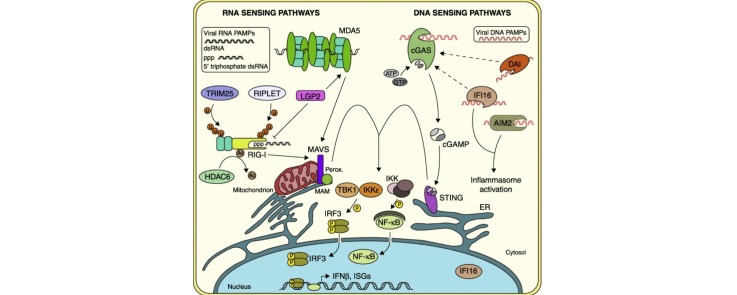
Graphical abstract
Highlights
-
•
Cells are equipped with pattern recognition receptors to sense invading viruses.
-
•
Nucleic acids of RNA viruses are sensed by RIG-I like receptors in the cytosol.
-
•
Foreign DNA is sensed by cGAS and other DNA sensors in the cytosol.
-
•
These pattern recognition receptors activate adaptor proteins to initiate antiviral innate immune responses.
Abstract
The ability to recognize invading viral pathogens and to distinguish their components from those of the host cell is critical to initiate the innate immune response. The efficiency of this detection is an important factor in determining the susceptibility of the cell to viral infection. Innate sensing of viruses is, therefore, an indispensable step in the line of defense for cells and organisms. Recent discoveries have uncovered novel sensors of viral components and hallmarks of infection, as well as mechanisms by which cells discriminate between self and non-self. This review highlights the mechanisms used by cells to detect viral pathogens in the cytosol, and recent advances in the field of cytosolic sensing of viruses.
Current Opinion in Virology 2017, 22:36–43
This review comes from a themed issue on Viral immunology
Edited by Jonathan W Yewdell and Guus F Rimmelzwaan
For a complete overview see the Issue and the Editorial
Available online 9th December 2016
http://dx.doi.org/10.1016/j.coviro.2016.11.012
1879-6257/© 2016 Elsevier B.V. All rights reserved.
Introduction
Human pathogenic viruses are a major global health concern, often leading to serious illness or death. Viral infection represents a significant challenge to host cells, as the ability to detect infection and inhibit viral replication is one of the key factors determining host susceptibility to infection. Many human pathogenic viruses have evolved strategies to avoid detection by the host cell, or to inhibit other antiviral factors, demonstrating the importance of antiviral innate immunity to protect against viral infection [1, 2].
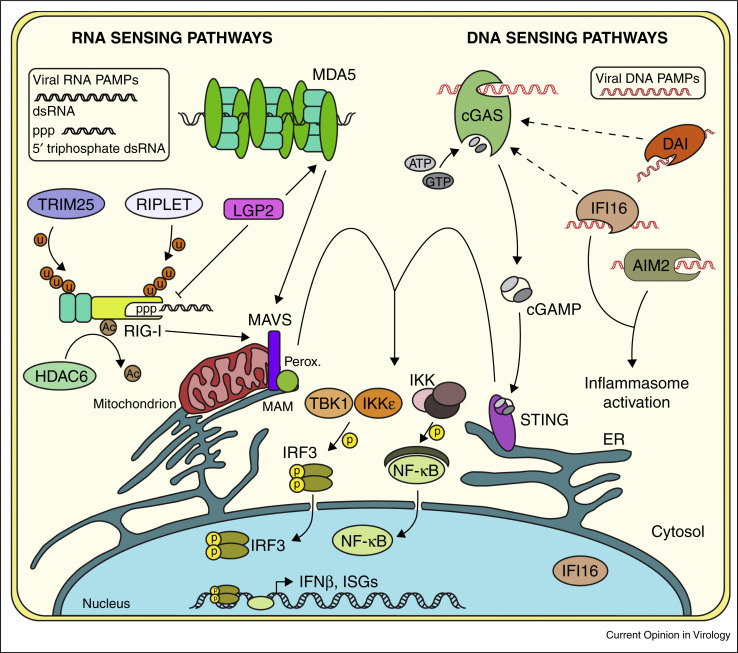
Pattern recognition receptors (PRRs) act as sensors for the products of viral infection, which are known as pathogen-associated molecular patterns (PAMPs). Viral PAMPs can include viral proteins or nucleic acids that are sensed by PRRs as non-self to elicit antiviral innate immune responses, primarily driven by type I and III interferons (IFN). This review will focus on the PRRs that sense viral nucleic acid PAMPs within the cytosol of the cell generated during RNA and DNA virus infection. We will describe how the cytosolic nucleic acid sensing PRRs, the RIG-I-like receptors (RLRs) and DNA sensors, discriminate between self and non-self to activate antiviral immune responses (Figure 1 ).
Figure 1.
Major pattern recognition receptors (PRRs) that sense RNA and DNA virus pathogen associated molecular patterns (PAMPs) in the cytosol. Following RIG-I sensing of short dsRNA, this sensor is further activated through K63-linked ubiquitination by TRIM25 and RIPLET, as well as through lysine deacetylation by HDAC6. Following sensing of long dsRNA, MDA5 oligomerizes along the dsRNA. Both RIG-I and MDA5 activate signaling through the adaptor MAVS located on mitochondria, mitochondrial-associated ER membranes (MAM), and peroxisomes (perox.), leading to the activation and nuclear translocation of IRF3 and NF-κB and the production of type I IFN and ISGs. Viral DNA PAMPs are sensed by cGAS which catalyzes the production of cGAMP after binding to DNA. cGAMP then signals through the adaptor STING, located on ER membranes to activate IRF3 and NF-κB. Other viral DNA sensors in the cytosol such as DAI and IFI16 are also postulated to function through this pathway. Activation of IFI16 or AIM2 following viral DNA detection leads to inflammasome activation.
RLRs sense cytosolic viral RNA and activate antiviral responses
The RLRs, members of the DExD/H box family of helicases, include retinoic acid-inducible gene I (RIG-I), melanoma differentiation factor 5 (MDA5), and laboratory of genetics and physiology 2 (LGP2). This family of cytosolic viral sensors is crucial for recognition of a large number of RNA viruses [3]. These sensors distinguish virus-associated RNAs from cellular RNAs to activate downstream signaling of antiviral innate immunity driven by mitochondrial antiviral signaling protein (MAVS), which aggregates into filaments following activation by PRRs [4]. Filamentous MAVS serves as a platform for interaction with other proteins involved in the signaling cascade, such as tumor necrosis factor receptor-associated factor (TRAF) proteins, which are important for MAVS signaling through TANK-binding kinase 1 (TBK1) and IκB-kinase-ɛ (IKKɛ), and the IκB kinase complex (IKK) [5]. TBK1/IKKɛ and IKK phosphorylate IRF3/IRF7 and the inhibitory subunit of NF-κB (IκBα), respectively. The transcription factors IRF3, IRF7, and NF-κB then translocate to the nucleus to induce transcription of type I IFNs (IFN-α and IFN-β). Type I IFN production drives autocrine and paracrine responses through the IFN-α/β receptor, which activates the JAK/STAT signaling pathway to ultimately induce the transcription of hundreds of IFN-stimulated genes (ISGs). These include many antiviral factors, which can inhibit viral replication in various ways [6, 7, 8].
RIG-I
RIG-I senses a number of RNA viruses including flaviviruses, alphaviruses, coronaviruses, reoviruses, paramyxoviruses, orthomyxoviruses, rhabdoviruses, arenaviruses, and bunyaviruses [9, 10]. RIG-I recognizes PAMPs, including short double-stranded RNA (dsRNA) containing either a 5′ triphosphate or 5′ diphosphate moiety that are generally unique to viral RNA [11, 12, 13•]. Interestingly, a recent report identified RIG-I as a PRR for Crimean-Congo hemorrhagic fever virus (CCHFV), whose RNA genome is 5′-monophosphorylated, implicating an additional ability of RIG-I to sense 5′-monophosphate-containing viral RNAs [14•]. Therefore, while canonical ligands of RIG-I have been identified, future research may uncover additional features of ligands, such as post-transcriptional RNA modifications, that allow RIG-I to distinguish self from non-self. Indeed, self-RNAs are distinguished from foreign RNAs by post-transcriptional modifications of their 5′ triphosphate ends, which contain the RNA cap structures. These include cap0: 7-methylguanosine addition to the gamma phosphate on the 5′ end of mRNAs; cap1: identical to cap0, with 2′-O-methylation of the first nucleotide following the 5′ triphosphate; and cap2: identical to cap1, with an additional 2′-O-methyl group on the second nucleotide. The 2′-O-methylation present in cap1 is crucial for avoiding recognition by RIG-I [15••, 16••]. In addition, 2′-O-methylation protects host mRNAs from sequestration by IFN-induced proteins with tetratricopeptide repeats (IFITs), which would otherwise inhibit the translation of these proteins [17]. In fact, certain viruses including flaviviruses, coronaviruses, and alphaviruses have co-opted cellular RNA capping strategies, likely to evade detection by RLRs [18, 19].
RIG-I contains several functional domains that regulate sensing of PAMPs and its subsequent activation. It is comprised of two N-terminal caspase activation and recruitment domains (CARDs), followed by two tandem helicase domains (Hel1, Hel2) separated by an insertion domain (Hel2i), as well as a C-terminal repressor domain (RD) [11, 20, 21]. In resting cells, Hel2i interacts with the second CARD, keeping RIG-I in an auto-inhibited state [22, 23]. The RD inhibits self-association of RIG-I, preventing its interaction with MAVS [20]. Upon sensing viral RNA, RIG-I undergoes a conformational change, in which the RD interacts with viral RNA and the helicase domains, breaking the interaction of Hel2i with the second CARD to release the CARDs for interaction with MAVS, which also contains a CARD motif [21, 22]. Following this conformational change, both the RD and CARDs are subject to post-translational modifications (PTMs). First, the RD of RIG-I is subject to Lysine (K) 63-linked poly-ubiquitination by the E3 ubiquitin ligase RIPLET [24, 25]. This promotes tripartite motif-containing protein 25 (TRIM25) K63-linked ubiquitination of the RIG-I CARDs [26]. Mex-3 RNA Binding Family Member C (MEX3C) was recently identified as an additional essential E3 ubiquitin ligase that mediates K63-linked ubiquitination on RIG-I CARDs [27], and TRIM4 was found to enhance RIG-I signaling via K63-linked ubiquitination that is redundant with TRIM25 and RIPLET [28]. Recent structural work has demonstrated that K63-linked ubiquitination of RIG-I stabilizes assembly of its CARDs into a helical tetramer. This tetramer of RIG-I CARDs facilitates interaction with MAVS, and nucleates MAVS filament formation [29]. Removal of specific PTMs, including lysine acetylation by histone deactylase 6 (HDAC6) and dephosphorylation of RIG-I CARDs by protein phosphatase 1 (PP1), is required for full RIG-I activation [30, 31, 32]. In addition to regulation by its structure and by PTMs, RIG-I is regulated at the cell biological level. In resting cells, RIG-I is localized to the cytoplasm where it can detect PAMPs of invading viruses. However, upon activation, RIG-I interacts with various proteins, including TRIM25 and 14-3-3ɛ, to form a translocon that facilitates RIG-I re-localization into intracellular membranes for interaction with MAVS [33, 34]. Taken together, these complex regulatory mechanisms prevent aberrant activation of RIG-I.
MDA5
While some overlap exists between the viruses sensed by RIG-I and MDA5 — such as flaviviruses, alphaviruses, coronaviruses, reoviruses, and paramyxoviruses — MDA5 plays an indispensable role in detection of picornaviruses and caliciviruses [35]. MDA5 has similar structural domains to RIG-I (CARDs-helicases-RD) and also signals downstream through MAVS. However, MDA5 senses long dsRNA, which are viral replication intermediates [36, 37]. Similar to RIG-I, 2′-O-methylation at the 5′ end of RNAs prevents MDA5 sensing [38]. While RIG-I recognizes the terminal end of dsRNA, MDA5 recognizes the internal duplex structure of dsRNA in a length-dependent fashion [37]. The crystal structure of MDA5 bound to dsRNA reveals that MDA5 stacks along dsRNA, forming filamentous structures, in a head-to-tail arrangement for signaling [39]. The ATP hydrolysis activity of the helicase domains is required for MDA5 filament formation [39, 40]. These filaments expose the CARDs of MDA5 for interaction with the CARD motif of MAVS [39]. PTMs also regulate MDA5 function. Like RIG-I, PP1 dephosphorylates MDA5 CARDs, leading to MDA5 activation [32]. However, unlike RIG-I, little is known about other PTMs that may regulate MDA5 activation, such as K63-linked ubiquitination [3]. In addition, little is known about the cell biology that regulates MDA5 activation. Future studies to address these aspects of the regulation of MDA5 will be of interest.
LGP2
LGP2 is less well characterized than RIG-I and MDA5, but increasing evidence suggests that LGP2 may act as a negative regulator of RIG-I-directed signaling and as an enhancer of MDA5-directed signaling [41, 42, 43•, 44]. LGP2 has similar domains to RIG-I and MDA5 (Helicase-RD), however it lacks the N-terminal CARDs required for interaction with MAVS. While it has been shown that LGP2 recognizes both dsRNA and single-stranded RNA (ssRNA), with a preference for RNA with a 5′ triphosphate, LGP2 is not able to independently activate downstream signaling to MAVS because it lacks N-terminal CARDs required for this interaction. In addition, multiple conflicting functions have been attributed to LGP2, including negative regulation of RIG-I [41, 42], as well as negative regulation of MDA5 [45], and positive regulation of MDA5 [43•, 45]. Recently, LGP2 was found to regulate the binding of MDA5 to RNA and regulate MDA5 filament assembly for enhanced signaling activity [43•]. Therefore, it seems LGP2 may serve multiple, diverse functions in response to different viruses. Further research will be required to appreciate the role and function of LGP2 in regulation of RNA virus sensing and downstream signaling.
Cytosolic DNA sensors activate antiviral responses
The presence of DNA in the cytosol is an indicator of pathogen infection or of cellular damage. Considering the multitude of intracellular pathogens capable of replicating in the cytosol, the detection of their nucleic acids is imperative for cellular defense against not only viruses, but also bacterial and eukaryotic pathogens. While sensors and pathways related to detection of RNA viruses are well defined, many sensors of viral DNA have only recently been identified. The roles of many of these proteins in initiating innate immune responses to DNA are not fully understood [46]. In addition, the signaling pathways leading to the production of IFN following detection of viral DNA PAMPs are less well defined than pathways activated by detection of RNA PAMPs. Nonetheless, the past decade has produced numerous discoveries uncovering key factors in DNA-sensing pathways, referred to as the IFN-stimulatory DNA (ISD) pathway [47, 48]. A number of cytosolic DNA sensors activate this pathway to signal through stimulator of interferon genes (STING). STING binds to and is activated by cyclic dinucleotides, such as cyclic GMP-AMP (cGAMP) [49]. Activated STING then translocates from the endoplasmic reticulum (ER) to perinuclear compartments, such as the Golgi, endosomes, and autophagy-related compartments [50, 51], which leads to its palmitoylation for signaling [52•]. It then recruits kinases that phosphorylate IRF3 and activate signaling in a fashion similar to MAVS [53••]. Interestingly, STING can also activate STAT6 for transcriptional induction of ISGs [54]. Ultimately, activation of STING signaling elicits type I IFN induction and similar antiviral response strategies as those seen in MAVS signaling. Thus, while cells use different sensors to detect RNA and DNA viruses, the signaling pathways ultimately converge in similar antiviral response strategies.
cGAS
Cyclic GMP-AMP synthase (cGAS) is the primary protein required for type I IFN induction in response to cytosolic DNA. cGAS detects DNA in the cytosol as a result of DNA virus infection or DNA transfection and synthesizes the second messenger cGAMP [55]. cGAMP subsequently binds to STING, leading to its activation [56]. While cGAS was only recently identified, it is already recognized as the primary cytosolic DNA sensor. Indeed, recent reports suggest that cGAS may mediate the ability of other DNA sensors to activate STING and the ISD pathway [57•]. cGAS senses DNA viruses such as herpesviruses, human papillomavirus, adenovirus, and hepatitis B virus, as well as retroviruses such as human immunodeficiency virus-1 (HIV-1), simian immunodeficiency virus, and murine leukemia virus [58, 59]. Additionally, cGAS plays a role in the innate immune response to a number of positive-sense RNA viruses, although it is not known whether cGAS can act as a true RNA sensor [60, 61].
Structural studies on cGAS have provided important insights into how it recognizes DNA and synthesizes the second messenger cGAMP. cGAS is composed of an N-terminal unstructured region, followed by a nucleotidyl transferase domain, and a C-terminal male abnormal 21 domain [55]. Resting cGAS exists in a bilobal conformation, with a zinc thumb located between the lobes. cGAS binds to dsDNA via this zinc thumb, which induces a conformational change in cGAS. The catalytic pocket of cGAS is then accessible for synthesis of cGAMP [59, 62, 63]. This synthesis generates 2′3′-cGAMP, an endogenous cGAMP, which contains two unique phosphodiester bonds [59, 64, 65, 66]. Interestingly, 2′3′-cGAMP binds STING with much higher affinity than cGAMP molecules with different phosphodiester linkages, demonstrating the importance of the specific product of cGAS to activate innate immunity [66]. This production of cGAMP is essential for STING activation by cGAS, as catalytically inactive cGAS does not induce type I IFN, despite its ability to bind DNA [55]. cGAMP activates STING by inducing a conformational change after which STING dimerizes and is subject to K63-linked ubiquitination by TRIM56 and TRIM32 [67, 68]. Further mechanisms governing cGAS activity, such as regulation by other PTMs, remain uncharacterized. Given its widespread or ubiquitous expression and the requirement of cGAS in the ISD pathway, future studies to uncover these regulatory mechanisms will be of importance.
DAI
DNA-dependent activator of IFN-regulatory factors (DAI) was the first cytosolic DNA sensor of antiviral innate immunity to be discovered [69]. DAI binds to cytosolic DNA derived from both viruses and host cells [69, 70]. Viruses sensed by DAI include herpes simplex virus-1 (HSV-1), human cytomegalovirus (HCMV), and mouse cytomegalovirus (MCMV) [69, 70, 71]. Interestingly, the ability of DAI to sense self-DNAs in the cytosol may play a role in the development of autoimmune disease. For example, DAI expression is upregulated in people with systemic lupus erythematosus (SLE), and in SLE mouse models [72]. The role of DAI in initiating the IFN response to cytosolic DNA appears to be either cell type-specific, or redundant, as DAI-deficient mice and cells derived from these mice elicited normal IFN responses to both DNA virus infection and synthetic DNA [73, 74].
The mechanism by which DAI senses cytosolic DNA is not well understood. A recent study used in vitro pull-down assays to show that DAI binds to DNA in a sequence-independent, but length-dependent manner [74]. Interestingly, this study also shows that DNA may serve as a scaffold upon which DAI can aggregate and that artificial dimerization of DAI induced type I IFN expression in the absence of DNA, suggesting that dimerization or oligomerization of DAI drives downstream signaling [74]. Further research will be required to gain a better understanding of the mechanisms by which DAI senses DNA. While STING is known as the major adaptor protein in DNA sensing pathways, it has not been clearly demonstrated that DAI signals through STING [75]. Though DAI may be dispensable for the innate immune response to viral DNA, future efforts should focus on dissecting the DAI pathway, given its possible roles in cell type-specific antiviral responses, and its contribution to autoimmune diseases.
AIM2-like receptors (ALRs)
The ALRs are a family of proteins that have also been suggested to acts as sensors of the ISD pathway [76, 77]. They are also known to be required for inflammasome activation in response to multiple pathogens [78, 79, 80]. The inflammasome is an inflammatory response induced following transcriptional activation of caspase-1, which then cleaves IL-1 cytokines into IL-1β and IL-18 to promote inflammation [81]. The ALR family consists of five members in humans: absent in melanoma 2 (AIM2), gamma-interferon-inducible protein 16 (IFI16), pyrin and HIN domain family member 1 (PYHIN1), myeloid cell nuclear differentiation antigen (MNDA), and pyrin domain-only protein 3 (POP3). AIM2 and IFI16 are the two most prominent members of the ALR family and are members of the pyrin and HIN domain (PYHIN) family. Both AIM2 and IFI16 have been shown to be required for inflammasome activation after DNA virus sensing. AIM2 activates inflammasomes in response to herpes simplex virus-1 (HSV-1), vaccinia virus, and mouse cytomegalovirus [80], and IFI16 activates inflammasomes in response to HSV-1 [82].
In addition to its role in inflammasome activation, IFI16 activates the ISD pathway by sensing non-self DNA in both the nucleus and cytosol [76, 77]. Depletion of IFI16 has been shown to dampen the IFN response to viruses such as the retrovirus HIV-1, HSV-1, and human cytomegalovirus (HCMV) [83, 84, 85]. While these studies have implicated IFI16 as an important sensor of both cytosolic and nuclear foreign DNA, a recent study demonstrated that in mice, ALRs are not required for the ISD response [86••]. Further, by using genetic knockouts, human IFI16 was shown to be non-essential for the IFN response to HCMV infection. Alternatively, cGAS was essential for the ISD response in both mice and human cells [86••]. Thus, similar to DAI, ALRs such as IFI16 either serve a cell type-specific role in initiating the ISD pathway, or have a redundant role with other factors. These recent findings highlight the uncertainty surrounding many putative DNA sensors, and the clear requirement of the recently discovered cGAS as the PRR of the ISD pathway.
Conclusion
Cytosolic sensing of foreign nucleic acids by PRRs is essential for an infected cell to mount antiviral responses to inhibit replication of invading viruses and prime an effective adaptive immune response [87]. Recent findings described here have contributed to our understanding of the mechanisms used by cells to recognize infection. New discoveries in the field of cytosolic nucleic acid sensing could yield important knowledge regarding how autoimmune diseases, in which aberrant sensing leads to inflammation and self-damage, are triggered. Additionally, future research will likely elucidate novel sensors of nucleic acids, regulatory factors in antiviral signaling pathways, and strategies used by viruses to antagonize these processes. These efforts could lead to novel treatment strategies for both autoimmune diseases and viral infections.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We would like to thank Dia Beachboard, Christine Vazquez, and Allison Roder for helpful discussion and reading of the manuscript. Research in the Horner Lab is supported by the National Institutes of Health (R01AI125416 and R21AI124100 (S.M.H), T32-CA009111 (M.J.M.)), as well as the Duke University Center for AIDS Research (5P30AI064518) and a Duke School of Medicine Whitehead Scholarship (S.M.H).
References
- 1.Beachboard D.C., Horner S.M. Innate immune evasion strategies of DNA and RNA viruses. Curr Opin Microbiol. 2016;32:113–119. doi: 10.1016/j.mib.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan Y.K., Gack M.U. Viral evasion of intracellular DNA and RNA sensing. Nat Rev Microbiol. 2016;14:360–373. doi: 10.1038/nrmicro.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gack M.U. Mechanisms of RIG-I-like receptor activation and manipulation by viral pathogens. J Virol. 2014;88:5213–5216. doi: 10.1128/JVI.03370-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hou F., Sun L., Zheng H., Skaug B., Jiang Q.X., Chen Z.J. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S., Chen J., Cai X., Wu J., Chen X., Wu Y.T., Sun L., Chen Z.J. MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. Elife. 2013;2:e00785. doi: 10.7554/eLife.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoggins J.W., Wilson S.J., Panis M., Murphy M.Y., Jones C.T., Bieniasz P., Rice C.M. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metz P., Dazert E., Ruggieri A., Mazur J., Kaderali L., Kaul A., Zeuge U., Windisch M.P., Trippler M., Lohmann V. Identification of type I and type II interferon-induced effectors controlling hepatitis C virus replication. Hepatology. 2012;56:2082–2093. doi: 10.1002/hep.25908. [DOI] [PubMed] [Google Scholar]
- 8.Schoggins J.W. Interferon-stimulated genes: roles in viral pathogenesis. Curr Opin Virol. 2014;6:40–46. doi: 10.1016/j.coviro.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen S., Thomsen A.R. Sensing of RNA viruses: a review of innate immune receptors involved in recognizing RNA virus invasion. J Virol. 2012;86:2900–2910. doi: 10.1128/JVI.05738-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kell A.M., Gale M., Jr. RIG-I in RNA virus recognition. Virology. 2015;479–480:110–121. doi: 10.1016/j.virol.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M., Taira K., Akira S., Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 12.Hornung V., Ellegast J., Kim S., Brzozka K., Jung A., Kato H., Poeck H., Akira S., Conzelmann K.K., Schlee M. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 13•.Goubau D., Schlee M., Deddouche S., Pruijssers A.J., Zillinger T., Goldeck M., Schuberth C., Van der Veen A.G., Fujimura T., Rehwinkel J. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates. Nature. 2014;514:372–375. doi: 10.1038/nature13590. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first study to identify RNAs bearing 5′-diphosphates as a non-canonical RIG-I ligand. In vitro transcribed 5′-diphosphate RNAs were sufficient to bind and activate RIG-I, and RIG-I recognition of 5′-diphosphate reovirus RNA was found to be essential to control reovirus infection.
- 14•.Spengler J.R., Patel J.R., Chakrabarti A.K., Zivcec M., Garcia-Sastre A., Spiropoulou C.F., Bergeron E. RIG-I mediates an antiviral response to Crimean-Congo hemorrhagic fever virus. J Virol. 2015;89:10219–10229. doi: 10.1128/JVI.01643-15. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified CCHFV RNA as a RIG-I ligand. This is the first study to implicate 5′-monophosphate RNA as a ligand for RIG-I, and demonstrates a need for future research on sensing of 5′-monophosphate viral RNA.
- 15••.Schuberth-Wagner C., Ludwig J., Bruder A.K., Herzner A.M., Zillinger T., Goldeck M., Schmidt T., Schmid-Burgk J.L., Kerber R., Wolter S. A conserved histidine in the RNA sensor RIG-I controls immune tolerance to N1-2′O-methylated self RNA. Immunity. 2015;43:41–51. doi: 10.1016/j.immuni.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors define mRNA capping modifications necessary to prevent RIG-I activation. 7-methylguanosine addition alone (cap0) cannot fully prevent RIG-I stimulation; 2′-O-methylation of the first nucleotide (cap1) of mRNAs is responsible for prevention of RIG-I stimulation. Further, the amino acid H830 in the RNA binding domain of RIG-I excludes cap1 RNAs, and mutation of this amino acid ablated the discriminatory ability of RIG-I.
- 16••.Devarkar S.C., Wang C., Miller M.T., Ramanathan A., Jiang F., Khan A.G., Patel S.S., Marcotrigiano J. Structural basis for m7G recognition and 2′-O-methyl discrimination in capped RNAs by the innate immune receptor RIG-I. Proc Natl Acad Sci U S A. 2016;113:596–601. doi: 10.1073/pnas.1515152113. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, the authors report similar findings about the function of 5′ mRNA capping strategies to avoid detection by RIG-I. Additionally, they found that 7-methylguanosine capping works synergistically with 2′-O-methylation to significantly weaken RIG-I and mRNA interaction. The same H830 amino acid residue was identified for exclusion of cap1 RNA.
- 17.Habjan M., Hubel P., Lacerda L., Benda C., Holze C., Eberl C.H., Mann A., Kindler E., Gil-Cruz C., Ziebuhr J. Sequestration by IFIT1 impairs translation of 2′O-unmethylated capped RNA. PLoS Pathog. 2013;9:e1003663. doi: 10.1371/journal.ppat.1003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daffis S., Szretter K.J., Schriewer J., Li J., Youn S., Errett J., Lin T.Y., Schneller S., Zust R., Dong H. 2′-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature. 2010;468:452–456. doi: 10.1038/nature09489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decroly E., Ferron F., Lescar J., Canard B. Conventional and unconventional mechanisms for capping viral mRNA. Nat Rev Microbiol. 2012;10:51–65. doi: 10.1038/nrmicro2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito T., Hirai R., Loo Y.M., Owen D., Johnson C.L., Sinha S.C., Akira S., Fujita T., Gale M., Jr. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc Natl Acad Sci U S A. 2007;104:582–587. doi: 10.1073/pnas.0606699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang F., Ramanathan A., Miller M.T., Tang G.Q., Gale M., Jr., Patel S.S., Marcotrigiano J. Structural basis of RNA recognition and activation by innate immune receptor RIG-I. Nature. 2011;479:423–427. doi: 10.1038/nature10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowalinski E., Lunardi T., McCarthy A.A., Louber J., Brunel J., Grigorov B., Gerlier D., Cusack S. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell. 2011;147:423–435. doi: 10.1016/j.cell.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 23.Ramanathan A., Devarkar S.C., Jiang F., Miller M.T., Khan A.G., Marcotrigiano J., Patel S.S. The autoinhibitory CARD2-Hel2i interface of RIG-I governs RNA selection. Nucleic Acids Res. 2016;44:896–909. doi: 10.1093/nar/gkv1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oshiumi H., Matsumoto M., Hatakeyama S., Seya T. Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I to promote interferon-beta induction during the early phase of viral infection. J Biol Chem. 2009;284:807–817. doi: 10.1074/jbc.M804259200. [DOI] [PubMed] [Google Scholar]
- 25.Oshiumi H., Miyashita M., Inoue N., Okabe M., Matsumoto M., Seya T. The ubiquitin ligase Riplet is essential for RIG-I-dependent innate immune responses to RNA virus infection. Cell Host Microbe. 2010;8:496–509. doi: 10.1016/j.chom.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Gack M.U., Shin Y.C., Joo C.H., Urano T., Liang C., Sun L., Takeuchi O., Akira S., Chen Z., Inoue S. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 27.Kuniyoshi K., Takeuchi O., Pandey S., Satoh T., Iwasaki H., Akira S., Kawai T. Pivotal role of RNA-binding E3 ubiquitin ligase MEX3C in RIG-I-mediated antiviral innate immunity. Proc Natl Acad Sci U S A. 2014;111:5646–5651. doi: 10.1073/pnas.1401674111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan J., Li Q., Mao A.P., Hu M.M., Shu H.B. TRIM4 modulates type I interferon induction and cellular antiviral response by targeting RIG-I for K63-linked ubiquitination. J Mol Cell Biol. 2014;6:154–163. doi: 10.1093/jmcb/mju005. [DOI] [PubMed] [Google Scholar]
- 29.Peisley A., Wu B., Xu H., Chen Z.J., Hur S. Structural basis for ubiquitin-mediated antiviral signal activation by RIG-I. Nature. 2014;509:110–114. doi: 10.1038/nature13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi S.J., Lee H.C., Kim J.H., Park S.Y., Kim T.H., Lee W.K., Jang D.J., Yoon J.E., Choi Y.I., Kim S. HDAC6 regulates cellular viral RNA sensing by deacetylation of RIG-I. EMBO J. 2016;35:429–442. doi: 10.15252/embj.201592586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H.M., Jiang F., Loo Y.M., Hsu S., Hsiang T.Y., Marcotrigiano J., Gale M., Jr. Regulation of retinoic acid inducible gene-I (RIG-I) activation by the histone deacetylase 6. EBioMedicine. 2016;9:195–206. doi: 10.1016/j.ebiom.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wies E., Wang M.K., Maharaj N.P., Chen K., Zhou S., Finberg R.W., Gack M.U. Dephosphorylation of the RNA sensors RIG-I and MDA5 by the phosphatase PP1 is essential for innate immune signaling. Immunity. 2013;38:437–449. doi: 10.1016/j.immuni.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horner S.M., Liu H.M., Park H.S., Briley J., Gale M., Jr. Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus. Proc Natl Acad Sci U S A. 2011;108:14590–14595. doi: 10.1073/pnas.1110133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H.M., Loo Y.M., Horner S.M., Zornetzer G.A., Katze M.G., Gale M., Jr. The mitochondrial targeting chaperone 14-3-3epsilon regulates a RIG-I translocon that mediates membrane association and innate antiviral immunity. Cell Host Microbe. 2012;11:528–537. doi: 10.1016/j.chom.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan Y.K., Gack M.U. RIG-I-like receptor regulation in virus infection and immunity. Curr Opin Virol. 2015;12:7–14. doi: 10.1016/j.coviro.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang D.C., Gopalkrishnan R.V., Wu Q., Jankowsky E., Pyle A.M., Fisher P.B. mda-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci U S A. 2002;99:637–642. doi: 10.1073/pnas.022637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato H., Takeuchi O., Mikamo-Satoh E., Hirai R., Kawai T., Matsushita K., Hiiragi A., Dermody T.S., Fujita T., Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zust R., Cervantes-Barragan L., Habjan M., Maier R., Neuman B.W., Ziebuhr J., Szretter K.J., Baker S.C., Barchet W., Diamond M.S. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat Immunol. 2011;12:137–143. doi: 10.1038/ni.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu B., Peisley A., Richards C., Yao H., Zeng X., Lin C., Chu F., Walz T., Hur S. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell. 2013;152:276–289. doi: 10.1016/j.cell.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 40.Motz C., Schuhmann K.M., Kirchhofer A., Moldt M., Witte G., Conzelmann K.K., Hopfner K.P. Paramyxovirus V proteins disrupt the fold of the RNA sensor MDA5 to inhibit antiviral signaling. Science. 2013;339:690–693. doi: 10.1126/science.1230949. [DOI] [PubMed] [Google Scholar]
- 41.Yoneyama M., Kikuchi M., Matsumoto K., Imaizumi T., Miyagishi M., Taira K., Foy E., Loo Y.M., Gale M., Jr., Akira S. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 42.Rothenfusser S., Goutagny N., DiPerna G., Gong M., Monks B.G., Schoenemeyer A., Yamamoto M., Akira S., Fitzgerald K.A. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J Immunol. 2005;175:5260–5268. doi: 10.4049/jimmunol.175.8.5260. [DOI] [PubMed] [Google Scholar]
- 43•.Bruns A.M., Leser G.P., Lamb R.A., Horvath C.M. The innate immune sensor LGP2 activates antiviral signaling by regulating MDA5-RNA interaction and filament assembly. Mol Cell. 2014;55:771–781. doi: 10.1016/j.molcel.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; A report addressing the role of LGP2 in MDA5 activation. LGP2 was shown to increase the initial rate of MDA5-RNA interaction, and also regulate MDA5 filament formation. LGP2 interaction resulted in shorter and more numerous MDA5 filaments, which could increase signaling relative to the longer filaments formed in the absence of LGP2.
- 44.Uchikawa E., Lethier M., Malet H., Brunel J., Gerlier D., Cusack S. Structural analysis of dsRNA binding to anti-viral pattern recognition receptors LGP2 and MDA5. Mol Cell. 2016;62:586–602. doi: 10.1016/j.molcel.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venkataraman T., Valdes M., Elsby R., Kakuta S., Caceres G., Saijo S., Iwakura Y., Barber G.N. Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses. J Immunol. 2007;178:6444–6455. doi: 10.4049/jimmunol.178.10.6444. [DOI] [PubMed] [Google Scholar]
- 46.Unterholzner L. The interferon response to intracellular DNA: why so many receptors? Immunobiology. 2013;218:1312–1321. doi: 10.1016/j.imbio.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Ishii K.J., Coban C., Kato H., Takahashi K., Torii Y., Takeshita F., Ludwig H., Sutter G., Suzuki K., Hemmi H. A toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 48.Stetson D.B., Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Burdette D.L., Monroe K.M., Sotelo-Troha K., Iwig J.S., Eckert B., Hyodo M., Hayakawa Y., Vance R.E. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishikawa H., Ma Z., Barber G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saitoh T., Fujita N., Hayashi T., Takahara K., Satoh T., Lee H., Matsunaga K., Kageyama S., Omori H., Noda T. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc Natl Acad Sci U S A. 2009;106:20842–20846. doi: 10.1073/pnas.0911267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Mukai K., Konno H., Akiba T., Uemura T., Waguri S., Kobayashi T., Barber G.N., Arai H., Taguchi T. Activation of STING requires palmitoylation at the Golgi. Nat Commun. 2016;7:11932. doi: 10.1038/ncomms11932. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors report a function for STING translocation from the ER to the Golgi. STING becomes palmitoylated at the Golgi, and this is required for the type I IFN response. These results are important for understanding autoimmune diseases involving constitutively active STING.
- 53••.Liu S., Cai X., Wu J., Cong Q., Chen X., Li T., Du F., Ren J., Wu Y.T., Grishin N.V. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 2015;347:aaa2630. doi: 10.1126/science.aaa2630. [DOI] [PubMed] [Google Scholar]; This study demonstrates a shared mechanism between MAVS, STING, and TRIF to specifically activate IRF3. MAVS, STING, and TRIF each recruit kinases to phosphorylate them following activation. This phosphorylation allows recruitment of IRF3 and its subsequent phosphorylation by TBK1.
- 54.Chen H., Sun H., You F., Sun W., Zhou X., Chen L., Yang J., Wang Y., Tang H., Guan Y. Activation of STAT6 by STING is critical for antiviral innate immunity. Cell. 2011;147:436–446. doi: 10.1016/j.cell.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 55.Sun L., Wu J., Du F., Chen X., Chen Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu J., Sun L., Chen X., Du F., Shi H., Chen C., Chen Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Orzalli M.H., Broekema N.M., Diner B.A., Hancks D.C., Elde N.C., Cristea I.M., Knipe D.M. cGAS-mediated stabilization of IFI16 promotes innate signaling during herpes simplex virus infection. Proc Natl Acad Sci U S A. 2015;112:E1773–E1781. doi: 10.1073/pnas.1424637112. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors report that cGAS interacts with IFI16 during HSV-1 infection, and stabilizes IFI16 protein. While IFI16 appears to be the direct of HSV-1, cGAS is required for full ISD signaling.
- 58.Ma Z., Damania B. The cGAS-STING defense pathway and its counteraction by viruses. Cell Host Microbe. 2016;19:150–158. doi: 10.1016/j.chom.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao D., Wu J., Wu Y.T., Du F., Aroh C., Yan N., Sun L., Chen Z.J. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341:903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schoggins J.W., MacDuff D.A., Imanaka N., Gainey M.D., Shrestha B., Eitson J.L., Mar K.B., Richardson R.B., Ratushny A.V., Litvak V. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505:691–695. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maringer K., Fernandez-Sesma A. Message in a bottle: lessons learned from antagonism of STING signalling during RNA virus infection. Cytokine Growth Factor Rev. 2014;25:669–679. doi: 10.1016/j.cytogfr.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Civril F., Deimling T., de Oliveira Mann C.C., Ablasser A., Moldt M., Witte G., Hornung V., Hopfner K.P. Structural mechanism of cytosolic DNA sensing by cGAS. Nature. 2013;498:332–337. doi: 10.1038/nature12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kranzusch P.J., Lee A.S., Berger J.M., Doudna J.A. Structure of human cGAS reveals a conserved family of second-messenger enzymes in innate immunity. Cell Rep. 2013;3:1362–1368. doi: 10.1016/j.celrep.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ablasser A., Goldeck M., Cavlar T., Deimling T., Witte G., Rohl I., Hopfner K.P., Ludwig J., Hornung V. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498:380–384. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diner E.J., Burdette D.L., Wilson S.C., Monroe K.M., Kellenberger C.A., Hyodo M., Hayakawa Y., Hammond M.C., Vance R.E. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep. 2013;3:1355–1361. doi: 10.1016/j.celrep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang X., Shi H., Wu J., Zhang X., Sun L., Chen C., Chen Z.J. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell. 2013;51:226–235. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsuchida T., Zou J., Saitoh T., Kumar H., Abe T., Matsuura Y., Kawai T., Akira S. The ubiquitin ligase TRIM56 regulates innate immune responses to intracellular double-stranded DNA. Immunity. 2010;33:765–776. doi: 10.1016/j.immuni.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 68.Zhang J., Hu M.M., Wang Y.Y., Shu H.B. TRIM32 protein modulates type I interferon induction and cellular antiviral response by targeting MITA/STING protein for K63-linked ubiquitination. J Biol Chem. 2012;287:28646–28655. doi: 10.1074/jbc.M112.362608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takaoka A., Wang Z., Choi M.K., Yanai H., Negishi H., Ban T., Lu Y., Miyagishi M., Kodama T., Honda K. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 70.DeFilippis V.R., Alvarado D., Sali T., Rothenburg S., Fruh K. Human cytomegalovirus induces the interferon response via the DNA sensor ZBP1. J Virol. 2010;84:585–598. doi: 10.1128/JVI.01748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Upton J.W., Kaiser W.J., Mocarski E.S. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang W., Zhou Q., Xu W., Cai Y., Yin Z., Gao X., Xiong S. DNA-dependent activator of interferon-regulatory factors (DAI) promotes lupus nephritis by activating the calcium pathway. J Biol Chem. 2013;288:13534–13550. doi: 10.1074/jbc.M113.457218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ishii K.J., Kawagoe T., Koyama S., Matsui K., Kumar H., Kawai T., Uematsu S., Takeuchi O., Takeshita F., Coban C. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 74.Wang Z., Choi M.K., Ban T., Yanai H., Negishi H., Lu Y., Tamura T., Takaoka A., Nishikura K., Taniguchi T. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc Natl Acad Sci U S A. 2008;105:5477–5482. doi: 10.1073/pnas.0801295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Radoshevich L., Dussurget O. Cytosolic innate immune sensing and signaling upon infection. Front Microbiol. 2016;7:313. doi: 10.3389/fmicb.2016.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Unterholzner L., Keating S.E., Baran M., Horan K.A., Jensen S.B., Sharma S., Sirois C.M., Jin T., Latz E., Xiao T.S. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kerur N., Veettil M.V., Sharma-Walia N., Bottero V., Sadagopan S., Otageri P., Chandran B. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011;9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hornung V., Ablasser A., Charrel-Dennis M., Bauernfeind F., Horvath G., Caffrey D.R., Latz E., Fitzgerald K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jones J.W., Kayagaki N., Broz P., Henry T., Newton K., O’Rourke K., Chan S., Dong J., Qu Y., Roose-Girma M. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci U S A. 2010;107:9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rathinam V.A., Jiang Z., Waggoner S.N., Sharma S., Cole L.E., Waggoner L., Vanaja S.K., Monks B.G., Ganesan S., Latz E. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo H., Callaway J.B., Ting J.P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnson K.E., Chikoti L., Chandran B. Herpes simplex virus 1 infection induces activation and subsequent inhibition of the IFI16 and NLRP3 inflammasomes. J Virol. 2013;87:5005–5018. doi: 10.1128/JVI.00082-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jakobsen M.R., Bak R.O., Andersen A., Berg R.K., Jensen S.B., Tengchuan J., Laustsen A., Hansen K., Ostergaard L., Fitzgerald K.A. IFI16 senses DNA forms of the lentiviral replication cycle and controls HIV-1 replication. Proc Natl Acad Sci U S A. 2013;110:E4571–E4580. doi: 10.1073/pnas.1311669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Orzalli M.H., DeLuca N.A., Knipe D.M. Nuclear IFI16 induction of IRF-3 signaling during herpesviral infection and degradation of IFI16 by the viral ICP0 protein. Proc Natl Acad Sci U S A. 2012;109:E3008–E3017. doi: 10.1073/pnas.1211302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li T., Chen J., Cristea I.M. Human cytomegalovirus tegument protein pUL83 inhibits IFI16-mediated DNA sensing for immune evasion. Cell Host Microbe. 2013;14:591–599. doi: 10.1016/j.chom.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86••.Gray E.E., Winship D., Snyder J.M., Child S.J., Geballe A.P., Stetson D.B. The AIM2-like receptors are dispensable for the interferon response to intracellular DNA. Immunity. 2016;45:255–266. doi: 10.1016/j.immuni.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors used genetic knockout systems to show that the ALRs in mice are not required for the type I IFN response to transfected DNA, DNA virus infection, or lentivirus infection. Additionally, IFI16 in primary human fibroblasts was dispensable for the ISD response to transfected DNA and HCMV infection. However, cGAS knockout cells did not generate an effective type I IFN response. This study further demonstrates the importance of cGAS as the primary DNA sensor in the ISD pathway.
- 87.Iwasaki A., Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]