Abstract
By convention, CD4+ T cells are activated predominantly by Major Histocompatibility Complex class II-bound peptides derived from extracellular (exogenous) antigens. It has been known for decades that alternative sources of antigen, particularly those synthesized within the antigen-presenting cell, can also supply peptides but the impact on TCD4+ responses, sometimes considerable, has only recently become appreciated. This review focuses on the contributions that studies of viral antigen have made to this shift in perspective, concluding with discussions of relevance to rational vaccine design, autoimmunity and cancer immunotherapy.
Introduction
CD4+ T cells (TCD4+) are key components in the adaptive immune responses to viral infections, receiving activation signals from antigen-derived peptides displayed at cell surfaces by major histocompatibility complex class II molecules (MHC-II). In elaborating a panoply of cytokines, TCD4+ help B cells differentiate into antibody producing plasma cells and enhance the functions of MHC class I (MHC-I)-restricted cytolytic CD8+ T cells (TCD8+) [1–4]. TCD4+, like TCD8+, can be cytolytic, an important capability in defense against several viruses including Epstein Barr virus (EBV) [5–7], ectromelia [8], influenza [9] and lymphocytic choriomeningitis virus [10]. Moreover, perforin+ TCD4+ are prevalent in the blood of human immunodeficiency virus (HIV) and EBV infected patients [11].
‘Professional’ antigen-presenting cells (pAPCs) — dendritic cells (DCs), macrophages and B cells — constitutively express MHC-II, allowing them to play many key and unique roles in host defense. Under inflammatory conditions, non-professional APCs can be induced to express MHC-II and serve as APCs [12,13].
By convention [14,15], the production of MHC-II-bound peptides begins with internalization of extracellular (exogenous) proteins by APCs [16] that are subsequently cleaved into peptides by acid proteases in the endosomal/lysosomal compartments. Simultaneously, newly synthesized MHC-II molecules in the endoplasmic reticulum (ER) are directed to the endosomal compartment by the non-covalently associated chaperone invariant chain (Ii). Upon reaching the late endosome Ii undergoes degradation, with only the portion termed Class II associated invariant chain peptide (CLIP) [17,18] surviving as it occupies the peptide-binding groove, which provides protection from proteolysis [19]. Processed peptides are exchanged for CLIP in a reaction that is catalyzed by H2-M (mouse) [20] or HLA-DM (humans) [21,22] heterodimer (hereafter referred to as DM).
Suggestions that there might be alternatives to this classical pathway were first reported over three decades ago in experiments that demonstrated the presentation of ‘self’ immunoglobulin proteins synthesized within the APC (‘endogenous’ processing) [23–26]. Subsequently, peptide elution and identification by mass spectrometry revealed that a large proportion of MHC-II-bound peptides are derived from cytosolic proteins [27–29]. Interspersed with these studies and from that time forward virus-based systems have substantially contributed to this issue in both reinforcing the notion of alternative MHC-II antigen processing and in providing mechanistic insights.
Alternative pathways involved in the generation of viral epitopes
Alternative exogenous processing — recycling MHC-II
An early study by Pinet et al. reported on an HLA-DR-restricted influenza hemagglutinin (HA) epitope whose presentation from input (exogenous) virus did not require Ii or MHC-II synthesis [30•]. Furthermore, truncating the cytoplasmic tails of DR, which prevented internalization, abrogated presentation [31]. In a subsequent publication the same group showed that the kinetics of presentation for this epitope were more rapid than another epitope processed by the classical pathway and required cysteine but not aspartic proteases (active in very low pH) evident from endosomal protease inhibitor experiments. Moreover, treatment with concanamycin B had no effect on its presentation but blocked presentation of the classically processed epitope, which suggested that presentation could take place in an early endocytic (EE) compartment [32]. Similar observations were made for a measles derived epitope, showing in addition that presentation is independent of DM expression [33]. While DM independence is generally the rule, DM dependence was described for an albumin derived peptide presented by recycling MHC-II [34].
We previously described an Ed-restricted peptide located within the stalk region of the influenza A/PR/8/34 HA that is, by all measures, presented by recycling MHC-II [35,36]. This is consistent with the biology of HA. Following internalization of virions, the stalk region of HA undergoes extensive unfolding in response to endosomal acidification, which triggers fusion of the viral and endosomal membranes and delivery of the viral genome to the cytosol. Since the class II peptide binding groove is open-ended the unfolded HA could ostensibly bind to recycling class II without any processing beyond separation from the virion. The extent to which recycling MHC-II contributes to the overall TCD4+ response to any complex pathogen has not yet been determined as the cellular mechanisms that underpin recycling are still being elucidated [37].
Endogenous processing — autophagy
Many studies have associated endogenous MHCII presentation of intracellular proteins with autophagy, a catabolic process where cytoplasmic elements are delivered to endolysosomal organelles.
Macroautophagy involves the engulfment of cytosolic materials into double membrane vesicles called autophagosomes which then fuse with lysosomes to deliver its cargo for further degradation by lysosomal enzymes [38,39]. Work from the Munz laboratory, utilizing both a small molecule inhibitor, 3-methyladenine, and short interfering RNAs demonstrated that recombinant vaccinia virus produced (endogenous) Epstein-Barr Nuclear Antigen-1 (EBNA1) activates TCD4+ via macroautophagy [5,40]. Of note, the presentation of three other epitopes within EBNA1 was substantially enhanced, in an autophagy dependent manner, upon ablation of the nuclear localization sequence, leading to the conclusion that location of the antigen governs whether macroautophagy is a viable processing mechanism [41]. In a Herpes Simplex Virus-1 mouse model, conditional knockout of the atg5 gene in conventional DCs, led to impaired TCD4+ responses in vivo [42]. Recently, autophagy-mediated presentation of ovalbumin (OVA) expressed by recombinant modified vaccinia ankara virus (MVA) was observed in an in vitro murine bone marrow derived DC (BMDC) model [43••]. Of note, Blanchet et al. reported that DCs provided with inactivated HIV virions (exogenous source) can produce the gag1 epitope from gag p24 via autophagy [44], indicating that autophagy-dependent MHC-II presentation is not restricted to endogenous antigens. Autophagy may not play a role in the endogenous processing of some viruses. Atg7 knockdown had no impact on the global MHC-II presentation of influenza epitopes [45••]. Additionally, it has been recently shown that endogenous processing of HIV gag2 and env proteins by monocyte derived DCs or HeLa cells transduced with class II associated transcriptional activator (CIITA) proceeds by an autophagy independent mechanism [46••].
In chaperone-mediated autophagy (CMA), proteins containing the relatively degenerate pentapeptide KFERQ motif are transferred from the cytoplasm into lysosomes in a lysosome associated membrane protein-2a (LAMP-2a)-dependent manner [47]. CMA has been shown to be important for the presentation of cytosolic autoantigens such as glutamate decarboxylase and a modified form of human immunoglobulin K chain [48]. In the case of microautophagy, microvesicular like bodies are produced through invaginations on lysosomal membranes that envelop cytosolic proteins and organelles [49]. To our knowledge, CMA has not yet been implicated in the generation of virus-derived epitopes and microautophagy has not been implicated in the MHC-II processing of any antigen.
Endogenous processing — bona fide cytosolic processing
Autophagy could be viewed as a variant on the classical scheme as antigen processing still takes place in the endosomal compartment. There are, however, many instances of endogenous processing where cytosolic machinery is involved. First indications of this were in the late 1980s, when many groups reported that endogenously expressed viral proteins were presented to TCD4+ [50–54].
The main engine for degradation of cytosolic proteins is the multicatalytic proteasome [55], which is best known for generating peptides conveyed via the transporter associated with antigen processing (TAP) into the endoplasmic reticulum for loading onto nascent MHC-I molecules and subsequent recognition by TCD8+. However, the proteasome can also be instrumental for the generation of MHC-II-restricted epitopes. We have demonstrated with several epitopes that in vitro presentation from endogenous sources of influenza proteins is essentially abrogated when highly specific proteasome inhibitor is provided [56,57••]. What is more, proteasome-dependent epitopes appear to drive a substantial portion of the TCD4+ response to influenza, on the order of 40–50% in the systems where analyses were conducted [56,57••]. Furthermore, we have reported that the vast majority of the TCD4+ response to influenza, much larger than 50%, is driven by endogenous sources of antigen [57••]. Our failure to implicate macroautophagy in the processing of influenza antigens [45••] implies the existence of yet unidentified endogenous processing machinery.
Earliest indications that TAP can play a role in MHC-II processing were reported by the Long laboratory who tracked presentation of a minigene-encoded peptide derived from influenza HA and expressed by a recombinant vaccinia virus. Presentation was substantially reduced in the absence of TAP [58]. The processing pathway differed from that of classical MHC-I in being inhibited by chloroquine, a lysosomotropic agent, implying involvement of the endosomal compartment in antigen processing and/or peptide loading [59,60]. Later, studies from our lab reported that endogenous production of epitopes (requiring delivery of antigen to the cytosol) from full-length forms of the influenza neuraminidase (NA) and HA glycoproteins require both proteasome and TAP function [56]. The involvement of TAP does appear to be exceptional. TAP function was dispensable for HLA-DR1-restricted presentation of a cytosolically targeted HA [58]. Moreover, our mapping of C57Bl/6 TCD4+ responses to live influenza led to the identification of thirteen I-Ab-restricted epitopes, all of which could be endogenously generated, and none requiring TAP [57••]. Of note, two of these epitopes, within the influenza nucleoprotein (NP), demonstrated full and partial proteasome dependence. Of even greater note, an NA-derived epitope was found to be dependent upon both gamma-interferon-inducible lysosomal thiol reductase (GILT) and proteasome function. Involvement of GILT is consistent with the epitope containing a cysteine residue that is disulfide bonded in the mature protein [61]. The mechanism by which a post-proteasomal intermediate is translocated into the EE, independent of TAP, for additional processing by GILT is currently under investigation. More recently, MVA-expressed OVA in BMDCs was also presented by MHC-II in a TAP-independent, proteasome dependent manner [43••].
The role for DM in loading of endogenously produced peptides appears to be quite variable. Dani et al. reported that the cytosolically targeted, proteasome dependent fusion protein I-Eα required DM for efficient loading on MHC-II [62]. On the other hand, data from our lab showed that presentation of the proteasome-, TAP-dependent, Ed-restricted epitopes within HA and NA do not require DM [56]. More recently we demonstrated with DM-knockout mice that nearly half of the thirteen IAb-restricted influenza epitopes that we mapped can be presented in a DM independent manner [57••].
Conclusion
We have proposed that the highly diverse alternative MHC-II processing pathways described in brief here (Figure 1) substantially increase the array of peptides that can be presented, consequently enhancing TCD4+ help to B cells and TCD8+ [57••]. This is consistent with our observation that immunization of mice with low doses of live influenza (enabling endogenous processing) vs. much higher doses of inactivated virus (restricting processing to exogenous pathways) results in higher titers of antibody that are more protective [57••], and reminiscent of the repeated observation that a live influenza vaccine is more protective than an inactivated vaccine in inexperienced (pediatric) individuals [63]. Such results suggest that the notion of maximizing access to alternative MHC-II processing pathways should deserve strong consideration in rational vaccine design.
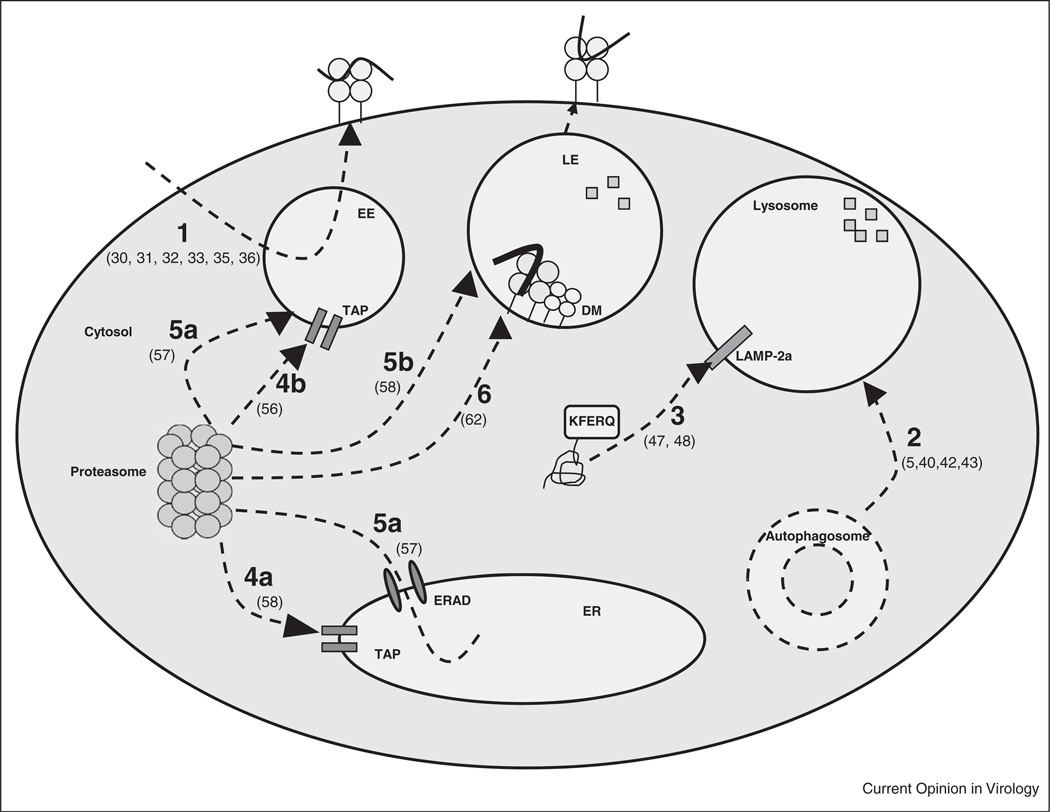
Figure 1. The Elucidation of Non-Classical MHC Class II Antigen Processing Through the Study of Viral A. Various alternative MHC-II processing pathways that have been described.
1. DM-independent recycling pathway [30•,31,32,33,35,36]; 2. Macroautophagy [5,40,42,43••]; 3. CMA [47,48]; 4a & 4b. Proteasome and TAP dependent pathway [58,56]; 5a & 5b. Proteasome dependent and TAP independent pathway [57••,58]; 6. Proteasome dependent, TAP independent and DM dependent pathway [62]. LE, late endosome; ERAD, ER associated degradation protein.
This review has focused on viral antigens but the same principles apply to the peptides that induce autoimmunity. As mentioned, targets of autoimmunity can be generated by chaperone-mediated autophagy [48] but endogenous processing has been reported for various autoantigens [64–68] and tumor antigens [69•,70]. If, as in the case of influenza, the majority of MHC-II-restricted self-peptides are produced by endogenous processing, systems that restrict candidate autoantigens to endosomal processing may miss opportunities for mechanistic insights and therapeutic strategies.
The importance of TCD4+ in cancer immunotherapy is becoming increasingly apparent [71,72]. As successful cancer immunotherapy is essentially controlled autoimmunity, principles derived in the study of autoantigen processing might be successfully transferred to this arena.
Much remains to be learned about the alternatives to conventional MHC-II antigen processing. Increased investigation in this regard could provide important insights into how host responses to viral infections, self-antigens and tumor-specific antigens evolve, and how enhanced therapeutic approaches to all three areas might be developed.
Acknowledgments
Our work is funded by grants R01AI113286 and R01AI123644 from the National Institutes of Health
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Riberdy JM, Christensen JP, Branum K, Doherty PC. Diminished primary and secondary influenza virus-specific CD8(+) T-cell responses in CD4-depleted Ig−/− mice. J Virol. 2000;74:9762–9765. doi: 10.1128/jvi.74.20.9762-9765.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belz GT, Wodarz D, Diaz G, Nowak MA, Doherty PC. Compromised influenza virus-specific CD8(+)-T-cell memory in CD4(+)-T-cell-deficient mice. J Virol. 2002;76:12388–12393. doi: 10.1128/JVI.76.23.12388-12393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 4.Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, Ye L, Sharpe AH, Freeman GJ, Ahmed R. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci USA. 2011;108:21182–21187. doi: 10.1073/pnas.1118450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munz C, Bickham KL, Subklewe M, Tsang ML, Chahroudi A, Kurilla MG, Zhang D, O’Donnell M, Steinman RM. Human CD4(+) T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J Exp Med. 2000;191:1649–1660. doi: 10.1084/jem.191.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paludan C, Bickham K, Nikiforow S, Tsang ML, Goodman K, Hanekom WA, Fonteneau JF, Stevanovic S, Munz C. Epstein-Barr nuclear antigen 1-specific CD4(+) Th1 cells kill Burkitt’s lymphoma cells. J Immunol. 2002;169:1593–1603. doi: 10.4049/jimmunol.169.3.1593. [DOI] [PubMed] [Google Scholar]
- 7.Su Z, Peluso MV, Raffegerst SH, Schendel DJ, Roskrow MA. Antigen presenting cells transfected with LMP2a RNA induce CD4+ LMP2a-specific cytotoxic T lymphocytes which kill via a Fas-independent mechanism. Leuk Lymphoma. 2002;43:1651–1662. doi: 10.1080/1042819021000002992. [DOI] [PubMed] [Google Scholar]
- 8.Fang M, Siciliano NA, Hersperger AR, Roscoe F, Hu A, Ma X, Shamsedeen AR, Eisenlohr LC, Sigal LJ. Perforin-dependent CD4(+) T-cell cytotoxicity contributes to control a murine poxvirus infection. Proc Natl Acad Sci U S A. 2012;109:9983–9988. doi: 10.1073/pnas.1202143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown DM, Lee S, Garcia-Hernandez ML, Swain SL. Multifunctional CD4 cells expressing gamma interferon and perforin mediate protection against lethal influenza virus infection. J Virol. 2012;86:6792–6803. doi: 10.1128/JVI.07172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jellison ER, Kim SK, Welsh RM. Cutting edge: MHC class II-restricted killing in vivo during viral infection. J Immunol. 2005;174:614–618. doi: 10.4049/jimmunol.174.2.614. [DOI] [PubMed] [Google Scholar]
- 11.Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, Easterbrook P, Grey P, Smith D, McMichael AJ, Cooper DA, Rowland-Jones SL, Kelleher AD. Characterization of CD4(+) CTLs ex vivo. J Immunol. 2002;168:5954–5958. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 12.Duraes FV, Thelemann C, Sarter K, cha-Orbea H, Hugues S, Reith W. Role of major histocompatibility complex class II expression by non-hematopoietic cells in autoimmune and inflammatory disorders: facts and fiction. Tissue Antigens. 2013;82:1–15. doi: 10.1111/tan.12136. [DOI] [PubMed] [Google Scholar]
- 13.Reith W, LeibundGut-Landmann S, Waldburger JM. Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immunol. 2005;5:793–806. doi: 10.1038/nri1708. [DOI] [PubMed] [Google Scholar]
- 14.Babbitt BP, Allen PM, Matsueda G, Haber E, Unanue ER. Pillars article: Binding of immunogenic peptides to Ia histocompatibility molecules. Nature. 1985;317:359–361. doi: 10.1038/317359a0. The Journal of Immunology 2005, 175:4163–4165. [DOI] [PubMed] [Google Scholar]
- 15.Shimonkevitz R, Kappler J, Marrack P, Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983;158:303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 17.Riese RJ, Wolf PR, Bromme D, Natkin LR, Villadangos JA, Ploegh HL, Chapman HA. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 1996;4:357–366. doi: 10.1016/s1074-7613(00)80249-6. [DOI] [PubMed] [Google Scholar]
- 18.Villadangos JA, Bryant RA, Deussing J, Driessen C, Lennon-Dumenil AM, Riese RJ, Roth W, Saftig P, Shi GP, Chapman HA, Peters C, Ploegh HL. Proteases involved in MHC class II antigen presentation. Immunol Rev. 1999;172:109–120. doi: 10.1111/j.1600-065x.1999.tb01360.x. [DOI] [PubMed] [Google Scholar]
- 19.Mouritsen S, Meldal M, Werdelin O, Hansen AS, Buus S. MHC molecules protect T cell epitopes against proteolytic destruction. J Immunol. 1992;149:1987–1993. [PubMed] [Google Scholar]
- 20.Wolf PR, Tourne S, Miyazaki T, Benoist C, Mathis D, Ploegh HL. The phenotype of H-2M-deficient mice is dependent on the MHC class II molecules expressed. Eur J Immunol. 1998;28:2605–2618. doi: 10.1002/(SICI)1521-4141(199809)28:09<2605::AID-IMMU2605>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 21.Denzin LK, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 22.Sherman MA, Weber DA, Jensen PE. DM enhances peptide binding to class II MHC by release of invariant chain-derived peptide. Immunity. 1995;3:197–205. doi: 10.1016/1074-7613(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 23.Bikoff E, Birshtein BK. T cell clones specific for IgG2a of the a allotype: direct evidence for presentation of endogenous antigen. J Immunol. 1986;137:28–34. [PubMed] [Google Scholar]
- 24.Bikoff EK, Eckhardt LA. Presentation of IgG2a antigens to class II-restricted T cells by stably transfected B lymphoma cells. Eur J Immunol. 1989;19:1903–1909. doi: 10.1002/eji.1830191022. [DOI] [PubMed] [Google Scholar]
- 25.Weiss S, Bogen B. B-lymphoma cells process and present their endogenous immunoglobulin to major histocompatibility complex-restricted T cells. Proc Natl Acad Sci USA. 1989;86:282–286. doi: 10.1073/pnas.86.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss S, Bogen B. MHC class II-restricted presentation of intracellular antigen. Cell. 1991;64:767–776. doi: 10.1016/0092-8674(91)90506-t. [DOI] [PubMed] [Google Scholar]
- 27.Rudensky AY, Preston-Hurlburt P, Hong SC, Barlow A, Janeway CA., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991;353:622–627. [PubMed] [Google Scholar]
- 28.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 29.Chicz RM, Urban RG, Gorga JC, Vignali DA, Lane WS, Strominger JL. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pinet V, Malnati MS, Long EO. Two processing pathways for the MHC class II-restricted presentation of exogenous influenza virus antigen. J Immunol. 1994;152:4852–4860. The data presented in this paper serves as the first clear evidence for processing of a viral antigen in the absence of newly synthesized protein and invariant chain.
- 31.Pinet V, Vergelli M, Martini R, Bakke O, Long E. Antigen presentation mediated by recycling of surface HLA-DR molecules. Nature. 1995;375:603–606. doi: 10.1038/375603a0. [DOI] [PubMed] [Google Scholar]
- 32.Pinet VMaLEO. Peptide loading onto recycling HLA-DR molecules occurs in early endosomes. Eur J Immunol. 1998;28:799–804. doi: 10.1002/(SICI)1521-4141(199803)28:03<799::AID-IMMU799>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 33.Demotz S. Processing of DR1-restricted determinants from the fusion protein of measles virus following two distinct pathways. Mol Immunol. 1996;33:387. doi: 10.1016/0161-5890(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 34.Pathak SS, Lich JD, Blum JS. Cutting edge: editing of recycling class II: peptide complexes by HLA-DM. J Immunol. 2001;167:632–635. doi: 10.4049/jimmunol.167.2.632. [DOI] [PubMed] [Google Scholar]
- 35.Chianese-Bullock KA, Russell HI, Moller C, Gerhard W, Monaco JJ, Eisenlohr LC. Antigen processing of two H2-IEd-restricted epitopes is differentially influenced by the structural changes in a viral glycoprotein. J Immunol. 1998;161:1599–1607. [PubMed] [Google Scholar]
- 36.Sinnathamby G, Eisenlohr LC. Presentation by recycling MHC class II molecules of an influenza hemagglutinin-derived epitope that is revealed in the early endosome by acidification. J Immunol. 2003;170:3504–3513. doi: 10.4049/jimmunol.170.7.3504. [DOI] [PubMed] [Google Scholar]
- 37.Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol. 2015;15:203–216. doi: 10.1038/nri3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berg TO, Fengsrud M, Stromhaug PE, Berg T, Seglen PO. Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. J Biol Chem. 1998;273:21883–21892. doi: 10.1074/jbc.273.34.21883. [DOI] [PubMed] [Google Scholar]
- 39.Liou W, Geuze HJ, Geelen MJ, Slot JW. The autophagic and endocytic pathways converge at the nascent autophagic vacuoles. J Cell Biol. 1997;136:61–70. doi: 10.1083/jcb.136.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, Munz C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 41.Leung CS, Haigh TA, Mackay LK, Rickinson AB, Taylor GS. Nuclear location of an endogenously expressed antigen, EBNA1, restricts access to macroautophagy and the range of CD4 epitope display. Proc Natl Acad Sci USA. 2010;107:2165–2170. doi: 10.1073/pnas.0909448107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee HK, Mattei LM, Steinberg BE, Alberts P, Lee YH, Chervonsky A, Mizushima N, Grinstein S, Iwasaki A. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity. 2010;32:227–239. doi: 10.1016/j.immuni.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thiele F, Tao S, Zhang Y, Muschaweckh A, Zollmann T, Protzer U, Abele R, Drexler I. Modified vaccinia virus ankara-infected dendritic cells present CD4+ T-cell epitopes by endogenous major histocompatibility complex class II presentation pathways. J Virol. 2015;89:2698–2709. doi: 10.1128/JVI.03244-14. This paper has used a recombinant MVA vector expressing OVA and demonstrated its immunogenicity in primary DCs. Endogenous processing of OVA was proteasome dependent but TAP independent and was also shown to be mediated by autophagy. Understanding the underlying presentation pathways can help in exploiting the efficacy of this vector for better vaccine development.
- 44.Blanchet FP, Moris A, Nikolic DS, Lehmann M, Cardinaud S, Stalder R, Garcia E, Dinkins C, Leuba F, Wu L, Schwartz O, Deretic V, Piguet V. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity. 2010;32:654–669. doi: 10.1016/j.immuni.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Comber JD, Robinson TM, Siciliano NA, Snook AE, Eisenlohr LC. Functional macroautophagy induction by influenza A virus without a contribution to major histocompatibility complex class II-restricted presentation. J Virol. 2011;85:6453–6463. doi: 10.1128/JVI.02122-10. Autophagy was a phenomenon associated with delivery of cytosolic cargo to lysosomal compartments; however, this paper described an autophagy independent pathway for the endogenous processing of influenza derived proteins.
- 46. Coulon PG, Richetta C, Rouers A, Blanchet FP, Urrutia A, Guerbois M, Piguet V, Theodorou I, Bet A, Schwartz O, Tangy F, Graff-Dubois S, Cardinaud S, Moris A. HIV-infected dendritic cells present endogenous MHC class II: restricted antigens to HIV-specific CD4+ T cells. J Immunol. 2016;197:517–532. doi: 10.4049/jimmunol.1600286. This work has used HIV to demonstrate endogenous MHC-II processing. Processing was independent of autophagy and proteasome function but susceptible to endosomal proteolysis. However, when epitopes were targeted to autophagosomes by LC3 fusion protein, the CD4+ T cell responses were greater in magnitude and in turn diversified the endogenous epitopes.
- 47.Dice JF. Chaperone-mediated autophagy. Autophagy. 2007;3:295–299. doi: 10.4161/auto.4144. [DOI] [PubMed] [Google Scholar]
- 48.Zhou D, Li P, Lin Y, Lott JM, Hislop AD, Canaday DH, Brutkiewicz RR, Blum JS. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity. 2005;22:571–581. doi: 10.1016/j.immuni.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 49.Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, Potolicchio I, Nieves E, Cuervo AM, Santambrogio L. Microautophagy of cytosolic proteins by late endosomes. Dev Cell. 2011;20:131–139. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobson S, Sekaly RP, Bellini WJ, Johnson CL, McFarland HF, Long EO. Recognition of intracellular measles virus antigens by HLA class II restricted measles virus-specific cytotoxic T lymphocytes. Ann N Y Acad Sci. 1988;540:352–353. doi: 10.1111/j.1749-6632.1988.tb27096.x. [DOI] [PubMed] [Google Scholar]
- 51.Jaraquemada D, Marti M, Long EO. An endogenous processing pathway in vaccinia virus-infected cells for presentation of cytoplasmic antigens to class II-restricted T cells. J Exp Med. 1990;172:947–954. doi: 10.1084/jem.172.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jean-Daniel Doucet, Marie-Andrée Forget, Cécile Grange, Ronan Nicolas Rouxel, Nathalie Arbour, Veronika von Messling, Réjean Lapointe. Endogenously expressed matrix protein M1 and nucleoprotein of influenza A are efficiently presented by class I and class II major histocompatibility complexes. J Gen Virol. 2011:1162–1171. doi: 10.1099/vir.0.029777-0. [DOI] [PubMed] [Google Scholar]
- 53.Eisenlohr LC, Hackett CJ. Class II major histocompatibility complex-restricted T cells specific for a virion structural protein that do not recognize exogenous influenza virus. Evidence that presentation of labile T cell determinants is favored by endogenous antigen synthesis. J Exp Med. 1989;169:921–931. doi: 10.1084/jem.169.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hegde NR, Dunn C, Lewinsohn DM, Jarvis MA, Nelson JA, Johnson DC. Endogenous human cytomegalovirus gB is presented efficiently by MHC class II molecules to CD4+ CTL. J Exp Med. 2005;202:1109–1119. doi: 10.1084/jem.20050162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kisselev AF, Akopian TN, Woo KM, Goldberg AL. The sizes of peptides generated from protein by mammalian 26 and 20 S proteasomes: implications for understanding the degradative mechanism and antigen presentation. J Biol Chem. 1999;274:3363–3371. doi: 10.1074/jbc.274.6.3363. [DOI] [PubMed] [Google Scholar]
- 56.Tewari MK, Sinnathamby G, Rajagopal D, Eisenlohr LC. A cytosolic pathway for MHC class II-restricted antigen processing that is proteasome and TAP dependent. Nat Immunol. 2005;6:287–294. doi: 10.1038/ni1171. [DOI] [PubMed] [Google Scholar]
- 57. Miller MA, Ganesan AP, Luckashenak N, Mendonca M, Eisenlohr LC. Endogenous antigen processing drives the primary CD4+ T cell response to influenza. Nat Med. 2015;21:1216–1222. doi: 10.1038/nm.3958. This work shows that majority of CD4+ T cell responses to influenza derived proteins are generated by endogenous processing. More than half of the epitopes were presented in DM-independent non-classical manner. Dissection of pathways revealed an interplay of network of pathways for efficient epitope generation. Moreover, from a vaccine standpoint, a single dose of live virus elicited better and durable antibody responses than inactivated virus.
- 58.Malnati MS, Marti M, LaVaute T, Jaraquemada D, Biddison W, DeMars R, Long EO. Processing pathways for presentation of cytosolic antigen to MHC class II-restricted T cells. Nature. 1992;357:702–704. doi: 10.1038/357702a0. [DOI] [PubMed] [Google Scholar]
- 59.Morrison LA, Lukacher AE, Braciale VL, Fan DP, Braciale TJ. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986;163:903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ziegler HK, Unanue ER. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci USA. 1982;79:175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arunachalam B, Phan UT, Geuze HJ, Cresswell P. Enzymatic reduction of disulfide bonds in lysosomes: characterization of a Gamma-interferon-inducible lysosomal thiol reductase (GILT) Proc Natl Acad Sci U S A. 2000;97:745–750. doi: 10.1073/pnas.97.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dani AA. The pathway for MHCII-mediated presentation of endogenous proteins involves peptide transport to the endolysosomal compartment. J Cell Sci. 2004;117:4219–4230. doi: 10.1242/jcs.01288. [DOI] [PubMed] [Google Scholar]
- 63.Johnson PRP. Comparison of long-term systemic and secretory antibody responses in children given live, attenuated, or inactivated influenza A vaccine. J Med Virol. 1985;17:325–335. doi: 10.1002/jmv.1890170405. [DOI] [PubMed] [Google Scholar]
- 64.Lich JD, Elliott JF, Blum JS. Cytoplasmic processing is a prerequisite for presentation of an endogenous antigen by major histocompatibility complex class II proteins. J Exp Med. 2000;191:1513–1524. doi: 10.1084/jem.191.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mukherjee P, Dani A, Bhatia S, Singh N, Rudensky AY, George A, Bal V, Mayor S, Rath S. Efficient presentation of both cytosolic and endogenous transmembrane protein antigens on MHC class II is dependent on cytoplasmic proteolysis. J Immunol. 2001;167:2632–2641. doi: 10.4049/jimmunol.167.5.2632. [DOI] [PubMed] [Google Scholar]
- 66.Costantino CM, Spooner E, Ploegh HL, Hafler DA. Class II MHC self-antigen presentation in human B and T lymphocytes. PLoS One. 2012;7:e29805. doi: 10.1371/journal.pone.0029805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Lierop MJC, den Hoed L, Houbiers J, Vencovsky J, Ruzickova S, Krystufkova O, van Schaardenburg M, van den Hoogen F, Vandooren B, Baeten D, de Keyser F, Sonderstrup G, Bos E, Boots AM. Endogenous HLA—DR-restricted presentation of the cartilage antigens human cartilage gp-39 and melanoma inhibitory activity in the inflamed rheumatoid joint. Arthritis Rheum. 2007;56:2150–2159. doi: 10.1002/art.22651. [DOI] [PubMed] [Google Scholar]
- 68.Katz-Levy Y, Neville KL, Girvin AM, Vanderlugt CL, Pope JG, Tan LJ, Miller SD. Endogenous presentation of self myelin epitopes by CNS-resident APCs in Theiler’s virus infected mice. J Clin Invest. 1999;104:599–610. doi: 10.1172/JCI7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Matsuzaki J, Tsuji T, Luescher I, Old LJ, Shrikant P, Gnjatic S, Odunsi K. Non-classical antigen processing pathways are required for MHC class II-restricted direct tumor recognition by NY-ESO-1-specific CD4(+) T cells. Cancer Immunol Res. 2014;2:341–350. doi: 10.1158/2326-6066.CIR-13-0138. This work is an example of non-canonical MHC-II presentation pathway of a tumor antigen, NY-ESO-1. It is processed in a proteasome dependent and TAP independent manner. Hence recognition of endogenous tumor antigens on MHC-II molecules could potentially evoke a robust CD4+ T cell response and can help in devising better therapies for cancer.
- 70.Tsuji T, Matsuzaki J, Caballero OL, Jungbluth AA, Ritter G, Odunsi K, Old LJ, Gnjatic S. Heat shock protein 90-mediated peptide-selective presentation of cytosolic tumor antigen for direct recognition of tumors by CD4+ T cells. J Immunol. 2012;188:3851–3858. doi: 10.4049/jimmunol.1103269. [DOI] [PubMed] [Google Scholar]
- 71.Maus MV, Fraietta JA, Levine BL, Kalos M, Zhao Y, June CH. Adoptive immunotherapy for cancer or viruses. Annu Rev Immunol. 2014;32:189–225. doi: 10.1146/annurev-immunol-032713-120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kamphorst AO, Ahmed R. CD4 T-cell immunotherapy for chronic viral infections and cancer. Immunotherapy. 2013;5:975–987. doi: 10.2217/imt.13.91. [DOI] [PMC free article] [PubMed] [Google Scholar]