Abstract
Objective
Surgery remains the cornerstone therapy for colorectal cancer (CRC). This study assesses CRC quality measures for surgical cases in Michigan.
Methods
In this retrospective cohort study, processes of care and outcomes for CRC resection cases were abstracted in 30 hospitals in the Michigan Surgical Quality Collaborative (2014- 2015). Measures were case-mix and reliability adjusted, using logistic regression models.
Results
For 871 cases (640 colon cancer, 231 rectal cancer), adjusted morbidity (27.4%) and mortality rates (1.5%) were low. Adjusted process measures showed gaps in quality of care. Mesorectal excision was documented in 59.4% of rectal cancer (RC) cases, 65% of RC cases had sphincter preserving surgery, 18.7% of cases had < 12 lymph nodes examined, 7.9% had a positive margin, 52.1% of stage II/III RC cases had neoadjuvant therapy, and 36% of ostomy cases had site marking.
Conclusion
This study finds gaps in quality of care measures for CRC, suggesting opportunity for regional quality improvement.
Keywords: colorectal cancer, quality improvement, process measures, outcome assessment, Michigan Surgical Quality Collaborative (MSQC)
Introduction
Surgical resection is the cornerstone of treatment for non-metastatic colorectal cancer (CRC). Thus, the quality of care provided by surgeons and their teams may have a substantial effect on CRC outcomes. Surgical technique and evidence-based perioperative care may affect short-term morbidity and mortality, while coordination of care and leadership of multidisciplinary teams may affect long-term cancer outcomes, through accurate staging and appropriate adjuvant and neoadjuvant therapy.
Unfortunately published evidence has suggested that care and outcomes vary for CRC in the United States and internationally. Cohort studies demonstrate variable short-term surgical outcomes, with complication rates ranging from 20% to 40%, and mortality rates from 3% to 6% [1–3]. Healthcare utilization also varies, including length of stay and readmission rates. Most importantly, there is variability in cancer-specific quality measures, such as adequate lymph node examination, rate of margin positivity, and use of mesorectal excision for rectal cancer [4]. However, hospitals in United States have limited ability to assess their quality of care for CRC, due to the lack of quality assessment programs, such as those established in Europe and Ontario [4]. The Institute of Medicine has called American cancer care a “system in crisis”, and calls for increased quality of care measurements to guide improvement in the United States [5].
In this context, we established a CRC-specific quality assessment program in the Michigan Surgical Quality Collaborative (MSQC) to better understand variability in postoperative outcomes, resource utilization, and CRC-specific process measures. After 18 months collecting data for this program, we present this prospective assessment of quality of care for CRC in 30 Michigan hospitals.
Methods
Study Setting
This was a retrospective cohort study of prospectively-collected data from the MSQC. The MSQC is a quality improvement organization funded by Blue Cross and Blue Shield of Michigan. MSQC participants are community and academic hospitals throughout the state. Details of MSQC data abstraction and data quality assurance have been previously described [6]. In brief, 72 Michigan hospitals participate in the MSQC collaborative, and these represent the state’s hospitals that perform major surgery. Specially trained data abstractors (nurses) prospectively collect patient characteristics, intraoperative data, laboratory results, and 30-day outcomes for patients undergoing specified general and vascular operations, utilizing a sampling algorithm that minimizes selection bias. For this study, 30 self-selected hospitals participated in a “colorectal cancer project” from 2014 to 2015. These hospitals abstracted additional data on colorectal cancer resections, and account for approximately 40% of all MSQC colorectal cancer cases. These hospitals are a mix of small and large hospitals, academic and community. Data collection and review for MSQC is institutional review board exempt. Per the data use agreement, all analyses are performed on the hospital level.
Patient Population
This study included colorectal cancer resections abstracted into the MSQC registry, with eligibility defined by CPT procedure and ICD diagnosis codes. All cases are adult patients, and eligible cases included colorectal resections for a primary (not recurrent) colorectal adenocarcinoma.
Outcomes
Data was abstracted from hospital medical records, on clinical and pathologic cancer stage, 30-day outcomes, operative and perioperative care processes, and utilization measures. CRC-specific quality measures included surgeon factors (sphincter preservation rate and documentation of performing mesorectal excision in rectal cancer resections, and use of minimally-invasive surgery), pathology factors (12 or more lymph nodes examined, positive margin rate), and multi-disciplinary factors (neoadjuvant radiation therapy for stage II and III rectal cancer and documentation of preoperative ostomy site marking in rectal cancer cases in which an ostomy was performed).
Statistical Analysis
Descriptive statistics were used to characterize the cohort of patients. Outcomes and utilization were summarized at the patient level, with point estimates and 95% confidence intervals (CI) based on logistic regression models. All case-mix adjustment models included the following covariates – surgical acuity, gender, BMI, ASA, tumor stage, age, and number of comorbidities present. Additional variables of clinical importance - or found empirically to be statistically associated with an outcome - were added to individual models. Specifically, mortality rate also adjusted for steroid/immunosuppressive use, ostomy creation, wound classification, nutritional status, operative duration, functional status, cardiac disease, and preoperative sepsis. The model for morbidity adjusted for steroid/immunosuppressive use, ostomy creation, wound classification, nutritional status, and operative duration. The length of stay model added to the mortality model the following variables: open wound, anemia, pneumonia, cirrhosis, ascites, lactic acidosis, COPD, and hyperglycemia. The operative duration model included nutritional status, ostomy creation, sleep apnea, bilirubin, and lactic acidosis. The readmission model included steroid use, ostomy creation, would classification, nutritional status, operative duration, and open wound. Reoperation and transfusion models used the same adjustments as readmission except that reoperation included cardiac disease and dialysis, and transfusion included cardiac disease and anemia.
To compare hospitals’ performance on the process of care measures, we generated hospitals’ case mix- and reliability-adjusted rates using two-stage logistic regression models. All of the models adjusted for surgical acuity, gender, BMI, ASA, tumor stage, age, tumor location, type of surgical resection, and number of comorbidities present. The adjustments were accomplished using a two stage approach. Stage one involved case-mix adjustment at case or patient level for case mix as a fixed effect. The second stage was reliability adjustment at the hospital level. Reliability adjustments were performed to ensure that risk-adjusted outcomes from hospitals with small case numbers were not skewed due to statistical “noise.” These calculations shift the estimate for complication rate back toward the average rate for the entire cohort, with the degree of shift proportional to the reliability measure of each hospital. Statistical analyses were performed using SAS Analytics Software version 9.4 (College Station, Texas).
Results
Patient Characteristics
871 patients underwent resection for CRC in 30 hospitals. 73% were colon and 27% were rectal cancer resections. Patient demographic, clinical, and tumor characteristics are presented in Table I. The majority of cases were Stage II and III. Most colon cancer resections were partial colectomies and most rectal cancer operations were low anterior resections. Of the rectal cancer resections, location was evenly distributed between upper, mid and low rectum. Surprisingly, 24% of resections were urgent or emergent (29% of colon cancer operations and 8% of rectal cancer resections).
Table I.
Patient and Tumor Characteristics (Unadjusted, n=871)
| Characteristics | Colon Cancer (n=640) | Rectal Cancer (n=231) |
|---|---|---|
| Age, mean (SD) | 68.0 (13.8) | 62.5 (12.8) |
| Gender, Male, n (%) | 320 (50.0) | 131 (56.7) |
| Race | ||
| White, n (%) | 532 (83.1) | 196 (84.8) |
| Black, n (%) | 77 (12.0) | 21 (9.1) |
| Other, n (%) | 31 (4.8) | 14 (6.1) |
| BMI, mean (SD) | 29.3 (6.6) | 27.8 (6.2) |
| Smoker, n (%) | 97 (15.2) | 49 (21.2) |
| Steroid/Immunosuppression Use, n (%) | 25 (3.9) | 9 (3.9) |
| ASA Classification | ||
| I, II, n (%) | 212 (33.1) | 102 (44.2) |
| III, n (%) | 362 (56.6) | 116 (50.2) |
| IV, V, n (%) | 66 (10.3) | 13 (5.6) |
| Stage | ||
| I, n (%) | 131 (20.5) | 51 (22.1) |
| II, n (%) | 190 (29.7) | 54 (23.4) |
| III, n (%) | 200 (31.3) | 70 (30.3) |
| IV, n (%) | 87 (13.6) | 30 (13.0) |
| Unknown, n (%) | 32 (5.0) | 26 (11.3) |
| Type of Surgery | ||
| Partial Colectomy, n (%) | 529 (82.7) | 18 (7.8) |
| Total Colectomy, n (%) | 7 (1.1) | 4 (1.7) |
| Total Proctocolectomy, n (%) | 3 (0.5) | 10 (4.3) |
| Anterior Resection, n (%) | 62 (9.7) | 131 (56.7) |
| Abdominoperineal Resection, n (%) | 0 (0.0) | 47 (20.3) |
| Hartmann’s Procedure, n (%) | 28 (4.4) | 7 (3.0) |
| Other, n (%) | 11 (1.7) | 14 (6.1) |
| Ostomy, n (%) | 55 (8.6) | 126 (54.5) |
| Tumor Location | ||
| Colon, n (%) | 640 (100.0) | N/A |
| Upper rectum, n (%) | N/A | 77 (33.3) |
| Mid rectum, n (%) | N/A | 74 (32.0) |
| Low rectum, n (%) | N/A | 80 (34.6) |
| Urgent/Emergent Surgery, n (%) | 187 (29.2) | 19 (8.2) |
30-day Outcomes
The adjusted mortality rate was 1.5% (95% CI 0.0%–3.1%) for colon cancer and 1.4% (95% CI 0.0%–3.5%) for rectal cancer. Morbidity rate for colon cancer was 26.2% (95% CI 20.3%–32.2%) and 30.7% (95% CI 23.4–38.1%) for rectal cancer. SSI rate was 7.6% for colon cancer and 11.0% for rectal cancer, with a SSI distribution for superficial, deep and organ space of 3.3%, 1.0%, and 2.8% for colon cancer and 1.2%, 4.5%, and 1.3% for rectal cancer, respectively. Anastomotic leak rates were 1.6% (95% CI 0.2%–3.0%) for colon cancer and 1.3% (95% CI 0.0%–3.3%) for rectal cancer.
In terms of resource utilization, the average length of stay was 7.6 days (95% CI 6.7–8.6). Readmission rates for colon cancer resection were 11.8% (95% CI 7.9%–15.7%) and 15.1% (9.9%–20.4%) for rectal cancer. Reoperation rates for colon cancer and rectal cancer were 7.7% (4.6%–10.8%) and 9.4% (5.2%–3.5%), respectively. Blood transfusions were required at a rate of 9.0% (95% CI 6.2%–11.8%) for colon cancer and 8.6% (95% CI 4.6%–12.5%) for rectal cancer.
CRC Quality Measures
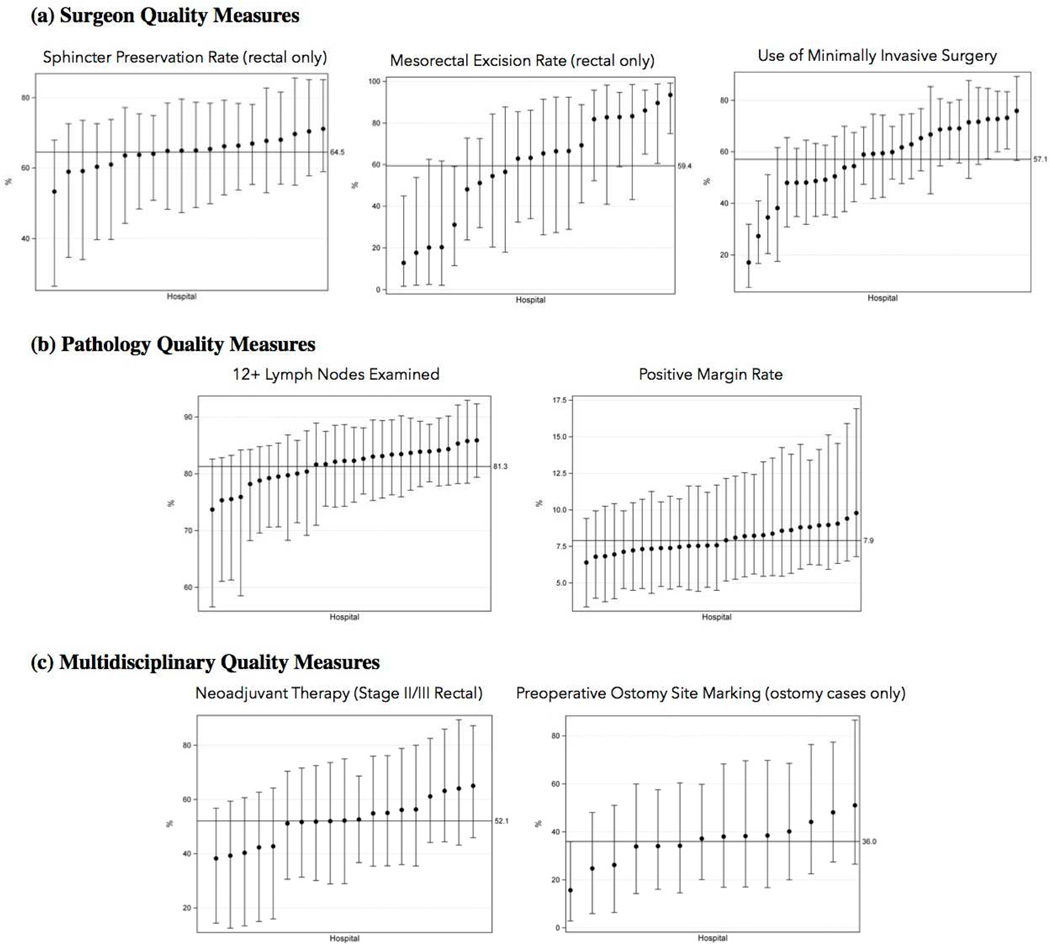
CRC care quality was assessed using process measures abstracted from hospital medical records, and organized into 3 domains: surgery quality, pathology quality, and multidisciplinary care quality. In terms of surgery quality (Figure Ia), there were gaps between optimal and observed performance. In rectal cancer cases, only 59.4% of operative reports documented performing a mesorectal excision. We also found underuse of minimally-invasive surgery for colon cancer, with 59% of cases performed MIS (95% CI 49.9%–68%). Finally, performance of sphincter preservation surgery (SPS) for rectal cancer was lower than expected at 64.5% (95% CI 41.1%–88.0%). For pathology quality (Figure 1b), we found that 12 or more lymph nodes were examined in 82.8% (95% CI 76.3%–89.3%) of colon cancer cases and 77.1% (95% CI 69.3%–84.9%) of rectal cancer cases. We also found that the positive margin rate for colon and rectal cancer was 7.9% (95% CI 5.8%–10.9% for colon cancer, 4.8%–12.9% for rectal cancer). Finally, in terms of multidisciplinary care quality (Figure 1c), we found that neoadjuvant therapy was used in only 52.1% (95% CI 21.9% – 82.2%) of clinical stage II and III rectal cancer cases. Preoperative ostomy site marking was also underused (36% of rectal cancer cases with an ostomy).
Figure I.
Hospitals’ Case-Mix-Adjusted Performance: (a) Surgeon Quality Measures, (b) Pathology Quality Measures, and (c) Multidisciplinary Quality Measures
Discussion
In 2014, the MSQC began a prospective cohort study of colorectal cancer surgery in Michigan, with the ultimate goal of understanding gaps in quality of care for this common condition. This study is the first to report on our findings. We find that modern surgery for CRC is associated with remarkably low risk of mortality, morbidity, and anastomotic leak. However, we also find significant gaps in CRC-specific quality of care, in surgical, pathology and multidisciplinary domains of quality. These results indicate the potential for performance improvement.
As a part of this CRC project, we examined mortality and morbidity, finding lower rates than previously reported (1.5% and 27.4%, respectively). These are in contrast to published morbidity rates ranging from 20% to 40%, and mortality rates from 3% to 6% [1–3]. Improved perioperative care and the increasing use of MIS are likely factors in these results. We were also pleasantly surprised to see low rates of anastomotic leak, which is concordant with recent randomized trials for rectal cancer surgery.
What is new in this study is the ability to measure and give feedback on cancer-specific quality measures. We have characterized CRC quality into 3 domains: surgery, pathology, and multidisciplinary. In terms of surgery factors, we find significant variation in use of mesorectal excision for rectal cancer. While incomplete documentation may be to blame, hospitals’ rates vary from less than 20% to more than 90% of cases, suggesting true variation in the importance placed on mesorectal excision between hospitals. Also, we found significant variability in the use of MIS surgery, ranging by hospital from less than 20% of cases to more than 70%. While there is controversy about the use of MIS for rectal cancer surgery, there is no controversy regarding the laparoscopic approach for colon cancer, and laparoscopy appears to be underused [7, 8]. Due to small numbers, there were not statistically-significant outlier hospitals for rates of SPS, but crude rates seem to vary by hospital (data not shown), and time will allow us to increase our power to know whether there are true outlier hospitals for SPS.
In terms of pathology factors, we find that nearly 20% of cases fail to meet the standard of 12 lymph nodes examined, which is an American Society of Clinical Oncology and National Quality Forum-endorsed measure. Other studies have reported poor rates of adequate node exam, ranging from 48% to 70% [9, 10]. While compliance with this measure is likely better than prior reports, there is still room for improvement. In an attempt to better understand poor compliance with the 12-node guideline, Nathan et al. investigated patient, hospital and physician practices, and found that the primary influencers were non-modifiable patient factors. They therefore recommend that if the lymph node benchmark is to be used as a quality standard, then reporting standards must be standardized to account for case-mix variation, which we have done in this study [9]. Our results also demonstrated a positive margin rate of 7.9%. Other studies have reported rates ranging from 2% to 10% [7, 8]. The goal of surgery should always be an R0 resection; thus further investigation is to begin to understand the factors associated with failure to attain negative margins (particularly for colon cancer cases).
Finally, we find that performance is poor for the multidisciplinary care measures, which focus on the rectal cancer portion of the cohort. Only 52.1% of cases of stage II and III rectal cancer received neoadjuvant radiation, and only 36% of cases in which an ostomy was performed had documentation of ostomy site marking, which is linked with decreased ostomy complications [11]. Differences in measures is likely not due to differences in patient case-mix, which has been adjusted for in all analyses.
One interesting finding was that nearly a quarter of the CRC resections were performed in the emergent/urgent setting. Previous studies have reported emergent CRC resection rates from 7–19%, and an associated increased risk of postoperative complications including mortality and SSI [2, 12]. Further study will be required to understand the reason for these high rates of emergent resection, and why alternatives of temporizing ostomy or stent performed are not used acutely, with cancer surgery performed electively, when cancer quality of care can be optimized.
There are several potential limitations to our study. First, all observational research studies may be influenced by unmeasured confounding factors. Also, these data are reliant on provider documentation which, when incomplete, could underreport complications or fail to document care processes that were actually performed. Some degree of variability in performance measures and outcomes can be attributed to the differences in patient populations between hospitals; however, we performed robust adjustment for case mix, so that where variability is demonstrated it is likely not due to differences in patient populations.
In conclusion, the purpose of this study was to identify quality gaps in the surgical management of CRC within a diverse group of hospitals. Our results show deficiencies in performance on surgical, pathology and multidisciplinary measures of care quality. These findings offer several opportunities for future, regional quality improvement.
Summary.
This paper assesses quality care measures associated with surgical management of colorectal cancer in the state of Michigan. We identified gaps in performance with regards to surgical technique as well as multidisciplinary team involvement. This suggests opportunities for regional quality improvement.
Acknowledgments
Sources of support: Dr. Kanters is supported by the NIH grant T32 HS000053-24. Dr. Hendren is supported by NIH/NCI 1K07 CA163665-22 and by the American Society of Colon and Rectal Surgeons Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No authors have disclosures related to the content of this study.
References
- 1.Schrag D, Cramer LD, Bach PB, et al. Influence of Hospital Procedure Volume on Outcomes Following Surgery for Colon Cancer. JAMA. 2000;284(23):3028–3035. doi: 10.1001/jama.284.23.3028. [DOI] [PubMed] [Google Scholar]
- 2.Alves A, Panis Y, Mathieu P, et al. Postoperative Mortality and Morbiditiy in French Patients Undergoing Colorectal Surgery. Arch Surg. 2005;140(3):278–283. doi: 10.1001/archsurg.140.3.278. [DOI] [PubMed] [Google Scholar]
- 3.Tekkis PP, Poloniecki JK, Thompson MR, Stamatakis JD. Operative mortality in colorectal cancer: prospective national study. BMJ. 2003;327(7425):1196–1201. doi: 10.1136/bmj.327.7425.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breugom AJ, Boelens PG, van den Broek CB, et al. Quality assurance in the treatment of colorectal cancer: the EURECCA initiative. Ann Oncol. 2014;25(8):1485–1492. doi: 10.1093/annonc/mdu039. [DOI] [PubMed] [Google Scholar]
- 5.Levit L, Balogh E, Nass S, Ganz PA. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: The National Academies Press; 2013. Committee on Improving the Quality of Cancer Care, Institute of Medicine. [PubMed] [Google Scholar]
- 6.Hendren S, Fritze D, Banerjee M, et al. Antibiotic choice is independently associated with risk of surgical site infection after colectomy. Ann Surg. 2013;257(3):469–475. doi: 10.1097/SLA.0b013e31826c4009. [DOI] [PubMed] [Google Scholar]
- 7.Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomized trial. Lancet oncol. 2005;6(7):477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 8.Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial) Lancet. 2005;365(9472):1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 9.Nathan H, Shore A, Anders RA, et al. Variation in lymph node assessment after colon cancer resection: patient, surgeon, pathologist or hospital? J Gastrointest Surg. 2011;15(3):471–479. doi: 10.1007/s11605-010-1410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nedrebo BS, Soreide K, Kesbakken A, et al. Risk factors associated with poor lymph node harvest after colon cancer surgery in a national cohort. Colorectal disease. 2013;15(6):301–308. doi: 10.1111/codi.12245. [DOI] [PubMed] [Google Scholar]
- 11.Person B, Ifargan R, Lachter J, et al. The impact of pre-operative stoma site marking on the incidence of complications, quality of life and patient’s independence. Dis Colon Rectum. 2012;55(7):783–787. doi: 10.1097/DCR.0b013e31825763f0. [DOI] [PubMed] [Google Scholar]
- 12.Aslar AK, Ozdemir S, Mahmoudi H, Kuzu MA. Analysis of 230 cases of emergent surgery for obstruction colon cancer – lessons learned. J Gastrointest Surg. 2011;15(1):110–119. doi: 10.1007/s11605-010-1360-2. [DOI] [PubMed] [Google Scholar]