Differences in acquisition and boosting of anti-malarial antibodies among African children exposed to malaria.
Keywords: P. falciparum, vaccines, serosurveillance, children
Abstract
Antibodies play a key role in acquired human immunity to Plasmodium falciparum (Pf) malaria and target merozoites to reduce or prevent blood-stage replication and the development of disease. Merozoites present a complex array of antigens to the immune system, and currently, there is only a partial understanding of the targets of protective antibodies and how responses to different antigens are acquired and boosted. We hypothesized that there would be differences in the rate of acquisition of antibodies to different antigens and how well they are boosted by infection, which impacts the acquisition of immunity. We examined responses to a range of merozoite antigens in 2 different cohorts of children and adults with different age structures and levels of malaria exposure. Overall, antibodies were associated with age, exposure, and active infection, and the repertoire of responses increased with age and active infection. However, rates of antibody acquisition varied between antigens and different regions within an antigen following exposure to malaria, supporting our hypothesis. Antigen-specific responses could be broadly classified into early response types in which antibodies were acquired early in childhood exposure and late response types that appear to require substantially more exposure for the development of substantial levels. We identified antigen-specific responses that were effectively boosted after recent infection, whereas other responses were not. These findings advance our understanding of the acquisition of human immunity to malaria and are relevant to the development of malaria vaccines targeting merozoite antigens and the selection of antigens for use in malaria surveillance.
Introduction
After repeated exposure to Pf malaria, individuals eventually develop effective immunity that controls blood-stage parasitemia and prevents symptomatic illness, as well as severe and life-threatening complications [1–3]. Antibodies are a key component of this acquired immunity, and the targets of protective antibodies include antigens expressed by merozoites, the form of malaria parasites that invade human erythrocytes. Antibody responses toward several merozoite antigens have been variably associated with protection in exposed populations [4–6]. and malaria vaccine efforts have long been focused on merozoite antigens thought to be involved in erythrocyte invasion [2, 7]. Protective antibodies are believed to act by inhibiting merozoite invasion of erythrocytes [8–12]; antibody-dependent, cell-mediated mechanisms [13–16]; and antibody-complement interactions to inhibit invasion [17]. However, currently, we have only a partial understanding of the targets of protective blood-stage antibodies and how responses to different antigens and overall immunity are acquired and maintained.
The complexity of the Pf merozoite presents a large array of antigens to the immune system. Protective humoral immunity is therefore likely to be comprised of an antibody repertoire directed toward multiple antigens [5, 6, 18]. Until recently, only a small number of the many potentially important merozoite antigens have been well studied as immune targets [2, 4], and even less have had their antibody function defined [7]. Recent studies have begun to identify additional candidate targets of protective immunity and show that there is a significant degree of relatedness between antibody responses and that antibodies to multiple, rather than single, antigens are typically associated with protection from clinical disease [5, 6, 18, 19–23]. Most previous epidemiologic studies of the acquisition of protective humoral immunity have focused on the response to selected single antigens or a very restricted number of antigens, particularly the leading merozoite vaccine candidate antigens, such as AMA1, MSP1, MSP2, and MSP3, or small combinations of antigens or allelic variants (see references cited in Fowkes et al. [4]), and only a small number of studies have included multiple merozoite antigens (e.g., see refs. [5, 6, 23, 24]) or antigens expressed on a microarray platform (e.g., see refs. [25, 26]).
Although an increasing number of merozoite antigens are now being assessed as potential targets of immunity, the rate at which antibodies to different antigens are acquired and boosted is less well defined. Additionally, differing malaria transmission levels and study population characteristics may influence responses to different antigens [27–29]. Antibodies to many merozoite antigens have been shown to increase with age, in parallel with acquisition of clinical immunity [22, 23, 27, 30, 31]. However, comparisons of the rates and patterns of acquisition of multiple antibody responses may provide a further understanding of clinical immunity and the importance of individual or combined responses in its development. A valuable application of a detailed knowledge of humoral responses to malaria blood-stage antigens is in the use of serology for malaria surveillance. This can be used for identifying populations with ongoing exposure, evaluating the impact of malaria control interventions, monitoring for the reintroduction of malaria after successful elimination, or monitoring population immunity to aid the identification of populations at high risk [27, 32, 33]. However, there is presently only limited understanding of which antigens are valuable for this approach and how sensitive antigen-specific responses are to recent malaria exposure. A number of factors need to be considered in selecting antigens for use in serosurveillance, including the sensitivity and specificity of antibodies to infection or exposure and the rate at which antibodies decline after exposure (reviewed in refs. [27, 32, 33]). Different antigens may be selected for different surveillance applications.
In light of these important gaps in our understanding, the major aims of this study were to evaluate the acquisition of antibodies to a broad range of different merozoite antigens, as well as advancing our understanding on the repertoire and relatedness of responses. We hypothesized that there are differences in the rates of acquisition of antibodies to different merozoite antigens and differences in the extent to which antibodies to antigens are boosted following infection. This knowledge is important for understanding how immunity to malaria is acquired and has implications for malaria vaccine development and for selection of antigens for use in serosurveillance approaches. More broadly, we aimed to advance our knowledge on human immune function, particularly in relation to the acquisition of antibodies to multiple antigens in children, which is an area that is poorly understood. To address these aims, we examined antibody responses to a broad range of merozoite antigens, including well-studied vaccine candidate antigens, as well less-studied merozoite antigens or antigen regions, such as the EBA140, EBA181, and EBA175 RIII-V (region III-V) and the PfRH and 6-cysteine domain antigens (Pf12, Pf41, Pf38). We examined responses in 2 cohorts from the same community, collected at different times and under different levels of malaria transmission. One cohort included children of all ages and adults, whereas the second focused on young children under 10 years of age. The use of 2 cohorts also enabled us to evaluate the effects of changing malaria transmission on acquisition of immunity and the repertoire of responses.
METHODS
Study site and populations
The study site of Ngerenya, North Kilifi district, coastal Kenya, has been described in detail previously [34, 35]. In 1997/1998, the predicted entomologic inoculation rate was 10 infective bites/person/yr [34]. The study sera used for investigations were collected cross-sectionally as part of 2 separate large longitudinal cohorts involving randomly selected households [35, 36]. The first set (n = 150) was selected randomly from the total number of samples collected in September 1998 from adults and children (n = 354) [35]. Following sample selection, study participants from this Ngerenya 1998 cohort were divided into 3 age groups, in broad agreement with age-associated incidence of clinical malaria in the area: 2–5, 6–14, and 15 yr or older (age range 18–81 yr; there were no 15- to 17-yr-olds). The second set of samples was collected from the same village in October 2002 and comprised 237 children aged 1–8 yr. This subset included all sera samples that were available from children ≥1 yr of age and was derived from a larger cohort of Ngerenya children (<8 yr, n = 297), followed continuously from 2001 to 2005 [36]. Six monthly cross-sectional bleeds were conducted, and the children were followed by active surveillance for symptomatic illness, involving weekly visits to record body temperature and symptom history, with blood films prepared from those who were febrile or symptomatic. Additionally, carers were encouraged to attend dedicated clinics if febrile illness occurred between visits. The longitudinal data available for the 12 mo before the October 2002 cross-sectional bleed were used to assess the association between disease episode or parasitemia and subsequent antibody response. Clinical disease among the children ≥1 yr of age was defined as the presence of measured fever (axillary temperature >37.5°C), in conjunction with parasitemia, >2500 parasites/μl. This parasite-density threshold provided optimal sensitivity and specificity for symptomatic malaria for this study population [35]. For both cohorts, parasitemic status, defined as the presence of blood stage Pf, excluding gametocytes, was ascertained by a thick smear at time of blood sampling. Characteristics of cohort study participants according to age, gender, and parasitemic status are summarized in Table 1. The malaria transmission rate in Ngerenya was lower in October 2002 [37], reflected by the lower proportion of parasitemic individuals in the 2002 cohort (39.3% in 1998 vs. 6.9% in 2002; Table 1).
TABLE 1.
Ngerenya 1998 and 2002 cohort characteristics, according to age, parasitemic status, and gender at the time of cross-sectional bleed
| Cohort |
Characteristics (P. falciparum) |
All |
Age group, yr |
Pa |
||
|---|---|---|---|---|---|---|
| 2–5 (n = 43) |
6–14 (n = 57) |
18–81 (n = 50) |
||||
| Ngerenya 1998 | Positive no. (%) | 59 (39.3) | 13 (30.2) | 34 (59.7) | 12 (24.0) | 0.001 |
| Densityb | 682 (225–1827) | 3712 (1151–12,354) | 762 (225–1452) | 255 (180–380) | 0.002 | |
| No. of males (%)c | 69 (46.0) | 23 (53.5) | 34 (60.0) | 12 (24.0) | 0.001 | |
| 1–4 (n = 131) |
5–8 (n = 106) |
|||||
| Ngerenya 2002 | Positive no. (%) | 16 (6.9)d | 7 (5.4) | 9 (8.6) | – | 0.347 |
| Densityb | 460 (180–1900) | 1640 (120–3560) | 320 (200–880) | – | 0.458 | |
| No. of males (%)c | 132 (55.7) | 66 (50.4) | 66 (62.3) | – | 0.087 | |
P values calculated using a χ2 test or Kruskal-Wallis test.
Parasites/microliter, median (25–75th percentiles), among individuals who were parasite positive.
No difference in rate of parasitemia was found for males compared with females within the Ngerenya 1998 or 2002 cohorts (P > 0.436).
n was 234 vs. 237 for parasitemic status, as a result of missing data.
In all cases, informed consent was obtained from donors or their parent/guardian(s). Ethical approval was obtained from the Ethics Committee of the Kenya Medical Research Institute (Nairobi, Kenya), Human Research Ethics Committee, Walter and Eliza Hall Institute (Melbourne, Australia), and Alfred Hospital Human Ethics Committee (Melbourne, Australia).
ELISAs
Serum antibodies to purified recombinant Pf antigens were assessed by ELISA, as described in ref. [38]. IgG was measured toward a panel of merozoite antigens with a known or potential role in Pf erythrocyte invasion [2] using recombinant antigens homologous to 3D7 or W2mef parasite genotypes, as well as the FC27 variant of MSP2, and against 3D7 schizont protein extract [38]. Details of the recombinant proteins, antigen, and antibody concentrations used are recorded in Supplemental Table 1, and these antigens have been previously validated [5]. Recombinant proteins used in ELISAs were based on the 3D7 genotype for all antigens; for 3 antigens, an additional allele was used. This is the most widely used approach in the published literature, and prior studies have shown that antigens encoded by the 3D7 genotype are widely recognized across the globe in different populations (e.g., see ref. [4]). We included a number of antigens that are conserved in sequence or have limited polymorphism. As such, most of the antibody response is to conserved epitopes, and therefore, antibody evaluation is not affected by possible strain differences in populations. These include MSP1-19, PfRH2, PfRH4, EBA140, EBA181, EBA175, Pf38, Pf41, and Pf12. For 3 antigens, we also included an additional allele: 1) For EBA175 RIII-V, we used the 3D7 and W2mef alleles, as this region of the protein has a dimorphic region, without other polymorphisms (e.g., see ref. [23]). Therefore, these 2 genotypes cover all antigenic diversity in this region. 2) For MSP2, we used proteins representing the 2 major allelic forms of this protein—3D7 and FC27; these 2 forms capture the great majority of antigenic diversity for this antigen (e.g., see ref. [39]). 3) For AMA1, we included the W2mef allele, in addition to 3D7, as this antigen has polymorphic epitopes, and the W2mef allele has antigenic differences from 3D7 (e.g., see ref. [40]).
Sera were tested in duplicate, together with 7—10 nonexposed United Kingdom or Melbourne control samples. Positive sera from adult malaria-exposed PNG (Papua New Guinea) donors (n = 3) and PBS blanks (n = 2) were included as plate controls to allow standardization of values to account for any plate-to-plate variation. The final sample absorbance value toward each antigen was the average of standardized duplicate values. For merozoite antigens expressed as fusion proteins, MBP or GST was tested in parallel and the average of standardized duplicate absorbance values subtracted from that of the appropriate merozoite antigen. For the September 1998 samples, absorbance against MBP protein and Plasmodium chabaudi MSP1-19, tested in parallel with Pf MSP1-19, was negative or very low (median 0; IQR 0–0.008, after deducting blank wells as background), and the final sample Pf MSP1-19 absorbance represents standardized Pf MSP1-19 minus standardized P. chabaudi MSP1-19 values [41]. His-tagged MSP1-19 was used for the Ngerenya 2002 cohort.
Statistical analysis
Statistical analysis was performed using Stata Version 9 (StataCorp, College Station, TX, USA). ELISA data showed non-normal distribution with a positive skew, so they were analyzed using nonparametric methods. The association between continuous variables and categorical variables was assessed using a Wilcoxon rank-sum test or Kruskal-Wallis test. The association among categorical variables was assessed using a χ2 or Fisher’s exact test. Spearman’s rank correlation coefficients were used to assess the association between 2 continuous variables. Individuals with ELISA antibody levels ≥75th percentile value were defined as "high responders."
Results were examined for all Ngerenya 1998 (n = 150) and Ngerenya 2002 (n = 237) cohort samples and then following stratification, according to age group and parasitemic status. Data exclusions for both cohorts were made as a result of insufficient sample volume for ELISA testing against all antigens; the total number of cohort samples tested against each antigen is listed in the relevant tables.
RESULTS
Differing rates of acquisition of antibodies to merozoite antigens in relation to age and exposure
We compared the pattern of antibody responses with different merozoite antigens across different age groups and relative to IgG with schizont extract (used as a broad marker of exposure to blood-stage malaria). We examined responses in 2 different cohorts performed at different times in the same community (Table 1). The 1998 cohort was designed to evaluate responses across a broad age range, including children of all ages and adults; the 2002 cohort was designed to examine responses in young children in more detail. Additionally, the comparison of the 2 cohorts allowed us to evaluate the effects of malaria transmission on antibody responses.
For the Ngerenya 1998 cohort of adults and children, the antibody levels to most merozoite antigens increased significantly with age group (Table 2), although different patterns of age association were seen. For example, age-associated acquisition of antibodies appeared strong for MSP1 and the EBA antigens but was weaker for both PfRH2 antigens, with a more prominent antibody response shown among the youngest children than seen for MSP1 and the EBA antigens. The median antibody levels to the MSP2 and MSP4 antigens were notably high among older children (6–14 yr) compared with younger children (2–5 yr). For the AMA1 antigens, antibody levels were significantly highest among 6- to 14-yr-old children (P < 0.001). The median antibody levels to MSP1 and the EBA antigens were notably high among adults rather than children (6–14 yr). In considering antibody levels to the 6-cys proteins (Pf38, Pf41, and Pf12), age association was seen only for Pf41 and Pf12 and when comparing children only (2–5 vs. 6–14 yr; P < 0.05; Table 2). In most cases within the cohort, similar age associations were seen when examining proportions of high antibody responders. For level of IgG, similar age acquisition patterns were seen for EBA175 RIII-V and EBA175-F2 and for PfRH2-2530 and PfRH2-2030, although the proportion of high responders to PfRH2-2530, but not PfRH2-2030, was greatest among the older children (6–14 yr; P = 0.021).
TABLE 2.
IgG responses among Ngerenya 1998 cohort members, according to age group and parasitemic status
| Antigensb | nc | Alla | Age group, yra |
Sign. | Pd | Parasitemic statusa |
Sign. | Pd | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2–5 | 6–14 | 18–81 | Apara. | Para. | |||||||
| MSP1-19 | 143 | 0.07 | 0.02 | 0.04 | 0.45 | * | <0.001 | 0.08 | 0.06 | 0.667 | |
| [0.01–0.49] | [0.00–0.11] | [0.01–0.21] | [0.11–0.88] | [0.00–0.51] | [0.02–0.47] | ||||||
| 35 (24.5) | 3 (7.7) | 10 (18.2) | 22 (44.9) | * | <0.001 | 22 (25.0) | 13 (23.6) | 0.854 | |||
| MSP1-42 | 150 | 0.22 | 0.05 | 0.22 | 0.63 | * | <0.001† | 0.17 | 0.24 | 0.140† | |
| [0.04–0.66] | [0.01–0.17] | [0.05–0.51] | [0.30–1.10] | [0.02–0.57] | [0.09–0.66] | ||||||
| 37 (24.7) | 2 (4.7) | 11 (19.3) | 24 (48.0) | * | <0.001 | 22 (24.2) | 15 (25.4) | 0.862 | |||
| MSP2 | 143 | 0.41 | 0.04 | 0.51 | 0.74 | * | <0.001† | 0.15 | 0.60 | * | <0.001† |
| [0.06–0.91] | [0.00–0.44] | [0.13–0.90] | [0.22–1.32] | [0.02–0.82] | [0.22–1.21] | ||||||
| 35 (24.5) | 3 (7.7) | 13 (23.6) | 19 (38.8) | * | 0.003 | 19 (21.6) | 16 (29.1) | 0.310 | |||
| MSP2(FC27) | 143 | 0.49 | 0.05 | 0.78 | 0.97 | * | <0.001† | 0.27 | 0.88 | * | <0.001† |
| [0.07–1.20] | [0.02–0.26] | [0.20–1.20] | [0.33–1.35] | [0.03–1.10] | [0.31–1.24] | ||||||
| 35 (24.5) | 4 (10.3) | 13 (23.6) | 18 (36.7) | * | 0.016 | 18 (20.5) | 17 (30.9) | 0.157 | |||
| MSP4 | 150 | 0.62 | 0.14 | 0.76 | 0.88 | * | <0.001† | 0.44 | 1.01 | * | <0.001† |
| [0.16–1.36] | [0.03–1.03] | [0.36–1.48] | [0.29–1.39] | [0.07–1.18] | [0.45–1.58] | ||||||
| 37 (24.7) | 8 (18.6) | 16 (28.1) | 13 (26.0) | 0.534 | 16 (17.6) | 21 (35.6) | * | 0.012 | |||
| AMA1 | 150 | 0.49 | 0.08 | 0.76 | 0.53 | * | <0.001† | 0.26 | 0.82 | * | <0.001† |
| [0.10–0.94] | [0.04–0.55] | [0.16–1.21] | [0.14–0.85] | [0.05–0.73] | [0.30–1.27] | ||||||
| 37 (24.7) | 7 (16.3) | 21 (36.8) | 9 (18.0) | * | 0.025 | 11 (12.1) | 26 (44.1) | * | <0.001 | ||
| AMA1(W2mef) | 150 | 0.47 | 0.08 | 0.76 | 0.63 | * | <0.001† | 0.37 | 1.02 | * | <0.001† |
| [0.08–1.08] | [0.01–0.82] | [0.18–1.31] | [0.17–1.08] | [0.03–0.87] | [0.18–1.46] | ||||||
| 37 (24.7) | 6 (14.0) | 19 (33.3) | 12 (24.0) | 0.083 | 12 (13.2) | 25 (42.4) | * | <0.001 | |||
| EBA140 RIII-V | 143 | 0.14 | 0.03 | 0.17 | 0.46 | * | <0.001† | 0.10 | 0.19 | * | 0.002† |
| [0.04–0.59] | [0.01–0.11] | [0.07–0.56] | [0.12–0.97] | [0.02–0.51] | [0.10–0.81] | ||||||
| 35 (24.5) | 2 (5.1) | 13 (23.6) | 20 (40.8) | * | 0.001 | 18 (20.5) | 17 (30.9) | 0.157 | |||
| EBA181 RIII-V | 143 | 0.10 | 0.06 | 0.10 | 0.27 | * | <0.001† | 0.10 | 0.12 | 0.488† | |
| [0.05–0.34] | [0.03–0.10] | [0.06–0.31] | [0.10–0.63] | [0.04–0.39] | [0.06–0.31] | ||||||
| 35 (24.5) | 2 (5.1) | 13 (23.6) | 20 (40.8) | * | 0.001 | 25 (28.4) | 10 (18.2) | 0.166 | |||
| EBA175 RIII-V | 143 | 0.10 | 0.04 | 0.11 | 0.46 | * | <0.001† | 0.08 | 0.15 | 0.065† | |
| [0.04–0.60] | [0.02–0.08] | [0.04–0.47] | [0.09–1.96] | [0.03–0.58] | [0.06–0.68] | ||||||
| 35 (24.5) | 3 (7.7) | 11 (20.0) | 21 (42.9) | * | <0.001 | 21 (23.9) | 14 (25.5) | 0.830 | |||
| EBA175-F2 | 150 | 0.18 | 0.05 | 0.24 | 0.34 | * | <0.001† | 0.16 | 0.25 | * | 0.031† |
| [0.06–0.51] | [0.01–0.09] | [0.13–0.47] | [0.11–0.95] | [0.04–0.43] | [0.08–0.84] | ||||||
| 37 (24.7) | 3 (7.0) | 13 (22.8) | 21 (42.0) | * | <0.001 | 18 (19.8) | 19 (32.2) | 0.085 | |||
| EBA175 | 150 | 0.04 | 0.01 | 0.06 | 0.18 | * | <0.001† | 0.03 | 0.05 | 0.053† | |
| (W2mef) | [0.01–0.25] | [0.00–0.02] | [0.02–0.22] | [0.04–0.79] | [0.01–0.20] | [0.02–0.29] | |||||
| 37 (24.7) | 3 (7.0) | 13 (22.8) | 21 (42.0) | * | <0.001 | 21 (23.1) | 16 (27.1) | 0.575 | |||
| RH2-2530 | 150 | 0.22 | 0.14 | 0.29 | 0.34 | * | 0.002† | 0.20 | 0.31 | 0.082† | |
| [0.13–0.63] | [0.08–0.44] | [0.17–0.78] | [0.17–0.77] | [0.12–0.55] | [0.16–0.86] | ||||||
| 37 (24.7) | 4 (9.3) | 18 (31.6) | 15 (30.0) | * | 0.021 | 16 (17.6) | 21 (35.6) | * | 0.012 | ||
| RH2-2030 | 150 | 0.23 | 0.14 | 0.26 | 0.30 | * | 0.013† | 0.21 | 0.27 | 0.073† | |
| [0.13–0.46] | [0.08–0.36] | [0.16–0.50] | [0.17–0.56] | [0.11–0.41] | [0.16–0.58] | ||||||
| 37 (24.7) | 6 (14.0) | 16 (28.1) | 15 (30.0) | 0.151 | 18 (19.8) | 19 (32.2) | 0.085 | ||||
| RH4-A3 | 150 | 0.12 | 0.08 | 0.11 | 0.22 | * | 0.005 | 0.10 | 0.15 | 0.164† | |
| [0.04–0.36] | [0.02–0.21] | [0.05–0.28] | [0.07–0.61] | [0.02–0.37] | [0.08–0.36] | ||||||
| 37 (24.7) | 4 (9.3) | 12 (21.1) | 21 (42.0) | * | 0.001 | 23 (25.3) | 14 (23.7) | 0.830 | |||
| SERA5 | 150 | 0.14 | 0.07 | 0.17 | 0.30 | * | <0.001† | 0.09 | 0.23 | * | 0.009† |
| [0.05–0.38] | [0.04–0.17] | [0.05–0.36] | [0.09–0.64] | [0.05–0.34] | [0.10–0.47] | ||||||
| 37 (24.7) | 2 (4.7) | 13 (22.8) | 22 (44.0) | * | <0.001 | 21 (23.1) | 16 (27.1) | 0.575 | |||
| Pf41 | 130 | 0.21 | 0.11 | 0.28 | 0.22 | 0.087† | 0.16 | 0.28 | 0.052 | ||
| [0.05–0.42] | [0.04–0.28] | [0.08–0.45] | [0.04–0.42] | [0.04–0.37] | [0.08–0.44] | ||||||
| 32 (24.6) | 4 (12.1) | 16 (31.4) | 12 (26.1) | 0.130 | 16 (20.0) | 16 (32.0) | 0.122 | ||||
| Pf38 | 130 | 0 | 0.00 | 0.01 | 0.01 | 0.277 | 0.00 | 0.03 | 0.131† | ||
| [0–0.12] | [0–0.04] | [0–0.13] | [0–0.20] | [0–0.10] | [0–0.15] | ||||||
| 32 (24.6) | 6 (18.2) | 13 (25.5) | 13 (28.3) | 0.581 | 18 (22.5) | 14 (28.0) | 0.479 | ||||
| Pf12 | 130 | 0.32 | 0.22 | 0.44 | 0.36 | 0.143† | 0.21 | 0.47 | * | <0.001† | |
| [0.07–0.70] | [0–0.61] | [0.15–0.89] | [0.12–0.57] | [0.04–0.57] | [0.24–1.05] | ||||||
| 32 (24.6) | 7 (21.2) | 17 (33.3) | 8 (17.39) | 0.166 | 12 (15.0) | 20 (40.0) | * | <0.001 | |||
| Schizont | 150 | 0.25 | 0.11 | 0.26 | 0.66 | * | <0.001† | 0.20 | 0.34 | 0.082† | |
| extract | [0.12–0.57] | [0.04–0.23] | [0.15–0.47] | [0.27–1.00] | [0.08–0.64] | [0.19–0.53] | |||||
| 37 (24.7) | 1 (2.3) | 8 (14.0) | 28 (56.0) | <0.001 | 25 (27.5) | 12 (20.3) | 0.322 | ||||
Apara., Aparasitemic; Para., parasitemic; RH, reticulocyte-binding homologue, Sign., significance.
For each antigen represented, figures reflect: median value (row 1); 25–75th percentile ([p25–p75]; row 2); and high responders n (%; row 3). High responders by ELISA are those with absorbance ≥75th percentile.
All alleles are 3D7 unless otherwise specified.
Number of samples tested by ELISA for each antigen.
P values calculated using a Kruskal-Wallis test or χ2 test.
P ≤ 0.05 is significant.
P ≤ 0.05 is significant, comparing antibody levels among children only (n = 100), children 2–5 and children 6–14 yr, using a Wilcoxon rank sum test (P values not shown).
With the exception of the 6-cys antigens, IgG levels to most antigens were significantly, positively correlated with age [median (IQR) of all rs values, 0.35 (0.23–0.44); Supplemental Table 2]. Correlations were strongest for the MSP1 antigens (MSP1-19 rs = 0.43, P < 0.001; MSP1-42 rs = 0.46, P < 0.001) and EBA antigens [EBA175 RIII-V rs = 43, P < 0.001; EBA175-F2 rs = 0.47, P < 0.001; EBA 175(W2mef) RIII-V rs = 0.54, P < 0.001] and weaker for AMA1 (rs = 0.23, P = 0.005), PfRH2-2030 (rs = 0.22, P = 0.006), and PfRH4 (A3 construct; rs = 0.24, P = 0.003). Similar associations were seen when analysis was done in children aged 1–15 yr.
These results suggest that antibodies are acquired to merozoite antigens at different rates in the Ngerenya 1998 cohort. An "early" rate of antibody acquisition, with relatively prominent responses present among older children, seemed to occur toward AMA1 and to some extent, MSP4, PfRH2, Pf41, and Pf12. In comparison, a "late" antibody response, which took longer to develop among children and was much more prominent among adults, was evident for MSP1-19 and the EBA(III-V) antigens.
We also examined the acquisition of antibodies within a second Ngerenya cohort consisting of young children (Ngerenya 2002). Although the levels of antibody within this cohort were generally lower, patterns of antigen-specific IgG acquisition with age were similar to those seen in the Ngerenya 1998 cohort (Table 3), despite the differing transmission levels (Table 1). Significant increases in antibody responses with age occurred for most antigens. However, no significant associations with age groups were seen for antibody responses to MSP1-19, EBA175, and EBA140; these are antigens that appeared to induce a late antibody response in the Ngerenya 1998 cohort, characterized by acquisition in older children and adults. With the consideration of the antibody response to different regions of the same antigen, significant age association was seen for the EBA175-F2 (P < 0.001) and EBA140 RII (P < 0.001) antigenic regions but not for the III-V regions of EBA175 (P = 0.361) or EBA140 (P = 0.065; Table 3). Associations with age groups were strongest for those antigens associated with an early antibody response [AMA1, MSP4, EBA140 RII, and EBA175-F2 (rs = 0.42–0.48, P < 0.001; Supplemental Table 3].
TABLE 3.
IgG responses in the Ngerenya 2002 cohort, according to age group and parasitemic status
| Antigensb | Age group, yra |
Sign. | Pc | Parasitemic statusa |
Sign. | Pc | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alla (n = 237) | 1–4 (n = 131) | 5–8 (n = 106) | All (n = 234)d | Apara. (n = 218) | Para. (n = 16) | |||||
| MSP1-19 | 0.02 | 0.02 | 0.04 | 0.567 | 0.02 | 0.02 | 0.09 | * | 0.031 | |
| [0–0.10] | [ 0–0.10] | [0–0.10] | [0–0.10] | [0.00–0.08] | [0.01–0.31] | |||||
| 62 (26.2) | 33 (25.2) | 29 (27.4) | 0.706 | 62 (26.5) | 54 (24.8) | 8 (50.0) | * | 0.039 | ||
| MSP2(3D7) | 0.04 | 0.03 | 0.06 | * | 0.001 | 0.04 | 0.04 | 0.26 | * | <0.001 |
| [0.02–0.12] | [0.01–0.06] | [0.03–0.20] | [0.02–0.12] | [0.02–0.09] | [0.05–0.74] | |||||
| 59 (24.9) | 18 (13.7) | 41 (38.7) | * | <0.001 | 58 (24.8) | 48 (22.0) | 10 (62.5) | * | 0.001 | |
| MSP2(FC27) | 0.02 | 0.02 | 0.03 | * | 0.002 | 0.02 | 0.02 | 0.46 | * | <0.001 |
| [0–0.08] | [ 0–0.06] | [0.01–0.13] | [0–0.08] | [0.10–0.06] | [0.10–0.89] | |||||
| 59 (24.9) | 24 (18.3) | 35 (33.0) | * | 0.009 | 58 (24.8) | 46 (21.1) | 12 (75.0) | * | <0.001 | |
| MSP4 | 0.12 | 0.07 | 0.36 | * | <0.001 | 0.12 | 0.11 | 1.01 | * | <0.001 |
| [0.02–0.48] | [0.01–0.22] | [0.07–0.75] | [0.02–0.48] | [0.02–0.41] | [0.40–1.64] | |||||
| 62 (26.2) | 20 (15.3) | 42 (39.6) | * | <0.001 | 61 (26.1) | 50 (22.9) | 11 (68.8) | * | <0.001 | |
| AMA1(3D7) | 0.05 | 0.02 | 0.15 | * | <0.001 | 0.05 | 0.04 | 0.51 | * | <0.001 |
| [0–0.31] | [0–0.14] | [0.03–0.55] | [0–0.31] | [0.01–0.24] | [0.16–1.32] | |||||
| 59 (24.9) | 18 (13.7) | 41 (38.7) | * | <0.001 | 58 (24.8) | 48 (22.0) | 10 (62.5) | * | 0.001 | |
| EBA140 | 0.02 | 0.02 | 0 | 0.065 | 0.02 | 0.02 | 0.04 | * | 0.016 | |
| [0–0.04] | [0–0.04] | [0–0.04] | [0–0.04] | [0–0.04] | [0.01–0.23] | |||||
| 60 (25.3) | 31 (23.7) | 29 (27.4) | 0.515 | 60 (25.6) | 52 (23.9) | 8 (50.0) | * | 0.034 | ||
| EBA181 | 0.07 | 0.06 | 0.08 | * | 0.032 | 0.07 | 0.06 | 0.08 | 0.296 | |
| [0.03–0.13] | [0.03–0.11] | 0.04–0.22] | [0.03–0.13] | [0.03–0.13] | [0.05–0.32] | |||||
| 61 (25.7) | 23 (17.6) | 38 (35.9) | * | 0.001 | 61 (26.1) | 55 (25.2) | 6 (37.5) | 0.374 | ||
| EBA175 | 0 | 0 | 0.01 | 0.361 | 0 | 0 | 0.03 | 0.063 | ||
| [0–0.02] | [0–0.02] | [0–0.03] | [0–0.02] | [0–0.018] | [0–0.35] | |||||
| 55 (23.2) | 25 (19.1) | 30 (28.3) | 0.095 | 55 (23.5) | 47 (21.6) | 8 (50.0) | * | 0.027 | ||
| EBA140 RIIe | 0.04 | 0.03 | 0.07 | * | <0.001 | 0.04 | 0.04 | 0.15 | * | <0.001 |
| [0.02–0.10] | [0.01–0.05] | [0.03–0.21] | [0.02–0.10] | [0.02–0.09] | [0.05–0.62] | |||||
| 59 (25.0) | 19 (14.6) | 40 (37.7) | * | <0.001 | 58 (24.9) | 49 (22.6) | 9 (56.3) | * | 0.003 | |
| EBA175-F2e | 0.16 | 0.12 | 0.26 | * | <0.001 | 0.16 | 0.14 | 0.67 | * | <0.001 |
| [0.09–0.35] | [0.07–0.20] | [0.13–0.69] | [0.09–0.35] | [0.08–0.28] | [0.34–1.18] | |||||
| 59 (25.0) | 15 (11.5) | 44 (41.5) | * | <0.001 | 58 (24.9) | 46 (21.2) | 12 (75.0) | * | <0.001 | |
| RH2-2030e | 0.05 | 0.04 | 0.10 | * | <0.001 | 0.05 | 0.05 | 0.17 | * | <0.001 |
| [0.03–0.10] | [0.02–0.07] | [0.04–0.21] | [0.03–0.10] | [0.03–0.10] | 0.07–0.35] | |||||
| 58 (24.6) | 18 (13.9) | 40 (37.7) | * | <0.001 | 56 (24.0) | 45 (20.7) | 11 (68.8) | * | <0.001 | |
| RH4-A3 | 0.02 | 0.01 | 0.04 | * | 0.026 | 0.02 | 0.02 | 0.15 | * | 0.002 |
| [0–0.09] | [0–0.07] | [0–0.12] | [0–0.09] | [0–0.08] | [0.04–0.28] | |||||
| 60 (25.3) | 27 (20.6) | 33 (31.1) | 0.064 | 58 (24.8) | 48 (22.0) | 10 (62.5) | * | 0.001 | ||
| RH4-BRe | 0.06 | 0.04 | 0.08 | * | <0.001 | 0.06 | 0.05 | 0.25 | * | <0.001 |
| [0.03–0.15] | [0.02–0.09] | [0.04–0.24] | [0.03–0.15] | [0.03–0.13] | [0.08–0.78] | |||||
| 59 (25.0) | 21 (16.2) | 38 (35.9) | * | 0.001 | 59 (25.3) | 48 (22.1) | 11 (68.8) | * | <0.001 | |
| Pf38e | 0.16 | 0.13 | 0.18 | * | <0.001 | 0.16 | 0.15 | 0.26 | * | 0.013 |
| [0.09–0.34] | [0.07–0.31] | [0.11–0.37] | [0.09–0.34] | [0.08–0.33] | [0.15–0.48] | |||||
| 59 (25.0) | 29 (22.3) | 30 (28.3) | 0.290 | 58 (24.9) | 52 (24.0) | 6 (37.5) | 0.227 | |||
| Schizont extract | 0.13 | 0.09 | 0.25 | * | <0.001 | 0.13 | 0.12 | 0.87 | * | <0.001 |
| [0.05–0.45] | [0.02–0.22] | [0.09–0.59] | [0.05–0.46] | [0.05–0.36] | [0.62–1.06] | |||||
| 62 (26.2) | 21 (16.0) | 41 (38.7) | * | <0.001 | 62 (26.5) | 49 (22.5) | 13 (81.3) | * | <0.001 | |
Sign., Significance.
For each antigen, represented figures reflect: median value (row 1); [p25–p75] (row 2); and high responders n (%; row 3). High responders are those with absorbance ≥75th percentile.
All alleles are 3D7 unless otherwise specified.
P values calculated using a Kruskal-Wallis test or χ2 test.
Three values are missing for parasitemic status.
For these antigens, insufficient serum volume resulted in missing data for a single child who was aparasitemic and in age group 1–4 yr.
P ≤ 0.05.
Effect of concurrent parasitemia on antibody levels and age associations
Within the Ngerenya 1998 cohort, median IgG responses were significantly higher among participants who were parasitemic rather than aparasitemic at the time of sample collection for MSP2(3D7), MSP2(FC27), MSP4, AMA1(3D7), AMA1(W2mef), EBA140 RIII-V, EBA175-F2, SERA5, and Pf12 (Table 2). A greater frequency of high responders was observed only for those antigens for which an early antibody response was seen within this cohort: MSP4, AMA1(3D7), AMA1(W2mef), PfRH2-2530, and Pf12. IgG responses to schizont extract did not differ according to the presence or absence of parasitemia. Higher antibody levels with parasitemia were more pronounced in children than in adults; among children only (n = 100), significant elevation with parasitemia was seen for IgG levels to all antigens other than MSP1-19 and Pf41 (Table 2). Additionally, among the Ngerenya 2002 children, higher antibody levels in association with parasitemia were observed for all merozoite antigens other than EBA181 and EBA175 (Table 3) and for schizont extract.
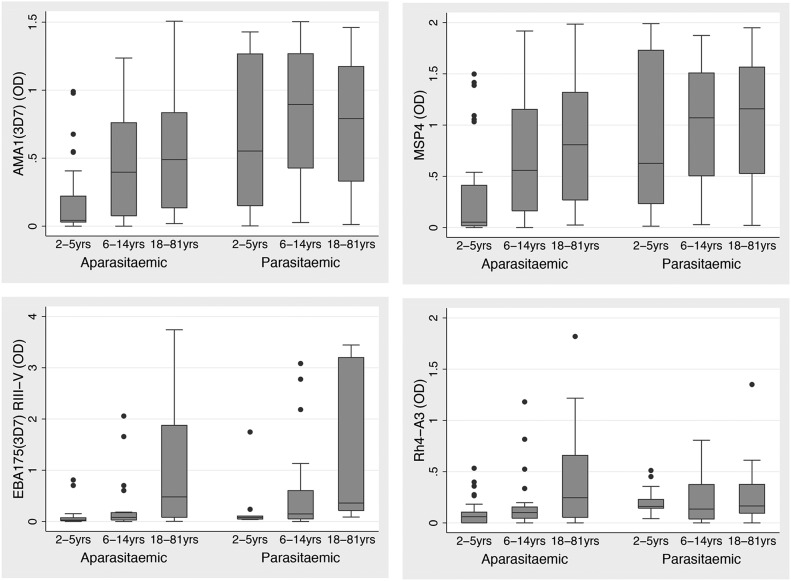
Age associations with IgG responses within the Ngerenya 1998 cohort were influenced by parasitemia status (Fig. 1 and Supplemental Table 4). An age-associated increase in IgG to most merozoite antigens was evident among individuals who were aparasitemic at sample collection. In contrast, among parasitemic individuals, associations between IgG responses and age were seen only for merozoite antigens that showed a late antibody response: MSP1-19, MSP1-42, EBA140 RIII-V, EBA175 RIII-V, EBA175-F2, and SERA5. A similar analysis was performed among Ngerenya 1998 children only (n = 100); among aparasitemic children, significant age association was no longer evident for IgG to MSP1-19, EBA175(W2mef) RIII-V, PfRH4-A3, and SERA5, reflecting the finding of a late antibody pattern of acquisition to these antigens, most prominent among adults and generally low among children (Supplemental Table 4); however the reduced sample size may have impacted power to detect differences.
Figure 1. Antibody responses according to age group and parasitemic status in the Ngerenya 1998 cohort for representative merozoite antigens.
IgG was measured by ELISA. Box plots display OD median levels and the IQR. Cohort individuals are separated according to parasitemic status at the time of sampling.
Antibody responses in association with recent malaria or parasitemic episodes
The longitudinal nature of the Ngerenya children’s cohort enabled us to examine the effect of recent malaria or parasitemia episodes on acquisition or boosting of antibodies to different antigens. Within the Ngerenya 2002 cohort, antibody levels were compared for children who had 0 versus 1 or more recorded episodes of parasitemia or symptomatic malaria in the 6 or 12 mo before the October 2002 sample collection and antibody testing (Table 4). Comparisons were made among aparasitemic children only (n = 218), as concurrent parasitemia was associated with raised antibody levels to most antigens, as shown above. The pattern of antibody response was similar when either prior parasitemic episodes or disease episodes were considered. However, the strength of associations was generally stronger for history of parasitemic rather than malaria episodes. Children who had experienced 1 or more episodes of parasitemia in the 6 mo before the sample collection had significantly higher antibody levels toward MSP2(FC27), MSP4, AMA1, EBA140 RII, EBA175-F2, and PfRH2-2030 than children who had experienced no episodes. Children who had experienced parasitemia within the 12 mo before the October 2002 bleed also showed higher antibody levels to MSP1-19, MSP2(3D7), PfRH4-BR, and Pf38, in addition to other antigens, compared with those who had not. Antibody levels to the EBA III-V antigens and PfRH4-A3 were generally low, and no differences were seen. Antibody levels to schizont extract were also highest for children who had experienced parasitemic or disease episodes.
TABLE 4.
IgG response among children of the Ngerenya 2002 cohort, according to parasitemia or malaria episode in the previous 6 or 12 moa
| Antigensb (Median [p25–p75]) | Parasitemic episodes (previous 6 mo) |
Malaria episodes (previous 6 mo) |
Parasitemic episodes
(previous 12 mo) |
Malaria episodes
(previous 12 mo) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 (n = 179) | ≥1 (n = 39) | Sign. | Pc | 0 (n = 197) | ≥1 (n = 21) | Sign. | Pc | 0 (n = 116) | ≥1 (n = 102) | Sign. | Pc | 0 (n = 169) | ≥1 (n = 49) | Sign. | Pc | |
| MSP1-19 | 0.02 | 0.04 | 0.262 | 0.02 | 0.06 | 0.075 | 0.01 | 0.05 | * | <0.001 | 0.02 | 0.05 | * | 0.039 | ||
| [0.00–0.08] | [0.01–0.11] | [0.00–0.07] | [0.01–0.22] | [0.00–0.05] | [0.01–0.22] | [0.00–0.06] | [0.01–0.17] | |||||||||
| MSP2 | 0.05 | 0.06 | 0.052 | 0.04 | 0.06 | 0.285 | 0.03 | 0.06 | * | <0.001 | 0.03 | 0.07 | * | 0.012 | ||
| [0.02–0.09] | [0.02–0.18] | [0.02–0.09] | [0.02–0.18] | [0.02–0.05] | [0.03–0.20] | [0.02–0.08] | [0.03–0.18] | |||||||||
| MSP2(FC27) | 0.02 | 0.05 | * | 0.005 | 0.02 | 0.07 | * | 0.007 | 0.02 | 0.03 | * | 0.003 | 0.02 | 0.05 | * | 0.008 |
| [0.01–0.06] | [0.02–0.11] | [0.01–0.06] | [0.02–0.12] | [0.01–0.04] | [0.01–0.10] | [0.01–0.05] | [0.01–0.10] | |||||||||
| MSP4 | 0.09 | 0.43 | * | <0.001 | 0.10 | 0.41 | * | 0.004 | 0.03 | 0.36 | * | <0.001 | 0.07 | 0.43 | * | <0.001 |
| [0.01–0.34] | [0.12–0.87] | [0.02–0.37] | [0.12–0.67] | [0.00–0.13] | [0.11–0.78] | [0.01–0.25] | [0.12–0.87] | |||||||||
| AMA1 | 0.03 | 0.17 | * | 0.002 | 0.04 | 0.17 | * | 0.009 | 0.01 | 0.16 | * | <0.001 | 0.02 | 0.18 | * | <0.001 |
| [0.00–0.10] | [0.02–1.00] | [0.00–0.24] | [0.04–0.44] | [0.00–0.07] | [0.03–0.90] | [0.00–0.15] | [0.05–0.61] | |||||||||
| EBA140 | 0.02 | 0.01 | 0.657 | 0.02 | 0.01 | 0.872 | 0.02 | 0.01 | 0.109 | 0.02 | 0.01 | 0.155 | ||||
| [0.00–0.04] | [0.00–0.04] | [0.00–0.04] | [0.00–0.07] | [0.00–0.04] | [0.00–0.04] | [0.00–0.04] | [0.00–0.03] | |||||||||
| EBA181 | 0.06 | 0.08 | 0.196 | 0.06 | 0.06 | 0.728 | 0.06 | 0.07 | 0.941 | 0.06 | 0.06 | 0.869 | ||||
| [0.03–0.12] | [0.04–0.23] | [0.03–0.12] | [0.03–0.14] | [0.03–0.11] | [0.03–0.14] | [0.03–0.13] | [0.03–0.13] | |||||||||
| EBA175 | 0.00 | 0.00 | 0.721 | 0.00 | 0.00 | 0.720 | 0.00 | 0.00 | 0.295 | 0.00 | 0.00 | 0.171 | ||||
| [0.00–0.02] | [0.00–0.03] | [0.00–0.02] | [0.00–0.01] | [0.00–0.02] | [0.00–0.02] | [0.00–0.02] | [0.00–0.01] | |||||||||
| EBA140 RIId | 0.03 | 0.07 | * | <0.001 | 0.03 | 0.09 | * | <0.001 | 0.02 | 0.06 | * | <0.001 | 0.03 | 0.09 | * | <0.001 |
| [0.01–0.07] | [0.04–0.17] | [0.01–0.07] | [0.05–0.17] | [0.01–0.04] | [0.04–0.19] | [0.01–0.06] | [0.05–0.23] | |||||||||
| EBA175-F2d | 0.14 | 0.29 | * | 0.003 | 0.14 | 0.31 | 0.059 | 0.11 | 0.23 | * | <0.001 | 0.14 | 0.19 | 0.054 | ||
| [0.07–0.25] | [0.12–0.54] | [0.08–0.26] | [0.10–0.55] | [0.06–0.19] | [0.13–0.57] | [0.08–0.26] | [0.10–0.54] | |||||||||
| RH2-2030d | 0.04 | 0.06 | * | 0.029 | 0.04 | 0.06 | 0.257 | 0.04 | 0.06 | * | <0.001 | 0.04 | 0.05 | 0.154 | ||
| [0.03–0.09] | [0.03–0.22] | [0.03–0.09] | [0.03–0.10] | [0.02–0.07] | [0.03–0.15] | [0.03–0.09] | [0.03–0.15] | |||||||||
| RH4-A3 | 0.02 | 0.00 | 0.247 | 0.02 | 0.00 | 0.161 | 0.02 | 0.02 | 0.686 | 0.02 | 0.01 | 0.386 | ||||
| [0.00–0.08] | [0.00–0.10] | [0.00–0.08] | [0.00 0.03] | [0.00–0.07] | [0.00–0.10] | [0.00–0.08] | [0.00–0.06] | |||||||||
| RH4-BRd | 0.05 | 0.06 | 0.123 | 0.05 | 0.06 | 0.806 | 0.04 | 0.06 | * | <0.001 | 0.05 | 0.06 | 0.061 | |||
| [0.02–0.11] | [0.03–0.17] | [0.03–0.12] | [0.03–0.13] | [0.02–0.09] | [0.03–0.19] | [0.02–0.11] | 0.04–0.19] | |||||||||
| Pf38d | 0.14 | 0.17 | 0.362 | 0.15 | 0.17 | 0.671 | 0.12 | 0.18 | * | 0.016 | 0.14 | 0.17 | 0.229 | |||
| [0.08–0.33] | [0.09–0.31] | [0.08–0.34] | [0.09–0.28] | [0.08–0.25] | [0.09–0.37] | [0.08–0.32] | [0.09–0.34] | |||||||||
| Schizont extract | 0.10 | 0.26 | * | <0.001 | 0.11 | 0.27 | * | 0.001 | 0.06 | 0.26 | * | <0.001 | 0.09 | 0.25 | * | <0.001 |
| [0.02–0.29] | [0.13–0.55] | [0.04–0.31] | [0.15–0.54] | [0.02–0.15] | [0.11–0.56] | [0.03–0.29] | [0.15–0.55] | |||||||||
Sign., Significance.
Comparisons were made using only cohort children who were aparasitemic at the time of blood collection (n = 218).
All alleles are 3D7 unless otherwise specified.
P values calculated using a Kruskal-Wallis test.
For these antigens, insufficient serum volume resulted in missing data for a single child with no history of parasitemia or malaria episodes in the previous 12 mo.
P ≤ 0.05.
Relatedness among responses to different antigens
Significant correlations existed among IgG levels to most merozoite antigens. However, the strength of correlation varied greatly for different antigen pairs and reflected the differing antigen-specific rates of antibody acquisition already noted (Supplemental Tables 2 and 3). For the Ngerenya 1998 cohort, a strong correlation of responses (P < 0.001) existed among variants of the same antigen [AMA1 (rs = 0.95), EBA175 (rs = 0.75), MSP2 (rs = 0.75), MSP1 (rs = 0.75), and PfRH2 (rs = 0.84)], among the 3D7 EBA proteins (ranging from rs = 0.60 to rs = 0.69), and among antigens for which an early antibody acquisition pattern was observed {MSP4 and AMA1 [AMA1(3D7), rs = 0.71, and AMA1(W2mef), rs = 0.70]}. In general, correlations for the IgG responses to the 6-cys antigens and to PfRH4 were weak compared with the responses to other merozoite antigens. However, correlations for IgG to Pf12 with IgG to AMA1, MSP4, and MSP2 were strong (rs range from 0.54 to 0.60; P < 0.001), reflecting an early antibody response for this antigen.
The breadth of antibody response toward multiple antigens
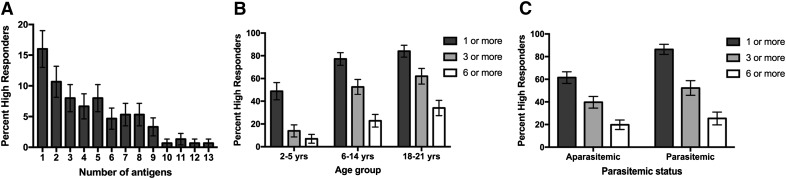
Within the Ngerenya 1998 cohort, ∼71% (n = 107) of individuals were high IgG responders (defined as responses in the top quartile) to at least 1 merozoite antigen, and 45% (n = 67) and 22% (n = 33) of individuals were high responders toward 3 or more or 6 or more antigens, respectively (Fig. 2A). The proportion of individuals who were high IgG responders increased with age group (P < 0.007; χ2 test), demonstrating an increasing breadth of antibody response with age (Fig. 2B). The proportion of individuals who were high responders to 1 or more antigens was positively associated with parasitemia (P < 0.001; Fig. 2C), but the association was not significant for ≥3 or ≥6 antigens (P > 0.118).
Figure 2. The proportion of high antibody responders to multiple antigens varies with age group within the Ngerenya 1998 cohort.
(A) The proportion of individuals who were high antibody responders to 0, 1, or multiple antigens. The response to 3D7 allelic variants and a single construct of each antigen were considered, and this totaled 13 different merozoite antigens. Error bars represent the se. (B) The proportion of high responders by age group to 1 or more, 3 or more, or 6 or more merozoite antigens, for a total of 13 different merozoite antigens. Error bars represent the se. (C) The proportion of high responders by parasitemic status to 1 or more, 3 or more, or 6 or more merozoite antigens, for a total of 13 different merozoite antigens. Error bars represent the se. P values are reported in the text. High responders were those individuals with ELISA IgG levels ≥75th percentile value.
DISCUSSION
We currently have only a partial knowledge of the complex humoral response directed toward the malaria merozoite. Until recently, the focus of studies of human immunity has been directed toward a small number of potential vaccine candidate antigens. The limited number of studies investigating the antibody responses against multiple merozoite antigens of Pf has restricted examination and comparison of the differing features of the antigen-specific responses. In this study, we used sera from a cohort of Kenyan adults and children to establish understanding of antibody profiles toward a panel of merozoite antigens. Similar investigations were also performed using samples collected at a time of lower malaria transmission from young children of the same community. This approach allowed comparison of antibody responses toward different antigens, between similar and dissimilar antigenic regions, as well as among children of both cohorts. An increase in antibody response was generally seen with age, exposure, and parasitemia. However, we showed varying profiles of antibody responses toward different merozoite antigens and in some cases, toward different regions of the same antigen. This variation in antibody response profiles occurred in an antigen-specific manner, despite differing malaria transmission levels for the 2 cohorts, and became most apparent as a result of our ability to assess responses in adults as well as children. These antigen-specific patterns of antibody response and their associations with epidemiologically relevant measures of exposure are summarized in Table 5.
TABLE 5.
Pattern of antibody acquisition toward merozoite antigens among the Ngerenya 1998 and 2002 cohorts
| Antigena | Rate of acquisition by ageb | Association with parasitemiac | Induction following episodesd |
|---|---|---|---|
| MSP1-19 | Late | Weak | Medium |
| MSP2 | Medium | Strong | Medium |
| MSP4 | Early | Strong | Strong |
| AMA1 | Early | Strong | Strong |
| EBA140 RIII-V | Late | Weak | Weak |
| EBA181 RIII-V | Late | Weak | Weak |
| EBA175 RIII-V | Late | Weak | Weak |
| EBA175-F2 | Late | Strong | Medium |
| EBA140 RII | – | Strong | Strong |
| RH2-2030 | Early | Strong | Medium |
| RH4-A3 | Late | Strong | Weak |
| SERA5 | Medium | Weak | – |
| Pf41 | Early | Weak | – |
All alleles were 3D7.
Determined by assessing antibody responses among adults and children of the Ngerenya 1998 cohort.
Determined by assessing antibody response results among children of the Ngerenya 1998 cohort
(2–14 yr old) and Ngerenya 2002 cohort (1–8 yr old).
Determined by assessing antibody responses among the Ngerenya 2002 cohort following a recorded episode of parasitemia or malaria in the previous 6 or 12 mo.
Age-associated increases in antibody levels have been shown toward merozoite antigens in many sero-epidemiologic studies and are thought to reflect cumulative exposure to malaria [42]. However, an early rate of antibody acquisition, with relatively prominent responses present among children was seen toward particular antigens, such as AMA1, and to a lesser extent, toward MSP4, PfRH2, Pf41, and Pf12. In comparison, a late development of the antibody response, with prominence of this response only among adults of the same cohort, was evident for other antigens, such as MSP1-19, PfRH4, and the EBA(III-V) antigens. Antibody levels were also strongly influenced by parasitemia, particularly among children, with a rise in antibody levels to merozoite antigens with parasitemia most prominent as part of the early antibody response among children, with a pattern suggesting that more stable seroprevalence develops subsequently among older children and adults. However, the antibody response associated with parasitemia also reflected antigen specificity, most evident in the Ngerenya 1998 cohort; among children, elevated antibody levels associated with parasitemia were seen only for those antigens, such as AMA1, that induced an early response, whereas among parasitemic adults and children, an age-associated antibody increase was seen for the late response antigens, such as MSP1-19 and the EBA RIII-V antigens, but not for early response-type antigens.
For this study, assay conditions were optimized for individual antigens, so antibody response patterns, but not absolute OD values, can be compared between different antigens and cohorts. Validation of protein regions used in ELISA assays has been carried out previously [5, 38], suggesting that the antigens used do, at least partially, reflect the native conformation of merozoite proteins. Additionally, responses to all antigens were associated, to varying degrees, with age and parasitemia, and there were little or no responses among unexposed Melbourne donors, further validating protein suitability for use in measurement of the malaria-specific immune response. Among the Ngerenya 1998 cohort, the levels of antibody to the 3 6-cys proteins varied greatly and were not associated with age. The response was particularly low toward Pf38, despite testing at high antigen and antibody concentrations. These merozoite surface antigens are exposed to the immune system [43], and Pf12 and Pf38 are present at copy numbers similar to well-characterized MSPs [44]. An SDS-PAGE of the recombinant proteins showed no major protein breakdown, and assay validity was suggested by the high level of reactivity toward each antigen, with positive control sera and some cohort samples, and little reactivity by samples from nonexposed donors. The folding of these recombinant proteins has been established in previous studies [43, 45], suggesting that the results with the 6-cys proteins are not explained by lack of folding.
To understand further the acquisition of antibodies and gain insights into the potential use of various antibodies as biomarkers of exposure for serosurveillance applications, we evaluated whether children with recent malaria or parasitemia episodes had higher antibody levels compared with children who had not had recent infection. The use of longitudinal data from the Ngerenya 2002 cohort showed that children who had experienced previous episodes of parasitemia or disease had higher antibody responses to those antigens that induced an early-type antibody response, such as MSP4 and AMA1. In comparison, no differences in antibody levels in relation to recent infection were observed toward the EBA III-V antigens in which responses were generally low among children (Table 5). Antibody responses directed toward the early response-type antigens therefore appear to provide a more sensitive marker of recent previous exposure among young children than that to other tested merozoite antigens. A number of recent studies have also provided new data on antigen-specific responses that may be valuable biomarkers of exposure. For example, these include MSP2, AMA1, and the EBAs [46–49] among the antigens we have examined in this study. Comparisons of merozoite antibodies between cohorts of children with different ages and therefore, different levels of exposure and immunity suggested that antibodies to merozoite antigens act as good biomarkers of malaria risk in populations with low-background immunity but evolve to become biomarkers of immunity in those with higher cumulative exposure and higher levels of immunity [46]. In young children, antibodies to merozoite antigens were also shown to be closely related to the molecular force of infection (the number of infections with new Pf genotypes during the period of follow-up) [46], supporting the principle that repeated infections lead to the gradual acquisition of high-level antibodies to merozoite antigens. A valuable extension of these findings would be to evaluate the acquisition of functional antibodies to specific antigens. Unfortunately, this is not presently possible, as assays to evaluate antigen-specific, functional antibodies to a range of merozoite antigens are lacking.
There are several potential reasons for the differing rates of antibody acquisition to merozoite antigens. Our study results suggest that there are differences in the inherent immunogenicity of different malarial antigens or epitopes, which has been previously proposed [25, 27, 50–54]. Observed variations in antibody response patterns to different antigens suggest differential recognition or handling of antigens by the immune system or differing antigen-specific sensitivities or requirements for antibody boosting or memory responses. The rate of antibody response may reflect antigen structural properties, including protein sequence features, secondary or tertiary structural features, or accessibility of epitopes to immune processing [50, 55, 56]. For example, the repetitive amino acid sequences, present in some antigens (e.g., MSP2), appear to be immunodominant [56], and the complex disulfide-bonded structure of antigens, such as MSP1-19, has been suggested to impede antigen processing or presentation [50, 55]. The rate of age-associated immune maturation [3] may also vary among antigens. The decay rates of antibodies toward merozoite epitopes are not fully understood. Age, transmission intensity, and exposure history are influencing factors [27, 49, 57, 58], although further studies are required to determine whether specific merozoite antigens elicit longer-lived responses [59]. The predominant subclass of antibodies produced has been shown to be antigen specific [22, 60], but broad antigen properties did not predict the antigen-specific profiles of IgG acquisition in this population (data not shown). The rates of conversion of B cells to secretory states or long-lived plasma cells [25, 42] and their propensity for apoptosis [61] or conversion to "exhausted" states [62–64] following malaria infection could also differ with antigen specificity, influencing age-seroprevalence profiles.
Several approaches were used to evaluate the repertoire and relatedness among antibody responses. Strong correlation of antibody responses was shown among those antigens found to have an early antibody acquisition pattern within both the Ngerenya 1998 and the Ngerenya 2002 cohorts. Additionally, the very strong correlations of IgG responses shown for the alternative allelic variants of MSP2 and AMA1 suggested coacquisition of antibodies to the different alleles. We explored the use of principle component analyses but did not find that this added substantially to our interpretation and analysis of data. Further identification and comparison of the varying antibody response rates to multiple merozoite antigens may contribute to understanding better the basis of these differing response patterns, including antigenic features that predict immunogenicity. Knowledge of these complex patterns of evolution of antibody responses against the pattern of clinical immune acquisition may aid in identifying immunologic responses that mediate anti-parasite or age-related immunity [1, 3, 65]; for example, the early-type IgG responses by young children to antigens, such as AMA1, may contribute to immune acquisition against severe and symptomatic malaria, whereas the later-type IgG responses apparent to multiple antigens could increase the levels of immunity and contribute to an overall effective response.
An increased understanding of the differing patterns of antibody acquisition to merozoite antigens could prove particularly valuable in the public health application of malaria serology [33]. As an increasing number of countries progress toward malaria elimination [66], there has been refocus on the potential role of malaria sero-epidemiology as a low-cost population screening tool, particularly when transmission levels are low [33, 67]. Population serological responses have been used to indicate malaria transmission levels and changes over time [27, 32, 68], as well as to identify foci of malaria transmission [69]. Currently, only a limited number of merozoite antigens have been assessed as sero-epidemiological markers of Pf malaria [27, 28, 46, 47, 49, 70]. A comprehensive understanding of the induction of antibodies, according to antigen specificity, level of exposure, and association with current or recent infection, could aid the tailored selection of antigens for serosurveillance, and these results have been summarized for our study in Table 5. For example, antibodies to MSP4, as well as AMA1, were acquired early and were readily inducible following malaria exposure, so testing against both of these antigens could increase sero-epidemiologic sensitivity in areas of low transmission. Alternatively, serological testing, including the EBA antigens, as well as MSP1-19, could inform the existence of established malaria transmission in a target population. Further studies, such as this one, which increase knowledge of the specific age-seroprevalence response profiles to multiple antigens, particularly among younger age groups and under conditions of differing transmission, may aid development of a robust sero-epidemiologic tool in which a suitable antigen or antigen combination could be selected according to epidemiologic circumstance or study-specific requirements [27, 33, 69, 71].
In conclusion, with the comparison among malaria-exposed adult and children of the antibody responses to a range of merozoite antigens or antigenic regions, we showed that the patterns of antibody acquisition varied in an antigen-specific manner, despite differing malaria-transmission levels. Our results suggested that antibodies to some antigens are acquired early in exposure to malaria, whereas other responses are acquired later, after greater exposure to malaria. We also identified particular responses as potentially sensitive markers of recent malaria infection among young children. Further, similar studies that allow comparison of antibody-response profiles to multiple antigenic regions, with similar as well as dissimilar features and across a variety of age groups and malaria transmission levels, may improve our ability to detect and predict these differing responses. This broad approach presents a rational and efficient use of epidemiologic studies and provides information that may aid development of sero-epidemiologic tools. Furthermore, the identification of proteins or protein regions that are naturally immunogenic in humans and the understanding of how they are acquired and boosted by recent infection may aid the selection of antigens for vaccine development and vaccine evaluation.
AUTHORSHIP
Study design was performed by F.J.M., C.K.M., B.S.C., T.N.W., K.M., and J.G.B. Experiments were done by F.J.M., K.E.M.P., and L.R. Samples and reagents were provided by J.S.R., T.N.W., P.R.G., A.N.H., P.R.S., R.F.A., D.L.N., C.C., B.S.C., K.M., and J.G.B. Data analysis and interpretation were implemented by F.J.M., L.R., F.J.I.F., C.K.M., J.A.S., B.S.C., K.M., and J.G.B. The manuscript was written by F.J.M. and J.G.B. with input from all authors.
Acknowledgments
Funding was provided by the National Health and Medical Research Council of Australia (project grant, program grant, and senior research fellowship to J.G.B.; Infrastructure for Research Institutes Support Scheme Grant; postgraduate research fellowships to F.J.M.; and Early Career Fellowship to J.S.R.); the Wellcome Trust (project grant to K.M. and J.G.B.; program grant to K.M.; and fellowships to K.M. and T.N.W.); Australian Research Council (Future Fellowship to F.J.I.F.); and Victorian State Government Operational Infrastructure Support grant. This research was supported, in part, by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, U.S. National Institutes of Health. The authors thank all study participants and staff at the Kenya Medical Research Institute (KEMRI; Kilifi), Ross Coppel (Monash University for providing recombinant MSP4), and Arzum Cubuk (Burnet Institute) for help with correcting the manuscript.
Glossary
- AMA1
apical membrane antigen 1
- EBA
erythrocyte-binding antigen
- IQR
interquartile range
- MBP
maltose-binding protein
- MSP
merozoite surface protein
- Pf
Plasmodium falciparum
- PfRH
Plasmodium falciparum reticulocyte-binding homologue
- rs
Spearman’s rho
- SERA5
serine repeat antigen 5
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflict of interest. This paper is published with the permission of the director of KEMRI. The views expressed in this report are those of the authors and do not reflect the policies or views of the Australian Defence Force.
REFERENCES
- 1.Marsh K., Kinyanjui S. (2006) Immune effector mechanisms in malaria. Parasite Immunol. 28, 51–60. [DOI] [PubMed] [Google Scholar]
- 2.Richards J. S., Beeson J. G. (2009) The future for blood-stage vaccines against malaria. Immunol. Cell Biol. 87, 377–390. [DOI] [PubMed] [Google Scholar]
- 3.Doolan D. L., Dobaño C., Baird J. K. (2009) Acquired immunity to malaria. Clin. Microbiol. Rev. 22, 13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowkes F. J., Richards J. S., Simpson J. A., Beeson J. G. (2010) The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: a systematic review and meta-analysis. PLoS Med. 7, e1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richards J. S., Arumugam T. U., Reiling L., Healer J., Hodder A. N., Fowkes F. J., Cross N., Langer C., Takeo S., Uboldi A. D., Thompson J. K., Gilson P. R., Coppel R. L., Siba P. M., King C. L., Torii M., Chitnis C. E., Narum D. L., Mueller I., Crabb B. S., Cowman A. F., Tsuboi T., Beeson J. G. (2013) Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J. Immunol. 191, 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osier F. H., Mackinnon M. J., Crosnier C., Fegan G., Kamuyu G., Wanaguru M., Ogada E., McDade B., Rayner J. C., Wright G. J., Marsh K. (2014) New antigens for a multicomponent blood-stage malaria vaccine. Sci. Transl. Med. 6, 247ra102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beeson J. G., Drew D. R., Boyle M. J., Feng G., Fowkes F. J., Richards J. S. (2016) Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol. Rev. 40, 343–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egan A. F., Burghaus P., Druilhe P., Holder A. A., Riley E. M. (1999) Human antibodies to the 19kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 21, 133–139. [DOI] [PubMed] [Google Scholar]
- 9.Hodder A. N., Crewther P. E., Anders R. F. (2001) Specificity of the protective antibody response to apical membrane antigen 1. Infect. Immun. 69, 3286–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu C. Y., Hodder A. N., Lin C. S., Hill D. L., Li Wai Suen C. S., Schofield L., Siba P. M., Mueller I., Cowman A. F., Hansen D. S. (2015) Antibodies to the Plasmodium falciparum proteins MSPDBL1 and MSPDBL2 opsonize merozoites, inhibit parasite growth, and predict protection from clinical malaria. J. Infect. Dis. 212, 406–415. [DOI] [PubMed] [Google Scholar]
- 11.Tran T. M., Ongoiba A., Coursen J., Crosnier C., Diouf A., Huang C. Y., Li S., Doumbo S., Doumtabe D., Kone Y., Bathily A., Dia S., Niangaly M., Dara C., Sangala J., Miller L. H., Doumbo O. K., Kayentao K., Long C. A., Miura K., Wright G. J., Traore B., Crompton P. D. (2014) Naturally acquired antibodies specific for Plasmodium falciparum reticulocyte-binding protein homologue 5 inhibit parasite growth and predict protection from malaria. J. Infect. Dis. 209, 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiling L., Richards J. S., Fowkes F. J., Wilson D. W., Chokejindachai W., Barry A. E., Tham W. H., Stubbs J., Langer C., Donelson J., Michon P., Tavul L., Crabb B. S., Siba P. M., Cowman A. F., Mueller I., Beeson J. G. (2012) The Plasmodium falciparum erythrocyte invasion ligand Pfrh4 as a target of functional and protective human antibodies against malaria. PLoS One 7, e45253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osier F. H., Feng G., Boyle M. J., Langer C., Zhou J., Richards J. S., McCallum F. J., Reiling L., Jaworowski A., Anders R. F., Marsh K., Beeson J. G. (2014) Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med. 12, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill D. L., Eriksson E. M., Li Wai Suen C. S., Chiu C. Y., Ryg-Cornejo V., Robinson L. J., Siba P. M., Mueller I., Hansen D. S., Schofield L. (2013) Opsonising antibodies to P. falciparum merozoites associated with immunity to clinical malaria. PLoS One 8, e74627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joos C., Marrama L., Polson H. E., Corre S., Diatta A. M., Diouf B., Trape J. F., Tall A., Longacre S., Perraut R. (2010) Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PLoS One 5, e9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouharoun-Tayoun H., Oeuvray C., Lunel F., Druilhe P. (1995) Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 182, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle M. J., Reiling L., Feng G., Langer C., Osier F. H., Aspeling-Jones H., Cheng Y. S., Stubbs J., Tetteh K. K., Conway D. J., McCarthy J. S., Muller I., Marsh K., Anders R. F., Beeson J. G. (2015) Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity 42, 580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dent A. E., Nakajima R., Liang L., Baum E., Moormann A. M., Sumba P. O., Vulule J., Babineau D., Randall A., Davies D. H., Felgner P. L., Kazura J. W. (2015) Plasmodium falciparum protein microarray antibody profiles correlate with protection from symptomatic malaria in Kenya. J. Infect. Dis. 212, 1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osier F. H., Fegan G., Polley S. D., Murungi L., Verra F., Tetteh K. K., Lowe B., Mwangi T., Bull P. C., Thomas A. W., Cavanagh D. R., McBride J. S., Lanar D. E., Mackinnon M. J., Conway D. J., Marsh K. (2008) Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect. Immun. 76, 2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray J. C., Corran P. H., Mangia E., Gaunt M. W., Li Q., Tetteh K. K., Polley S. D., Conway D. J., Holder A. A., Bacarese-Hamilton T., Riley E. M., Crisanti A. (2007) Profiling the antibody immune response against blood stage malaria vaccine candidates. Clin. Chem. 53, 1244–1253. [DOI] [PubMed] [Google Scholar]
- 21.Meraldi V., Nebié I., Tiono A. B., Diallo D., Sanogo E., Theisen M., Druilhe P., Corradin G., Moret R., Sirima B. S. (2004) Natural antibody response to Plasmodium falciparum Exp-1, MSP-3 and GLURP long synthetic peptides and association with protection. Parasite Immunol. 26, 265–272. [DOI] [PubMed] [Google Scholar]
- 22.Stanisic D. I., Richards J. S., McCallum F. J., Michon P., King C. L., Schoepflin S., Gilson P. R., Murphy V. J., Anders R. F., Mueller I., Beeson J. G. (2009) Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect. Immun. 77, 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards J. S., Stanisic D. I., Fowkes F. J., Tavul L., Dabod E., Thompson J. K., Kumar S., Chitnis C. E., Narum D. L., Michon P., Siba P. M., Cowman A. F., Mueller I., Beeson J. G. (2010) Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin. Infect. Dis. 51, e50–e60. [DOI] [PubMed] [Google Scholar]
- 24.Osier F. H., Weedall G. D., Verra F., Murungi L., Tetteh K. K., Bull P., Faber B. W., Remarque E., Thomas A., Marsh K., Conway D. J. (2010) Allelic diversity and naturally acquired allele-specific antibody responses to Plasmodium falciparum apical membrane antigen 1 in Kenya. Infect. Immun. 78, 4625–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crompton P. D., Kayala M. A., Traore B., Kayentao K., Ongoiba A., Weiss G. E., Molina D. M., Burk C. R., Waisberg M., Jasinskas A., Tan X., Doumbo S., Doumtabe D., Kone Y., Narum D. L., Liang X., Doumbo O. K., Miller L. H., Doolan D. L., Baldi P., Felgner P. L., Pierce S. K. (2010) A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc. Natl. Acad. Sci. USA 107, 6958–6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doolan D. L., Mu Y., Unal B., Sundaresh S., Hirst S., Valdez C., Randall A., Molina D., Liang X., Freilich D. A., Oloo J. A., Blair P. L., Aguiar J. C., Baldi P., Davies D. H., Felgner P. L. (2008) Profiling humoral immune responses to P. falciparum infection with protein microarrays. Proteomics 8, 4680–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drakeley C. J., Corran P. H., Coleman P. G., Tongren J. E., McDonald S. L., Carneiro I., Malima R., Lusingu J., Manjurano A., Nkya W. M., Lemnge M. M., Cox J., Reyburn H., Riley E. M. (2005) Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc. Natl. Acad. Sci. USA 102, 5108–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noland G. S., Hendel-Paterson B., Min X. M., Moormann A. M., Vulule J. M., Narum D. L., Lanar D. E., Kazura J. W., John C. C. (2008) Low prevalence of antibodies to preerythrocytic but not blood-stage Plasmodium falciparum antigens in an area of unstable malaria transmission compared to prevalence in an area of stable malaria transmission. Infect. Immun. 76, 5721–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pratt-Riccio L. R., Lima-Junior J. C., Carvalho L. J., Theisen M., Espíndola-Mendes E. C., Santos F., Oliveira-Ferreira J., Goldberg A. C., Daniel-Ribeiro C. T., Banic D. M. (2005) Antibody response profiles induced by Plasmodium falciparum glutamate-rich protein in naturally exposed individuals from a Brazilian area endemic for malaria. Am. J. Trop. Med. Hyg. 73, 1096–1103. [PubMed] [Google Scholar]
- 30.Osier F. H., Polley S. D., Mwangi T., Lowe B., Conway D. J., Marsh K. (2007) Naturally acquired antibodies to polymorphic and conserved epitopes of Plasmodium falciparum merozoite surface protein 3. Parasite Immunol. 29, 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Persson K. E. (2010) Erythrocyte invasion and functionally inhibitory antibodies in Plasmodium falciparum malaria. Acta Trop. 114, 138–143. [DOI] [PubMed] [Google Scholar]
- 32.Cook J., Reid H., Iavro J., Kuwahata M., Taleo G., Clements A., McCarthy J., Vallely A., Drakeley C. (2010) Using serological measures to monitor changes in malaria transmission in Vanuatu. Malar. J. 9, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elliott S. R., Fowkes F. J., Richards J. S., Reiling L., Drew D. R., Beeson J. G. (2014) Research priorities for the development and implementation of serological tools for malaria surveillance. F1000Prime Rep. 6, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mbogo C. N., Snow R. W., Khamala C. P., Kabiru E. W., Ouma J. H., Githure J. I., Marsh K., Beier J. C. (1995) Relationships between Plasmodium falciparum transmission by vector populations and the incidence of severe disease at nine sites on the Kenyan coast. Am. J. Trop. Med. Hyg. 52, 201–206. [DOI] [PubMed] [Google Scholar]
- 35.Mwangi T. W., Ross A., Snow R. W., Marsh K. (2005) Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J. Infect. Dis. 191, 1932–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nyakeriga A. M., Troye-Blomberg M., Dorfman J. R., Alexander N. D., Bäck R., Kortok M., Chemtai A. K., Marsh K., Williams T. N. (2004) Iron deficiency and malaria among children living on the coast of Kenya. J. Infect. Dis. 190, 439–447. [DOI] [PubMed] [Google Scholar]
- 37.O’Meara W. P., Mwangi T. W., Williams T. N., McKenzie F. E., Snow R. W., Marsh K. (2008) Relationship between exposure, clinical malaria, and age in an area of changing transmission intensity. Am. J. Trop. Med. Hyg. 79, 185–191. [PMC free article] [PubMed] [Google Scholar]
- 38.Persson K. E., McCallum F. J., Reiling L., Lister N. A., Stubbs J., Cowman A. F., Marsh K., Beeson J. G. (2008) Variation in use of erythrocyte invasion pathways by Plasmodium falciparum mediates evasion of human inhibitory antibodies. J. Clin. Invest. 118, 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishnarjuna B., Andrew D., MacRaild C. A., Morales R. A., Beeson J. G., Anders R. F., Richards J. S., Norton R. S. (2016) Strain-transcending immune response generated by chimeras of the malaria vaccine candidate merozoite surface protein 2. Sci. Rep. 6, 20613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terheggen U., Drew D. R., Hodder A. N., Cross N. J., Mugyenyi C. K., Barry A. E., Anders R. F., Dutta S., Osier F. H., Elliott S. R., Senn N., Stanisic D. I., Marsh K., Siba P. M., Mueller I., Richards J. S., Beeson J. G. (2014) Limited antigenic diversity of Plasmodium falciparum apical membrane antigen 1 supports the development of effective multi-allele vaccines. BMC Med. 12, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson D. W., Fowkes F. J., Gilson P. R., Elliott S. R., Tavul L., Michon P., Dabod E., Siba P. M., Mueller I., Crabb B. S., Beeson J. G. (2011) Quantifying the importance of MSP1-19 as a target of growth-inhibitory and protective antibodies against Plasmodium falciparum in humans. PLoS One 6, e27705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss G. E., Traore B., Kayentao K., Ongoiba A., Doumbo S., Doumtabe D., Kone Y., Dia S., Guindo A., Traore A., Huang C. Y., Miura K., Mircetic M., Li S., Baughman A., Narum D. L., Miller L. H., Doumbo O. K., Pierce S. K., Crompton P. D. (2010) The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLoS Pathog. 6, e1000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanders P. R., Gilson P. R., Cantin G. T., Greenbaum D. C., Nebl T., Carucci D. J., McConville M. J., Schofield L., Hodder A. N., Yates J. R. III, Crabb B. S. (2005) Distinct protein classes including novel merozoite surface antigens in Raft-like membranes of Plasmodium falciparum. J. Biol. Chem. 280, 40169–40176. [DOI] [PubMed] [Google Scholar]
- 44.Gilson P. R., Nebl T., Vukcevic D., Moritz R. L., Sargeant T., Speed T. P., Schofield L., Crabb B. S. (2006) Identification and stoichiometry of glycosylphosphatidylinositol-anchored membrane proteins of the human malaria parasite Plasmodium falciparum. Mol. Cell. Proteomics 5, 1286–1299. [DOI] [PubMed] [Google Scholar]
- 45.Taechalertpaisarn T., Crosnier C., Bartholdson S. J., Hodder A. N., Thompson J., Bustamante L. Y., Wilson D. W., Sanders P. R., Wright G. J., Rayner J. C., Cowman A. F., Gilson P. R., Crabb B. S. (2012) Biochemical and functional analysis of two Plasmodium falciparum blood-stage 6-cys proteins: P12 and P41. PLoS One 7, e41937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanisic D. I., Fowkes F. J., Koinari M., Javati S., Lin E., Kiniboro B., Richards J. S., Robinson L. J., Schofield L., Kazura J. W., King C. L., Zimmerman P., Felger I., Siba P. M., Mueller I., Beeson J. G. (2015) Acquisition of antibodies against Plasmodium falciparum merozoites and malaria immunity in young children and the influence of age, force of infection, and magnitude of response. Infect. Immun. 83, 646–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Helb D. A., Tetteh K. K., Felgner P. L., Skinner J., Hubbard A., Arinaitwe E., Mayanja-Kizza H., Ssewanyana I., Kamya M. R., Beeson J. G., Tappero J., Smith D. L., Crompton P. D., Rosenthal P. J., Dorsey G., Drakeley C. J., Greenhouse B. (2015) Novel serologic biomarkers provide accurate estimates of recent Plasmodium falciparum exposure for individuals and communities. Proc. Natl. Acad. Sci. USA 112, E4438–E4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baum E., Badu K., Molina D. M., Liang X., Felgner P. L., Yan G. (2013) Protein microarray analysis of antibody responses to Plasmodium falciparum in western Kenyan highland sites with differing transmission levels. PLoS One 8, e82246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ondigo B. N., Hodges J. S., Ireland K. F., Magak N. G., Lanar D. E., Dutta S., Narum D. L., Park G. S., Ofulla A. V., John C. C. (2014) Estimation of recent and long-term malaria transmission in a population by antibody testing to multiple Plasmodium falciparum antigens. J. Infect. Dis. 210, 1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egan A., Waterfall M., Pinder M., Holder A., Riley E. (1997) Characterization of human T- and B-cell epitopes in the C terminus of Plasmodium falciparum merozoite surface protein 1: evidence for poor T-cell recognition of polypeptides with numerous disulfide bonds. Infect. Immun. 65, 3024–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L., Richie T. L., Stowers A., Nhan D. H., Coppel R. L. (2001) Naturally acquired antibody responses to Plasmodium falciparum merozoite surface protein 4 in a population living in an area of endemicity in Vietnam. Infect. Immun. 69, 4390–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woehlbier U., Epp C., Kauth C. W., Lutz R., Long C. A., Coulibaly B., Kouyaté B., Arevalo-Herrera M., Herrera S., Bujard H. (2006) Analysis of antibodies directed against merozoite surface protein 1 of the human malaria parasite Plasmodium falciparum. Infect. Immun. 74, 1313–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polley S. D., Mwangi T., Kocken C. H., Thomas A. W., Dutta S., Lanar D. E., Remarque E., Ross A., Williams T. N., Mwambingu G., Lowe B., Conway D. J., Marsh K. (2004) Human antibodies to recombinant protein constructs of Plasmodium falciparum apical membrane antigen 1 (AMA1) and their associations with protection from malaria. Vaccine 23, 718–728. [DOI] [PubMed] [Google Scholar]
- 54.Ford L., Lobo C. A., Rodriguez M., Zalis M. G., Machado R. L., Rossit A. R., Cavasini C. E., Couto A. A., Enyong P. A., Lustigman S. (2007) Differential antibody responses to Plasmodium falciparum invasion ligand proteins in individuals living in malaria-endemic areas in Brazil and Cameroon. Am. J. Trop. Med. Hyg. 77, 977–983. [PubMed] [Google Scholar]
- 55.Hensmann M., Li C., Moss C., Lindo V., Greer F., Watts C., Ogun S. A., Holder A. A., Langhorne J. (2004) Disulfide bonds in merozoite surface protein 1 of the malaria parasite impede efficient antigen processing and affect the in vivo antibody response. Eur. J. Immunol. 34, 639–648. [DOI] [PubMed] [Google Scholar]
- 56.Anders R. F., Coppel R. L., Brown G. V., Kemp D. J. (1988) Antigens with repeated amino acid sequences from the asexual blood stages of Plasmodium falciparum. Prog. Allergy 41, 148–172. [PubMed] [Google Scholar]
- 57.Akpogheneta O. J., Duah N. O., Tetteh K. K., Dunyo S., Lanar D. E., Pinder M., Conway D. J. (2008) Duration of naturally acquired antibody responses to blood-stage Plasmodium falciparum is age dependent and antigen specific. Infect. Immun. 76, 1748–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fowkes F. J., McGready R., Cross N. J., Hommel M., Simpson J. A., Elliott S. R., Richards J. S., Lackovic K., Viladpai-Nguen J., Narum D., Tsuboi T., Anders R. F., Nosten F., Beeson J. G. (2012) New insights into acquisition, boosting, and longevity of immunity to malaria in pregnant women. J. Infect. Dis. 206, 1612–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fowkes F. J., Boeuf P., Beeson J. G. (2016) Immunity to malaria in an era of declining malaria transmission. Parasitology 143, 139–153. [DOI] [PubMed] [Google Scholar]
- 60.Tongren J. E., Drakeley C. J., McDonald S. L., Reyburn H. G., Manjurano A., Nkya W. M., Lemnge M. M., Gowda C. D., Todd J. E., Corran P. H., Riley E. M. (2006) Target antigen, age, and duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect. Immun. 74, 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wykes M. N., Zhou Y. H., Liu X. Q., Good M. F. (2005) Plasmodium yoelii can ablate vaccine-induced long-term protection in mice. J. Immunol. 175, 2510–2516. [DOI] [PubMed] [Google Scholar]
- 62.Pierce S. K. (2009) Understanding B cell activation: from single molecule tracking, through Tolls, to stalking memory in malaria. Immunol. Res. 43, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiss G. E., Crompton P. D., Li S., Walsh L. A., Moir S., Traore B., Kayentao K., Ongoiba A., Doumbo O. K., Pierce S. K. (2009) Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J. Immunol. 183, 2176–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Illingworth J., Butler N. S., Roetynck S., Mwacharo J., Pierce S. K., Bejon P., Crompton P. D., Marsh K., Ndungu F. M. (2013) Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J. Immunol. 190, 1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Filipe J. A., Riley E. M., Drakeley C. J., Sutherland C. J., Ghani A. C. (2007) Determination of the processes driving the acquisition of immunity to malaria using a mathematical transmission model. PLOS Comput. Biol. 3, e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feachem R. G., Phillips A. A., Hwang J., Cotter C., Wielgosz B., Greenwood B. M., Sabot O., Rodriguez M. H., Abeyasinghe R. R., Ghebreyesus T. A., Snow R. W. (2010) Shrinking the malaria map: progress and prospects. Lancet 376, 1566–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Drakeley C., Cook J. (2009) Chapter 5. Potential contribution of sero-epidemiological analysis for monitoring malaria control and elimination: historical and current perspectives. Adv. Parasitol. 69, 299–352. [DOI] [PubMed] [Google Scholar]
- 68.Stewart L., Gosling R., Griffin J., Gesase S., Campo J., Hashim R., Masika P., Mosha J., Bousema T., Shekalaghe S., Cook J., Corran P., Ghani A., Riley E. M., Drakeley C. (2009) Rapid assessment of malaria transmission using age-specific sero-conversion rates. PLoS One 4, e6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bejon P., Williams T. N., Liljander A., Noor A. M., Wambua J., Ogada E., Olotu A., Osier F. H., Hay S. I., Färnert A., Marsh K. (2010) Stable and unstable malaria hotspots in longitudinal cohort studies in Kenya. PLoS Med. 7, e1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Segeja M. D., Mmbando B. P., Seth M. D., Lusingu J. P., Lemnge M. M. (2010) Acquisition of antibodies to merozoite surface protein 3 among residents of Korogwe, north eastern Tanzania. BMC Infect. Dis. 10, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corran P., Coleman P., Riley E., Drakeley C. (2007) Serology: a robust indicator of malaria transmission intensity? Trends Parasitol. 23, 575–582. [DOI] [PubMed] [Google Scholar]