Abstract
Exposure to ethanol (EtOH) or methamphetamine (MA) can lead to increase in extracellular glutamate concentration in the brain. Although studies from ours showed the effects of EtOH exposure on key glial glutamate transporters, there is less known about the effects of sequential exposure to EtOH and MA or MA alone on certain glial glutamate transporters. In this study, we investigated the effects of sequential exposure to EtOH and MA on the expression of the major glutamate transporter, glutamate transporter 1 (GLT-1), as well as cystine/glutamate antiporter (xCT) and glutamate aspartate transporter (GLAST) in striatum and hippocampus. We also tested the effects of ceftriaxone (CEF), known to upregulate GLT-1, in animals administered EtOH and MA. Wistar rats were orally gavaged with EtOH (6 g/kg) or water for seven days. On the following day (Day 8), rats received four intraperitoneal (i.p.) injections of MA (10 mg/kg) or saline (vehicle) occurring every 2 hours. Rats were then treated with CEF (200 mg/kg/day, i.p.) or saline on Day 8, 9 and 10. EtOH or MA exposure caused a significant downregulation of GLT-1 expression as compared to control groups in striatum and hippocampus. Furthermore, sequential exposure of EtOH and MA caused a significant downregulation of GLT-1 expression as compared to either drug administered alone in both brain regions. Importantly, GLT-1 expression was restored following CEF treatment. These findings demonstrated that sequential exposure to EtOH and MA has synergistic effect in downregulation of GLT-1 and this effect can be attenuated by CEF treatment.
Keywords: GLT-1, methamphetamine, ethanol, glutamate, xCT, GLAST, gavage
INTRODUCTION
Evidence demonstrated increases in ethanol (EtOH) intake with methamphetamine (MA) exposure, which was associated with elevation on the rewarding effects when compared to either drug administered alone (Kirkpatrick et al., 2012; Bujarski et al., 2014). Concomitant exposure to EtOH and MA increased the reward effects and decreased impairments in sleep and performance (Kirkpatrick et al., 2012), and stimulated the release of dopamine in striatum (Nishiguchi et al., 2002; Yamauchi et al., 2000). Thus, concomitant exposure to EtOH and MA can lead to clinical challenges for diagnosis and treatment because of their interactive effects (Sepehrmanesh et al., 2014).
Several studies focused on the role of glutamate in MA abuse (Abekawa et al., 1994; Nash and Yamamoto, 1992; Qi et al., 2009). MA increased the extracellular glutamate concentrations in striatum (Nash and Yamamoto, 1992) and hippocampus (Raudensky and Yamamoto, 2007). It has been shown that repeated high dose treatment with MA (10 mg/kg every 2 hrs × 4) can lead to damage of dopaminergic terminals and increases of extracellular glutamate concentration (Thomas et al., 2004; Halpin et al., 2013; Bowyer et al., 1994). In addition, studies reported that repeated MA exposure can lead to increase in both extracellular concentrations of dopamine and glutamate in the striatum (Nash and Yamamoto, 1992) and hippocampus (Rocher and Gardier, 2001). Although, it is now well known about the effects of MA exposure in the increase of extracellular glutamate in specific brain regions, there is little known about its effects on glutamate transporters after MA administration or MA administered sequentially with EtOH.
Moreover, it has been demonstrated that EtOH exposure can decrease glutamate uptake and increase extracellular glutamate concentrations in nucleus accumbens (Das et al., 2015; Melendez et al., 2005). Also, chronic EtOH consumption downregulated the expression of major glutamate transporters such as glutamate transporter 1 (GLT-1, its homolog is excitatory amino acid 2) and cystine/glutamate exchanger (xCT) (Rao and Sari, 2014; Sari and Sreemantula, 2012; Aal-Aaboda et al., 2015; Alasmari et al., 2015; Alhaddad et al., 2014; Das et al., 2015). In addition, binge EtOH gavage (4 g/kg, 3 to 4 times a day) decreases the expression of GLT-1 and produces withdrawal signs upon cessation to EtOH exposure (Abulseoud et al., 2014; Das et al., 2016). Glutamate homeostasis is regulated by a number of transporters, including GLT-1, xCT, and glutamate aspartate transporter (GLAST). The present study was aimed to examine the effects of sequential exposure of EtOH and MA as well as the drug alone on GLT-1, xCT, and GLAST expression. We suggested that hyperglutamatergic state induced by MA or EtOH might be associated with deficit in glutamate transporters. We hypothesized that CEF, known GLT-1 upregulator, would normalize the expression of these glutamate transporters after exposure to sequential EtOH and MA in both striatum and hippocampus.
Materials and Methods
Drugs
(+) MA hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO). CEF (Sandoz Inc., Princeton, NJ) was purchased from The University of Toledo Medical Center Pharmacy. Both drugs were dissolved in saline solution (0.9% NaCl). EtOH (190 proof, Decon Lab) was diluted with water for oral gavage.
Animals
Male Wistar rats were used in this study to examine the effects of sequential EtOH and MA exposure on selective glutamate transporters. Wistar rats were chosen due to their higher EtOH threshold effect compared to other rats (Abulseoud et al., 2014). Wistar rats were received from Harlan Laboratories. Rats were housed in standard plastic tube with free access to food and water throughout the experiments. Rats were kept in 21°C with relative humidity of 50% on a regular 12 hrs light/dark cycle. The experiments and housing procedures were approved by the Institutional Animal Care and Use committee at The University of Toledo with guidelines followed by the Institutional Animal Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory.
EtOH and MA exposure as well as CEF treatment
Timeline of the experimental design is illustrated in Table 1. Wistar rats were divided randomly into six groups: (a) Water gavaged group received i.p saline (i.p.) then saline (i.p.); (b) Water gavaged group received MA (i.p.) then saline (i.p.); (c) Water gavaged group received MA (i.p.) then CEF (i.p.); (d) EtOH gavaged group received saline (i.p.) then saline (i.p.); (e) EtOH gavaged group received MA (i.p.) then saline (i.p.); (f) EtOH gavaged group received MA (i.p.) then CEF (i.p.). Rats received oral gavage of water (control) or EtOH (6 g/kg) for seven days. At Day 8, rats received total of four injections of saline or MA (10 mg/kg) every 2 hrs; and saline or CEF (200 mg/kg, i.p.) was administered 30 minutes following the last MA i.p. injection. At Day 9, rats received saline or CEF 24 hrs following the last MA i.p. injections. At Day 10, rats received last saline or CEF dose, and then rats were euthanized with CO2 inhalation followed by decapitation.
Table 1.
Timeline of experimental design
| Days | Days 1 – 7 | Day 8 | Day 9 and 10 |
|---|---|---|---|
| Treatment | EtOH (6 g/kg) or water oral gavage |
MA (10 mg/kg, i.p) or saline every 2 hrs, total 4 doses |
CEF (200 mg/kg, i.p) or saline |
| CEF (200 mg/kg, i.p) or saline |
Brain tissue dissection
Dorsal striatum and Hippocampus were isolated using a cryostat (Leica CM1950) with guidance of rat brain atlas (Paxinos, 2006). Brain regions were kept at −80°C for immunoblotting to detect the selected protein targets.
Immunoblotting procedures
Immunoblotting procedures were done as previously described (Sari et al., 2010; Sari et al., 2009). In brief, striatum and hippocampus samples were lysed in lysis buffer. Quantified proteins were separated through 10% polyacrylamide gels in 1× laemmli buffer. Proteins were then transferred onto PVDF membrane (Bio-Rad Laboratories). Membranes were further blocked with 3 % blocking buffer (fat free milk, LabScientific) in 1× TBST. The membranes were then incubated with primary antibodies (GLT-1, xCT or GLAST) overnight at 4°C. β-tubulin was used as a loading control. Membranes were incubated with chemiluminescent substrate (Super Signal West Pico, Thermo Scientific) and exposed to autoradiography films (Denvilla Scientific Inc.). The membranes were then developed using X-Ray film processor (Konica SRX101A – Tabletop). Images of immunoblots were quantified using MCID Digital Imaging Software.
Statistical analysis
Analyses of the effects of MA, EtOH, and CEF on GLT-1, xCT, and GLAST expression were performed using one-way analysis of variance (ANOVA) followed by Newman-Keuls multiple comparison tests. An independent t-test was performed for further comparison between individual groups. All statistical analyses were done using p value less than 0.05 (p<0.05) as level of significance.
Results
Effects of MA and CEF on GLT-1 expression in striatum and hippocampus in water or EtOH oral gavaged rats
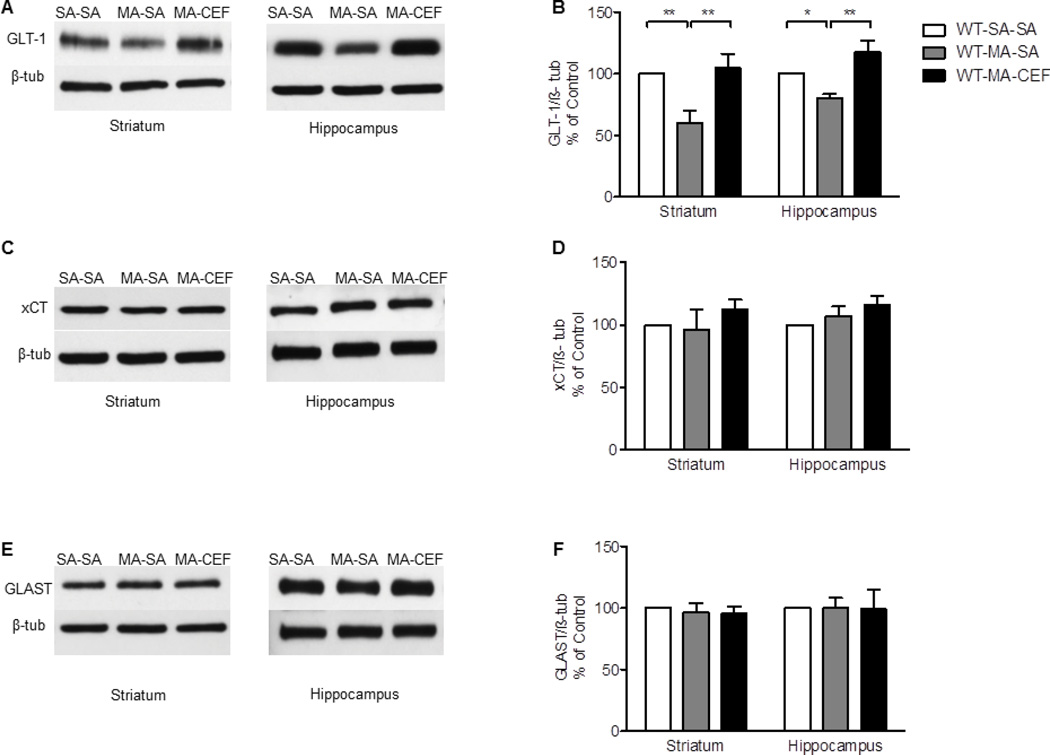
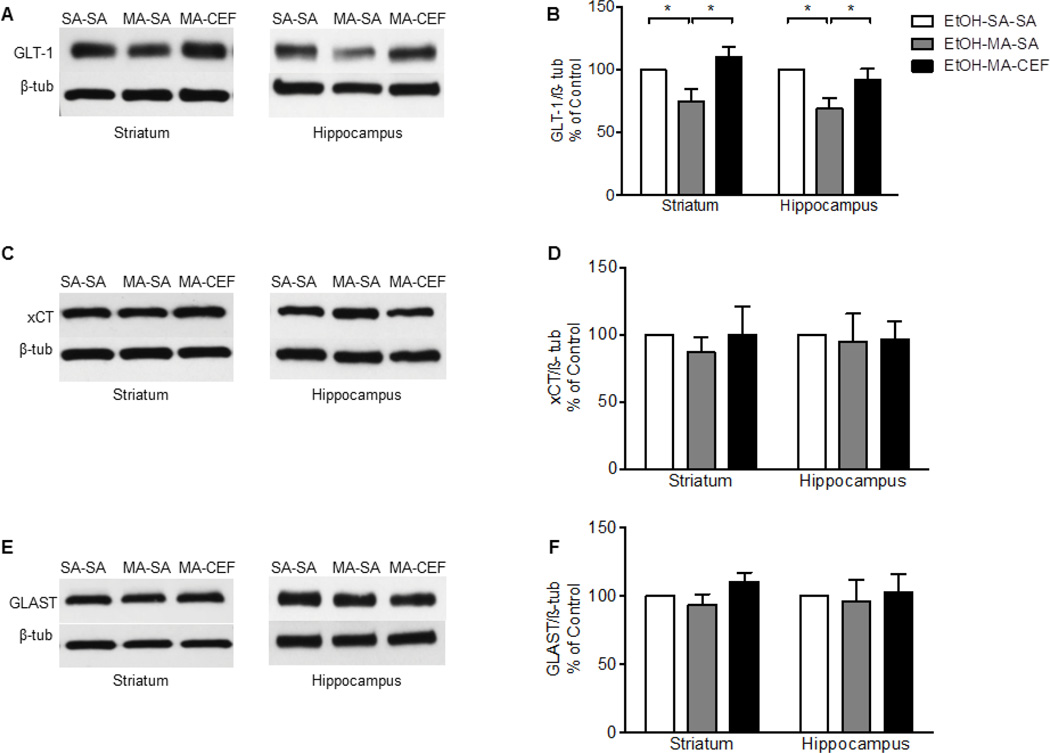
We investigated the effects of CEF on GLT-1 expression in striatum and hippocampus in rats that received water (WT) or EtOH through oral gavage procedure and challenged with repeated high dose of MA. One-way ANOVA revealed a significant main effect among water saline-saline (WT-SA-SA), water methamphetamine-saline (WT-MA-SA), and water methamphetamine-ceftriaxone (WT-MA-CEF) groups in striatum [F (2, 15) = 7.587, p= 0.0053] and hippocampus [F (2, 15) = 9.662, p= 0.002]. Newman-Keuls multiple comparison post-hoc test demonstrated a significant increase in the expression of GLT-1 in WT-MA-CEF treated groups compared to WT-MA-SA treated groups in striatum and hippocampus (p<0.01; Fig. 1A, B. Alternatively, quantitative analysis showed a significant downregulation of GLT-1expression in WT-MA-SA treated groups as compared to control groups in striatum and hippocampus (p<0.05; Fig. 1A, B. Statistical analysis revealed no significant effects between WT-SA-SA and WT-MA-CEF-treated groups rats in striatum and hippocampus. Furthermore, one-way ANOVA revealed a significant effect between ethanol-saline-saline (EtOH-SA-SA), ethanol-methamphetamine-saline (EtOH-MA-SA), and ethanol-methamphetamine-ceftriaxone (EtOH-MA-CEF) in striatum [F (2, 15) = 6.092, p=0.0116] and hippocampus [F (2, 15) = 5.467, p=0.0165]. Newman-Keuls multiple comparison post-hoc test revealed a significant increase in the expression of GLT-1 in EtOH-MA-CEF treated groups compared to EtOH-MA-SA in striatum and hippocampus (p<0.05, Fig. 2A, B. However, no significant effects were found between EtOH-SA-SA and EtOH-MA-CEF treated groups in striatum and hippocampus (Fig. 2A, B.
Figure 1.
Effects of MA (10 mg/kg i.p. every 2 hrs ×4) and CEF (200 mg/kg) on GLT-1, xCT and GLAST expression in water groups in both striatum and hippocampus. (A, C, and E) Immunoblots for GLT-1, xCT and GLAST, respectively. (B) Quantitative analysis showed a significant increase in the ratio of GLT-1/β-tubulin in WT-MA-CEF groups compared to WT-MA-SA groups in striatum and hippocampus. Significant downregulation of GLT-1 expression was observed in WT-MA-SA groups compared to control WT-SA-SA groups in striatum and hippocampus. No significant difference in GLT-1 expression was found in WT-MA-CEF groups compared to WT-SA-SA group in both brain regions. (D) Quantitative analysis showed no significant difference in the ratio of xCT/β-tubulin between all tested groups in striatum and hippocampus. (F) Quantitative analysis showed no significant difference in the ratio of GLAST/β-tubulin between all tested groups in striatum and hippocampus. Values shown as means ± S.E.M (*p < 0.05 **p < 0.01) (n = 6 for each group).
Figure 2.
Effects of MA (10 mg/kg i.p. every 2 hrs ×4), EtOH (6 g/kg), and CEF (200 mg/kg) on GLT-1, xCT and GLAST in EtOH groups in both striatum and hippocampus. (A, C, and E) Immunoblots for GLT-1, xCT and GLAST, respectively. (B) Quantitative analysis showed a significant increase in the ratio of GLT-1/β-tubulin in EtOH-MA-CEF groups compared to EtOH-MA-SA group in striatum and hippocampus. A significant downregulation of GLT-1 expression was found in EtOH-MA-SA groups compared to control EtOH-SA-SA groups in both brain regions. No significant difference in GLT-1 expression was found in EtOH-MA-CEF group compared to EtOH-SA control group. (D) Quantitative analysis did not reveal any significant difference in the ratio of xCT/β-tubulin between all tested groups in both brain regions. (F) Quantitative analysis did not reveal any significant difference in the ratio of GLAST/β-tubulin between all tested groups in both brain regions. Values shown as means ± S.E.M (*p < 0.05) (n=6–7 for each group).
Effects of MA and CEF on xCT expression in striatum and hippocampus in water or EtOH oral gavaged rats
We examined the effects of CEF on xCT expression in striatum and hippocampus in rats received water or EtOH through oral gavage and challenged with repeated high dose of MA. One-way ANOVA followed by Newman-Keuls multiple comparison post-hoc test showed no significant effects between WT-SA-SA, WT-MA-SA, and WT-MA-CEF groups in striatum [F (2, 15) = 0.2770, p=0.7619, Fig. 1C, D] and hippocampus [F (2, 15) = 1.724, p=0.2119, Fig. 1C, D]. One-way ANOVA followed by Newman-Keuls multiple comparison post-hoc test demonstrated no significant changes in the expression of xCT between EtOH-SA-SA, EtOH-MA-SA, and EtOH-MA-CEF in striatum [F (2, 15) = 0.3416, p=0.3416, Fig. 2 C, D] and hippocampus [F (2, 15) = 0.03487, p=0.9658, Fig. 2 C, D].
Effects of MA and CEF on GLAST expression in striatum and hippocampus in water or EtOH gavaged rats
GLAST expression was investigated to evaluate the effects of CEF in striatum and hippocampus in rats received water or EtOH through oral gavage and challenged with repeated high dose of MA. One-way ANOVA followed by Newman-Keuls multiple comparison post-hoc test revealed no significant changes in the expression of GLAST between WT-SA-SA, WT-MA-SA, and WT-MA-CEF in both striatum [F (2, 15) = 0.1772, p=0.8393, Fig. 1 E, F] and hippocampus [F (2, 18) = 0.1153, p=0.8917, Fig. 1 E, F]. One-way ANOVA followed by Newman-Keuls multiple comparison post-hoc test revealed no significant changes in the expression of GLAST between EtOH-SA-SA, EtOH-MA-SA, and EtOH-MA-CEF [F (2, 15) = 2.151, p=0.1509, Fig. 2 F] and hippocampus [F (2, 15) = 0.08096, p=0.9226, Fig. 2 E, F].
Effects of exposure of EtOH alone or with MA on GLT-1 expression in striatum and hippocampus
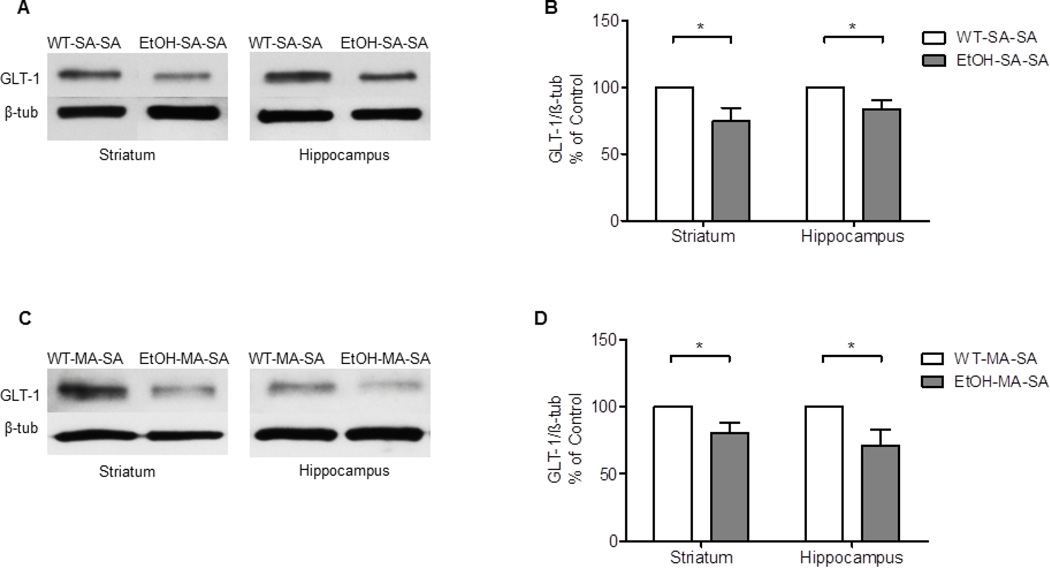
The effects of sequential exposure of EtOH and MA on the expression of GLT-1 in were examined in both striatum and hippocampus. An Independent t-test showed a significant decrease in the expression of GLT-1 in EtOH-SA-SA groups compared to WT-SA-SA in striatum (p=0.0285, Fig. 3A, B and hippocampus (p=0.0432, Fig. 3A, B. The effects of EtOH and MA on the expression of GLT-1 were examined in both striatum and hippocampus. An Independent t-test showed a significant decrease of GLT-1 expression in EtOH-MA-SA groups compared to WT-MA-SA in striatum (p<0.0255, Fig. 3C, D and hippocampus (p<0.0399, Fig. 3C, D.
Figure 3.
Effects of MA (10 mg/kg i.p. every 2 hrs ×4) and EtOH (6 g/kg) on GLT-1 expression in striatum and hippocampus. (A, C) Immunoblots of GLT-1 in WT-SA-SA and EtOH-SA-SA groups as well as in WT-MA-SA and EtOH-MA-SA groups, respectively. (B) Quantitative analysis revealed a significant downregulation of GLT-1 expression in EtOH-SA-SA groups compared to WT-SA-SA groups in striatum and hippocampus, respectively. (D) Quantitative analysis revealed a significant downregulation of GLT-1 expression in EtOH-MA-SA groups compared to WT-MA-SA groups in striatum and hippocampus, respectively. Values shown as means ± S.E.M (*p < 0.05) (n = 6 for each group).
Discussion
Several studies have reported the negative effects of MA and EtOH exposure in the brain (Kirkpatrick et al., 2012; Kim et al., 2006). Concomitant exposure of EtOH and MA might be lethal, as MA can produce stimulating effects that counteract the depressing effects of EtOH and consequently increases EtOH seeking behavior (Bujarski et al., 2014). The rewards effects have been reported to be greater when MA is consumed with EtOH (Kirkpatrick et al., 2012). Studies from our laboratory reported that chronic EtOH consumption disturbs glutamate homeostasis via downregulation of GLT-1and xCT (Aal-Aaboda et al., 2015; Rao and Sari, 2014; Sari and Sreemantula, 2012; Sari et al., 2013; Das et al., 2015). However, little is known about GLT-1 and the other glutamate transporters (xCT and GLAST) following MA exposure. Importantly, the present study revealed for the first time that repeated high dose MA (10 mg/kg every 2 hrs, × 4 i.p.) downregulated GLT-1 expression in both the striatum and hippocampus, which might be associated with increases in extracellular glutamate levels as reported in previous studies (Nash and Yamamoto, 1992; Halpin et al., 2013; Bowyer et al., 1994). However, it has been reported that MA at lower dose (2.0 mg/kg) can increase GLT-1 expression in prefrontal cortex in mice, but not in hippocampus (Qi et al., 2012). We suggest that downregulation of GLT-1 expression may occur only when repeated high dose of MA is applied.
Oral gavage of EtOH alone was found to downregulate GLT-1 expression in striatum and hippocampus. In contrast, the use of low EtOH dose (1g/kg) for 7 days has been reported to decrease glutamate uptake but did not show any changes in the expression of GLT-1 or GLAST (Melendez et al., 2005). In the present study, however, rats were gavaged with EtOH (6 g/kg) for 7 days, which represent the repeated binge dosing paradigm of EtOH (Faingold, 2008). In addition, it has been shown that rats administered EtOH using a binge gavage protocol (4 g/kg, 3 to 4 times a day) reduced GLT-1 expression in the nucleus accumbens, medial prefrontal cortex and striatum (Das et al., 2016; Abulseoud et al., 2014). Interestingly, MA and EtOH exposure has synergistic effect in the downregulation of GLT-1 expression as compared to either drug administered alone. GLT-1 is responsible for the majority of glutamate uptake in the brain (Danbolt, 2001). Thus, inhibition of GLT-1 synthesis or blockade of GLT-1 can lead to increase glutamate extracellular concentration (Rothstein et al., 1996; Das et al., 2015). In fact, GLT-1 has been linked to many neurodegenerative diseases such as Alzheimer’s, Huntington’s, Amyotrophic lateral sclerosis (ALS) and epilepsy (Tanaka et al., 1997; Lievens et al., 2001; Lauderback et al., 2001; Rothstein et al., 1995). The elevation in glutamate extracellular concentration also activates post synaptic receptors such as N-methyl-D-aspartate receptor (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA), and Kainate receptors (KA) (Choi, 1992; Meldrum, 2000; Sattler and Tymianski, 2001). Excessive extracellular glutamate concentrations can lead to overstimulation of postsynaptic receptors, which is pivotal in neurodegenerative diseases and neural death (Maragos et al., 1987; Scott et al., 2002; Howland et al., 2002; Gao et al., 2000). This suggests that MA and EtOH may exacerbate glutamatergic excitotoxicity as compared to either drug administered alone.
CEF is known to upregulate GLT-1 expression in different brain regions, including both striatum (Sari et al., 2010) and hippocampus (Aal-Aaboda et al., 2015). It is important to note that several β-lactam antibiotics have been reported to upregulate GLT-1 (Rothstein et al., 2005); and CEF, in particular, was able to attenuate cocaine seeking through normalizing GLT-1 and restoring glutamate homeostasis (Knackstedt et al., 2010). In addition, it has also been found that CEF reduced cocaine seeking in animal model through upregulation of GLT-1 in nucleus accumbens and prefrontal cortex (Sari et al., 2009). In addition, CEF attenuated the hyperactivity and behavioral changes associated with amphetamine (Rasmussen et al., 2011). Furthermore, studies from our laboratory have demonstrated the ability of CEF to decrease EtOH consumption and normalize GLT-1 expression (Sari et al., 2013; Rao and Sari, 2014). Therefore, CEF was one of the best medications in attenuating glutamatergic excitotoxicity that might be associated with EtOH and MA co-abuse. Therefore, we examined the ability of CEF on restoring GLT-1 expression following MA and EtOH. Remarkably, CEF successfully restored GLT-1expression following MA exposure in all tested water and EtOH groups in both striatum and hippocampus.
The expression of xCT and GLAST following METH and EtOH were also investigated; however, no significant changes were found in either striatum or hippocampus. It is well known that xCT plays an important role in regulating extracellular glutamate concentration in striatum (Baker et al., 2002) and drug seeking [for review see Ref. (Reissner and Kalivas, 2010). It is noteworthy, that we did not find any significant changes in xCT expression in EtOH compared with water group which is consistent with our previous findings that xCT was not downregulated in binge EtOH drinking (Das et al., 2016). There is possibility that ETOH or MA may induce dysfunction of xCT without affecting its expression. Studies are warranted to investigate the effects of MA and EtOH in the function of xCT in specific areas of the brain. Furthermore, GLAST is also responsible for clearing extracellular glutamate concentration; however, this transporter is mainly expressed in other brain regions such as cerebellum and inner ear (Lehre and Danbolt, 1998). Studies have shown that GLAST was not altered in chronic EtOH exposure (Alhaddad et al., 2014) and ALS neurodegenerative disease (Rothstein et al., 1995). However, these studies demonstrated that GLT-1 was downregulated, but GLAST was not affected. Together, we suggest that GLT-1 was more vulnerable to the insults of EtOH and MA exposure as compared to xCT and GLAST.
In conclusion, this study revealed for the first time that repeated high dose of MA downregulated GLT-1 expression in striatum and hippocampus, which may provide a possible pharmacological mechanism in the increase of extracellular glutamate concentrations in specific brain regions. This study also revealed for the first time the effects of sequential EtOH and MA administration on downregulation of GLT-1 but not xCT and GLAST expression in striatum and hippocampus. In addition, there was a synergistic effect of EtOH and MA exposure in downregulation of GLT-1 in striatum and hippocampus as compared to drug administered alone. Importantly, CEF was able to restore GLT-1 expression in MA administered alone or sequentially administered with EtOH. These findings suggest that GLT-1 might be a potential target in EtOH and MA co-abuse, and CEF might alleviate EtOH and MA induced hyperglutamatergic state through the upregulation of GLT-1 expression.
Acknowledgments
This work was supported in part by Award Number R01AA019458 (Y.S.) from the National Institutes on Alcohol Abuse and Alcoholism and also by start-up funds from the University of Toledo. The authors would like to thank Alqassem Hakami, Atiah Almalki, Fawaz Alasmari, and Sujan Das for their help in the experiments.
Footnotes
Author Contributions
FSA participated in study design and conceptualization, drafted and revised the manuscript, collected and analyzed the data. YSA collected the data and helped with the editing of manuscript. YS conceptualized and designed the study, critically revised the manuscript for intellectual content, and approved the final version of the manuscript.
Disclosure Statements
The authors declare no conflict of interest.
References
- Aal-Aaboda M, Alhaddad H, Osowik F, Nauli SM, Sari Y. Effects of (R)-(−)-5-methyl-1-nicotinoyl-2-pyrazoline on glutamate transporter 1 and cysteine/glutamate exchanger as well as ethanol drinking behavior in male, alcohol-preferring rats. J Neurosci Res. 2015 doi: 10.1002/jnr.23554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abekawa T, Ohmori T, Koyama T. Effects of repeated administration of a high dose of methamphetamine on dopamine and glutamate release in rat striatum and nucleus accumbens. Brain Res. 1994;643:276–281. doi: 10.1016/0006-8993(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Abulseoud OA, Camsari UM, Ruby CL, Kasasbeh A, Choi S, Choi D-S. Attenuation of Ethanol Withdrawal by Ceftriaxone-Induced Upregulation of Glutamate Transporter EAAT2. Neuropsychopharmacology. 2014;39:1674–1684. doi: 10.1038/npp.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari F, Abuhamdah S, Sari Y. Effects of ampicillin on cystine/glutamate antiporter and glutamate transporter 1 isoforms as well as ethanol drinking in male P rats. Neuroscience letters. 2015;600:148–152. doi: 10.1016/j.neulet.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Das SC, Sari Y. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology. 2014;231:4049–4057. doi: 10.1007/s00213-014-3545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, Xi Z-X, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. The Journal of Neuroscience. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. Journal of Pharmacology and Experimental Therapeutics. 1994;268:1571–1580. [PubMed] [Google Scholar]
- Bujarski S, Roche DJ, Lunny K, Moallem NR, Courtney KE, Allen V, Hartwell E, Leventhal A, Rohrbaugh T, Ray LA. The relationship between methamphetamine and alcohol use in a community sample of methamphetamine users. Drug Alcohol Depend. 2014;142:127–132. doi: 10.1016/j.drugalcdep.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. Journal of neurobiology. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Das SC, Althobaiti YS, Alshehri FS, Sari Y. Binge ethanol withdrawal: Effects on post-withdrawal ethanol intake, glutamate-glutamine cycle and monoamine tissue content in P rat model. Behav Brain Res. 2016 doi: 10.1016/j.bbr.2016.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, Sari Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold CL. The Majchrowicz binge alcohol protocol: an intubation technique to study alcohol dependence in rats. Current Protocols in Neuroscience. 2008 doi: 10.1002/0471142301.ns0928s44. 9.28. 21–29.28. 12. [DOI] [PubMed] [Google Scholar]
- Gao X-M, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. American Journal of Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- Halpin LE, Northrop NA, Yamamoto BK. Ammonia Mediates Methamphetamine-Induced Increases in Glutamate and Excitotoxicity. Neuropsychopharmacology. 2013;39:1031–1038. doi: 10.1038/npp.2013.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proceedings of the National Academy of Sciences. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon Sung Y, Kim J, Kwon D-H, Chang KH, Renshaw PF. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. The International Journal of Neuropsychopharmacology. 2006;9:221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Levin FR, Foltin RW, Hart CL. Acute and residual interactive effects of repeated administrations of oral methamphetamine and alcohol in humans. Psychopharmacology. 2012;219:191–204. doi: 10.1007/s00213-011-2390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biological psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer's disease brain: the role of Aβ1–42. Journal of neurochemistry. 2001;78:413–416. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. The Journal of Neuroscience. 1998;18:8751–8757. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievens J-C, Woodman B, Mahal A, Spasic-Boscovic O, Samuel D, Kerkerian-Le Goff L, Bates G. Impaired glutamate uptake in the R6 Huntington's disease transgenic mice. Neurobiology of disease. 2001;8:807–821. doi: 10.1006/nbdi.2001.0430. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Greenamyre JT, Penney JB, Jr, Young AB. Glutamate dysfunction in Alzheimer's disease: an hypothesis. Trends in Neurosciences. 1987;10:65–68. [Google Scholar]
- Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130:1007s–1015s. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcoholism: Clinical and Experimental Research. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3, 4-methylenedioxymethamphetamine. Brain Res. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- Nishiguchi M, Kinoshita H, Taniguchi T, Utsumi T, Ouchi H, Minami T, Hishida S. Effects of chronic alcohol administration on changes of extracellular dopamine and serotonin concentration induced by methamphetamine--comparison of two different alcohol preference rat lines. Nihon Arukoru Yakubutsu Igakkai zasshi= Japanese journal of alcohol studies & drug dependence. 2002;37:555–576. [PubMed] [Google Scholar]
- Paxinos G. The Rat Brain in Stereotaxic Coordinates. Academic Press; 2006. [Google Scholar]
- Qi J, Han WY, Yang JY, Wang LH, Dong YX, Wang F, Song M, Wu CF. Oxytocin regulates changes of extracellular glutamate and GABA levels induced by methamphetamine in the mouse brain. Addiction biology. 2012;17:758–769. doi: 10.1111/j.1369-1600.2012.00439.x. [DOI] [PubMed] [Google Scholar]
- Qi J, Yang J-Y, Wang F, Zhao Y-N, Song M, Wu C-F. Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology. 2009;56:856–865. doi: 10.1016/j.neuropharm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Rao PS, Sari Y. Effects of ceftriaxone on chronic ethanol consumption: a potential role for xCT and GLT1 modulation of glutamate levels in male P rats. J Mol Neurosci. 2014;54:71–77. doi: 10.1007/s12031-014-0251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen B, Unterwald EM, Rawls SM. Glutamate transporter subtype 1 (GLT-1) activator ceftriaxone attenuates amphetamine-induced hyperactivity and behavioral sensitization in rats. Drug and alcohol dependence. 2011;118:484–488. doi: 10.1016/j.drugalcdep.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudensky J, Yamamoto BK. Effects of chronic unpredictable stress and methamphetamine on hippocampal glutamate function. Brain Res. 2007;1135:129–135. doi: 10.1016/j.brainres.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Kalivas PW. Using glutamate homeostasis as a target for treating addictive disorders. Behav Pharmacol. 2010;21:514–522. doi: 10.1097/FBP.0b013e32833d41b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocher C, Gardier AM. Effects of repeated systemic administration of d-Fenfluramine on serotonin and glutamate release in rat ventral hippocampus: comparison with methamphetamine using in vivo microdialysis. NAUNYN SCHMIEDEBURGS ARCHIVES OF PHARMACOLOGY. 2001;363:422–428. doi: 10.1007/s002100000381. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Hoberg MD, Vidensky S, Chung DS. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Annals of neurology. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- Sari Y, Prieto AL, Barton SJ, Miller BR, Rebec GV. Ceftriaxone-induced up-regulation of cortical and striatal GLT1 in the R6/2 model of Huntington’s disease. J Biomed Sci. 2010;17:2426–2435. doi: 10.1186/1423-0127-17-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. The Journal of Neuroscience. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN. Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience. 2012;227:327–335. doi: 10.1016/j.neuroscience.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN, Lee MR, Choi DS. Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. J Mol Neurosci. 2013;51:779–787. doi: 10.1007/s12031-013-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Molecular neurobiology. 2001;24:107–129. doi: 10.1385/MN:24:1-3:107. [DOI] [PubMed] [Google Scholar]
- Scott HL, Pow DV, Tannenberg A, Dodd PR. Aberrant expression of the glutamate transporter excitatory amino acid transporter 1 (EAAT1) in Alzheimer's disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:RC206–RC206. doi: 10.1523/JNEUROSCI.22-03-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehrmanesh Z, Ahmadvand A, Moraveji A. Comorbidity and Pattern of Substance Use in Hospitalized Psychiatric Patients. Iranian Red Crescent medical journal. 2014;16 doi: 10.5812/ircmj.19282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. Journal of Pharmacology and Experimental Therapeutics. 2004;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Yamauchi J, Marukawa S, Hishida S. Simultaneous administration of ethanol emphasizes the effect of methamphetamine on central nervous system in rat with high alcohol preference. Nihon Arukoru Yakubutsu Igakkai zasshi= Japanese journal of alcohol studies & drug dependence. 2000;35:28–47. [PubMed] [Google Scholar]