and then a Butterworth lowpass fourth order filter of 6Hz. A similar method was reported by Hallal and colleagues [8]. Walking stride was determined based on heel contacts recorded by the footswitches. Each stride EMG was resampled to 1000 points and a mean EMG signal of all the strides in the walk was obtained. The mean of the maximum EMG signal amplitude of each muscle group for all strides in a walking trial was also determined. The mean stride EMG for each muscle group was then normalized as a percentage of this mean maximum amplitude.
Measurement of Muscle Co-contraction and Functional Outcomes
EMG signals during each stride were averaged across all strides in a subject’s walk to get an overall EMG stride pattern. Muscle co-contraction was quantified for this averaged stride. Muscle co-contraction was measured as the percentage of total muscle activity when antagonist muscle groups (the anterior tibialis and lateral gastrocnemius muscles) were simultaneously activated [3, 15–17] and calculated using the following equation:
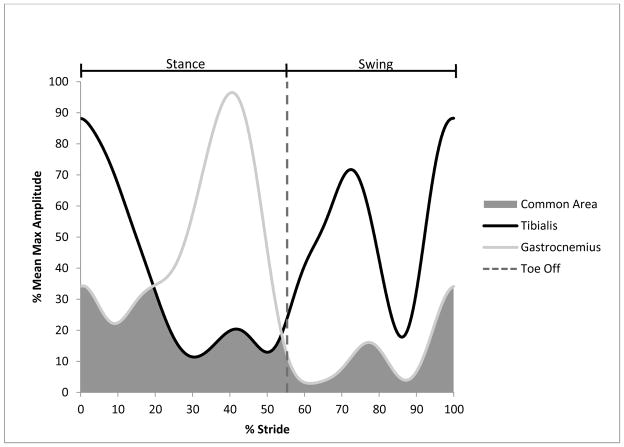
Area A is the area under the EMG curve of muscle A, area B is the area under the EMG curve of muscle B, and the common area A & B is the area under the curves shared by both muscle A and muscle B during an average stride [3] (Figure 1). The stance co-contraction was calculated from the heel strike to toe-off of the averaged stride EMG signal; the swing co-contraction was calculated from the toe-off to heel strike of the averaged stride EMG signal (Figure 1).
Figure 1.
Calculation of lower limb muscle co-contraction. The EMG signals of the anterior tibialis and lateral gastrocnemius muscles were extracted from each stride of each walking trial and normalized to 100%. The common area is the overlapping region under the curves of the EMG signals and represents the amount of muscle co-contraction. The toe off divides the stride into the stance and swing phases of the gait cycle.
Gait speed and phase times were determined for each 90 second walking trial under normal walking and dual task walking conditions. Gait speed was measured by dividing the total distance walked by the trial duration (90 seconds). Stride, stance, and swing times were calculated from the footswitch data. Stride time was measured as the time between each heel strike of the same foot. Stance time was measured as the time between the heel strike and toe-off of the same foot; this is the time during the gait cycle when the foot is in contact with the ground. The swing time was measured as the time between the toe-off and heel strike of the same foot; this is the time during the gait cycle when the foot is off the ground.
The TMT is a pen and paper test that is used to measure cognitive flexibility, processing speed, and set-shifting [18–20]. TMT contains two parts: TMT A and TMT B. TMT A required participants to draw a continuous line connecting 25 encircled numbers scattered across a sheet of paper in sequential order (1 to 25). TMT B required participants to connect scattered numbers (1 to 13) and letters (A to L) in sequential, yet alternating order (i.e. 1-A, 2-B, 3-C, etc). The time to complete each test was recorded. Set-shifting ability was corrected for performance speed by calculating the difference in completion time between TMT B and TMT A (ΔTMT = TMT B − TMT A), a measure based on previous literature [18].
The SPPB test was used to quantify physical function by measuring standing balance, normal walking speed, and the ability to sit and stand from a chair five times in a row. Each of these tasks was given a score that corresponded to quartiles of time it took to complete the task. The scores ranged from 0 to 4, with 0 indicating inability to perform the task and 4 indicating the fastest quartile. The maximum SPPB score is 12, indicating the highest lower extremity physical function [21, 22].
The BBS measured static and dynamic balance. The assessment consisted of 14 items involving balance while standing, reaching, turning, and transferring that were scored from a scale of 0 to 4, with 0 indicating the lowest level of function and 4 indicating the highest level of function. The maximum BBS score is 56, indicating the highest functional balance [23].
The ABC assessed self-efficacy for balance. Participants were asked to rate their confidence in performing 16 ambulatory tasks from a scale of 0 to 100, with 0 indicating not at all confident and 100 indicating completely confident. The overall score was determined by summing item scores and dividing by the total number of items [24].
Data Analysis
Percent co-contraction data were analyzed across 56 out of the 65 recruited participants during normal and dual task walking. Missing co-contraction data was due to missing or corrupted EMG files and/or missing footswitch data (n=9). A bivariate correlation analysis was used to examine the association between muscle co-contraction and age. Due to the significant correlation between muscle co-contraction and age, age was then included as a covariate in the following multiple linear regression models. Multiple linear regression analyses, with age used as a covariate, were performed to examine the associations between muscle co-contraction and SPPB, BBS, ABC, ΔTMT, gait speed, and stride, stance, and swing times. Multiple linear regression analyses were also used to examine the associations between the percent change in co-contraction and the percent change in gait speed and stride, stance, and swing times. The percent change was defined as the difference from NW condition to the DT walking condition divided by the NW. In all of the analyses, a series of model diagnostic plots were examined to ensure proper model fit and all assumptions were met. Functional measures greater than 2 standard deviations above or below the mean were removed from the analyses. A p value < 0.05 was considered statistically significant. All the statistical analyses were performed using SAS (version 9.4, SAS Institute).
RESULTS
Participant characteristics are summarized in Table 1. For participant study flow please refer to Manor et al. (2014) [14].
Table 1.
Participant Characteristics and Functional Measures (n = 56).
| Mean ± SD | |
|---|---|
| Age | 85.4 ± 5.9 |
| Sex (M/F) | 13/43 |
| Caucasian n (%) | 54 (96.4%) |
| College educated n (%) | 34 (60.7%) |
| Use of assisted device n (%) | 22 (39.3%) |
| BMI (kg/m^2) | 27.3 ± 4.2 |
| SPPB | 8.4 ± 2.5 |
| BERG | 46.6 ± 5.3 |
| ABC | 78.2 ± 17.4 |
| TMT (s) | |
| TMT A | 63.6 ± 40.5 |
| TMT B | 146.8 ± 72.6 |
| ΔTMT | 86.5 ± 52.1 |
| NW Co-Contraction (%) | |
| Stride | 55.2 ± 14.2 |
| Stance | 57.1 ± 14.4 |
| Swing | 50.5 ± 16.8 |
| DT Co-Contraction (%) | |
| Stride | 56.4 ± 13.4 |
| Stance | 58.7 ± 13.9 |
| Swing | 51.1 ± 16.5 |
| Gait Speed (m/s) | |
| NW | 0.95 ± 0.24 |
| DT | 0.87 ± 0.21 |
| Stride Time (s) | |
| NW | 1.13 ± 0.12 |
| DT | 1.20 ± 0.14 |
| Stance Time (s) | |
| NW | 0.65 ± 0.08 |
| DT | 0.71 ± 0.10 |
| Swing Time (s) | |
| NW | 0.46 ± 0.04 |
| DT | 0.48 ± 0.04 |
Note: BMI = Body Mass Index; SPPB = Short Physical Performance Battery; BBS = Berg Balance Scale; ABC = Activities-specific Balance Confidence; TMT = Trail Making Test. NW = normal walking; DT = dual task walking.
The bivariate correlation analysis revealed that there was a significant and positive association between co-contraction and age in the stride and swing phases during normal walking (r=0.305, p=0.023; r=0.384, p=0.004, respectively) and in the swing phase during dual task walking (r=0.381, p=0.004) (Table 2). Therefore, we used age as a covariate in subsequent multiple linear regression analyses to examine the relationships between co-contraction and other variables (ΔTMT, ABC, gait speed, and stride, stance, and swing time), as well as the relationship between percent change from NW to DT in co-contraction and gait parameters.
Table 2.
Bivariate correlation analysis between lower limb muscle co-contraction during walking and age.
| Normal Walking | ||
|---|---|---|
|
| ||
| Age (years) | ||
|
| ||
| Co-Contraction (%) | r | p-val |
|
| ||
| Stride | 0.305 | 0.023 |
| Stance | 0.192 | 0.160 |
| Swing | 0.384 | 0.004 |
|
| ||
| Dual Task Walking | ||
|
| ||
| Age (years) | ||
|
| ||
| Co-Contraction (%) | r | p-val |
|
| ||
| Stride | 0.243 | 0.074 |
| Stance | 0.094 | 0.496 |
| Swing | 0.381 | 0.004 |
The multiple linear regressions revealed significant associations between co-contraction and cognitive and physical measures. Swing co-contraction levels were positively associated with the ΔTMT times during both the normal and dual task walking conditions (R2=0.251, p=0.022; R2= 0.266, p=0.010, respectively) (Table 3). Stride and stance co-contraction levels were inversely associated with gait speed during the dual task walking but not the normal walking condition (R2=0.379, p=0.038; R2=0.378, p=0.04, respectively) (Table 4). Stride co-contraction levels were also positively associated with stride time during the dual task walking condition (R2=0.157, p=0.034) (Table 4). Co-contraction levels increased to a small extent during dual task walking conditions (Table 1); however, the percent changes in co-contraction were associated with the percent changes in stride, stance, and swing times after adjusting for age (R2=0.323, p<0.001; R2=0.245, p=0.008; R2=0.09, p=0.04, respectively).
Table 3.
Multiple linear regression analysis of the relationship between lower limb muscle co-contraction during walking and ΔTMT, adjusted for age.
| Normal Walking | |||
|---|---|---|---|
|
| |||
| ΔTMT (s) | |||
|
| |||
| Co-Contraction (%) | R2 | β | p-val |
|
| |||
| Stride | 0.113 | 0.049 | 0.246 |
| Stance | 0.026 | 0.007 | 0.870 |
| Swing | 0.251 | 0.110 | 0.022 |
|
| |||
| Dual Task Walking | |||
|
| |||
| ΔTMT (s) | |||
|
| |||
| Co-Contraction (%) | R2 | β | p-val |
|
| |||
| Stride | 0.096 | 0.058 | 0.151 |
| Stance | 0.012 | 0.023 | 0.582 |
| Swing | 0.266 | 0.121 | 0.010 |
Note: R2 = measures the proportion of variance in the outcome variable explained by the predictor variable; β = magnitude of the slope of the relationship; p-val = probability of whether or not β is significantly different than 0 (p-val < 0.05 was considered statistically significant).
Table 4.
Multiple linear regression analyses of the relationships between lower limb muscle co-contraction and ABC, gait speed, and stride, stance, and swing times during normal and dual task walking, adjusted for age.
| Normal Walking | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| ABC (%) | Gait Speed (m/s) | Stride Time (s) | Stance Time (s) | Swing Time (s) | |||||||||||
|
| |||||||||||||||
| Co-Contraction (%) | R2 | β | p-val | R2 | β | p-val | R2 | β | p-val | R2 | β | p-val | R2 | β | p-val |
| Stride | 0.147 | −0.394 | 0.021 | 0.352 | −0.001 | 0.466 | 0.140 | 0.001 | 0.218 | - | - | - | - | - | - |
| Stance | 0.131 | −0.342 | 0.036 | 0.349 | −0.001 | 0.573 | - | - | - | 0.129 | 0.0005 | 0.511 | - | - | - |
| Swing | 0.152 | −0.362 | 0.018 | 0.361 | −0.002 | 0.273 | - | - | - | - | - | - | 0.034 | −0.0004 | 0.256 |
|
| |||||||||||||||
| Dual Task Walking | |||||||||||||||
|
| |||||||||||||||
| ABC (%) | Gait Speed (m/s) | Stride Time (s) | Stance Time (s) | Swing Time (s) | |||||||||||
|
| |||||||||||||||
| Co-Contraction (%) | R2 | β | p-val | R2 | β | p-val | R2 | β | p-val | R2 | β | p-val | R2 | β | p-val |
|
| |||||||||||||||
| Stride | 0.170 | −0.456 | 0.010 | 0.379 | −0.004 | 0.038 | 0.157 | 0.003 | 0.034 | - | - | - | - | - | - |
| Stance | 0.140 | −0.374 | 0.027 | 0.378 | −0.004 | 0.04 | - | - | - | 0.190 | 0.002 | 0.08 | - | - | - |
| Swing | 0.151 | −0.360 | 0.018 | 0.360 | −0.003 | 0.097 | - | - | - | - | - | - | 0.030 | 0.0001 | 0.821 |
Note: R2 = measures the proportion of variance in the outcome variable explained by the predictor variable; β = magnitude of the slope of the relationship; p-val = probability of whether or not β is significantly different than 0 (p-val < 0.05 was considered statistically significant).
The multiple linear regressions also reviewed that ABC measures were inversely related to co-contraction under both normal and dual task conditions in all gait phases (Table 4). Other functional outcomes, SPPB and BBS, were not significantly related to co-contraction after adjusting for age.
DISCUSSION
The present study examined the relationship between lower extremity muscle co-contraction during walking and cognitive and gait performances. The bivariate correlations showed that increasing age was associated with higher co-contraction levels in the stride and swing phases of the gait cycle. The multiple linear regressions showed that longer ΔTMT times were associated with higher co-contraction levels during the swing phase. The multiple linear regressions also indicated that high co-contraction during stride and stance phases were related to slower gait speed only during the dual task condition. No other known studies have examined co-contraction levels during different gait phases and their relationship with gait and cognitive impairments, particularly in very old adults.
Our findings are consistent with previous studies showing that older adults exhibit greater muscle co-contraction than younger adults [3, 4, 8], but extend these observations into a population far advanced in age. This is a rapidly enlarging and costly population that is especially vulnerable to cognitive dysfunction, mobility disability, and falls. One interpretation of this finding is that older adults utilize co-contraction to stiffen joints in order to compensate for poor postural control [4]. Adults suffering from neurological impairment, such as stroke, were shown to have increased duration of muscle co-contraction, most likely as a strategy to improve walking stability [17]. Although co-contraction may be compensatory, excessive levels of co-contraction in older adults may lead to higher energy expenditure during ambulation [6, 7] and further impair physical function and postural control, and increase the risk of falls [12, 25].
Our data also revealed that co-contraction was associated with gait parameters only during the cognitive stress of dual task walking. Furthermore, the small increase in co-contraction from single to dual task walking conditions was significantly associated with an increase in stride, stance, and swing time. Rankin et al. suggested that dual tasking reduces the attentional processing resources available for balance control and has a much larger impact on postural control in older than in younger adults, presumably because older adults have less attentional reserve [11].
The relationship between ΔTMT and co-contraction also revealed associations between cognitive function and lower limb muscle co-contraction. Higher co-contraction levels during the swing phase of the gait cycle were associated with poorer executive function. The swing phase may require greater cognitive demands than other gait phases. Plummer-D’Amato et al. showed that dual tasking had a significant effect on the nonparetic limb swing duration of stroke patients [13]. Under dual task conditions, these patients had longer double limb support time and reduced swing times on the nonparetic leg, suggesting that “balance control during dual task walking may be significantly compromised in independent ambulators who have suffered a stroke” [13].
Higher co-contraction levels were also inversely associated with low balance confidence (ABC score) during both normal and dual task walking. This finding is supported by Hallal et al. who found that when older women walked on a treadmill containing obstacles that may disturb balance, they had higher lower limb co-contraction compared to younger women [8]. As balance confidence is predictive of fall risk [26], excessive co-contraction may mediate this relationship.
Our results on co-contraction during specific gait phases suggest that different phases require different levels of cognitive and motor activation [27]. Co-contraction in the stance phase had significant associations with physical measures, while co-contraction in the swing phase had significant associations with cognitive measures. Supporting the body’s mass, as well as lifting and lowering the foot, recruit distinct muscle groups and may also require various levels of cognitive resources. Abbud et al. demonstrated that a cognitive task only interfered with gait during single-leg stance, suggesting that attentional resources are used differently across gait phases [27]. Thus, in future studies the stance and swing phases of the gait cycle should be considered independently when measuring lower limb muscle co-contraction during walking.
Study limitations include measuring only relative muscle activity and not absolute muscle activity. The relatively small sample size also limits our power to detect functional associations and our ability to generalize our results to larger populations. The advanced age of the study cohort increased the variability of our measures and limited our ability to generalize our results to all elderly individuals. Also, the relationships we observed were relatively weak, probably reflecting the multiple unmeasured factors that influence gait or balance and the heterogeneity of the population. Only future intervention studies that manipulate the degree of co-contraction, while keeping other independent variables constant, can determine the full clinical significance of our findings. Nevertheless, this study is unique in measuring co-contraction during different gait phases and showing associations with cognitive and physical measures. Our findings suggest that lower extremity muscle co-contraction may have important functional implications and may partially explain some of the mobility impairments associated with advanced age.
HIGHLIGHTS.
Lower leg muscle co-contraction correlated with executive function and gait.
Co-contraction in each gait phase had different cognitive and gait associations.
Co-contraction may contribute to the link between executive function and gait.
Acknowledgments
This work was supported by grants R01-AG041785, R01-AG025037, and T32-AG023480 to Dr. Lipsitz from the National Institute on Aging. Drs. Lo and Olson were recipients of postdoctoral training grants from T32-AG023480. Dr. Manor was supported by 1-K01-AG044543-01A1 from the National Institute on Aging. Dr. Lipsitz holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife.
Footnotes
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakano MM, Otonari TS, Takara KS, Carmo CM, Tanaka C. Physical performance, balance, mobility, and muscle strength decline at different rates in elderly people. J Phys Ther Sci. 2014;26:583–6. doi: 10.1589/jpts.26.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busse ME, Wiles CM, van Deursen RW. Co-activation: its association with weakness and specific neurological pathology. J Neuroeng Rehabil. 2006;3:26. doi: 10.1186/1743-0003-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hallal ZC, Marques NR, Vieira ER, Brunt D, Spinoso DH, Castro A, Cardozo AC, Goncalves M. Lower limb muscle coactivation levels in healthy younger and older adults during functional dual-task gait. Motriz Revista de Educação Física. 2013;19:620–6. [Google Scholar]
- 4.Benjuya N, Melzer I, Kaplanski J. Aging-induced shifts from a reliance on sensory input to muscle cocontraction during balanced standing. J Gerontol A Biol Sci Med Sci. 2004;59:166–71. doi: 10.1093/gerona/59.2.m166. [DOI] [PubMed] [Google Scholar]
- 5.Hortobagyi T, Solnik S, Gruber A, Rider P, Steinweg K, Helseth J, DeVita P. Interaction between age and gait velocity in the amplitude and timing of antagonist muscle coactivation. Gait Posture. 2009;29:558–64. doi: 10.1016/j.gaitpost.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Peterson DS, Martin PE. Effects of age and walking speed on coactivation and cost of walking in healthy adults. Gait Posture. 2010;31:355–9. doi: 10.1016/j.gaitpost.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Mian OS, Thom JM, Ardigo LP, Narici MV, Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol (Oxf) 2006;186:127–39. doi: 10.1111/j.1748-1716.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- 8.Hallal CZ, Marques NR, Spinoso DH, Vieira ER, Goncalves M. Electromyographic patterns of lower limb muscles during apprehensive gait in younger and older female adults. J Electromyogr Kinesiol. 2013;23:1145–9. doi: 10.1016/j.jelekin.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33:195–200. doi: 10.1097/00003677-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005;164:541–8. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- 11.Rankin JK, Woollacott MH, Shumway-Cook A, Brown LA. Cognitive influence on postural stability: a neuromuscular analysis in young and older adults. J Gerontol A Biol Sci Med Sci. 2000;55:M112–9. doi: 10.1093/gerona/55.3.m112. [DOI] [PubMed] [Google Scholar]
- 12.Nagai K, Yamada M, Mori S, Tanaka B, Uemura K, Aoyama T, Ichihashi N, Tsuboyama T. Effect of the muscle coactivation during quiet standing on dynamic postural control in older adults. Arch Gerontol Geriatr. 2013;56:129–33. doi: 10.1016/j.archger.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Plummer-D’Amato P, Altmann LJ, Behrman AL, Marsiske M. Interference between cognition, double-limb support, and swing during gait in community-dwelling individuals poststroke. Neurorehabil Neural Repair. 2010;24:542–9. doi: 10.1177/1545968309357926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manor B, Lough M, Gagnon MM, Cupples A, Wayne PM, Lipsitz LA. Functional benefits of tai chi training in senior housing facilities. J Am Geriatr Soc. 2014;62:1484–9. doi: 10.1111/jgs.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Nardo F, Mengarelli A, Maranesi E, Burattini L, Fioretti S. Assessment of the ankle muscle co-contraction during normal gait: a surface electromyography study. J Electromyogr Kinesiol. 2015;25:347–54. doi: 10.1016/j.jelekin.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Wang R, Gutierrez-Farewik EM. Compensatory strategies during walking in response to excessive muscle co-contraction at the ankle joint. Gait Posture. 2014;39:926–32. doi: 10.1016/j.gaitpost.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Rosa MC, Marques A, Demain S, Metcalf CD. Lower limb co-contraction during walking in subjects with stroke: A systematic review. J Electromyogr Kinesiol. 2014;24:1–10. doi: 10.1016/j.jelekin.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Coppin AK, Shumway-Cook A, Saczynski JS, Patel KV, Ble A, Ferrucci L, Guralnik JM. Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing. 2006;35:619–24. doi: 10.1093/ageing/afl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlson MC, Fried LP, Xue QL, Bandeen-Roche K, Zeger SL, Brandt J. Association between executive attention and physical functional performance in community-dwelling older women. J Gerontol B Psychol Sci Soc Sci. 1999;54:S262–70. doi: 10.1093/geronb/54b.5.s262. [DOI] [PubMed] [Google Scholar]
- 20.Gordon NG. The Trail Making Test in neuropsychological diagnosis. J Clin Psychol. 1972;28:167–9. doi: 10.1002/1097-4679(197204)28:2<167::aid-jclp2270280212>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 21.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 23.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring Balance in the Elderly: Validation of an Instrument. Canadian Journal of Public Health. 1992;83:S7–S11. [PubMed] [Google Scholar]
- 24.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A:M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 25.Cenciarini M, Loughlin PJ, Sparto PJ, Redfern MS. Stiffness and damping in postural control increase with age. IEEE Trans Biomed Eng. 2010;57:267–75. doi: 10.1109/TBME.2009.2031874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunter KB, De Costa J, White KN, Hooker KA, Hayes WC, Snow CM. Balance Self-Efficacy Predicts Risk Factors for Side Falls and Frequent Falls in Community-Dwelling Elderly. Journal of aging and physical activity. 2003;11:28–39. [Google Scholar]
- 27.Abbud GA, Li KZ, DeMont RG. Attentional requirements of walking according to the gait phase and onset of auditory stimuli. Gait Posture. 2009;30:227–32. doi: 10.1016/j.gaitpost.2009.05.013. [DOI] [PubMed] [Google Scholar]