Abstract
The study examined the effects of a social stressor (Trier Social Stress Test) on 24 male and 32 female college students’ affective and physiological reactivity and their subsequent performance on a decision-making task (Iowa Gambling Task). The 56 participants were randomly assigned to a social stressor or a control condition. Compared to controls, participants in the stress condition responded with higher heart rates and skin conductance responses, reported more negative affect, and on the decision-making task made less advantageous choices. An exploratory regression analysis revealed that among men higher levels of heart rate were positively correlated with riskier choices on the Iowa Gambling Task, whereas for women this relationship was curvilinear. Exploratory correlational analyses showed that lower levels of skin conductance within the stress condition were associated with greater levels of substance use and gambling. The results suggest that the presence of a stressor may generally result in failure to attend to the full range of possible consequences of a decision. The relationship pattern between the degree of stress responding and successful decision making may be different for men and women.
Keywords: Stress, Decision Making, Heart Rate, Skin Conductance, Gender, Trier Social Stress Test, Iowa Gambling Task
Introduction
Stress connotes events that the individual finds physically, physiologically or emotionally challenging. It involves several different processes that describe adaptations to a wide array of situations, spanning from daily hassles to traumatic life experiences (McEwen, 2008). The autonomic nervous system is central to these adaptations and encompasses a comprehensive regulatory system that controls visceral functions such as heart rate, respiration, salivation, and perspiration (Sherwood, 2004). Prolonged exposure to stress can produce chronically elevated stress levels that cause wear and tear on immune functioning and various physiological systems. In addition, stress also affects cognitive function. As Mather and Lighthall (2012) pointed out, most research on stress and cognitive function has been limited to memory, whereas other cognitions, such as decision making, have received less attention. Decision making is a particularly important area to study because many decisions are made under stressful conditions, such as when circumstances demand a quick course of action. Indeed, the process of decision making in and of itself can be stressful, such as when a decision involves high risk and its outcome is uncertain. Thus, the relationship between stress and decision making can be bidirectional because stress may affect the quality of the decision and also be evoked by the decision-making process.
In a study on the effects of stress on decision making (Preston, Buchanan, Stansfield, & Bechara, 2007), participants were exposed to a stressful public speaking task before being administered a commonly used decision task involving a virtual card game, the Iowa Gambling Task (IGT). The study found that individuals who were experiencing stress took longer than controls to learn which choices led to more advantageous outcomes on the gambling task. However, the relationship between stress and performance followed an inverted U-shaped curve indicating that performance under stress was enhanced up to a point and then began to deteriorate. This finding is consistent with the Yerkes-Dodson Law (Yerkes & Dodson, 1908) as well as other research showing a curvilinear relationship between arousal and performance on cognitive tasks (Eysenck, 1975a; Eysenck, 1975b).
Preliminary research suggests that gender may also play a role in how a person responds to stress. For example, Preston et al. (2007) reported some preliminary evidence that the relationship between physiological arousal and decision making may differ by gender as they found that stress-induced increases in heart rate were related to poorer performance on the IGT in men but to better performance in women. Another study using cortisol levels as the stress indicator replicated the inverse linear relationship between stress and performance on the IGT in men, but in women the relationship was curvilinear (van den Bos, Harteveld, & Stoop, 2009). The findings from these studies were preliminary and more research is needed to shed light on the effects of stress on cognitive performance.
There is some evidence that the effects of stress on decision making may also have implications for substance use and gambling. We have argued that decisions under uncertainty are in most cases in and of themselves stressful. Hence, the quality of such decisions is affected by a person’s ability to cope with stress. Sinha (2008) has shown that individuals who are prone to addiction are at increased risk of developing an addictive behavior when they experience chronic stressful life events for which they lack effective coping strategies. An additional complicating factor is that decisions made under uncertainty are more susceptible to the effects of reward and less so to the effects of punishment, as a recent comprehensive review of the literature by Starcke and Brand (2012) has shown. Individuals who use substances or gambling to cope may pay less attention to the negative consequences of their substance abuse when stressed and instead favor the immediate reward (e.g., relapse) (Sinha, 2008). But the more immediately satisfying decision may lead to further adverse consequences, which may result in a vicious cycle (Sinha, 2011). A laboratory study supported this assumption showing that individuals with nicotine dependence were less able to resist cigarette smoking after completing a stressful compared to a neutral imagery task (McKee et al., 2011). Therefore, research on how stress impacts decision making may have implications for both intervention in and prevention of problematic substance use.
To our knowledge, only one study to date has examined the effects of an acute social stressor on decision making under uncertainty in individuals who are addiction prone. Gullo and Stieger (2011) examined whether heavy drinkers in a stressful situation are more susceptible to the effects of reward and less so to the effects of punishment. They randomized heavy and light drinkers either to an anticipatory stressor (a public speaking task) or a control condition. They found that heavy drinkers in the control condition made more disadvantageous decisions on the IGT than light drinkers, but these differences disappeared in the stress condition because the heavy drinkers significantly improved their decision-making performance under stress. The authors suggested that the anticipatory stress increased heavy drinkers’ attention to losses, which effectively rebalanced their reward and punishment sensitivity. However, as the participants only expected to make a public speech, this manipulation was not as stressful as if they had been required to actually give a speech before completing the IGT. Hence, further research is needed to clarify how the degree of stress affects decision making in individuals who use substances.
The aim of the current study was to assess the effects of stress on decision making under uncertainty. We hypothesized that college students’ subjective mood state and physiological arousal in response to an acute social stressor would predict risk taking on the IGT. Given that higher physiological reactivity may be associated with greater risk taking in men, but less risk taking in women (Preston et al, 2007), the second aim of the study was to conduct exploratory analyses to examine whether gender moderates the relationship between stress response and decision making among measures of physiological reactivity (i.e., skin conductance and heart rate). The final aim was to explore whether the participants’ substance use and gambling were related to their stress-elicited physiological reactivity and their performance on the decision-making task.
Method
Participants
Fifty-six college students (32 women and 24 men) recruited from introductory psychology classes at a public Northeastern university participated in the study in partial fulfillment of a research requirement. Participants were informed that the purpose of the study would be to evaluate the effect of a performance task and a computerized game on people’s physiological (skin conductance and heart rate) and subjective reactions (emotional response). The students’ mean age was 20.5 years (range 18-33) and 91.1% were single. The majority identified as Caucasian (46.4%), followed by Asian (17.9%), Hispanic (16.1%), African American (10.7%) and “other” (8.9%).
Research Design
The study used a two-group experimental design. The independent variable was stress induction. The dependent variables were non-specific skin conductance response (NS-SCR, measured as the frequency of responses per minute) and skin conductance level (SCL, measured as the overall conductivity of the skin over time), heart rate, subjective mood, and performance on the IGT.
Measures
Current mood states
The Positive and Negative Affect Schedule (PANAS; Watson, 1988) was used to assess affective reactions to a social stress task. The questionnaire is comprised of a positive and a negative affect subscale, with ten items each rating present mood states (e.g., enthusiasm, anxiousness) on a Likert-type scale from 1 (very slightly or not at all) to 4 (very much). Participants were instructed to indicate to what extent they felt this way at the present moment. The PANAS has been validated on a college sample and has good internal consistency (negative affect: α=.87; positive affect: α=.88). For the three administrations of the PANAS in the present sample (detailed in the procedure section below), Cronbach’s alpha was .86, .89, and .90 (positive affect) and .80, .89, and .68 (negative affect).
In addition to the PANAS, all participants were asked to verbally rate subjective stress at two time points during the stress induction. This was to ensure that the stress induction relative to the control condition did indeed increase subjective feelings of stress. Specifically, participants were asked how stressed they felt at this moment on a scale from 1 (not at all) to 10 (the most stress ever experienced). Cronbach’s alpha for these items was .96. Because the participants might feel pressured to answer a certain way given the verbal nature of the administration, we only used this scale as a manipulation check and interpreted subjective distress primarily with negative affect rating on the PANAS.
Substance use and gambling frequency
The CORE Alcohol and Drug Survey measures college students’ substance use habits and related information (CORE Institute, 2004). This self-report instrument has been administered nationally since 1989 at over 800 universities and demonstrates adequate reliability and validity (Presley, Meilman & Lyerla, 1994). For the purpose of the present study only questions about past-year and past-30-day frequency of alcohol, cigarette, and marijuana use and gambling for money were used. Questions were answered on a 9-point Likert scale ranging from “0 days” (no use) up to “all 30 days” for the past month, and from “Didn’t use” to “Every day” for the past year. Past research on the CORE estimates the test-retest reliability for alcohol, cigarette, and marijuana frequency is estimated to be .98 for all three items and a Cronbach’s alpha ranging between .37 to .55 (Presley, Meilman, & Lyerla, 1993). The Cronbach’s alpha for this sample was .57 for past month frequency and .62 for past year frequency.
Skin conductance
Skin conductance levels provide information about internal emotional states due to increased sympathetic activation (Dawson, Schell, & Filion, 2007). Decision making and stress increase skin conductance (Critchley, Elliot, Mathias, & Dolan, 2000), which makes it a useful tool in experimental designs employing stress and cognitive tasks. Skin conductance is comprised of general levels (tonic) and responses to both internal and external stimuli (phasic). Tonic skin conductance levels (SCL) can provide useful information about levels of arousal and attentional processes (Dawson et al., 2007). The frequency of skin conductance responses (SCR) can be useful when studying individual differences related to behavior or psychopathology, specifically rates of non-specific SCRs (NS-SCR). NS-SCRs occur in the presence of both external stimuli and internal states, such as in response to cognitions and emotions, that occur absent of external stimuli (Turpin & Grandfield (2009).
Skin conductance was measured using the palm Q sensor, a portable electrodermal activity monitor (Affectiva, Inc). The sampling rate was set at a maximum 16 Hz/second. The data were downloaded from the palm sensor to a computer with Q software and visually analyzed for any aberrant readings. If the data suddenly dropped to .00 μSiemens and returned to original levels within a few seconds, it was marked with an event marker and then treated with artifact correction after being imported into Ledalab 3.4.5 (Benedek & Kaernbach, 2010). In Ledalab, the skin conductance data was decomposed into continuous phasic and tonic components. Dawson and colleagues (2007) recommend a minimum amplitude for NS-SCR between .01-.05 μSiemens. For the current study, we elected to use the most conservative cut-off of .05 μSiemens. Within this software, the frequency of NS-SCRs per minute and SCLs were generated for the acclimation period, all three portions of the TSST (preparatory, speech, and math), and the risk task. SCLs were averaged for each of the three periods in the study: the acclimation period (5 minutes), the TSST period (13 minutes), and finally the risk-taking task (IGT). The equivalent time periods to the TSST were calculated in the control condition.
Heart rate
Both branches of the autonomic nervous system are activated reciprocally. Activation of the sympathetic nervous system results in heart rate acceleration under conditions of stress, whereas activation of the parasympathetic nervous system is generally associated with heart rate deceleration under resting conditions (Thayer & Lane, 2009). The autonomic nervous system allows for appropriate regulation of heart rate to a variety of environmental demands, such as a stressful situation. Heart rate is estimated to have an average rise in normal samples between 15-25 beats per minute (bpm) in response to a social stressor when participants are standing (Kudielka, Hellhammer, & Kirschbaum, 2007); however, another study found a difference between 10-15 bpm, although it is unclear whether participants were standing or sitting during the procedures (Kelly, Tyrka, Anderson, Price, & Carpenter, 2008).
Heart rate was measured with a Polar WearLink+ heart-rate monitor (Polar Electro Inc.). The data were wirelessly transmitted to a heart-rate receiver module (RS400; Polar Electro Inc.) at a sampling rate of 1 Hz. The heart rate data were transferred from the receiver module into the Polar ProTrainer 5 software (Version 5.40.172; Polar Electro Inc.) via a Polar IrDA USB adapter. Similar to SCL, heart rate was averaged within each of the five periods in the study in the TSST condition. The equivalent periods were calculated in the control condition.
Decision making
The Iowa Gambling Task (IGT) is one of the most commonly used decision-making tasks that has been employed with clinical (Bechara, Damasio, Damasio, & Anderson, 1994) and non-clinical populations (Shurman, Horan, & Nuechterlein, 2005). It is comprised of four virtual decks of cards (A, B, C, or D) presented on a computer screen. On each of a total of 100 trials, participants select one card from one of the four decks and win some play money, but every so often they will also lose some money. The aim is to win as many play dollars as possible. Unbeknownst to the participants, two “bad” decks deliver larger immediate wins (e.g., $100) but also larger penalties (e.g., $250), resulting in a net loss over time; two “good” decks deliver smaller wins (e.g., $50) and also smaller penalties (e.g., $100), resulting in a net gain over the 100 trials.
Thus, the IGT mimics real-life decision making in that participants must weigh risks and benefits of each choice by paying attention to the wins and losses associated with the various decks and using this information in subsequent decisions. Normal individuals develop a preference for advantageous decks after completing approximately 40 trials (Bechara, Tranel, & Damasio, 2000). In contrast, impulsive individuals continue to choose more cards from the disadvantageous decks (Hooper, Luciana, Conklin, & Yarger, 2004; Carlson, Zayas, & Guthormsen, 2009; Burdick, Roy, & Raver, 2013). Collectively, this research suggests that latter trials, particularly after trial 40, provide a better index of adaptive decision making. In support of this, one study found that better criterion and construct validity were achieved when the last 60 trials of the IGT were used (Gansler, Jerram, Vannorsdall, & Schretlen, 2011). We therefore disregarded the first 40 acquisition trials and evaluated the number of choices from advantageous decks during the last 60 trials.
A large body of literature has shown that individuals with a history of addictive behaviors show deficits in decision making and engage in riskier behaviors, not unlike patients with specific frontal lobe dysfunctions (Clark & Robbins, 2002; Goudriaan, Oosterlaan, de Beurs, & van den Brink, 2005; Goudriaan, Oosterlaan, de Beurs, & van den Brink, 2006). Research has also found a relationship between greater risk taking on the IGT and cocaine and marijuana abuse (Verdejo-Garcia, Benbrook, Funderburk, Cadet, & Bolla, 2007) as well as pathological gambling and alcohol dependency (Goudriaan et al., 2005; Lawrence, Luty, Bogdan, Sahakian, & Clark, 2009). The IGT therefore serves as a useful measure for risky decision making.
Procedure
In the laboratory, after providing informed consent, the participants were randomly assigned to either a stress or a no-stress control condition. They were instructed on how to fit the heart rate monitor under their clothing in privacy. For the skin conductance monitor, the experimenter placed sensors on the participants’ non-dominant palm. Participants then walked for five minutes to raise their heart rate and maximize the connection of the skin conductance monitor, and subsequently they completed the PANAS. Then they sat quietly for a 10-minute acclimation period before undergoing the Trier Social Stress Test (experimental condition) or reading an informative article unrelated to the study (control condition) for an equivalent amount of time (~13 min).
Stress condition
The stress manipulation was based on an adapted version of the Trier Social Stress Test (TSST), as outlined in Kirschbaum and colleagues (1993). Participants received written instructions and were given three minutes to prepare a five-minute speech for a mock job interview within their chosen profession during which they had to convince a panel of two judges that they were the best person for the job. They were informed that the panel would be evaluating their performance. They received paper and pencil to organize their thoughts but were not allowed to use their notes during the presentation. After the preparatory period, the participants were led to a room and seated on a stool in front of two confederates who were acting as judges. Although the original TSST requires participants to stand on a marked spot, we asked participants to remain seated to allow for a more direct comparison to the control condition. The confederates wore white lab coats, held clipboards and maintained a neutral and unresponsive manner throughout the procedures. The experimenter set a time marker on both physiological recording devices. The participants were seated on a tall stool in front of the judges and began their speech. If they finished their speech before the allotted time, after 15 seconds the experimenter prompted them to continue. If the participants paused again, the experimenter probed with standardized questions (“What are your strengths/weaknesses relative to your profession?”). Another time marker on both recording devices was set to mark the end of the first stress task. At this time, participants were asked to provide subjective ratings of their current level of stress.
After this task, participants were instructed in a mental arithmetic task consisting of serially subtracting 13 from 1022 as quickly and as accurately as possible. When participants made a mistake, the experimenter told them to begin again from 1022. After five minutes, the task was stopped, and a third time marker was set on both physiological recording devices. The second subjective stress rating was recorded at this time and then the participants completed a second PANAS.
Control condition
Following the acclimation period, participants randomized to the control condition were led into another room, seated at a desk, and instructed to read an informative article on “South Africa’s Teeming Seas” from National Geographic for thirteen minutes to approximate the time for the TSST in the experimental condition. After the first 3 minutes, the experimenter entered the room and set a time marker on the heart rate and skin conductance monitors that corresponded in time with the end of the preparation period in the TSST condition. At the 8-minute mark, a second time marker was set to correspond with the end of the speech task in the experimental condition and participants were asked to provide a subjective rating of their current level of stress. Then the participants were asked to continue reading the article. After 13 minutes had elapsed, a third time marker was recorded on the physiological devices to correspond with the end of the mental arithmetic task of the TSST. Participants were again asked to rate their subjective stress level and then completed the second PANAS.
Decision-making task, final questionnaires, and debriefing
Thereafter participants were instructed to sit for approximately seven minutes, as previous research has shown that decision-making deficits are most robust when the catecholamine levels are allowed to peak (Pabst, Brand, & Wolf, 2013). The participants then completed the decision-making task (as described above). Following participation in the IGT, participants completed a final PANAS and the CORE-R to obtain an assessment of their substance use habits and gambling. At the end of the experiment, all participants were debriefed.
Results
Preliminary Analyses
Nine participants (three men and six women) were removed from the analyses involving the experimental manipulation due to malfunction of the physiological recording devices. Data from 47 participants (23 in the experimental and 24 in the control group) were used in these analyses. This resulted in 10 men and 13 women in the stress condition, and 11 men and 13 women in the control condition. The data of all 56 participants were used for exploratory analyses whenever available.
Variables showed appropriate distributions, except “past-year gambling,” which was positively skewed and logarithmically transformed. Previous literature has indicated that raw skin conductance values tend to be positively skewed and transformations are recommended (Dawson et al., 2007). Only tonic SCL values were positively skewed and were logarithmically transformed. Variables were assessed for univariate and multivariate outliers using z-scores and Mahalanobis’ distance, respectively. No univariate outliers were indicated; one multivariate outlier was removed from analyses concerning negative affect. For the regression analyses, we calculated baseline-corrected heart rate, skin conductance levels and responses, and negative affect by creating a composite averaged level combining all three-stress periods, then subtracting the baseline levels from that averaged number.
There were no statistically significant differences between the experimental and the control group in gender [χ2 (1, N = 47) = 0.026, p = .87], age [F (1, 46) = 0.34, p = .56], or ethnicity/race [χ2 (4, N = 47) = 8.23, p = .084].
Physiological Data
Heart rate and both skin conductance measures were not significantly correlated during any assessment period (p > .05). However, SCL and NS-SCRs were significantly correlated. We therefore conducted two separate repeated measures analyses of variance (ANOVAs), one for heart rate and another one for the two skin conductance measures together. Descriptive statistics (means, standard deviation, minimum, and maximum) can be found in Table 1.
Table 1.
Descriptive statistics for physiological and subjective reactivity by condition.
| Stress |
Control |
|||||
|---|---|---|---|---|---|---|
| Mean (SD) | Min | Max | Mean (SD) | Min | Max | |
|
|
|
|||||
| Heart Rate | ||||||
| Baseline | 79.56 (12.59) | 47.49 | 107.94 | 79.49 (11.64) | 59.17 | 102.72 |
| Prep | 88.89 (16.85) | 48.49 | 126.61 | 77.83 (10.16) | 61.56 | 92.99 |
| Speech | 92.81 (20.95) | 61.29 | 138.97 | 78.93 (10.28) | 58.85 | 93.77 |
| Math | 88.64 (18.02) | 52.25 | 119.01 | 77.92 (10.78) | 54.63 | 93.88 |
| IGT | 74.96 (12.10) | 46.64 | 90.23 | 77.46 (9.40) | 57.95 | 91.52 |
|
| ||||||
| SCL | ||||||
| Baseline | 4.30 (4.71) | .082 | 16.55 | 3.26 (3.91) | .13 | 17.1 |
| Prep | 5.83 (5.11) | .12 | 19.30 | 4.92 (4.58) | .28 | 20.73 |
| Speech | 6.02 (4.01) | .54 | 14.96 | 4.79 (4.68) | .27 | 20.41 |
| Math | 5.74 (3.63) | .83 | 14.06 | 5.48 (4.95) | .46 | 19.80 |
| IGT | 4.83 (3.77) | .12 | 13.59 | 6.05 (6.00) | .35 | 23.65 |
|
| ||||||
| NS-SCR | ||||||
| Baseline | 23.69 (19.50) | 0 | 64.50 | 18.26 (16.20) | 0 | 51.50 |
| Prep | 23.35 (16.78) | 0 | 52.33 | 20.42 (17.81) | 0 | 62.67 |
| Speech | 38.08 (15.10) | .65 | 62.87 | 30.83 (18.06) | .43 | 61.25 |
| Math | 43.71 (13.59) | 0 | 62.57 | 28.06 (18.85) | 0 | 59.16 |
| IGT | 33.98 (16.41) | 0 | 59.50 | 29.33 (17.35) | .46 | 56.78 |
|
| ||||||
| Negative Affect |
||||||
| Baseline | 12.88 (3.23) | 10.00 | 21.00 | 14.11 (5.07) | 10.00 | 29.00 |
| Post-Stress | 20.80 (8.49) | 10.00 | 42.00 | 14.07 (4.33) | 10.00 | 24.00 |
| Post-IGT | 13.14 (3.08) | 10.00 | 22.00 | 12.61 (3.07) | 10.00 | 22.00 |
|
| ||||||
| Self-reported Stress |
||||||
| Post-Speech | 6.70 (2.13) | 2.00 | 10.00 | 3.35 (2.51) | 1.00 | 9.00 |
| Post-Math | 6.54 (2.15) | 2.00 | 10.00 | 3.93 (2.75) | 1.00 | 9.00 |
Note:
SCL = Skin conductance levels, NS-SCR = Non-specific skin conductance responses, IGT=Iowa Gambled Task.
Heart rate
We conducted a 5 (time) × 2 (condition) mixed design ANOVA with the five experimental periods as the repeated-measures factor (baseline, preparation, speech, math, and IGT) and condition (stress, control) as the between-subjects factor. The dependent variable was heart rate in beats per minute averaged within each time period. Box’s M test was significant (p < .001) and therefore Pillai’s trace is reported below (Tabachnick & Fidell, 2013). As Mauchly’s test indicated that the assumption of sphericity was not met, we used a Greenhouse-Geisser correction.
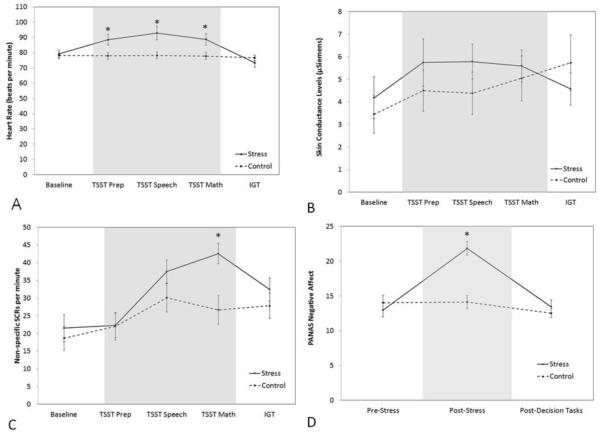
The condition by heart rate interaction was significant [Pillai’s trace = .41, F(1.2, 26.59) = 7.33, p = .009, ηp2 = 0.41], as was the main effect for time [Pillai’s trace = .45, F(2.53, 26.59) = 8.62, p = .006, ηp2 = 0.45], but it only approached significance for condition (p = .075). Examining the time period within each condition showed a significant effect of stress on heart rate, F(2.53, 113.89) = 29.99, p < .001, ηp2 = 0.40, whereas the equivalent time period in the control condition was not significant (p = .864) (see Figure 1A). Heart rate was significantly higher in the TSST group compared to the control group only during the TSST time periods [Preparation: F(.63, 28.49) = 6.61, p = .027, ηp2 = 0.13; Speech: F(.63, 28.49) = 8.83, p = .013, ηp2 = 0.16; Math: F(.63, 28.49) = 6.07, p = .032, ηp2 = 0.12].
Figure 1.
Averaged heart rate (A), skin conductance levels (B), non-specific skin conductance response per minute (C), and subjective negative affect (D) during each experimental time period. Errors bars indicate SEM.
Note: * indicates p < .05.
Skin conductance
We conducted a 5 (time) × 2 (condition) Doubly MANOVA with experimental period as the repeated-measures factor (acclimation, preparation, speech, math, and IGT) and condition (stress, control) as the between-subjects factor. Averaged SCL and frequency of NS-SCR served as the dependent variables. The assumption of homogeneity of regression was supported. The Bartlett test of sphericity was not significant (p = .123).
The condition by time interaction was significant, Wilks’ λ = .78, F(8, 358) = 5.91, p < .001, ηp2 = 0.12, indicating that the skin conductance measures differed for the two conditions over time. The main effect of condition was not significant (p = .347), but the main effect of time was [Wilks’ λ = .203, F(8, 38) = 18.62, p < .001, ηp2 = 0.80]. The main effect of time on SCL revealed that levels generally rose regardless of condition (Figure 1B). The interaction effect showed that both skin conductance measures changed over time based on condition [SCL: F(4, 180) = 5.34, p < .001, ηp2 = 0.11; NS-SCR: F(4, 180) = 6.91, p < .001, ηp2 = 0.13]. The interaction effect within each time period revealed a significant effect only during the math task [Wilks’ λ = .774, F(2, 44) = 6.41, p = .004, ηp2 = 0.23]. While the SCL did not achieve significance, p = .181 (Figure 1B), the NS-SCR was significantly higher during the math portion of the TSST, F(1, 45) = 9.96, p = .001, ηp2 = 0.181 (Figure 1C).
Subjective mood states
We then examined the effect of condition on subjective stress reports and the three PANAS assessments. To determine that the social stressor indeed increased subjective stress levels, we averaged the two subjective stress levels and used an independent t-test for unequal variances to compare the two conditions. A significant effect for condition was revealed, t(46.27)=5.07, p<.001. Individuals in the stress condition reported feeling significantly more stressed compared to individuals in the control condition (Table 1).
To further assess the effect of the stressor on general affect, we conducted a Doubly MANOVA using the positive affect and negative affect subscales of the PANAS as the within-subjects factor and condition (stress, control) as the between-subjects factor. The assumption of homogeneity of regression was upheld. Assumptions of normality and multicollinearity were met, as was the assumption of sphericity (Bartlett’s test; p = .338).
The time by condition interaction was significant [Wilks’ λ = .59, F(4, 174) = 9.29, p < .001, ηp2 = 0.18], indicating that mood states changed over time based on condition. The main effect of condition was marginally significant (p = .051); the repeated-measures main effect was significant [F(4, 174) = 21.21, p < .001, ηp2 = 0.33]. The time by condition interaction revealed that positive affect did not change over time (p = .64), whereas negative affect changed based on condition F(2, 88) = 19.05, p < .001, ηp2 = 0.30 (see Figure 1D). Analysis of the effect of condition within each time point revealed that negative affect significantly differed by condition [Wilks’ λ = .75, F(2, 43) = 7.31, p < .001, ηp2 = 0.25], but only following the TSST [F(1, 44) = 14.59, p < .001, ηp2 = 0.25].
Iowa Gambling Task
We conducted a one-way ANOVA to assess the effect of condition on the number of advantageous cards chosen on the IGT from trials 41 to 100, which was significant, F(1, 45) = 4.11, p < .05, ηp2 = 0.084. Consistent with our hypothesis, participants in the stress condition showed impaired decision making by choosing significantly fewer cards from the advantageous decks (M = 29.04, SD = 8.34) than those in control condition (M = 34.96, SD = 11.37).
Exploratory Analyses
Gender differences
A study by van den Bos et al. (2009) indicated that there may be a gender difference in the relationship of stress-induced arousal (cortisol in their study) and decision making. We therefore conducted an exploratory regression analysis using linear and quadratic predictor variables to assess the arousal-performance relationship within each gender in the current sample. In four separate regression equations, we used baseline-corrected heart rate, SCL, NS-SCR, and the PANAS negative affect subscale to predict advantageous choices on the last sixty trials of the IGT. We tested the significance of the unstandardized regression coefficients using bootstrapping procedures to reduce the influence of bias (e.g., heteroskedasticity, effect of the distribution) on our results (Efron & Tibshirani, 1993). Confidence intervals were generated using 1,000 bootstrapped samples. In step 1, we included the linear independent variables and in step 2, the quadratic-transformed variables to assess how much they predicted performance on the IGT beyond the linear regression.
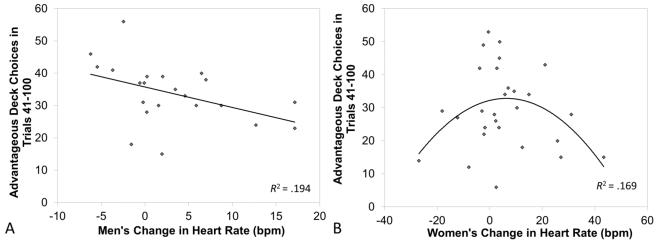
In men, increases in heart rate were linearly related to decreases in advantageous choices on the IGT [Adjusted R =.441, ΔF(1,20) = 4.82, p = .04, B=−.64, 95% CI: −1.12, −.12; Figure 2A] and accounted for 19.4% of the variance on the IGT; the quadratic relationship was not significant [Adjusted R =.475, ΔF(1,19) = .77, p = .392, B=.035, 95% CI: −.035, .142]. In contrast, for women the linear relationship between heart rate and performance was not significant [Adjusted R =.082, ΔF(1,26) = .175, p = .679, B=−.068, 95% CI: −.385, .275], but the quadratic relationship was [Adjusted R =.419, ΔF(1,25) = 5.12, p = .033, B=−.015, 95% CI: −.031, −.004; Figure 2B] and accounted for 16.9% of the variance on the IGT. Neither of the skin conductance measures predicted performance on the IGT. For SCL, neither the linear (men, p = .888; women, p = .528) nor the quadratic model (men, p = .336; women, p = .071) achieved statistical significance, although for women the quadratic relationship approached significance, suggesting that the analysis was likely underpowered (R2 = .114). Similarly, for NS-SCR neither the linear (men, p = .592; women, p = .422) nor the quadratic model (men, p = .334; women, p = .669) was significant. Finally, change in negative affect did also not predict performance (linear model: men, p = .703; women, p = .213; quadratic model: men, p = .737; women, p = .524).
Figure 2.
Total choices of advantageous decks on the IGT under stress-induced heart rate arousal in men (A) and women (B).
Addictive Behaviors
Previous research has demonstrated that decreased arousal to an acute stressor is related to more frequent substance use (Thomas, Bacon, Sinha, Uhart, & Adinoff, 2012; Wemm et al., 2013). To examine this issue in the present sample, we conducted a correlational analysis between substance use and physiological arousal. We created a composite substance use variable (by averaging participants’ self-reported past-month tobacco, alcohol, and marijuana use on the CORE-R) and found substance use to be inversely related to changes in SCL during the TSST [SCL: r (26) = −.523, p = .006]. NS-SCR and heart rate were not significantly related to substance use although the direction of the relationship was also negative [heart rate: r (24)= −.280, p = .185; NS-SCR: r (26)= −.250, p = .217]. Using the entire sample, we also examined whether substance use was correlated with performance on the IGT, but found no significant correlation in this non-clinical sample, r (56) = −.066, p = .630.
Finally, we examined the possible effects of a non-substance related addictive behavior, i.e., gambling. Given the relatively low involvement in gambling in this sample, we used past-year frequency. Within the stress condition, the relationship between gambling and SCL was significant, r (26)= −.509, p = .008. As before, heart rate and NS-SCR were not significant [heart rate: r (24)= −.141, p = .512; NS-SCR: r (26)= −.034, p = .869]. Similarly, using the entire sample, we also found no significant correlation between gambling frequency and performance on the IGT, r (56) = .011, p = .934.
Discussion
The results of the present study suggest that exposure to an acute social-evaluative stressor may negatively impact decision making. Participants who underwent the TSST showed significant stress-induced increases in physiological arousal (heart rate and SCL) as well as negative affect (PANAS). These stress reactions were associated with selecting more cards from the less advantageous decks on the IGT compared to participants in the control condition. These results are consistent with previous studies showing that stress induction has a deleterious effect on adaptive decision making. On the IGT, participants must learn which decks are more profitable in the long term by attending to the proportion of wins and losses across trials. The presence of an acute stressor apparently delays learning of these contingencies, which suggests that participants under stress may show deficits in processing of feedback.
Notably, only frequency of skin conductance responses was significantly different between the two conditions, whereas skin conductance levels were not. The pattern of results can be explained by research indicating overlapping, yet different neural mechanisms that correspond to skin conductance responses and levels. Increased attentional and emotional load elicit fast-acting (less than five seconds) skin conductance responses via prefrontal cortex and amygdala activation, respectively (Critchley, Elliot, Mathias, Dolan, 2000). In contrast, cognitively demanding tasks result in increases in skin conductance levels over several minutes through activity in the ventromedial prefrontal cortex (vmPFC; Nagai, Critchley, Featherstone, Trimble, & Dolan, 2004). The control task required the participants to read a document, a cognitively demanding task, whereas the TSST capitalizes on both cognitive and emotional demands. Therefore, the primary difference between the two conditions may be the result of using neural pathways related to emotional load, and therefore the stress condition specifically targets skin conductance response. For this reason, the social stressor may have an effect on skin conductance responses more than skin conductance levels.
Of note, there was a main effect of time on skin conductance levels. Skin conductance levels, particularly within the control condition, showed a tendency to rise as participants progressed through the procedures and reached a peak during the IGT. This phenomenon has two explanations. First, the IGT comprises the most cognitively demanding aspect for the control condition. Second, the IGT was developed specifically to target activity in the vmPFC (Bechara, Tranel, & Damasio, 2000), an area of the brain found to mediate the relationship between skin conductance levels and attentionally demanding tasks (Nagai et al., 2004). Therefore, this task likely led to increase in skin conductance levels comparable to those in the experimental condition.
Based on the evidence in the literature, it is possible that acute stress enhances learning of positive outcomes and inhibits learning of negative outcomes (Mather & Lighthall, 2012). The activation of the reward pathway may increase the salience of reward-associated behaviors while decreasing learning of punishment-associated behaviors. Due to this so-called reward sensitivity, individuals who are stressed may fail to pay attention to the higher losses associated with certain decks on the IGT and focus more on decks with higher rewards, which will translate itself into less advantageous choices on the IGT.
The failure to attend to the full range of possible consequences of behavior may also play a role in increased use of substances and gambling. For example, stress may amplify the rewarding effects of substances in individuals with substance use disorders and blunt adaptive responses to stressors (Mather & Lighthall, 2012; Sinha, 2009). Our exploratory analyses suggest that increased gambling is associated with blunted physiological reactivity, similar to past research and our own findings related to substance use. Furthermore, research comparing problems gamblers and alcohol dependent individuals has shown similar deficits in decision making in both groups (Goudriaan, Ooserlaan, de Beurs, & van den Brink, 2005). Collectively, our preliminary results and others’ past research support the hypothesis that there may be common biological processes involved in substance and behavioral addictions (Shaffer et al., 2004). Although we were unable to directly link the blunted physiological reactivity to poor decision making due to both the low sample size and the non-clinical nature of our sample, we speculate that stress may have effects on the decision-making process of problem gamblers similar to individuals who abuse substances. It would therefore be valuable if future research examined the effects of stress on decision making in individuals who abuse substances to those who gamble problematically.
We also found preliminary support for a possible gender difference in the stress-performance relationship, which was first reported by van den Bos et al. (2009). These researchers showed that in women increases in the stress levels (measured via cortisol) were related to performance on the IGT in an inverted U-shaped curve whereas the relationship in men was linear. Similarly, in our study, heart rate arousal in men was linearly related to poorer decision making on the IGT. In contrast, the relationship for women was curvilinear, such that increases in heart rate were associated with more advantageous decision making up to a certain point, after which the performance began to deteriorate. It has been hypothesized that gender differences may arise from differential processing of rewards (Mather & Lighthall, 2012). To illustrate, fMRI results found that men exposed to a stressful cold-pressor task showed increased responsivity to a decision-making task in the anterior insula and dorsal striatum, areas critical to the integration of sensorimotor, cognitive, and emotional signals. In contrast, a decreased response in these same areas was observed in women. Additionally, relative to women, men under stress tended to make quicker decisions, engage in more automatic processing and tended to focus more on the rewarding aspects of the task.
We also examined in an exploratory analysis the relationship between substance use and physiological arousal, given that previous research has shown blunted physiological responses to stressors in individuals with addictive behaviors. For example, in one study alcohol dependent patients displayed a blunted heart rate response to a stressor (Sinha et al., 2009). Consistent with this research, we found an inverse correlation between participants’ level of self-reported substance use and their SCL arousal in response to a psychosocial stressor. Heart rate, in contrast, was not correlated with substance use. A hypoactive stress response measured as cortisol levels has also been noted in previous research with pathological gamblers (Paris, Franco, Sodano, Frye, & Wulfert, 2010). In the present study, a blunted SCL response was associated with greater gambling frequency; however, heart rate and NS-SCR did not show a significant relationship. We may have replicated the findings from previous studies only partially because the participants comprising the present sample were, on average, light to moderate social drinkers who used other substances only sparingly and who also showed an overall low level of gambling involvement. In contrast, previous studies were conducted with alcohol-dependent drinkers (Sinha et al., 2009) and high-frequency gamblers (Paris et al., 2010). Nevertheless, even with a college student sample we were able to show that higher levels of substance use and gambling were associated with a blunted SCL. This finding, particularly as it pertains to gambling, is of interest because researchers have recently reported finding blunted cortisol and heart rate responses to a stress task in women with another so-called behavioral addiction, i.e., probable exercise dependence (Heaney, Ginty, Carroll, & Phillips, 2011). Behavioral addictions such as excessive gambling or exercising may be particularly well-suited for elucidating the role of stress in addiction because underlying processes and mechanisms can be explored without the confounding effects of substances (alcohol, drugs, nicotine) on the body’s physiology. We therefore recommend that future studies examine the relationship between stress and addiction by comparing substance-related and behavioral addictions.
Limitations and Future Directions
When interpreting the present findings, it is important to keep in mind that the study was conducted with a convenience sample of college students. As we cannot be sure that the results will generalize to other samples, future research is needed with community samples of varying ages and backgrounds.
Another limitation was the relatively small sample size. As nine participants were lost to analyses involving the physiological measures because of equipment malfunction, there was unfortunately insufficient power to investigate possible interactions between substance use and stress reactivity with decision making. The lack of power also limited the interpretation of the exploratory analyses.
We also point out that the IGT is not a uniformly accepted task to measure decision making under uncertainty. One group of researchers has criticized its underlying general assumptions (Dunn, Dalgleish, & Lawrence, 2006). Another group has questioned the validity of the IGT’s key assumptions for normal populations (Steingroever, Wetzels, Horstmann, Neumann, & Wagenmakers, 2013), even though it has widely been employed with non-clinical populations. A caveat regarding the present study is therefore indicated. Although participants exposed to a social stressor made less advantageous choices on the IGT, as we hypothesized, future research should seek to replicate the present findings with other decision making tasks.
Finally, the present study only allowed us to conduct an exploratory analysis of the relationship between physiological arousal and decision making as a function of participants’ involvement in addictive behaviors (here substance use and gambling). Research in this area is still in its incipient stages. For future studies it would be beneficial to investigate how stress may differentially affect risk taking in clinical and non-clinical populations. Of particular interest might be to compare individuals with substance-related versus behavioral addictions as this might identify predisposing mechanisms regardless of the form an addictive behavior takes. In addition, given the preliminary evidence that men and women differ when they make decisions in stressful conditions, more research is needed to elucidate possible gender differences.
Conclusions
In summary, our study has shown that stress increases arousal and negative emotions and adversely impacts decision-making, but differently for men and women. We speculate that under stress men rely more on automatic processing and thus are biased toward immediate rewards, whereas women – up to a certain stress level – become more conservative before their behavior deteriorates. Our findings also suggest that the hypoactive SCL response associated with increased substance use may reflect stress dysregulation noted in substance use disorders. Together these results emphasize the importance of stress reactivity as a potential point of intervention in reducing risk taking.
Footnotes
Conflict of Interest Statement:
The authors declare they have no conflict of interest.
References
- Bechara A, Damasio A, Damasio H, Anderson S. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1-3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123(11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Benedek M, Kaernbach C. A continuous measure of phasic electrodermal activity. Journal of Neuroscience Methods. 2010;190:80–91. doi: 10.1016/j.jneumeth.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick JD, Roy AL, Raver CC. Evaluating the Iowa Gambling Task as a direct assessment of impulsivity with low-income children. Personality and individual differences. 2013;55(7):771–776. doi: 10.1016/j.paid.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM, Zayas V, Guthormsen A. Neural correlates of decision making on a gambling task. Child development. 2009;80(4):1076–1096. doi: 10.1111/j.1467-8624.2009.01318.x. [DOI] [PubMed] [Google Scholar]
- Clark L, Robbins T. Decision-making deficits in drug addiction. Trends in Cognitive Sciences. 2002;6(9):361. doi: 10.1016/s1364-6613(02)01960-5. [DOI] [PubMed] [Google Scholar]
- CORE Institute . National Reference Group: Cross- Tabulation. Core Institute; Carbondale, IL: 2004. [Google Scholar]
- Critchley H, Elliott R, Mathias C, Dolan R. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. The Journal of Neuroscience. 2000;20(8):3033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. Descartes' error: Emotion, reason, and the human brain. Putnam; New York: 1994. [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The Electrodermal System. In: Cacioppo JT, Tassinary LG, Bernston GG, editors. Handbook of Psychophysiology. New York: 2007. pp. 159–223. New York. [Google Scholar]
- Dunn B, Dalgleish T, Lawrence A. The somatic marker hypothesis: a critical evaluation. Neuroscience and Biobehavioral Reviews. 2006;30(2):239–271. doi: 10.1016/j.neubiorev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. An introduction to the bootstrap. Chapman and Hall; 1993. [Google Scholar]
- Eysenck MW. Extraversion, arousal, and speed of retrieval from secondary storage1. Journal of Personality. 1975;43(3):390–401. doi: 10.1111/j.1467-6494.1975.tb00712.x. [DOI] [PubMed] [Google Scholar]
- Eysenck MW. Effects of noise, activation level, and response dominance on retrieval from semantic memory. Journal of Experimental Psychology: Human Learning and Memory. 1975;1(2):143. [PubMed] [Google Scholar]
- Gansler DA, Jerram MW, Vannorsdall TD, Schretlen DJ. Does the Iowa Gambling Task measure executive funciton? Archives of Clinical Neuropsychology. 2011;26(8):706–717. doi: 10.1093/arclin/acr082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan A, Oosterlaan J, de Beurs E, van den Brink W. Decision making in pathological gambling: a comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and normal controls. Cognitive Brain Research. 2005;23(1):137–151. doi: 10.1016/j.cogbrainres.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Decision making in pathological gambling: a comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and normal controls. Cognitive Brain Research. 2005;23(1):137–151. doi: 10.1016/j.cogbrainres.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Goudriaan A, Oosterlaan J, de Beurs E, van den Brink W. Psychophysiological determinants and concomitants of deficient decision making in pathological gamblers. Drug and Alcohol Dependence. 2006;84(3):231–239. doi: 10.1016/j.drugalcdep.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Gullo MJ, Stieger AA. Anticipatory stress restores decision-making deficits in heavy drinkers by increasing sensitivity to losses. Drug and alcohol dependence. 2011;117(2):204–210. doi: 10.1016/j.drugalcdep.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Heaney J, Ginty A, Carroll D, Phillips A. Preliminary evidence that exercise dependence is associated with blunted cardiac and cortisol reactions to acute psychological stress. International Journal of Psychophysiology. 2011;79(2):323–329. doi: 10.1016/j.ijpsycho.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, Yarger RS. Adolescents' performance on the Iowa Gambling Task: implications for the development of decision making and ventromedial prefrontal cortex. Developmental psychology. 2004;40(6):1148. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Tyrka AR, Anderson GM, Price LH, Carpenter LL. Sex differences in emotional and physiological responses to the Trier Social Stress Test. Journal of behavior therapy and experimental psychiatr. 2008;39(1):87–98. doi: 10.1016/j.jbtep.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K, Hellhammer D. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1-2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C. Ten Years of Research with the Trier Social Stress Test--Revisited. In: Winkielman EH-JP, editor. Social neuroscience: Integrating biological and psychological explanations of social behavior. Guilford Press; New York, NY, US: 2007. pp. 56–83. [Google Scholar]
- Lawrence A, Luty J, Bogdan N, Sahakian B, Clark L. Problem gamblers share deficits in impulsive decision-making with alcohol-dependent individuals. Addiction. 2009;104(6):1006–1015. doi: 10.1111/j.1360-0443.2009.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Lighthall N. Both Risk and Reward are Processed Differently in Decisions Made Under Stress. Current Directions in Psychological Science. 2012;21(2):36–41. doi: 10.1177/0963721411429452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583(2-3):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Critchley HD, Featherstone E, Trimble MR, Dolan RJ. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological acount of a “default mode” of brain function. NeuroImage. 2004;22:243–251. doi: 10.1016/j.neuroimage.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Pabst S, Brand M, Wolf OT. Stress and decision making: A few minutes make all the difference. Behavioral Brain Research. 2013;250:39–45. doi: 10.1016/j.bbr.2013.04.046. [DOI] [PubMed] [Google Scholar]
- Paris J, Franco C, Sodano R, Frye C, Wulfert E. Gambling pathology is associated with dampened cortisol response among men and women. Physiology and Behavior. 2010;99(2):230–233. doi: 10.1016/j.physbeh.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley CA, Meilman PW, Cashin JR, Lyerla R. Alcohol and drugs on American college campuses: Use, consequences, and perceptions of the campus environment: Volume I: 1989-91. Southern Illinois University; Carbondale, IL: 1993. [Google Scholar]
- Presley CA, Meilman PW, Lyerla R. Development of the Core Alcohol and Drug Survey: initial findings and future directions. Core Institute, Southern Illinois University; 1994. [DOI] [PubMed] [Google Scholar]; Journal of American College Health. 42(6):248–55. doi: 10.1080/07448481.1994.9936356. [DOI] [PubMed] [Google Scholar]
- Preston S, Buchanan T, Stansfield R, Bechara A. Effects of anticipatory stress on decision making in a gambling task. Behavioral Neuroscience. 2007;121(2):257–263. doi: 10.1037/0735-7044.121.2.257. [DOI] [PubMed] [Google Scholar]
- Shaffer HJ, LaPlante DA, LaBrie RA, Kidman RC, Donato AN, Stanton MV. Toward a syndrome model of addiction: Multiple expressions, common etiology. Harvard review of psychiatry. 2004;12(6):367–374. doi: 10.1080/10673220490905705. [DOI] [PubMed] [Google Scholar]
- Sherwood L. Human physiology: from cells to systems. 8th ed. Cengage Learning; Belmont, CA: 2004. The Peripheral Nervous System: Efferent Division; pp. 239–256. [Google Scholar]
- Shurman B, Horan WP, Nuechterlein KH. Schizophrenia patients demonstrate a distinctive pattern of decision-making impairment on the Iowa Gambling Task. Schizophrenia Research. 2005:72. doi: 10.1016/j.schres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Stress and addiction: a dynamic interplay of genes, environment, and drug intake. Biological psychiatry. 2009;66(2):100–101. doi: 10.1016/j.biopsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox H, Hong K, Bergquist K, Bhagwagar Z, Siedlarz K. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34(5):1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KIA, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress-and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Archives of general psychiatry. 2011;68(9):942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcke K, Brand M. Decision making under stress: A selective review. Neuroscience and Biobehavioral Reviews. 2012;36(4):1228–1248. doi: 10.1016/j.neubiorev.2012.02.003. doi: http://dx.doi.org/10.1016/j.neubiorev.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Steingroever H, Wetzels R, Horstmann A, Neumann J, Wagenmakers EJ. Performance of healthy participants on the Iowa Gambling Task. Psychological Assessment. 2013;25(1):180. doi: 10.1037/a0029929. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 6th ed Allyn and Bacon; Boston: 2013. [Google Scholar]
- Thayer J, Lane R. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews. 2009;33(2):81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Thomas S, Bacon A, Sinha R, Uhart M, Adinoff B. Clinical laboratory stressors used to study alcohol-stress relationships. Alcohol Research-Current Review. 2012;34(4):459–467. [PMC free article] [PubMed] [Google Scholar]
- Turpin G, Grandfield T. Electrodermal Activity. In: Fink G, editor. Stress Science: Neuroendocrinology. 1st ed Academic Press; 2009. pp. 313–316. [Google Scholar]
- van den Bos R, Harteveld M, Stoop H. Stress and decision-making in humans: performance is related to cortisol reactivity, albeit differently in men and women. Psychoneuroendocrinology. 2009;34(10):1449–1458. doi: 10.1016/j.psyneuen.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet J-L, Bolla K. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug and Alcohol Dependence. 2007;90(1):2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark L, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wemm S, Fanean A, Baker A, Blough E, Mewaldt S, Bardi M. Problematic drinking and physiological responses among female college students. Alcohol. 2013;47(2):149–157. doi: 10.1016/j.alcohol.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit- formation. Journal of comparative neurology and psychology. 1908;18(5):459–482. [Google Scholar]