Abstract
The lethality of infection by Bacillus anthracis is largely due to its plasmid-encoded toxins, which consist of a carrier protein, the protective antigen (PA), in combination with either the lethal-factor or edema-factor moiety. During B. anthracis infections, PA secreted by bacteria binds to membrane receptors of susceptible cells, is cleaved proteolytically, attaches to lethal factor or edema factor, undergoes oligomerization and internalization, and transports its toxin partners to acidic endosomes where they are released into the cytosol. To identify specific host functions that mediate these events, we used RNA encoded by a lentivirus-based library of ≈40,000 human ESTs to inactivate chromosomal genes in a human cell population, and we isolated clones that survived PA-dependent toxin-induced death. This phenotypic screen and subsequent analysis identified ARAP3, which is a phosphoinositide-binding protein implicated previously in membrane vesicle trafficking and cytoskeletal organization, as a mammalian host-cell gene that is essential for normal anthrax toxicity. ARAP3 deficiency produced by antisense expression of an ARAP3 EST impaired entry of PA and its bound toxigenic moieties into both human and mouse cells, resulting in reduced toxin sensitivity. Our results demonstrate the usefulness of antisense EST libraries for global chromosomal gene inactivation, establish the practicality of loss-of-function phenotypic screens for the identification of genomic loci required for pathogen effects in mammalian cells, and reveal an important role for ARAP3 in cellular internalization of anthrax toxin.
Keywords: antisense, endocytosis, pathogen
Whereas efforts at modulating the propagation and pathogenicity of microorganisms commonly have been directed at the pathogens, there is increasing recognition that host-gene products essential for these events may also be potential targets for countermeasures against pathogenic microbes. Earlier work (e.g., refs. 1–4) has resulted in the identification of a limited number of such host-gene products, largely from studies of the effects of individual pathogens or toxins on targeted cells. However, the possible applicability of direct phenotype-based screens for the identification of host genes that mediate or modulate pathogen or toxin functions has been suggested by evidence that the mammalian cell gene tsg101, which was identified initially in a tumorigenesis-based genetic screen (5), can also affect the release of budding viruses (6, 7).
Bacillus anthracis, a Gram-positive and spore-forming bacterium, poses a major public health threat from its potential use as an agent of bioterrorism (8–10). The lethality of infection by the bacterium largely is due to its toxin, which consists of three plasmid-encoded protein moieties: protective antigen (PA), lethal factor (LF), and edema factor (EF) (11–13). Acting as a carrier protein, PA (83 kDa) binds its cognate receptor on the plasma membrane of susceptible cells and is cleaved subsequently by a furin-like protease (2); the larger of the two cleavage products, PA63, remains bound to the cell surface where it oligomerizes to form a ring-shaped heptamer. Internalization of this hepatmer transports the PA-bound EF and LF cargo into acidic endosomes, where LF and EFs are released into the cytosol to elicit cytotoxicity. Although two plasma membrane proteins that can act as receptors for PA have been identified (3, 4), little is known about the host proteins that are required for later steps in the toxigenic pathway.
We sought to develop an approach for the de novo identification of cellular genes that affect the lethality of anthrax toxin, and to do this, we designed a gene-inactivation approach that uses a library of EST–mRNA segments that are used commonly for gene mapping and the construction of DNA microarrays. Because collections of ESTs contain defined sequences that are represented equally and mostly unique, we reasoned that EST-based libraries would be useful for gene inactivation at a genome-wide level. By using RNAs encoded by EST sequences to inactivate corresponding genes in a population of human cells, together with loss-of-function genetic screening of this population for toxin resistance, we identified ARAP3 as a cellular gene affecting susceptibility to anthrax toxin. Here, we show that ARPA3 deficiency results in defective internalization of PA and consequent resistance to anthrax toxicity. Our results demonstrate the usefulness of EST libraries for global gene inactivation in mammalian cells and reveal ARAP3 as a potential target for countermeasures against the lethal effects of anthrax toxin.
Materials and Methods
pLEST Vector. We constructed the EST expression vector pLEST by using a parental vector (pRRLsinPPT.CMV.MCS.Wpre, kindly provided by L. Naldini, University of Torino Medical School, Candiolo, Italy), which has been used for gene therapy (14). We replaced the CMV promoter in the original vector backbone with a fused DNA fragment containing a neomycin-resistance expression cassette and a tetracycline-regulated tetracycline-responsive element (TRE)-CMV promoter. We obtained the neo cassette by SalI and BamHI digestion from the pCDNA-neo vector (Clontech) and the TRE-CMV promoter by XhoI and BamHI digestion from pRevTRE (Clontech). The neo cassette was placed in an orientation opposite to the direction of lentiviral gene transcription to prevent truncation of viral genomic RNA transcripts by the neo mRNA termination signal. Detailed cloning information and a vector map are available upon request.
EST Library Construction. We obtained a human EST collection (Invitrogen) containing DNAs of ≈40,000 sequence-verified ESTs from the IMAGE Consortium. We removed ≈100 ng of DNA from each sample, pooled these DNAs into 96 subfractions that each contained 417 ESTs, and amplified the pooled EST DNA by PCR (18 cycles of 95°C for 30 sec, 55°C for 1 min, and 72°C for 2 min; Hot-start, Qiagen, Valencia, CA). We used the following primers: ESTF_NheI, 5′-TCTGCTAGCCACACAGGAAACAGCTATG; and ESTR_NheI, 5′-TCTGCTAGCTTGTAAAACGACGGCCAGTG. The PCR products from the 96 sub-EST fractions were collected into 10 final groups, digested with NheI, and cloned by using the pLEST vector. We introduced the ligated DNA mixtures into XL2 blue Super-competent bacteria cells (Stratagene) and transferred the transformation mixtures into liquid LB medium containing ampicillin. A small fraction of the mixture was removed to estimate the size of the library (i.e., number of independent clones), and 3 ml of the culture was frozen as stock. The remaining portion of the culture was used for DNA preparation (Maxi DNA kit, Qiagen). Before carrying out the procedures described above, the ability of Escherichia coli libraries containing a collection of human ESTs to maintain the initial EST representation during library construction was estimated in a pilot experiment by using small subpool containing 100 ESTs. Sequencing of EST inserts from 20 randomly selected individual pLEST-containing bacterial clones after amplification of the subpool revealed one repeat sequence among this population.
Genomic DNA Extraction and PCR. We isolated genomic DNA from 1–2 million cultured cells of individual clones by using the Gentra genomic-DNA-extraction kit (Gentra Systems). Genomic DNA usually was dissolved in 50 μl of the DNA-hydration buffer. PCR-amplification of the EST insert used the following primers: ESTF, 5′-CACACAGGAAACAGCTATG; and ESTR, 5′-TTGTAAAACGACGGCCAGTG. Gel-purified PCR products were sequenced by using either one of the EST primers. To determine the orientation of the inserted EST, we performed genomic PCR by using one of the EST primers and Lenti3 primer (5′-TGTTGCTCCTTTTACGCTATG), which is located 3′ of the EST insert in the pLEST vector.
Mammalian Cell Culture and Transfection. We maintained the prostate cancer cell line M2182 (kindly provided by J. L. Ware, Medical College of Virginia, Richmond) in RPMI 1640 medium (Invitrogen) by using supplements as described in ref. 15. We cultured the Raw 264.7 mouse macrophage and 293T cell lines in DMEM (Invitrogen) containing 10% FBS. We performed DNA transfections with Lipofectamine 2000 (Invitrogen) or FuGene6 (Roche) according to the manufacturer's recommended protocols.
Lentivirus Production and Infection. We produced lentivirus by transient transfection of 293T cells (calcium phosphate precipitation method) by using library DNA along with DNAs of packaging and VSVG envelope constructs as described (14). Cells were supplied with fresh medium 24 h after transfection, and virus-containing supernatant collected 24 h after this medium change was filtered through a 0.22-μm low-protein binding filter (Millipore). Infection of cells by the filtered lentivirus was carried out in suspensions containing polybrene at 37°C for 6–18 h; selection for virus-infected cells was carried out by adding the antibiotic G418 (Invitrogen) 48 h after the start of infection (the G418 dosage was 350 μg/ml for M2182 cells and 500 μg/ml for Raw 264.7 cells). G418-resistant clones were pooled 10–14 d later. Library size was estimated by counting the number of independent G418-resistant clones on each plate before the pooling.
Western Blotting. Rabbit polyclonal anti-PA antibody (1:1,000 dilution) and goat polyclonal anti-ARAP3 antibodies (1:1,000 dilution) were kindly provided by S. Leppla (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda) and P. Hawkins (The Babraham Institute, Cambridge, United Kingdom), respectively. Mouse anti-tubulin mAb and horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology. Western blotting was performed essentially as described (16). Chemiluminescence of Western blot bands was quantitated by using a Versadoc 1000 instrument (Bio-Rad).
Toxin Treatment. PA and LF were purchased from List Biological Laboratories (Campbell, CA). FR59 was a gift from S. Leppla. We exposed cells to toxins for 48 h unless otherwise indicated; we used 50 ng/ml PA plus 50 ng/ml FP59 to treat M2182 cells. We used 500 ng/ml PA plus 500 ng/ml LF to constitute the native anthrax toxin in experiments employing Raw 264.7 cells. After toxin treatment, cells were washed with PBS and cultured in fresh growth medium for up to 2 weeks to identify surviving clones or for 1 d before testing in 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assays.
MTT Viability Assay. Cells were seeded and treated the next day with the indicated amount of toxin. After incubation at 37°C (2 d for M2182 cells, and 3 h for Raw 264.7 cells), cells were washed, supplied with fresh medium, and cultured for additional 24 h. We then added 10 μl of MTT (Sigma) freshly prepared at 10 mg/ml in PBS to cells, incubated the cells at 37°C for 2 h, removed the supernatant, added 50 μl of lysis buffer (10% SDS/0.01 M HCl), and continued incubation at 37°C for 10 min. We added 200 μl of PBS to cell lysates, and absorbance readings at 570 nm (Tecan Technologies, Research Triangle Park, NC) were obtained immediately.
Assays for Processing of PA. We performed these assays according to a protocol in ref. 17. To assess binding of PA to the cell surface, cells plated 1 d previously at ≈70–80% confluence were cooled to room temperature for 15 min, washed with PBS once, and incubated with 1 μg/ml PA in binding buffer (DMEM without carbonate/25 mM Hepes/50 μg/ml gentamycin/0.5 mg/ml BSA, pH 7.4) at 4°C for 2 h. We then washed the cells with cold PBS four to five times and disrupted them in lysis buffer (150 mM NaCl/50 mM Tris·HCl, pH 7.5/0.5% Nonidet P-40). Lysates were examined by Western blotting using anti-PA antibody and anti-tubulin antibody as probes. For PA internalization, we treated cells at 70–80% confluence with 1 μg/ml PA at 37°C for 30 min, rinsed the cells with cold PBS once, and trypsinized and washed the cells with PBS three times. Cellular lysates were made and examined by Western blotting as indicated above.
Fluorescence Confocal Microscopy. PA protein was first labeled with Alexa Fluor 488 by using the A-10235 protein-labeling kit (Molecular Probes). The potency of PA after labeling proved to be retained, as determined by MTT assay. Immunostaining was performed as follows. In brief, cells were grown on cover slips, incubated with or without 0.5 μg/ml PA-Alexa 488 for 30 min at 4°C for PA-binding analysis and at 37°C for PA-processing analysis, washed with PBS for three times, fixed in 4% paraformaldehyde, and permeabilized by addition of 0.2% Triton X-100. The cells were then mounted onto slides and examined by using an LSM confocal microscope (Zeiss).
Results
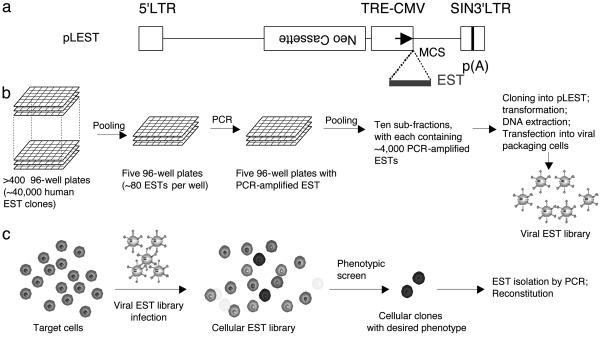
Construction of Lentivirus-Based Antisense EST Libraries. To enable efficient and controllable expression of ESTs, we constructed the lentivirus-based expression vector pLEST. A schematic diagram of the vector is shown in Fig. 1a. The backbone of pLEST is derived from lentiviral vector pRRLsinPPT.CMV.MCS.Wpre (14) but lacks its constitutive promoter. Instead, pLEST contains a TRE-regulated CMV promoter, allowing tetracycline-regulated gene transcription of ESTs introduced into the vector. pLEST also carries a neomycin (neo)-resistance cassette, which confers G418 drug resistance in mammalian cells and, thus, can act as a selectable marker for stable integration of pLEST into the chromosomes of vector-infected cells. The neo cassette is placed in an orientation opposite to the direction of transcription of the RNA that comprises the lentiviral genome so that the viral genome transcript will not be terminated prematurely. In addition, pLEST contains a multiple cloning site (MCS) for insertion of ESTs.
Fig. 1.
Construction and use of lentivirus-based EST expression vector and derived libraries. (a) Schematic diagram of the lentiviral expression vector pLEST. The backbone of pLEST was derived from a lentiviral gene therapy vector pRRLsinPPT.CMV.MCS.Wpre (14). The CMV promoter in the original vector was replaced by a tetracycline controllable promoter (TRE-CMV). A neomycin-resistance expression cassette (Neo) that was used as a selectable marker was placed in an orientation opposite to the direction of lentivirus gene transcription. A multiple cloning site (MCS) 3′ to the TRE-CMV promoter allows subsequent cloning and transcription of EST inserts. p(A), polyadenylation signal. (b) Procedure for construction of pLEST-based EST libraries. See Materials and Methods for details. (c) General scheme for EST library-screening process.
By using pLEST, we constructed a library of lentiviruses that express ≈40,000 previously cloned ESTs representing ≈28,000 unique human genes (Fig. 1b). Because the EST sequences were inserted bidirectionally in the expression vector, we anticipated that the lentivirus-based EST library would be capable of inactivating complementary mRNAs by antisense mechanisms and, possibly, also of interfering with the functions of some proteins by the production of dominant-negative peptide fragments encoded by ESTs transcribed in the sense direction.
Isolation of Cellular Clones Resistant to PA-Dependent Toxicity. The genetic screen that we used was designed to identify human cell clones that show reduced toxin sensitivity after infection with the lentivirus-based EST library described above (Fig. 1c). A human prostate cancer cell line M2182 (15) that was engineered to express the tetracycline-dependent transcriptional activator (tTA) was infected with this library, yielding approximately 1 million independent G418-resistant clones. Our initial screenings used a hybrid toxin consisting of PA and a recombinant cytotoxin FP59; FP59 is a fusion protein containing the N-terminal PA-binding domain of LF and the ADP-ribosylation domain of Pseudomonas aeruginosa exotoxin A (18). Because the lethality of FP59 requires PA-mediated cellular entry of the exotoxin component, we anticipated that survivors would include clones in which this function of PA is defective. After exposure of the M2182 cellular EST library to PA plus FP59, 20 surviving cell colonies were observed, whereas fewer than five survivors were present in similarly sized control populations infected with a lentivirus vector lacking EST inserts. Retesting of survivors from the EST-expressing population indicated maintenance of toxin resistance in 15 of the 20 EST-infected isolates.
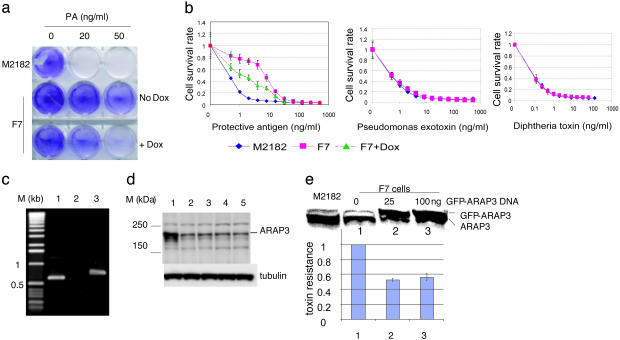
Three of the PA/FP59-resistant clones, including one that we designated as F7, showed decreased resistance in the presence of doxycycline, which is a tetracycline analog that down-regulates the TRE-CMV promoter (Fig. 2 a and b Left). Reversal of the resistance phenotype was incomplete, possibly because repression of the promoter is only partial (cf., ref. 19). To determine the specificity of the toxin-resistance phenotype in these clones, we performed MTT cell-viability assays by using serially diluted toxins. Although all three tetracycline-reversible clones were resistant to the PA/FP59 hybrid toxin, only clone (F7) exhibited a phenotype specific for PA/FP59; the clone F7 cells were as sensitive as naïve wild-type cells to native Pseudomonas exotoxin and diphtheria toxin, neither of which depends on PA for cellular entry (Fig. 2b). These results suggested that the decreased PA/FP59 toxin sensitivity observed for clone F7 results from interference with functions specifically mediated by the PA component of the hybrid toxin.
Fig. 2.
Characterization of cell clone F7. (a) Plate assay for resistance to PA/FP59 toxin. M2182-tTA cells and clone F7 cells seeded in a six-well plate at 2 × 105 cells per well were treated with the indicated dosage of PA plus a constant amount of FP59 (50 ng/ml) for 48 h in the presence or absence of doxycycline. After removal of toxins by replacement of culture media, cells were allowed to grow for 2 d and were then stained with crystal violet. (b) Specificity of toxin resistance in F7 cells. M2182-tTA cells or clone F7 cells plated at 1 × 104 cells per well in 96-well plates were treated with serially diluted toxins (PA/FP59, Pseudomonas exotoxin, or diphtheria toxin) for 2 d. MTT cell-viability assays were performed, and data were plotted as shown. Each data point represents an average of eight wells, with error bars showing standard deviations. (c) PCR analysis of genomic DNA from clone F7. PCR was performed by using clone F7 genomic DNA and different primer sets. Lanes: 1, ESTF plus ESTR; 2, ESTF plus Lenti3; 3, ESTR plus Lenti3. (d) Western blot analysis of lysates of different cell populations. Lanes: 1, naïve M2182-tTA cells; 2, clone F7; 3–5, three clonal isolates of reconstituted M2182-tTA cells expressing ARAP3 antisense EST. (e) Effect of transient overexpression of GFP-ARAP3 in clone F7 cells. Two sets of clone F7 cells seeded at 2 × 105 cells per well (six-well plate) were transfected with indicated amount of GFP-ARAP3 construct. (Upper) Lysates were made from M2182-tTA cells (as control) and one set of transfected cells and used for anti-ARAP3 Western blot analysis. (Lower) The other set of transfected F7 cells was tested for resistance to PA/FP59 by MTT assays. Resistance of F7 cells transfected with an empty vector was assigned a value of 1. The data in each bar represent the average data from three experiments. Bars 1–3 correspond to lanes 1–3.
Clone F7 Expresses Antisense ARAP3 EST and Contains Reduced ARAP3 Protein. The EST expressed in clone F7 was amplified by PCR from genomic DNA using two primers complementary to vector sequences bracketing EST inserts. Sequence analysis of the single PCR product that we obtained (Fig. 2c, lane 1) indicated that it corresponds to a segment (nucleotides 998–1,458; IMAGE clone no. 809620) of cDNA encoding ARAP3, a recently described phosphoinositide-binding protein that includes a GTPase-activating protein (GAP) domain for Arf6-GTPase and another GAP domain for Rho GTPase (20, 21). ARAP3 has been shown to be a specific phosphoinositide-stimulated Arf6 GAP, and both of its GAP domains play a role in mediating PI3K-dependent rearrangements in the cell cytoskeleton and cell shape (20). Further analysis of the PCR product amplified from F7 indicated that the ARAP3 EST in this toxin-resistant cell clone was oriented in antisense direction relative to the TRE-CMV promoter (Fig. 2c, lanes 2 and 3). Western blotting quantitated by chemiluminescence densitometry showed that ARAP3 protein expression in F7 was reduced to ≈30% of the level observed in the parental M2182 cell line (Fig. 2d, lanes 1 and 2). Transient overexpression of an ARAP3 protein fused at the N terminus with GFP partially reversed the increased resistance of F7 cells to PA/FP59 (Fig. 2e).
Expression of Antisense ARAP3 EST in Naïve Cells Recapitulates Toxin-Resistance Phenotype. The role of ARAP3 deficiency in toxin resistance was confirmed by experiments in which the ARAP3 EST was cloned in antisense orientation in the pLEST vector and introduced into naïve M2182-tTA cells. Whereas control cells infected with the vector virus alone or expressing the ARAP3 EST in the sense direction were killed efficiently by PA/FP59, M2182 cells transcribing this EST in the antisense direction had reduced toxin susceptibility (Fig. 3a). Three randomly picked toxin-resistant clones that were isolated from this reconstitution experiment showed ≈70% reduction in ARAP3 protein (Fig. 2d, lanes 3–5). We were unable to reduce the ARAP3 protein to a level that was comparable with that in F7 cells by using stable small interfering RNA in naïve M2182 cells. Although we obtained partial reversion of toxin resistance in clone F7 by transient overexpression of ARAP3 (Fig. 2e), we were unable to establish stable F7-derived cell lines that expressed ARAP3 to a level that was sufficient to overcome the effects of antisense-mediated inhibition of ARAP3 expression fully.
Fig. 3.
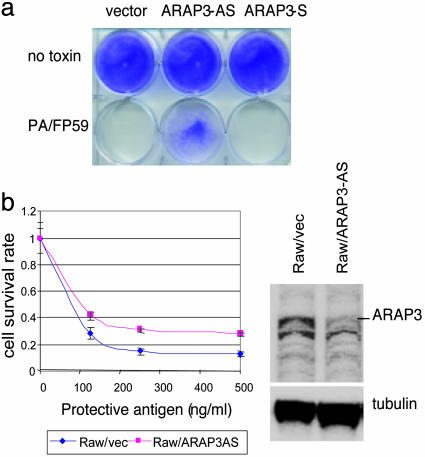
Toxin insensitivity resulting from antisense ARAP3 EST expression in naive cells. (a) Effects of PA/FP59 toxin on M2182-tTA cells infected with lentivirus containing empty pLEST vector, ARAP3 EST in antisense orientation (ARAP3-AS) or ARAP3 EST in sense orientation (APRAP3-S). (b) Effect of ARAP3 antisense EST expression on susceptibility of mouse macrophage cells to anthrax toxin. Raw 264.7/tTA cells were infected with the lentivirus containing pLEST vector alone or with a lentivirus expressing antisense ARAP3 EST (ARAP3-AS). Pooled G418-resistant cells were used for MTT assay (3 h of toxin treatment, LF fixed at 500 ng/ml; Left) and Western blot analysis (Right). The values shown in MTT assay represent the fraction of surviving cells.
In vivo, macrophages are one of the targets of anthrax infection, and in culture, they are susceptible to killing by anthrax lethal toxin (LeTx) formed by the interaction of PA with LF (22, 23). Paralleling its role in the internalization of FP59 in the experiments described above, PA mediates entry of LF into macrophages. We introduced the tTA element into the mouse macrophage cell line Raw 264.7, infected these cells with a lentivirus expressing the ARAP3 human EST in antisense orientation, and investigated both macrophage susceptibility to LeTx and the effect of these manipulations on the cellular level of ARAP3 protein. Antisense expression of the human EST sequence, which has 92% identity to the corresponding segment of mouse ARAP3 transcript resulted in ≈60% reduction of ARAP3 protein and ≈2-fold enhancement of cellular resistance to LeTx treatment as determined by MTT assay (Fig. 3b).
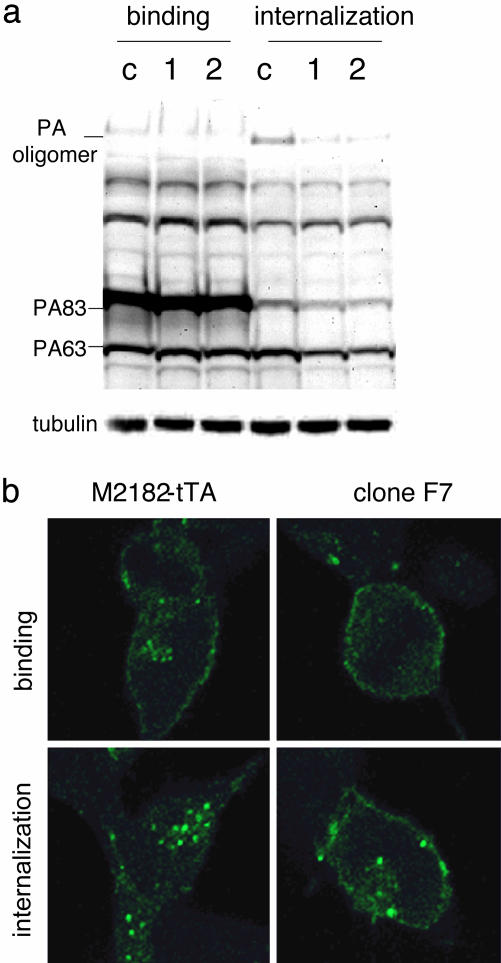
ARAP3-Deficient Cells Exhibit Impaired PA Internalization. Collectively, the above results argue strongly that the toxin-resistance phenotype produced by ARAP3 deficiency results from impaired functioning of PA, which in our experiments was required as a carrier by both FP59 and LF. To evaluate this interpretation further and to understand the mechanism(s) underlying our findings, we investigated the effects of ARAP3 deficiency on certain parameters of PA function (membrane binding, cleavage, and internalization of PA oligomers). We observed no detectable alteration of PA membrane binding in either clone F7 or reconstituted M2182 cells expressing antisense RNA to the ARAP3 EST, as indicated by the intensity of the unprocessed 83-kDa PA band (Fig. 4a). However, in ARAP3-deficient cells that were incubated with PA at 37°C, which ordinarily enables internalization of oligomers of the cleaved 63-kDa PA subunit (17), the intracellular level of PA oligomers was reduced to approximately one-third of normal (Fig. 4a), as determined by densitometry analysis of the Western blot. Defective internalization of PA in F7 cells was confirmed by fluorescence microscopy using FITC-labeled PA (Fig. 4b), whereas nearly all of the PA-associated fluorescence signal entered the cytoplasm in naïve cells and was detectable in the form of cytosolic aggregates after a 30-min period of incubation at 37°C, consistent with the results given in ref. 24, more than one-half of the PA signal remained on the cell surface in cells of clone F7.
Fig. 4.
PA-processing assay in ARAP3-deficient cells. (a) Western blot analysis of bound and internalized PA. PA binding and internalization assays were done as described in Material and Methods. Lanes: c, naïve M2182-tTA cells; 1, F7 cells; 2, pooled M2182-tTA cells expressing ARAP3 antisense EST. PA83 is the full-length form of PA (83 kDa), and PA63 is the furin-cleaved form of PA (63 kDa); the oligomer represents the homoheptamer of PA63. Tubulin Western blotting was used as a control for equal loading. (b) Confocal microscopy imaging. Binding and internalization of Alexa Fluor 488-labeled PA83 were examined as described in Materials and Methods.
Discussion
Identification of the host factors that are required for the propagation or actions of microbial pathogens or toxins is essential for an understanding of pathogenesis and may also provide useful information for the development of host-oriented therapies for diseases of microbial etiology. Phenotypic screens based on loss or gain of genetic functions that affect toxin sensitivity offer a potential approach for the systematic identification of such host factors. This article describes the development of an EST-based approach for global inactivation of host genes and the use of this approach to identify ARAP3 as a cellular gene affecting the internalization and toxicity of anthrax toxin. Our results suggest that EST libraries would be useful in other loss-of-function genetic screens.
Other approaches that have been developed in recent years for nondirected inactivation of gene function in mammalian cells include infection with vectors containing libraries of cDNAs expressed in antisense orientation (25, 26), the use of retrovirus constructs to insert a regulated promoter and generate antisense RNA at random locations in mammalian cell chromosomes (5), the construction of ribozyme libraries (27), and most recently, the production of libraries containing collections of small interfering RNAs (28, 29). Potential advantages of the EST-based gene-inactivation approach described here are the predefined composition of and approximately equal representation of genes in EST libraries, in addition to the opportunity for genome-wide coverage by a single library prepared from already available ESTs corresponding to variably spliced transcripts from multiple tissues. ESTs producing a phenotype of interest can be identified rapidly by using one-step PCR amplification of genomic DNA from the functionally altered cells. Microarray analysis of gene expression in several independent clones of cells targeted by our antisense EST libraries showed no detectable evidence of induction of IFN-response genes (Q.L. and S.N.C., unpublished data).
There is ample evidence that PA and its associated LF or EF moieties are delivered into affected cells by endocytic vesicles (12, 24, 30, 31). The findings reported here indicate that ARAP3 function is required for this process to occur normally. Given the previously discovered ability of ARAP3 to regulate Arf6 GTPase (20), and the demonstrated role of Arf (ADP ribosylation factor) proteins in endocytic trafficking from cell surfaces (32–34), we suggest that the effects of ARAP3 depletion on the internalization of toxigenic cargo bound to PA and, consequently, to cell surface receptors may be mediated through Arf-related pathways. In this context, we note that independent EST-based loss-of-function screens recently carried out in our laboratory have identified two other members of the Arf GTPase family as cellular factors that affect the lethality of anthrax toxin (unpublished data).
Acknowledgments
We thank Drs. P. Hawkins, S. Krugmann (The Babraham Institute, Cambridge, U.K.), and S. Leppla for kind gifts of reagents and helpful discussion, and L. Shapiro and A. Friedlander for comments on the manuscript. This work was supported by a grant from the Defense Advanced Research Projects Agency (DARPA) of the U.S. Department of Defense (to S.N.C.).
Abbreviations: PA, protective antigen; LF, lethal factor; EF, edema factor; TRE, tetracycline-responsive element; tTA, tetracycline-dependent transcriptional activator; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide.
References
- 1.Kounnas, M. Z., Morris, R. E., Thompson, M. R., FitzGerald, D. J., Strickland, D. K. & Saelinger, C. B. (1992) J. Biol. Chem. 267, 12420–12423. [PubMed] [Google Scholar]
- 2.Klimpel, K. R., Molloy, S. S., Thomas, G. & Leppla, S. H. (1992) Proc. Natl. Acad. Sci. USA 89, 10277–10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley, K. A., Mogridge, J., Mourez, M., Collier, R. J. & Young, J. A. (2001) Nature 414, 225–229. [DOI] [PubMed] [Google Scholar]
- 4.Scobie, H. M., Rainey, G. J., Bradley, K. A. & Young, J. A. (2003) Proc. Natl. Acad. Sci. USA 100, 5170–5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li, L. & Cohen, S. N. (1996) Cell 85, 319–329. [DOI] [PubMed] [Google Scholar]
- 6.Garrus, J. E., von Schwedler, U. K., Pornillos, O. W., Morham, S. G., Zavitz, K. H., Wang, H. E., Wettstein, D. A., Stray, K. M., Cote, M., Rich, R. L., et al. (2001) Cell 107, 55–65. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Serrano, J., Zang, T. & Bieniasz, P. D. (2001) Nat. Med. 7, 1313–1319. [DOI] [PubMed] [Google Scholar]
- 8.Dixon, T. C., Meselson, M., Guillemin, J. & Hanna, P. C. (1999) N. Engl. J. Med. 341, 815–826. [DOI] [PubMed] [Google Scholar]
- 9.Mock, M. & Fouet, A. (2001) Annu. Rev. Microbiol. 55, 647–671. [DOI] [PubMed] [Google Scholar]
- 10.Lane, H. C., Montagne, J. L. & Fauci, A. S. (2001) Nat. Med. 7, 1271–1273. [DOI] [PubMed] [Google Scholar]
- 11.Smith, H. (2000) Trends Microbiol. 8, 199–200. [DOI] [PubMed] [Google Scholar]
- 12.Collier, R. J. & Young, J. A. (2003) Annu. Rev. Cell Dev. Biol. 19, 45–70. [DOI] [PubMed] [Google Scholar]
- 13.Moayeri, M. & Leppla, S. H. (2004) Curr. Opin. Microbiol. 7, 19–24. [DOI] [PubMed] [Google Scholar]
- 14.Follenzi, A., Ailles, L. E., Bakovic, S., Geuna, M. & Naldini, L. (2000) Nat. Genet. 25, 217–222. [DOI] [PubMed] [Google Scholar]
- 15.Jackson-Cook, C., Bae, V., Edelman, W., Brothman, A. & Ware, J. (1996) Cancer Genet. Cytogenet. 87, 14–23. [DOI] [PubMed] [Google Scholar]
- 16.Harlow, E. & Lane, D. (1999) Using Antibodies: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 17.Liu, S. & Leppla, S. H. (2003) J. Biol. Chem. 278, 5227–5234. [DOI] [PubMed] [Google Scholar]
- 18.Arora, N., Klimpel, K. R., Singh, Y. & Leppla, S. H. (1992) J. Biol. Chem. 267, 15542–15548. [PubMed] [Google Scholar]
- 19.Zhu, Z., Ma, B., Homer, R. J., Zheng, T. & Elias, J. A. (2001) J. Biol. Chem. 276, 25222–25229. [DOI] [PubMed] [Google Scholar]
- 20.Krugmann, S., Anderson, K. E., Ridley, S. H., Risso, N., McGregor, A., Coadwell, J., Davidson, K., Eguinoa, A., Ellson, C. D., Lipp, P., et al. (2002) Mol. Cell 9, 95–108. [DOI] [PubMed] [Google Scholar]
- 21.Santy, L. C. & Casanova, J. E. (2002) Curr. Biol. 12, R360–R362. [DOI] [PubMed] [Google Scholar]
- 22.Weinrauch, Y. & Zychlinsky, A. (1999) Annu. Rev. Microbiol. 53, 155–187. [DOI] [PubMed] [Google Scholar]
- 23.Dixon, T. C., Fadl, A. A., Koehler, T. M., Swanson, J. A. & Hanna, P. C. (2000) Cell Microbiol. 2, 453–463. [DOI] [PubMed] [Google Scholar]
- 24.Abrami, L., Liu, S., Cosson, P., Leppla, S. H. & van der Goot, F. G. (2003) J. Cell Biol. 160, 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gudkov, A. V., Kazarov, A. R., Thimmapaya, R., Axenovich, S. A., Mazo, I. A. & Roninson, I. B. (1994) Proc. Natl. Acad. Sci. USA 91, 3744–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimchi, A. (2003) Methods Mol. Biol. 222, 399–412. [DOI] [PubMed] [Google Scholar]
- 27.Pierce, M. L. & Ruffner, D. E. (1998) Nucleic Acids Res. 26, 5093–5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berns, K., Hijmans, E. M., Mullenders, J., Brummelkamp, T. R., Velds, A., Heimerikx, M., Kerkhoven, R. M., Madiredjo, M., Nijkamp, W., Weigelt, B., et al. (2004) Nature 428, 431–437. [DOI] [PubMed] [Google Scholar]
- 29.Paddison, P. J., Silva, J. M., Conklin, D. S., Schlabach, M., Li, M., Aruleba, S., Balija, V., O'Shaughnessy, A., Gnoj, L., Scobie, K., et al. (2004) Nature 428, 427–431. [DOI] [PubMed] [Google Scholar]
- 30.Bhatnagar, R. & Batra, S. (2001) Crit. Rev. Microbiol. 27, 167–200. [DOI] [PubMed] [Google Scholar]
- 31.Abrami, L., Lindsay, M., Parton, R. G., Leppla, S. H. & Van Der Goot, F. G. (2004) J. Cell Biol. 166, 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Randazzo, P. A., Nie, Z., Miura, K. & Hsu, V. W. (2000) Sci. STKE 2000, RE1. [DOI] [PubMed] [Google Scholar]
- 33.Donaldson, J. G. (2002) Methods Mol. Biol. 189, 191–198. [DOI] [PubMed] [Google Scholar]
- 34.Donaldson, J. G. (2003) J. Biol. Chem. 278, 41573–41576. [DOI] [PubMed] [Google Scholar]