Abstract
Anesthetic agents are often administered in the neonatal period, a time of rapid brain development and synaptogenesis. Mounting evidence suggests that anesthetics can disrupt neurocognitive development, particularly in cases of multiple or prolonged anesthetic exposure. Previous studies have shown that administering multiple doses of ketamine-xylazine (KX) anesthesia to neonatal mice can induce long-term changes to synaptic plasticity in the cortex, but the effect on neurocognitive function remains unclear. In this study, we exposed neonatal mice to single dose and multiple doses of KX anesthesia in the neonatal period (postnatal days 7, 9, 11), and conducted a series of behavioral tests in young adulthood (1 month of age). Mice receiving multiple doses of KX anesthesia showed deficits in novel object recognition, sociability, preference for social novelty and contextual fear response, but no effect on auditory-cued fear response. Single dose of KX anesthesia had no effect on these behaviors except for contextual fear response. We also observed that multiple exposures to KX anesthesia were associated with decreased CaMKII phosphorylation, which is known to play a role in synapse development and long-term potentiation, likely contributing to learning impairment.
Keywords: Anesthetics, Ketamine, Neonate, Behavior, CaMKII
1. Introduction
Anesthetics were once thought to have a completely reversible effect on cognition, with brain structure and function returning to its previous state at the conclusion of surgery. Recent literature suggests that anesthetics can have lasting effects on the brain, especially when exposure occurs during peak synaptogenesis in the neonatal period (Rice and Barone, 2000). The effect of anesthetics on the developing brain has long been disputed. Beginning in the 1970s, early work showed disruption of neural development in a rodent model with chronic exposure to halothane (Chang et al., 1974; Levin et al., 1991). Early studies were primarily concerned with chronic exposure by operating room staff (Whitcher et al., 1971). As surgeries became more common in the neonatal period, there has been renewed interest in the impact of anesthetics on neural development, particularly in relation to the duration and frequency of anesthetic exposure.
Recent literature has focused on whether neonatal anesthetic exposure is sufficient to cause disruption in neural development and cause cognitive changes later in life (Amrock et al., 2015; Flick et al., 2011; Haberny et al., 2002; Wilder et al., 2009). In a sentinel neurotoxicity study, Jevtovic et al showed that a single exposure to nitrous oxide, isoflurane, and midazolam is sufficient to cause neurocognitive deficits in rats (Jevtovic-Todorovic et al., 2003). Such cognitive disruption with anesthetic agents occurs across several animal models (Fredriksson et al., 2007; Kodama et al., 2011; Satomoto et al., 2009; Shen et al., 2013; Viberg et al., 2008), including primates. For example, early ketamine exposure in rhesus monkeys is associated with cognitive deficits as adults (Paule et al., 2011; Zou et al., 2011). Human studies of anesthetic effect on neurocognitive development are largely limited to retrospective studies (DiMaggio et al., 2009; DiMaggio et al., 2011; Flick et al., 2011; Ing et al., 2012; Sprung et al., 2012; Sun, 2010; Wilder et al., 2009), in which prior anesthetic exposure was studied in children known to have grossly abnormal cognitive development. Therefore, it is difficult to extrapolate the nature of specific neurocognitive abnormalities in children on the basis of these studies. Current prospective studies include the PANDA study, which follows neurocognitive outcomes for children exposed to anesthesia prior to three years of age and the GAS study, which follows neurocognitive outcomes for children exposed to general vs. regional anesthesia for hernia repair (Miller et al., 2014). Thus far, results show that a single exposure to volatile anesthetic does not have a significant effect on IQ in sibling pairs (Sun et al., 2016). Similarly, neonates with 1 hour of sevoflurane exposure vs. regional anesthesia showed no difference in cognitive function at two years of age (Davidson et al., 2016). However, little is known about the effect of prolonged or repeated anesthetic exposure on neurocognitive development.
Ketamine-xylazine (KX) anesthesia is commonly used in mice. Ketamine, a dissociative anesthetic, is sometimes used in pediatric patients. Ketamine acts as an antagonist at the N-methyl-D-aspartate (NMDA) receptor, which is known to play a crucial role in Hebbian learning and synaptic plasticity (Haberny et al., 2002; Harris et al., 2003). Mice require higher doses of ketamine than humans to achieve the same anesthetic effect. Ketamine is administered in combination with xylazine, an alpha-2 adrenergic agonist, in order to induce general anesthesia. We have previously shown that multiple exposures, but not a single exposure of neonatal mice, to KX (20 mg/kg ketamine and 3 mg/kg xylazine) anesthesia resulted in impaired motor learning in rotarod and treadmill motor learning tasks (Huang et al., 2016). In this study, we aimed to further characterize the behavioral effects of neonatal KX anesthesia in mice. Specifically, we investigated whether a single or multiple exposures of neonatal mice to KX anesthesia cause deficits in novel object recognition, social behavior as well as fear conditioning. The behavior effects of repeated exposure to either ketamine or xylazine alone were also examined.
Finally, calcium/calmodulin-dependent kinase II (CaMKII) is a molecular substrate for learning and memory (Coultrap and Bayer, 2012; Lisman et al., 2012). Activated CaMKII is critically involved in the induction of long-term potentiation and has been shown to be essential for a variety of learning tasks (Giese et al., 1998; Irvine et al., 2005). We therefore examined the levels of CaMKII and phospho-CaMKII in adult mouse brain after multiple exposures to KX anesthesia in the neonatal period.
2. Materials and Methods
2.1 Experimental animals and anesthetic treatment
The animal protocol was approved by Institutional Animal Care and Use Committee at New York University Medical Center (New York, NY, USA). C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and group-housed in the New York University Skirball animal facility. Mice of both sexes were randomly assigned to three treatment groups, with half male and half female in each group. The control group received saline injections. The single-exposure group received one intraperitoneal injection of KX [ketamine (20 mg/kg) and xylazine (3 mg/kg)] on postnatal day 7 (P7). The multiple-exposure group received KX injection on P7, 9 and 11, a total of three injections. The dosage of KX was selected on the basis that one injection of ketamine (20 mg/kg) and xylazine (3 mg/kg) produces general anesthesia in neonatal mice for ~0.5–1 hour. We observed that ~20% of mice vocalized and pivoted to tail pinch 30 min after injection and ~80% of mice showed voluntary movement within one hour. During anesthesia, a heating pad was used to maintain the animal’s body temperature at about 37°C. We measured the blood gas values at 1 h after KX injection. Consistent with our previous study, 1-hour KX anesthesia did not induce significant alteration in blood gas values including pH and partial pressure of oxygen and carbon dioxide (Yang et al., 2011).
2.2. Novel object recognition test
In all behavioral experiments, the animals were acclimatized before the experiments and researchers were blind to the experimental condition. Mice were tested for novel object recognition in custom-built opaque plexiglas boxes (25 × 25 × 25 cm). Behavior was videotaped using standard webcams and images were analyzed with Quicktime software (Apple). Mice were handled by the same researcher for 3 days and then habituated to the testing environment for 30 min with no objects in the chamber. Twenty four hours after the habituation, mice were placed into the testing environment with two identical sample objects (familiar object) placed in opposite corners for 10 min. Mice were removed and returned to their home cages. Twenty four hours later, mice were returned to the testing environment for 5 min where one of the sample objects was replaced by a novel object, which differs from the familiar object in the shape and texture. Measures of interaction were taken of the amount of time that the animal spent with its head and nose oriented toward and within 2 cm of the object. Different types of interactions such as the animal accidentally touching, sitting or standing on the object were not considered exploratory activity. Discrimination index was calculated as the time spent interacting with the novel object divided by the total time spent exploring both objects.
2.3. Sociability and preference for social novelty
The tests for sociability and preference for social novelty were conducted in a custom-built three-chambered box (Moy et al., 2004). Each chamber was 20 × 30 × 30 cm and the dividing walls were made from clear plexiglas, with small rectangular doors (5 × 8 cm) allowing comunication between chambers. Behavior was videotaped using standard webcams and images were analyzed with Quicktime software (Apple). The test mouse was first placed in the middle chamber and allowed to explore all three chambers for 10 min. After this habituation period, an unfamiliar mouse (stranger 1) that had no prior contact with the test mouse was randomly placed in one of the side chambers. The stranger mouse was placed in a cylinder-shaped, mesh-wire enclosure (10 cm in diameter and 15 cm high) that allowed nose contact between the test mouse and the stanger mouse, but prevented fighting. The animals serving as strangers were of the same sex and had been previously habituated to the mesh-wire enclosure. Both doors to the side chambers were then unblocked and the test mouse was allowed to explore the entire social test box in a 10-min session. Measures were taken of the amount of time spent in each chamber. Sociability was calculated as the time spent in the social chamber divided by the total time spent in social and empty chambers. Following the sociability test, the test mouse was assessed for preference for social novelty in another 10-min session. A novel, unfamiliar mouse (stranger 2) was placed in the chamber that had been empty during the sociability test. Stranger 2 was placed in an identical, mesh-wire enclosure. The test mouse then had a choice between the first, already-interacted mouse (stranger 1), vs. the novel unfamiliar mouse (stranger 2). Measures were taken of the amount of time spent in each chamber. Preference for social novelty was calculated as the time that the test mouse spent in the chamber with the novel, unfamiliar mouse divided by the total time spent in two side chambers.
2.4. Cued and contextual fear conditioning
Fear conditioning was performed in a FreezeFrame fear conditioning system (Coulbourn Instruments, Whitehall, PA). Mice were placed in a cleaned chamber for 2 min before they were presented with a auditory cue (a 400-Hz, 80-dB tone) for 30 sec. A mild foot shock (0.5-mA) was administered during the last 2 sec of the tone presentation and co-terminated with the tone. A total of three trials were repeated with a 60–210 sec intertrial interval on the training day. The next day, contextual fear memory was tested by returning mice to the same chamber for 5 min without applying the shock. Contextual fear memory was measured by the percent of time that the animals spent on freezing in response to re-presentation of the context. Auditory-cued fear memory was tested by placing mice to a different chamber and presenting the auditory cue. Auditory-cued fear memory was measured by the percent of time that the animals spent on freezing duirng the tone presentation.
2.5. Isolation of synaptosome fractions and Western blot
Mice were deeply anesthetized and perfused with 40 ml of Ca2+/Mg2+-free Dulbecco’s phosphate buffered saline. The brain was dissected and homogenized with a dounce homogenizer in buffer A (320 mM sucrose, 1 mM NaHCO3, 1 mM MgCl2, and 0.5 mM CaCl2) and cleared of nuclei and insoluble material by centrifugation at 3000g. The resulting supernatant was centrifuged at 30,000g to pellet membranes, and the pellet was subsequently resuspended in buffer B (320 mM sucrose and 1 mM NaHCO3). Membranes were fractionated using a discontinuous sucrose gradient consisting of 1.0 and 1.2 M sucrose at 120,000g for 2 hours at 4°C. After centrifugation, the synaptosomal fraction was isolated at the 1.0/1.2 interface, diluted in buffer B, and pelleted at 120,000g for 45 min at 4°C. The resulting synaptosomes were solubilized in 25 mM tris (pH 7.4) and 2% SDS. Protein content was determined by bicinchoninic acid assay (Thermo Scientific), and 10 μg of total protein was loaded per lane on a SDS-PAGE gel. Separated proteins were transferred to polyvinylidene difluoride membrane (Millipore) and blocked for 30 min with 2% bovine serum albumin in tris-buffered saline–Tween 20. Blocked membranes were probed overnight with the following antibodies: CaMKII (ThermoFisher), p-CaMKII (phosphoSolutions), and actin (Sigma). After washing, membranes were incubated with anti-rabbit or anti-mouse IgG-HRP secondary antibodies (Jackson ImmunoResearch), washed, and incubated with enhanced chemiluminescence reagent (GE Life Sciences) before exposure to film. Densitometry analysis was performed by manual scanning and digitalization of film, and quantified using the gel analysis plugin for NIH ImageJ software (Parkhurst et al., 2013).
2.6. Statistics
Prism software (GraphPad 6.0) was used to conduct the statistical analysis. Data were presented as means ± SEM. Tests for differences between populations were performed using a one-way ANOVA followed by Tukey’s post hoc test, or Student’s t test as specified in the text. Significant levels were set at P < 0.05.
3. Results
3.1. Multiple, but not single, neonatal exposures to KX anesthesia impair novel object recognition
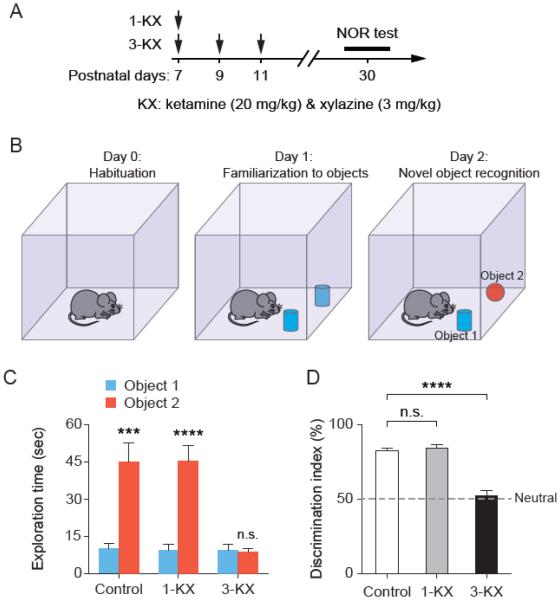
To determine whether single and multiple exposures of neonatal mice to KX anesthesia cause abnormal behavior, we performed a series of behavioral tests in young adult mice that had been exposed to KX anesthesia during early brain development. Specifically, the single-exposure group (1-KX) received one injection of KX [ketamine (20 mg/kg) and xylazine (3 mg/kg)] at P7; the multiple-exposure group (3-KX) received three injections of KX at P7, P9 and P11; control mice received saline injections (Fig. 1A). At 1 month of age, mice were first tested for novel object recognition (NOR) (Fig. 1B). NOR test is based on the spontaneous tendency of animals to spend more time exploring a novel object than a familiar object. The preference to explore the novel object reflects the animal’s learning and recognition memory. Both control and single-exposure groups showed a preference toward the novel object during the NOR test, but the multiple-exposure group showed no such preference at 24 h after training (Fig. 1C). Performance on the NOR task was measured by the discrimination index, which is the percentage of time that mice spent with novel object relative to the total time exploring both objects. We found that, as compared to saline-treated controls (81.9% ± 1.9%), the discrimination index is significantly lower in the multiple-exposure group (52.3 ± 3.1%; P < 0.0001 vs. control by Tukey’s post hoc test), but not in the single-exposure group (83.9 ± 2.2%; P = 0.86 vs. control) (Fig. 1D). Our results indicate that repeated exposure, but not single exposure, to KX anesthesia at neonatal age results in persistent impairment in recognition memory.
Fig. 1.
Multiple, but not single, exposures to KX anesthesia at neonatal age result in impaired novel object recognition. (A) Experimental design. Animals received one or three injections of KX [ketamine (20 mg/kg) and xylazine (3 mg/kg)] at P7–11, and were tested for novel object recognition (NOR) at 1 month of age. (B) Diagram for NOR training and testing. (C) The amount of time that the animals spent exploring each object on day 2. Control: t(9) = 5.610, P = 0.0003; 1-KX: t(9) = 6.790, P < 0.0001; 3-KX: t(9) = 0.6827, P = 0.512, paired t test. n = 10 mice for each group. (D) Analysis of novel object preference on day 2. Preference to novel object was significantly reduced in 3-KX group as compared to control. F(2, 27) = 47.25, P < 0.0001 by one-way ANOVA. P = 0.8577 for 1-KX vs. control, P < 0.0001 for 3-KX vs. control, Tukey’s post hoc test. Data are presented as means ± SEM. ***P < 0.001, ****P < 0.0001, n.s., not significant.
3.2. Multiple, but not single, exposures to KX anesthesia impair social behavior
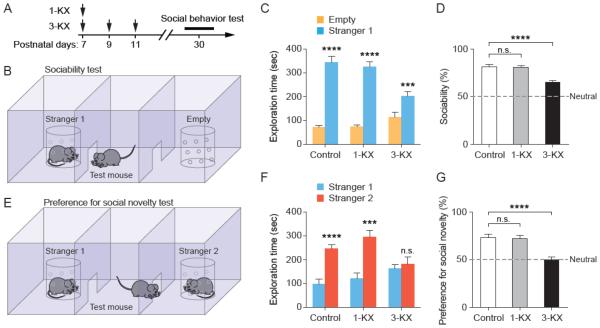
Normal mice exhibit social interaction behavior and have a tendency to preferentially explore unfamiliar mice. To determine whether mice with neonatal exposure to KX anesthesia have abnormal social behavior, we examined the animals’ sociability and preference for social novelty at one month of age (Fig. 2A). In the test for sociability, the test mouse was given a choice between spending time in the side with an unfamiliar mouse vs. an empty side (Fig. 2B). All groups of mice spent more time with the unfamiliar mouse than the empty chamber (Fig. 2C), but the sociability of 3-KX mice was significantly lower than that of control mice (Fig. 2D). Sociability was expressed as the percent of time that the test mouse spent in the chamber with unfamiliar mouse relative to the total time spent in both side chambers. There was no difference in sociability between 1-KX mice and control mice. Following the sociability test, test of preference for social novelty was performed (Fig. 2E). Again, we found that control and single-exposure groups spent more time exploring the novel mouse, while the multiple-exposure group spent similar amount with both familiar and unfamiliar mice (Fig. 2F). Thus, the preference for social novelty was significantly lower in 3-KX mice (P < 0.0001 vs. control by Tukey’s post hoc test), but not in mice with single KX exposure (P = 0.96 vs. control) (Fig. 2G). Together, these results show that multiple, but not single, neonatal exposures to KX anesthesia results in persistent impairment in social behavior.
Fig. 2.
Multiple, but not single, exposures to KX anesthesia at neonatal age result in impaired social behavior in adulthood. (A) Timeline for neonatal anesthetic exposure and social behavior tests in young adulthood. (B) Diagram of three-chambered sociability test. (C) The amount of time that the test mice spent exploring empty chamber and an unfamiliar mouse. Control: t(9) = 8.811, P < 0.0001; 1-KX: t(9) = 12.34, P < 0.0001; 3-KX: t(9) = 5.545, P = 0.0004, paired t test. n = 10 mice for each group. (D) Sociability was expressed the percentage of time that the test mouse spent in the side with an unfamiliar mouse (stranger 1) relative to the total time spent in both sides. Sociability was significantly reduced in 3-KX, but not 1-KX group, as compared to control. F(2, 27) = 20.07, P < 0.0001 by one-way ANOVA. P = 0.99 for 1-KX vs. control, P < 0.0001 for 3-KX vs. control, Tukey’s post hoc test. (E) Preference for social novelty test was conducted immediately after sociability test. (F) The amount of time that the test mouse spent exploring each stranger. Control: t(9) = 8.899, P < 0.0001; 1-KX: t(9) = 5.279, P = 0.0005; 3-KX: t(9) = 0.7485, P = 0.4733, paired t test. n = 10 mice for each group. (G) Social preference was expressed the percentage of time that the test mouse spent with the unfamiliar mouse (stranger 2) relative to the total time spent with both sides. Social preference was significantly reduced in 3-KX, but not 1-KX group, as compared to control. F(2, 27) =15.95, P < 0.0001 by one-way ANOVA. P = 0.9646 for 1-KX vs. control, P < 0.0001 for 3-KX vs. control, Tukey’s post hoc test. Data are presented as means ± SEM. ***P < 0.001, ****P < 0.0001, n.s., not significant.
3.3. Exposure to KX anesthesia impairs contextual, but not auditory-cued fear response
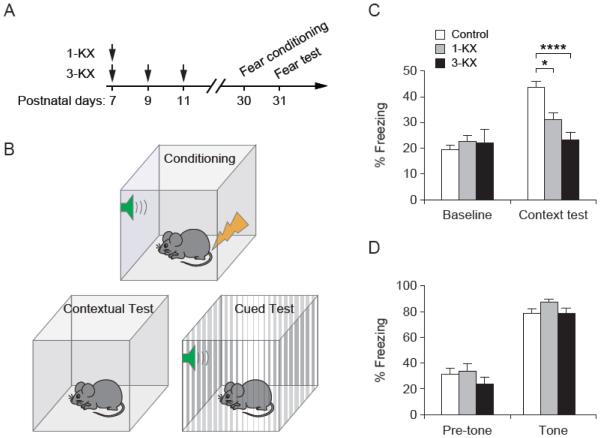
To further examine the effect of neonatal anesthetic exposure on learning and memory, we compared KX- and saline-treated mice in fear conditioning at 1 month of age (Fig. 3A). Specifically, mice were trained to associate an unconditioned stimulus (the foot shock) with a context (the shock chamber) or a tone. Twenty-four hours after fear conditioning, mice were returned to the same chamber to test for the contextual fear response, or were presented with the same tone in a new chamber to test for the auditory-cued fear response (Fig. 3B). We found that the levels of freezing upon re-exposure to the training context were significant lower in KX-treated mice as compared to saline-treated controls [F(2, 32) = 12.62, P < 0.0001 by one-way ANOVA] (Fig. 3C). However, both control and KX-treated mice showed similar freezing behavior upon re-exposure to the tone during auditory-cued fear test [F(2, 27) = 1.905, P = 0.1683 by one-way ANOVA] (Fig. 3D). These results indicate that exposure of neonatal mice to KX anesthesia resulted in impaired contextual fear learning in young adulthood, but had no effect on auditory-cued fear response.
Fig. 3.
Exposure to KX anesthesia at neonatal age impairs contextual, but not auditory-cued fear memory. (A) Timeline for anesthetic exposure and fear conditioning test. (B) Diagram for contextual and auditory-cued fear conditiong and test. (C) Contextual fear response in mice with various treatment (n = 13 mice for control group, n = 8 mice for 1-KX group, n = 14 mice for 3-KX group). F(2, 32) = 12.62, P < 0.0001 by one-way ANOVA. P = 0.0392 for 1-KX vs. control, P < 0.0001 for 3-KX vs. control, Tukey’s post hoc test. (D) Auditory-cued fear response in mice with various treatment (n = 13 mice for control group, n = 8 mice for 1-KX group, n = 9 mice for 3-KX group). F(2, 27) = 1.905, P = 0.1683 by one-way ANOVA. Data are presented as means ± SEM. *P < 0.05, ****P < 0.0001.
3.4. Multiple exposures to high-dose ketamine impair contextual fear response.
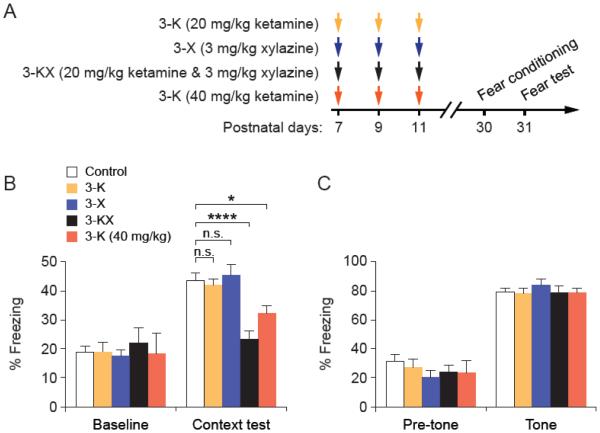
To investigate the behavioral effect of ketamine and xylazine individually, we administered mice with ketamine (20 mg/kg) or xylazine (3 mg/kg) alone at P7, P9 and P11 (3-K and 3-X groups) (Fig. 4A). Although administration of KX, a mixture of ketamine (20 mg/kg) and xylazine (3 mg/kg), reliably induces general anesthesia and provides immobilization in neonatal mice for about 1 hour, administration of ketamine (20 mg/kg) or xylazine (3 mg/kg) alone does not immobilize the animals. When these mice were subjected to fear conditioning and testing at 1 month of age, we found that repeated exposure to either ketamine or xylazine had no significant effects on both contextual and cued fear response (Fig. 4B, C). Repeated exposure to low-dose ketamine (20 mg/kg) had no effects on fear learning, but repeated exposure to a higher dosage of ketamine (40 mg/kg) resulted in impaired contextual fear response at 1 month of age (Fig. 4B). Similarly to KX anesthesia, multiple exposures to high-dose ketamine had no effects on the animals’ auditory-cued fear memory (Fig. 4C).
Fig. 4.
Multiple exposures to high-dose ketamine result in contextual fear learning deficits. (A) Experimental design. Fear conditioning and testing were conducted in 1-month-old mice that have received vaious treatments at P7–11. (B) Contextual fear response in mice with various treatment. 3-K vs. Control: t(16) = 0.2535, P = 0.8031; 3-X vs. Control: t(17) = 0.3303, P = 0.7452; 3-KX vs. Control: t(25) = 4.759, P < 0.0001; 3-K (40 mg/kg) vs. Control: t(19) = 2.382, P = 0.0278; unpaired t test. (C) Auditory-cued fear response in mice with various treatment. 3-K vs. Control: t(16) = 0.2216, P = 0.8274; 3-X vs. Control: t(17)= 0.9536, P = 0.3536; 3-KX vs. Control: t(20) = 0.04205, P = 0.9669; 3-K (40 mg/kg) vs. Control: t(17)= 0.1164, P = 0.9087; unpaired t test. Data are presented as means ± SEM. *P < 0.05, ****P < 0.0001, n.s., not significant.
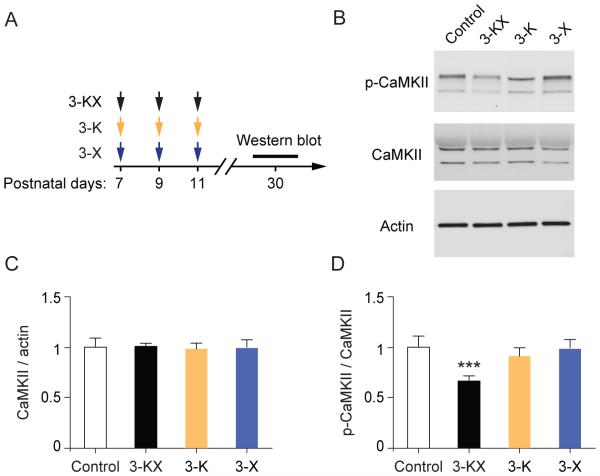
3.5. Persistent reduction of p-CaMKII after multiple exposures to KX anesthesia
CaMKII plays an important role in learning and memory (Coultrap and Bayer, 2012; Lisman et al., 2012). To determine whether mice with neonatal anesthetic exposure have abnormal levels of CaMKII in the brain, we examined protein levels of CaMKII using Western blot analysis (Fig. 5A). We found that repeated KX anesthesia had no significant effect on total amounts of CaMKII at synapses (Fig. 5B and C), but caused a significant decrease in the level of CaMKII phosphorylated at Thr286 (p-CaMKII) (active form) as compared to saline-treated controls (0.65 ± 0.066 vs. 1 ± 0.128, P = 0.0002) (Fig. 5B and D). Repeated exposure to either ketamine or xylazine alone had no significant effects on both CaMKII and p-CaMKII (Fig. 5B-D). These results demonstrate that multiple exposures to KX anesthesia at the neonatal stage cause long-lasting reduction of CaMKII phosphorylation in the brain.
Fig. 5.
Multiple exposures to KX anesthesia at neonatal age result in impaired CaMKII signaling. (A) Timeline for antiesthetic exposure and Western blot analysis. (B) Synaptosome fractions were generated from the brains of 1-month-old mice, and probed with antibodies for CaMKII and p-CaMKII by Western blot. (C) Densitometric quantification of Western blots (n = 5 mice per group). The amount of total CaMKII is comparable between control and anesthetic groups. 3-KX vs. Control: t(8) = 0.1944, P = 0.8507; 3-K vs. Control: t(8) =0.3920, P =0.7053; 3-X vs. Control: t(8) =0.1649, P =0.8731; unpaired t test. (D) Phosphorylation levels of CaMKII were significantly decreased in mice with repeated exposure to KX anesthesia. 3-KX vs. Control: t(8) = 6.296, P = 0.0002, 3-K vs. Control: t(8) =1.429, P =0.1910; 3-X vs. Control: t(8) =0.2479, P =0.8105; unpaired t test. Data are presented as means ± SEM. ***P < 0.001.
4. Discussion
Neonatal mice with multiple exposures to KX anesthesia had deficits in novel object recognition, sociability and social novelty, and contextual fear response at age one month, but no deficits for auditory-cued fear response. Our results suggest that some cognitive processes are more vulnerable to disruption by KX anesthesia than others. Learning deficits (except for contextual fear) were dependent of frequency of exposure, and were noted after multiple doses, but not single dose of KX anesthesia. The observed behavioral changes correlate with alterations in synaptic structural and functional plasticity as detected in previous in vivo imaging studies, which also occurred only with multiple exposures (Huang et al., 2016; Huang and Yang, 2015). Similar selective cognitive impairment has also been shown in mice exposed to volatile anesthetics. For example, isoflurane exposure in neonatal mice caused deficits in contextual, spatial, and social recognition tasks but not in novel object recognition (Lee et al., 2014; Satomoto et al., 2009). Further characterization to identify specific cognitive process vulnerable to disturbance by anesthetic agents may prove useful to focus neurocognitive testing to identify subtle behavioral changes.
In conjunction with deficits in social behavior and learning, we observed that multiple exposures to KX anesthesia resulted in decreased phosphorylation levels of CaMKII. There were no changes in the abundance of CaMKII in the synapses. CaMKII is a key mediator of long-term potentiation and learning, and is a major component of postsynaptic dendritic spines (Lee et al., 2009). Dendritic spine plasticity is thought to play an integral role in learning and memory formation (Bailey and Kandel, 1993; Bhatt et al., 2009; Lai et al., 2012; Yang et al., 2009). Decreased CaMKII phosphorylation, together with decreases in other synaptic proteins such as glutamate NMDA and AMPA receptor subunits after repeated KX anesthesia (Huang et al., 2016), could account for the abnormal dendritic spine development observed in previous studies (Huang et al., 2016; Huang and Yang, 2015), and contribute to learning deficits. Of note, CaMKII is translocated to synapses by NMDA receptor binding (Shen and Meyer, 1999), which is the pharmacological target of ketamine.
KX anesthesia was administered at P7, P9, and P11, corresponding to neonatal development. Mice are less developmentally mature at birth, and P7 is thought to represent a similar stage of neurodevelopment to human newborn (Deng et al., 2014). We chose this time point because animal models for ketamine exposure have shown abnormalities in development including neural apoptosis with exposure before 2–3 weeks of age (Liu et al., 2011; Paule et al., 2011; Slikker et al., 2007; Young et al., 2005). Exposure at this time period is of interest to both pediatric and obstetrical anesthesia. Ketamine is sometimes used in obstetric anesthesia during cesarean section, and is known to cross the placental barrier (Ellingson et al., 1977). Our study did not observe obvious learning and social deficits in mice with a single anesthetic exposure, as would typically be the case for a child delivered by cesarean section. Further studies in mice before P7 may also be useful to investigate the effects of similar anesthetics on fetal development.
Our study observed decreased performance on cognitive tasks when xylazine was administered with ketamine compared with ketamine alone. Although xylazine is used only as a veterinary anesthetic, it functions as an alpha-2 agonist similar to dexmedetomidine, which is commonly used in humans. Dexmedetomidine has been increasingly used in children as an anesthetic agent, and for the prevention of emergence delirium (Ibacache et al., 2004). Therefore, it is plausible that combination of ketamine and an alpha-2 agonist may be used clinically. Of note, studies in rats have shown both in vivo and in vitro that dexmedetomidine decreased cortical apoptosis from prolonged exposure to isoflurane (Sanders et al., 2010). Further study is warranted to study the effect of alpha-2 agonists on cognitive performance in combination with other anesthetics.
The use of a mouse model to study anesthetic neurotoxicity has both advantages and limitations. The use of an animal model allows for control of environment, removing many of the confounding variables that are difficult to control in human studies. In both children and mice, an enriched home environment also influences cognitive development (Huang and Yang, 2015; Shih et al., 2012). A limitation of our study is that mice are less sensitive to ketamine than humans, such that xylazine is also administered to induce general anesthesia. Ketamine alone at 20 mg/kg for three doses was not sufficient to induce the behavioral changes, but we did see impairment in contextual fear learning with ketamine 40 mg/kg for three doses. Recent literature are consistent with our findings, as neonatal exposure across several animal models is associated with learning deficits (Huang et al., 2012; Paule et al., 2011) and neuronal apoptosis (Ikonomidou et al., 1999; Soriano et al., 2010). Similarly, there is some evidence that children with multiple exposures to ketamine may show abnormalities in later neurocognitive testing (Harris et al., 2003; Sprung et al., 2012; Wilder et al., 2009).
Our study provides further evidence that multiple neonatal exposures to KX anesthesia or high-dose ketamine can lead to abnormal neurocognitive development in mice. Behavioral changes were observed only in mice with multiple anesthetic exposures in the neonatal period, and occurred in conjunction with abnormally low levels of phosphorylated CaMKII, a key mediator for learning and memory. While it is reassuring that a single exposure to KX anesthesia in the neonatal period did not result in significant abnormalities in most behavioral tests, our findings suggest that multiple anesthetic doses in the neonatal period may have long-lasting behavioral effects.
Highlights.
Single dose of ketamine-xylazine anesthesia has no obvious behavior effects in mice.
Multiple doses of neonatal anesthesia impair learning and social behavior.
Multiple doses of neonatal anesthesia reduce the phosphorylation level of CaMKII.
Acknowledgments
We thank Dr. Juan Mauricio Garré for the assistance in the social behavior tests, Drs. Thomas Blanck and Esperanza Recio-Pinto for helpful discussions. This study was supported by National Institutes of Health GM107469 and AG048410 (to G.Y.), Guangdong Natural Science Foundation (grant no.2016A030313531) (to L.H) and NYU Department of Anesthesiology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: The authors declare no competing financial interests.
References
- Amrock LG, Starner ML, Murphy KL, Baxter MG. Long-term effects of single or multiple neonatal sevoflurane exposures on rat hippocampal ultrastructure. Anesthesiology. 2015;122(1):87–95. doi: 10.1097/ALN.0000000000000477. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER. Structural-Changes Accompanying Memory Storage. Annual review of physiology. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261–282. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- Chang LW, Dudley AW, Jr., Lee YK, Katz J. Ultrastructural changes in the nervous system after chronic exposure to halothane. Experimental neurology. 1974;45(2):209–219. doi: 10.1016/0014-4886(74)90113-7. [DOI] [PubMed] [Google Scholar]
- Coultrap SJ, Bayer KU. CaMKII regulation in information processing and storage. Trends Neurosci. 2012;35(10):607–618. doi: 10.1016/j.tins.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Disma N, de Graaff JC, Withington DE, Dorris L, Bell G, Stargatt R, Bellinger DC, Schuster T, Arnup SJ, Hardy P, Hunt RW, Takagi MJ, Giribaldi G, Hartmann PL, Salvo I, Morton NS, von Ungern Sternberg BS, Locatelli BG, Wilton N, Lynn A, Thomas JJ, Polaner D, Bagshaw O, Szmuk P, Absalom AR, Frawley G, Berde C, Ormond GD, Marmor J, McCann ME, consortium GAS. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387(10015):239–250. doi: 10.1016/S0140-6736(15)00608-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M, Hofacer RD, Jiang C, Joseph B, Hughes EA, Jia B, Danzer SC, Loepke AW. Brain regional vulnerability to anaesthesia-induced neuroapoptosis shifts with age at exposure and extends into adulthood for some regions. British journal of anaesthesia. 2014;113(3):443–451. doi: 10.1093/bja/aet469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. Journal of neurosurgical anesthesiology. 2009;21(4):286–291. doi: 10.1097/ANA.0b013e3181a71f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113(5):1143–1151. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson A, Haram K, Sagen N, Solheim E. Transplacental passage of ketamine after intravenous administration. Acta anaesthesiologica Scandinavica. 1977;21(1):41–44. doi: 10.1111/j.1399-6576.1977.tb01191.x. [DOI] [PubMed] [Google Scholar]
- Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128(5):e1053–1061. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson A, Ponten E, Gordh T, Eriksson P. Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107(3):427–436. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279(5352):870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Haberny KA, Paule MG, Scallet AC, Sistare FD, Lester DS, Hanig JP, Slikker W., Jr. Ontogeny of the N-methyl-D-aspartate (NMDA) receptor system and susceptibility to neurotoxicity. Toxicological sciences : an official journal of the Society of Toxicology. 2002;68(1):9–17. doi: 10.1093/toxsci/68.1.9. [DOI] [PubMed] [Google Scholar]
- Harris LW, Sharp T, Gartlon J, Jones DN, Harrison PJ. Long-term behavioural, molecular and morphological effects of neonatal NMDA receptor antagonism. Eur J Neurosci. 2003;18(6):1706–1710. doi: 10.1046/j.1460-9568.2003.02902.x. [DOI] [PubMed] [Google Scholar]
- Huang L, Cichon J, Ninan I, Yang G. Post-anesthesia AMPA receptor potentiation prevents anesthesia-induced learning and synaptic deficits. Science translational medicine. 2016;8(344) doi: 10.1126/scitranslmed.aaf7151. 344ra385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Liu Y, Jin W, Ji X, Dong Z. Ketamine potentiates hippocampal neurodegeneration and persistent learning and memory impairment through the PKCgamma-ERK signaling pathway in the developing brain. Brain Res. 2012;1476:164–171. doi: 10.1016/j.brainres.2012.07.059. [DOI] [PubMed] [Google Scholar]
- Huang L, Yang G. Repeated exposure to ketamine-xylazine during early development impairs motor learning-dependent dendritic spine plasticity in adulthood. Anesthesiology. 2015;122(4):821–831. doi: 10.1097/ALN.0000000000000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibacache ME, Munoz HR, Brandes V, Morales AL. Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children. Anesthesia and analgesia. 2004;98(1):60–63. doi: 10.1213/01.ANE.0000094947.20838.8E. table of contents. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Ing C, Dimaggio C, Whitehouse A, Hegarty MK, Brady J, von Ungern-Sternberg BS, Davidson A, Wood AJ, Li G, Sun LS. Long-term Differences in Language and Cognitive Function After Childhood Exposure to Anesthesia. Pediatrics. 2012;130(3):e476–485. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Vernon J, Giese KP. AlphaCaMKII autophosphorylation contributes to rapid learning but is not necessary for memory. Nat Neurosci. 2005;8(4):411–412. doi: 10.1038/nn1431. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23(3):876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama M, Satoh Y, Otsubo Y, Araki Y, Yonamine R, Masui K, Kazama T. Neonatal desflurane exposure induces more robust neuroapoptosis than do isoflurane and sevoflurane and impairs working memory. Anesthesiology. 2011;115(5):979–991. doi: 10.1097/ALN.0b013e318234228b. [DOI] [PubMed] [Google Scholar]
- Lai CS, Franke TF, Gan WB. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483(7387):87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- Lee BH, Chan JT, Hazarika O, Vutskits L, Sall JW. Early exposure to volatile anesthetics impairs long-term associative learning and recognition memory. PloS one. 2014;9(8):e105340. doi: 10.1371/journal.pone.0105340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458(7236):299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Uemura E, Bowman RE. Neurobehavioral toxicology of halothane in rats. Neurotoxicology and teratology. 1991;13(4):461–470. doi: 10.1016/0892-0362(91)90096-f. [DOI] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13(3):169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Paule MG, Ali S, Wang C. Ketamine-induced neurotoxicity and changes in gene expression in the developing rat brain. Current neuropharmacology. 2011;9(1):256–261. doi: 10.2174/157015911795017155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TL, Park R, Sun LS. Report of the fourth PANDA Symposium on "Anesthesia and Neurodevelopment in Children". Journal of neurosurgical anesthesiology. 2014;26(4):344–348. doi: 10.1097/ANA.0000000000000109. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes, brain, and behavior. 2004;3(5):287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia Promote Learning-Dependent Synapse Formation through Brain-Derived Neurotrophic Factor. Cell. 2013;155(7):1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA, Slikker W, Jr., Wang C. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicology and teratology. 2011;33(2):220–230. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental health perspectives 108 Suppl. 2000;3:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RD, Sun P, Patel S, Li M, Maze M, Ma D. Dexmedetomidine provides cortical neuroprotection: impact on anaesthetic-induced neuroapoptosis in the rat developing brain. Acta anaesthesiologica Scandinavica. 2010;54(6):710–716. doi: 10.1111/j.1399-6576.2009.02177.x. [DOI] [PubMed] [Google Scholar]
- Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110(3):628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284(5411):162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118(3):502–515. doi: 10.1097/ALN.0b013e3182834d77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J, May LD, Gonzalez HE, Lee EW, Alvi RS, Sall JW, Rau V, Bickler PE, Lalchandani GR, Yusupova M, Woodward E, Kang H, Wilk AJ, Carlston CM, Mendoza MV, Guggenheim JN, Schaefer M, Rowe AM, Stratmann G. Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology. 2012;116(3):586–602. doi: 10.1097/ALN.0b013e318247564d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slikker W, Jr., Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP, Paule MG, Wang C. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98(1):145–158. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- Soriano SG, Liu Q, Li J, Liu JR, Han XH, Kanter JL, Bajic D, Ibla JC. Ketamine activates cell cycle signaling and apoptosis in the neonatal rat brain. Anesthesiology. 2010;112(5):1155–1163. doi: 10.1097/ALN.0b013e3181d3e0c2. [DOI] [PubMed] [Google Scholar]
- Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, Bojanic K, Welch TL, Olson MD, Hanson AC, Schroeder DR, Wilder RT, Warner DO. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clinic proceedings. Mayo Clinic. 2012;87(2):120–129. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L. Early childhood general anaesthesia exposure and neurocognitive development. British journal of anaesthesia. 2010;105(Suppl 1):i61–68. doi: 10.1093/bja/aeq302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Li G, Miller T, Salorio C. Association Between a Single General Anesthesia Exposure Before Age 36 Months and Neurocognitive Outcomes in Later Childhood. JAMA. 2016;315(21):2312–2320. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viberg H, Ponten E, Eriksson P, Gordh T, Fredriksson A. Neonatal ketamine exposure results in changes in biochemical substrates of neuronal growth and synaptogenesis, and alters adult behavior irreversibly. Toxicology. 2008;249(2-3):153–159. doi: 10.1016/j.tox.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Whitcher CE, Cohen EN, Trudell JR. Chronic exposure to anesthetic gases in the operating room. Anesthesiology. 1971;35(4):348–353. doi: 10.1097/00000542-197110000-00006. [DOI] [PubMed] [Google Scholar]
- Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110(4):796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Chang PC, Bekker A, Blanck TJ, Gan WB. Transient effects of anesthetics on dendritic spines and filopodia in the living mouse cortex. Anesthesiology. 2011;115(4):718–726. doi: 10.1097/ALN.0b013e318229a660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462(7275):920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C, Jevtovic-Todorovic V, Qin YQ, Tenkova T, Wang H, Labruyere J, Olney JW. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. British journal of pharmacology. 2005;146(2):189–197. doi: 10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Liu F, Zhang X, Patterson TA, Callicott R, Liu S, Hanig JP, Paule MG, Slikker W, Jr., Wang C. Inhalation anesthetic-induced neuronal damage in the developing rhesus monkey. Neurotoxicology and teratology. 2011;33(5):592–597. doi: 10.1016/j.ntt.2011.06.003. [DOI] [PubMed] [Google Scholar]