Abstract
In separate prior studies, physical topographic surface modification or nitric oxide (NO) release has been demonstrated to each be an effective approach to inhibit and control bacterial adhesion and biofilm formation on polymeric surfaces. Such approaches can prevent biomaterial-associated infection without causing the antibiotic resistance of the strain. In this work, both techniques were successfully integrated and applied to a polyurethane (PU) biomaterial surface that bears ordered pillar topographies (400/400 nm and 500/500 nm patterns) at the top surface and a S-nitroso-N-acetylpenicillamine (SNAP, NO donor) doped sub-layer in the middle, via a soft lithography two-stage replication process. Upon placing the SNAP textured PU films into PBS at 37°C, the decomposition of SNAP within polymer film initiates NO release with a lifetime of up to 10 days at flux levels > 0.5 ×10−10 mol min−1 cm−2 for a textured polyurethane layer containing 15 wt% SNAP. The textured surface reduces the accessible surface area and the opportunity of bacteria-surface interaction, while the NO release from the same surface further inhibits bacterial growth and biofilm formation. Such dual functionality surfaces are shown to provide a synergistic effect on inhibition of Staphylococcus epidermidis bacterial adhesion that is significantly greater than the inhibition of bacterial adhesion achieved by either single treatment approach alone. Longer term experiments to observe biofilm formation demonstrate that the SNAP doped-textured PU surface can inhibit the biofilm formation for > 28 d and provide a practical approach to improve the biocompatibility of current biomimetic biomaterials and thereby reduce the risk of pathogenic infection.
Keywords: Texturing, Nitric Oxide Release, Bacterial Adhesion, Biofilm, S-nitroso-N-acetylpenicillamine (SNAP), Antimicrobial Infection
Graphical abstract

1. Introduction
The long-term use of biomaterials designed for fabricating implantable medical devices such as intravascular catheters, urinary catheters, indwelling blood pumps, vascular assist devices, and orthopedic implants is complicated by the potential for microbial infection due to pathogenic bacterial adhesion and biofilm formation on biomaterial surfaces.[1–3] Due to the difficulty in treating bacterial biofilms via antibiotics and the increasing levels of antibiotic resistance of pathogens, surgical removal and replacement of the implanted devices is often the only treatment for device-centered infections, causing a significant increase in morbidity and cost.[4] Combatting device-associated infections has been a great challenge in the field of implant-associated health care.[5]
Bacterial adhesion to the device surface is the first and critical step in the pathogenesis of implant related infections. As an alternative to traditional methods in which antibiotics or biocides are released or infused (e.g., catheter lock solutions), methods to design and control material properties to reduce/control bacterial adhesion and thereby reduce microbial infection are more attractive approaches.[6, 7] One promising method is the generation of functional surfaces through topographic modifications (e.g., surface texturing) that significantly reduce the initial attachment of microorganisms and the number of persistent pathogens to the surfaces of the implant.[8] By analogy to natural antifouling surfaces such as shark skin, shell or lotus leaf, etc., the physical topographic surface modification with micro- or nano-size features reduces the surface contact area and changes the surface energy, and this approach has been shown to be effective in controlling bacterial adhesion and biofilm formation.[9–12] With interest in this area and the related research increasing, over the past two decades biomimetic or bio-inspired materials have been developed to prepare the sterile biomaterials for the use in medical devices.[13, 14] For example, the topographical features mimicking the shark’s skin were applied into polydimethylsiloxane (PDMS) elastomer and it was found that such surfaces disrupt biofilm formation of Staphylococcus aureus (S. aureus), suggesting the possibility for application of this approach in biomedical devices.[15] Furthermore, an in vitro study showed that the micro-patterned surface inhibited the bacterial colonization and migration of uropathogenic Escherichia coli (E. coli); thereby it could be applied to reduce the risk of catheter-associated urinary tract infection.[16] Our group has previously developed submicron-textured polyurethane biomaterial surfaces featuring patterns with pillars of diameter and spacing of 400/400 nm and 500/500 nm, and demonstrated that both textured surfaces decreased the adhesion of Staphylococcal epidermidis (S. epidermidis) and S. aureus and inhibited biofilm formation under shear and static conditions.[17] Such submicron-textured surfaces have also been shown to reduce platelet adhesion, thereby reducing the potential of implant associated blood clotting/thrombosis of the device if implanted in the blood stream.[18] These in vitro successes of textured materials have demonstrated the great potential for their use in clinical applications to combat health-care infections and thrombosis caused by catheters and other implanted devices.
Nitric oxide (NO) releasing biomaterials represent another biomimetic strategy with great potential for clinical use. NO is an endogenous gas molecule and its continuous release from the endothelial cells that line all blood vessels can effectively prevent the adhesion/activation of platelets on normal blood vessel walls.[19] NO also plays an important role in the immune response as an antimicrobial agent and host defense against pathogenic bacteria.[20] As a diatomic free radical, NO can cross the membranes to enter the microbial cell readily and kill the microbe by directly damaging DNA, proteins, and lipids through production of potent nitrosating species or by combining with reactive oxygen species (e.g., superoxide, peroxide) and oxidizing the same targets.[21, 22] Furthermore, the rapid reduction of microbial loads reduces the pressure for the evolution and spreading of variant bacteria and limits the possibility of promoting NO resistant strains.[23] For these reasons, polymeric materials that mimic endogenous NO release provides a potential solution against medical device-associated microbial infection and also can prevent platelet activation and thereby reduce risk of thrombus formation. Extensive studies have already demonstrated that NO release can effectively inhibit bacterial adhesion and reduce biofilm development on material surfaces.[24–27]
Since NO is highly reactive and short-lived under physiological conditions, NO donor molecules with functional groups that can store and release NO are necessary. These NO donors are incorporated into materials either by blending discrete NO donors within polymeric films, or by covalently attaching them to polymer backbones and/or to the inorganic polymeric filler particles that are often employed to enhance the strength of biomedical polymers (e.g., fumed silica or titanium dioxide).[28] N-diazeniumdiolates[26, 29, 30] and S-nitrosothiols[30–32] are commonly used to prepare NO releasing polymeric matrices for improved biocompatibility of blood-contacting medical devices. The N-diazeniumdiolates are generally synthesized by reaction of amines with NO gas to form relatively stable compounds that release NO when in contact with bodily fluids through proton or thermally driven mechanisms. The S-nitrosothiols are generally formed by reaction of nitrous acid with the parent thiol, and undergo thermal decomposition to release NO, which are generally catalyzed by metal ions (e.g., copper), and light.[30] Nitric oxide can also be generated via other techniques, such as electrochemical reduction of nitrite,[33] or reduction of sodium nitroprusside.[34] However, the current NO release strategies have the challenges in terms of storage, stability, costly synthesis or short NO release lifetime. Among the NO donors reported to date, S-nitroso-N-acetylpenicillamine (SNAP) is among the most attractive ones in terms of its long-term NO release capability and enhanced stability when incorporated into low water uptake biomedical polymers. Indeed, SNAP has already been incorporated into a number of low water uptake polymers to yield promising new biomaterials. For example, it was reported that SNAP-doped polyurethane Elast-eon E2As polymer retained 82% of the initial SNAP after 2 month storage at 37°C [35] and released NO slowly at the physiological flux level for 3 weeks. Similarly, SNAP in CarboSil 20 80A also exhibits a stability at 88.5 % ± 4.3% of the initial amount after 8 months storage in the dark at 37 °C.[36] The increased stability of SNAP within these polymers is believed to be due to the intermolecular hydrogen bonds between crystallized SNAP molecules. For example, long-term storage stability of SNAP in the CarboSil polymer was found to originate from the formation of a polymer-crystal composite during the solvent evaporation. This composite led to sustained NO release at the physiological flux levels that can last for >3 weeks with the cross-linked silicone rubber as a topcoat.[36]
The SNAP-doped polymers exhibit good blood compatibility. The E2As catheters doped with SNAP significantly reduced the amount of thrombus and bacterial adhesion compared the E2As control catheters when they were implanted in sheep veins for 7 d.[37] An in vitro experiment of S. aureus biofilm formation over a 7 d period showed that SNAP-doped CarboSil 20 80A intravascular catheters had 5 log units reduction of viable cell count on their surfaces.[36] All results reported to date suggest that SNAP-doped low water uptake polyurethane copolymers could be attractive for clinical applications, providing long-term storage stability, long-term NO release capability in vivo, and increased biocompatibility and antimicrobial activity.
Although each biomimetic approach described above (surface texturing modification or NO release) has been shown to exhibit significant antimicrobial and antithrombotic effects, the use of a single approach is still far from ideal for achieving more complete control of thrombosis and microbial infection on implanted biomedical devices. Each strategy has its inherent drawbacks. For example, physical surface defects such as missed pillars during fabrication is always unavoidable, leading to local adhesion and accumulation of platelets or bacteria, while the NO release polymers have the limit of lifetime and storage stability. To overcome the shortcomings of each individual approach, the combination of surface texturing and NO release to control bacterial adhesion and biofilm formation would provide potentially additive or synergetic effects to decrease bacterial adhesion and/or platelet activation. In this study we fabricated new polyurethane films that bear ordered pillar topographies at the top surface as well as a NO releasing material in the sublayer. Such a combination produces dual functional materials with the reduced accessible surface contact area for bacteria adhesion and providing continuous NO release. Experimental data demonstrate that the dual functional surfaces provide a further improvement in the surface antimicrobial activity and anti-biofilm functionality than either of the approaches can provide when employed alone. Results show that textured surfaces with NO release inhibit biofilm formation over a relatively long term (> 28 d) and this provides a practical approach to improve the biocompatibility of current biomimetic biomaterials and reduce the risk of pathogenic infection. The successful implementation of such materials in clinical practice could ultimately reduce costs by decreasing the number of implant replacements and/or amputation/mortality.
2. Materials and Methods
2.1 Materials
CarboSil® 20 80A silicone-polycarbonate-urethane (PU, DSM) was supplied as a solid and dissolved in N,N-dimethylacetamide (DMAc, Sigma-Aldrich, St. Louis, MO) at a concentration of 15 w/v% (1.5 g PU in 10 ml DMAc) for preparing the films. The SNAP-doped PU solutions were prepared by dissolving SNAP at 5, 10, or 15 w/w% relative to solid polymer within 15 w/v% Carbosil PU DMAc solutions. After extensive mixing, films can then be cast from these solutions by a spin coater (Specialty Coating System Inc., Indianapolis, IN). CarboSil PU contains 4,4′-methylene bisphenyl diisocyanate (MDI) as a hard segment and poly(dimethylsiloxane) (PDMS) and polycarbonate as soft segments. N-Acetyl-D-penicillamine was purchased from Sigma-Aldrich for the synthesis of SNAP, which has been described in previous publications.[35, 37] All aqueous solutions were prepared with Millipore water (18.2 MΩ) and phosphate buffered saline (PBS 0.01 M, pH 7.4, Sigma-Aldrich) was used for in vitro experiments. All culture media and containers for bacteria study were autoclaved at 121°C for 20 min, and the Carbosil films were sterilized by washing with 70% ethanol prior to experiments. All films were prepared in a clean room to avoid contamination.
2.2 Preparation of textured and NO releasing PU films
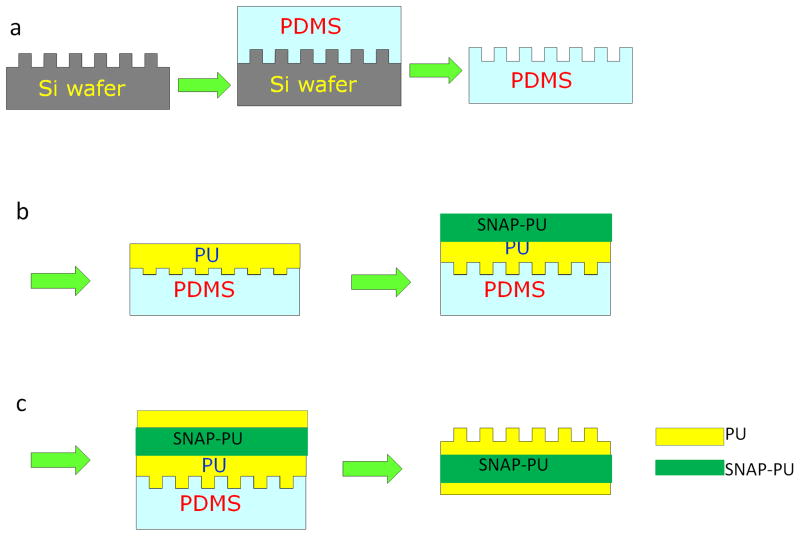
The CarboSil 20 80A PU film surfaces were textured with ordered arrays of pillars using a modified soft lithography two-stage replication molding technique that has been described previously.[17, 18] Briefly, a master pattern with ordered arrays of pillars was first fabricated on a silicon wafer. Then a silicone mold (PDMS) was cast against the master pattern to provide the negative silicone mold (see Fig. 1a). To obtain the highest replication efficiency of surface topography, the PU films were prepared by spin casting CarboSil onto a PDMS mold at 1200 rpm for 1 min in one thin layer first, followed by degassing and curing overnight at room temperature under vacuum. Then, additional layers of CarboSil PU were added by spin casting at 600 rpm for 1 min, respectively. Each layer was degassed and cured at room temperature under vacuum. The CarboSil PU solutions doped with different concentrations of SNAP (5, 10, 15 % (w/w) were repeatedly added on the PU films by spin casting at 600 rpms for 1 min, followed with degassing and curing overnight under vacuum (Fig. 1b). Finally, the regular CarboSil PU without SNAP was added on the SNAP-PU layer by spin casting, and cured under vacuum, so that the SNAP-doped textured PU films were composed of 3 main layers with a base of PU, a middle layer containing SNAP, and a textured PU top layer with patterns and having a total film thickness of approximately 700 μm (Fig. 1c). Two patterns of silicon wafers with pillar geometries having diameter (d) and separation (s) and d/s values of 400/400 nm and 500/500 nm were used as the masters for replicating PU films. The height of pillars in both patterns was 600 nm. Textured silicon wafers were fabricated by RTI International (Research Triangle Park, NC) based on requirements.
Figure 1.
Schematic diagrams of a soft lithography two-stage replication molding technique employed to prepare textured films examined in this study. (a) Transferring the pattern from a Si wafer onto the PDMS mold; (b) Adding polyurethane to PDMS mold and the SNAP-doped PU by spin casting; (c) Spin casting the PU onto the base of SNAP-textured surfaces.
2.2 NO release measurements
Nitric oxide release from the films was measured using a Sievers chemiluminescence Nitric Oxide Analyzer (NOA) 280i (Boulder, CO) using a method described previously.[35, 38] Briefly, films with diameter of ~10 mm were placed in the sample vessel immersed in PBS containing 100 μM EDTA at 37°C. NO was continuously purged from the buffer and swept from the headspace using nitrogen gas and a bubbler into the chemiluminescence detection chamber of the NOA. When not being tested with the NOA, the SNAP-doped samples were incubated in PBS under same conditions avoiding exposure to light. The NO flux was calculated based on assuming a flat surface area of two sides of films exposed to solution. All experiments were conducted in triplicate.
2.3 Surface water wettability measurement
The water wettability of CarboSil PU films was determined as the advancing water contact angle measured by sessile drop method using a Krüss contact angle goniometer. All measurements were made using water as a probe liquid and an ~ 8 μl of water droplet was used for the measurement. Advancing contact angles were obtained by a minimum of eight independent measurements and are presented as mean ± standard deviation.
2.4 Characterization of surface topography by atomic force microscopy (AFM)
A Multimode AFM with a Nanoscope IIIa control system (software version 5.12r3, Veeco, Santa Barbara, CA) was used to examine the surface textures of PU films operated in tapping mode (intermittent contact) using Si probes having aspect ratio of ~ 4:1 (TETRA, K-Tek Nanotechnology, Wilsonville, OR) in air. AFM was also used to image the bacterial adhesion on CarboSil PU surfaces. AFM images were treated and analyzed by off-line AFM software (version 5.12r3, Veeco, Santa Barbara, CA).
2.5 Bacterial growth, adhesion, and biofilm formation on SNAP-doped textured PU surfaces
S. epidermidis RP62A is a strongly adherent, slime-producing, pathogenic strain isolated from a patient with intravascular catheter-associated sepsis.[39] S. epidermidis RP62A (ATCC 35984) was used to test the bacterial growth inhibition, and adhesion to various CarboSil surfaces in this study. The culturing and collection of bacteria have been reported in a previous publication.[17] Bacterial colonies of the strain were cultured in tryptic soy broth (TSB) at 37 °C for 24 h and collected by centrifuge at 1360g for 10 min. The pellet was re-suspended in PBS and the concentration of bacteria was measured via a spectrophotometer at 600 nm (turbidity measurement). To test the effect of NO release on bacterial growth, the polymer films were cut into round disks with 9 mm diameter, and soaked in PBS for 1 h, and placed in 24 well plates. Two ml of TSB culture medium containing S. epidermidis RP62A with a concentration of 1×106 CFU/ml was added and incubated at 37°C with shaking at 180 rpm. At 6, 24, and 48 h time points, 0.25 ml medium was drawn and diluted into 1 ml with sterile TSB, and the optical density (OD) was measured at 600 nm, where OD600 values indicated the growth of bacteria.
To test bacterial adhesion, the bacterial suspension was diluted in PBS to a final concentration of 1×108 CFU/ml. PU films were cut into the round pieces with diameter of 10 mm and pre-hydrated in Millipore water for 24 h and conditioned in PBS for 1 h, to fully equilibrate the outermost surface of the polymer films. Such pre-hydration also washes away any SNAP on the surface of the films that would lead to an initial burst of NO release from the surface of the film. This provides a more reliable approach to evaluate the effect of stable NO release on bacterial adhesion over longer periods by removing the initial NO burst. Bacterial adhesion was carried out in a micro-well plate with the volume of ~ 0.5 ml at 37°C for 1 h at near static condition with slight orbital shaking. After adhesion, the bacteria suspension was exchanged with PBS for 3 times, and then the samples were fixed in 2.5 % glutaraldehyde for 2 h. After being rinsed with PBS, bacteria on the PU films were stained with Hoechst 33258 (Invitrogen) and analyzed under a fluorescence optical microscope (Nikon, Eclipse 80i).
Biofilm formation was assessed under static and shear conditions. PU films were incubated with S. epidermidis RP62A in tryptic soy broth medium containing bacteria at an initial concentration of 1×108 CFU/ml in a 12 well plate for 2 days at 37°C. A rotating disk system with a speed control (model: AFMSRX, Pine Instrument, PA) was used to assess biofilm formation and slime production on surfaces under shear condition.[17] PU films were incubated in TSB under same conditions and run at speed of 431 rpm. Every 2 days, 25 % of media was replaced for supplement of nutrients. After desired time periods, the samples were washed with PBS by sequential addition and aspiration, stained with 100 μg/ml wheat germ agglutinin-FITC (Sigma) for 1 min, and fixed in 1% paraformaldehyde for 30 min. The samples were then examined by fluorescence optical microscopy.
2.6 Statistical Analysis
Statistical analysis was performed using SAS software (version 9.4). Means of experimental data were compared by 2-sample t-test and differences were considered statistically significant for p< 0.05. Significance is denoted in figures of this paper with one symbol denoting p< 0.05, two symbols p < 0.01, and three symbols denoting p< 0.001. The bacterial adhesion was further regressed with the factors by ANOVA general linear model (GLM), and differences in bacterial adhesion means were analyzed by the least mean square method.
3. Results and Discussion
3.1 Preparation SNAP-textured polyurethane films
In a previous study,[35] we examined the NO release and stability of 5wt% and 10 wt% SNAP-doped Elast-eon E2As polymers. In this study, we examined a different polymer, CarboSil 20 80A, and combined the SNAP-doped PU in the middle layer and the top layer with textured pattern to make the “sandwich-like” polymer films. To study the NO release profiles and the response of bacterial adhesion to the polymer films that contain different concentrations of NO donor SNAP, a series of SNAP-textured films with CarboSil 20 80A polyurethane, in which the middle layer of PU was doped with 5 wt%, 10 wt%, and 15 wt% SNAP and the top surface layer was textured with patterns of 400/400 nm or 500/500 nm, were fabricated by a soft lithography two-stage replication molding technique. Generally, we obtained regular textured patterns on the outer polyurethane surfaces, similar to the features that we described in previous publications for the regular textured surface (without NO release).[17, 40] Both the diameter of pillars and distance of inter pillars are less than the dimensions of bacterial cells, ensuring a reduction of available contact area when bacterial cells contact the surface, with minimal opportunities for bacteria to become lodged in the spaces between pillars.
Initially we observed some shallow areas on the textured surfaces after we applied only a thin layer at the top surface (composed with PU layers which were spin coated at 1200 rpm and 600 rpm for 1 min, respectively) and with SNAP-doped polyurethane added as the middle layer (Figure 2a). The shallow areas are believed to be SNAP that diffused into the thin top polyurethane layers during fabrication, leading to a reduction of pillar height. To improve the texturing pattern, two more layers were added to the top textured layer by spin coating at 600 rpm for 1 min. The thicker top layer of PU significantly limited the diffusion of SNAP into the textured layer during drying/curing and the resulting textured surface had a more uniform distribution of pillars, with a pillar yield of > 99.8% (see Fig. 2b). To ensure that the NO release can be controlled, a base layer of PU was subsequently applied onto the middle SNAP-PU layer by spin coating at 400 rpm for 1 min, and application of such layers was repeated for 3 times. In this manner, the sandwich-like PU films with SNAP and texturing were fabricated. The subsequent measurement of NO generation showed that a thicker top layer of PU extended the lifetime of NO release, suggesting that the thickness of top layer can control the release rate of NO (see Section 3.3). Further, a thicker top layer of PU can also reduce the leaching of SNAP, NAP and NAP dimer from the films.
Figure 2.
3D atomic force microscope (AFM) image of NO releasing 500/500 nm textured polyurethane film surface; (a) thin top layer showing the diffusion of SNAP onto top surface, and (b) thick top layer showing normal textured surface feature (scan size: 20×20 μm2, height 1000 nm).
The SNAP-textured polymer film was prepared by solvent evaporation under vacuum. In this study, DMAc was used as the solvent to dissolve the polyurethane and/or SNAP to form the thin layer on the mold by spin casting. Such process enables easy control of the thickness of films and produces well textured patterns with high yield of pillars, but is time-consuming. Brisbois et al.[35] used the alternative solvent tetrahydrofuran (THF) to prepare the SNAP-doped Elast-eon E2As polymer as a homogenous coating on a Teflon plate. However, it appears that this approach is unsuitable for spin casting in a soft lithography two-stage replication process since the fast evaporation of THF often causes a heterogeneous film thickness during the spinning process. Furthermore, the pattern produced was found to be unacceptable and pillar yield was low because the PU polymer solution did not have good access into the submicron size holes within the PDMS mold.
3.2 Surface wettability
The regular smooth CarboSil 2080A PU surface is hydrophobic with a water contact angle of 91.0±1.4°. Surface texturing increased the hydrophobicity with water contact angle of 139.3 ±1.6° due to air captured in the spaces between pillars (Figure 3). This is similar to the BioSpan® MS/0.4 polyurethane textured surfaces that have been described elsewhere.[40] Adding the SNAP-doped polyurethane as the middle layer of films did not affect the water contact angle significantly for the smooth surfaces while it appeared to decrease the water contact angles for the textured surfaces as the SNAP content increased. This is probably due to the diffusion of SNAP into top layer of PU film. However, since the observed differences of water contact angles between textured surfaces having 0, 5 and 10 wt% SNAP was only approximately 5°, this is generally within the variability of the measurement method.
Figure 3.
Water contact angle of smooth and textured (500/500 nm pattern) PU films with SNAP-doped PU in middle layer.
3.3 NO generation from SNAP-doped PU films
S-Nitrosothiols (RSNOs) are one of the most widely used NO donors. SNAP can decompose into the disulfide, dimer of NAP or (NAP)2, and release NO in the presence of light, heat, or moisture. In the presence of certain metal ions (e.g. Cu(II)) a reductive catalytic reaction is possible that leads to formation of initially NAP (and then (NAP)2 after oxidation of NAP thiols) and NO. SNAP is a synthetic tertiary RSNO and is more stable than most physiological RSNOs due to the steric hindrance of the sulfur atom.[41, 42] Upon placing the SNAP-doped CarboSil PU films into PBS at 37°C, the decomposition of SNAP within polymer film occurs simultaneously to release NO owing to the combination of moisture and temperature.
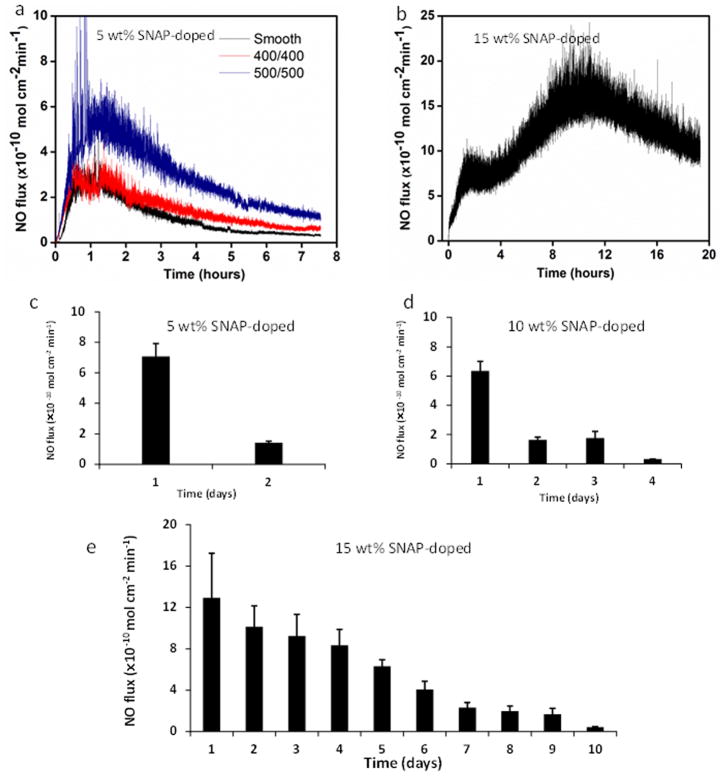
The NO release flux rate depends on the topcoat polymer layer thickness, the surface topography, and level of SNAP content within the ‘sandwich’ middle layer of the polymer film. We found that the NO release was fast for the SNAP-polymer with a thin top layer and lasted only 3–4 days. Also, we found that NO flux was generally higher in the presence of the textured surface than on the normal smooth surface, likely due to the increased water contacting surface area of the textured film (Figure 4a). To control the NO release flux, we fabricated CarboSil PU films containing 5, 10, and 15 wt% SNAP in a middle layer sandwiched by a base PU and a thick textured PU surface. Figure 4b illustrates the NO flux release from 15 wt% SNAP-doped surface in contact with water at 37°C. It was found that the NO flux release was delayed initially, then gradually increased with time and then reached a peak. The average NO flux was as high as 15 ×10−10 mol min−1 cm−2 on day 1, and then decreased in the following days. The lifetime of NO release varied with the concentration of SNAP-doped within the films. The NO flux release from SNAP textured surfaces with 500/500 nm patterns was found to last only about 2 and 4 days for 5 wt% and 10 wt% SNAP-doped films, respectively, while the lifetime of NO release for 15 wt% SNAP-doped materials can last up to 10 d at an NO flux > 0.5 ×10−10 mol min−1 cm−2 (see Figures 4c–4e). Healthy endothelial cells produce NO fluxes generally in the range of 0.5–4.0 ×10−10 mol min−1 cm−2 to prevent platelet activation and subsequent thrombosis.[43]
Figure 4.
NO release from SNAP-doped textured polyurethane film in PBS at 37°C without light. (a) smooth, 400/400nm, and 500/500nm textured PU films with 5 wt% SNAP-doped on day 1; (b) NO release on day 1 from 500/500 nm textured thicker PU films with 15 wt% SNAP doped; and the average NO flux released from surface as the function of time (days) from 500/500 nm textured PU films with (c) 5 wt%, (d) 10 wt%, and (e) 15 wt% SNAP doped. Data collection ended when the flux went below physiological levels of 0.5 ×10−10 mol min−1 cm−2.
The sustained NO release originates from the formation of a composite of SNAP crystals within the polymer phase. Wo et al.[36] proposed a 2-step mechanism of NO release from the SNAP-doped CarboSil polymer. Upon hydration at 37°C SNAP in the polymer matrix decomposes and releases NO, primarily in the water-rich regions near the polymer/solution interface, and the dissolved SNAP in the bulk polymeric phase becomes unsaturated, resulting in the dissolution of crystalline SNAP within the bulk of the polymer. The SNAP-textured CarboSil polyurethane film examined in this study consists of top, middle, and base layers with same CarboSil 20 80A polymer and the SNAP doped in the middle layer. Comparing the long-term NO release from SNAP-doped E2As polymer[35] or silicon rubber top coated CarboSil polymer,[36] the lifetime of NO release from SNAP-textured PU films in this study is relatively shorter (10 d) vs. these prior reports. One probable reason for this is that the top layer in the textured films is a relatively thin film formed by the spin coating process, and SNAP from the underlying layer can enter this outermost layer during the preparation process leading to a greater release rate of NO at the initial time point (e.g., first day). Additional spin coating of layers within the top portion of the film reduces the diffusion of SNAP into top textured layer during film preparation, and increases the lifetime of NO release (Figure 4e). Another plausible explanation for reduced longevity of NO release from the textured films that have relatively thin top layers is that the repeated dissolution and recrystallizing of SNAP during fabrication process may lead to the instability and partial decomposition of SNAP within polymer. Clearly, the fabrication process of preparing optimal SNAP-textured films still needs to be optimized.
3.4 Surface topography of SNAP-doped PU films
NO release from SNAP-doped polymer film changes the nano-topography of top surface. Figure 5 illustrates the AFM surface image of a smooth film containing 15 wt% SNAP in middle layer before and after hydration. Surprisingly there are particle-like spots distributed on the smooth surface before hydration, and these spots had diameters in the range of 250–350 nm and height of 35–60 nm. These spots were likely due to the SNAP diffusion into the very top thin layer of PU during fabrication and some of SNAP crystals extending out from the surface. We did not observe such particle-like spots on the films in absence of SNAP. After hydration in PBS for 24 h at 37°C, there were few particle-like spots found on surface. Instead, only small pits with a depth of around 5–30 nm were observed, suggesting that SNAP decomposed/dissolved and generated NO flux, which led to the formation of these observed pits.
Figure 5.
Atomic force microscope (AFM) images of smooth polyurethane surface with 15 wt% SNAP in the middle layer (a) before hydration and (b) after hydration (Scale bar = 2 μm).
3.5 Antimicrobial properties of SNAP-doped PU films
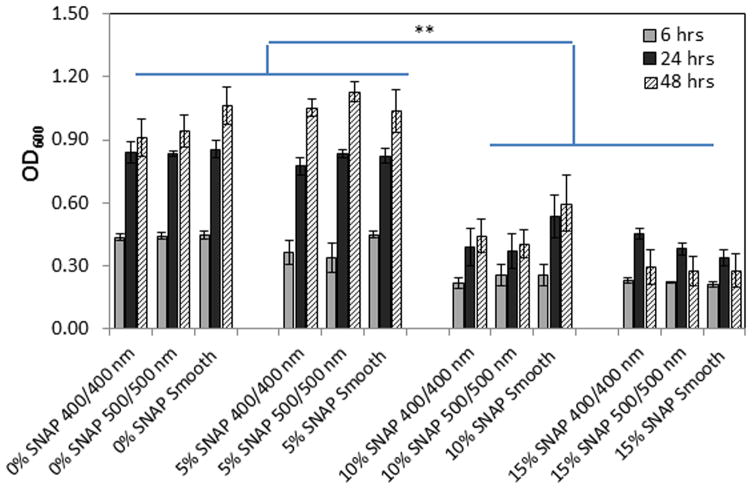
The antimicrobial properties of polymers were evaluated by the measurements of bacterial turbidity of media inoculated with bacterial strains and incubated with polymer films. Figure 6 shows that the values of OD600 of media incubated with polymers without SNAP or doped with 5 wt% SNAP increased sharply after 24 h, suggesting that the polymers with 5 wt% SNAP doped in middle layer had no significant inhibition on bacterial growth in the bulk of the culture; however, the OD600 values of media incubated with 10 wt% and 15 wt% SNAP-doped polymers increased only slightly at 24 and 48 h. The values were about half of the values of OD600 for polymers without SNAP or with 5 wt% SNAP, suggesting that bacteria in the bulk media solution were inhibited by the NO released by the SNAP-doped films. Of interest is that the OD600 values of media in contact with the 15 wt% SNAP-doped polymers decreased at 48 h (p-values =0.08 and 0.13 for textured 400/400nm and 500/500 nm surfaces, respectively) compared to the values at 24 h, indicating that bacteria were inhibited by the high NO flux released from these polymers during the initial 2 d of incubation.
Figure 6.
Optical density of bacterial culture medium measured at 600 nm wavelength after being incubated with various CarboSil films for 6, 24, and 48 h. The significance, indicated by asterisk, was compared between 0% or 5% SNAP doped films and 10% or 15% SNAP doped films at corresponding culture time and show the p-values <0.01.
3.6 Bacterial adhesion on SNAP-doped PU film surfaces
Bacterial adhesion to the various PU film configurations was assessed under static condition in PBS and evaluated by the bacteria counts per unit area. Generally, both the textured surfaces alone and the use of SNAP alone (without texturing (smooth)), significantly reduced bacterial adhesion on CarboSil PU film surfaces (Figure 7a). Without NO release from polymers (0% SNAP), surface texturing alone (400/400 nm and 500/500 nm) reduced bacterial adhesion by ca. 61–64%, when compared to the smooth samples. With SNAP present in the middle layer of the polymer films, the reduction rate increased to up to 88% on the films containing 15 wt% SNAP and the 500/500 nm pattern surface texture. The response of bacterial adhesion to the NO release is dependent on the concentration of SNAP doped in the middle layer of polymer films. Bacterial adhesion generally decreased with the increase of SNAP concentration due to the increase of NO release level, as expected (Figure 7b). We used the general linear model (GLM) to regress the factors of SNAP content, surface texturing, and interaction of SNAP×texturing, and found that both surface texturing and SNAP were significant factors influencing S. epidermidis bacterial adhesion (both p-values <0.0001). Furthermore, the interaction of SNAP and texturing also significantly affected bacterial adhesion (p-value <0.0001), strongly suggesting that the bacterial adhesion linearly decreased with increasing SNAP concentration, but the slopes for different surfaces (Smooth, 400/400 nm, and 500/500 nm patterns) were significantly different (Figure 7b), suggesting that the combination of texturing and SNAP produced synergistic effect on inhibition of S. epidermidis bacterial adhesion.
Figure 7.
(a) Bacterial adhesion and reduction rates (against smooth regular PU polymer) on NO releasing textured polyurethane surfaces under static condition at 37°C for 1 h; (b) linear regression of bacterial adhesion against SNAP content. Asterisks in graph represent a statistically significant difference in bacterial adhesion relative to non-textured surface, with one symbol denoting p< 0.05, two symbols denoting p < 0.01, and three symbols denoting p< 0.001.
These differences in bacterial adhesion were further compared by the least square means method. Table 1 shows that the differences of means between smooth and textured surfaces (either 400/400 nm or 500/500 nm) at SNAP levels of 0 wt%, 5 wt%, and 10 wt% are significant, but not significant at SNAP level of 15 wt% (all p-values >0.05 for smooth and textured surfaces). Results suggest that both NO release and texturing contributed to inhibition of bacterial adhesion at lower levels of SNAP content, but the NO release dominates the degree of reduction in bacterial adhesion at high levels of SNAP (15 wt%), indicating that the effect of surface texturing becomes less significant when the concentration of SNAP is as high as 15 wt%. A comparison of bacterial adhesion between two textured surfaces with different dimensions, 400/400 nm and 500/500 nm, indicates that there is no statistically significant difference of mean for these two differently patterned films (all p-values >0.05) regardless of the levels of SNAP concentration present. This is due to the similar pillar geometry of the patterns with each having the submicron dimensions (400 nm and 500 nm). The effect of significantly larger or smaller pattern sizes on bacterial adhesion in combination with NO release still needs to be examined in the future.
Table 1.
Statistical analysis of difference of least square means in bacterial adhesion Staphylococcus epidermidis between every two groups of biomaterial surfaces examined in this work.
| SNAP (wt%) | Comparison | P value |
|---|---|---|
| 0 | Smooth vs 400/400 | < 0.0001 |
| Smooth vs 500/500 | < 0.0001 | |
| 400/400 vs 500/500 | 0.2841 | |
| 5.0 | Smooth vs 400/400 | < 0.0001 |
| Smooth vs 500/500 | < 0.0001 | |
| 400/400 vs 500/500 | 0.4711 | |
| 10.0 | Smooth vs 400/400 | 0.0002 |
| Smooth vs 500/500 | < 0.0001 | |
| 400/400 vs 500/500 | 0.8381 | |
| 15.0 | Smooth vs 400/400 | 0.9991 |
| Smooth vs 500/500 | 0.4598 | |
| 400/400 vs 500/500 | 0.4605 |
The above bacterial adhesion experiment was carried out in PBS, although the presence of proteins are potentially quite important in influencing bacterial adhesion on implanted medical devices surfaces. When a foreign material is implanted in the body, plasma proteins rapidly adsorb on the surfaces and form a layer of proteins. The nature of adsorbed proteins modulates the bacterial adhesion, aggregation and biofilm formation. In vitro studies have shown that the presence of the serum proteins generally suppresses the initial bacterial adhesion due to the nonspecific interactions of albumin and the surfaces of bacteria [44] while adhesive proteins (e.g., fibrinogen and fibronectin) were reported to increase adhesion via forming the specific ligand/receptor bonds between proteins and the surfaces of bacterial cells.[45, 46] In our previous study of bacterial adhesion on submicron textured polyurethane surfaces, a greater decrease in adhesion was observed when textured surfaces were incubated in solutions containing plasma or serum compared with the adhesion in PBS.[17] Similarly, we expect a decrease in bacterial adhesion on SNAP-textured PU surfaces in the solution containing serum proteins compared to the adhesion in PBS (Figure 7). We also expect that surface texturing and NO release could exert the similar ability in controlling bacterial growth and adhesion on SNAP-textured surfaces in solutions containing plasma proteins. Such studies will be conducted during the next phase of this research project.
3.7 Biofilm formation on SNAP-Textured PU films
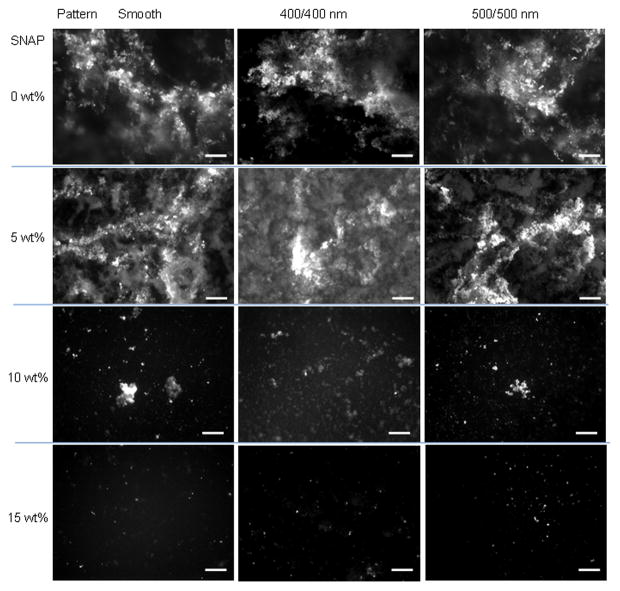
S. epidermidis RP62A biofilm formation on dual functionalized CarboSil films was assessed in TSB culture under static conditions as well as shear conditions over different periods, respectively. Under static conditions, biofilm was observed on un-doped (0 wt% SNAP) and 5 wt% SNAP doped CarboSil film surfaces, either smooth or textured, within 2 d (Figure 8); however, there were only a few of clusters of bacteria or small biofilms formed on 10 wt% SNAP (both smooth and textured surfaces), and no biofilms were observed on the film surfaces with 15 wt% SNAP, for both smooth and textured surfaces. This suggests that the higher rates of NO release inhibit the growth of bacteria biofilm on the polymeric surfaces. This is consistent with the antimicrobial properties of these new materials (Figure 6), where bacteria growth was inhibited in culture medium. Similar bacterial growth and biofilm formation were observed on PU surfaces with the same SNAP-doping level, suggesting that texturing had no significant effect on inhibition of biofilm formation under static conditions. Under static conditions, NO release exerts the more powerful ability to inhibit bacterial growth. In contrast, the combination of texturing and NO release is much more effective in controlling bacterial growth and biofilm formation under shear conditions (see Figure 9).
Figure 8.
Fluorescent optical microscopy images of biofilms formed on a variety of PU film surfaces under static condition for 2 d at 37°C (scale bar = 50 μm).
Figure 9.
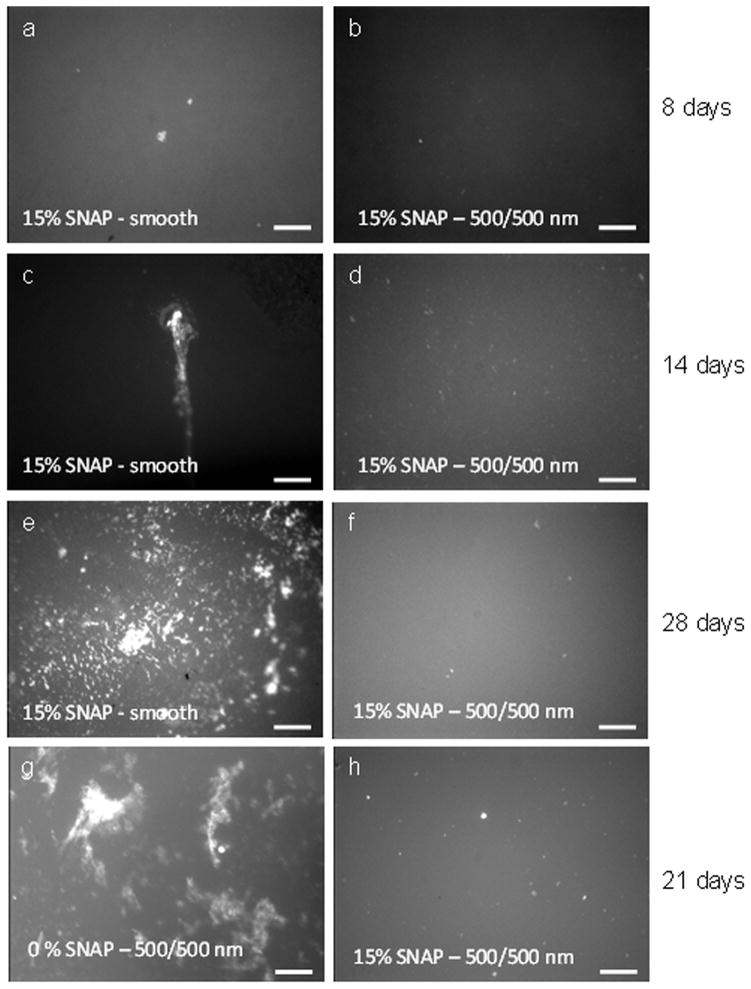
Fluorescent optical microscopic images show the biofilm formation on polyurethane film surfaces at 37°C under shear, (a) 15 wt% SNAP – smooth and (b) 15 wt% SNAP and 500/500 nm textured surfaces for 8 d; (c) 15 wt% SNAP – smooth and (d) 15 wt% SNAP and 500/500 nm textured surfaces for 14 d; (e) 15 wt% SNAP-smooth and (f) 15 wt% SNAP-500/500 nm textured surfaces for 28 d; (g) un-doped textured 500/500 nm surface and (h) 15 wt% SNAP-500/500 nm textured surface for 21 d (scale bar = 50 μm).
The polymer films were further examined for biofilm formation under shear conditions by using a rotating disk system for different periods. There was no significant biofilm observed on 15 wt% SNAP-doped CarboSil film surfaces that were either smooth or textured (with 500/500 nm pattern) over an 8 d period (Figure 9a and 9b). A small area with biofilm slime was observed on the 15 wt% SNAP smooth surface (Figure 9c) after sample was run in culture medium for 14 d, while no biofilm was observed on the 15 wt% SNAP-textured surface under same condition (Figure 9d). The duration of experiments was extended to 28 d with the smooth and textured films containing 15 wt% SNAP. It is obvious that there was no biofilm formation on the textured surface while biofilm did form on the smooth surfaces (see Figures 9e and 9f). To further examine the effect of NO release on biofilm formation, the textured films with or without SNAP were run in culture medium for 21 d. Results show biofilm/slime formed on the regular (0 wt% SNAP) textured surface while no biofilm/slime was observed on 15 wt% SNAP-textured surface (Figures 9g and 9h). Overall, CarboSil 20 80A PU films containing 15 wt% SNAP doped in the middle layer and the 500/500 nm textured pattern as the top layer can inhibit the biofilm growth for > 28 d. Since the NO release from 15 wt% SNAP-doped PU films can only last up to 10 d, the long term (> 28 d) inhibition of biofilm growth is attributed to the combination of early NO release and the physical surface topography modification created by the pillars. The high NO flux inhibits or kills the bacteria in the initial period and protects the material from biofilm formation. As the NO flux decreases, the surface textured topography continues reducing the bacterial adhesion and inhibiting biofilm formation on surface for longer periods. Results suggest that the combination of NO release and topography modification provides a promising approach to greatly inhibit biofilm growing for long-term on the polymer surface.
This study has demonstrated the fabrication of the PU films that bear ordered pillar topographies at the top surface and NO release PU in the sublayer. Such a unique combination of surface texturing and NO release can provide a biomimetic surface that exhibits substantial antimicrobial/antibiofilm activity. On one hand, the topographical feature reduces the accessible surface contact area for potential bacterial adhesion. The fractions of total top surface areas for these textured patterns are ~ 25% of the nominal surface area and these textured surfaces alone reduce adhesion of S. epidermidis by 61–64% under near static condition in PBS solution for 1 h. The reduction of bacterial adhesion by topographical surface modification may also be attributed to the change of surface energy and surface wettability. The textured pattern makes the surface more hydrophobic due to the Cassie – Baxter effect.[47] Our previous studies showed that the increase of surface hydrophobicity is necessary for the inhibition of bacterial adhesion and biofilm formation on textured surfaces, especially when the size of textured pattern is larger than the dimension of bacterial cells.[40] The submicron textured CarboSil PU surface examined in this study physically reduced the availability of surface contact area and increased the surface hydrophobicity, which both contribute to lowering bacterial adhesion. On the other hand, the addition of SNAP-doped sublayer between base layer and the outer textured surface layer can provide controlled release of NO over an extended period of time. This NO release largely inhibited the growth of bacteria (Figure 6) and reduced the bacterial adhesion on the surface overall. Without surface texturing, the 15 wt% SNAP in middle layer increased the bacterial adhesion inhibition rates up to 75 % after 1 h (Figure 7a) and there was no biofilm formed on smooth surface after 2 d under static conditions (Figure 8). This clearly suggests the importance of NO release in control of bacterial adhesion and biofilm formation on biomaterial surfaces. Under shear conditions, biofilm formation was found on the smooth surfaces with 15 wt% SNAP within 14 d and on the textured 500/500 nm surface within 21 d, suggesting that each individual treatment alone, either NO release or physical topography modification, can only protect polymer materials less than 21 d. However, integration of NO release and surface texturing together can synergistically inhibit S. epidermidis biofilm formation for the longer 28 d period. Since the lifetime of NO release from SNAP-textured films employed in this study only lasted about 10 d, much shorter than the NO release lifetime in other studies,[35, 36] there is also room for improvement in the preparation and design of these new biomimetic polymeric surfaces that may further increase useful lifetime.
Bacterial adhesion is the critical step in the pathogenesis of biomaterial associated infection. Once implanted, a biomaterial surface faces a competition between integration of the material into the surrounding tissue and the adhesion of bacteria. As a successful implant, tissue integration should occur prior to bacterial adhesion and colonization. Therefore, the prevention of bacteria adhesion within the initial hours following implantation, especially in the first 6 h of post-implantation “decisive period”, is critical for the long-term success of an implant since the implant is particularly susceptible to surface colonization during this period.[48, 49] The NO possesses a broad-spectrum antibacterial activity against both Gram-positive and Gram-negative bacteria. The NO flux released from SNAP doped films controls the bacteria growth and inhibits the bacterial adhesion within the initial hours (Figure 6), and this helps accelerate the integration of tissue onto the material’s surface thereby inhibiting microbial infection. The other benefit of using NO to control bacterial adhesion is the very limited possibility of it promoting bacterial resistance, a common issue for the antibiotic therapy. It has not been reported that NO treatment would cause potential antibiotic resistance.[50] Therefore, the controlled release of NO over a long period (>10 d) is desirable and will provide additional benefits for the long-term success of polymeric implants.
4. Conclusion
SNAP-doped and textured CarboSil 20 80A polyurethane films were fabricated with the top layer bearing ordered submicron pillars and a SNAP-doped sublayer for NO release, via a two-stage replication process. Controlling the thickness of top layer can limit and control the diffusion of SNAP during fabrication process and produce the desired uniform pillars on the outermost surface. The NO release rate depends on the SNAP concentration in the sublayer. The 15 wt% SNAP-doped textured polyurethane film release NO for up to 10 d above the lower end of the physiological flux level, 0.5 ×10−10 mol min−1 cm−2. The combination of NO release and surface texturing produced a synergistic effect on inhibition of S. epidermidis bacterial adhesion, significantly greater than the inhibition of bacterial adhesion achieved by either the use of NO release or texturing alone. The long-term study of biofilm formation on biomaterial surface showed that the biomimetic SNAP-textured CarboSil PU surface reduced the bacterial adhesion and inhibited biofilm formation for at least 28 d, providing a practical approach to improve the biocompatibility of current biomaterials to control and potentially reduce the rate of pathogenic infections caused by implanted polymeric biomedical devices.
Statement of Significance.
Microbial infection remains a significant barrier to development and implementation of advanced blood-contacting medical devices. Clearly, method to design and control material properties to control and reduce microbial infection is a central question to biomaterial researchers. In separate prior studies, physical topographic surface modification or nitric oxide (NO) release has been demonstrated each to be effective approach to inhibit and control bacterial adhesion and biofilm formation on polymeric surfaces. Such approach can prevent biomaterial-associated infection without causing the antibiotic resistance of the strain. However, efficiency of antimicrobial property of each approach is still limited and far from sufficient for widespread clinical use. This work successfully integrated both techniques and applied them to a polyurethane (PU) biomaterial surface that bears dual functions, surface topographic modification and NO release. The former reduced the surface contact area and changed surface wettability, resulting in reduction of bacterial adhesion, and NO release further inhibited bacteria growth. Such dual functionalized surfaces provide a synergistic effect on inhibition of Staphylococcus epidermidis bacterial adhesion which was significantly greater than the inhibition of bacterial adhesion achieved by either single treatment approach alone. Furthermore, longer term experiments showed that the dual functionalized surface can inhibit biofilm formation for > 28 days. The success of this work provides a practical approach to improve the biocompatibility of current biomaterials and thereby reduce the risk of pathogenic infection.
Acknowledgments
This study is partially supported from National Institutes of Health (HL-069965 and HL-128337) and Surgery Feasibility Grant of Penn State College of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Donlan RM. Biofilms and device-associated infections. Emerging Infectious Diseases. 2001;7:277–81. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francolini I, Donelli G. Prevention and control of biofilm-based medical-device-related infections. Fems Immunology and Medical Microbiology. 2010;59:227–38. doi: 10.1111/j.1574-695X.2010.00665.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Gowardman J, Rickard CM. Impact of microbial attachment on intravascular catheter-related infections. International Journal of Antimicrobial Agents. 2011;38:9–15. doi: 10.1016/j.ijantimicag.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Klevens RM, Edwards JR, Richards CL, Horan TC, Gaynes RP, Pollock DA, Cardo DM. Estimating health care-associated infections and deaths in US hospitals, 2002. Public Health Reports. 2007;122:160–6. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wenzel RP. Health care-associated infections: Major issues in the early years of the 21st century. Clinical Infectious Diseases. 2007;45:85–8. doi: 10.1086/518136. [DOI] [PubMed] [Google Scholar]
- 6.Campoccia D, Montanaro L, Arciola CR. A review of the clinical implications of anti-infective biomaterials and infection-resistant surfaces. Biomaterials. 2013;34:8018–29. doi: 10.1016/j.biomaterials.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 7.Campoccia D, Montanaro L, Arciola CR. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 2013;34:8533–54. doi: 10.1016/j.biomaterials.2013.07.089. [DOI] [PubMed] [Google Scholar]
- 8.Estrela AB, Heck MG, Abraham WR. Novel Approaches to Control Biofilm Infections. Current Medicinal Chemistry. 2009;16:1512–30. doi: 10.2174/092986709787909640. [DOI] [PubMed] [Google Scholar]
- 9.Scardino AJ, Hudleston D, Peng Z, Paul NA, de Nys R. Biomimetic characterisation of key surface parameters for the development of fouling resistant materials. Biofouling. 2009;25:83–93. doi: 10.1080/08927010802538480. [DOI] [PubMed] [Google Scholar]
- 10.Scardino AJ, Zhang H, Cookson DJ, Lamb RN, de Nys R. The role of nano-roughness in antifouling. Biofouling. 2009;25:757–67. doi: 10.1080/08927010903165936. [DOI] [PubMed] [Google Scholar]
- 11.Barthlott W, Neinhuis C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta. 1997;202:1–8. [Google Scholar]
- 12.Bhushan B, Jung YC. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Progress in Materials Science. 2011;56:1–108. [Google Scholar]
- 13.Sun T, Qing G, Su B, Jiang L. Functional biointerface materials inspired from nature. Chemical Society Reviews. 2011;40:2909–21. doi: 10.1039/c0cs00124d. [DOI] [PubMed] [Google Scholar]
- 14.Shin S, Seo J, Han H, Kang S, Kim H, Lee T. Bio-Inspired Extreme Wetting Surfaces for Biomedical Applications. Materials. 2016;9:116. doi: 10.3390/ma9020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung KK, Schumacher JF, Sampson EM, Burne RA, Antonelli PJ, Brennan AB. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases. 2007;2:89–94. doi: 10.1116/1.2751405. [DOI] [PubMed] [Google Scholar]
- 16.Reddy ST, Chung KK, McDaniel CJ, Darouiche RO, Landman J, Brennan AB. Micropatterned Surfaces for Reducing the Risk of Catheter-Associated Urinary Tract Infection: An In Vitro Study on the Effect of Sharklet Micropatterned Surfaces to Inhibit Bacterial Colonization and Migration of Uropathogenic Escherichia coli. Journal of Endourology. 2011;25:1547–52. doi: 10.1089/end.2010.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L-C, Siedlecki CA. Submicron-textured biomaterial surface reduces staphylococcal bacterial adhesion and biofilm formation. Acta Biomaterialia. 2012;8:72–81. doi: 10.1016/j.actbio.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Milner KR, Snyder AJ, Siedlecki CA. Sub-micron texturing for reducing platelet adhesion to polyurethane biomaterials. Journal of Biomedical Materials Research Part A. 2006;76A:561–70. doi: 10.1002/jbm.a.30554. [DOI] [PubMed] [Google Scholar]
- 19.Radomski MW, Palmer RMJ, Moncada S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochemical and Biophysical Research Communications. 1987;148:1482–9. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- 20.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–16. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 21.Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. Journal of Clinical Investigation. 1997;99:2818–25. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones ML, Ganopolsky JG, Labbé A, Wahl C, Prakash S. Antimicrobial properties of nitric oxide and its application in antimicrobial formulations and medical devices. Applied Microbiology and Biotechnology. 2010;88:401–7. doi: 10.1007/s00253-010-2733-x. [DOI] [PubMed] [Google Scholar]
- 23.Privett BJ, Broadnax AD, Bauman SJ, Riccio DA, Schoenfisch MH. Examination of bacterial resistance to exogenous nitric oxide. Nitric Oxide. 2012;26:169–73. doi: 10.1016/j.niox.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seabra AB, Martins D, Simoes M, da Silva R, Brocchi M, de Oliveira MG. Antibacterial Nitric Oxide-Releasing Polyester for the Coating of Blood-Contacting Artificial Materials. Artificial Organs. 2010;34:E204–E14. doi: 10.1111/j.1525-1594.2010.00998.x. [DOI] [PubMed] [Google Scholar]
- 25.Park J, Kim J, Singha K, Han D-K, Park H, Kim WJ. Nitric oxide integrated polyethylenimine-based tri-block copolymer for efficient antibacterial activity. Biomaterials. 2013;34:8766–75. doi: 10.1016/j.biomaterials.2013.07.064. [DOI] [PubMed] [Google Scholar]
- 26.Cai W, Wu J, Xi C, Meyerhoff ME. Diazeniumdiolate-doped poly(lactic-co-glycolic acid)-based nitric oxide releasing films as antibiofilm coatings. Biomaterials. 2012;33:7933–44. doi: 10.1016/j.biomaterials.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barraud N, Kelso J, Rice MA, Kjelleberg SS. Nitric Oxide: A Key Mediator of Biofilm Dispersal with Applications in Infectious Diseases. Current Pharmaceutical Design. 2015;21:31–42. doi: 10.2174/1381612820666140905112822. [DOI] [PubMed] [Google Scholar]
- 28.Frost MC, Reynolds MM, Meyerhoff ME. Polymers incorporating nitric oxide releasing/generating substances for improved biocompatibility of blood-contacting medical devices. Biomaterials. 2005;26:1685–93. doi: 10.1016/j.biomaterials.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Handa H, Brisbois EJ, Major TC, Refahiyat L, Amoako KA, Annich GM, Bartlett RH, Meyerhoff ME. In vitro and in vivo study of sustained nitric oxide release coating using diazeniumdiolate-oped poly(vinyl chloride) matrix with poly(lactide-co-glycolide) additive. Journal of materials chemistry B, Materials for biology and medicine. 2013;1:3578–87. doi: 10.1039/C3TB20277A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varu VN, Tsihlis ND, Kibbe MR. Basic science review: nitric oxide--releasing prosthetic materials. Vasc Endovascular Surg. 2009;43:121–31. doi: 10.1177/1538574408322752. [DOI] [PubMed] [Google Scholar]
- 31.Coneski PN, Schoenfisch MH. Synthesis of nitric oxide-releasing polyurethanes with S-nitrosothiol-containing hard and soft segments. Polymer Chemistry. 2011;2:906–13. doi: 10.1039/C0PY00269K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laver JR, McLean S, Bowman LA, Harrison LJ, Read RC, Poole RK. Nitrosothiols in bacterial pathogens and pathogenesis. Antioxid Redox Signal. 2013;18:309–22. doi: 10.1089/ars.2012.4767. [DOI] [PubMed] [Google Scholar]
- 33.Ren H, Wu J, Xi C, Lehnert N, Major T, Bartlett RH, Meyerhoff ME. Electrochemically modulated nitric oxide (NO) releasing biomedical devices via copper(II)-Tri(2-pyridylmethyl)amine mediated reduction of nitrite. ACS Appl Mater Interfaces. 2014;6:3779–83. doi: 10.1021/am406066a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore CM, Nakano MM, Wang T, Ye RW, Helmann JD. Response of Bacillus subtilis to Nitric Oxide and the Nitrosating Agent Sodium Nitroprusside. Journal of Bacteriology. 2004;186:4655–64. doi: 10.1128/JB.186.14.4655-4664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brisbois EJ, Handa H, Major TC, Bartlett RH, Meyerhoff ME. Long-term nitric oxide release and elevated temperature stability with S-nitroso-N-acetylpenicillamine (SNAP)-doped Elast-eon E2As polymer. Biomaterials. 2013;34:6957–66. doi: 10.1016/j.biomaterials.2013.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wo Y, Li Z, Brisbois EJ, Colletta A, Wu J, Major TC, Xi C, Bartlett RH, Matzger AJ, Meyerhoff ME. Origin of long-term storage stability and nitric oxide release behavior of carboSil polymer doped with S-Nitroso-N-acetyl-d-penicillamine. ACS Appl Mater Interfaces. 2015;7:22218–27. doi: 10.1021/acsami.5b07501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brisbois EJ, Davis RP, Jones AM, Major TC, Bartlett RH, Meyerhoff ME, Handa H. Reduction in Thrombosis and Bacterial Adhesion with 7 Day Implantation of S-Nitroso-N-acetylpenicillamine (SNAP)-Doped Elast-eon E2As Catheters in Sheep. Journal of materials chemistry B, Materials for biology and medicine. 2015;3:1639–45. doi: 10.1039/C4TB01839G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colletta A, Wu J, Wo Y, Kappler M, Chen H, Xi C, Meyerhoff ME. S-Nitroso-N-acetylpenicillamine (SNAP) Impregnated Silicone Foley Catheters: A Potential Biomaterial/Device To Prevent Catheter-Associated Urinary Tract Infections. ACS Biomaterials Science & Engineering. 2015;1:416–24. doi: 10.1021/acsbiomaterials.5b00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, Ravel J, Paulsen IT, Kolonay JF, Brinkac L, Beanan M, Dodson RJ, Daugherty SC, Madupu R, Angiuoli SV, Durkin AS, Haft DH, Vamathevan J, Khouri H, Utterback T, Lee C, Dimitrov G, Jiang L, Qin H, Weidman J, Tran K, Kang K, Hance IR, Nelson KE, Fraser CM. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. Journal of Bacteriology. 2005;187:2426–38. doi: 10.1128/JB.187.7.2426-2438.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu LC, Siedlecki CA. Staphylococcus epidermidis adhesion on hydrophobic and hydrophilic textured biomaterial surfaces. Biomedical Materials. 2014;9:035003. doi: 10.1088/1748-6041/9/3/035003. [DOI] [PubMed] [Google Scholar]
- 41.Wang PG, Xian M, Tang X, Wu X, Wen Z, Cai T, Janczuk AJ. Nitric Oxide Donors: Chemical Activities and Biological Applications. Chemical Reviews. 2002;102:1091–134. doi: 10.1021/cr000040l. [DOI] [PubMed] [Google Scholar]
- 42.Lin C-E, Richardson SK, Wang W, Wang T, Garvey DS. Preparation of functionalized tertiary thiols and nitrosothiols. Tetrahedron. 2006;62:8410–8. [Google Scholar]
- 43.Vaughn MW, Kuo L, Liao JC. Estimation of nitric oxide production and reaction rates in tissue by use of a mathematical model. Am J Physiol. 1998;274:H2163–76. doi: 10.1152/ajpheart.1998.274.6.H2163. [DOI] [PubMed] [Google Scholar]
- 44.Patel JD, Ebert M, Ward R, Anderson JM. S-epidermidis biofilm formation: Effects of biomaterial surface chemistry and serum proteins. Journal of Biomedical Materials Research Part A. 2007;80A:742–51. doi: 10.1002/jbm.a.31103. [DOI] [PubMed] [Google Scholar]
- 45.Xu CP, Boks NP, de Vries J, Kaper HJ, Norde W, Busscher HJ, van der Mei HC. Staphylococcus aureus-Fibronectin Interactions with and without Fibronectin-Binding Proteins and Their Role in Adhesion and Desorption. Applied and Environmental Microbiology. 2008;74:7522–8. doi: 10.1128/AEM.00948-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartford OM, Wann ER, Hook M, Foster TJ. Identification of residues in the Staphylococcus aureus fibrinogen-binding MSCRAMM clumping factor A (ClfA) that are important for ligand binding. Journal of Biological Chemistry. 2001;276:2466–73. doi: 10.1074/jbc.M007979200. [DOI] [PubMed] [Google Scholar]
- 47.Cassie A, Baxter S. Wettability of porous surfaces. Transactions of the Faraday Society. 1944;40:546–51. [Google Scholar]
- 48.Poelstra KA, Barekzi NA, Rediske AM, Felts AG, Slunt JB, Grainger DW. Prophylactic treatment of gram-positive and gram-negative abdominal implant infections using locally delivered polyclonal antibodies. Journal of Biomedical Materials Research. 2002;60:206–15. doi: 10.1002/jbm.10069. [DOI] [PubMed] [Google Scholar]
- 49.Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35:780–9. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 50.Wo Y, Brisbois EJ, Bartlett RH, Meyerhoff ME. Recent advances in thromboresistant and antimicrobial polymers for biomedical applications: just say yes to nitric oxide (NO) Biomaterials Science. 2016;4:1161–83. doi: 10.1039/c6bm00271d. [DOI] [PMC free article] [PubMed] [Google Scholar]