Background
Macrolides are a class of broad spectrum antibiotics of large molecular size. The class includes erythromycin, clarithromycin, and azithromycin, among others [1]. Macrolides are used to treat both local and systemic infections, including infections of the skin, respiratory tract, gastrointestinal tract, and genital tract [2]. They also exhibit anti-inflammatory and immunomodulatory properties [3].
Macrolides are divided into categories based on chemical structure. Erythromycin and clarithromycin are 14-membered lactone rings. Clarithromycin [6-O-methylerythromycin] differs from erythromycin by a methoxy group instead of a hydroxyl group at position 6 on the carbon ring [2]. Azithromycin [9-deoxo-9a-aza-9a-methyl-9a-homoerythromycin] is a part of the azalide subclass and contains a 15-membered ring, with a methyl-substituted nitrogen instead of a carbonyl group at the 9a position on the aglycone ring, which prevents metabolism by the mechanism undergone by other macrolides [2, 4]. Erythromycin was the first macrolide discovered and has been in use since the 1950’s, whereas clarithromycin and azithromycin were approved more recently by the FDA in 1991 and 1992, respectively [2, 5]. Due to its structural differences, azithromycin does not interact with CYP3A4, SLCO1B1 or SLCO1B3, and therefore has a longer half-life and fewer drug interactions than other macrolides [6, 7].
Since erythromycin, clarithromycin and azithromycin are the most commonly described macrolide antibiotics, this review will focus on the pharmacokinetics, pharmacodynamics and pharmacogenomics of these three drugs (Figure 1). Zuckerman, et al 2011 and Periti, et al 1989 provide an overview of the pharmacokinetics and pharmacodynamics of other macrolides, such as telithromycin, tigecycline, and roxithromycin [1, 2].
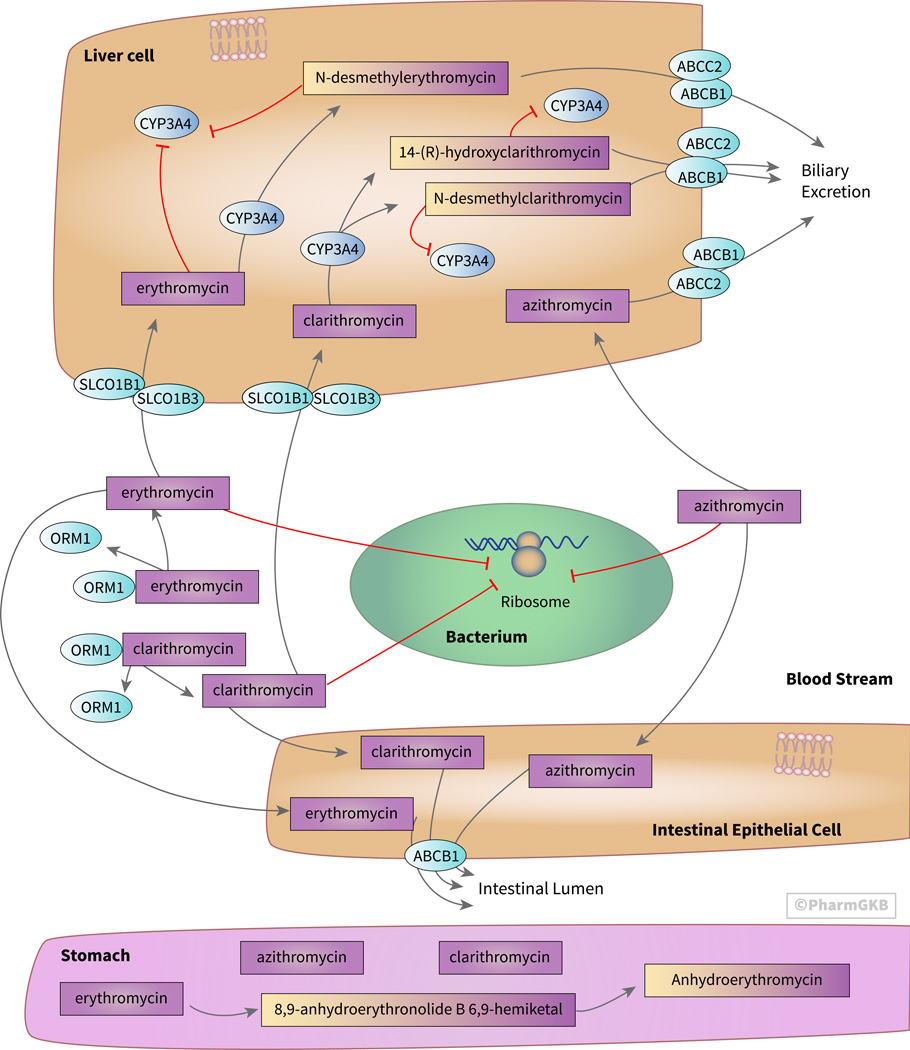
Figure 1. Stylized cells depicting the transport, metabolism and mechanism of action of the macrolide antibiotics erythromycin, clarithromycin, and azithromycin.
The bacteriostatic and bactericidal properties of macrolide antibiotics result from inhibition of the 50S subunit of the 70S bacterial ribosome. Erythromycin is degraded in low pH environments, such as in the stomach, whereas clarithromycin and azithromycin are more acid stable, leading to increased bioavailability. Erythromycin and clarithromycin interact more extensively with drug transporters and metabolizing enzymes than does azithromycin due to different chemical properties. An interactive version of this pathway is available at PharmGKB [https://www.pharmgkb.org/pathway/PA166160731].
Pharmacokinetics
The pharmacokinetic properties of macrolide antibiotics differ based on their chemical structure. In low pH environments, such as in the stomach, erythromycin is degraded (figure 1). The 8,9-anhydro-6,9-hemiketal intermediate is inactive as an antibiotic but may cause the gastrointestinal adverse effects that have been associated with erythromycin [5]. This intermediate is then further metabolized into the inactive anhydroerythromycin, erythromycin-6,9;9,12-spiroketal [4, 8]. Clarithromycin is more acid-stable than erythromycin and is not degraded as extensively in the stomach [5]. Azithromycin is even more stable at low pH, resulting in a longer serum half-life and increased concentrations in tissues compared to erythromycin [2]. As a result of their better stability at low pH, azithromycin has an oral bioavailability of 37% and clarithromycin has an oral bioavailability of 55%, compared to an oral bioavailability of 25% for erythromycin [9, 10]. Peak serum concentrations of azithromycin and clarithromycin are lower than for erythromycin of the same dose [8].
Macrolide absorption in the intestine is thought to be limited by P-glycoprotein (ABCB1) efflux transporters, which are encoded by the ABCB1 gene (figure 1) [9, 11]. ABCB1 is also believed to mediate excretion of macrolides into the bile. Because ABCB1 is involved in the transport of many other drugs, erythromycin and clarithromycin participate in drug-drug interactions [6]. One example is that erythromycin and clarithromycin have been found to increase the uptake and concentrations of pravastatin and simvastatin by inhibiting their efflux through ABCB1 [6]. As discussed later, macrolide inhibition of CYP3A4 may also decrease statin metabolism and increase concentrations.
Macrolides are lipophilic and are widely distributed in blood and tissues [5, 10]. Once in the bloodstream, macrolides preferentially bind alpha-1-acid glycoprotein (AGP) (encoded by the gene ORM1), the binding protein found in the highest concentration after albumin [10]. Erythromycin is 70–80% bound to AGP in the plasma [10]. However, azithromycin is 93% unbound in the plasma, but only 16% unbound in liver tissue [12].
Macrolides concentrate in phagocytes, which then transport the drug to the site of infection [7]. Concentrations in phagocytes of clarithromycin and azithromycin are 400 times and 800 times that of what is found in the serum, respectively [5]. Macrolide concentrations in tissues are 50 times that of what is found in the plasma, and macrolides partition especially into the spleen, liver, lungs, and kidneys [13]. Macrolides are found in the peritoneal fluids and breast milk, but do not partition greatly into the cerebrospinal fluid, where they are found at only 2–13% that of plasma concentrations [13].
Erythromycin and clarithromycin are substrates of SLCO1B1 and SLCO1B3 for uptake into the hepatocytes [6]. Erythromycin undergoes extensive metabolism by CYP3A4 in the liver, with 80% inactivated through demethylation before ~60% is excreted in the bile and ~40% in the urine [5]. The major metabolite is N-desmethylerythromycin. Clarithromycin is also thought to be metabolized by CYP3A4 in the liver into the inactive metabolite N-desmethylclarithromycin and the active metabolite 14-(R)-hydroxyclarithromycin [5]. Clarithromycin and erythromycin are thought to inhibit CYP3A4 by forming inactive complexes with CYP3A4 through their nitrosoalkane metabolites [14, 15]. Due to its inhibition of CYP3A4 and ABCB1, erythromycin has been shown to result in a sixfold increase in the AUC of simvastatin, which is metabolized by CYP3A4. Additionally, rhabdomyolosis is associated with the concomitant use of erythromycin and lovastatin, presumably due to increased concentrations of lovastatin due to reduced metabolism and reduced efflux [14].
In contrast to erythromycin and clarithromycin, azithromycin does not seem to interact with SLCO1B1 or SLCO1B3 [6]. Azithromycin has been shown to be a weak substrate for CYP3A4, to be minimally metabolized by the enzyme, and to neither induce nor inhibit CYP3A4 activity [16]. Only about 6% of azithromycin is recovered in the urine, with most being excreted unchanged in the bile, through both MRP2 (encoded by the gene ABCC2) and ABCB1 [5, 12]. MRP2 is thought to play a smaller role in excretion of azithromycin into the bile than does ABCB1 [12].
Pharmacodynamics
Macrolides stop bacterial growth by inhibiting protein synthesis [17]. Through reversibly binding to the 50S subunit of the 70S bacterial ribosomes, macrolides block further translation of proteins [17]. As a result, macrolides are effective against actively dividing organisms. Depending on the organism and the drug concentration, macrolides may be bacteriostatic, stopping bacterial growth, or bactericidal, killing the bacteria [18]. Efficacy of the drug is optimized according to the percentage of time of a dosing interval that is spent with concentrations above the minimum inhibitory concentration [13]. This mechanism of action creates a low barrier for bacteria developing resistance to the drugs if ribosomal structure or the affinity of the macrolide change, or if efflux of the drug increases through modification of the bacterial macrolide efflux (mef) genes [2, 7]. A summary of the mechanisms through which macrolides can cause resistance can be found in Zuckerman, et al. 2011 [2]. In 2001, rates of resistance to erythromycin were 16.3% in Canada and 31.5% in the United States [5].
Though rare, macrolide treatment can cause liver injury, resulting in the inability to secrete bile and in inflammation [14]. The risk factors for whether this reaction will occur are not known, but the mechanism may be through an immunological reaction such as concomitant infection of the liver, through the production of hepatotoxic metabolites, or through the induction of cell signaling pathways leading to hepatocyte death [19]. Hepatotoxicity is seen much more rarely in patients taking azithromycin than in patients taking erythromycin [14]. Clarithromycin has better activity against gram positive bacteria compared to azithromycin, and azithromycin has better activity against gram negative bacteria compared to clarithromycin. Because of the low permeability of the cell walls of gram negative bacteria, the greater stability and tissue concentrations of azithromycin is thought to improve its ability to penetrate gram negative bacteria and to stop bacterial gene translation and growth [2]. Both of these newer macrolides are better against both gram negative and gram positive bacteria than erythromycin is, and cause fewer side effects [2].
Pharmacogenomics
Variability in genes encoding drug transporters and metabolizing enzymes may lead to differing interindividual drug responses and systemic concentrations. Genetic variants in ABCB1 (P-gp) and ABCC2 (MRP2) have been reported to affect the transport and clearance of erythromycin [20, 21]. Patients with both the 2677GG (rs2032582) and 3435CC (rs1045642) diplotypes in ABCB1 reportedly had higher maximum concentrations of azithromycin compared to patients with the 2677TT/3435TT diplotypes [16]. Patients with the TT diplotype at rs717620 in ABCC2 may experience increased clearance of erythromycin compared to patients with the CC and CT diplotypes [20].
Potentially as a result of genetic variation in the ORM1 gene encoding AGP, differing AGP concentrations may affect the unbound circulating drug concentration, with lower AGP concentration associated with increased unbound drug concentration, and thereby altered drug distribution and clearance [10]. Perhaps, though not necessarily, due to differing allele frequencies of the ORM1 gene, people of Asian, Iranian and African descent have been shown to have 10–20% less AGP than do Caucasians [10]. Genetic variation in ORM1 has been shown to affect the blood concentration and clearance of antiretroviral therapies, though no effect has been seen specifically with macrolide treatment [22].
SLCO1B1 transports erythromycin into the liver, and the SLCO1B1*5 (rs4149056) variant reportedly results in a 50% reduction in erythromycin transport, explaining about 10% of the variability in erythromycin demethylation as assessed with the erythromycin breath test [21]. Additionally, variation in the SLCO1B3 gene has been associated with altered accumulation of erythromycin in the liver, with a variant at the 334 locus (rs4149117) increasing transporter activity and increasing erythromycin uptake [20].
Variation in CYP3A4 may affect the metabolism of macrolides. While not necessarily the result of genetic variation, people of Asian descent reportedly exhibit less CYP3A4 activity than do Caucasians, and Koreans reportedly had a 65% higher AUC for erythromycin compared to Caucasians on the same dose [10]. While pharmacogenomic information also is not specifically reported, Caucasians were found to have a lower AUC and shorter half-life for azithromycin compared to Mexican, Thai, Chinese, Japanese, and Jordanian patients [10].
Limited data on pharmacogenetic relationships of macrolide antibiotics can be found in published literature. However, the potential impacts of genetic variability on the pharmacokinetics and pharmacodynamics of macrolides are important to consider as sources of interindividual differences in macrolide response. Variants in genes involved in macrolide pathways have been associated with altered dose and response related to other drugs. For example, variation in the SLCO1B1 gene has been associated with altered concentrations and clearance of statins resulting in clinically meaningful implications for risk of myopathy [23]. Variation increasing the activity of the ABCB1 gene has been associated with multi-drug resistance to chemotherapy to treat metastatic cancer [24]. Thus, variants in these genes likely also have effects on macrolide absorption, distribution, concentrations, and metabolism.
Conclusion
The pharmacokinetic and pharmacodynamic pathways of macrolides suggest that genetic variation affects both their drug transport and metabolism. Because of differences in the structure of each macrolide, the impacts of genetic variation will vary. Erythromycin, the first of the macrolides to be approved by the FDA, interacts with more proteins than does azithromycin, whose structure results in different transport and metabolism of the drug. As a result, azithromycin exhibits a lower number of interactions with proteins, likely is less affected by genetic variation, and has increased activity against gram negative bacteria as a result of higher tissue concentrations. Clarithromycin, also a newer macrolide with better performance than erythromycin, may have improved activity against some strains of bacteria compared to azithromycin, though it is more subject to drug-drug interactions and genetic variation in genes encoding transporters and metabolizing enzymes.
Acknowledgments
Sources of funding: NIH/NIGMS R24 GM61374
Footnotes
Disclaimers: none
Conflicts of Interest: RBA is a stockholder in Personalis Inc. and a paid advisor for Pfizer and Karius. TEK is a paid scientific advisor to Rxight Pharmacogenetics.
References
- 1.Periti P, et al. Clinical pharmacokinetic properties of the macrolide antibiotics. Clinical Pharmacokinetics. 1989;16:193–214. doi: 10.2165/00003088-198916040-00001. [DOI] [PubMed] [Google Scholar]
- 2.Zuckerman JM, Qamar F, Bono BR. Review of macrolides (azithromycin, clarithromycin), ketolids (telithromycin) and glycylcyclines (tigecycline) Med Clin North Am. 2011;95(4):761–791. doi: 10.1016/j.mcna.2011.03.012. viii. [DOI] [PubMed] [Google Scholar]
- 3.Zarogoulidis P, et al. Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur J Clin Pharmacol. 2012;68(5):479–503. doi: 10.1007/s00228-011-1161-x. [DOI] [PubMed] [Google Scholar]
- 4.Fiese EF, Steffen SH. Comparison of the acid stability of azithromycin and erythromycin A. Journal of Antimicrobial Chemotherapy. 1990;25:39–47. doi: 10.1093/jac/25.suppl_a.39. [DOI] [PubMed] [Google Scholar]
- 5.Zuckerman JM. Macrolides and ketolides: azithromycin, clarithromycin, telithromycin. Infect Dis Clin North Am. 2004;18(3):621–649. doi: 10.1016/j.idc.2004.04.010. xi. [DOI] [PubMed] [Google Scholar]
- 6.Seithel A, et al. The influence of macrolide antibiotics on the uptake of organic anions and drugs mediated by OATP1B1 and OATP1B3. Drug Metab Dispos. 2007;35(5):779–786. doi: 10.1124/dmd.106.014407. [DOI] [PubMed] [Google Scholar]
- 7.Parnham MJ, et al. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther. 2014;143(2):225–245. doi: 10.1016/j.pharmthera.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Bahal N, Nahata MC. The new macrolide antibiotics: azithromycin, clarithromycin, dirithromycin, and roxithromycin. Ann Pharmacother. 1992;26(1):46–55. doi: 10.1177/106002809202600112. [DOI] [PubMed] [Google Scholar]
- 9.Garver E, et al. Involvement of intestinal uptake transporters in the absorption of azithromycin and clarithromycin in the rat. Drug Metab Dispos. 2008;36(12):2492–2498. doi: 10.1124/dmd.108.022285. [DOI] [PubMed] [Google Scholar]
- 10.Tsai D, et al. Interethnic differences in pharmacokinetics of antibacterials. Clin Pharmacokinet. 2015;54(3):243–260. doi: 10.1007/s40262-014-0209-3. [DOI] [PubMed] [Google Scholar]
- 11.Kurnik D, Wood AJ, Wilkinson GR. The erythromycin breath test reflects P-glycoprotein function independently of cytochrome P450 3A activity. Clin Pharmacol Ther. 2006;80(3):228–234. doi: 10.1016/j.clpt.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Sugie M, et al. Possible Involvement of the Drug Transporters P Glycoprotein and Multidrug Resistance-Associated Protein Mrp2 in Disposition of Azithromycin. Antimicrobial Agents and Chemotherapy. 2004;48(3):809–814. doi: 10.1128/AAC.48.3.809-814.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baietto L, et al. A 30-years Review on Pharmacokinetics of Antibiotics: Is the time right for pharmacogenetics? Current Drug Metabolism. 2014;15:581–598. doi: 10.2174/1389200215666140605130935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westphal JF. Macrolide-induced clinically relevant drug interactions with cytochrome p-450A (CYP) 3A4: an update focused on clarithromycin, azithromycin and dirithromycin. Br J Clin Pharmacol. 2000;50:285–295. doi: 10.1046/j.1365-2125.2000.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi HY, et al. Effects of erythromycin on voriconazole pharmacokinetics and association with CYP2C19 polymorphism. Eur J Clin Pharmacol. 2010;66(11):1131–1136. doi: 10.1007/s00228-010-0869-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He X-J, et al. Influence of ABCB1 gene polymorphisms on the pharmacokinetics of azithromycin among healthy Chinese Han ethnic subjects. Pharmacological Reports. 2009;61:843–850. doi: 10.1016/s1734-1140(09)70140-9. [DOI] [PubMed] [Google Scholar]
- 17.Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1–40. doi: 10.1016/B978-0-12-800173-8.00001-5. [DOI] [PubMed] [Google Scholar]
- 18.Wishart DS, et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. [Accessed June 28, 2016];Nucleic Acids Res. 2006 34(Database issue):D668–D672. doi: 10.1093/nar/gkj067. "Drug Bank: Erythromycin. DB00199". [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrade RJ, Tulkens PM. Hepatic safety of antibiotics used in primary care. J Antimicrob Chemother. 2011;66(7):1431–1446. doi: 10.1093/jac/dkr159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franke RM, et al. Effect of ABCC2 (MRP2) transport function on erythromycin metabolism. Clin Pharmacol Ther. 2011;89(5):693–701. doi: 10.1038/clpt.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lancaster CS, et al. OATP1B1 polymorphism as a determinant of erythromycin disposition. Clin Pharmacol Ther. 2012;92(5):642–650. doi: 10.1038/clpt.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colombo S, et al. Orosomucoid (alpha1-acid glycoprotein) plasma concentration and genetic variants: effects on human immunodeficiency virus protease inhibitor clearance and cellular accumulation. Clin Pharmacol Ther. 2006;80(4):307–318. doi: 10.1016/j.clpt.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Ramsey LB, et al. The clinical pharmacogenetics implementation consortium guideline for SLCO1B1 and simvastatin-induced myopathy: 2014 update. Clin Pharmacol Ther. 2014;96(4):423–428. doi: 10.1038/clpt.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205(2):275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]