Abstract
The objective of this study was to examine the influence of BMI on the passive-elastic properties of the ankle plantarflexors in older women. Twenty-three women, 65–80 yr, were separated into normal weight (NW, BMI < 25.0 kg m−2, n = 11) and overweight-obese (OW, BMI ≥ 25.0 kg m−2, n = 12) groups. Resistive torque of the ankle plantarflexors was recorded on an isokinetic dynamometer by passively moving the ankle into dorsiflexion. Stiffness, work absorption, and hysteresis were calculated across an ankle dorsiflexion angle of 10 – 15 deg. Maximal plantarflexor strength was assessed, then participants walked at maximal speed on an instrumented gait analysis treadmill while muscle activation (EMG) was recorded. Plantarflexor stiffness was 34% lower in OW (26.4 ± 12.7 Nm rad−1) than NW (40.0 ± 15.7 Nm rad−1, p = 0.032). Neither work absorption nor hysteresis were different between OW and NW. Stiffness per kg was positively correlated to strength (r = 0.66, p < 0.001), peak vertical ground reaction force during walking (r = 0.72, p < 0.001), weight acceptance rate of force (r = 0.51, p = 0.007), push-off rate of force (r = 0.41, p = 0.026), maximal speed (r = 0.61, p = 0.001), and inversely correlated to BMI (r = −0.61, p = 0.001), and peak plantarflexor EMG (r = −0.40, p = 0.046). Older women who are OW have low plantarflexor stiffness, which may limit propulsive forces during walking and necessitate greater muscle activation for active force generation.
Keywords: Muscle, Materials Testing, Gait, Mobility Limitation, Obesity, Older Adults
1. Introduction
Older adults who are overweight or obese (OW) have preferred and maximal walking speeds that are slower than older adults who are normal weight (NW) placing them at greater risk for mobility disability [1, 2]. Poor mobility and functional limitation in OW are related to low muscle strength per kilogram of body mass, low aerobic capacity, heightened muscle activation, and a mass-specific energy cost of walking that is 20% greater than NW [1, 3–5].
During walking and running, lower-extremity propulsive forces result from both active muscle force generation and passive-elastic energy (work absorption) stored in the muscle-tendon unit (MTU) [6, 7]. With each step, the muscles that work at the hip, knee, and ankle absorb work to conserve energy so that active muscle force generation and the energy cost of walking are minimized [8]. The ability to absorb work during deformation of the MTU is proportional to its stiffness, that is, stiffer muscle has the potential to absorb and return more elastic energy. However, modeling suggests that MTU stiffness has an optimal range of values that maximize the efficiency of walking [9]. The author theorizes that in OW excess fat mass arithmetically reduces the ratio of MTU stiffness to body mass to suboptimal levels. This may result in an attenuation of lower-extremity forces, greater motor unit recruitment for active force generation, and an elevated metabolic energy cost that contribute to walking difficulty in older adults with obesity.
Continuous overloading of muscles and tendons by excess body weight could lead to tissue degeneration if the microtraumas of repetitive stress exceed tendon regeneration. This, in addition to dyslipidemia and the systemic, chronic, low-grade inflammation that accompanies obesity, may lead to the reduction of collagen fibrils, disorganization of tendon architecture, and intratendinous lipid deposition shown in obese animal models [10]. These histological changes likely diminish the quality of the MTU and could further reduce its capacity to store and return elastic energy. Studying the impact of body weight on MTU passive-elastic properties is important as observational studies demonstrate that obesity increases the risk for tendinopathy, particularly Achilles tendinopathy and plantar fasciopathy [11, 12]. In fact, the incidence of Achilles tendon rupture has increased by 15% over 20 yr, largely driven by a doubling of injury rate in people over the age of 50 yr [13].
Despite the clinical and functional importance of MTU passive-elastic properties, it is currently unclear how they are affected by adiposity in older adults [14]. Therefore, the purpose of this study was to examine the association of body mass index (BMI) with ankle plantarflexor stiffness, work absorption, and hysteresis and relate these passive-elastic properties to walking performance in older women. It was hypothesized that plantarflexor muscle stiffness and work absorption would be lower in OW than in NW, and hysteresis higher in OW. Second, it was hypothesized that plantarflexor stiffness and work absorption would be positively related to walking speed and vertical ground reaction forces (vGRF), and inversely related to plantarflexor muscle activation.
2. Methods
2.1 Participants
Twenty-three older women, between the ages of 65 – 80 yr were recruited to the study by newspaper advertisement and were separated by BMI into a NW group (BMI < 25 kg m−2, n = 11) and an OW group (BMI ≥ 25 kg m−2, n = 12, of which one participant had BMI ≥ 30 kg m−2). Participants were included if they were able to walk independently and had no physical limitations that prevented participation. The study was approved by the University of New Hampshire Intuitional Review Board and all participants gave their written, informed consent.
2.2 Visit 1 - Familiarization
Upon arrival to the laboratory, the Rapid Assessment of Physical Activity was used to determine participation in aerobic activity (maximum score of 7) and strengthening activities (maximum score of 3). Body composition was assessed through the use of whole-body air-displacement plethysmography (Bod Pod, Life Measures, Inc. Concord, CA, USA). Next, participants were familiarized to strength and passive muscle properties procedures on a commercial isokinetic dynamometer (HUMAC NORM, CSMI, Stoughton, MA, USA). Subjects were then familiarized to the treadmill walking task which was performed on an instrumented, gait analysis treadmill (Gaitway II, Kistler Instrument Corp., Amherst, NY, USA).
2.3 Visit 2 – Data Collection
2.3.1 Plantarflexor strength
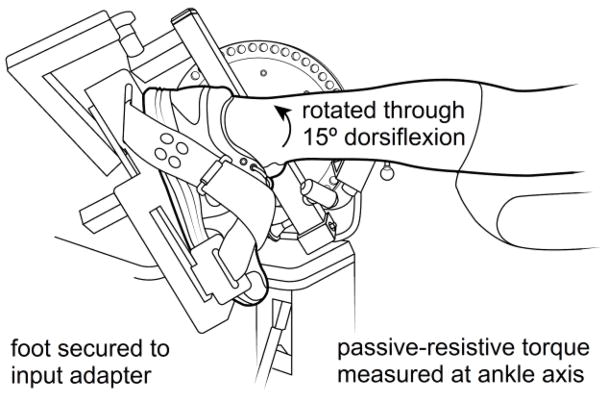
At visit 2, participants were placed on the dynamometer in the prone position and the left foot was secured to the dynamometer’s resistance arm using the manufacturer’s ankle adapter and nylon straps (Figure 1). Participants’ plantarflexor strength (active torque) was measured by performing two maximal, voluntary, isometric contractions (MVC) at an ankle angle of 90 deg (i.e. neutral, 0 deg of dorsiflexion). The analog torque of the dynamometer was recorded using a data acquisition system and the two peak values were averaged (BIOPAC MP100, Biopac Systems Inc, Goleta, California, USA).
Figure 1.
Isokinetic dynamometer adapted for the measurement of passive and active ankle torque.
2.3.2 Plantarflexor passive-elastic properties
To measure passive-elastic properties of the ankle plantarflexor MTU, the relaxed ankle was moved from 15 degrees of plantarflexion, through the neutral position (0 degrees), to 15 degrees of ankle dorsiflexion (Figure 1) and back to the original starting position at an angular velocity of 15 deg s−1. MTU material properties were calculated across an ankle dorsiflexion angle of 10 – 15 deg (Δ = 5 deg or 0.087 rad). This range was chosen as it is in the linear portion of the passive torque-angle curve and it approximates the ankle range of motion seen during the push-off phase of walking in older adults [15]. Peak passive torque was recorded as the highest torque observed. Plantarflexor stiffness was determined as the slope of the torque-angle curve expressed as Nm·rad−1. In this model, ankle torque serves as a surrogate measure of muscle stress and ankle angle serves as a measure of muscle strain [16]. Work absorption, a measure of elastic energy storage, was calculated by integrating the torque-angle curve across the same range of motion and is expressed as Nm·rad. Hysteresis, the loss of energy over time due to viscoelastic stress relaxation, was calculated as the percent reduction in work absorption between the muscle lengthening and shortening phases.
2.3.3 Walking gait kinetics
Participants began walking on the instrumented treadmill at a speed of 0.8 m s−1, which was then increased linearly at a rate of 0.015 m s−1 each second. Self-selected maximal walking speed was determined by asking participants to identify their fastest speed as if they were late for an important event. Participants then walked at this speed for two minutes while vGRF were recorded from the treadmill’s force plates at 100 Hz by its accompanying software (Gaitway v.2.0.8.50, Kistler Instrument Corp., Amherst, NY, USA). The peak vGRF, weight acceptance rate of force, and push-off rate of force were averaged over 20 steps for analysis.
2.3.4 Muscle activation
Surface electromyography (EMG) was used to assess activation of the ankle plantarflexors during the MVC and walking tasks. A preamplified surface electrode with a gain of 300× (B&L Engineering, Santa Ana, CA, USA) was placed over the medial head of the gastrocnemius muscle of the left leg. The EMG signal was sampled by the data acquisition system at 1,000 Hz, bandpass filtered (30–500 Hz), rectified, and integrated every 20 samples. During walking, the peak amplitude of the integrated EMG was recorded for five steps, averaged, and expressed as a percentage of the peak EMG measured during the MVC.
2.4 Statistical analysis
Multivarirate analysis of variance (MANOVA) was used to compare subject characteristics and dependent measures between NW and OW groups. Because groups differed by age, it was tested as a covariate. The Pearson product-moment statistic was used to evaluate the relationship between muscle stiffness, work absorption, body composition, muscle strength, walking speed, ground reaction forces, and muscle activation during walking. Multiple linear regression was used to determine the independent associations of BMI, stiffness, and strength predictor variables with self-selected maximal speed and peak vGRF dependent variables. The critical p-value for statistical significance was set to p < 0.05 for all tests.
3. Results
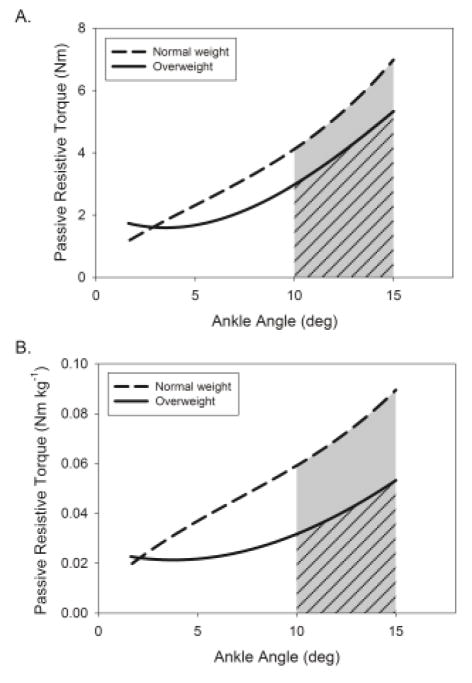
OW were significantly younger, heavier, had a higher BMI and percent body fat, and had slower self-selected maximal walking speeds than NW (Table 1). Plantarflexor stiffness (Figure 2A, slope) was 34% lower in OW (26.4 ± 12.7 Nm rad−1) than NW (40.0 ± 15.7 Nm rad−1, p = 0.032) and stiffness per kg (Figure 2B) was 47% lower in OW (0.37 ± 0.16 vs. 0.70 ± 0.28 Nm rad−1 kg−1, p = 0.002). Work absorption (Figure 2, shaded area under curve) was not different between OW (0.36 ± 0.16 Nm rad) and NW (0.48 ± 0.29 Nm rad, p = 0.31) nor was it different when expressed per kg (0.0050 ± 0.0023 vs. 0.0083 ± 0.0052 Nm rad kg−1, p = 0.078). There was no difference in hysteresis between OW (48 ± 22%) and NW (42 ± 23%, p = 0.56). Age did not affect these group comparisons.
Table 1.
Participant descriptive characteristics.
| Normal weight | Overweight | P | |
|---|---|---|---|
| Age (yr) | 74.1 ± 4.3 (66 – 81) | 69.8 ± 4.1 (65 – 76) | 0.021 |
| Mass (kg) | 57.8 ± 4.1 (49.4 – 63.5) | 70.6 ± 6.9 (61.9 – 86.1) | < 0.001 |
| Height (m) | 1.63 ± 0.04 (1.56 – 1.69) | 1.58 ± 0.07 (1.52 – 1.73) | 0.058 |
| Body Mass Index (kg m−2) | 21.8 ± 1.9 (17.8 – 24.8) | 28.4 ± 2.0 (25.6 – 33.3) | < 0.001 |
| Percent Body Fat (%) | 30.1 ± 9.0 (17.3 – 45.8) | 43.5 ± 3.6 (37.7 – 48.0) | < 0.001 |
| RAPA Aerobic Score | 5.6 ± 1.4 (3 – 7) | 5.1 ± 1.6 (3 – 7) | 0.413 |
| RAPA Strength Score | 2.3 ± 1.2 (0 – 3) | 1.5 ± 1.2 (0 – 3) | 0.118 |
| Maximal Walking Speed (m s−1) | 1.54 ± 0.25 (1.00 – 1.86) | 1.26 ± 0.19 (1.03 – 1.53) | 0.007 |
Values are mean ± SD (range)
RAPA = Rapid Assessment of Physical Activity, maximum score of 7 for aerobic activities and 3 for strengthening activities.
Figure 2.
Stress-strain curves of the ankle plantarflexors for the comparison of passive-elastic properties between normal weight (dashed) and overweight (solid) older adults in absolute terms (A) and per kilogram of body mass (B). Stiffness was determined as the slope of the angle-torque curve from 10 – 15 degrees of dorsiflexion and work absorption was determined as the area under the curve shown in the shaded region; diagonal lines identify the overweight group work absorption.
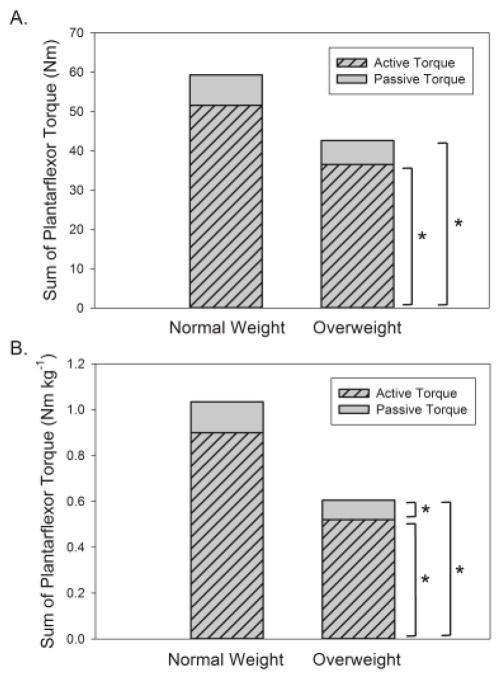
Plantarflexor strength (Figure 3A, active torque) was 29% lower in OW (p = 0.018) as was strength per kilogram (Figure 3B, −43%, p < 0.001). Peak passive torque was similar between OW and NW when expressed in absolute terms (Figure 3A, p = 0.28), but was 37% lower in OW when expressed per kilogram (Figure 3B, p = 0.042). The sum of ankle plantarflexor strength (active torque) and peak passive torque was 28% lower in OW (p = 0.035), and 42% lower per kilogram (p = 0.001).
Figure 3.
Comparison of peak active torque, peak passive torque, and their sum between normal weight and overweight older adults in absolute terms (A) and per kilogram of body mass (B). * = significantly different between groups, p < 0.05.
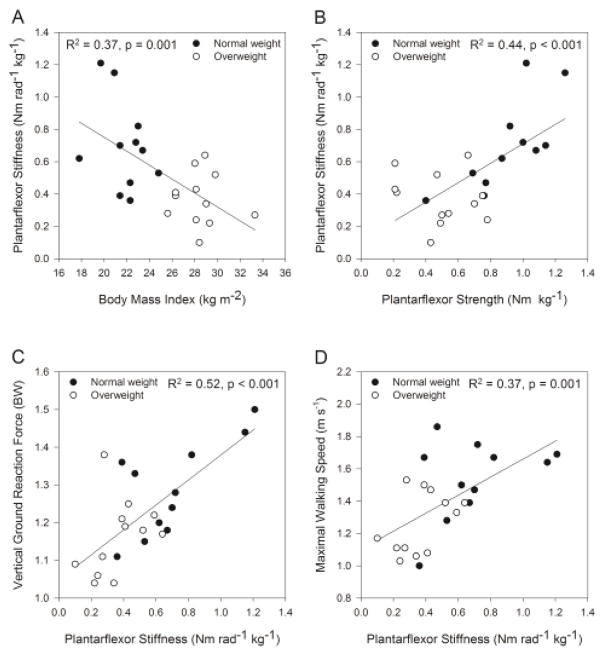
Stiffness per kg was inversely correlated to BMI (Figure 4A), percent body fat (r = −0.60, p = 0.003), and peak plantarflexor EMG (r = −0.40, p = 0.046). Stiffness per kg was positively correlated to strength (Figure 4B), peak vGRF during walking (Figure 4C), weight acceptance rate of force (r = 0.51, p = 0.007), push-off rate of force (r = 0.41, p = 0.026), and maximal speed (Figure 4D).
Figure 4.
Relationship between body mass index and plantarflexor stiffness (A), ankle plantarflexor strength and stiffness (B), plantarflexor stiffness and peak vertical ground reaction force during walking (C), plantarflexor stiffness and self-selected maximal walking speed (D). BW = body weights
Linear regression showed that together BMI, stiffness per kg, and plantarflexor strength per kg explained 59% of the variance in peak vGRF (p = 0.001). By controlling for each of the other independent variables in the model, partial correlations indicated that stiffness per kg explained more of the variability (r = 0.56, p = 0.008) in peak vGRF than BMI (r = −0.38, p = 0.086) or plantarflexor strength (r = −0.21, p = 0.37). In a second regression, BMI, stiffness per kg, and plantarflexor strength per kg together explained 46% of the variance in maximal walking speed (p = 0.008). Partial correlations for maximal walking speed showed that BMI (r = −0.32, p = 0.164) and stiffness per kg (r = 0.31, p = 0.17) each explained similar variance in speed with little explained by plantarflexor strength (r = 0.095, p = 0.682). Collinearity diagnostics including Eigenvalues near zero, a condition index of 29, and variance inflation factors near 2 indicate that moderate dependencies exist among the predictor variables BMI, stiffness, and strength [17].
4. Discussion
4.1 Passive-elastic MTU properties
The results of this study support the hypothesis that there is a disproportionately low ratio of plantarflexor MTU stiffness to body mass in OW, but failed to show differences in work absorption and hysteresis between groups. In fact, plantarflexor stiffness was inversely related to adiposity as both body fat percentage and BMI each explained 37% of its variance. Faria and colleagues showed an elevated stiffness in subjects with obesity when expressed in absolute terms, but only a trend toward lower stiffness for the participants with the greatest obesity when expressed per kg of body mass [18]. The lack of agreement between our studies may have occurred because the previous study’s participants were younger (mean 58 yr), OW had lower strength than NW in the current study, but not in Faria’s; and they measured stiffness based on damped frequency of force oscillation that prevents a direct comparison our results.
Two proposed mechanisms for altered connective tissue properties in OW have been reported by Abate, 1) greater stress placed on connective tissue elicits microstructural damage, and 2) the dyslipidemia and chronic inflammation of obesity reduce connective tissue quality [10]. The author proposes that sarcopenic and dynapenic obesity [19, 20], as a consequence of poor nutrition, limited physical activity, and sedentary behavior [21], is a third possible explanation for low MTU stiffness in older adults who are OW. With sarcopenic obesity, the lower muscle mass and cross-sectional area than expected for body size in OW would not only decrease capacity for active force generation, but would also impair the ability to harness passive-elastic energy [22, 23]. The cross-sectional area of muscle and tendon is the primary factor that determines its stiffness, and stiffness is proportional to a material’s capacity for work absorption. It is therefore not surprising that in this study plantarflexor strength explained 44% of the variance in stiffness. Fortunately, passive stiffness is a modifiable property of muscle that has been shown to increase by 65% in older adults who completed a resistance exercise program [24].
4.2 Active and Passive Torque
During dynamic movements, both actively generated muscle force and passive-elastic force contribute to the net torque about a joint. This study showed that active and passive torque, and their sum, are substantially lower in OW than NW. These findings are problematic because low plantarflexion torque capacity in OW is related to reduced mobility and history of falls [2, 25]. If the MTU of OW is overly compliant, lower-extremity forces may be limited, or a greater degree of active contraction may be necessary to produce the forces required for locomotion. A limitation of this study is that the dynamometer measurements of muscle stiffness, work absorption, and peak passive torque were isolated, slow, and submaximal, yet they provide a characterization of MTU compliance under load that should relate to its behavior during walking. Another limitation is that OW participants were younger than NW, although when tested as a covariate age did not significantly affect group differences for the passive muscle properties.
4.3 Walking performance
Plantarflexor stiffness per kg explained 52% of the variance in peak vGRF and 37% of the variance in maximal walking speed. These findings support the study’s second hypothesis that passive-elastic properties of muscle may be related to walking ability in older adults. The associations show that older women with greater muscle stiffness were able to impart a larger force to the ground and obtain faster walking speeds. The link between stiffness and vGRF is important because there is linear relationship between vGRF and walking speed, as vGRF makes up the largest component of the ground reaction force vector responsible for locomotion [26].
In addition to MTU stiffness, it is known that BMI and strength contribute to walking performance in older adults. Thus, partial correlations were used to examine the association between stiffness and peak vGRF independent of BMI and plantarflexor strength. After controlling for these variables the relationship remained significant, but the amount of variance in peak vGRF explained by plantarflexor stiffness per kg was reduced from 52% to 31%. Similarly, controlling for BMI and strength reduced the amount of variance in maximal walking speed explained by stiffness from 37% to 10%. The partial correlations associated with BMI, stiffness, and strength should be interpreted with caution because collinearity statistics indicated that there are moderate associations among these predictor variables that affects the ability to accurately assign relative importance [17]. Nevertheless, these same collinearity statistics indicate that there is enough independence between BMI, stiffness, and strength such that each explains some of the variance in walking performance of older adults. In a similar fashion, Stenroth and colleagues used regression models to isolate the relation of muscle strength and Achilles tendon stiffness to mobility and showed that each was independently associated with six-minute walk distance in older adults [27]. These authors hypothesized that a stiffer Achilles tendon may reduce shortening velocity of gastrocnemius allowing it to operate in in the optimal range of the length-tension relationship. This hypothesis provides a theoretical link between low muscle stiffness, low ground reaction forces and rates, and slow maximal walking speed observed in OW in the current study.
It was previously thought that the long duration of stance during walking limits the potential for energy storage and return in MTU connective tissues [8]. However, Ishikawa et al. measured Achilles tendon force and fascicle lengths of the plantarflexors in vivo during natural walking [6]. They demonstrated that the MTU is stretched during weight acceptance and rapidly recoils during push-off contributing to mechanical power at the ankle in a catapult-like fashion [6]. The results of the current study support this assertion as push-off rates were positively correlated to MTU stiffness in older adult walkers. Farris and Sawicki further demonstrated the importance of passive-elastic MTU properties by showing that gastronemius contracts isometrically during midstance as the Achilles tendon elongates during ankle dorsiflexion [7]. Remarkably, they determined that the Achilles tendon contributed similar force, and twice the power, as muscle fascicles during push-off. Researchers and clinicians should appreciate the role passive-elastic muscle properties play in walking energetics and should recognize that a poor ability to harness passive-elastic energy will require greater active force generation.
4.4 Muscle Activation
When EMG during walking was expressed as a percentage of the peak EMG obtained from a maximal contraction, OW had nearly double the plantarflexor activation of NW, despite walking 19% slower. A novel finding was that there was a weak, inverse correlation between muscle stiffness and plantarflexor activation. The author hypothesizes that the elevated muscle activation in OW may have occurred to increase active force generation to compensate for a poor ability to utilize elastic energy, and, because OW possessed low plantarflexor strength and were operating at a high percentage of capacity. Greater muscle activation is problematic because it elevates the energy cost of walking, increases the likelihood for fatigue as high-threshold motor units are recruited, and contributes to a greater sense of effort during ambulation [28, 29]. Previous research in the author’s laboratory demonstrated that the mass-specific energy cost of walking was 20% greater in OW compared to NW [3]. While a number of biomechanical gait alterations in OW walkers explain some of their elevated energy cost [30], it is interesting to consider that impaired ability to utilize MTU elastic energy could also contribute to greater energy cost of walking.
5. Conclusions
OW demonstrated lower MTU stiffness than NW that hinders their capacity to passively store elastic energy. Low plantarflexor stiffness and work absorption were associated with adiposity, low strength, diminished lower-extremity forces and rates during walking, slower walking speed, and elevated muscle activation. This study presents preliminary evidence that the passive-elastic properties of muscle differ between OW and NW and that these properties are related to the maximal walking performance in older adults.
Highlights.
Passive-elastic properties of skeletal muscle may be altered by age and adiposity.
Ankle plantarflexor stiffness is lower in overweight and obese older adults than normal weight.
Stiffness and work absorption are related to propulsive forces and maximal speed during walking.
Muscle activation during walking is greater in obese older adults who possess low plantarflexor stiffness.
Acknowledgments
D.P. LaRoche was supported by the National Center for Advancing Translational Sciences via NIH Grant L30 TR000588. The National Institutes of Health had no involvement in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest
The author reports no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stenholm S, Alley D, Bandinelli S, Griswold ME, Koskinen S, Rantanen T, et al. The effect of obesity combined with low muscle strength on decline in mobility in older persons: results from the InCHIANTI study. Int J Obes. 2009;33:635–44. doi: 10.1038/ijo.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaRoche DP, Kralian RJ, Millett ED. Fat mass limits lower-extremity relative strength and maximal walking performance in older women. Journal of Electromyography and Kinesiology. 2011;21:754–61. doi: 10.1016/j.jelekin.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaRoche DP, Marques NR, Shumila HN, Logan CR, Laurent RS, Goncalves M. Excess body weight and gait influence energy cost of walking in older adults. Med Sci Sports Exerc. 2015;47:1017–25. doi: 10.1249/MSS.0000000000000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ko SU, Stenholm S, Ferrucci L. Characteristic gait patterns in older adults with obesity--Results from the Baltimore Longitudinal Study of Aging. J Biomech. 2010;43:1104–10. doi: 10.1016/j.jbiomech.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maslow AL, Price AE, Sui X, Lee DC, Vuori I, Blair SN. Fitness and adiposity as predictors of functional limitation in adults. J Phys Act Health. 2011;8:18–26. doi: 10.1123/jpah.8.1.18. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa M, Komi PV, Grey MJ, Lepola V, Bruggemann GP. Muscle-tendon interaction and elastic energy usage in human walking. J Appl Physiol (1985) 2005;99:603–8. doi: 10.1152/japplphysiol.00189.2005. [DOI] [PubMed] [Google Scholar]
- 7.Farris DJ, Sawicki GS. Human medial gastrocnemius force-velocity behavior shifts with locomotion speed and gait. Proc Natl Acad Sci U S A. 2012;109:977–82. doi: 10.1073/pnas.1107972109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawicki GS, Lewis CL, Ferris DP. It pays to have a spring in your step. Exerc Sport Sci Rev. 2009;37:130–8. doi: 10.1097/JES.0b013e31819c2df6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtwark GA, Wilson AM. Optimal muscle fascicle length and tendon stiffness for maximising gastrocnemius efficiency during human walking and running. J Theor Biol. 2008;252:662–73. doi: 10.1016/j.jtbi.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Abate M. How obesity modifies tendons (implications for athletic activities) Muscles Ligaments Tendons J. 2014;4:298–302. [PMC free article] [PubMed] [Google Scholar]
- 11.Franceschi F, Papalia R, Paciotti M, Franceschetti E, Di Martino A, Maffulli N, et al. Obesity as a risk factor for tendinopathy: a systematic review. Int J Endocrinol. 2014;2014:670262. doi: 10.1155/2014/670262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaida JE, Ashe MC, Bass SL, Cook JL. Is adiposity an under-recognized risk factor for tendinopathy? A systematic review. Arthritis Rheum. 2009;61:840–9. doi: 10.1002/art.24518. [DOI] [PubMed] [Google Scholar]
- 13.Ganestam A, Kallemose T, Troelsen A, Barfod KW. Increasing incidence of acute Achilles tendon rupture and a noticeable decline in surgical treatment from 1994 to 2013. A nationwide registry study of 33,160 patients. Knee Surg Sports Traumatol Arthrosc. 2015 doi: 10.1007/s00167-015-3544-5. [DOI] [PubMed] [Google Scholar]
- 14.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 15.Ko SU, Tolea MI, Hausdorff JM, Ferrucci L. Sex-specific differences in gait patterns of healthy older adults: Results from the Baltimore Longitudinal Study of Aging. Journal of biomechanics. 2011;44:1974–9. doi: 10.1016/j.jbiomech.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaRoche DP, Connolly DA. Effects of stretching on passive muscle tension and response to eccentric exercise. Am J Sports Med. 2006;34:1000–7. doi: 10.1177/0363546505284238. [DOI] [PubMed] [Google Scholar]
- 17.Mason CH, Perreault WD. Collinearity, Power, and Interpretation of Multiple-Regression Analysis. J Marketing Res. 1991;28:268–80. [Google Scholar]
- 18.Faria A, Gabriel R, Abrantes J, Bras R, Moreira H. Triceps-surae musculotendinous stiffness: relative differences between obese and non-obese postmenopausal women. Clin Biomech. 2009;24:866–71. doi: 10.1016/j.clinbiomech.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Bouchard DR, Dionne IJ, Brochu M. Sarcopenic/obesity and physical capacity in older men and women: data from the Nutrition as a Determinant of Successful Aging (NuAge)-the Quebec longitudinal Study. Obesity (Silver Spring) 2009;17:2082–8. doi: 10.1038/oby.2009.109. [DOI] [PubMed] [Google Scholar]
- 20.Bouchard DR, Janssen I. Dynapenic-obesity and physical function in older adults. J Gerontol A Biol Sci Med Sci. 2010;65:71–7. doi: 10.1093/gerona/glp159. [DOI] [PubMed] [Google Scholar]
- 21.Lord S, Chastin SF, McInnes L, Little L, Briggs P, Rochester L. Exploring patterns of daily physical and sedentary behaviour in community-dwelling older adults. Age Ageing. 2011;40:205–10. doi: 10.1093/ageing/afq166. [DOI] [PubMed] [Google Scholar]
- 22.Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2:819–29. doi: 10.1016/S2213-8587(14)70034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muraoka T, Muramatsu T, Fukunaga T, Kanehisa H. Elastic properties of human Achilles tendon are correlated to muscle strength. J Appl Physiol (1985) 2005;99:665–9. doi: 10.1152/japplphysiol.00624.2004. [DOI] [PubMed] [Google Scholar]
- 24.Reeves ND, Narici MV, Maganaris CN. Strength training alters the viscoelastic properties of tendons in elderly humans. Muscle Nerve. 2003;28:74–81. doi: 10.1002/mus.10392. [DOI] [PubMed] [Google Scholar]
- 25.LaRoche DP, Cremin KA, Greenleaf B, Croce RV. Rapid torque development in older female fallers and nonfallers: a comparison across lower-extremity muscles. J Electromyogr Kinesiol. 2010;20:482–8. doi: 10.1016/j.jelekin.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 26.LaRoche DP, Millett ED, Kralian RJ. Low strength is related to diminished ground reaction forces and walking performance in older women. Gait & posture. 2011;33:668–72. doi: 10.1016/j.gaitpost.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stenroth L, Sillanpaa E, McPhee JS, Narici MV, Gapeyeva H, Paasuke M, et al. Plantarflexor Muscle-Tendon Properties are Associated With Mobility in Healthy Older Adults. J Gerontol A Biol Sci Med Sci. 2015;70:996–1002. doi: 10.1093/gerona/glv011. [DOI] [PubMed] [Google Scholar]
- 28.Hortobagyi T, Finch A, Solnik S, Rider P, DeVita P. Association between muscle activation and metabolic cost of walking in young and old adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2011;66:541–7. doi: 10.1093/gerona/glr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laurin J, Pertici V, Dousset E, Marqueste T, Decherchi P. Group III and IV muscle afferents: role on central motor drive and clinical implications. Neuroscience. 2015;290:543–51. doi: 10.1016/j.neuroscience.2015.01.065. [DOI] [PubMed] [Google Scholar]
- 30.Peyrot N, Thivel D, Isacco L, Morin JB, Duche P, Belli A. Do mechanical gait parameters explain the higher metabolic cost of walking in obese adolescents? J Appl Physiol (1985) 2009;106:1763–70. doi: 10.1152/japplphysiol.91240.2008. [DOI] [PubMed] [Google Scholar]