Abstract
Flaviviruses are among the most diverse viruses with over 85 species recognized. Taxonomically, this genus is one of the 4 recognized genera within the family Flaviviridae. Most flaviviruses of human public health significance, e.g. dengue, yellow fever and Zika viruses, are arthropod-borne (arboviruses) and have two evolutionarily and ecologically distinct transmission cycles: a sylvatic transmission cycle, where the virus circulates between zoonotic vertebrate reservoir and amplification hosts and arboreal mosquitoes; and an urban transmission cycle, where the virus circulates between humans and peridomestic Aedes spp. mosquitoes. Zika virus (ZIKV), a flavivirus closely related to West Nile, dengue, Spondweni, Japanese encephalitis and yellow fever viruses, remained in obscurity since its discovery in 1947, but has recently emerged to cause a series of epidemics in the South Pacific, and most recently reaching nearly pandemic levels with its introduction in the Americas. Available epidemiologic and experimental evidence points to Aedes aegypti as the principal urban vector, possibly supplemented by Ae. albopictus in some locations. Unfortunately, the former is one of the most difficult mosquitoes to control owing to its highly anthropophilic behavior.
Introduction
Zika virus (ZIKV), a flavivirus closely related to West Nile, dengue and yellow fever viruses, remained in relative obscurity since its discovery in 1947 in Ziika (note original forest spelling was inadvertantly changed for the virus) Forest preserve, Uganda [1]. A year later the virus was isolated from arboreal Aedes africanus mosquitoes [1] and the first described human cases following ZIKV infection were reported in Nigeria in 1952 [2]. The first direct detection of ZIKV outside Africa and the first evidence of transmission by a domestic (urban) vector occurred when the virus was isolated from Ae. aegypti mosquitoes in Malaysia in 1966 [3]. A decade later, the first human infections in Asia were diagnosed in Indonesia in patients presenting with fever, malaise, stomach ache and anorexia [4]. However, throughout this time period no estimates of incidence in Africa or Asia were available. In 2007 ZIKV re-emerged to cause a series of epidemics in Africa, Southeast Asia [5–7], and more recently has reached nearly pandemic levels with its introduction into the Americas in 2013 [8,9].
Flaviviruses like dengue (DENV) and yellow fever viruses (YFV), are closely related and remarkably similar in some aspects of their natural history to ZIKV (Figure 1). All belong to the genus Flavivirus, family Flaviviridae. All of the approximately 80+ recognized species of flaviviruses share an approximately 10.7 kb, single-stranded, positive sense RNA genome, comprising three structural protein genes and seven non-structural protein genes. Species in this genus cluster within one of four major clades based upon the taxonomy of their hosts and their mode of transmission [10–13]: (i) transmission among vertebrate hosts by mosquitoes, (ii) transmission among vertebrate hosts by ticks, (iii) transmission among vertebrates without any known vector (no known vector), and (iv) restricted host range transmission among arthropods (insect-specific) without the involvement of vertebrates. Viruses of public health importance cluster within the mosquito-borne group and belong to a subgroup primarily transmitted by Aedes spp. mosquitoes. As discussed below, all of these viruses originated in sylvatic, enzootic cycles (Figures 1, 2), in Asia and Africa respectively, maintained in non-human primates and forest-dwelling Aedes mosquitoes, and have a history of successful emergence into sustained transmission among humans by Ae. aegypti (reviewed in [14–18]).
Figure 1.
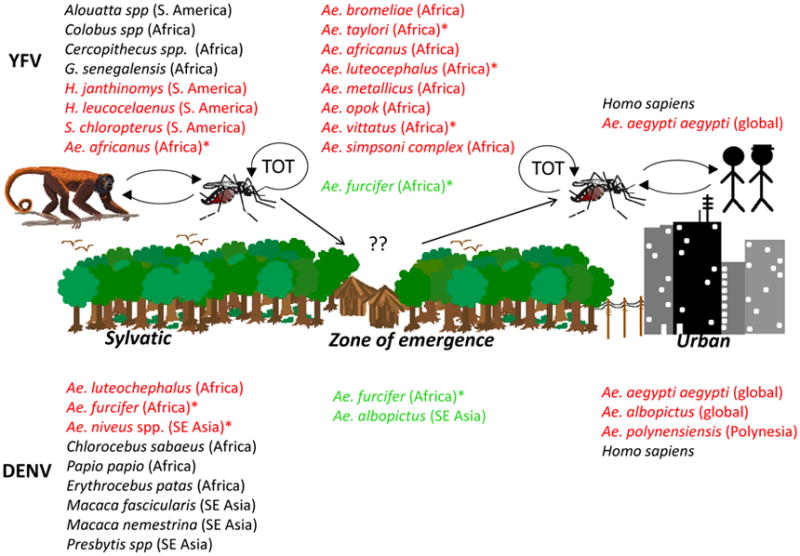
Transmission cycles of dengue and yellow fever viruses, flaviviruses with significant human health impact. TOT – transovarial transmission; *-indicates major vectors; in red: vectors in either transmission cycle; in green: vectors implicated as bridge vectors
Figure 2.
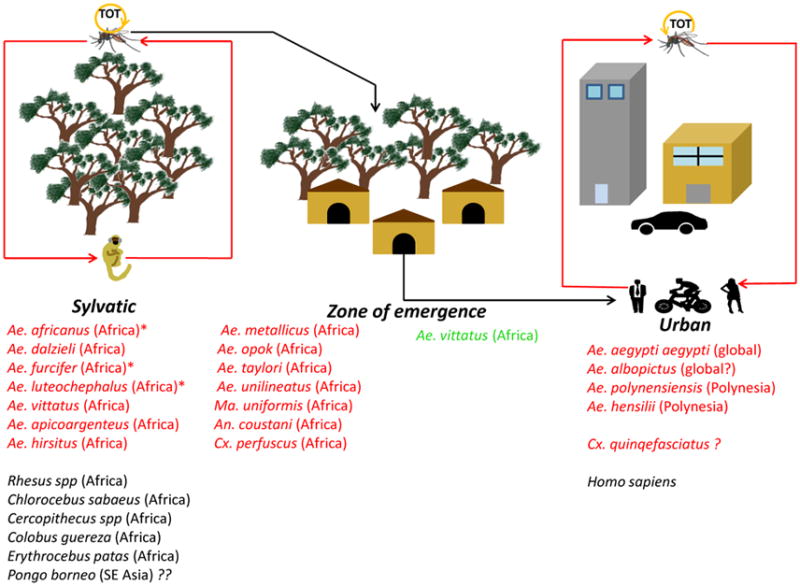
Transmission cycles of Zika virus. TOT – transovarial transmission; *-indicates major vectors; in red: vectors in either transmission cycle; in green: vectors implicated as bridge vectors
Transmission Cycles
ZIKV circulation has been documented in two ecologically and evolutionarily distinct transmission cycles: an enzootic, sylvatic cycle, where the virus circulates between arboreal Aedes spp. mosquitoes and non-human primates; and a human or urban cycle, between humans and peridomestic/domestic Aedes spp. mosquitoes. The former has been documented in Africa [1] and there is indirect and unconfirmed evidence that ZIKV may be circulating in forests of Southeast Asia [19]. Until 2007, our knowledge of ZIKV derived almost exclusively from the sylvatic transmission cycle. The first documented ZIKV isolation was from Ae. (Stegomyia) africanus mosquitoes in Uganda [1,20], and subsequently in the Central African Republic [21] (CAR) and Senegal [22] from this species, suggesting its central role as a principal enzootic vector [the other being Ae. (Stegomyia) luteocephalus](Figure 2). ZIKV has been also isolated from Ae. (Stegomyia) opok in the CAR [21], Ae. (Stegomyia) apicoargenteus in Uganda, [23] Ae. (Stegomyia) luteocephalus in Nigeria [24] and Senegal [25] and Ae. (Fredwardsius) vittatus, Ae. (Diceromyia) furcifer, and Ae. (Stegomyia) aegypti formosus in Côte d’Ivoire [26] and Senegal [22]. In addition to these mosquitoes, ZIKV has also been isolated from other Aedine species [e.g. Ae. (Aedimorphus) dalzieli, Ae. (Aedimorphus) hirsutus, Ae. (Stegomyia) unilineatus, Ae. (Stegomyia) metallicus], as well as non-Aedene species mosquitoes such as, Anopheles (Meigen) coustani and Mansonia (Mansonioides) uniformis, mosquitoes that inhabit various rural ecotypes. Lastly, there is a report of isolation from a single pool of Culex (Culex) perfuscus mosquitoes, an observation that requires further verification [22]. The isolation of ZIKV from Ae. (Fredwardsius) vittatus mosquitoes sampled in an agricultural village within the ‘zone of emergence’ supports its putative role as a bridge vector of enzootic ZIKV strains into the human transmission cycle [22] (Figure 2).
ZIKV transmission in the urban cycle mainly involves the anthropophilic Ae. (Stegomyia) aegypti mosquito, as documented by limited field surveillance [3,4,9] and experimental studies with geographically diverse populations [27–34]; Ae. (Stegomyia) hensilli [35] and/or Ae. (Stegomyia) polynesiensis [7,31] may serve as secondary vectors in niche ecotypes. However, experimental studies have yielded mixed results, with some suggesting relative refractoriness of Ae. aegypti mosquitoes [33]. This has led to speculation that other mosquitoes common in tropical cities, such as Ae. (Stegomyia) albopictus, Ae. (Protomacleaya) triseriatus [36] and Cx. (Culex) quinquefasciatus, could be potential ZIKV vectors.
Aedes (Stegomyia) albopictus [37] was implicated as a vector of peridomestic transmission in Gabon in 2007, and subsequent experimental studies [38] supported the role of Asian Ae. albopictus populations as competent ZIKV vectors. Aedes albopictus is a highly invasive species, fueled by global trade. This species expanded significantly its global geographic distribution in tropical as well as temperate settings, thus positioning it to become a significant ZIKV vector if conditions permit.
To explain the spectacular global spread of ZIKV, it was suggested that ZIKV underwent adaptive evolution for more efficient urban transmission by Ae. aegypti mosquitoes. Although phylogenetic analyses [9,39] suggest that this adaptive evolution could have occurred in Southeast Asia or the South Pacific where the virus has been circulating since the 1960’s [3], experimental infections with laboratory colonies [32] or feral populations [34] of Ae. aegypti from diverse geographic settings fail support this hypothesis. While both studies have demonstrated that various strains of ZIKV (from either Asian/American or African lineages) have the capacity to infect and replicate in American Ae. aegypti populations, none have exhibited an enhanced replicative fitness or virus adaptation to these mosquito populations [32,34]. Thus, the extent and intensity of ZIKV transmission in the Americas may be influenced by other factors, certainly including the immunologically naïve human populations.
Although a perspective publication [40] and unsubstantiated reports in the Brazilian news media suggested that Cx. quinquefasciatus mosquitoes may be competent vectors of ZIKV, several peer-reviewed studies utilizing mosquito populations from Brazil [41], Italy [42], Tunisia [43], the U.S. [36,43,44] demonstrated that these mosquitoes as well as Cx. pipiens are refractory to ZIKV infection and incapable of transmission. However, a recent report by Guo et al. [45] suggested that Chinese Cx. quinquefasciatus are competent vectors in the laboratory setting. While this report contradicts other experimental studies, it is possible that factors such as the mosquito virome and/or microbiome or genetic differences in geographic mosquito populations may contribute to the conflicting results. Also, laboratory vector competence is only meaningful if a mosquito species repeatedly feeds on humans, and widely divergent results have been obtained by studies of Cx. quinquefasciatus feeding patterns [46–49].
In summary, several experimental studies have demonstrated that Culex spp. mosquitoes are incompetent vectors of ZIKV transmission, and no evidence of natural transmission during outbreaks in the Americas [9], Southeast Asia [5] and Africa [37] has been reported.
Challenges to vector control
The absence of licensed vaccines and therapeutics indicates that very limited options exist in the short term to control the explosive global spread of ZIKV. The only currently viable methods include reduction of contact between the vector and susceptible humans, and the elimination and/or reduction of mosquito populations. While historical attempts to eradicate Ae. aegypti were successful, today they are considered to be environmentally unacceptable due to the highly toxic nature of the persistent insecticides used, such as DDT. Fueled by uncontrolled urban development and weakening of vector control measures, many neotropical cities are now fully reinfested with Ae. aegypti, and the prospects for renewed eradication efforts face formidable and probably overwhelming challenges. Reductions in Ae. aegypti populations can most feasibly be accomplished by the use of cost-effective approaches such as: community engagement and personal responsibility for eliminating or treating larval habitats, e.g. standing water in flower pots, water storage containers and refuse that holds standing water; use of larvicides to eliminate mosquito larvae from these habitats; application of insecticide aerosols within homes or other places where people are exposed to biting vectors and thus virus infection (although penetration of aerosols applied from trucks or airplanes into indoor resting sites favored by the adult females is challenging); release of genetically modified mosquitoes that express a dominant lethal gene resulting in the death of all offspring from mating with wild females, thus eliminating the risk for persistence of the transgene in nature; release of Ae. aegypti harboring the endosymbiotic Wolbachia bacteria, which suppress viral transmission by interfering with replication in the mosquito; and use of use of inexpensive and relatively maintenance-free lethal traps (reviewed in [39,50–52]). Collectively all these methods may be faced with logistical, technical and financial challenges and regardless which source(s) of vector reduction are implemented, they will need to be sustained over a long period of time if they are to be successful in reducing the risk of human exposure to human arboviruses.
Conclusion and future directions
Various Aedine spp. are the main vectors of ZIKV transmission in either sylvatic or urban transmission cycle, whereas Culex species are generally refractory for transmission. Given the recent controversy surrounding the role of Culex species in ZIKV transmission, other factors influencing vector competence, such as the mosquito gut mibrobiome and/or virome, should be investigated further. Moreover, the introduction of ZIKV and the explosive epidemic underway in the Americas beg the question whether the peridomestic Ae. (Stegomyia) albopictus has a potential role in ZIKV transmission in urban and rural settings, especially in temperate regions where Ae. aegypti cannot survive the cold winters. This vector has been implicated in Asia as a potential bridge vector for sylvatic DENV into the urban cycle (reviewed in [15,53]). Thus could Ae. (Stegomyia) albopictus serve as a bridge vector for spillback infections from humans to nonhuman primates, leading to establishment of an enzootic ZIKV transmission cycle in the Americas [54]? Several arboreal New World mosquitoes involved in the enzootic transmission of yellow fever virus, including Haemagogus albomaculatus, Hg. spegazzini, Hg. janthinomys, Sabethes chloropterus, Sa. albipivus, Sa. glaucodaemon, Sa. soperi, and Sa. cyaneus, Psorophora ferox and Ae. (Ochlerotatus) serratus (reviewed in [53]) could serve as enzootic ZIKV vectors and should be evaluated experimentally. Importantly, establishment of a ZIKV sylvatic transmission cycle in the Americas would render future eradication efforts practically impossible, and also might inhibit our ability to control the ongoing outbreak of congenital Zika syndrome.
Highlights.
Zika virus is a member of the genus Flavivirus, family Flaviviridae
Transmitted in two ecologically and phylogenetically distinct transmission cycles
Vectored mainly by Aedes spp. mosquitoes in either transmission cycle
Its host range recently expanded to include the Western Hemisphere
Acknowledgments
This work was supported in part by NIH grants R24AI120942, 1U01AI115577 and grants by the Brown Foundation of Houston and Institute of Human Infection and Immunity (IHII). We would also like to thank Shannan Rossi and Sasha Azar for expert graphic design. The funding agencies had no involvement in the writing of the report or in the decision to submit this article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1**.Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. Seminal study that described the first isolation and characterization of Zika virus. [DOI] [PubMed] [Google Scholar]
- 2.Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48:139–145. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- 3**.Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg. 1969;18:411–415. doi: 10.4269/ajtmh.1969.18.411. Seminal study describing the first isolation of Zika virus in the domestic Aedes aegypti mosquitoes. [DOI] [PubMed] [Google Scholar]
- 4*.Olson JG, Ksiazek TG, Suhandiman, Triwibowo Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg. 1981;75:389–393. doi: 10.1016/0035-9203(81)90100-0. Seminal study describing the first human isolation of Zika virus in Asia. [DOI] [PubMed] [Google Scholar]
- 5.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 6.Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry AL, Mallet HP, Sall AA, Musso D. Zika virus, French polynesia, South pacific, 2013. Emerg Infect Dis. 2014;20:1085–1086. doi: 10.3201/eid2006.140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect. 2014;20:O595–596. doi: 10.1111/1469-0691.12707. [DOI] [PubMed] [Google Scholar]
- 8.Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110:569–572. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Guerbois M, Fernandez-Salas I, Azar SR, Danis-Lozano R, Alpuche-Aranda CM, Leal G, Garcia-Malo IR, Diaz-Gonzalez EE, Casas-Martinez M, Rossi SL, et al. Outbreak of Zika Virus Infection, Chiapas State, Mexico, 2015; and First Confirmed Transmission by Aedes aegypti Mosquitoes in the Americas. J Infect Dis. 2016 doi: 10.1093/infdis/jiw302. First study demonstrating the isolation of Zika virus in Ae. aegypti mosquitoes in the Americas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook S, Holmes EC. A multigene analysis of the phylogenetic relationships among the flaviviruses (Family: Flaviviridae) and the evolution of vector transmission. Arch Virol. 2006;151:309–325. doi: 10.1007/s00705-005-0626-6. [DOI] [PubMed] [Google Scholar]
- 11.Blitvich BJ, Firth AE. Insect-specific flaviviruses: a systematic review of their discovery, host range, mode of transmission, superinfection exclusion potential and genomic organization. Viruses. 2015;7:1927–1959. doi: 10.3390/v7041927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolling BG, Weaver SC, Tesh RB, Vasilakis N. Insect-Specific Virus Discovery: Significance for the Arbovirus Community. Viruses. 2015;7:4911–4928. doi: 10.3390/v7092851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simmonds P, Becher P, Collett MS, Could EA, Heinz FX, Meyers G, Monath TP, Plentev A, Rice CM, Stiasny K, et al. Flaviviridae. In: King AMQ, Adams MJ, Carstens EB, editors. Virus Taxonomy-Classification and Nomenclature of Viruses. Ninth Report of the International Committee on Taxonomy of Viruses. Lefkowitz EJ: Elsevier Academic Press; 2012. pp. 1003–1020. [Google Scholar]
- 14.Hanley KA, Monath TP, Weaver SC, Rossi SL, Richman RL, Vasilakis N. Fever versus fever: The role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect Genet Evol. 2013;19C:292–311. doi: 10.1016/j.meegid.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasilakis N, Cardosa J, Hanley KA, Holmes EC, Weaver SC. Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat Rev Microbiol. 2011;9:532–541. doi: 10.1038/nrmicro2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryant JE, Holmes EC, Barrett AD. Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog. 2007;3:e75. doi: 10.1371/journal.ppat.0030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaver SC, Barrett AD. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat Rev Microbiol. 2004;2:789–801. doi: 10.1038/nrmicro1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfe ND, Kilbourn AM, Karesh WB, Rahman HA, Bosi EJ, Cropp BC, Andau M, Spielman A, Gubler DJ. Sylvatic transmission of arboviruses among Bornean orangutans. Am J Trop Med Hyg. 2001;64:310–316. doi: 10.4269/ajtmh.2001.64.310. [DOI] [PubMed] [Google Scholar]
- 20.Haddow AJ, Williams MC, Woodall JP, Simpson DI, Goma LK. Twelve Isolations of Zika Virus from Aedes (Stegomyia) Africanus (Theobald) Taken in and above a Uganda Forest. Bull World Health Organ. 1964;31:57–69. [PMC free article] [PubMed] [Google Scholar]
- 21.Berthet N, Nakoune E, Kamgang B, Selekon B, Descorps-Declere S, Gessain A, Manuguerra JC, Kazanji M. Molecular characterization of three Zika flaviviruses obtained from sylvatic mosquitoes in the Central African Republic. Vector Borne Zoonotic Dis. 2014;14:862–865. doi: 10.1089/vbz.2014.1607. [DOI] [PubMed] [Google Scholar]
- 22.Diallo D, Sall AA, Diagne CT, Faye O, Faye O, Ba Y, Hanley KA, Buenemann M, Weaver SC, Diallo M. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS ONE. 2014;9:e109442. doi: 10.1371/journal.pone.0109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCrae AW, Kirya BG. Yellow fever and Zika virus epizootics and enzootics in Uganda. Trans R Soc Trop Med Hyg. 1982;76:552–562. doi: 10.1016/0035-9203(82)90161-4. [DOI] [PubMed] [Google Scholar]
- 24**.Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond) 1979;83:213–219. doi: 10.1017/s0022172400025997. Seminal study describing the fisrt documented Zika outbreak in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornet M, Robin Y, Chateau R, Heme G, Adam C. Isolementd’arbovirus au Senegal oriental a partir de moustiques (1972–1977) et note surl’epidemiologie des virus transmis par les Aedes, en particulier du virus amaril. ORSTOM, Entomol Med Parasitol. 1979;17:149–163. [Google Scholar]
- 26.Akoua-Koffi C, Diarrassouba S, Benie VB, Ngbichi JM, Bozoua T, Bosson A, Akran V, Carnevale P, Ehouman A. Investigation surrounding a fatal case of yellow fever in Cote d’Ivoire in 1999. Bull Soc Pathol Exot. 2001;94:227–230. [PubMed] [Google Scholar]
- 27.Li MI, Wong PS, Ng LC, Tan CH. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. PLoS Negl Trop Dis. 2012;6:e1792. doi: 10.1371/journal.pntd.0001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boorman JP, Porterfield JS. A simple technique for infection of mosquitoes with viruses; transmission of Zika virus. Trans R Soc Trop Med Hyg. 1956;50:238–242. doi: 10.1016/0035-9203(56)90029-3. [DOI] [PubMed] [Google Scholar]
- 29.Cornet M, Robin Y, Adam C, Valade M, Calvo M. Comparison between experimental transmission of yellow fever and zika viruses in Aedes aegypti] Cah ORSTOM ser Ent med et Parasitol. 1979;17:47–53. [Google Scholar]
- 30.Hall-Mendelin S, Pyke AT, Moore PR, Mackay IM, McMahon JL, Ritchie SA, Taylor CT, Moore FA, van den Hurk AF. Assessment of Local Mosquito Species Incriminates Aedes aegypti as the Potential Vector of Zika Virus in Australia. PLoS Negl Trop Dis. 2016;10:e0004959. doi: 10.1371/journal.pntd.0004959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richard V, Paoaafaite T, Cao-Lormeau VM. Vector Competence of French Polynesian Aedes aegypti and Aedes polynesiensis for Zika Virus. PLoS Negl Trop Dis. 2016;10:e0005024. doi: 10.1371/journal.pntd.0005024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weger-Lucarelli J, Ruckert C, Chotiwan N, Nguyen C, Garcia Luna SM, Fauver JR, Foy BD, Perera R, Black WC, Kading RC, et al. Vector Competence of American Mosquitoes for Three Strains of Zika Virus. PLoS Negl Trop Dis. 2016;10:e0005101. doi: 10.1371/journal.pntd.0005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, Dupont-Rouzeyrol M, Lourenco-de-Oliveira R, Failloux AB. Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus. PLoS Negl Trop Dis. 2016;10:e0004543. doi: 10.1371/journal.pntd.0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roundy CM, Azar SR, Rossi SL, Huang JH, Leal G, Yun R, Fernandez-Salas I, Vitek CJ, Paploski IAD, Kitron U, et al. Variation in Aedes aegypti competence for Zika virus transmission as a function of viral strain, blood meal type, and mosquito geographic origin. Emerg Infect Dis. 2016 doi: 10.3201/eid2304.161484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ledermann JP, Guillaumot L, Yug L, Saweyog SC, Tided M, Machieng P, Pretrick M, Marfel M, Griggs A, Bel M, et al. Aedes hensilli as a potential vector of Chikungunya and Zika viruses. PLoS Negl Trop Dis. 2014;8:e3188. doi: 10.1371/journal.pntd.0003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aliota MT, Peinado SA, Osorio JE, Bartholomay LC. Culex pipiens and Aedes triseriatus Mosquito Susceptibility to Zika Virus. Emerg Infect Dis. 2016;22:1857–1859. doi: 10.3201/eid2210.161082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, Fontenille D, Paupy C, Leroy EM. Zika virus in Gabon (Central Africa)--2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8:e2681. doi: 10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong PS, Li MZ, Chong CS, Ng LC, Tan CH. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl Trop Dis. 2013;7:e2348. doi: 10.1371/journal.pntd.0002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, Shi PY, Vasilakis N. Zika Virus: History, Emergence, Biology, and Prospects for Control. Antiviral Res. 2016 doi: 10.1016/j.antiviral.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ayres CF. Identification of Zika virus vectors and implications for control. Lancet Infect Dis. 2016;16:278–279. doi: 10.1016/S1473-3099(16)00073-6. [DOI] [PubMed] [Google Scholar]
- 41.Fernandes RS, Campos SS, Ferreira-de-Brito A, Miranda RM, Barbosa da Silva KA, Castro MG, Raphael LM, Brasil P, Failloux AB, Bonaldo MC, et al. Culex quinquefasciatus from Rio de Janeiro Is Not Competent to Transmit the Local Zika Virus. PLoS Negl Trop Dis. 2016;10:e0004993. doi: 10.1371/journal.pntd.0004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boccolini D, Toma L, Di Luca M, Severini F, Romi R, Remoli ME, Sabbatucci M, Venturi G, Rezza G, Fortuna C. Experimental investigation of the susceptibility of Italian Culex pipiens mosquitoes to Zika virus infection. Euro Surveill. 2016:21. doi: 10.2807/1560-7917.ES.2016.21.35.30328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amraoui F, Atyame-Nten C, Vega-Rua A, Lourenco-de-Oliveira R, Vazeille M, Failloux AB. Culex mosquitoes are experimentally unable to transmit Zika virus. Euro Surveill. 2016:21. doi: 10.2807/1560-7917.ES.2016.21.35.30333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang YJ, Ayers VB, Lyons AC, Unlu I, Alto BW, Cohnstaedt LW, Higgs S, Vanlandingham DL. Culex Species Mosquitoes and Zika Virus. Vector Borne Zoonotic Dis. 2016;16:673–676. doi: 10.1089/vbz.2016.2058. [DOI] [PubMed] [Google Scholar]
- 45.Guo XX, Li CX, Deng YQ, Xing D, Liu QM, Wu Q, Sun AJ, Dong YD, Cao WC, Qin CF, et al. Culex pipiens quinquefasciatus: a potential vector to transmit Zika virus. Emerg Microbes Infect. 2016;5:e102. doi: 10.1038/emi.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Molaei G, Andreadis TG, Armstrong PM, Bueno R, Jr, Dennett JA, Real SV, Sargent C, Bala A, Randle Y, Guzman H, et al. Host feeding pattern of Culex quinquefasciatus (Diptera: Culicidae) and its role in transmission of West Nile virus in Harris County, Texas. Am J Trop Med Hyg. 2007;77:73–81. [PubMed] [Google Scholar]
- 47.de Carvalho GC, dos Malafronte RS, Miti Izumisawa C, Souza Teixeira R, Natal L, Marrelli MT. Blood meal sources of mosquitoes captured in municipal parks in Sao Paulo, Brazil. J Vector Ecol. 2014;39:146–152. doi: 10.1111/j.1948-7134.2014.12081.x. [DOI] [PubMed] [Google Scholar]
- 48.Azmi SA, Das S, Chatterjee S. Seasonal prevalence and blood meal analysis of filarial vector Culex quinquefasciatus in coastal areas of Digha, West Bengal, India. J Vector Borne Dis. 2015;52:252–256. [PubMed] [Google Scholar]
- 49.Guo XX, Li CX, Wang G, Zheng Z, Dong YD, Zhang YM, Xing D, Zhao TY. Host feeding patterns of mosquitoes in a rural malaria-endemic region in hainan island, china. J Am Mosq Control Assoc. 2014;30:309–311. doi: 10.2987/14-6439R.1. [DOI] [PubMed] [Google Scholar]
- 50.Ritchie SA, Devine G. Conventional Vector Control: Evidence it controls Arboviruses. In: Vasilakis N, Gubler DJ, editors. Arboviruses: Molecular Biology, Evolution and Control. Caister Academic Press; 2016. pp. 281–290. [Google Scholar]
- 51.Walker T, Sinkins SP. Biological control of arbovirus vectors. In: Vasilakis N, Gubler DJ, editors. Arboviruses: Molecular Biology, Evolution and Control. Caister Academic Press; 2016. pp. 291–302. [Google Scholar]
- 52.Olson KE, Franz AWE. Geneticaly modified vectors for control of arboviruses. In: Vasilakis N, Gubler DJ, editors. Arboviruses: Molecular Biology, Evolution and Control. Caister Academic Press; 2016. pp. 315–336. [Google Scholar]
- 53.Hanley KA, Monath TP, Weaver SC, Rossi SL, Richman RL, Vasilakis N. Fever versus fever: The role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect Genet Evol. 2013 doi: 10.1016/j.meegid.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Althouse BM, Vasilakis N, Sall AA, Diallo M, Weaver SC, Hanley KA. Potential for Zika virus to establish a sylvatic transmission cycle in the Americas. PLoS Negl Trop Dis. 2016 doi: 10.1371/journal.pntd.0005055. [DOI] [PMC free article] [PubMed] [Google Scholar]


