Summary
FoxO proteins are major targets of insulin action. Adipose triacylglycerol (TAG) lipase mediates the first step in TAG hydrolysis and FoxO1 stimulates ATGL expression in adipose tissue. Here, we report that FoxO1 also stimulates ATGL and suppresses expression of its inhibitor, the G0/G1 switch gene protein 2 (G0S2), in the liver. Studies in FoxO transgenic and knockout mice and hepatocytes show that FoxO proteins mediate effects of insulin on ATGL and G0S2 expression. Further, ATGL-dependent lipolysis mediates effects of FoxOs on glycolytic, lipogenic and gluconeogenic gene expression and metabolism, including fatty acid oxidation, which is required for effects of FoxO1 on glucose homeostasis. These results reveal that ATGL-dependent lipolysis and fatty acid oxidation play a critical role in integrating the regulation of glucose and lipid metabolism downstream from FoxO proteins in the liver, and suggest that targeting ATGL-dependent lipolysis and its downstream effectors may provide an effective strategy for improving the treatment of patients with diabetes and hepatic insulin resistance.
Introduction
FoxO transcription factors are major targets of insulin action. Insulin stimulates Akt phosphorylation of FoxO proteins and promotes their translocation from the nucleus and thereby suppresses their effects on gene expression (Brunet et al., 1999; Zhao et al., 2004). In the liver, FoxO proteins contribute to the regulation of multiple metabolic pathways involved in the response to fasting, including gluconeogenesis, glycolysis and lipogenesis (Haeusler et al., 2014; Xiong et al., 2013; Zhang et al., 2012; Zhang et al., 2006), and suppressing hepatic FoxO1 function is critical for the ability of insulin to regulate hepatic glucose production and maintain glucose homeostasis (Dong et al., 2008; O-Sullivan et al., 2015; Titchenell et al., 2015). However, little is known regarding the role of FoxO proteins in regulating triacylglycerol catabolism in the liver.
FoxO proteins promote TAG catabolism in adipose tissue by stimulating the expression of adipose triacylglycerol (TAG) lipase (ATGL) (Chakrabarti and Kandror, 2009), which mediates the first step in lipolysis, after which other lipases, including hormone sensitive lipase (HSL) and monoacylglycerol lipase (MAGL) promote the removal of additional fatty acids from the glycerol backbone of TAG (Coleman and Mashek, 2011; Zechner et al., 2009). Initially identified in adipose tissue (Zimmermann et al., 2004), ATGL plays an important role in regulating TAG turnover in many tissues. In the liver, overexpressing ATGL decreases TAG content and promotes fatty acid oxidation (FAO) (Ong et al., 2011; Turpin et al., 2011). Conversely, disrupting hepatic ATGL expression promotes steatosis while improving glucose tolerance and insulin sensitivity (Kienesberger et al., 2009; Ong et al., 2013), indicating that regulating intrahepatic TAG catabolism may be important for the ability of insulin to maintain glucose homeostasis. FoxO proteins stimulate ATGL expression in adipose tissue and interact with response elements in the ATGL promoter (Chakrabarti and Kandror, 2009). However, the role of FoxO proteins in regulating hepatic ATGL expression and activity, and the role of ATGL in mediating effects of FoxO proteins on other aspects of metabolism in the liver have not been previously reported.
ATGL activity is inhibited by interaction with the G0/G1 switch-2 protein (G0S2) (Yang et al., 2010), and altering G0S2 expression in the liver also is associated with changes in TAG content and FAO (Wang et al., 2013; Zhang et al., 2014), consistent with its role as a physiologically relevant inhibitor of ATGL. Insulin has been reported to stimulate G0S2 expression in adipose tissue but the mechanism mediating this effect remain unknown (Heckmann et al., 2013). Gene profiling studies we performed in transgenic mice suggested that hepatic expression of G0S2 may be suppressed by FoxO1 (Zhang et al., 2006). Based on these observations, we asked whether FoxO proteins stimulate ATGL and suppress G0S2 expression, and whether ATGL-dependent lipolysis contributes to other effects of FoxO proteins in the liver. Our results show that FoxO proteins stimulate ATGL and suppress G0S2 expression in the liver, and that ATGL-dependent lipolysis plays an important role in mediating effects of FoxO proteins on both lipid and glucose metabolism in the liver, including effects on glycolysis, lipogenesis and gluconeogenesis.
Experimental Procedures
Animal Studies
Animal studies were approved by the Institutional Animal Care Committese at the Jesse Brown VA Medical Center and University of Minnesota. Transgenic mice expressing constitutively active FoxO1 (CA-FoxO1) in liver were previously described (Zhang et al., 2006). FoxO knockout (FoxO KO) mice were made by crossing FoxO floxed (FoxOfl/fl) (from Dr. Ron DePinho) with albumin-Cre (Jackson Lab) mice and disruption of liver FoxO1, FoxO3 and FoxO4 was confirmed by real-time qPCR and western blotting (Fig S1A–C). Wild type male FVB/N mice were purchased (Harlan, Madison). Studies were performed on 8–12 week old male mice unless otherwise stated. Mice were housed on a 12:12 hr light:dark cycle with lights off at 18:00. Adenoviral vectors (0.5–1×109 pfu/mouse) were injected by tail vein 5–7 d before studies.
For glucose and pyruvate tolerance tests, 4-hr fasted mice were treated with glucose (2 g/kg ip) or sodium pyruvate (2 g/kg ip) in phosphate buffered saline (PBS) or PBS alone and tail blood glucose levels were measured with a OneTouch Ultra glucose meter (Lifespan) at t = 0, 15, 30, 60 and 90 min. For studies in refed mice, chow was removed at 16:00 and replaced 18 hr later, and mice were sacrificed 6 hr later by decapitation following brief sedation with isoflurane. Livers were snap frozen in liquid nitrogen. Liver and serum samples from cervical blood were stored at −80°C. To assess liver triacylglycerol (TAG) secretion, mice were injected by tail vein with Tyloxapol (350 mg/kg, Sigma-Aldrich) 4 hr after refeeding and serial tail blood samples were collected in heparinized capillary tubes at appropriate time points for analysis of TAG levels. For analysis of de novo lipogenesis, 18-hr fasted mice were allowed to refeed for 2 hr prior to treatment with deuterated water (25 µl/g, to achieve 4% enrichment) and were sacrificed 4 hr later.
Hepatocytes
Primary hepatocytes were isolated by collagenase perfusion and transfected with adenoviral vectors as previously reported (Bu et al., 2009). For studies of insulin effects, hepatocytes were cultured in M199 media 10% FBS, 10 nM insulin and 10 nM dexamethasone for 4 hr after plating, and then in serum-free medium with/without 100 nM insulin for 4 hr, or as otherwise noted, before cell extracts were collected for analysis.
Pulse-chase studies were performed to measure TAG turnover and fatty acid oxidation (FAO) of fatty acids in the TAG pool as previously described(Sapiro et al., 2009). Hepatocytes were exposed to 500 µM [1-14C]oleate for 2 h (pulse), followed by a 6 hr chase period with media devoid of fatty acids. Radiolabelled TAG was quantified in cell extracts following separation by thin layer chromatography and media acid-soluble metabolites (ASMs) were measured as markers of FAO as previously described (Sapiro et al, 2009). To visualize lipid droplets, hepatocytes were harvested 24 hr following transfection with adenoviral vectors, rinsed with PBS, fixed with 3.7% formaldehyde and stained with Oil Red O.
To measure glucose production, hepatocytes were cultured in glucose-free DMEM without phenol red, supplemented with 20 mM sodium lactate and 2 mM sodium pyruvate (pH 7.4) and 10 nM insulin for 3 hr. Glucose concentration in medium was measured by glucose assay (Sigma) and normalized to total protein from cell lysates.
Biochemical analysis
Plasma levels of β-hydroxybutyrate (BHB), non-esterified free fatty acids (NEFAs), TAG, and cholesterol were measured with commercially available kits (Wako). Hepatic TAG content was measured following extraction with chloroform-methanol after the method of Bligh and Dyer (40). Liver acylcarnitines were analyzed using stable isotope dilution techniques (An et al., 2004). Measurements were made by flow injection tandem mass spectrometry using sample preparation methods described previously. The data were acquired using a Waters Acquity™ UPLC system equipped with a TQ (triple quadrupole) detector and a data system controlled by MassLynx 4.1 operating system (Waters, Milford, MA)
Lipoproteins in plasma were analyzed in the University of Cincinnati Mouse Metabolic Phenotype Center. Plasma samples were fractionated by FPLC using two Superose 6 columns linked in tandem as reported and 0.5 ml fractions were collected for analysis of TAG and cholesterol concentration as previously reported (Bu et al., 2009). Two samples from each treatment group were analyzed, and the average is presented for each group.
Gene expression and western blotting
RNA was isolated using available kits (Qiagen), and cDNA transcripts were prepared (Superscript Vilo) for quantitative real-time PCR (qPCR) (MyiQ, BioRad) using SYBR green. mRNA levels were adjusted for variances in L32 ribosomal protein mRNA abundance. Primer sequences for qPCR are shown in Table S1.
For western blotting, tissue lysates were prepared with T-Per (Pierce) supplemented with protease and phosphatase inhibitors (Pierce). Proteins were resolved by denaturing SDS/Laemmli gel electrophoresis with 4–15% gradient acrylamide gels. Membranes were blocked for 2 hr at room temperature with 5% milk in PBST, rinsed 3 times with and probed with antibodies against ATGL (tubulin, actin, HSP90, FoxO1, FoxO3 (from Cell Signaling), glucokinase or SREBP-1c (from Santa Cruz Biotechnology) at a 1:1000 dilution, or an antibody against G0S2 (provided by Jun Liu (Yang et al., 2010)) at a 1:500 dilution. Western blotting for apolipoprotein B was performed in the Cincinnati Mouse Metabolic Phenotyping Center using goat anti-rat apoB antibody diluted 1:4000 (Nauli et al., 2006).
Statistical analysis
Experiments were performed with 3–6 mice per group and mean and SEM are reported. Statistical significance (P<0.05) of differences between groups was determined by Student’s t test (2 groups) or analysis of variance (ANOVA) with post-hoc testing (least mean square) (≥ 3 groups).
Results
FoxO proteins regulate ATGL and G0S2 in liver
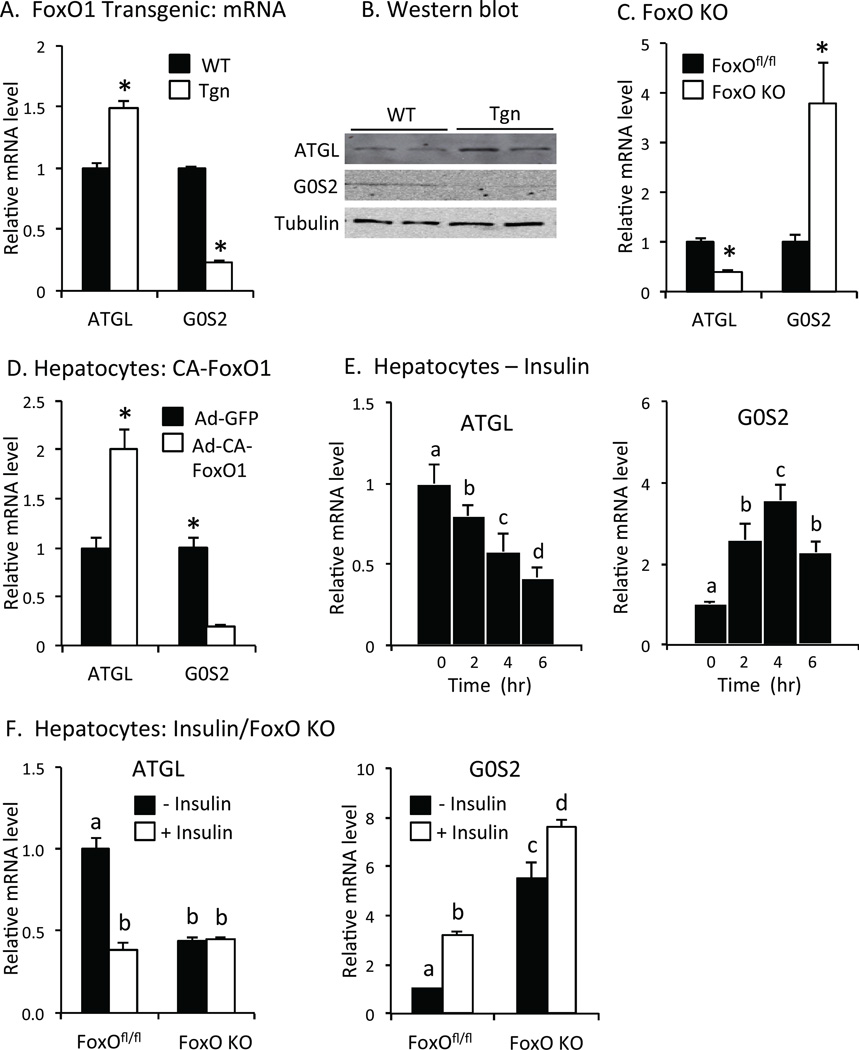
ATGL mRNA and protein levels were increased and G0S2 expression was decreased in FoxO1 transgenic mice (Tgn) expressing constitutively active FoxO1 (CA-FoxO1) in the liver compared to wild type (WT) littermates (Figs 1A and 1B). Conversely, ATGL expression was reduced and G0S2 expression was increased in mice lacking FoxO1, FoxO3 and FoxO4 expression in the liver (FoxO KO) compared to floxed controls (FoxOfl/fl) (Fig 1C), demonstrating that endogenous FoxO proteins promote ATGL and suppress G0S2 expression in the liver.
Fig. 1. ATGL and G0S2 gene expression.
A. FoxO1 transgenic mice. Relative ATGL and G0S2 mRNA levels in liver of wild type (WT, solid bar) and FoxO1 transgenic (Tgn, open bar) 6 hr after refeeding (n = 4–6). B. Western blot. ATGL, G0S2 and tubulin proteins in liver were measured by western blot in refed WT and Tgn mice. C. FoxO KO mice. Relative ATGL and G0S2 mRNA levels in FoxOfl/fl (solid bar) and FoxO KO (open bar) mice (n = 5). D. Effects of FoxO1 in hepatoctyes. Relative ATGL and G0S2 mRNA levels in primary hepatocytes from wild type mice transfected with adenovirus expressing constitutively active FoxO1 (Ad-CA-FoxO1, open bar) or green fluorescent protein (Ad-GFP, solid bar) (n = 4). E. Regulation by insulin. ATGL (left panel) and G0S2 (right panel) mRNA levels in primary hepatocytes treated with/without 100 nM insulin (n = 3). F. FoxO KO hepatocytes. ATGL (left) and G0S2 (right) mRNA levels were measured in hepatocytes isolated from FoxOfl/fl or FoxO KO mice following 4 hr treatment with (open bar) or without insulin (n = 3). Statistical significance (P<0.05) was determined by Student’s t test (*) or ANOVA, where bars with unlike letters differ. Mean + SEM.
Constitutively active FoxO1 (CA-FoxO1) stimulated ATGL and suppressed G0S2 expression in primary hepatocytes from wild type mice (Fig 1D), showing that FoxO1 promotes ATGL and inhibits G0S2 expression in a cell autonomous fashion. Conversely, insulin, which inhibits the effects of FoxO proteins on gene expression, reduced ATGL mRNA levels by ~60% and increased G0S2 expression ~4-fold in wild type hepatocytes (Fig 1E). ATGL expression was decreased in hepatocytes from FoxO KO vs. FoxOfl/fl mice, and there was no additional effect of insulin on ATGL mRNA levels in FoxO KO hepatocytes (Fig 1F, left panel). Conversely, the expression of G0S2 was increased ~6-fold in FoxO KO vs. FoxOfl/fl hepatocytes, and the ability of insulin to stimulate G0S2 expression was markedly reduced in hepatocytes from FoxO KO (~36% increase) vs. FoxOfl/fl (~3-fold increase) mice (Fig 1F, right panel). These results show that FoxO proteins regulate, and mediate effects of insulin, on ATGL and G0S2 expression in the liver.
ATGL-dependent effects of FoxO1 on TAG turnover and FAO in hepatocytes
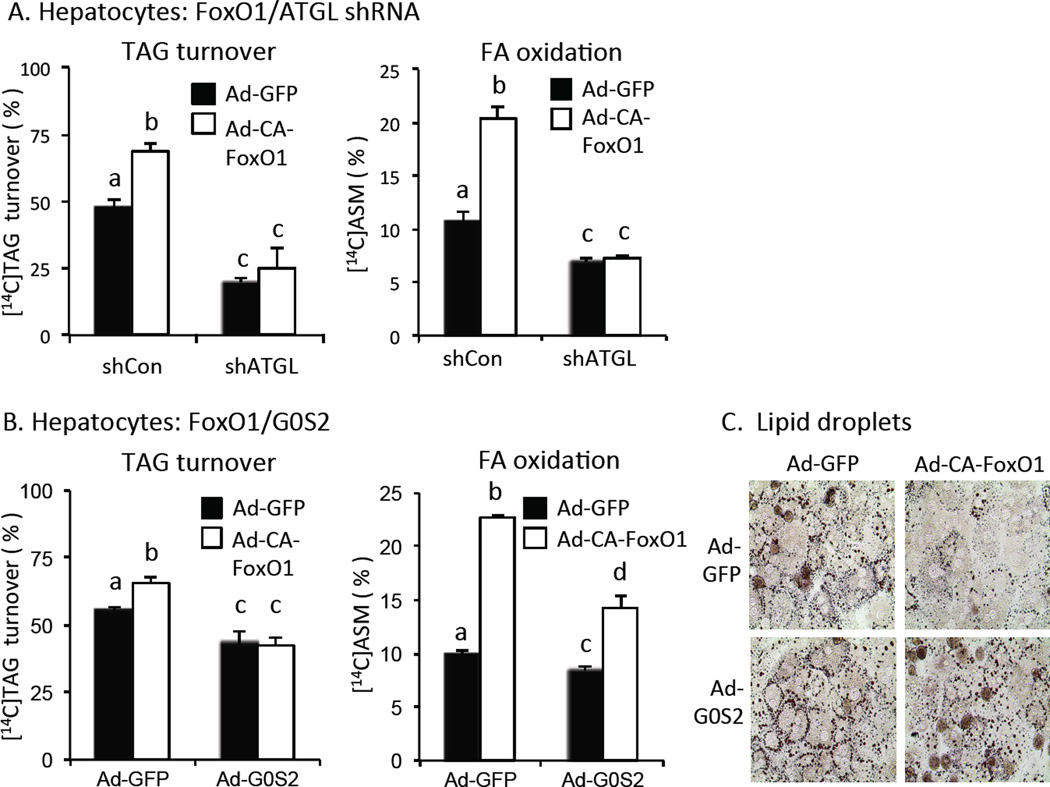
Pulse-chase studies showed that constitutively active FoxO1 (CA-FoxO1) stimulated [1-14C]oleate-labeled TAG turnover (Fig 2A, left panel) and the oxidation of hydrolyzed fatty acids (FAO) (Fig 2A, right panel) in isolated hepatoctyes. Knocking down ATGL reduced basal TAG turnover and FAO by 50–60% and disrupted the effects of CA-FoxO1. Co-expressing G0S2, which inhibits ATGL activity, also reduced basal TAG turnover and FAO, but not to the same extent as disrupting ATGL expression (Fig 2B). Rescuing G0S2 expression also disrupted the effect of FoxO1 on TAG turnover, and reduced the effect of FoxO1 on FAO by ~75% (Fig 2B). FoxO1 also promoted depletion of lipid droplets in hepatocytes and co-expression of G0S2 also blocked this effect (Fig 2C). These results show that FoxO1 promotes TAG turnover and FAO in liver cells in an ATGL-dependent fashion.
Fig. 2. TAG catabolism in hepatocytes.
A. ATGL-dependent effects of FoxO1. TAG turnover (left panel) and fatty acid oxidation (accumulation of acid soluble metabolites (ASM) in hepatocyte conditioned medium) (right panel) were measured in [1-14C]oleate-loaded hepatocytes following transfection adenovirus expressing control/scrambled shRNA or ATGL shRNA plus adenovirus expressing CA-FoxO1 (open bars) or GFP (solid bars) (n = 3). B. Co-expression of G0S2. TAG turnover (left panel) and fatty acid oxidation (right panel) in hepatocytes transfected with adenovirus ATGL shRNA or control/scrambled shRNA plus adenovirus expressing CA-FoxO1 (open bars) or GFP (solid bars) (n = 3). C. Lipid droplets. Hepatocytes expressing CA-FoxO1 or GFP plus G0S2 or GFP were stained with Oil red O to visualize lipid droplets. Statistical significance (P<0.05) was determined by ANOVA, where bars with unlike letters differ. Mean + SEM.
ATGL-dependent effects of FoxO1 on TAG catabolism and FAO in vivo
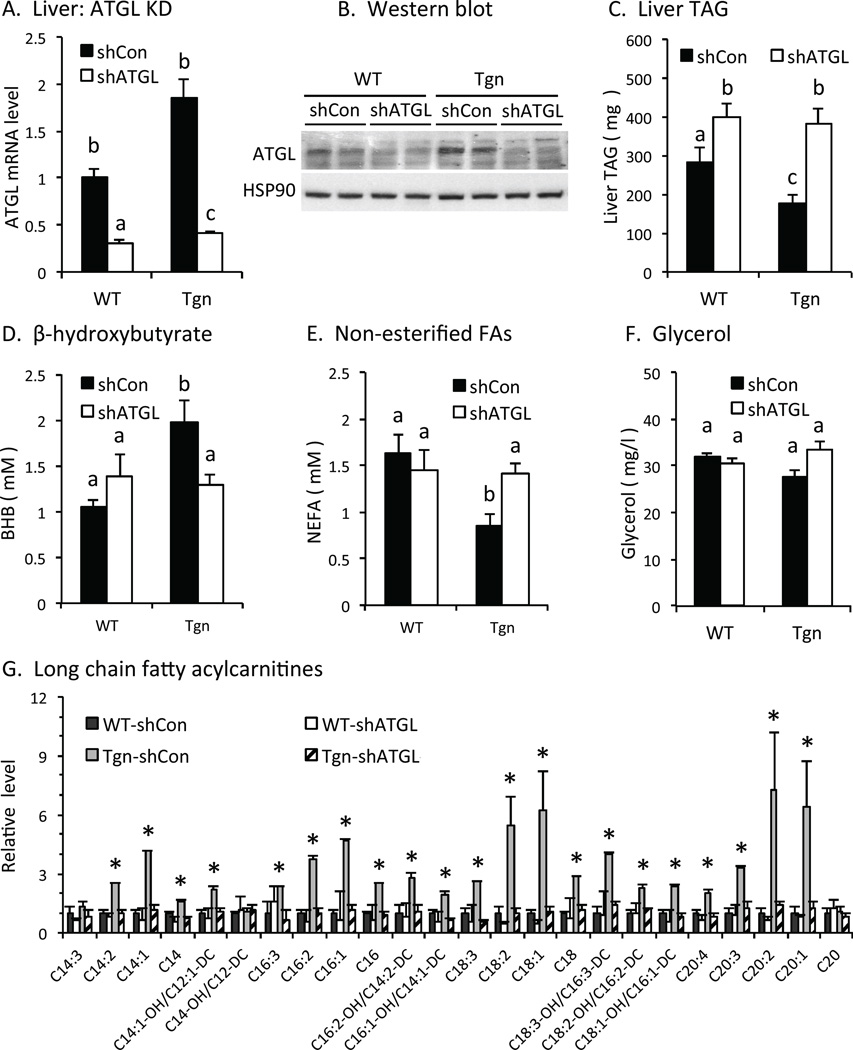
Treatment with adenovirus expressing ATGL-specific shRNA suppressed ATGL mRNA and protein levels in the liver of FoxO1 transgenic and wild type mice (Figs 3A, 3B), but not in adipose tissue (Fig S2), confirming that the ATGL knockdown (KD) is liver-specific, as expected. ATGL KD increased liver TAG content in WT mice (Fig 3C), consistent with previous studies (Ong et al., 2011; Ong et al., 2013). Liver TAG content was low in Tgn vs. WT mice and was restored to WT levels by ATGL KD (Fig 3C), indicating that ATGL-dependent lipolysis contributes to effects of FoxO1 on liver TAG content.
Fig. 3. TAG catabolism in liver.
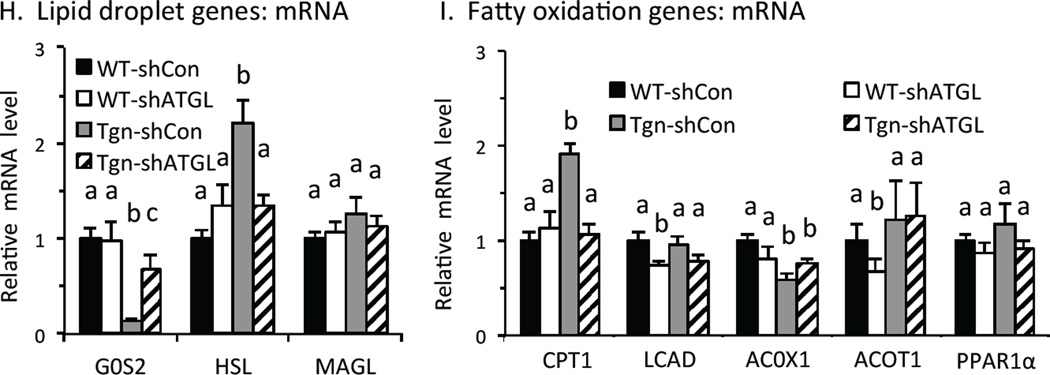
A. ATGL knockdown in vivo. ATGL mRNA levels were measured in liver in wildtype (WT) and transgenic (Tgn) mice 7 d after tail vein injection with adenovirus expressing control/scrambled shRNA (shCON, solid bars) or ATGL shRNA (shATGL, open bars) or (n = 4–5). B. Western blot. ATGL protein level in liver from WT and Tgn mice w/wo ATGL KD. C. Liver TAG. TAG in refed WT and Tgn mice 7 d after ATGL KD (n = 4–5). D-F. Plasma levels of β-hydroxybutyrate (D), non-esterified fatty acids (E) and glycerol (F). Plasma was collected from briefly (4-hr) fasted mice from WT and Tgn mice 5 days after treatment with control (solid bar) or shATGL (open bar) adenovirus. Plasma BHB levels in 4-hr fasted WT and Tgn mice 5 d post ATGL KD (n = 6–8). G. Fatty acylcarnitines. Long chain fatty acyl carnitines in liver from refed WT and Tgn mice 7 d after treatment with adenovirus expressing scrambled/control or ATGL shRNA (n = 4–5). H. Lipid droplet genes. Relative level of G0S2, hormone sensitive lipase (HSL) and monoacylglycerol lipase (MAGL) mRNA in WT and Tgn mice 7d post ATGL shRNA or control adenovirus treatment (n = 4–5). I. Fatty acid oxidation genes. Carnitine palmitoyltransferase-1 (CPT-1), very long chain acyl-CoA dehydrogenase (LCAD), acyl-CoA oxidase (ACOX1), acyl-CoA thioesterase1 (ACOT1) and PPARα mRNA levels 7 d post treatment with ATGL shRNA or control adenovirus are shown (n = 4–5). Statistical significance (P<0.05) was determined by ANOVA, where bars with unlike letters differ, or Student’s t test (*). Mean + SEM.
Plasma beta-hydroxybutyrate (BHB) levels were increased in briefly (4 hr) fasted Tgn vs. WT mice and knocking down ATGL reversed this effect (Fig 3D), indicating that FoxO1 promotes FAO in the liver in an ATGL-dependent fashion. Circulating levels of non-esterified fatty acids (NEFAs) were reduced in Tgn vs. WT mice and knocking down ATGL reversed this effect (Fig 3E), indicating that increased FAO in Tgn mice was not due to increased availability of circulating NEFAs. Glycerol levels were not altered in Tgn vs. WT mice (Fig 3F), suggesting that lipolysis in white adipose tissue (WAT) was not reduced and that decreased NEFAs may reflect increased re-esterification in WAT (Wolfe and Peters, 1987) and/or uptake and utilization by the liver in Tgn vs. WT mice. We also considered whether FAO is increased in Tgn mice 6 hr after refeeding, when endogenous FoxO proteins are inactive but the constitutively active FoxO1 expressed in Tgn mice remains active. Although levels of BHB levels are suppressed in both WT and Tgn refed mice (Zhang et al., 2006), long chain fatty acylcarnitine levels were increased in the liver of Tgn vs. WT, and knocking down ATGL disrupted this effect (Fig 3G). This result indicates that FAO also is increased in refed Tgn vs. WT mice in an ATGL-dependent fashion, and that suppressing function is required to fully suppress intrahepatic lipolysis and FAO in the refed state.
We also examined the expression of other genes related to TAG turnover and FAO. The expression of G0S2 was suppressed in refed Tgn vs. WT mice and was partially restored to WT levels by ATGL KD in Tgn mice (Fig 3H). This result indicates that FoxO1 suppresses G0S2 expression through both ATGL-dependent and – independent mechanisms. Conversely, expression of hormone sensitive lipase, which catabolizes diacyglycerols, was increased in Tgn vs. WT mice and ATGL KD also reversed this effect. These results indicate that, in addition to its direct effect as a lipase on TAG turnover, ATGL also promotes intrahepatic lipolysis by altering G0S2 and HSL expression downstream from FoxO1.
The expression of carnitine palmitoyl transferase 1 (CPT1), which promotes translocation of fatty acylCoAs into mitochondria, was increased 2-fold in Tgn vs. WT mice, and this effect was reversed by ATGL KD (Fig 3I). Although fatty acid activation of peroxisome proliferator receptor-α (PPARα) can promote the expression of CPT1, the expression of other PPARα-regulated genes involved in promoting FAO was not increased in Tgn vs. WT mice (Fig 3I), including very long chain acyl dehydrogenase (LCAD), acyl-CoA oxidase 1 (ACOX1) and acyl-CoA thioesterase 1 (ACOT1). This suggests that other mechanisms may mediate effects of FoxO1 and ATGL on CPT1 expression.
Together, these results indicate that ATGL promotes FAO in the liver downstream from FoxO proteins by multiple mechanisms, including direct effects on TAG turnover and effects on gene expression.
ATGL-dependent and –independent effects on lipid levels and lipogenesis
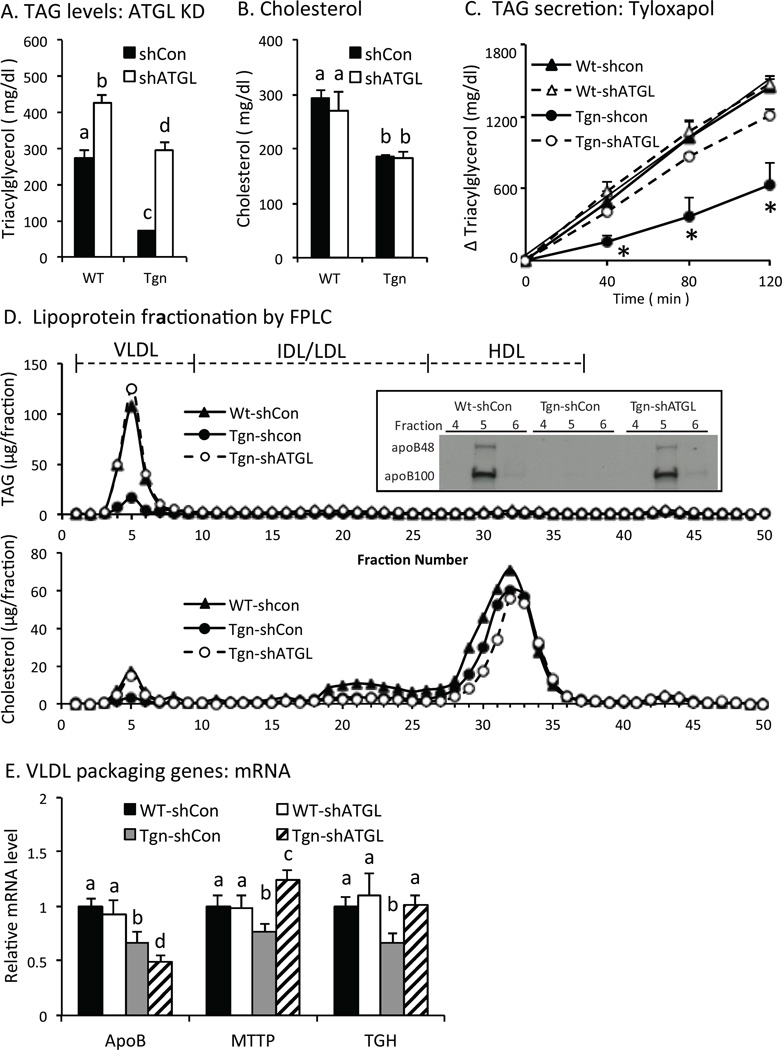
We also examined circulating TAG levels in Tgn and WT mice 6 hr after refeeding, when de novo lipogenesis is stimulated in the liver. Previous studies indicate that FoxO proteins suppress de novo lipogenesis in the liver and circulating TAG levels (Haeusler et al., 2014; Zhang et al., 2012; Zhang et al., 2006). As shown in Fig 4A, TAG levels are reduced in Tgn vs. WT mice treated with control adenovirus, and ATGL KD increased TAG levels in Tgn mice ~3-fold increase, indicating that ATGL contributes to effects of FoxO1 on TAG levels. At the same time, ATGL KD also increased TAG levels in WT mice by ~40% (Fig 4A), so that TAG levels were still higher in WT vs. Tgn mice after ATGL KD (Fig 4A). Similarly, adenoviral expression of G0S2 also improved TAG levels in Tgn mice by ~3-fold and increased TAG levels by ~30% in WT mice (Fig S3A). These results indicate that ATGL-dependent lipolysis contributes to effects of FoxO on TAG levels, and that FoxO suppresses TAG levels by both ATGL-dependent and – independent mechanisms. Cholesterol levels also were low in Tgn vs. WT, but were not affected by ATGL KD (Fig 4B) or G0S2 expression (Fig S3B), indicating the effect of ATGL was specific for TAGs.
Fig. 4. Lipid levels and lipogenesis.
A. Serum TAG levels in refed male WT and Tgn mice w/wo ATGL knock down (KD) (n = 4–5). B. Cholesterol levels in refed mice w/wo ATGL KD (n = 4–5). C. Hepatic TAG secretion. WT and Tgn mice w/wo ATGL KD were treated with Tyloxapol 4 hr after refeeding. Plasma TAG level was determined 0, 40, 80 and 120 min after Tyloxapol treatment, and change in TAG level is shown. (n = 4) D. Lipoprotein fractionation. Plasma from refed WT and Tgn mice w/wo ATGL KD was fractionated by FPLC and TAG (upper panel) and cholesterol (lower panel) content was determined in FPLC fractions. Average values are shown for two samples from each group. Insert. Western blot of apolipoprotein B in FPLC fractions 4–6 is shown. E. VLDL packaging genes. Liver apoB, microsomal triglyceride transfer protein (MTTP), and triacylglycerol hydrolase (TGH) mRNA levels in refed mice 7 d post ATGL KD are shown (n = 4–5). F. Lipogenic genes. Liver SREBP-1c, ATP citrate lyase, acetyl-CoA carboxylase, fatty acid synthase, stearoyl-CoA desaturase-1, and glycerol-3-phosphate acyltransferase-1 mRNA levels in refed mice are shown (n = 4–5). G. Glycolytic genes. Liver mRNA levels for Gck and pyruvate kinase in refed mice (n = 4–5). H. Western blot. Liver SREBP-1c, Gck and tubulin protein levels in refed mice. I-K. Lipogenesis. Plasma TAG levels (I), and malonyl-CoA in liver (J) were measured in refed female WT and Tgn mice 7 d after treatment with adenovirus expressing ATGL shRNA or control/scrambled shRNA. Deuterated water was administered 2 hr after refeeding and tissue was harvested 4 hr later for determination of newly synthesized palmitate in liver (K) (n = 4–5). Statistical significance (P<0.05) was determined by ANOVA, where bars with unlike letters differ. Mean + SEM.
Studies with Tyloxapol, which inhibits endovascular lipolysis of TAG, showed that the appearance of newly secreted TAG was reduced in Tgn vs. WT mice and was restored by ATGL KD (Fig 4C). Fractionation of plasma lipoproteins in refed mice revealed that VLDL TAG content was decreased in Tgn vs. Wt mice and restored by ATGL KD (Fig 4D, upper panel), and western blotting showed that VLDL apolipoprotein B (apoB) levels also were reduced in Tgn vs. WT mice and restored by ATGL KD (insert). Since each VLDL particle contains one apoB molecule, this result indicates that changes in TAG levels in Tgn mice reflect ATGL-dependent effects on hepatic secretion of VLDL particles. VLDL (but not HDL) cholesterol content also was reduced in Tgn vs. WT mice, and restored by knocking down ATGL (Fig 4D, lower panel), consistent with changes in VLDL particle number. These results indicate that suppressing FoxO1 and ATGL function is important in promoting hepatic VLDL production in the postprandial state.
Liver apoB mRNA levels were modestly reduced in Tgn vs. WT mice (Fig 4E), but remained low after ATGL KD (Fig 4E), indicating that changes in apoB expression were not responsible for the recovery of VLDL production in Tgn mice treated with ATGL shRNA. In contrast, expression of microsomal TAG transfer protein (MTTP), which promotes the packaging of TAG in VLDL, and triacyglycerol hydrolase (TGH)/ carboxylesterase 3, which is required for VLDL secretion (Lian et al., 2012), were reduced in Tgn vs. WT mice and restored by ATGL KD. These results indicate that changes in MTTP and TGH expression may contribute to improved VLDL secretion and TAG levels after ATGL is knocked down in Tgn mice.
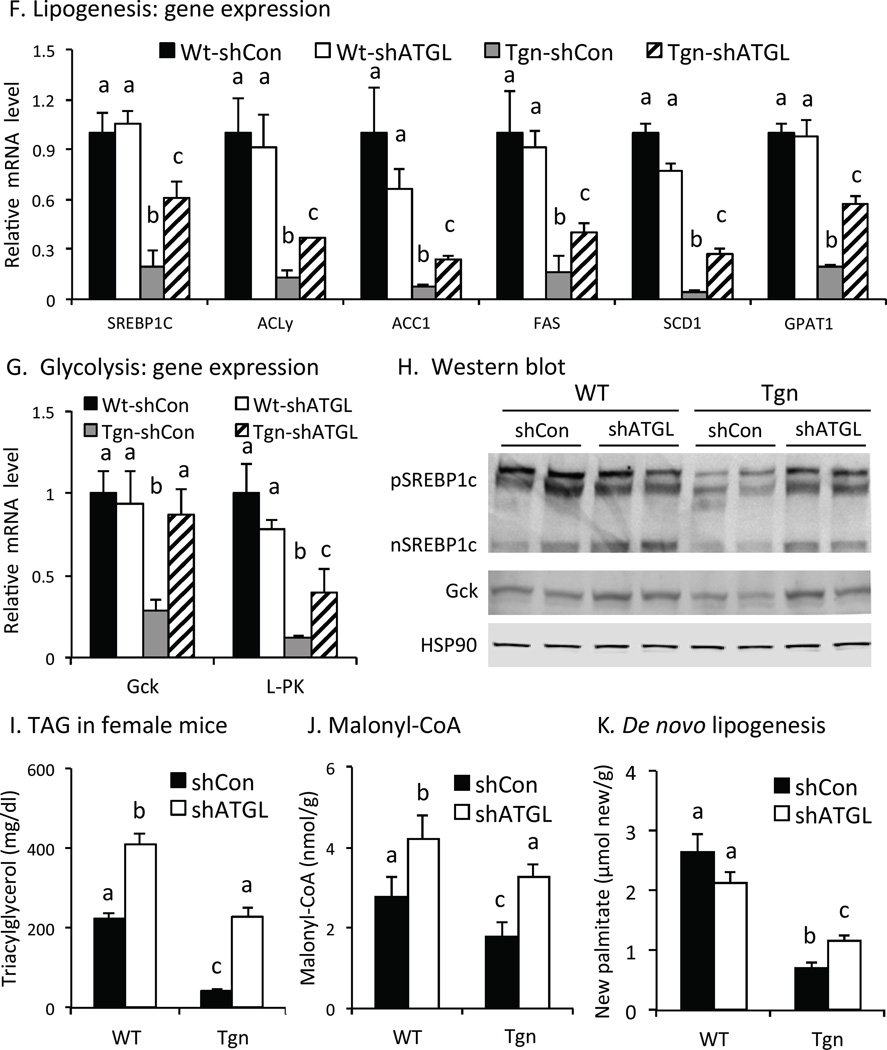
Previous studies have shown that FoxO proteins can suppress de novo lipogenesis by reducing glycolytic and/or lipogenic gene expression and metabolism in the liver (Haeusler et al., 2014; Zhang et al., 2006). As shown in Fig 4F, the expression of sterol response element binding protein-1c, a major regulator of lipogenic gene expression, and several of its downstream target genes (including ATP citrate lyase, acetylCoA carboxylase-1, fatty acid synthase, stearoylCoA desaturase-1 and glycerol-3 phosphate acyltransferase-1) were suppressed in Tgn mice and partially restored by ATGL KD. Glucokinase (Gck) and pyruvate kinase (L-PK) expression also were suppressed in Tgn vs. WT mice and largely restored by ATGL KD (Fig 4G). Western blotting confirmed that SREBP-1c and Gck protein levels also were restored in Tgn mice by ATGL KD (Fig 4H). Expression of G0S2 also restored Gck and improved lipogenic gene expression in Tgn mice (Fig S3C), supporting the concept that ATGL-dependent lipolysis contributes to the regulation of glycolytic and lipogenic gene expression by FoxO proteins.
In a separate study, we examined effects on TAG levels and de novo lipogenesis in female mice. TAG levels were decreased in refed female Tgn vs. WT mice and were partially restored to WT levels by ATGL KD (Fig 4I), similar to males (above). Liver levels of malonyl-CoA levels were decreased in refed Tgn vs. WT mice, and were increased by ATGL KD (Fig 4J), consistent with increased glycolytic and lipogenic metabolism. Studies with deuterated water showed that newly synthesized palmitate in liver also was reduced in refed Tgn vs. WT liver, consistent with previous studies (Zhang et al., 2006), and was improved by knocking down ATGL (Fig 4K). The increase in newly synthesized may be underestimated in Tgn mice, since hepatic secretion VLDL also is markedly improved ATGL KD in Tgn mice (above). These results indicate the concept that ATGL contributes to the suppression of de novo lipogenesis by FoxO1. At the same time, since ATGL KD does not fully restore levels of TAG, malonyl-CoA, or newly synthesized palmitate, both ATGL-dependent and –independent effects appear to contribute to effects of FoxO1 on TAG levels and de novo lipogenesis.
ATGL-dependent and –independent effects of FoxO1 on glucose homeostasis
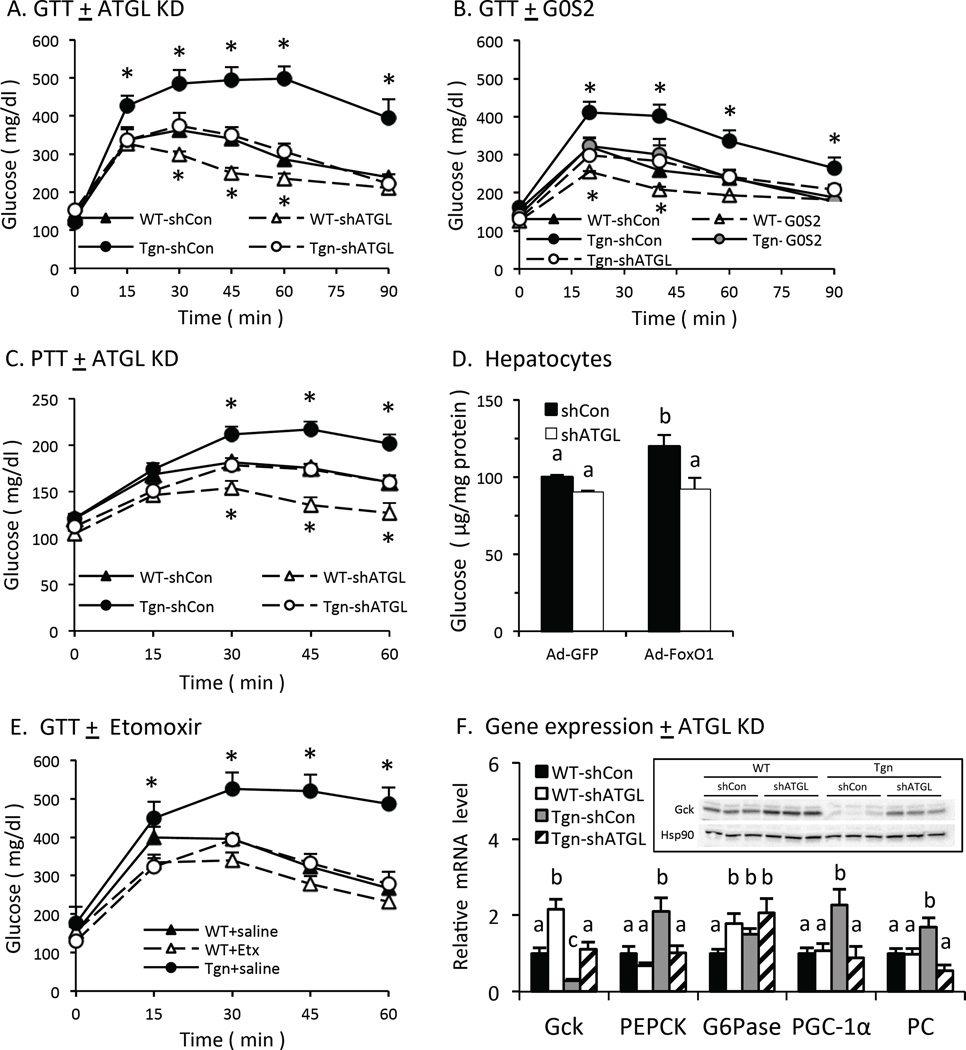
We also asked if ATGL contributes to FoxO effects on glucose homeostasis. Glucose tolerance was impaired in 4-hr fasted Tgn vs. WT mice and knocking down ATGL restored glucose tolerance in Tgn mice (Fig 5A), indicating that ATGL-dependent lipolysis contributes to effects of FoxO1 on glucose homeostasis. At the same time, ATGL KD also improved glucose tolerance in WT mice, consistent with previous reports (Ong et al., 2013), so that glucose tolerance remained significantly different in Tgn vs. WT mice after ATGL KD (Fig 5A). This result indicates that ATGL-independent mechanisms also contribute to the effects of FoxO1 on glucose tolerance. Adenoviral expression G0S2 in the liver of female Tgn and WT mice yielded similar results (Fig 5B), supporting the concept that ATGL-dependent lipolysis contributes to effects of FoxO1 on glucose homeostasis and that FoxO1 impairs glucose tolerance by both ATGL-dependent and –independent mechanisms.
Fig. 5. Glucose tolerance and glucose production.
A. Glucose tolerance:ATGL KD. Glucose tolerance tests were performed in 4-hr fasted WT and Tgn male mice 5 d after treatment with adenovirus expressing ATGL shRNA or control shRNA (n = 4–5). B. Glucose tolerance:G0S2 expression. GTTs were performed in 4-hr fasted WT and Tgn female mice 5 d after treatment with adenovirus expressing G0S2 or GFP (control) (n = 4–5). C. Pyruvate tolerance. Pyruvate tolerance tests were performed in 4-hr fasted WT and Tgn mice 5 d post ATGL KD (n = 4–5). D. Hepatocytes. Production of glucose from pyruvate and lactate was measured in hepatocytes co-transfected with adenovirus expressing CA-FoxO1 or GFP, plus adenovirus expressing ATGL shRNA (open bars) or control shRNA (solid bars) (n = 3–4). E. Etomoxir. GTTs in 4-hr fasted WT and Tgn mice 30 min after treatment with etomoxir or PBS (n = 4–6). F. Gene expression. PEPCK, G6Pase, PGC-1α and PC mRNA levels in liver from refed mice 5 d after treatment with adenovirus expression ATGL shRNA or control shRNA (n = 4). Statistical significance (P<0.05) was determined by Student’s t test (*) or ANOVA, where bars with unlike letters differ. Mean + SEM.
Pyruvate tolerance also was impaired in Tgn vs. WT mice and restored to normal by knocking down ATGL (Fig 5C), indicating that gluconeogenesis is increased in FoxO1 transgenic mice and that ATGL contributes to this effect. FoxO1 also increased glucose production from pyruvate in isolated hepatocytes and knocking down ATGL disrupted this effect (Fig 5D), supporting the concept that ATGL contributes to the ability of FoxO1 to promote hepatic glucose production in a cell autonomous fashion.
Since ATGL promotes FAO downstream from FoxO1 and FAO promotes gluconeogenesis in the liver (Lewis et al., 1997; Perry et al., 2015), we also asked whether FAO is required for FoxO1 to promote glucose intolerance in transgenic mice. Treatment with etomoxir (3 mg/kg), which is sufficient to suppress FAO in the liver (Satapati et al., 2012) and BHB levels in briefly fasted FoxO1 transgenic mice (Fig S4A), restored normal glucose tolerance in FoxO1 transgenic mice (Fig 5E), indicating that FAO is required for effects of FoxO1 on glucose tolerance.
We also asked whether ATGL contributes to effects of FoxO proteins on glycolytic and/or gluconeogenic gene expression in briefly fasted mice (Fig 5F). Glucokinase (Gck) mRNA and protein levels are suppressed in FoxO1 Tgn mice and restored by ATGL KD (Fig 5F). At the same time, ATGL KD increased Gck expression in WT mice so that Gck mRNA levels were still lower in Tgn vs. WT mice when ATGL expression is suppressed (Fig 5F). These results indicate that FoxO1 suppresses Gck expression by both ATGL-dependent and –independent mechanisms. Conversely, the expression of several genes which promote gluconeogenesis (GNG), including phosphoenolpyruvate carboxykinase (PEPCK), peroxisome proliferator receptor gamma coactivator-1α (PGC-1α), and pyruvate carboxylase (PC), was increased in Tgn vs. WT mice and normalized in an ATGL-dependent fashion (Fig 5F). The expression of glucose-6 phosphatase (G6pase) also was increased in Tgn vs. WT, but was not dependent on ATGL.
Adenoviral expression of G0S2 also restored the expression of Gck and suppressed PEPCK expression in Tgn mice (Figs S3C–D), supporting the concept that that ATGL-dependent lipolysis contributes to effects of FoxO1 on glycolytic and gluconeogenic gene expression. In contrast, inhibition of FAO with etomoxir failed to disrupt effects of FoxO1 on glycolytic (Gck, PK), lipogenic (SREBP-1c, SCD-1) or gluconeogenic (PEPCK) gene expression in Tgn mice (Fig S4E), indicating that FAO is not required for these ATGL-dependent effects of FoxO1 on gene expression.
Discussion
The results of this study provide several novel insights regarding the role of ATGL and its inhibitor, G0S2, in mediating effects of FoxO proteins on gene expression and metabolism in the liver. Key findings include: 1) FoxO proteins regulate and mediate effects of insulin on ATGL and G0S2 expression in the liver in a cell autonomous fashion; 2) FoxO proteins promote intrahepatic TAG catabolism and FAO in an ATGL-dependent fashion; and 3) ATGL-dependent FAO contributes to effects of FoxO proteins on glucose homeostasis; and 4) ATGL-dependent lipolysis also contributes to effects of FoxO proteins on glycolytic, lipogenic and gluconeogenic gene expression and metabolism. Together, these findings reveal that ATGL-dependent lipolysis plays an important role in mediating effects of FoxO proteins on multiple aspects of glucose and lipid metabolism in the liver.
We found that FoxO proteins stimulate ATGL expression in the liver and are required for insulin to regulate ATGL in hepatocytes. FoxO1 directly targets the ATGL promoter and promotes ATGL expression in adipose tissue (Chakrabarti and Kandror, 2009). While other pathways also appear to contribute to the ability of insulin to regulate of ATGL in adipose tissue (Chakrabarti et al., 2013), our results indicate that FoxO proteins play a major role in regulating and mediating effects of insulin on ATGL expression in the liver.
We also found that FoxO proteins suppress the expression of G0S2 and play an important role in mediating effects of insulin on G0S2 expression in the liver. Insulin stimulates G0S2 expression in adipose tissue (Yang et al., 2010), yet the mechanism mediating this effect has not been identified. Our data indicates that insulin stimulates the expression of G0S2, at least in part, by disrupting negative effects of FoxO proteins on G0S2 expression in the liver, and possibly other tissues. Interestingly, knocking down ATGL partially restored the expression of G0S2 in FoxO1 transgenic mice to WT levels, indicating that ATGL-dependent mechanisms contribute to the effect of FoxO proteins on G0S2 expression and that ATGL promotes its own activity by suppressing the expression of its inhibitor, G0S2.
ATGL-dependent lipolysis also may promote effects of FoxO proteins on TAG catabolism in the liver by other mechanisms. For example, promoting the expression of HSL, an important diacylglycerol lipase, would enhance intrahepatic lipolysis, and increasing the expression of CPT1, which is required for translocation of long chain fatty acyl-CoAs into the mitochondria, would enhance β-oxidation. Suppression of stearoyl CoA desaturase-1 and glycerol-3-phosphate acyltransferase would help to ensure that free fatty acids derived from either extrahepatic sources or intrahepatic lipolysis would be partitioned towards FAO rather than storage as TAGs in lipid droplets. Further, suppressing glycolytic and lipogenic gene expression, and lowering levels of malonyl-CoA, an important inhibitor of CPT1, also would contribute to increased FAO. Together, these effects would contribute to the ability of FoxO1 and ATGL to promote TAG turnover and FAO in the liver.
Our results also indicate that ATGL-dependent lipolysis and FAO play an important role in mediating effects of FoxO proteins on glucose homeostasis. Recent studies indicate that ATGL influences hepatic glucose metabolism (Brown et al., 2010; Ong et al., 2013; Zhang et al., 2014). Our results show that ATGL-dependent effects contribute to the regulation of hepatic glucose metabolism by multiple mechanisms. We found that FoxO1 promotes hepatic TAG turnover and FAO in an ATGL-dependent manner, and studies with etomoxir showed that FAO is important in mediating effects of FoxO1 on glucose homeostasis. FAO promotes gluconeogenesis by providing ATP and reducing equivalents required for glucose production, and acetyl CoA, which increases the activity of pyruvate carboxylase (Freedman and Kohn, 1964; Perry et al., 2015; Williamson et al., 1966). Conversely, FAO suppresses glycolysis at multiple levels, including the activity of pyruvate dehydrogenase (Freedman and Kohn, 1964; Garland and Randle, 1964; Hue and Taegtmeyer, 2009). Thus, FoxO proteins may limit glycolysis and the diversion of pyruvate into other pathways and promote hepatic glucose production, at least in part, by promoting FAO.
We also found that ATGL-dependent lipolysis plays an important role in mediating effects of FoxO1 on gene expression related to glycolytic/lipogenic and gluconeogenic metabolism, including the expression of glucokinase (Gck) and PEPCK. Early studies showed that FoxO1 suppresses glucokinase expression (Zhang et al., 2006), and recent reports indicate that glucokinase plays an important role in mediating effects of FoxO proteins on glycolytic and lipogenic metabolism and glucose tolerance (Haeusler et al., 2014; Xiong et al., 2013; Zhang et al., 2012; Zhang et al., 2006). Previous studies indicate that FoxO1 may suppress glucokinase expression by interacting with other trans-acting factors (Ganjam et al., 2009; Hirota et al., 2003; Hirota et al., 2008), and stimulate promote PEPCK expression through direct interaction with a cis-acting element located in the PEPCK promoter (Hall et al., 2000; Yeagley et al., 2001). Our results indicate that ATGL-dependent lipolysis also plays an important role in mediating effects of FoxO1 on hepatic gene expression, including the expression of glucokinase and PEPCK, and glucose utilization and production in the liver.
ATGL-dependent lipolysis can exert effects on gene expression via activation of PPARα by fatty acids (Haemmerle et al., 2011; Sapiro et al., 2009), and PPARα may contribute to some effects of FoxO1 on gene expression, including the expression of CPT1. However, the expression of several PPARα-regulated genes involved in promoting FAO (LCAD, ACOX1 and ACOT1) was not increased in FoxO1 transgenic mice. Also, the expression of G0S2, which is induced by fatty acids and PPARα (Jaeger et al., 2015; Zandbergen et al., 2005), was strongly suppressed in FoxO1 transgenic mice and partially restored by knocking down ATGL. These findings indicate that other mechanisms must be involved in mediating ATGL-dependent effects of FoxO1 on gene expression in the liver. Additional studies are needed to examine the role that other intracellular pathways emanating from the lipid droplet (Khan et al., 2015; Mullins et al., 2014; Tang et al., 2013) may play in mediating effects of FoxO proteins on hepatic gene expression and metabolism.
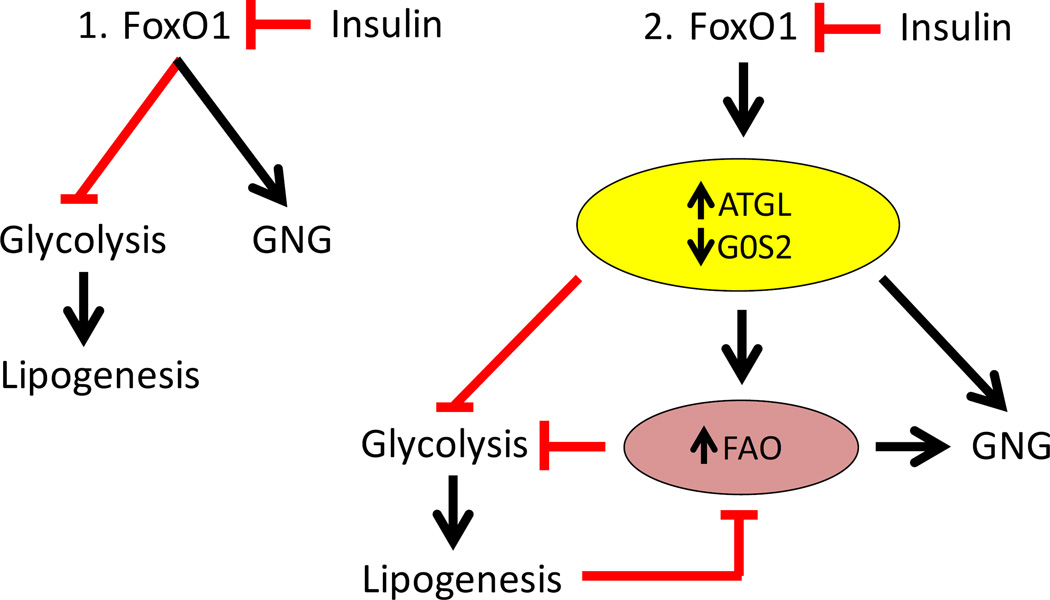
Fig 6 summarizes these findings. As indicated in panel 1, previous studies indicate that FoxO proteins can suppress glycolysis/lipogenesis and promote gluconeogenesis metabolism through direct effects on gene expression. As shown in panel 2, our results indicate that FoxO proteins also increase ATGL and decrease G0S2 expression, and thereby enhance ATGL-dependent lipolysis and FAO in the liver. FAO promotes gluconeogenesis (GNG) and suppresses glycolysis. ATGL-dependent lipolysis also contributes to negative effects of FoxO on glycolytic/lipogenic gene expression and metabolism, and reduced levels of malonyl-CoA, which, in combination with increased availability of free fatty acids, promotes increased FAO. Further, ATGL-dependent lipolysis also contributes to effects of FoxO1 on gluconeogenic gene expression. Insulin suppresses the function of FoxO proteins, including effects on ATGL-dependent lipolysis and FAO, which serves to increases glycolysis and lipogenesis, and decreases gluconeogenesis.
Fig. 6. Integrated regulation of glucose and lipid metabolism by FoxO proteins and ATGL-dependent TAG hydrolysis.
1. Previous studies have shown that FoxO proteins suppress glycolytic/lipogenic metabolism and promote gluconeogenic metabolism in the liver, and that insulin disrupts this effect of FoxO proteins. 2. FoxO proteins promote ATGL and suppress G0S2 expression, and promote intrahepatic lipolysis and FAO in an ATGL-dependent fashion. In addition to direct effects of FoxO proteins on gene expression, ATGL-dependent mechanisms also contribute to effects of FoxO proteins on reduced glycolytic/lipogenic and increased gluconeogenic gene expression and metabolism. Increased FAO promotes gluconeogenesis (GNG) and suppresses glycolytic/lipogenic metabolism downstream from FoxO1 and ATGL. At the same time, reduced glycolysis and synthesis of malonyl-CoA may promote FAO.
In summary, these studies demonstrate that FoxO proteins regulate ATGL and G0S2 expression in the liver, and reveal a novel role for ATGL-dependent lipolysis in mediating effects of FoxO proteins on glycolytic, gluconeogenic and lipogenic gene expression and metabolism in the liver. Since FoxO proteins are major targets of insulin action, these findings indicate that regulation of intrahepatic lipolysis also may be important in mediating effects of insulin on multiple aspects of glucose and lipid metabolism in the liver, and suggest that targeting ATGL-dependent lipolysis and its downstream effectors may provide an effective strategy for improving the treatment of diabetes and hepatic insulin resistance.
Supplementary Material
Acknowledgments
This research was supported in part by grants from the Department of Veterans Affairs Merit Review Program (TGU) and NIH grants DK58398 (CBN), DK085008 (DGM), DK059630 (University of Cincinnati Mouse Metabolic Phenotyping Center), UL1TR000439 (CASE MMPC) and DK050456 (Minnesota Obesity Center). The authors thank Dr. Jacob Bartana for helpful comments and discussion, and Dr. Michelle Pucholwicz for guidance in planning and analysis of studies related to lipogenesis.
Footnotes
Author Contributions
WZ, SYB, MTM, SAK and OI performed experiments. IO-S developed mouse models. ZS contributed to data analysis. CN contributed to data analysis and interpretation. DM and TU designed studies and wrote the manuscript.
References
- An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, Koves TR, Stevens R, Millington D, Newgard CB. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nature medicine. 2004;10:268–274. doi: 10.1038/nm995. [DOI] [PubMed] [Google Scholar]
- Brown JM, Betters JL, Lord C, Ma Y, Han X, Yang K, Alger HM, Melchior J, Sawyer J, Shah R, et al. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J Lipid Res. 2010;51:3306–3315. doi: 10.1194/jlr.M010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Bu SY, Mashek MT, Mashek DG. Suppression of long chain acyl-CoA synthetase 3 decreases hepatic de novo fatty acid synthesis through decreased transcriptional activity. The Journal of biological chemistry. 2009;284:30474–30483. doi: 10.1074/jbc.M109.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti P, Kandror KV. FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. The Journal of biological chemistry. 2009;284:13296–13300. doi: 10.1074/jbc.C800241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti P, Kim JY, Singh M, Shin YK, Kim J, Kumbrink J, Wu Y, Lee MJ, Kirsch KH, Fried SK, et al. Insulin inhibits lipolysis in adipocytes via the evolutionarily conserved mTORC1-Egr1-ATGL-mediated pathway. Molecular and cellular biology. 2013;33:3659–3666. doi: 10.1128/MCB.01584-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Mashek DG. Mammalian triacylglycerol metabolism: synthesis, lipolysis, and signaling. Chemical reviews. 2011;111:6359–6386. doi: 10.1021/cr100404w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell metabolism. 2008;8:65–76. doi: 10.1016/j.cmet.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman AD, Kohn L. PYRUVATE METABOLISM AND CONTROL: FACTORS AFFECTING PYRUVIC CARBOXYLASE ACTIVITY. Science. 1964;145:58–60. doi: 10.1126/science.145.3627.58. [DOI] [PubMed] [Google Scholar]
- Ganjam GK, Dimova EY, Unterman TG, Kietzmann T. FoxO1 and HNF-4 are involved in regulation of hepatic glucokinase gene expression by resveratrol. The Journal of biological chemistry. 2009;284:30783–30797. doi: 10.1074/jbc.M109.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland PB, Randle PJ. Control of pyruvate dehydrogenase in the perfused rat heart by the intracellular concentration of acetyl-coenzyme A. The Biochemical journal. 1964;91:6c–7c. [PubMed] [Google Scholar]
- Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nature medicine. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler RA, Hartil K, Vaitheesvaran B, Arrieta-Cruz I, Knight CM, Cook JR, Kammoun HL, Febbraio MA, Gutierrez-Juarez R, Kurland IJ, et al. Integrated control of hepatic lipogenesis versus glucose production requires FoxO transcription factors. Nature communications. 2014;5:5190. doi: 10.1038/ncomms6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RK, Yamasaki T, Kucera T, Waltner-Law M, O’Brien R, Granner DK. Regulation of phosphoenolpyruvate carboxykinase and insulin-like growth factor-binding protein-1 gene expression by insulin. The role of winged helix/forkhead proteins. The Journal of biological chemistry. 2000;275:30169–30175. doi: 10.1074/jbc.M004898200. [DOI] [PubMed] [Google Scholar]
- Heckmann BL, Zhang X, Xie X, Liu J. The G0/G1 switch gene 2 (G0S2): regulating metabolism and beyond. Biochimica et biophysica acta. 2013;1831:276–281. doi: 10.1016/j.bbalip.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Daitoku H, Matsuzaki H, Araya N, Yamagata K, Asada S, Sugaya T, Fukamizu A. Hepatocyte nuclear factor-4 is a novel downstream target of insulin via FKHR as a signal-regulated transcriptional inhibitor. The Journal of biological chemistry. 2003;278:13056–13060. doi: 10.1074/jbc.C200553200. [DOI] [PubMed] [Google Scholar]
- Hirota K, Sakamaki J, Ishida J, Shimamoto Y, Nishihara S, Kodama N, Ohta K, Yamamoto M, Tanimoto K, Fukamizu A. A combination of HNF-4 and Foxo1 is required for reciprocal transcriptional regulation of glucokinase and glucose-6-phosphatase genes in response to fasting and feeding. The Journal of biological chemistry. 2008;283:32432–32441. doi: 10.1074/jbc.M806179200. [DOI] [PubMed] [Google Scholar]
- Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. American journal of physiology. Endocrinology and metabolism. 2009;297:E578–E591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger D, Schoiswohl G, Hofer P, Schreiber R, Schweiger M, Eichmann TO, Pollak NM, Poecher N, Grabner GF, Zierler KA, et al. Fasting-induced G0/G1 switch gene 2 and FGF21 expression in the liver are under regulation of adipose tissue derived fatty acids. Journal of hepatology. 2015;63:437–445. doi: 10.1016/j.jhep.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Sathyanarayan A, Mashek MT, Ong KT, Wollaston-Hayden EE, Mashek DG. ATGL-catalyzed lipolysis regulates SIRT1 to control PGC-1alpha/PPAR-alpha signaling. Diabetes. 2015;64:418–426. doi: 10.2337/db14-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienesberger PC, Lee D, Pulinilkunnil T, Brenner DS, Cai L, Magnes C, Koefeler HC, Streith IE, Rechberger GN, Haemmerle G, et al. Adipose triglyceride lipase deficiency causes tissue-specific changes in insulin signaling. The Journal of biological chemistry. 2009;284:30218–30229. doi: 10.1074/jbc.M109.047787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GF, Vranic M, Harley P, Giacca A. Fatty acids mediate the acute extrahepatic effects of insulin on hepatic glucose production in humans. Diabetes. 1997;46:1111–1119. doi: 10.2337/diab.46.7.1111. [DOI] [PubMed] [Google Scholar]
- Lian J, Wei E, Wang SP, Quiroga AD, Li L, Di Pardo A, van der Veen J, Sipione S, Mitchell GA, Lehner R. Liver specific inactivation of carboxylesterase 3/triacylglycerol hydrolase decreases blood lipids without causing severe steatosis in mice. Hepatology (Baltimore, Md.) 2012;56:2154–2162. doi: 10.1002/hep.25881. [DOI] [PubMed] [Google Scholar]
- Mullins GR, Wang L, Raje V, Sherwood SG, Grande RC, Boroda S, Eaton JM, Blancquaert S, Roger PP, Leitinger N, et al. Catecholamine-induced lipolysis causes mTOR complex dissociation and inhibits glucose uptake in adipocytes. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:17450–17455. doi: 10.1073/pnas.1410530111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, Vonlehmden SB, Lee D, Jandacek RJ, Abumrad NA, Tso P. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 2006;131:1197–1207. doi: 10.1053/j.gastro.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O-Sullivan I, Zhang W, Wasserman DH, Liew CW, Liu J, Paik J, DePinho RA, Stolz DB, Kahn CR, Schwartz MW, et al. FoxO1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization. Nature communications. 2015;6:7079. doi: 10.1038/ncomms8079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology (Baltimore, Md.) 2011;53:116–126. doi: 10.1002/hep.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KT, Mashek MT, Bu SY, Mashek DG. Hepatic ATGL knockdown uncouples glucose intolerance from liver TAG accumulation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:313–321. doi: 10.1096/fj.12-213454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Camporez JP, Kursawe R, Titchenell PM, Zhang D, Perry CJ, Jurczak MJ, Abudukadier A, Han MS, Zhang XM, et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160:745–758. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapiro JM, Mashek MT, Greenberg AS, Mashek DG. Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity. J Lipid Res. 2009;50:1621–1629. doi: 10.1194/jlr.M800614-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satapati S, Sunny NE, Kucejova B, Fu X, He TT, Mendez-Lucas A, Shelton JM, Perales JC, Browning JD, Burgess SC. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J Lipid Res. 2012;53:1080–1092. doi: 10.1194/jlr.M023382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T, Abbott MJ, Ahmadian M, Lopes AB, Wang Y, Sul HS. Desnutrin/ATGL activates PPARdelta to promote mitochondrial function for insulin secretion in islet beta cells. Cell metabolism. 2013;18:883–895. doi: 10.1016/j.cmet.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titchenell PM, Chu Q, Monks BR, Birnbaum MJ. Hepatic insulin signalling is dispensable for suppression of glucose output by insulin in vivo. Nature communications. 2015;6:7078. doi: 10.1038/ncomms8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin SM, Hoy AJ, Brown RD, Rudaz CG, Honeyman J, Matzaris M, Watt MJ. Adipose triacylglycerol lipase is a major regulator of hepatic lipid metabolism but not insulin sensitivity in mice. Diabetologia. 2011;54:146–156. doi: 10.1007/s00125-010-1895-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang Y, Qian H, Lu J, Zhang Z, Min X, Lang M, Yang H, Wang N, Zhang P. The g0/g1 switch gene 2 is an important regulator of hepatic triglyceride metabolism. PloS one. 2013;8:e72315. doi: 10.1371/journal.pone.0072315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JR, Kreisberg RA, Felts PW. Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proceedings of the National Academy of Sciences of the United States of America. 1966;56:247–254. doi: 10.1073/pnas.56.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe RR, Peters EJ. Lipolytic response to glucose infusion in human subjects. The American journal of physiology. 1987;252:E218–E223. doi: 10.1152/ajpendo.1987.252.2.E218. [DOI] [PubMed] [Google Scholar]
- Xiong X, Tao R, DePinho RA, Dong XC. Deletion of hepatic FoxO1/3/4 genes in mice significantly impacts on glucose metabolism through downregulation of gluconeogenesis and upregulation of glycolysis. PloS one. 2013;8:e74340. doi: 10.1371/journal.pone.0074340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Lu X, Lombes M, Rha GB, Chi YI, Guerin TM, Smart EJ, Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell metabolism. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeagley D, Guo S, Unterman T, Quinn PG. Gene- and activation-specific mechanisms for insulin inhibition of basal and glucocorticoid-induced insulin-like growth factor binding protein-1 and phosphoenolpyruvate carboxykinase transcription. Roles of forkhead and insulin response sequences. The Journal of biological chemistry. 2001;276:33705–33710. doi: 10.1074/jbc.M101215200. [DOI] [PubMed] [Google Scholar]
- Zandbergen F, Mandard S, Escher P, Tan NS, Patsouris D, Jatkoe T, Rojas-Caro S, Madore S, Wahli W, Tafuri S, et al. The G0/G1 switch gene 2 is a novel PPAR target gene. The Biochemical journal. 2005;392:313–324. doi: 10.1042/BJ20050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res. 2009;50:3–21. doi: 10.1194/jlr.R800031-JLR200. [DOI] [PubMed] [Google Scholar]
- Zhang K, Li L, Qi Y, Zhu X, Gan B, DePinho RA, Averitt T, Guo S. Hepatic suppression of Foxo1 and Foxo3 causes hypoglycemia and hyperlipidemia in mice. Endocrinology. 2012;153:631–646. doi: 10.1210/en.2011-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. The Journal of biological chemistry. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Xie X, Heckmann BL, Saarinen AM, Czyzyk TA, Liu J. Targeted disruption of G0/G1 switch gene 2 enhances adipose lipolysis, alters hepatic energy balance, and alleviates high-fat diet-induced liver steatosis. Diabetes. 2014;63:934–946. doi: 10.2337/db13-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Gan L, Pan H, Kan D, Majeski M, Adam SA, Unterman TG. Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. The Biochemical journal. 2004;378:839–849. doi: 10.1042/BJ20031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.