Abstract
Background
Fixed combination calcipotriol as hydrate (Cal) 50 µg/g plus betamethasone as dipropionate (BD) 0.5 mg/g aerosol foam is an alcohol-free treatment for psoriasis. Betamethasone 17-valerate 2.25 mg (BV)-medicated plasters are recommended for treating psoriasis plaques localized in difficult-to-treat (DTT; elbow, knee, anterior face of the tibia) areas.
Objective
The aim of this study was to compare the efficacy of Cal/BD foam with BV-medicated plaster in patients with plaque psoriasis.
Methods
In this phase IIa, randomized, single-center, investigator-blinded, 4-week study, both Cal/BD foam and BV-medicated plaster were applied once daily to six test sites (three for each treatment). The primary efficacy endpoint was absolute change in total clinical score (TCS; sum of erythema, scaling, and infiltration); secondary endpoints were changes from baseline in each individual clinical score, ultrasonographic changes (total skin and echo-poor band thickness), and safety; and post hoc analysis was change from baseline in TCS on DTT areas.
Results
Thirty-five patients were included. Least-squares mean change in TCS from baseline was significantly greater for Cal/BD foam (−5.8) than BV-medicated plaster (−3.7; difference −2.2; 95% confidence interval −2.6 to −1.8; p < 0.001); greater changes for Cal/BD foam were observed from day 8 for each clinical sign. Absolute total skin and echo-poor band thickness change was significantly greater for Cal/BD foam than for BV-medicated plaster (both p < 0.001). Post hoc analyses showed that Cal/BD foam was significantly more effective than BV-medicated plaster on DTT areas after 4 weeks (p < 0.001), and both treatments were well tolerated.
Conclusion
Cal/BD foam demonstrated superior efficacy versus BV-medicated plasters, including on DTT areas, in patients with plaque psoriasis.
Clinical trial registration number: NCT02518048.
Key Points
| Fixed combination calcipotriol as hydrate (Cal) 50 µg/g plus betamethasone as dipropionate (BD) 0.5 mg/g aerosol foam and betamethasone 17-valerate 2.25 mg (BV)-medicated plasters are efficacious and safe topical treatments for plaque psoriasis; the latter is also recommended for treating plaques localized on difficult-to-treat (DTT; elbow, knee, anterior face of the tibia) areas. |
| Cal/BD aerosol foam demonstrated significantly greater improvement in clinical signs of psoriatic plaques compared with BV-medicated plasters. Furthermore, Cal/BD aerosol foam was significantly more effective than BV-medicated plasters on DTT areas, as shown in post hoc analyses. |
| Cal/BD aerosol foam may provide a more successful treatment outcome in patients with plaque psoriasis than some of the currently available topical therapies, regardless of plaque location. |
Introduction
Psoriasis vulgaris is an immune-mediated, chronic inflammatory disorder characterized by plaques of scaly, thickened skin [1–3]. The most common topical therapies for treating mild-to-moderate psoriasis vulgaris are corticosteroids and vitamin D3 analogs, which are used either alone or in combination [4, 5]. Fixed combination calcipotriol 50 µg/g (Cal) and betamethasone dipropionate 0.5 mg/g (BD) in ointment and gel formulations are established first-line topical treatments [6]. However, patient adherence to topical therapies remains an issue, mostly because of the time-consuming and inconvenient nature of daily treatment application [7, 8].
In order to address the different needs of patients, alternative topical formulations and methods of administration have been developed. Cal/BD aerosol foam (Enstilar®; LEO Pharma, Copenhagen, Denmark), an alcohol-free, paraffin-based formulation with emollient properties, was developed with the aim of addressing these needs [9–14]; this aerosol formulation becomes supersaturated when applied to the skin, leading to increased bioavailability and improved clinical efficacy [14]. A previous plaque test study demonstrated that Cal/BD aerosol foam had significantly greater efficacy over 4 weeks of treatment than Cal/BD ointment [12]. In the recent phase III, randomized PSO-ABLE study, Cal/BD aerosol foam demonstrated significantly greater efficacy at 4 weeks compared with 8 weeks of treatment with Cal/BD gel [15]. Betamethasone 17-valerate (BV) is a corticosteroid used in the treatment of psoriasis vulgaris and other inflammatory skin disorders. Potent BV-medicated plasters, containing 2.25 mg BV (Betesil® medicated plaster, manufactured by Altergon, Morra De Sanctis, Italy), are marketed as being suitable for plaques localized in difficult-to-treat (DTT) areas such as knees, elbows, and the anterior face of the tibia. The plaster can be trimmed to cover psoriatic plaques exactly and deliver the active ingredient to the affected area specifically.
The aim of this study was to compare the efficacy of Cal/BD aerosol foam with BV-medicated plasters using a psoriasis plaque test design modified from the method developed by Dumas and Scholtz [16].
Patients and Methods
Patients
Eligible patients (aged ≥18 years) with psoriasis vulgaris, with preferably three (or at least two) plaques on their arms, legs, and/or trunk, were recruited. In patients with three plaques, each plaque had to be large enough to accommodate two test sites, while patients with two plaques needed to have one plaque that could accommodate four test sites and another that could accommodate two test sites. Each test site had an area of 5 cm2, with at least 2 cm between the test sites. Patients were required to have stable psoriasis based on the difference in total clinical score (TCS; sum score of erythema, scaling, and infiltration for a particular test site) between screening and baseline visits (maximum 28 days), as well as plaques with a TCS of 4–9 (inclusive) and a score of ≥1 for each item. Patients with a change of more than one point in any of the clinical signs were excluded.
Patients receiving systemic treatment with biological therapies with a possible effect on psoriasis (etanercept within 4 weeks prior to randomization; adalimumab or infliximab within 8 weeks; ustekinumab within 16 weeks; other biological therapies within 4 weeks or five half-lives [whichever was longer]) were excluded from the study. Other exclusion criteria included systemic treatment (other than with biological therapy, e.g. retinoids, immunosuppressants) within 4 weeks prior to randomization; treatment with psoralen combined with ultraviolet A therapy within 4 weeks or ultraviolet B therapy within 2 weeks; potent or very potent (WHO group III–IV) corticosteroids within 4 weeks; WHO group I–II corticosteroids within 2 weeks, except if used for scalp and/or facial psoriasis treatment; any concomitant medical or dermatological disorders that might preclude accurate evaluation of psoriasis on test areas; use of emollients within 1 week; and initiation of, or expected changes to, concomitant medication that may affect psoriasis, such as β-blockers, antimalarial drugs, lithium and angiotensin-converting enzyme (ACE) inhibitors, within 2 weeks.
Study Design
This was a phase IIa, single-center, investigator-blinded, within-patient controlled, randomized, 4-week study (ClinicalTrials.gov identifier NCT02518048) conducted in France (Centre de Pharmacologie Clinique Appliquée à la Dermatologie, Nice, France). The protocol was approved by the relevant independent Ethics Committee and appropriate regulatory authorities. The study was performed in accordance with the Declaration of Helsinki and all patients provided written informed consent prior to enrolment.
Each patient received both Cal/BD aerosol foam and BV-medicated plaster once daily (6 days per week, excluding Sundays, for a total of 28 days) to enable intraindividual comparisons. Both treatments were applied by clinical personnel to a total of six test sites (three for each treatment) and, to ensure blinding, were later removed by someone other than the investigator. BV-medicated plasters were cut into test site size for application and maintained with hypoallergenic dressing. The amount of Cal/BD aerosol foam (50 mg) was sprayed onto test sites that were delimited by an adhesive ring, gently rubbed into the skin using a gloved finger, and covered with a non-occlusive gauze.
During the study, patients were not allowed to use any medication that could interfere with the trial results, including those requiring washout. Treatments that were permitted during the study included inhaled corticosteroids, neutral emollients on psoriasis plaques that were not treated in the trial, and WHO group I–II corticosteroids for the treatment of scalp and facial psoriasis.
Assessments
The primary efficacy endpoint was the absolute change in TCS of Cal/BD aerosol foam and BV-medicated plaster from baseline to the end of treatment (day 29). The severity of clinical signs (erythema, scaling, and infiltration) for each target plaque and test site was assessed by the investigator using a 7-point scale (range 0 [no evidence], 1.5 [mild-to-moderate] to 3.0 [severe]; TCS total range 0–9), and the absolute change in TCS was calculated from the mean TCS of the three test sites per treatment.
Secondary efficacy endpoints comparing Cal/BD aerosol foam and BV-medicated plaster included TCS change at each visit (i.e. days 4, 8, 11, 15, 18, 22, and 25) compared with baseline; change from baseline in clinical signs at each visit; and change in ultrasound measurements of total skin and echo-poor band thickness (mm) from baseline to end of treatment (day 29).
A post hoc analysis on the TCS data was conducted to compare the effect of the two treatments from baseline to end of treatment on DTT (elbow, knee, and anterior face of the tibia) and other areas (arm, trunk/back, and areas of the lower leg, except anterior).
Adverse events (AEs), with a focus on local cutaneous AEs, were also assessed throughout treatment to evaluate the safety and tolerability of Cal/BD aerosol foam and BV-medicated plaster. An AE was defined as any unfavorable and unintended sign, symptom, or disease temporally associated with the use of the investigated treatments, regardless of its cause. The severity of the AE was classified into mild, moderate, and severe categories according to the investigator or sub-investigator’s clinical judgment.
Statistical Analysis
Assuming the standard deviation (SD) for the difference in TCS of 2.0 and a mean difference of 0.75, a sample size of 33 patients and three within-subject replicates of each treatment was required to detect a difference between Cal/BD aerosol foam and BV-medicated plaster with 95% power. All statistical analyses were performed on the full analysis set (FAS), which comprised all randomized patients. The absolute changes in TCS (overall and for the post hoc analysis), total skin thickness, and echo-poor band thickness from baseline to the end of treatment were analyzed using a mixed-model analysis of variance (ANOVA), with treatment as the fixed effect and subject as the random effect. All significance tests were two-sided using the 5% significance level and 95% confidence intervals (CIs). Missing data were imputed using the method of last observation carried forward (LOCF). All data presented are LOCF and mean values are least-square means calculated from the ANOVA analysis.
Results
Patient Characteristics
Thirty-five patients were enrolled and randomized in the study between September and December 2015 (Fig. 1; Table 1). All but one patient completed the study (voluntary discontinuation).
Fig. 1.
CONSORT diagram. BD betamethasone as dipropionate 0.5 mg/g, Cal calcipotriol 50 µg/g, BV betamethasone 17-valerate 2.25 mg, CONSORT Consolidated Standards Of Reporting Trials
Table 1.
Patient demographics and baseline characteristics
| Characteristic | All patients (n = 35) |
|---|---|
| Mean age ± SD, years | 51.5 ± 12.9 |
| Males:females, n | 25:10 |
| Mean disease duration ± SD, years | 24.0 ± 12.9 |
| Mean TCSa ± SD | 6.6 ± 0.6 |
SD standard deviation, TCS total clinical score
aSum of three scores (erythema, scaling, lesional thickness)
Efficacy
Clinical Assessment
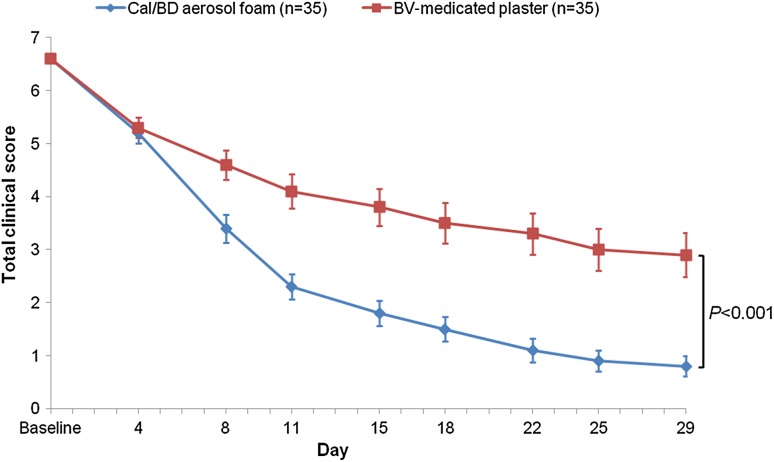
The mean (±SD) baseline TCS was 6.6 ± 0.6 (Table 1). At all visits after day 4, the change in TCS was greater at sites treated with Cal/BD aerosol foam compared with BV-medicated plaster (Fig. 2). The mean change in TCS from baseline to the end of treatment for Cal/BD aerosol foam was significantly greater (−5.8) than for BV-medicated plaster (−3.7; difference −2.2; 95% CI −2.6 to −1.8; p < 0.001).
Fig. 2.
Mean TCS and 95% CI from baseline to end of treatment (LOCF): FAS. The p value is calculated for the mean change in TCS from baseline to end of treatment for Cal/BD aerosol foam versus BV-medicated plaster. BD betamethasone as dipropionate 0.5 mg/g, BV betamethasone 17-valerate 2.25 mg, Cal calcipotriol 50 µg/g, CI confidence interval, FAS full analysis set, LOCF last observation carried forward, TCS total clinical score
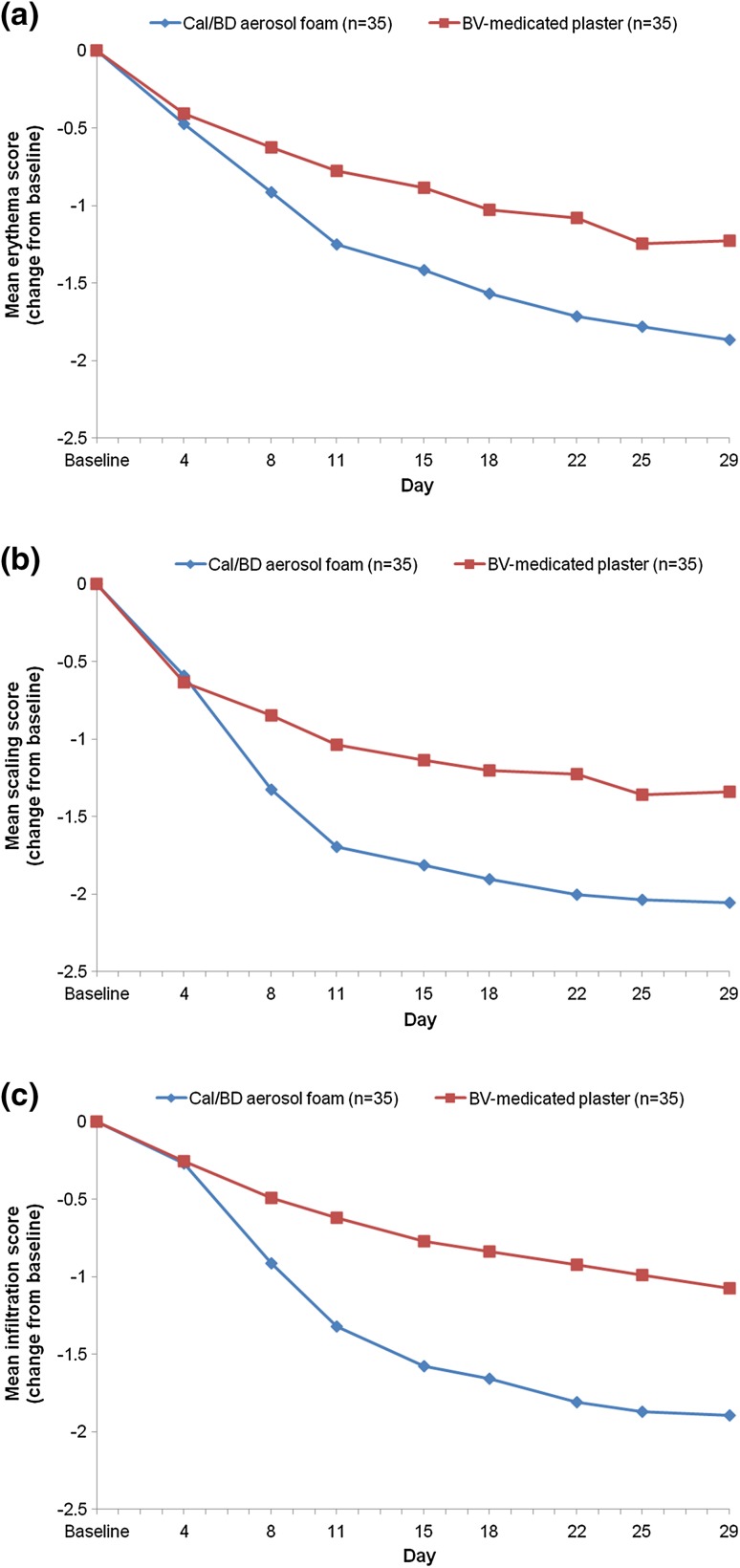
All three clinical signs improved over the course of the study in both groups. From day 8 to the end of treatment, a numerically larger change in mean TCS in each of the clinical signs (erythema, scaling, and infiltration) was observed in the Cal/BD aerosol foam group compared with the BV-medicated plaster group (Fig. 3).
Fig. 3.
Mean change in a erythema, b scaling, and c infiltration from baseline to the end of treatment (LOCF): FAS. BD betamethasone as dipropionate 0.5 mg/g, Cal calcipotriol 50 µg/g, BV betamethasone 17-valerate 2.25 mg, FAS full analysis set, LOCF last observation carried forward
Ultrasound Assessment
Mean change in total skin thickness from baseline to the end of treatment was significantly greater in the Cal/BD aerosol foam group (−1.0 mm) compared with BV-medicated plaster treatment (−0.6 mm; difference −0.4 mm; 95% CI −0.5 to −0.3; p < 0.001). A significantly larger decrease from baseline to the end of treatment in echo-poor band thickness was also observed with Cal/BD aerosol foam (−1.3 mm) compared with BV-medicated plaster (−0.7 mm; difference −0.6 mm; 95% CI −0.7 to −0.4; p < 0.001).
Difficult-to-Treat Areas
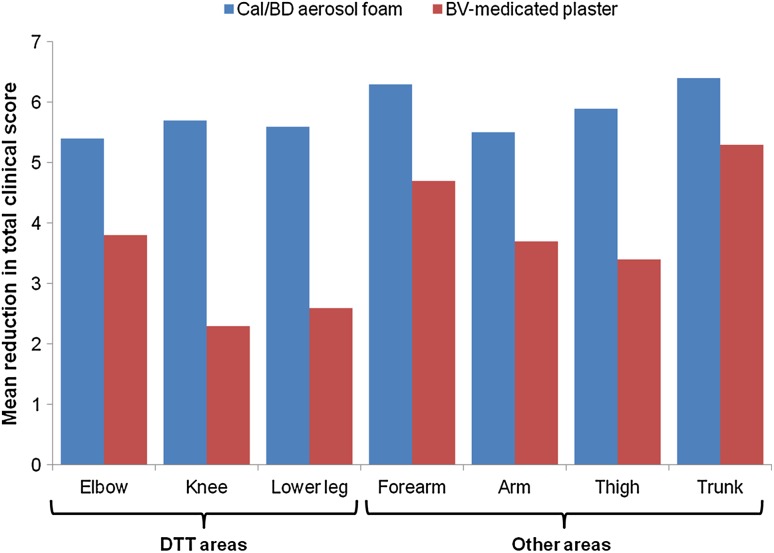
After 4 weeks of treatment, Cal/BD aerosol foam was more effective than BV-medicated plaster on all individual body areas assessed (Fig. 4). When assessing efficacy on DTT areas (i.e. elbow, knee, and anterior face of the tibia combined), treatment with Cal/BD aerosol foam led to significantly greater decreases in TCS compared with BV-medicated plaster at week 4 (mean change from baseline in TCS −5.5 vs. −3.4; difference −2.1; 95% CI −2.7 to −1.6; p < 0.001). Cal/BD aerosol foam was also significantly more efficacious on the other areas than BV-medicated plaster (mean change from baseline in TCS −6.2 vs. −4.4; difference −1.8; 95% CI −2.4 to −1.2; p < 0.001).
Fig. 4.
Mean reduction in TCS between baseline and week 4 on DTT and other areas (LOCF): FAS. All lower-leg sites were considered as DTT areas, except for six sites on two patients, which were not on the anterior face of the tibia. BD betamethasone as dipropionate 0.5 mg/g, Cal calcipotriol 50 µg/g, BV betamethasone 17-valerate 2.25 mg, DTT difficult-to-treat, FAS full analysis set, LOCF last observation carried forward, TCS total clinical score
Safety and Tolerability
A total of 15 patients (42.9%) experienced 20 AEs during the trial; none were severe. The most frequent AEs were headache (n = 8; 22.9%), influenza (n = 3; 8.6%), and oropharyngeal pain (n = 3; 8.6%). No cutaneous AEs were observed and no other AEs occurred in more than one patient. No deaths, serious AEs or other significant AEs were observed.
Discussion
Results from this plaque test study demonstrated that Cal/BD aerosol foam was significantly more efficacious, as shown by TCS and ultrasound skin thickness assessments, compared with BV-medicated plaster. The psoriasis plaque test is a well-established, safe, and relatively low-cost method for evaluating the efficacy of topical treatments in psoriasis [12, 16, 17]. As the method combines the assessment of TCS and ultrasound measurements of skin thickness, an accurate evaluation of treatment efficacy can be achieved [12, 16, 17]. Furthermore, the plaque test enables intraindividual comparisons and increases the probability of detecting clinically relevant differences with a limited sample size [18]. The TCS has been used in several plaque trials and has proven to be both reliable and useful for the evaluation of selected test sites [12, 16, 17].
In this study, the rate of improvement in TCS and the individual TCS components (i.e. erythema, scaling, and infiltration) was more rapid with Cal/BD aerosol foam than with BV-medicated plaster; a difference was observed early into treatment (from day 8) and was maintained throughout the study. The mean change in TCS and total skin thickness from baseline to day 29, and rapid onset of symptom improvements for areas treated with Cal/BD aerosol foam, was similar to that observed in a previous 4-week plaque test study [12]. Occlusion of corticosteroids is a widely accepted method to enhance the penetration of the medication and improve its efficacy [19]. BV-medicated plasters are occlusive and are specifically recommended for use on psoriasis plaques localized to DTT areas (i.e. the knees, elbows, and anterior side of the tibia) [20]. A post hoc analysis showed that Cal/BD aerosol foam was significantly more efficacious than BV-medicated plasters, even on these DTT areas (p < 0.001). Cal/BD aerosol foam and BV-medicated plasters were both well tolerated throughout the study.
Conclusion
Cal/BD aerosol foam demonstrated superior efficacy compared with BV-medicated plasters, including on DTT areas of the body. Cal/BD aerosol foam may therefore provide a more successful treatment outcome in patients with psoriasis vulgaris than some of the currently available topical therapies, regardless of plaque location.
Acknowledgements
The authors thank Florence Préaud from LEO Pharma for clinical trial management, and Magali Procacci-Babled from CPCAD for study coordination. Medical writing support was provided by Mai Kurihara, PhD, from Mudskipper Business Ltd, and was funded by LEO Pharma.
Compliance with Ethical Standards
Funding
This study was sponsored by LEO Pharma.
Conflict of interest
Catherine Queille-Roussel has no conflicts of interest to declare; Monika Rosen, Fabrice Clonier, and Kasper Nørremark are employees of LEO Pharma; Jean-Philippe Lacour has received grants from AbbVie, Boehringer, Celgene, Janssen, LEO Pharma, Lilly, Novartis and Roche, and has been a consultant for AbbVie, Celgene, LEO Pharma, Lilly and Novartis.
All procedures in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- 1.Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–850. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 2.Schön MP, Boehncke W-H. Psoriasis. N Engl J Med. 2005;352:1899–1912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 3.Segaert S, Røpke M. The biological rationale for use of vitamin D analogs in combination with corticosteroids for the topical treatment of plaque psoriasis. J Drugs Dermatol. 2013;12:e129–e137. [PubMed] [Google Scholar]
- 4.Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643–659. doi: 10.1016/j.jaad.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 5.Samarasekera E, Sawyer L, Parnham J, Smith CH. Assessment and management of psoriasis: summary of NICE guidance. BMJ. 2012;345:e6712. doi: 10.1136/bmj.e6712. [DOI] [PubMed] [Google Scholar]
- 6.Laws PM, Young HS. Topical treatment of psoriasis. Expert Opin Pharmacother. 2010;11:1999–2009. doi: 10.1517/14656566.2010.492778. [DOI] [PubMed] [Google Scholar]
- 7.Puig L, Carrascosa JM, Belinchon I, Fernandez-Redondo V, Carretero G, Ruiz-Carrascosa JC, et al. Adherence and patient satisfaction with topical treatment in psoriasis, and the use, and organoleptic properties of such treatments: a Delphi study with an expert panel and members of the Psoriasis Group of the Spanish Academy of Dermatology and Venereology. Actas Dermosifiliogr. 2013;104:488–496. doi: 10.1016/j.ad.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Tan X, Feldman SR, Chang J, Balkrishnan R. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263–1271. doi: 10.1517/17425247.2012.711756. [DOI] [PubMed] [Google Scholar]
- 9.Koo J, Tyring S, Werschler WP, Bruce S, Olesen M, Villumsen J, et al. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris: a randomized phase II study. J Dermatol Treat. 2016;27:120–127. doi: 10.3109/09546634.2015.1083935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebwohl M, Tyring S, Bukhalo M, Alonso-Llamazares J, Olesen M, Lowson D, et al. Fixed combination aerosol foam calcipotriene 0.005% (Cal) plus betamethasone dipropionate 0.064% (BD) is more efficacious than Cal or BD aerosol foam alone for psoriasis vulgaris: a randomized, double-blind, multicenter, three-arm, phase II study. J Clin Aesthet Dermatol. 2016;9:34–41. [PMC free article] [PubMed] [Google Scholar]
- 11.Leonardi C, Bagel J, Yamauchi P, Pariser D, Xu Z, Olesen M, et al. Efficacy and safety of calcipotriene plus betamethasone dipropionate aerosol foam in patients with psoriasis vulgaris: a randomized phase III study (PSO-FAST) J Drugs Dermatol. 2015;14:1468–1477. [PubMed] [Google Scholar]
- 12.Queille-Roussel C, Olesen M, Villumsen J, Lacour JP. Efficacy of an innovative aerosol foam formulation of fixed combination calcipotriol plus betamethasone dipropionate in patients with psoriasis vulgaris. Clin Drug Investig. 2015;35:239–245. doi: 10.1007/s40261-015-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taraska V, Tuppal R, Olesen M, Bang Pedersen C, Papp K. A novel aerosol foam formulation of calcipotriol and betamethasone has no impact on HPA axis and calcium homeostasis in patients with extensive psoriasis vulgaris. J Cutan Med Surg. 2015;20:44–51. doi: 10.1177/1203475415597094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lind M, Nielsen KT, Schefe LH, Noerremark K, Eriksson AH, Norsgaard H, et al. Supersaturation of calcipotriene and betamethasone dipropionate in a novel aerosol foam formulation for topical treatment of psoriasis provides enhanced bioavailability of the active ingredients. Dermatol Ther (Heidelb). 2016;6:413–425. doi: 10.1007/s13555-016-0125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul C, Stein Gold L, Cambazard F, Kalb RE, Lowson D, Bang B, et al. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy versus gel in patients with psoriasis vulgaris: randomized, controlled PSO-ABLE study. J Eur Acad Dermatol Venereol. 2016. doi:10.1111/jdv.13859. [DOI] [PMC free article] [PubMed]
- 16.Dumas KJ, Scholtz JR. The psoriasis bio-assay for topical corticosteroid activity. Acta Derm Venereol. 1972;52:43–48. [PubMed] [Google Scholar]
- 17.Queille-Roussel C, Hoffmann V, Enevold A, Ganslandt C. Use of a psoriasis plaque test in the development of a gel formulation of calcipotriol and betamethasone dipropionate for scalp psoriasis. J Dermatol Treat. 2013;24:250–254. doi: 10.3109/09546634.2011.641936. [DOI] [PubMed] [Google Scholar]
- 18.Kvist PH, Svensson L, Hagberg O, Hoffmann V, Kemp K, Røpke MA. Comparison of the effects of vitamin D products in a psoriasis plaque test and a murine psoriasis xenograft model. J Transl Med. 2009;7:107. doi: 10.1186/1479-5876-7-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhai H, Maibach HI. Effects of skin occlusion on percutaneous absorption: an overview. Skin Pharmacol Appl Skin Physiol. 2001;14:1–10. doi: 10.1159/000056328. [DOI] [PubMed] [Google Scholar]
- 20.Genus Pharmaceuticals. Betesil 2.250 mg medicated plaster. Betamethasone valerate. Prescribing information. 2016. http://www.medicines.org.uk/emc/PIL.22221.latest.pdf?documentid=22221. Accessed 30 June 2016.