Abstract
Background
Despite increased efforts to control and ultimately eradicate human malaria, Plasmodium ovale malaria is for the most part outside the focus of research or public health programmes. Importantly, the understanding of P. ovale—nowadays regarded as the two distinct species P. ovale wallikeri and P. ovale curtisi—largely stems from case reports and case series lacking study designs providing high quality evidence. Consecutively, there is a lack of systematic evaluation of the clinical presentation, appropriate treatment and relapse characteristics of P. ovale malaria. The aim of this systematic review is to provide a systematic appraisal of the current evidence for severe manifestations, relapse characteristics and treatment options for human P. ovale malaria.
Methods and results
This systematic review was performed according to the PRISMA guidelines and registered in the international prospective register for systematic reviews (PROSPERO 2016:CRD42016039214). P. ovale mono-infection was a strict inclusion criterion. Of 3454 articles identified by the literature search, 33 articles published between 1922 and 2015 met the inclusion criteria. These articles did not include randomized controlled trials. Five prospective uncontrolled clinical trials were performed on a total of 58 participants. P. ovale was sensitive to all tested drugs within the follow-up periods and on interpretable in vitro assays. Since its first description in 1922, only 18 relapsing cases of P. ovale with a total of 28 relapse events were identified in the scientific literature. There was however no molecular evidence for a causal relationship between dormant liver stages and subsequent relapses. A total of 22 severe cases of P. ovale malaria were published out of which five were fatal. Additionally, two cases of congenital P. ovale malaria were reported.
Conclusions
Current knowledge of P. ovale malaria is based on small trials with minor impact, case reports and clinical observations. This systematic review highlights that P. ovale is capable of causing severe disease, severe congenital malaria and may even lead to death. Evidence for relapses in patients with P. ovale malaria adds up to only a handful of cases. Nearly 100 years after P. ovale’s first description by Stephens the evidence for the clinical characteristics, relapse potential and optimal treatments for P. ovale malaria is still scarce.
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-017-1759-2) contains supplementary material, which is available to authorized users.
Keywords: Plasmodium ovale, Treatment evaluation, Relapse characteristics, Severe Plasmodium ovale malaria, Congenital Plasmodium ovale malaria
Background
In 2015, 214 million new cases of clinical malaria accounting for around 438,000 deaths were identified worldwide [1]. Although these numbers are decreasing, they remain striking, as most were preventable. Malaria is among the “big three” infectious diseases and receives relatively much attention and funding. However, research focuses almost entirely on the most prevalent malaria parasite Plasmodium falciparum, whereas the other Plasmodium species are widely neglected.
Plasmodium ovale has so far received comparatively little attention in medical research. The primary focus after its first description by Stephens in 1922 was the characterization of its microscopic morphology [2–4]. Interestingly, it has been demonstrated recently by molecular methods that P. ovale essentially consists of two distinct sympatric species termed P. ovale curtisi and P. ovale wallikeri [5]. So far, only few clinical, epidemiological and therapeutic studies report specific data for P. ovale subspecies. However, based on molecular analysis the geographic distribution of P. ovale seems larger than previously thought [6, 7].
Although considered to induce only mild disease of minor importance, case reports indicate its potential of evoking severe disease and even death [8, 9]. A systematic evaluation of potential complications of P. ovale malaria is currently missing. Treatment of P. ovale was historically developed based on the empiric use of anti-malarial drugs administered for P. falciparum and Plasmodium vivax malaria. Since then, no systematic drug evaluation or development programme has been undertaken for P. ovale malaria.
One of the cornerstones of today’s understanding of ovale malaria is its potential to lead to hypnozoite induced relapse. This feature of tertian malaria is the reason for recommending the use of the antihypnozoite drug primaquine in P. ovale infections. Interestingly, this concept has been challenged recently based on a lack of experimental and clinical data supporting the hypnozoite model in ovale malaria [10–12].
These important gaps in the perception of the basic biology of P. ovale, of the potential to cause severe disease, of the evidence behind current treatment recommendations and of its potential to cause relapse were the principal reasons to endeavor for a systematic evaluation of all available evidence of P. ovale research since its description in 1922.
Methods
This systematic review was conducted following the PRISMA guidelines [13]. The protocol was registered at the international prospective register of systematic reviews (PROSPERO 2016:CRD42016039214). The scientific databases MEDLINE, EMBASE, Cochrane Library, Scopus, CINAHL, Conference Proceedings Citation Index, Web of Science/Science Citation Index Expanded and DARE were searched for publications between 1922 and 2015 using “P. ovale” as search term. Additionally, Google Scholar was searched for publications between 1922 and 1971 to increase the coverage for the pre-internet era. Furthermore, ClinicalTrials.gov and the EU Clinical Trials Register were checked for unpublished studies on P. ovale.
Data extraction
Screening, selection and data extraction were performed independently by the first and the second author. Disagreements and uncertainties at any stage of the process were discussed and resolved by consensus. If needed, the last author was consulted for a final decision. Only English, German and French articles were included in this analysis unless there was clear indication for relevant information of publications in other languages. Full texts of potentially relevant articles were obtained and articles from other sources were included in the pool of articles. Articles were matched to three different categories: treatment, severity and relapse.
Plasmodium ovale mono-infection of a human subject, defined by diagnosis based on microscopy and/or polymerase chain reaction (PCR) was a strict eligibility criterion for all categories. Additionally, separate definitions as follows were applicable for each category. Case reports were not used for treatment evaluation, otherwise there were no inclusion restrictions regarding types of studies. For in vitro studies, only assays with interpretable results were considered. Severe P. ovale malaria was determined on the basis of the 2014 WHO criteria for severe falciparum malaria [14] and other serious or life threatening clinical conditions as defined by the authors. As parasite counts are generally lower in severe P. ovale than in severe P. falciparum malaria [7] no threshold was determined for parasitaemia. A relapse was defined as a reappearing P. ovale parasitaemia following an initially diagnosed and adequately treated P. ovale “primary infection” (regardless of 8-aminoquinoline application) and subsequent permanent residence in a non-endemic country. The term “primary infection” was used to describe the first reported P. ovale infection in the article, which was adequately treated (important for malaria infection studies, where patients were mostly not treated in case of self-limiting infection). The period between primary infection and relapse and two relapse events, respectively, was counted as time between the date of treatment and the first mentioned date of reappearance. In order not to confuse delayed primary attacks with relapses, articles where the primary infection was not explicitly stated to have been a P. ovale infection were excluded.
Outcomes
Primary outcomes were adequate clinical and parasitological response on day 28, frequency of severe complications and the number of reported true relapses. Secondary outcomes were to obtain relapse characteristics, treatment regimen used, parasite clearance time (PCT), fever clearance time (FCT) and treatment outcome.
Data synthesis and risk of bias assessment
References were compiled in EndNote X6 (Thomson Reuters) and extracted data was collected in a standardized Microsoft® Excel® 2013 datasheet. Descriptive statistics were performed using IBM® SPSS® Statistics 20. Applicable risk of bias was assessed in applicable studies using the Cochrane collaboration’s tool for assessing risk of bias in combination with the methods guide for comparative effectiveness reviews [15, 16]. To assess overall quality of reporting an evaluation tool was created uniformly for all included study designs following the study quality assessment of the case series studies tool of the National Institute of Health [17].
Results
Study selection
The search yielded 3454 publications. After elimination of duplicates and screening of available titles and abstracts for relevance, 212 articles were selected for full review (Fig. 1). Two articles were added from other sources and a total of 36 articles met the required criteria. Of this pool, two articles reporting severe cases were excluded due to incomplete data and one because of double reporting, leaving 33 articles for data extraction.
Fig. 1.
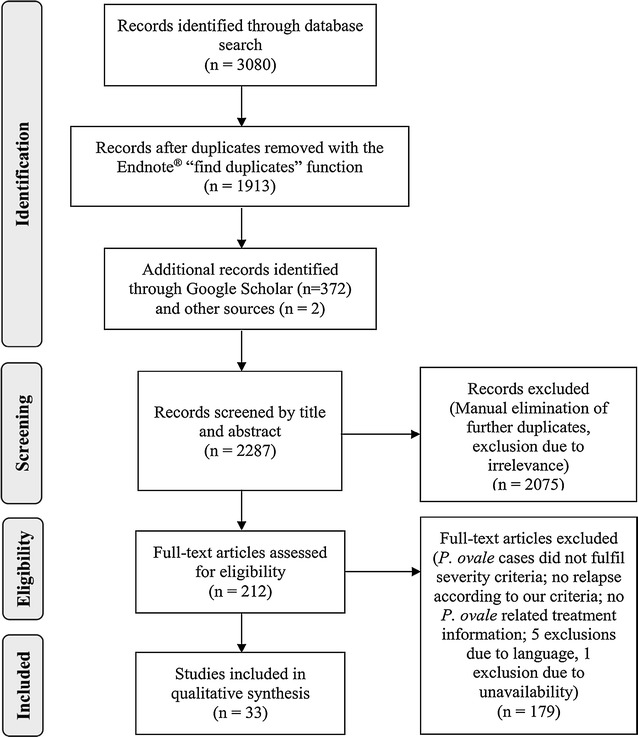
PRISMA [13] adapted flow diagram
No report was rated as having a low risk of bias due to the underlying study design. There were many case reports which made the systematic review especially vulnerable to selection bias and publication bias. To deal with publication bias, results from Clinical Trial Registers were included. As data were not used for a meta-analysis, missing data items did not influence individual risk of bias assessment. Individual risk of bias within studies as well as completeness of reporting are given in the Additional files 1, 2 and 3.
Study population
The study population of the included articles was heterogeneous. It consisted of residents in malaria-endemic areas, individuals visiting friends or relatives (VFRs), tourists, professionals temporarily residing in endemic countries, neurosyphilis patients treated with iatrogenic malaria infection, experimental malaria infections and one case of malaria transmission by blood transfusion. Study designs were diverse, but lacked designs judged to provide high quality evidence. There were no randomized controlled clinical trials (RCTs) and small sample size case series and case reports dominated the findings. The majority of reports did not distinguish between the two sympatric P. ovale species.
Not all endemic areas of the world were represented by the data that was found. The majority of cases was from sub-Saharan Africa. Asia was represented by Indonesia, Papua New Guinea and India. There were no eligible P. ovale reports from South America. Detailed information about treatment, severe disease and relapse is subsequently described.
Treatment
The literature search yielded five prospective studies evaluating treatment for P. ovale in a total of 58 participants. Baseline characteristics are outlined in Table 1. One study was conducted in Indonesia [18], two in Cameroon [19, 20], one in Gabon [21] and one in France on returnees from sub-Saharan Africa [22]. One trial was exclusively designed for P. ovale infected individuals [19]. Artesunate, atovaquone, chloroquine, mefloquine and pyronaridine were used as study drugs. Two prospective clinical trials with 13 participants in total chose chloroquine as study drugs [18, 21]. In general, sample sizes were small and control groups were missing in all 5 prospective studies. In fact, the largest study recruiting 30 patients evaluated artesunate therapy. Although the authors classified it as randomized trial, neither a placebo group nor a second treatment arm were described [19].
Table 1.
Baseline characteristics in prospective uncontrolled clinical trials
| Authors | No of P. ovale cases | Age (years) | Sex | Patients’ status | Origin of infection |
|---|---|---|---|---|---|
| Siswantoro et al. [18] | 11 | 28 (median) | 8 M, 3 F | R | Indonesia |
| Same-Ekobo et al. [19] | 30 | – | – | R | Cameroon |
| Ringwald et al. [20] | 8 | 17 (median) | – | R | Cameroon |
| 2 | 8 (median) | – | R | Cameroon | |
| Radloff et al. [21] | 3 | >10 years | – | R | Gabon |
| Danis et al. [23] | 4 | 19–32 | – | T | Sub-Saharan Africa |
No number, M male, F female, R resident, T tourist, – not mentioned in the original publication
The longest follow-up period was 28 days, therefore, treatment success could not be obtained for days 42 and 63. Besides Siswantoro et al. (eight male, three female) [18], no publication reported the participants’ sex. For further details see Table 2. Two clinical trials additionally performed in vitro drug sensitivity testing. Interpretable assays showed no resistances of P. ovale against amodiaquine, artesunate, chloroquine, mefloquine, piperaquine or pyronaridine [18, 20].
Table 2.
Treatment characteristics
| Authors | Diagnostics | Parasitaemia | Drug | Dosing (total) | Period | Adverse events | Mean FCT (h) | Mean PCT (h) | Cure | Last day of observation |
|---|---|---|---|---|---|---|---|---|---|---|
| Siswantoro et al. | MIC + PCR | 645 p/µl | Chloroquine | 25 mg/kg (+150 mg base) | Over 3 days | – | 24 | 48 | Y~ | 28 |
| Same-Ekobo et al. | MIC | 534,642 p/µl (total) | Artesunate | 600 mg | Over 5 days | Vertigo, non-severe transient decrease of reticulocytes in 1 participant | 36.6 | 38.8 | Y | 14 |
| Ringwald et al. | MIC | 2250–40,680 p/µl | Pyronaridine | 32 mg/kg | Over 3 days | Mild gastrointestinal disturbances, pruritus | 45 | 57 | Y | 14 |
| MIC | 6656–13,680 p/µl | Chloroquine | 25 mg/kg | Over 3 days | – | 24 | 60 | Y | 14 | |
| Radloff et al. | MIC | 700–5000 p/µl | Atovaquone/proguanil | 3000 mg/1200 mg | Over 3 days | – | – | 72–168 | Y | 28 |
| Danis et al. | – | – | Mefloquine | 0.5–1.25 mg | Once and twice | – | – | 72–120 | Y | – |
MIC microscopy, PCR polymerase chain reaction, Y yes, –not mentioned in the original publication, ~ adequate clinical and parasitological response of P. ovale at day 28, however, reappearing P. vivax in follow up period at days 14 and 23
Description of complicated and severe P. ovale malaria
Twenty two cases of severe P. ovale malaria were identified in scientific literature. Nigeria was the most commonly reported place of potential infection in travel histories (4 times) followed by Ghana, Cameroon and the Democratic Republic of Congo (3 times), and Ivory Coast and Niger (twice). The only non-African country reporting a complicated disease course was India (once). Mean age was 35.8 ± 13.6 years standard deviation (SD), with a range from 17 to 75 years. Fourteen cases were male, six female, for two sex was unknown. In Table 3, baseline characteristics are displayed in more detail. In 15 cases, P. ovale was diagnosed by microscopy. Seven patients were diagnosed by microscopy and PCR, out of which 2 cases were microscopically negative with a positive PCR result [24]. Species specific PCR was performed for four cases. Two were positive for P. ovale curtisi [8, 24], 1 for P. ovale wallikeri and for 1 species differentiation could not be deducted from the article [24, 25].
Table 3.
Baseline characteristics of severely diseased P. ovale cases
| Authors, year of publication | Age | Sex | Patient status | Travel history | Chemoprophylaxis |
|---|---|---|---|---|---|
| Tomar et al. [61], 2015 | 75 | M | R | None, resident of India | NA |
| Lemmerer et al. [62], 2015 | 29 | M | W | Democratic Republic of Congo | – |
| Strydom et al. [36], 2014 | 42 | M | W | Guinea, Mozambique | None |
| Rojo-Marcos et al. [24], 2014 | 17 | F | – | – | – |
| 31 | M | – | – | – | |
| Lau et al. [8], 2013 | 59 | M | T | Nigeria | Mefloquine |
| Hachimi et al. [42], 2013 | 31 | M | – | Democratic Republic of Congo | – |
| Lahlou et al. [41], 2012 | 28 | M | W | Democratic Republic of Congo | – |
| Roze et al. [63], 2011 | 24 | M | W | Chad, Ivory Coast | Doxycycline |
| Coton et al. [64], 2011 | 33 | M | W | Djibouti | – |
| Haydoura et al. [65], 2010 | 46 | F | B | NA | NA |
| Cinquetti [66], 2010 | 34 | M | W | Ivory Coast, Senegal | Doxycycline |
| Rojo-Marcos et al. [25], 2008 | 43 | M | V | Nigeria | None |
| Rubinstein et al. [67], 2005 | 23 | M | – | Nigeria | – |
| Filler et al. [68], 2003 | 39 | F | T | Cameroon, Botswana, Zimbabwe, South Africa | Yes, drug unknown |
| Lee et al. [69], 1999 | 31 | F | T | Ghana | Mefloquine |
| Patel [70], 1993 | 42 | M | T | Central and southern Africa | NA |
| Facer et al. [9], 1991 | 51 | F | T | Ghana | None |
| Monlun, et al. [71], 1989 | 38 | M | T | Niger | Chloroquine |
| Bock [72], 1939 | 23 | – | W | Western Africa, Cameroon | Chinoplasmine |
| 20 | – | W | Western Africa, Cameroon | Quinine (irregular) | |
| Fairley [73], 1933 | 28 | M | T | Nigeria, Ghana, Gambia, Sierra Leone | Quinine |
M male, F, female, T tourist, R resident, W work, B blood transfusion, V visiting friends or relatives, NA not applicable, – not mentioned in the original publication
For the 22 patients with severe clinical conditions, 15 different features of severity could be identified. Taking the patients together, 35 severe conditions were reported. Acute respiratory distress syndrome (ARDS) was reported in five patients and therefore was the most prevalent severe condition. It was followed by anaemia with a hemoglobin level <7 g/dl, and pulmonary edema which occurred in 4 patients. 5 of the reported cases died and 3 patients had organic sequelae, however, 64% of the reported cases (n = 14) survived without sequelae. The majority of deaths occurred following onset of ARDS. Further details are displayed in Table 4.
Table 4.
Characteristics of severe P. ovale disease
| Authors | Diagnostics | Parasitaemia | Body temperature (°C) | Features of severity | Treatment | Concomitant medication | Outcome | Comment |
|---|---|---|---|---|---|---|---|---|
| Tomar et al. [61] | MIC + PCR | – | 39 | Bilirubin >50 µm/l, creatinine >265 µm/l, systolic blood pressure <80 mmHg, hemoglobinuria | Artesunate iv | Ceftriaxone iv, antipyretics | Survival without sequelae | |
| Lemmerer et al. [62] | MIC | – | 40.5 | Splenic rupture | Chloroquine, 2325 mg po over 2 days | – | Survival with sequelae | |
| Strydom et al. [36] | MIC + PCR | 1.4% | 39.5 | Bilirubin >50 µm/l, systolic blood pressure <80 mmHg | Quinine, 600 mg iv eight hourly and doxycycline 100 mg twelve hourly | Ceftriaxone | Survival without sequelae | |
| Rojo-Marcos et al. [24] | MIC + PCR (microscopy neg, PCR positive) | Nega | – | Hemoglobin <7 g/dla | – | – | Survival without sequelaea | |
| MIC + PCR (microscopy neg, PCR positive) | Nega | – | Hemoglobin <7 g/dla | – | – | Survival without sequelaea | ||
| Lau et al. [8] | MIC + PCR | 0.18% | 40.8 | Creatinine >265 µm/l, acidosis, ARDS | Chloroquine 150 mg base for 2 days, quinine for 1 day and artesunate for 7 days | Ceftriaxone, piperacillin/tazobactam, vancomycin, imipenem, meropenem | Death | The first antibiotic was started on day 4 despite negative blood cultures; Enterobacter cloacae was found on day 15 (nosocomial sepsis); seizures were described on day 17; atrial fibrillation on day 22; asystole on day 32 |
| Hachimi et al. [42] | MIC | <0.2% | 39 | ARDS | Quinine | – | Death | History of tuberculosis 11 years ago |
| Lahlou et al. [41] | MIC | 0.2% | – | ARDS | Quinine | Ciprofloxacin | Death | History of treated pulmonary tuberculosis 10 years ago |
| Roze et al. [63] | MIC | 0.2% | – | ARDS | Chloroquine and quinine | – | Survival without sequelae | History of tuberous sclerosis and spontaneous pneumothorax |
| Coton et al. [64] | MIC | – | 40 | Acute pericarditis | Chloroquine 30 mg/kg over 6 days | Ketoprophen, omeprazol, aspirin | Survival without sequelae | |
| Haydoura et al. [65] | MIC | 1.1% | 40 | Oxygen saturation <92%, ARDS | Quinine iv for 7 days and doxycycline po for 7 days | Warfarin | Survival without sequelae | History of MTHFR, secondary portal vein thrombosis and severe lower gastrointestinal bleeding from hemorrhoids requiring 7 units of red blood cells |
| Cinquetti [66] | MIC + PCR | 0.001% | 39.5 | Splenic infarction | Quinine 8 mg/kg iv three times daily | – | Survival with sequelae | |
| Rojo-Marcos et al. [25] | MIC + PCR | 6000 trophozoites + gametocytes/µl | 40.5 | ARDS | Chloroquine for 3 days | – | Survival without sequelae | History of diabetes mellitus and hypertension; incomplete left bundle block in the predose ECG followed by left ventricular hypertrophy in the day 30 ECG; presence of Mansonella perstans; nosocomial Acinetobacter baumanii in broncho-alveolar aspirate |
| Rubinstein et al. [67] | MIC | 0.2% | – | Bilirubin >50 µm/l | Quinine for 7 days and doxycycline for 7 days | – | Survival without sequelae | |
| Filler et al. [68] | MIC | – | – | Hemoglobin <7 g/dl, splenic rupture, cardiac arrest | Quinine sulfate and doxycycline, followed by quinidine iv | – | Death | History notably of MS |
| Lee et al. [69] | MIC | 0.1% | 39 | Oxygen saturation <92%, pulmonary edema | Chloroquine | – | Survival without sequelae | Negative blood cultures |
| Patel [70] | MIC | – | – | Splenic rupture | Chloroquine | – | Survival with sequelae | |
| Facer et al. [9] | MIC | 1.8% | NA | Splenic rupture | NA | NA | Death | Diagnosis post mortem; absence of P. falciparum confirmed with “DNA analysis” |
| Monlun et al. [71] | MIC | – | 41 | Cardiomyopathy | Chloroquine 500 mg/day for 5 days | – | Survival without sequelae | Cardiomyopathy resolved without additional specific treatment |
| Bock [72] | MIC | – | – | Hemoglobin <7 g/dl | Mepacrine | – | Survival without sequelae | Case 2 |
| MIC | – | 40 | Cardiac arrhythmia | Mepacrine | – | Survival without sequelae | Case 15 | |
| Fairley [73] | MIC | – | 38.3 | Hemoglobinuria | Mepacrine 0.1 g three times daily for 6 days and quinine bihydrochloride 7.5 g/day iv for 5 days | Saline | Survival without sequelae |
MIC microscopy, PCR polymerase chain reaction, po per os, iv intravenous, NA not applicable, ARDS acute respiratory distress syndrome, MTHFR methylentetrahydrofolate reductase defect, MS multiple sclerosis, – not mentioned in the original publication
aInformation provided by the author
Congenital malaria
Besides the clinically severe cases described above, two independent cases of congenital P. ovale malaria were identified presenting with severe anaemia [26, 27]. The two mothers (both secundigravidae) had resided in an African country prior to birth but gave birth to their children in Europe and also remained there during the observation period. Both had a history of treated malaria of unknown species. The respective children were delivered by Cesarean, one because of a treated human immunodeficiency virus (HIV) infection of the mother, the other one as an emergency cesarean section. Being healthy at birth, malaria was diagnosed 5 and 3 weeks post-partum. Detailed information is presented in Table 5.
Table 5.
Characteristics of severe congenital malaria
| Authors, year of publication | Sex | Birth weight (kg) | Previous residence of mother | Country of birth | Diagnostics | Parasitaemia | Body temperature (°C) | Hemoglobin level (g/dl) | Treatment | Outcome | Concomitant medication | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Penazzato et al. [26], 2007 | – | 3.13 | Nigeria | Italy | MIC + PCR | 0.01% | – | 5.4 | Quinine sulphate 20 mg/kg/d for 5 days | Recovered without sequelae | Zidovudine | Mother HIV positive, no materno-foetal transmission of HIV |
| Jenkins et al. [27], 1957 | M | 4 | East Africa | England | MIC | – | 40 | 6.8 | Proguanil 0.25 g daily for 5 days followed by: chloroquine sulphate ¼ tablet twice daily for 2 days, then ¼ tablet daily for 2 days, then ¼ tablet weekly for 3 weeks | Recovered without sequelae | Penicillin, potassium permanganate baths, local aqueous gentian violet 0.66% | Pubic rash after day 3 |
| Erythromycin | Given on suspicion with proguanil | |||||||||||
| Ferrous sulphate |
MIC microscopy, PCR polymerase chain reaction, M male, – not mentioned in the original publication
Relapse
From the description of P. ovale as distinct species in 1922 up to 2015 a total of 18 cases with potentially relapsing P. ovale parasitaemia according to the inclusion criteria applied for this systematic review were reported in scientific literature. These patients were described to have experienced a total of 28 potential relapse events. 4 cases (22%) occurred in tourists, 14 (78%) in malaria infection studies. Sex was specified in 44% of the patients, all of them were male. Fever was mentioned in five episodes, other clinical information about relapse characteristics was missing. The most commonly used drugs to treat primary infections and relapses were chloroquine and quinine sulfate. Median time between primary infections and first potential relapses was 17 weeks (min–max 2–60 weeks). The median time between first and second potential relapse was also 17 weeks, ranging from 5 to 72 weeks. The time between second and third relapse was not reported. Six relapses occurred despite previous primaquine treatment. Eight individuals presented with two relapses and one individual relapsed three times. Details can be found in Tables 6 and 7.
Table 6.
Baseline characteristics of potentially relapsing patients
| Authors, year of publication | Patient’s status | Age (years) | Sex | Probable origin of infection | Chemoprophylaxis | Parasitaemia (parasites/µl) |
|---|---|---|---|---|---|---|
| Bottieau [28], 2005 | T | 17 | M | Ghana | Mefloquine | – |
| T | 22 | M | Nigeria | Mefloquine | – | |
| T | 14 | M | Uganda | None | – | |
| Collins et al. [29], 2002 | I | – | – | NA | NA | 3780 |
| I | – | – | NA | NA | 2220 | |
| I | – | – | NA | NA | 8560 | |
| I | – | – | NA | NA | 9810 | |
| I | – | – | NA | NA | 5376 | |
| Nathwani et al. [30], 1991 | T | 24 | M | Papua New Guinea | Chloroquine, pyrimethamine | – |
| Chin et al. [31], 1971 | E | – | M | NA | NA | – |
| E | – | M | NA | NA | – | |
| E | – | M | NA | NA | – | |
| E | – | M | NA | NA | – | |
| E | – | M | NA | NA | – | |
| Garnham et al. [32], 1955 | I | – | – | NA | NA | – |
| I | – | – | NA | NA | – | |
| Jeffery [33], 1954 | I | – | – | NA | NA | – |
| I | – | – | NA | NA | – |
T tourist, E sporozoite induced experimental infection, I malaria infection therapy in neurosyphilis patients, M male, F female, NA not applicable, – not mentioned in the original publication
Table 7.
Relapse characteristics
| Authors | Diagnostic method primary infection | Treatment primary infection | Dosage primary infection | PQ therapy primary infection? | Time between primary infection and 1st relapse (weeks) | Diagnostic method 1st relapse | Treatment 1st relapse | PQ therapy 1st relapse? | Time between 1st and 2nd relapse (weeks) | Diagnostic method 2nd relapse | Treatment 2nd relapse | PQ therapy 2nd relapse? | Time between 2nd and 3rd relapse (weeks) | Treatment 3rd relapse |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bottieau [28] | MIC | Quinine | 1.5 g/day for 5 days | Y, “standard regimen” (presumably 15 mg/day for 14 days) | 7 | MIC | Chloroquine 1.5 g over 3 days | Y, 7 mg/kg over 3 weeks | NA | NA | NA | NA | NA | NA |
| Doxycycline | 100 mg/day for 7 days | |||||||||||||
| – | Quinine–doxycycline | – | Y, “standard regimen” | 2 | – | Chloroquine, dosage unknown | Y, 10 mg/kg over 4 weeks | NA | NA | NA | NA | NA | NA | |
| – | Chloroquine | – | N | 60 | – | Atovaquone–proguanil, dosage unknown | Y, 5 mg/kg over 6 weeks | 78 | MIC | Atovaquone–proguanil, then chloroquine | Y, 8 mg/kg over 3 weeks | NA | NA | |
| Collins et al. [29] | MIC | Chloroquine | 1.5 g over 3 days | N | 22 | MIC | Chloroquine, dosage unknown | N | 17 | MIC | Chloroquine, dosage unknown | – | NA | NA |
| MIC | Chloroquine | 1.5 g over 3 days | N | 10 | MIC | – | – | NA | NA | NA | NA | NA | NA | |
| MIC | Chloroquine | 1.5 g over 3 days | N | 15 | MIC | – | – | NA | NA | NA | NA | NA | NA | |
| MIC | Chloroquine | 1.5 g over 3 days | N | 24 | MIC | – | – | NA | NA | NA | NA | NA | NA | |
| MIC | Chloroquine | 1.5 g over 3 days | N | 20 | MIC | – | – | NA | NA | NA | NA | NA | NA | |
| Nathwani et al. [30] | MIC | Chloroquine | 15 mg/day for 14 days | Y, 15 mg/day for 14 days | 17 | MIC | Chloroquine 1.5 g over 3 days | Y, 30 mg/day for 21 days | NA | NA | NA | NA | NA | NA |
| Chin et al. [31] | MIC | Quinine sulphate | 650 mg 8-hourly for 5 days | N | – | MIC | Quinine sulphate 650 mg 8-hourly for 5 days | N | 5 | MIC | Quinine sulphate 650 mg 8-hourly for 5 days | N | – | Quinine sulphate 650 mg 8-hourly for 5 days |
| MIC | Quinine sulphate | 650 mg 8-hourly for 5 days | N | 2 | MIC | Quinine sulphate 650 mg 8-hourly for 5 days | N | 20 | MIC | Quinine sulphate 650 mg 8-hourly for 5 days | N | NA | NA | |
| MIC | Quinine sulphate | 650 mg 8-hourly for 5 days | N | 36 | MIC | Quinine sulphate 650 mg 8-hourly for 5 days | N | NA | NA | NA | NA | NA | NA | |
| MIC | Quinine sulphate | 650 mg 8-hourly for 5 days | N | – | MIC | Quinine sulphate 650 mg 8-hourly for 5 days | N | – | MIC | Quinine sulphate 650 mg 8-hourly for 5 days | N | NA | NA | |
| MIC | Chloroquine | 600 mg single dose | N | – | MIC | Chloroquine 600 mg single dose | N | – | MIC | Chloroquine 600 mg single dose | Y, 15 mg/day for 14 days | NA | NA | |
| Garnham et al. [32] | MIC | Chloroquine | – | N | 15 | MIC | No treatment | N | 10 | MIC | No treatment | N | NA | NA |
| MIC | Chloroquine | – | N | 14 | MIC | No treatment | N | 14 | MIC | No treatment | N | NA | NA | |
| Jeffery [33] | MIC | Chloroquine | – | N | 21 | MIC | Chloroquine, dosage unknown | N | 22a | MIC | – | – | NA | NA |
| MIC | chloroquine | – | N | 34 | MIC | chloroquine, dosage unknown | Y, dosage unknown | NAa | NA | NA | NA | NA | NA |
MIC microscopy, Y yes, N no, NA not applicable, – not mentioned in the original publication
aThe patient who did not receive primaquine treatment for his 1st relapse developed a second one, however it was not clear from the article, which one of the two patients developed the described second relapse
Diagnostics relied exclusively on microscopy. PCR correction of the infective species was not performed. Furthermore, there were no articles proving a causal relationship between dormant liver stages and reappearances of P. ovale infections in the human host.
Discussion
Several small literature reviews focusing on specific but limited aspects of P. ovale malaria have been previously published, most often appended to case reports. The epidemiology of ovale malaria in a high endemic setting has been demonstrated with long-term surveillance data [34, 35]. P. ovale has also been addressed in the context of other infectious diseases [36, 37]. However, to date, the scientific literature does not provide a systematic overview focusing on clinical, therapeutic and relapse characteristics of P. ovale. As to the dimorphism of P. ovale, too few articles distinguished between the sympatric species to suggest potential differences. This systematic review therefore combines data from both P. ovale species.
Evaluation of current treatment recommendations
Chloroquine has been the recommended treatment for P. ovale malaria for many years. In the latest guideline for the treatment of malaria, the WHO strongly recommends to treat P. ovale and other non-falciparum Plasmodium species with artemisinin-based combination therapy or chloroquine on the basis of “high-quality evidence”. Following elaborations of underlying studies in the WHO guideline however rather break this down to experience [38]. In this systematic review, no high-quality studies supporting current treatment recommendations were identified. Not a single randomized controlled clinical trial on P. ovale malaria has been published in scientific literature. This finding is supported by a report by Visser et al. [37]. Although chloroquine has been tested in small prospective uncontrolled trials, one might question whether this small number of participants and a lack of control groups in all studies provide enough evidence for unequivocal treatment recommendations. Summing up all published reports and clinical experience, it becomes evident that anti-malarial drugs employed for P. falciparum are also effective for P. ovale. However, scientifically sound evidence for this is currently missing.
Severe disease
In 1932, James and coworkers stated that it was unlikely that another malignant species besides P. falciparum would be discovered [39]. Since then Plasmodium knowlesi was found to be infective for humans leading to life-threatening quotidian malaria. Also the previously considered benign malaria species P. malariae, P. vivax and P. ovale were reported to cause severe disease and even death in a small minority of patients. To date little is known on the specific pathogenesis of severe diseases in these non-falciparum malarias. The results of this systematic review support this understanding.
It is of interest that ARDS was the main feature of severe disease in P. ovale malaria as it was described in returning travellers with P. vivax malaria [40]. The potential coincidence that the two patients with a history of tuberculosis 10 years and more ago both died from ARDS raises the question whether a preexisting pulmonary condition may be a risk factor for respiratory complications of P. ovale infection [41, 42]. Anaemia was also reported as a feature of severe P. ovale malaria, however due to its multifactorial aetiology it is difficult to attribute this with confidence to P. ovale infection. Nevertheless it has been reported concordantly in paediatric patients with P. vivax infection in Asia [43].
An important limitation in the description of severe cases of P. ovale infection is the only partly performed molecular assessment of blood samples. Although light microscopy forms the current gold standard for malaria diagnostics, its sensitivity is inferior to most molecular methods. Additionally, species determination and distinction, especially between P. ovale and P. vivax can be challenging most notably in low parasitaemic smears [44, 45]. It is, therefore, not possible to entirely exclude the possibility of coinfection with other Plasmodium species including P. falciparum in these cases.
Congenital malaria is a rare finding in non-endemic countries. Even more surprising was the identification of two cases of congenital P. ovale malaria with severe anaemia in Europe. Both mothers had been living in an endemic country in the past. Interestingly, one of the infants was born to a HIV positive mother. An association between HIV and the incidence of P. falciparum in pregnancy has already been shown [46] and it might be speculated that the same is true for P. ovale. In 2008, Vottier et al. reported another congenital P. ovale infection transmitted by an HIV positive mother which was however not severe [47].
The concept of hypnozoite-induced relapse in P. ovale malaria
Although the concept of hypnozoite-induced relapse in all tertian malarias seemed as a unanimous concept until recently, molecular evidence supportive for this model is scarce. A recent experimental study in mice engrafted with human hepatocytes observed uninucleate parasitic structures measuring ~5 µm (day 8) and late schizonts (day 21) after P. ovale sporozoite inoculation [48, 49]. The description of these histological structures resembles the findings of Krotoski described for Plasmodium cynomolgi bastianelli in Rhesus monkeys (average diameter 4.5 µm) and for P. vivax in chimpanzees (approximately 4–5 µm diameter) [50, 51]. However, this analogy does not constitute proof that these uninucleate structures truly represent hypnozoites or rather retarded forms. Furthermore it does not provide evidence for these structures to cause relapse events [48]. Based on this lack of firm experimental evidence and the scarcity of clinical reports a recent perspective article challenged the current concept of P. ovale relapse caused by liver hypnozoites proposing a gradual dormancy concept [52].
The presence of dormancies as such can be assumed as data from malaria elimination settings suggests their important role for sustained malaria transmission, along with P. vivax [53].
Plasmodium ovale hypnozoites have not yet been unequivocally demonstrated in the human host. As evidenced by this systematic review a total of 18 reported cases of P. ovale relapse in nearly 100 years do not provide solid evidence for the current relapse theory. On the other hand, experiments and malaria treatment of neurosyphilis patients have shown that in case of repetitive inoculation with the same strain, immunity to this homologous challenge develops fast and subsequent infections remain often asymptomatic [54, 55]. Hence, it may be speculated that a true relapse may lead to mitigated symptoms or may even be sub-clinical.
In this context, it is of interest to note that six potential relapses occurred despite previous primaquine treatment. However, intake of primaquine has not been evaluated in these patients.
Historically, the concept of treatment of P. ovale relapses with an 8-aminoquinoline is based on the observation that quinine and pamaquine (the first synthetic 8-aminoquinoline) together were more effective in the treatment of certain malaria cases than quinine alone. When Sinton and Bird observed that pamaquine reduced the relapse rate of P. vivax malaria [56] several 8-aminoquinoline derivatives were synthesized and tested for this purpose. Primaquine finally showed a higher anti-relapse effect than pamaquine with reduced toxicity among the most promising substances, but effectiveness for P. ovale relapses has since then only been presumed and never demonstrated [57]. Importantly, from a methodological point of view, to prove the effectiveness of a medication it is necessary to first unequivocally demonstrate the existence of the condition to be treated—in this case hypnozoite-induced relapse.
Richter et al. questioned the existence of relapses in P. ovale in a review in 2010. They stated that “it may be difficult to differentiate a true relapse from a primary malaria attack with a long latency” [10]. To overcome that difficulty, the analysis was restricted to cases, which did not reside in a malaria endemic area between the occurrences of primary infection and relapse. In addition, the species of the primary infection had to be explicitly mentioned to be P. ovale and treated with anti-malarial chemotherapy. Comparing the results of this systematic review with those of Richter et al. [52], these strict criteria are the main reason why even fewer cases of potential relapses were observed here.
Finally, only one potential case of relapse that was investigated with molecular methods could be identified in the literature [58, 59]. As this case occurred in an endemic area, the report did not fit the criteria of this systematic review and was therefore not included in the primary analyses. After personal communication with one of the authors (Fuehrer) the confirmation for this potential relapse case was based on the sequence homology of partial cox1, SSU rRNA, and porbp2 loci between the primary and the potential relapse isolate [60]. These markers are usually not used for intraspecific distinction but for differentiation between the species. The multigene approach, however, enhances the significance of the result. In summary, the identification of highly sensitive genetic markers or techniques that can discriminate between hypnozoite induced relapse and other sources of recurrent infections is still a work in progress.
Limitations of this systematic review are the low strength of evidence of the included studies based on their study design. At the same time, they form the only available evidence to address the review questions and form the basis of current recommendations.
Conclusion
In conclusion, this review of the scientific literature between 1922 and 2015 did not reveal a single high quality randomized controlled clinical trial. The reported evidence indicates that P. ovale is capable of evoking severe disease, severe congenital malaria and even death. Evidence for P. ovale related recommendations, however, seems to be scarce and is often based on clinical experience rather than on solid scientific evidence. Accordingly, this underlines the importance for clinical trials with larger sample size to obtain the efficacy of several treatment options for P. ovale.
Evidence for relapses in P. ovale malaria is poor. Relapses in the human host have so far only once been studied with molecular methods. Hence, there is a need to further explore the P. ovale relapse theory and find scientifically sound evidence that proves or disproves the existence of relapses and of hypnozoites as origin of such potential P. ovale relapse events. With that knowledge, one might also gain a new perspective on the adequate management for the radical cure of tertian ovale malaria—a neglected malaria, which in the future may gain in public health importance in the setting of successful elimination campaigns for falciparum malaria.
Authors’ contributions
MG conceptualization, data curation, formal analysis, investigation, methodology, project administration, visualization, writing-original draft preparation. HSF data curation, formal analysis, investigation, methodology, resources, writing-review and editing. LV investigation, validation, writing-review and editing. AL investigation, validation, writing-review and editing. MR conceptualization, funding acquisition, methodology, supervision, writing-review and editing. All authors read and approved the final manuscript.
Acknowledgements
The authors gratefully acknowledge the following individuals for their contribution: Dr. Thierry Coton, Dr. Chi-Tai Fang, Dr. Christian Rabe and Dr. Hans-Peter Fuehrer for sharing their manuscripts as well as further details; Javier Ibañez, MSc for his help with a translation;
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analysed during this study are included in this published article (and its Additional files 1, 2 and 3).
Funding
This work was financially supported by the Karl Landsteiner Gesellschaft and the Austrian Federal Ministry of Science, Research and Economy as part of the EDCTP2 programme. This study is part of the EDCTP2 programme supported by the European Union. The funders did not play any role in study design, collection of data, data analysis, preparation and publishing of the manuscript. Support by the Deutsche Forschungsgemeinschaft and the Open Access Publishing fund of University Tuebingen is acknowledged.
Abbreviations
- ARDS
acute respiratory distress syndrome
- cox1
cytochrome c oxidase subunit 1
- FCT
fever clearance time
- HIV
human immunodeficiency virus
- MTHFR
methylentetrahydrofolate reductase defect
- PCT
parasite clearance time
- porbp2
Plasmodium ovale reticulocyte binding protein 2
- RCT
randomized controlled trial
- SSU rRNA
small subunit ribosomal ribonucleic acid
- VFR
visiting friends and relatives
- WHO
World Health Organization
Additional files
Additional file 1. Alphabetic list of included articles with overall quality of reporting and risk of bias assessment. -, not determined; overall risk of bias was declared “high” for case reports and case series; for applicable trials, overall risk of bias results from the detailed risk of bias assessment outlined in the Additional file 2; detailed completeness of reporting assessment is displayed in the Additional file 3.
Additional file 2. Detailed risk of bias assessment of prospective uncontrolled clinical trials. Key: 3 or more bias high risk: over all high risk of bias; 3 or more bias medium risk, 2 or less bias high risk: over all medium risk of bias.
Additional file 3. Detailed completeness of reporting assessment with a focus on P. ovale relevant information. NA, not applicable; CD, cannot be determined; *, for hyper endemic areas adequate length of follow-up was 14 days, otherwise 28 days; ~, total parasitaemia of patients given, else well described; Key: one partial or CD, else yes and NA: good; one no and one partial or CD, else yes: medium; 2-3 times partial and CD, else yes: medium; more than 3 partial and CD: poor; more than 2 no: poor.
Contributor Information
Mirjam Groger, Email: michael.ramharter@medizin.uni-tuebingen.de.
Hannah S. Fischer, Email: michael.ramharter@medizin.uni-tuebingen.de
Luzia Veletzky, Email: michael.ramharter@medizin.uni-tuebingen.de.
Albert Lalremruata, Email: michael.ramharter@medizin.uni-tuebingen.de.
Michael Ramharter, Email: michael.ramharter@medizin.uni-tuebingen.de.
References
- 1.WHO. World malaria report 2015. Geneva: World Health Organization; 2015. http://www.who.int/malaria/media/world-malaria-report-2015/en/. Accessed 02 Aug 2016.
- 2.Stephens J. A new malaria parasite of man. Ann Trop Med Parasitol. 1922;16:383–386. doi: 10.1080/00034983.1922.11684331. [DOI] [Google Scholar]
- 3.Blair DM. Infections with Plasmodium ovale Stephens in Southern Rhodesia. Trans R Soc Trop Med Hyg. 1938;32(229–31):33–36. [Google Scholar]
- 4.Stephens J, Owen DU. Plasmodium ovale. Ann Trop Med Parasitol. 1927;21:293–302. doi: 10.1080/00034983.1927.11684538. [DOI] [Google Scholar]
- 5.Sutherland CJ, Tanomsing N, Nolder D, Oguike M, Jennison C, Pukrittayakamee S, et al. Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J Infect Dis. 2010;201:1544–1550. doi: 10.1086/652240. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland CJ. Persistent parasitism: the adaptive biology of malariae and ovale malaria. Trends Parasitol. 2016;32:808–819. doi: 10.1016/j.pt.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Mueller I, Zimmerman PA, Reeder JC. Plasmodium malariae and Plasmodium ovale—the “bashful” malaria parasites. Trends Parasitol. 2007;23:278–283. doi: 10.1016/j.pt.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau YL, Lee WC, Tan LH, Kamarulzaman A, Syed Omar SF, Fong MY, et al. Acute respiratory distress syndrome and acute renal failure from Plasmodium ovale infection with fatal outcome. Malar J. 2013;12:389. doi: 10.1186/1475-2875-12-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Facer CA, Rouse D. Spontaneous splenic rupture due to Plasmodium ovale malaria. Lancet. 1991;338:896. doi: 10.1016/0140-6736(91)91562-9. [DOI] [PubMed] [Google Scholar]
- 10.Richter J, Franken G, Mehlhorn H, Labisch A, Haussinger D. What is the evidence for the existence of Plasmodium ovale hypnozoites? Parasitol Res. 2010;107:1285–1290. doi: 10.1007/s00436-010-2071-z. [DOI] [PubMed] [Google Scholar]
- 11.Markus MB. The hypnozoite concept, with particular reference to malaria. Parasitol Res. 2011;108:247–252. doi: 10.1007/s00436-010-2072-y. [DOI] [PubMed] [Google Scholar]
- 12.Markus MB. Do hypnozoites cause relapse in malaria? Trends Parasitol. 2015;31:239–245. doi: 10.1016/j.pt.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Severe malaria. Trop Med Int Health. 2014;19:7–131. [DOI] [PubMed]
- 15.Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters M, et al. Assessing the risk of bias of individual studies in systematic reviews of health care interventions. In Methods guide for effectiveness and comparative effectiveness reviews. Rockville; 2008. [PubMed]
- 16.The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions Version 5.1.0. 2011. www.handbook.cochrane.org. Accessed 15 Aug 2016.
- 17.National Institutes of Health Quality Assessment Tool for Case Series Studies. https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/case_series. Accessed 11 Nov 2016.
- 18.Siswantoro H, Russell B, Ratcliff A, Prasetyorini B, Chalfein F, Marfurt J, et al. In vivo and in vitro efficacy of chloroquine against Plasmodium malariae and P. ovale in Papua, Indonesia. Antimicrob Agents Chemother. 2011;55:197–202. doi: 10.1128/AAC.01122-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Same-Ekobo A. Rapid resolution of Plasmodium ovale malaria using artesunate (arsumax®) Med Trop (Mars) 1999;59:43–45. [PubMed] [Google Scholar]
- 20.Ringwald P, Bickii J, Same Ekobo A, Basco LK. Pyronaridine for treatment of Plasmodium ovale and Plasmodium malariae infections. Antimicrob Agents Chemother. 1997;41:2317–2319. doi: 10.1128/aac.41.10.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radloff PD, Philipps J, Hutchinson D, Kremsner PG. Atovaquone plus proguanil is an effective treatment for Plasmodium ovale and P. malariae malaria. Trans R Soc Trop Med Hyg. 1996;90:682. doi: 10.1016/S0035-9203(96)90435-6. [DOI] [PubMed] [Google Scholar]
- 22.Danis M, Legros F, Gay F, Brousse G, Bricaire F, Gentilini M. Imported malaria in France. Med Mal Infect. 1999;29:257S–273S. doi: 10.1016/S0399-077X(00)88263-3. [DOI] [Google Scholar]
- 23.Danis M, Felix H, Brucker G, Druilhe P, Datry A, Richard-Lenoble D, et al. Mefloquine: comparative therapy of Plasmodium falciparum, Plasmodium vivax and Plasmodium ovale malaria. Med Trop (Mars) 1982;42:427–432. [PubMed] [Google Scholar]
- 24.Rojo-Marcos G, Rubio-Munoz JM, Ramirez-Olivencia G, Garcia-Bujalance S, Elcuaz-Romano R, Diaz-Menendez M, et al. Comparison of imported Plasmodium ovale curtisi and P. ovale wallikeri infections among patients in Spain, 2005–2011. Emerg Infect Dis. 2014;20:409–416. doi: 10.3201/eid2003.130745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rojo-Marcos G, Cuadros-Gonzalez J, Mesa-Latorre JM, Culebras-Lopez AM, de Pablo-Sanchez R. Acute respiratory distress syndrome in a case of Plasmodium ovale malaria. Am J Trop Med Hyg. 2008;79:391–393. [PubMed] [Google Scholar]
- 26.Penazzato M, Rampon O, De Canale E, De Rossi A, Mazza A, D’Elia R, et al. Congenital Plasmodium ovale malaria in an infant born to HIV positive mother. J Pediatr Infect Dis. 2007;2:167–169. [Google Scholar]
- 27.Jenkins HG. Congenital malaria in England; Plasmodium ovale. BMJ. 1957;1:88–89. doi: 10.1136/bmj.1.5010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bottieau E, Van Gompel A, Peetermans WE. Failure of primaquine therapy for the treatment of Plasmodium ovale malaria. Clin Infect Dis. 2005;41:1544–1545. doi: 10.1086/497378. [DOI] [PubMed] [Google Scholar]
- 29.Collins WE, Jeffery GM. A retrospective examination of sporozoite-induced and trophozoite-induced infections with Plasmodium ovale: development of parasitologic and clinical immunity during primary infection. Am J Trop Med Hyg. 2002;66:492–502. doi: 10.4269/ajtmh.2002.66.492. [DOI] [PubMed] [Google Scholar]
- 30.Nathwani D, Currie PF, Smith CC, Khaund R. Recurrent Plasmodium ovale infection from Papua New Guinea-chloroquine resistance or inadequate primaquine therapy? J Infect. 1991;23:343–345. doi: 10.1016/0163-4453(91)93548-Q. [DOI] [PubMed] [Google Scholar]
- 31.Chin W, Coatney GR. Relapse activity in sporozoite-induced infections with a West African strain of Plasmodium ovale. Am J Trop Med Hyg. 1971;20:825–827. doi: 10.4269/ajtmh.1971.20.825. [DOI] [PubMed] [Google Scholar]
- 32.Garnham PC, Bray RS, Cooper W, Lainson R, Awad FI, Williamson J. The pre-erythrocytic stage of Plasmodium ovale. Trans R Soc Trop Med Hyg. 1955;49:158–167. doi: 10.1016/0035-9203(55)90042-0. [DOI] [PubMed] [Google Scholar]
- 33.Jeffery GM, Young MD, Wilcox A. The Donaldson strain of malaria. 1. History and characteristics of the infection in man. Am J Trop Med Hyg. 1954;3:628–637. doi: 10.4269/ajtmh.1954.3.628. [DOI] [PubMed] [Google Scholar]
- 34.Roucher C, Rogier C, Sokhna C, Tall A, Trape JF. A 20-year longitudinal study of Plasmodium ovale and Plasmodium malariae prevalence and morbidity in a West African population. PLoS ONE. 2014;9:e87169. doi: 10.1371/journal.pone.0087169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faye FB, Spiegel A, Tall A, Sokhna C, Fontenille D, Rogier C, et al. Diagnostic criteria and risk factors for Plasmodium ovale malaria. J Infect Dis. 2002;186:690–695. doi: 10.1086/342395. [DOI] [PubMed] [Google Scholar]
- 36.Strydom KA, Ismail F, Frean J. Plasmodium ovale: a case of not-so-benign tertian malaria. Malar J. 2014;13:85. doi: 10.1186/1475-2875-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visser BJ, Wieten RW, Kroon D, Nagel IM, Belard S, van Vugt M, et al. Efficacy and safety of artemisinin combination therapy (ACT) for non-falciparum malaria: a systematic review. Malar J. 2014;13:463. doi: 10.1186/1475-2875-13-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO: guidelines for the treatment of malaria. Geneva: World Health Organization. 2015. http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf?ua=1&ua=1. Accessed 02 Aug 2016.
- 39.James S, Nicol W, Shute P. A study of induced malignant tertian malaria. Proc R Soc Med. 1932;25:1153. doi: 10.1177/003591573202500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price L, Planche T, Rayner C, Krishna S. Acute respiratory distress syndrome in Plasmodium vivax malaria: case report and review of the literature. Trans R Soc Trop Med Hyg. 2007;101:655–659. doi: 10.1016/j.trstmh.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 41.Lahlou H, Benjelloun S, Khalloufi A, Moudden EL, Hachimi MA, Errami M, et al. An exceptional observation of acute respiratory distress associated with Plasmodium ovale infection. Clin Chem Lab Med. 2012;50:A141. [Google Scholar]
- 42.Hachimi MA, Hatim EA, Moudden MK, Elkartouti A, Errami M, Louzi L, et al. The acute respiratory distress syndrome in malaria: is it always the prerogative of Plasmodium falciparum? Rev Pneumol Clin. 2013;69:283–286. doi: 10.1016/j.pneumo.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Price RN, Douglas NM, Anstey NM. New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr Opin Infect Dis. 2009;22:430–435. doi: 10.1097/QCO.0b013e32832f14c1. [DOI] [PubMed] [Google Scholar]
- 44.Chavatte JM, Tan SB, Snounou G, Lin RT. Molecular characterization of misidentified Plasmodium ovale imported cases in Singapore. Malar J. 2015;14:454. doi: 10.1186/s12936-015-0985-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alemu A, Fuehrer HP, Getnet G, Kassu A, Getie S, Noedl H. Comparison of Giemsa microscopy with nested PCR for the diagnosis of malaria in North Gondar, north–west Ethiopia. Malar J. 2014;13:174. doi: 10.1186/1475-2875-13-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duffy PE, Fried M. Malaria in the pregnant woman. Curr Top Microbiol Immunol. 2005;295:169–200. doi: 10.1007/3-540-29088-5_7. [DOI] [PubMed] [Google Scholar]
- 47.Vottier G, Arsac M, Farnoux C, Mariani-Kurkdjian P, Baud O, Aujard Y. Congenital malaria in neonates: two case reports and review of the literature. Acta Paediatr. 2008;97:505–509. doi: 10.1111/j.1651-2227.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 48.Soulard V, Bosson-Vanga H, Lorthiois A, Roucher C, Franetich JF, Zanghi G, et al. Plasmodium falciparum full life cycle and Plasmodium ovale liver stages in humanized mice. Nat Commun. 2015;6:7690. doi: 10.1038/ncomms8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markus MB. Mouse-based research on quiescent primate malaria parasites. Trends Parasitol. 2016;32:271–273. doi: 10.1016/j.pt.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Krotoski WA, Collins WE, Bray RS, Garnham PC, Cogswell FB, Gwadz RW, et al. Demonstration of hypnozoites in sporozoite-transmitted Plasmodium vivax infection. Am J Trop Med Hyg. 1982;31:1291–1293. doi: 10.4269/ajtmh.1982.31.1291. [DOI] [PubMed] [Google Scholar]
- 51.Krotoski WA, Krotoski DM, Garnham PC, Bray RS, Killick-Kendrick R, Draper CC, et al. Relapses in primate malaria: discovery of two populations of exoerythrocytic stages. BMJ. 1980;280:153–154. doi: 10.1136/bmj.280.6208.153-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richter J, Franken G, Holtfreter MC, Walter S, Labisch A, Mehlhorn H. Clinical implications of a gradual dormancy concept in malaria. Parasitol Res. 2016;115:2139–2148. doi: 10.1007/s00436-016-5043-0. [DOI] [PubMed] [Google Scholar]
- 53.Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Li Wai Suen CSN, et al. Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PLoS Med. 2015;12:e1001891. doi: 10.1371/journal.pmed.1001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinton JA. Studies of infections with Plasmodium ovale. V. The effects of multiple inoculations upon the degree and nature of the immunity developed. Trans R Soc Trop Med Hyg. 1940;33:585–595. doi: 10.1016/S0035-9203(40)90018-9. [DOI] [Google Scholar]
- 55.Sinton JA, Hutton EL, Shute PG. Studies of infections with Plasmodium ovale. II. Acquired resistance to ovale infections. Trans R Soc Trop Med Hyg. 1939;33:47–68. doi: 10.1016/S0035-9203(39)90162-8. [DOI] [Google Scholar]
- 56.Sinton JA, Bird W. Studies in malaria, with special reference to Treatment. IX. Plasmoquine in the treatment of malaria. Indian J Med Res. 1928;16:159–178. [Google Scholar]
- 57.Desjardins RE, Doberstyn EB, Wernsdorfer WH. Principles and practice of malariology. London: Churchill Livingstone Inc; 1988. [Google Scholar]
- 58.Fuehrer HP, Starzengruber P, Swoboda P, Khan WA, Matt J, Ley B, et al. Indigenous Plasmodium ovale malaria in Bangladesh. Am J Trop Med Hyg. 2010;83:75–78. doi: 10.4269/ajtmh.2010.09-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Starzengruber P, Fuehrer HP, Swoboda P, Khan WA, Yunus EB, Hossain SM, et al. The first case of Plasmodium ovale malaria from Bangladesh. BMJ Case Rep. 2010;2010:02865. doi: 10.1136/bcr.03.2010.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fuehrer HP, Habler VE, Fally MA, Harl J, Starzengruber P, Swoboda P, et al. Plasmodium ovale in Bangladesh: genetic diversity and the first known evidence of the sympatric distribution of Plasmodium ovale curtisi and Plasmodium ovale wallikeri in southern Asia. Int J Parasitol. 2012;42:693–699. doi: 10.1016/j.ijpara.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 61.Tomar LR, Giri S, Bauddh NK, Jhamb R. Complicated malaria: a rare presentation of Plasmodium ovale. Trop Doct. 2015;45:140–142. doi: 10.1177/0049475515571989. [DOI] [PubMed] [Google Scholar]
- 62.Lemmerer R, Unger M, Vossen M, Forstner C, Jalili A, Starzengruber P, et al. Case report: spontaneous rupture of spleen in patient with Plasmodium ovale malaria. Wien Klin Wochenschr. 2015. [DOI] [PubMed]
- 63.Rozé B, Lambert Y, Gelin E, Geffroy F, Hutin P. Plasmodium ovale malaria severity. Med Mal Infect. 2011;41:216–217. doi: 10.1016/j.medmal.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 64.Coton T, Debourdeau P, Schoenlaub P, Grassin F, Maslin J. Péricardite aigue associée à un accès palustre de reviviscence un Plasmodium ovale. Med Trop (Mars) 2011;71:79–80. [PubMed] [Google Scholar]
- 65.Haydoura S, Mazboudi O, Charafeddine K, Bouakl I, Baban TA, Taher AT, et al. Transfusion-related Plasmodium ovale malaria complicated by acute respiratory distress syndrome (ARDS) in a non-endemic country. Parasitol Int. 2011;60:114–116. doi: 10.1016/j.parint.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 66.Cinquetti G, Banal F, Rondel C, Plancade D, de Saint Roman C, Adriamanantena D, et al. Splenic infarction during Plasmodium ovale acute malaria: first case reported. Malar J. 2010;9:288. doi: 10.1186/1475-2875-9-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rubinstein J, Fischer RA, Newman RD, Parise ME, Johnston SP, Young J. Late relapse of Plasmodium ovale as malaria-Philadelphia, Pennsylvania, November 2004. MMWR Morb Mortal Wkly Rep. 2005;54:1231–1233. [PubMed] [Google Scholar]
- 68.Filler S, Causer LM, Newman RD, Barber AM, Roberts JM, MacArthur J, et al. Malaria surveillance-United States, 2001. Morb Mortal Wkly Rep Surveill Summ. 2003;52:1–14. [PubMed] [Google Scholar]
- 69.Lee EY, Maguire JH. Acute pulmonary edema complicating ovale malaria. Clin Infect Dis. 1999;29:697–698. doi: 10.1093/clinids/29.2.468. [DOI] [PubMed] [Google Scholar]
- 70.Patel MI. Spontaneous rupture of a malarial spleen. Med J Aust. 1993;159:836–837. doi: 10.5694/j.1326-5377.1993.tb141386.x. [DOI] [PubMed] [Google Scholar]
- 71.Monlun E, Christmann D, Kremer M, Rey D, Storck D. Myocardial involvement in malaria. Rev Med Interne. 1989;13:433–436. [Google Scholar]
- 72.Bock E. Epidemiological, clinical and parasitological features of malaria produced by Plasmodium ovale Stephens, 1922. Archiv für Schiffs-und Tropenhygiene. 1939;43:327–353. [Google Scholar]
- 73.Fairley NH. A case of malaria due to Plasmodium ovale Stephens 1922. BMJ. 1933;2:101–102. doi: 10.1136/bmj.2.3784.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Additional files 1, 2 and 3).


