Introduction
Heart failure (HF) is a rapidly growing health concern affecting nearly 6 million patients and families in the United States (US) alone.1,2 From a health systems perspective, HF is the primary reason for hospitalization and rehospitalization among older adults in the US3,4 and comes with exorbitant health expenditures.1 Persons with HF also live with poor quality of life (QOL) and disabling symptoms, and 50% of patients will die within 5 years of diagnosis.2,5 Although patients are the focus of the vast majority of research in HF, living with HF is typically a shared experience, and there are considerable implications for caregiver well-being.6,7 For example, informal caregivers of patients with HF experience significant strain8,9 and depression related to caregiving.10,11 Higher caregiver strain is especially concerning because of the link between strain and caregiver morbidity/mortality.12,13 Hence, there is increasing interest in examining both caregiver- and patient-level factors associated with HF and the treatment thereof.
Although HF patient and caregiver characteristics are often studied at the individual level rather than in the context of a dyadic relationship, there is evidence from the broader caregiving literature that the patient-caregiver dyad is transactional in nature (i.e. bi-directional influence of one member on the other).14,15 Quantifying these important transactional influences in HF fills an important gap for researchers and clinicians who are interested in supporting the health of patients, caregivers, and the caregiving dyad. A meta-analytic approach is a particularly rigorous way to advance the science of caregiving dyads by synthesizing effects observed across multiple studies and providing insight into the design of future dyadic research.16 Accordingly, the purpose of this meta-analysis was to synthesize the results of HF studies focused on relationships between caregiver well-being and patient-oriented and clinical outcomes.
Methods
Study selection and data extraction
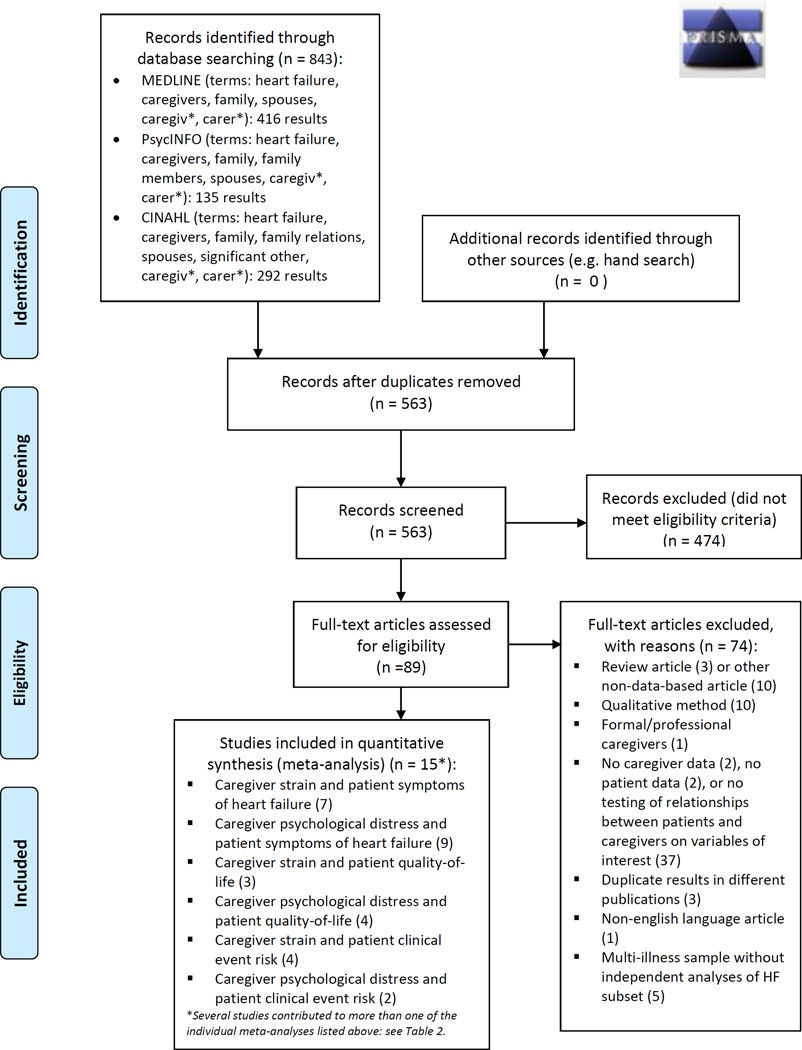
This study was a random-effects meta-analysis of published observational studies, conducted in accordance with PRISMA and MOOSE guidelines.17,18 Studies were considered eligible for inclusion if they met the following characteristics: 1) the sample consisted of adult HF patients and their informal caregivers, 2) data on measures of interest were collected on both members of the dyad, and 3) the results included tests of association between patient and caregiver measures of interest. We did not exclude studies based on date of publication. Non-English language studies were excluded. MEDLINE, CINAHL, and PsycInfo databases were searched for eligible studies; full search strategies are presented within the PRISMA flow diagram (Figure 1). The original search was conducted in November 2013 and was updated in March 2015 to ensure no new studies met inclusion criteria.
Figure 1.
PRISMA Flow Diagram
Study screening and evaluation for eligibility and inclusion into the meta-analysis was conducted by the first author with guidance from the senior author. Data were extracted into Excel format, then re-extracted, compared, and corrected for any errors. For each study, the following variables were collected: 1) study authors, 2) date of publication, 3) journal/source of publication, 4) funding source, 5) number of patient-caregiver dyads in sample, 6) patient-caregiver dyad relationship type, 7) demographic characteristics of patients and caregiver (age, gender, race), 8) instrument used to measure outcomes of interest, 9) analytic approach used to test the association between patient and caregiver outcomes, 10) result of statistical test of association for given relationships of interest. If clarification on published findings was needed, this was requested from the corresponding author, who was also queried about other available data in accordance with current guidelines.
Analysis
A random-effects meta-analytic approach was selected for this analysis because of substantial differences across studies in terms of measurement and sampled populations. In random-effects meta-analysis, it is not assumed that there is one true effect size across all studies. Rather, the effect sizes of the observed studies are considered to be a random sample of all possible effect sizes.19,20 Under this assumption, the summary effect is the weighted average of all studies, with the weight of each study being the inverse of variance within each study plus the variance between studies. As this was a meta-analysis of correlations from observational studies, the summary effect was estimated in the metric of Fisher’s z; Pearson’s r are also provided for ease of interpretation.
Significant heterogeneity across studies can reduce precision in meta-analyses. In this analysis, heterogeneity in effect sizes was examined using the Q and I2 statistics. A significant Q indicates excess dispersion in effect sizes across studies. The I2 is a “signal-to-noise” ratio of excess dispersion to total dispersion, and therefore indicates the proportion of heterogeneity that is concerning and warrants additional investigation (signal), versus “spurious” heterogeneity that is due to chance (noise). Typically, an I2 of <25% is considered a low amount of “real” heterogeneity and is not considered problematic, while I2 values of 50% or 75% are considered moderate and high amounts of “real” heterogeneity, respectively, and should be investigated further to identify the source.21 In the instance of concurrent significant Q and I2 greater than 25%, subgroup analysis can be conducted in an effort to explain sources of heterogeneity, provided the n (number of studies included) is adequate.
Publication bias (bias in the summary effect due to unpublished studies) was assessed visually using funnel plots. Additionally, bias from small-study effects (bias in the summary effect due to studies that have a very large effect, but a very small n) was assessed using Egger’s test; a non-significant Egger’s test indicates limited concern of bias from small-study effects.
Results
Results from the process of study identification, screening, eligibility, and inclusion are outlined in the flow diagram (Figure 1). As a whole, a total of 15 articles were included across the six meta-analyses we conducted (Table 1). However, most studies contributed to more than one individual meta-analysis, as shown in Table 2.9,22–35
Table 1.
Study Characteristics
| Author, Date | Journal | Funding | Dyad n and Relationship Type |
Caregiver Characteristics |
Patient Characteristics |
|---|---|---|---|---|---|
| Agren, 2010 | Eur J CardiovascNurs | Linköping University; Swedish Institute for Health Sciences; Sweden; Swedish Research Council; Vårdal Foundation |
n=135 100% Spousal |
Age 69±12 75% Female Race not reported |
Age 71±12 25% Female Race not reported |
| Barnes, 2006 | Int J PalliatNurs | UK Department of Health |
n=213 73% Spousal |
70% > 60 years 76% Female Race not reported |
100% > 60 years 36% Female Race not reported |
| Chung, 2009 | J Psychosom Res | NIH/NINR; University of Kentucky |
n=58 100% Spousal |
Age 58±12 Gender not reported Race not reported |
Age 62±13 26% Female 93% White |
| Chung, 2010 | J of CardiovascNurs | University of Kentucky; NIH/NINR |
n=109 79% Spousal |
Age 57±13 75% Female 94% White |
Age 61±12 40% Female Race not reported |
| Hooley, 2005 | Congest Heart Fail | Undergraduate Internal Medicine Research Foundation, Dalhousie University |
n=50 66% Spousal |
Age 61±14 80% Female Race not reported |
Age 72±11 28% Female Race not reported |
| Hwang, 2011 | Am J Crit Care | University of California, San Francisco |
n=76 74% Spousal |
Age 53±16 71% Female 63% White |
Age 54±14 45% Female 66% White |
| Luttik, 2007 | Eur J Heart Fail | Netherlands Heart Foundation |
n=357 100% Spousal |
Age 67±12 75% Female Race not reported |
Age 68±11 25% Female Race not reported |
| Martensson, 2003 | J Heart Lung Transplant | Research Council of Southeastern Sweden; Swedish Heart and Lung Foundation;AHA |
n=48 100% Spousal |
Age 57±10 100% Female 83% White |
Age 61±9 0% Female 77% White |
| Pihl, 2005 | Eur J Heart Fail | Funding source not reported |
n=47 100% Spousal |
Age 75±7 72% Female Race not reported |
Age 78±5 28% Female Race not reported |
| Pressler, 2013 | J CardiovascNurs | Indiana University; NIH/NINR |
n=63 68% Spousal |
Age 60±15 76% Female 84% White |
Age 69±13 46% Female 81% White |
| Rohrbaugh, 2002 | J Fam Psych | NIH |
n=181 100% Spousal |
Age 52±11 Gender not reported 83% White* |
Age 53±10 27% Female 83% White* |
| Rohrbaugh, 2009 | Heart Lung | AHA |
n=60 100% Spousal |
Age 66±11 72% Female Race not reported |
Age 67 ±12 28% Female 85% White |
| Saunders, 2008 | Home Healthc Nurse | Funding source not reported |
n=41 46% Adult child of patient, 46% Spousal |
Age 59±15 85% Female 68% White |
Age 78±10 51% Female 85% White |
| Schwarz, 2003 | Heart Lung | Funding source not reported |
n=128 61.7% Spousal, 27.3% Adult child of patient |
Age 65±15 74% Female 89% White |
Age 77±6 50% Female 89% White |
| Trivedi, 2012 | J CardiovascNurs | VA Puget Sound; Durham VA |
n=23 100% Spousal |
Age not reported 100% Female Race not reported |
Age 66±7 100% Male 61% White |
This publication reported race for the whole sample (patients and caregivers together), rather than for patients and caregivers separately
All journal abbreviations per National Library of Medicine; Age reported as Mean±SD; UK: United Kingdom; NIH: National Institutes of Health; NINR: National Institute of Nursing Research; AHA: American Heart Association; VA: Veterans Affairs
Table 2.
Study Measures by Meta-Analysis Aim
| Author, Date |
Caregiver Strain and Patient HF Symptoms |
Caregiver Psychological Distress and Patient HF Symptoms |
Caregiver Strain and Patient QOL |
Caregiver Psychological Distress and Patient QOL |
Caregiver Strain and Patient Clinical Event Risk |
Caregiver Psychological Distress and Patient Clinical Event Risk |
|---|---|---|---|---|---|---|
| Agren, 2010 | CG: CBS (Swedish) |
CG: CBS (Swedish) |
||||
| Pt: NYHA | Pt: SF-36 PCS | |||||
| Barnes, 2006 | CG: CSI (dichotomized) |
|||||
| Pt: NYHA (dichotomized) | ||||||
| Chung, 2009 | CG: BSI – Depression |
|||||
| Pt: MLHFQ | ||||||
| Chung, 2010 | CG: BDI-II | |||||
| Pt: NYHA | ||||||
| Hooley, 2005 | CG: ZBI (dichotomized) |
CG: BDI-II (dichotomized) |
CG: ZBI | CG: ZBI (dichotomized) |
CG: BDI-II | |
| Pt: NYHA (dichotomized) |
Pt: NYHA (dichotomized) |
Pt: MLHFQ | Pt: hospitalization or death 6 months after enrollment |
Pt: hospitalization or death 6 months after enrollment |
||
| Hwang, 2011 | CG: CRA (schedule) |
CG: CRA (physical health) |
||||
| Pt: NYHA | Pt: days since last hospital discharge |
|||||
| Luttik, 2007 | CG: CRA (schedule) |
|||||
| Pt: RAND-36 PF | ||||||
| Martensson, 2003 | CG: BDI | CG: BDI | ||||
| Pt: NYHA | Pt: SF-12 PCS | |||||
| Pihl, 2005 | CG: Zung | CG: Zung | ||||
| Pt: NYHA | Pt: SF-12 PCS | |||||
| Pressler, 2013 | CG: OCBS | CG: PHQ-8 | ||||
| Pt: NYHA (dichotomized) |
Pt: NYHA (dichotomized) |
|||||
| Rohrbaugh, 2002 | CG: HSC-25 | |||||
| Pt: NYHA | ||||||
| Rohrbaugh, 2009 | CG: HSC-25 | CG: HSC-25 | ||||
| Pt: NYHA | Pt: SF-36 composite PCS/MCS |
|||||
| Saunders, 2008 | CG: CRA (schedule) |
CG: CES-D | CG: CRA (family) |
|||
| Pt: NYHA (caregiver evaluated) |
Pt: NYHA | Pt: Number of hospitalizations past 12 months |
||||
| Schwarz, 2003 | CG: PSS | CG: CES-D | ||||
| Pt: Hospitalization or death 3 months after enrollment |
Pt: Hospitalization or death 3 months after enrollment |
|||||
| Trivedi, 2012 | CG: ZBI | CG: CES-D | ||||
| Pt: HCS (HF- specific) |
Pt: HCS (HF- specific) |
CG: Caregiver; Pt: Patient; CBS (Swedish): Swedish Caregiver Burden Scale; NYHA: New York Heart Association Classification; SF-12/36 PCS/MCS: Short Form-12/36 Physical Component Summary/Mental Component Summary; CSI: Carer Strain Index; BSI: Brief Symptom Inventory; MLHFQ: Minnesota Living with Heart Failure Questionnaire; BDI: Beck Depression Inventory; ZBI: Zarit Burden Interview; CRA: Caregiver Reaction Assessment; RAND-36 PF: RAND version of the SF-36, Physical Function subscale; Zung: Zung Depression Scale; OCBS: Oberst Caregiving Burden Scale; PHQ-8: Patient Health Questionnaire-8; HSC-25: Hopkins Symptom Checklist-25; CES-D: Center for Epidemiologic Studies Depression Scale; PSS: Perceived Stress Scale; HCS: Health Complaints Scale
Caregiver Well-being and Patient Heart Failure Symptoms
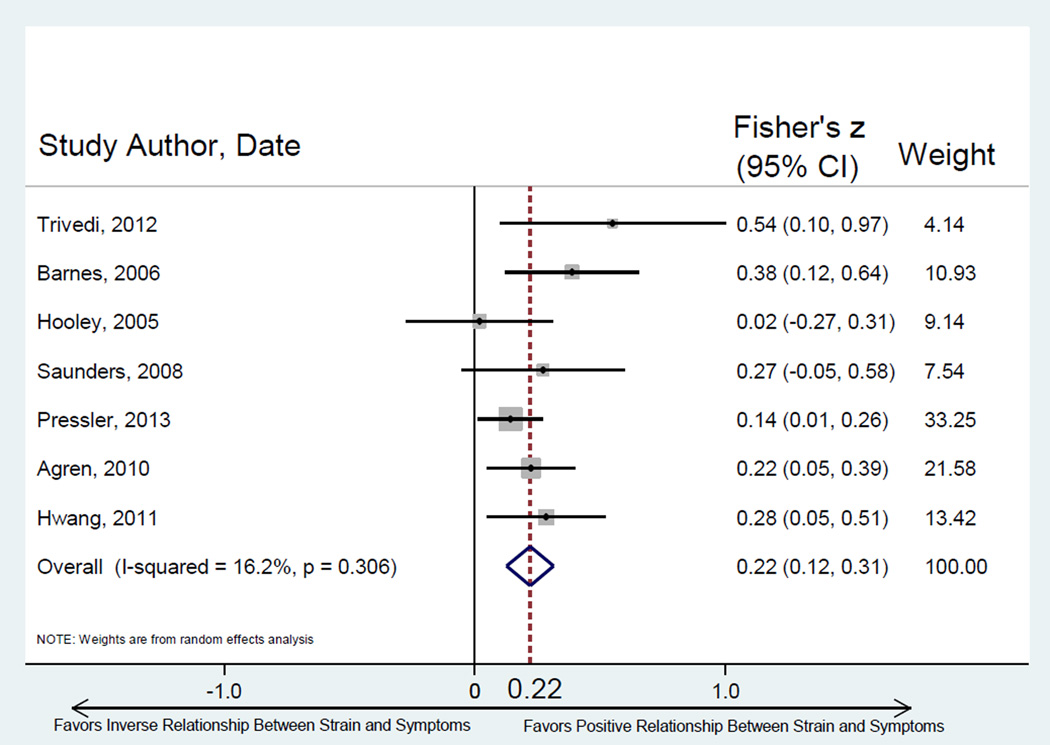
Seven studies tested the relationship between caregiver strain and patient HF symptoms (Table 2). Selected strain measures across studies were variable, while HF symptom measures were fairly consistent with most studies reporting New York Heart Association (NYHA) Class. Higher caregiver strain was significantly associated with worse HF patient symptoms across studies (Figure 2). There was limited between-study heterogeneity (Q = 7.16, p = 0.306) and minimal small sample bias.
Figure 2.
Random effects meta-analysis of relationship between caregiver strain and patient symptoms of HF. Note: Fisher’s z converted to metric of Pearson’s r = 0.213. Egger’s test for bias of small study effects: t = 1.60, p = 0.170.
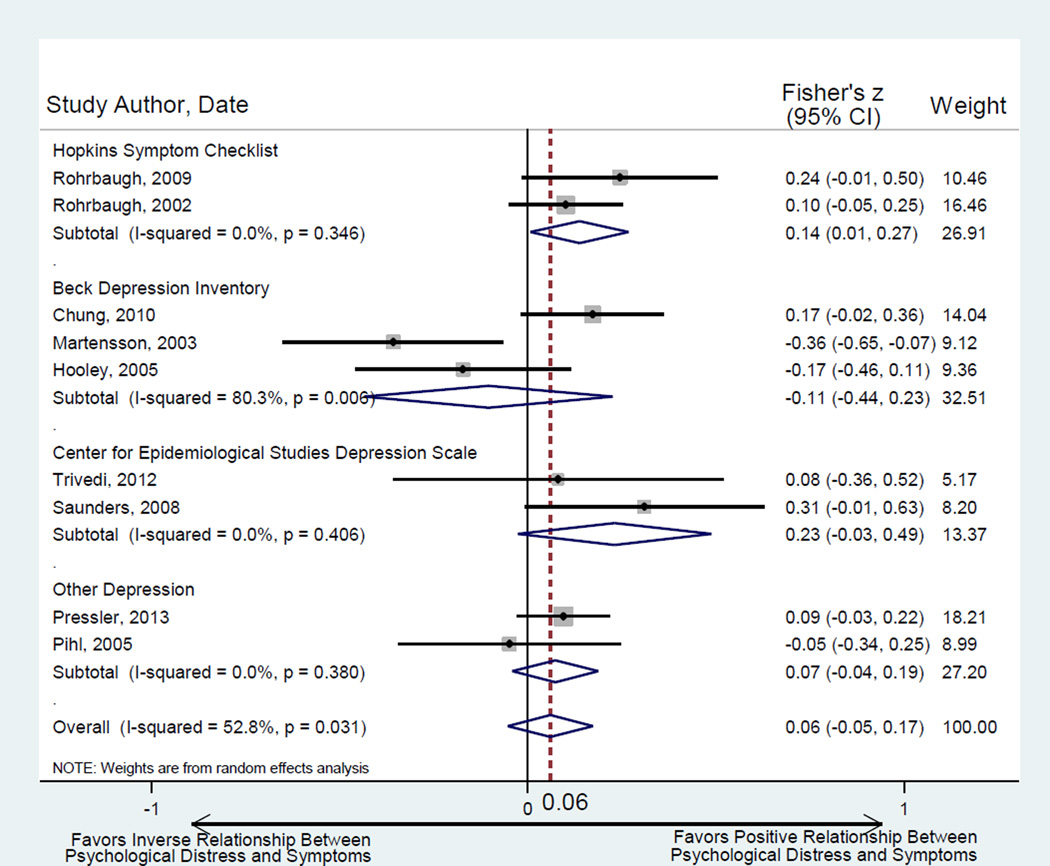
Nine studies tested the relationship between caregiver psychological distress and patient HF symptoms (Table 2). For caregiver pyschological distress, depression instruments were the most commonly used measures. Measures of patient HF symptoms were consistent (largely NYHA Class). Overall, there was significant heterogeneity across studies (Q = 16.96, p = 0.031, I2 = 52.8%) and no precise estimate of the relationship between caregiver psychological distress and patient HF symptoms could be quantified. We conducted a four-subgroup analysis of studies by measure (Hopkins Symptom Checklist, Beck Depression Inventory, Center for Epidemiologic Studies Depression Scale, and remaining depression measures) (Figure 3). Greater caregiver psychological distress was associated with worse patient symptoms across studies that used the Hopkins Symptom Checklist; otherwise, there was no significant relationship between caregiver psychological distress and patient HF symptoms.
Figure 3.
Random effects meta-analysis of relationship between caregiver psychological distress and patient symptoms of HF. Note: Fisher’s Z converts to the metric of Pearson’s r as follows: for HSC summary effect r = 0.136; for BDI summary effect r = −0.105; for CES-D summary effect r = 0.226; for Other Depression summary effect r = 0.073; for Overall summary effect r = 0.060. Egger’s test for bias of small study effects: t = −0.71, p =0.502.
Caregiver Well-being and Patient Quality-of-Life
Three studies tested the relationship between caregiver strain and patient QOL (Table 2). Across studies, three different measures of caregiver strain and three different measures of QOL were utilized. One study was not amenable for inclusion in the analysis due to missing data, leaving two studies appropriate for synthesis. There was a significant relationship between higher caregiver strain and worse patient QOL (Fisher’s z = −0.356 ± 0.08, z-score = 4.76, p < 0.001); however, results from this analysis are limited by the small number of included studies.
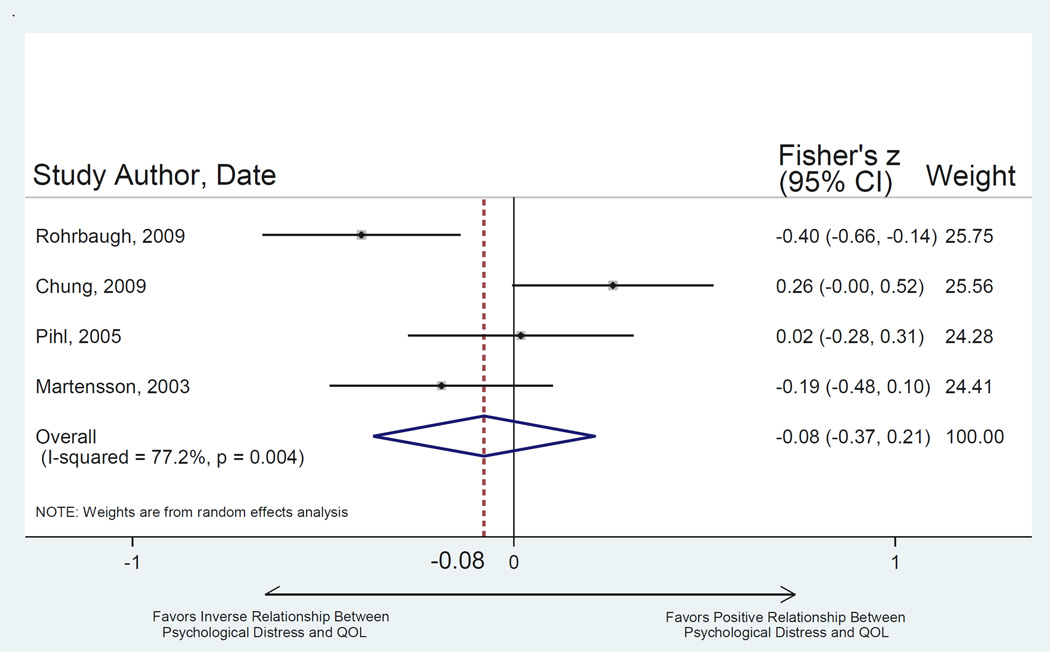
Four studies tested the relationship between caregiver psychological distress and patient QOL (Table 2). Across studies, there was substantial variability in both the selection of caregiver and patient measures and in study design. This substantial between-study heterogeneity (Q = 13.15, p = 0.004, I2 = 77.2%) prevented a precise estimation of effect size (Fisher’s z = −0.08 ± 0.15, z-score = 0.53, p = 0.595) (Figure 4). Due to the small number of identified studies testing this relationship it was not possible to run additional analyses by measure subgroups.
Figure 4.
Random-effects meta-analysis of relationship between caregiver psychological distress and patient QOL. Note: Fisher’s Z when converted to metric of Pearson’s r = −0.079. Egger’s test for bias of small study effects: t = 0.15, p = 0.898.
Caregiver Well-being and Patient Clinical Event Risk
Four studies tested the relationship between caregiver strain and patient clinical event risk (Table 2). Across studies there was substantial variability in measures of caregiver strain and definitions of clinical event risk. Although the number of studies was adequate for attempting meta-analysis, the variation in type of event (hospitalization only, mortality only, combined hospitalization/mortality) and time-to-event (days since last event, number of events in past year, event occurrence within 6 months post-enrollment, event occurrence within 3 months post-enrollment) made the examination of this relationship non-conducive to meta-analysis. Of these four studies, all found a significant relationship between greater caregiver strain and clinical events, regardless of how those events were quantified or modeled in the original papers (Table 2). In order to estimate a summary effect for this relationship, however, studies with congruent type of event and time-to-event measures are needed.
Two studies tested the relationship between caregiver psychological distress and patient clinical event risk (Table 2). As with the examination of strain and clinical events, differences in type of event and time-to-event made the examination of this relationship inappropriate for meta-analysis. Both studies found a significant relationship between worse caregiver psychological distress and higher clinical event risk. However, in order to estimate an accurate and informative summary effect, congruence in type of event and time-to-event in future studies are needed.
Discussion
Although a small body of literature has examined relationships between caregiver well-being and patient outcomes in HF, this analysis is the first to combine existing quantitative knowledge in this domain using meta-analytic methods. In accordance with sections 24–26 of the PRISMA guidelines,17 this discussion will summarize the main findings of each meta-analysis, consider relevance and implications of each to clinical practice, health policy, and research, and discuss study limitations.
Caregiver Well-Being and Patient HF Symptoms
In this meta-analysis, we found that higher caregiver strain was significantly associated with worse patient symptoms. Strengthening our confidence in this finding, there was no evidence of excess heterogeneity (despite differences in measures) or small study effects. This is not surprising, given that similar relationships between strain and disease severity have been observed in other illness contexts.36–38 In contrast, we observed no significant association between caregiver psychological distress and patient symptoms in HF. Given that a positive association has been demonstrated between patient symptoms and caregiver depression in cancer,39 Parkinson’s,40 and dementia36 dyads, this lack of significant finding was somewhat unexpected.
In terms of measurement of patient symptoms, it is possible that differences in cancer/ Parkinson’s/ dementia symptoms or the utilization of NYHA Classification – a global measure of symptom severity – as a proxy for more nuanced HF symptom measures may explain both the variability in study results and the lack of a significant summary effect. Although NYHA Class quantifies severity of symptoms in general, we have no way of quantifying types of symptoms in particular or the degree to which those symptoms are bothersome to the patient – both aspects of HF symptomatology that may be pertinent to the caregiver experience. For example, there may be particular symptoms or clusters of symptoms (e.g. breathlessness) that are particularly distressing to family members.
In terms of measurement of caregiver psychological distress, it is notable that our subgroup analysis by caregiver measures found significant associations between the HSC (which measures both depression and anxiety) and patient symptoms, but not between patient symptoms and depression-only measures of caregiver distress. Congruent with our findings, the landmark Caregiver Health Effects Study reported that increases in patient physical impairment were associated with increases in caregiver anxiety, but not depression, over time.12 Similarly, within the context of HF, several qualitative studies have identified caregiver anxiety as a major theme of the caregiving experience and a common response to increasing patient symptoms.41 Thus, there may be particular utility in adding measures of anxiety to future studies involving patient-caregiver dyads.
Caregiver Well-Being and Patient Quality of Life
We found that higher caregiver strain was significantly associated with worse patient QOL. However, our analysis was constrained by sample size, as few studies examined this relationship. Thus, our confidence in this finding is somewhat limited by our inability to adequately test for bias. In contrast, we observed no significant association between caregiver psychological distress and patient QOL, but again, relatively few studies exist that test this relationship. Moreover, the substantial amount of between-study heterogeneity – possibly related to a high degree of variability in measuring both caregiver psychological distress and patient QOL across studies – precluded identification of a summary effect. Thus, there may indeed be a significant relationship between caregiver psychological distress and patient QOL that is otherwise obscured by differences in measurement or sampling across studies, as well as the relative paucity of studies. As QOL becomes an increasingly important outcome in HF,42 it is essential to understand the important role caregiver factors may have on patient QOL. Furthermore, given that patients with HF often report substantial QOL impairment,43 it is important to support caregivers who may experience associated increases in strain or psychological distress.
Caregiver Well-Being and Patient Clinical Event Risk
Although there were multiple studies that tested the relationship between caregiver strain and caregiver psychological distress and patient clinical event risk, we were unable to summarize them using meta-analytic methods. With substantial variation in type and time-to-event, a summary effect would be uninterpretable. However, it should be emphasized that this does not mean that no relationship between caregiver well-being and patient clinical event risk exists. To the contrary, these were the only relationships in this analysis in which all studies reported significant positive findings between worse caregiver well-being and higher patient clinical event-risk. This level of consensus on such critical outcomes – hospitalization and death – clearly warrants continuing investigation, ideally utilizing more congruent measures of clinical event risk.
Implications for Clinical Practice, Health Policy, and Research
There are several notable clinical, research, and policy implications from these findings. As HF symptoms worsen and patient QOL declines, caregivers may be at increased risk of strain and its sequelae, namely, increased morbidity and mortality.12,13 However, because the synthesis of cross-sectional observational studies precludes conclusions about directionality/causality of relationships, it might also be said that assessment of increased strain in caregivers may signal higher patient symptom burden or QOL impairment. In either case, this first meta-analysis of patient-caregiver relationships in HF demonstrates that the experiences of patient and caregiver are clearly transactional, providing support for dyadic approaches to research and clinical management. For example, researchers and clinicians interested in studying and supporting self-care in HF patients recognize that caregiver strain may have a negative impact on self-care behaviors.44 This is particularly concerning if caregiver strain increases commensurate with patient symptoms, since caregivers may be less able to assist advanced HF patients who are at greatest risk for exacerbation if self-care is compromised. Therefore, the patient and caregiver as a dyad may benefit jointly from research, clinical care, and health policies that recognize and support the health and well-being of both members of the caregiving dyad, rather than focusing solely on either patient or caregiver. This is reflected in the recent interventions in HF that have successfully integrated patients and caregivers together to improve outcomes for both members of the dyad.45,46 However, our ability to make definitive clinical recommendations for managing patient-caregiver dyads together is hindered by the current state of the literature in HF, which consists largely of analyses conceptualized and conducted at the individual level (e.g. individual patient/caregiver endpoints and limited examination of within-dyad interdependence). Thus, although this meta-analysis provides important information on how individual caregiver and patient outcomes are related on average, we are almost completely bereft of insight into how patients and caregivers experience and manage HF together within the context of their relationship to one another. Therefore, in order to better support patients and caregivers together within a dyadic context, we must expand research in HF to include studies that conceptualize and analyze research questions at the level of the dyad.
Dyadic research approaches may also contribute to a more holistic understanding of clinical event-risk. Despite emphasis on reducing hospitalizations,47 HF-associated hospitalizations in the United States have not declined,48 and there is some concern that over-avoidance of hospitalization may sacrifice potentially associated survival benefits.49 Most nursing interventions that aim to reduce clinical event risk do not include the caregiver or take a dyadic approach, and almost half have no success in reducing hospitalization or death.50 Furthermore, most risk prediction models in HF do not include social support variables.51 Although we were not able to statistically synthesize relationships between caregiver well-being and patient clinical event risk due to variation in event risk measures, all the studies we identified found significant positive relationships between worse caregiver well-being and patient clinical events. Given the clinical and research gaps in explaining variability in HF risk, it may be useful to examine caregiver factors as potential predictors of patient clinical event risk. However, the limitations of the cross-sectional nature of these studies cannot be understated. It is equally, if not more, plausible that the directionality of the relationship runs opposite – namely, that higher odds of patient clinical events influences higher strain in caregivers. Regardless, this is clearly a relationship that warrants further investigation, as both event-risk in patients and strain in caregivers are important clinical outcomes.13,42 Thus, developing a better understanding of the patient-caregiver dyad as a whole in HF may assist researchers in designing more efficacious models and interventions, guide clinicians in providing care that is more closely aligned with the real-world context of caregiving relationships in outpatient settings, and help policy makers to develop policies that support better outcomes for both patients and caregivers.
Limitations and Future Recommendations
The findings of this study have several limitations. Firstly, this analysis required integrating studies that used differing measures (e.g. strain, psychological distress, clinical event risk). In some instances this did not appear problematic (e.g. strain and patient symptoms), but in other cases this contributed to substantial heterogeneity in the analysis (e.g. caregiver psychological distress) or precluded analysis entirely (patient clinical event risk). Secondly, in the meta-analyses involving patient symptoms, although we used NYHA Class as a proxy for symptom severity, it is not a robust symptom measure; however, this is a readily available clinical characteristic frequently collected on patients in studies of HF caregivers. This is a reflection of the current state of the literature in HF, which predominantly consists of studies whose central focus is either patients or caregivers, rather than both members of the dyad. That is to say, although studies may include measures of both patient and caregiver characteristics, typically only one member is extensively measured and few characteristics of the other member are included. Thus, there is an opportunity to advance the science by explicitly acknowledging and examining the transactional nature of the patient-caregiver dyad, collecting robust data on clinical- and person-oriented measures (ideally using the same measure for patient and caregiver to facilitate dyadic analysis) from both members of the dyad, and using appropriate dyadic methods in future study designs and analysis. Thirdly, some of our analyses were hindered by the size and number of available studies. Although a minimum of two studies is required for meta-analysis, our confidence in the summary effect is strengthened when the sample size is adequate for rigorous tests of bias. Again, the lack of studies examining the interrelationship of patients and caregivers in HF is an important limitation of the current state of the science in HF. As more research is done at the level of the dyad, dyadic interdependence, covarying outcomes, and dyadic archetypes can be more fully elucidated. Furthermore, when more studies are available for synthesis, techniques such as meta-regression can be utilized to more rigorously examine relationships of interest. And finally, although every effort was made to ensure inclusion of all available studies in this meta-analysis, there is always a possibility of bias from missed studies.
Conclusions
In this meta-analysis, higher caregiver strain was associated with worse patient symptoms and worse patient QOL. Although we found no significant relationship between caregiver psychological distress and patient symptoms or QOL, substantial heterogeneity was present in both analyses. Finally, studies examining relationships between caregiver well-being and patient clinical events were not amenable to meta-analysis due to variations in event-risk estimation. Future research involving patients and caregivers should include robust measures of clinical- and person-oriented outcomes from both members of the dyad. In particular, measures of psychological (anxiety and depression) and physical health and QOL should be included, as well as comprehensive measures of patient HF symptoms and caregiver strain. Most importantly, in order to better address the needs of patients and caregivers together, we must advance the science through research that is conceptualized and conducted at the level of the dyad.
Acknowledgments
Funding
This work was supported by the National Institutes of Health/ National Institute of Nursing Research (1F31NR014760).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health.
Footnotes
Conflicts of Interest
None declared.
Contributor Information
Julie T. Bidwell, Oregon Health & Science University School of Nursing.
Karen S. Lyons, Oregon Health & Science University School of Nursing.
Christopher S. Lee, Oregon Health & Science University School of Nursing; Oregon Health & Science University Knight Cardiovascular Institute.
References
- 1.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: A report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. NEJM. 2009;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 4.Liu L. Changes in cardiovascular hospitalization and comorbidity of heart failure in the United States: Findings from the National Hospital Discharge Surveys 1980–2006. Int J Cardiol. 2011;149(1):39–45. doi: 10.1016/j.ijcard.2009.11.037. [DOI] [PubMed] [Google Scholar]
- 5.Zambroski CH, Moser DK, Bhat G, Ziegler C. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur J Cardiovasc Nurs. 2005;4(3):198–206. doi: 10.1016/j.ejcnurse.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Joo H, Fang J, Losby JL, Wang G. Cost of informal caregiving for patients with heart failure. Am Heart J. 2015;169(1):142–148. e142. doi: 10.1016/j.ahj.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gure TR, Kabeto MU, Blaum CS, Langa KM. Degree of disability and patterns of caregiving among older Americans with congestive heart failure. J Gen Intern Med. 2008;23(1):70–76. doi: 10.1007/s11606-007-0456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang B, Luttik ML, Dracup K, Jaarsma T. Family caregiving for patients with heart failure: Types of care provided and gender differences. J Card Fail. 2010;16(5):398–403. doi: 10.1016/j.cardfail.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Luttik ML, Jaarsma T, Veeger N, Tijssen J, Sanderman R, van Veldhuisen DJ. Caregiver burden in partners of Heart Failure patients; limited influence of disease severity. Eur J Heart Fail. 2007;9(6–7):695–701. doi: 10.1016/j.ejheart.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Agren S, Evangelista L, Davidson T, Stromberg A. The influence of chronic heart failure in patient-partner dyads – A comparative study addressing issues of health-related quality of life. J Cardiovasc Nurs. 2011;26(1):65–73. doi: 10.1097/JCN.0b013e3181ec0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinquart M, Sorensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a meta-analysis. Psychol Aging. 2003;18(2):250–267. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- 12.Beach SR, Schulz R, Yee JL, Jackson S. Negative and positive health effects of caring for a disabled spouse: longitudinal findings from the caregiver health effects study. Psychol Aging. 2000;15(2):259–271. doi: 10.1037//0882-7974.15.2.259. [DOI] [PubMed] [Google Scholar]
- 13.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282(23):2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 14.Berg CA, Upchurch R. A developmental-contextual model of couples coping with chronic illness across the adult life span. Psychol Bull. 2007;133(6):920–954. doi: 10.1037/0033-2909.133.6.920. [DOI] [PubMed] [Google Scholar]
- 15.Chung ML, Lennie TA, Mudd-Martin G, Dunbar SB, Pressler SJ, Moser DK. Depressive symptoms in patients with heart failure negatively affect family caregiver outcomes and quality of life. Eur J Cardiovasc Nurs. 2014 doi: 10.1177/1474515114535329. [published online before print] [DOI] [PubMed] [Google Scholar]
- 16.Borenstein M, Hedges LV, HIggins JPT, Rothstein HR. Introduction to Meta-Analysis. Cornwall, UK: Wiley; 2009. [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychol Methods. 1998;3(4):486–504. [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agren S, Evangelista L, Stromberg A. Do partners of patients with chronic heart failure experience caregiver burden? Eur J Cardiovasc Nurs. 2010;9(4):254–262. doi: 10.1016/j.ejcnurse.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes S, Gott M, Payne S, et al. Characteristics and views of family carers of older people with heart failure. Int J Palliat Nurs. 2006;12(8):380–389. doi: 10.12968/ijpn.2006.12.8.380. [DOI] [PubMed] [Google Scholar]
- 24.Chung ML, Moser DK, Lennie TA, Rayens MK. The effects of depressive symptoms and anxiety on quality of life in patients with heart failure and their spouses: Testing dyadic dynamics using Actor-Partner Interdependence Model. J Psychosom Res. 2009;67(1):29–35. doi: 10.1016/j.jpsychores.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung ML, Pressler SJ, Dunbar SB, Lennie TA, Moser DK. Predictors of depressive symptoms in caregivers of patients with heart failure. J Cardiovasc Nurs. 2010;25(5):411–419. doi: 10.1097/JCN.0b013e3181d2a58d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hooley PJ, Butler G, Howlett JG. The relationship of quality of life, depression, and caregiver burden in outpatients with congestive heart failure. Congest Heart Fail. 2005;11(6):303–310. doi: 10.1111/j.1527-5299.2005.03620.x. [DOI] [PubMed] [Google Scholar]
- 27.Hwang B, Fleischmann KE, Howie-Esquivel J, Stotts NA, Dracup K. Caregiving for patients with heart failure: Impact on patients' families. Am J Crit Care. 2011;20(6):431–441. doi: 10.4037/ajcc2011472. [DOI] [PubMed] [Google Scholar]
- 28.Martensson J, Dracup K, Canary C, Fridlund B. Living with heart failure: Depression and quality of life in patients and spouses. J Heart Lung Transplant. 2003;22(4):460–467. doi: 10.1016/s1053-2498(02)00818-5. [DOI] [PubMed] [Google Scholar]
- 29.Pihl E, Jacobsson A, Fridlund B, Stromberg A, Martensson J. Depression and health-related quality of life in elderly patients suffering from heart failure and their spouses: A comparative study. Eur J Heart Fail. 2005;7(4):583–589. doi: 10.1016/j.ejheart.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Pressler SJ, Gradus-Pizlo I, Chubinski SD, et al. Family caregivers of patients with heart failure: A longitudinal study. J Cardiovasc Nurs. 2013;28(5):417–428. doi: 10.1097/JCN.0b013e3182563877. [DOI] [PubMed] [Google Scholar]
- 31.Rohrbaugh MJ, Cranford JA, Shoham V, Nicklas JM, Sonnega JS, Coyne JC. Couples coping with congestive heart failure: Role and gender differences in psychological distress. J Fam Psychol. 2002;16(1):3–13. doi: 10.1037//0893-3200.16.1.3. [DOI] [PubMed] [Google Scholar]
- 32.Rohrbaugh MJ, Shoham V, Cleary AA, Berman JS, Ewy GA. Health consequences of partner distress in couples coping with heart failure. Heart Lung. 2009;38(4):298–305. doi: 10.1016/j.hrtlng.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Saunders MM. Family caregiver support and hospitalizations of patients with heart failure. Home Healthc Nurse. 2008;26(10):624–632. doi: 10.1097/01.NHH.0000341226.40640.ad. [DOI] [PubMed] [Google Scholar]
- 34.Schwarz KA, Elman CS. Identification of factors predictive of hospital readmissions for patients with heart failure. Heart Lung. 2003;32(2):88–99. doi: 10.1067/mhl.2003.15. [DOI] [PubMed] [Google Scholar]
- 35.Trivedi RB, Piette J, Fihn SD, Edelman D. Examining the interrelatedness of patient and spousal stress in heart failure: Conceptual model and pilot data. J Cardiovasc Nurs. 2012;27(1):24–32. doi: 10.1097/JCN.0b013e3182129ce7. [DOI] [PubMed] [Google Scholar]
- 36.Ornstein K, Gaugler JE. The problem with "problem behaviors": A systematic review of the association between individual patient behavioral and psychological symptoms and caregiver depression and burden within the dementia patient-caregiver dyad. Int Psychogeriatr. 2012;24(10):1536–1552. doi: 10.1017/S1041610212000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mioshi E, Foxe D, Leslie F, et al. The impact of dementia severity on caregiver burden in frontotemporal dementia and Alzheimer disease. Alzheimer Dis Assoc Disord. 2013;27(1):68–73. doi: 10.1097/WAD.0b013e318247a0bc. [DOI] [PubMed] [Google Scholar]
- 38.Burke T, Elamin M, Galvin M, Hardiman O, Pender N. Caregiver burden in amyotrophic lateral sclerosis: a cross-sectional investigation of predictors. J Neurol. 2015 doi: 10.1007/s00415-015-7746-z. [published online before print] [DOI] [PubMed] [Google Scholar]
- 39.Given B, Wyatt G, Given C, et al. Burden and depression among caregivers of patients with cancer at the end of life. Oncol Nurs Forum. 2004;31(6):1105–1117. doi: 10.1188/04.ONF.1105-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter JH, Stewart BJ, Lyons KS, Archbold PG. Do motor and nonmotor symptoms in PD patients predict caregiver strain and depression? Mov Disord. 2008;23(9):1211–1216. doi: 10.1002/mds.21686. [DOI] [PubMed] [Google Scholar]
- 41.Kang X, Li Z, Nolan MT. Informal caregivers' experiences of caring for patients with chronic heart failure: systematic review and metasynthesis of qualitative studies. J Cardiovasc Nurs. 2011;26(5):386–394. doi: 10.1097/JCN.0b013e3182076a69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 43.Lesman-Leegte I, Jaarsma T, Coyne JC, Hillege HL, Van Veldhuisen DJ, Sanderman R. Quality of life and depressive symptoms in the elderly: A comparison between patients with heart failure and age- and gender-matched community controls. J Card Fail. 2009;15(1):17–23. doi: 10.1016/j.cardfail.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Foebel AD, Hirdes JP, Heckman GA. Caregiver status affects medication adherence among older home care clients with heart failure. Aging Clin Exp Res. 2012;24(6):718–721. doi: 10.3275/8475. [DOI] [PubMed] [Google Scholar]
- 45.Dunbar SB, Clark PC, Reilly CM, et al. A trial of family partnership and education interventions in heart failure. J Card Fail. 2013;19(12):829–841. doi: 10.1016/j.cardfail.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung ML, Lennie TA, Mudd-Martin G, Moser DK. Adherence to a low-sodium diet in patients with heart failure is best when family members also follow the diet: A multicenter observational study. J Cardiovasc Nurs. 2015;30(1):44–50. doi: 10.1097/JCN.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindenauer PK, Remus D, Roman S, et al. Public reporting and pay for performance in hospital quality improvement. NEJM. 2007;356(5):486–496. doi: 10.1056/NEJMsa064964. [DOI] [PubMed] [Google Scholar]
- 48.Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure-associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61(12):1259–1267. doi: 10.1016/j.jacc.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heidenreich PA, Sahay A, Kapoor JR, Pham MX, Massie B. Divergent trends in survival and readmission following a hospitalization for heart failure in the Veterans Affairs health care system 2002 to 2006. J Am Coll Cardiol. 2010;56(5):362–368. doi: 10.1016/j.jacc.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 50.Allen JK, Dennison CR. Randomized trials of nursing interventions for secondary prevention in patients with coronary artery disease and heart failure: systematic review. J Cardiovasc Nurs. 2010;25(3):207–220. doi: 10.1097/JCN.0b013e3181cc79be. [DOI] [PubMed] [Google Scholar]
- 51.Rahimi K, Bennett D, Conrad N, et al. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail. 2014;2(5):440–446. doi: 10.1016/j.jchf.2014.04.008. [DOI] [PubMed] [Google Scholar]