Abstract
Background
Vitamin D insufficiency (serum 25-OH vitamin D > 10 ng/ml and < 30 ng/ml) is prevalent in the obese (body mass index (BMI) > 30 kg/m2), yet relationships between the two are poorly understood. Objectives of this study include identification of the impact of obesity on reducing serum 25-OH vitamin D concentration, particularly in response to altered vitamin D3 supplementation, and to elucidate the longitudinal impact of serum 25-OH vitamin D on body mass index.
Methods
Twenty four-week-old lean and obese male C57BL/6 J mice were fed low, standard, or high levels of cholecalciferol supplementation and followed for 24 weeks. Longitudinal measurements include serum 25-OH and 1,25-(OH)2 vitamin D, intact PTH, and calcium concentrations, as well as BMI, bone density and body fat/lean mass.
Results
Baseline serum 25-OH concentrations were not different in lean and obese mice (lean 32.8 ± 4.4 ng/ml versus obese 30.9 ± 1.6 ng/ml p = 0.09). Lean mice receiving low supplementation exhibited rapid declines in serum 25-OH vitamin D concentrations, falling from 33.4 ± 5.4 ng/ml to 14.5 ± 3.4 ng/ml after 2 weeks, while obese mice declined at a lower rate, falling from 30.9 ± 1.5 to 19.0 ± 0.9 ng/ml within the same time period. Surprisingly, high vitamin D3 supplementation did not substantially increase serum vitamin D concentrations above standard supplementation, in either lean or obese mice. No differences in serum 1,25-(OH)2 vitamin D, intact parathyroid hormone (PTH) or serum calcium were observed between lean and obese mice within the same vitamin D supplementation group. Yet obese mice exhibited lower serum calcitriol, higher serum PTH, and lower bone mineral density (BMD) than did lean mice. Additionally, neither body mass index nor body fat % was significantly correlated with vitamin D concentrations. Interestingly, lean mice with high vitamin D supplementation consumed significantly more food than did lean mice with standard or low supplementation (14.6 ± 1.7 kcal/mouse/day versus 11.8 ± 1.4 and 12.3 ± 1.7 respectively, p < 0.0001 for both).
Conclusions
Low cholecalciferol supplementation in both lean and obese mice significantly and sustainably reduces serum 25-OH vitamin D concentrations. Interestingly, obesity slowed the rate of decline. Over the period of the study, vitamin D insufficiency was not subsequently correlated with greater BMI/body fat, although lean mice with high supplementation consumed greater calories with no apparent BMI increase.
Keywords: Vitamin D, PTH, Insufficiency, Obesity, Weight, BMI, Body Composition, Mice, Fat, High fat diet
Background
Vitamin D insufficiency (serum 25-OH vitamin D >10 ng/ml and <30 ng/ml) is a prevalent condition with an estimated 70% of the population considered at risk [1, 2]. Concurrent with widespread vitamin D insufficiency, the Centers for Disease Control also reports that a large percentage of the population (>35%) has a body mass index (BMI) considered obese. Several human studies have explored the possibility that these two phenomena may be linked [3–6], however, it remains unclear if vitamin D insufficiency promotes weight gain and/or if obesity modulates serum vitamin D concentration. Interestingly, studies seeking to modulate body weight by correcting vitamin D status have been unsuccessful [7–9], although other beneficial effects, including reduced body fat [7], reduced inflammatory profile [9], and improved insulin resistance [10] were seen. Contrary to what is seen in human trials, animal studies appear to show that vitamin D may promote weight gain. Vitamin D receptor (VDR) knock-out mice were shown to be resistant to weight gain [11, 12], as were diet-induced vitamin D deficient/insufficient mice to high fat [13] and “western” diets [14]. Additionally, over-expression of VDR in adipocytes induced weight gain in mice [15]. Although interpretation of these data suggests that vitamin D, through VDR, increases weight gain, other studies have shown that administration of 1,25-(OH)2 vitamin D, the active form of vitamin D and direct ligand to VDR, decreases weight and has other beneficial effects [16, 17].
Alternatively, data from several studies suggest obesity may impair the effectiveness of vitamin D supplementation. Supplementation in the obese has resulted in less than expected supplementation, [18–21] and these findings are further supported by a meta-analysis of greater than 12,000 patients showing that BMI negatively correlates with the effect of supplementation [22]. Additionally, mice given 2,000 IU vitamin D3/kg chow in a “western” diet had similar serum 25-OH vitamin D concentration as their lean counterparts given 1,000 IU vitamin D3/kg chow at the conclusion of the study [14]. It has been speculated that fat sequestration of vitamin D leads to lower serum concentrations [21], although this traditional view has recently been challenged by one study suggesting dilution by greater body mass and not fat sequestration leads to the dampening effects [23].
Studies examining the interrelationships between serum vitamin D insufficiency and obesity frequently focus on human populations where differing lifestyle and/or genetic factors may obfuscate findings. Animal models, on the other hand, predominately make use of receptor knockouts or complete dietary removal to simulate vitamin D deficient conditions, which may not accurately reflect the more prevalent condition of insufficiency in humans. To further explore the relationships between 25-OH vitamin D status and weight/body fat, we induced vitamin D insufficiency in lean and obese mice and examined food consumption, body weight, and body composition. Our data indicate that higher BMI modestly buffers serum 25-OH vitamin D concentration in response to lower supplementation. Further, our findings also suggest that neither high or low vitamin D3 supplementation modulates body weight or body fat.
Methods
Mice
All studies and experimental protocols were approved by and in compliance with guidelines of the Miami VA Animal Care and Use Committee. At all times mice were provided ad libitum access to chow/water and covered to avoid exposure to facility lighting. 8-week-old male C57BL/6 mice were randomly assigned to either high fat (60% FDC, Dyets Inc, Bethlehem, PA, ID# D180988) or low fat (10% FDC, ID# D180989) feeding for 16 weeks. Mice failing to gain more than 10% body weight were removed from the study. At 24 weeks of age, lean mice were randomly assigned to low fat diets containing 125, 1000 or 4000 IU vitamin D3/kg chow and obese mice to high fat diets containing 162, 1282 or 5169 IU vitamin D3/kg chow (Table 1), and were followed for 24 additional weeks. Numbers of animals were nine for lean-low & lean-standard, eight for lean-high, six for obese-low & obese-standard, and seven for obese-high. Mice and chow from cages were weighed on a weekly basis. Additionally, mouse length was measured at the end of the study by anesthetizing mice and measuring from nose to the base of the tail. BMI was calculated as g/cm2 [24].
Table 1.
Diet compositions
| Component (g/kg) | Low fat Diet | High fat diet |
|---|---|---|
| Vitamin Free Casein | 200 | 258 |
| L-Cystine | 3 | 4 |
| Dyetrose | 35 | 162 |
| Sucrose | 349 | 88 |
| Cornstarch | 315 | 0 |
| Soybean oil | 25 | 32 |
| Lard | 20 | 317 |
| t-Butylhydroquinone (TBHQ) | 0.005 | 0.006 |
| Cellulose | 50 | 65 |
| Dicalcium Phosphate | 13 | 17 |
| Calcium Carbonate | 5.5 | 7.1 |
| Potassium Citrate | 16.5 | 21.3 |
| Choline Bitartrate | 2 | 2.6 |
| Vitamin/Mineral Mix (Vit D free) | 20 | 26 |
| Vitamin D3 | 125/1000/4000 IU/kg | 162/1282/5169 IU/kg |
| 3.1/25.0/100.0 μg/kg | 4.0/32.3/129.0 μg/kg |
Serum analysis
Blood was extracted through the sub-mandibular vein using a mouse lancet (MEDIpoint, inc., Mineola, NY.) and collected into microcentrifuge tubes. Samples were held at room temperature for 10 min to allow coagulation and then centrifuged at 13,000 RPM for 10 min to allow separation of serum. Analysis of serum was performed using specific ELISA kits for 25-OH vitamin D (ImmunoDiagnostic Systems, Inc., Scottsdale, AZ), 1,25-(OH)2 vitamin D (MyBioSource, San Diego, CA.) and intact PTH (MyBioSource), while colorimetric assays were used to assess calcium (Biovision, San Francisco, CA.) according to manufacturer protocols.
Dual-energy X-Ray absorptiometry
Analysis of bone mineral density, body fat % and lean mass was performed using a Lunar PIXImus II (GE Healthcare, United Kingdom). Animals were anesthetized and then analyzed with a single scan at baseline and every 8 weeks thereafter.
Statistics
Two-way ANOVA, followed by post hoc Tukey’s Multiple Comparisons test when applicable, was used for comparisons between all vitamin D and diet groups at each time point as well as between vitamin D groups and time points, separately for obese and lean (Ian E. Holliday, 2012, Two-Way ANOVA (v1.0.3) in Free Statistics Software (v1.1.23-r7), Office for Research Development and Education, http://www.wessa.net/rwasp_Two%20Factor%20ANOVA.wasp). An * indicates statistically significant results corresponding to * for p < 0.05, ** for p < 0.01, *** for p < 0.001 and **** for p < 0.0001. All data were screened for potential outliers using GraphPad online software (La Jolla, CA.), using a significance cut-off of 0.05. All data are presented as mean ± standard deviation.
Results and Discussion
Serum 25-OH vitamin D concentrations fall rapidly with low supplementation to a constant insufficient level, while greater supplementation leads to little increase; obesity tempers decline in response to low vitamin D chow
In order to understand the varied interrelationships between vitamin D and body weight/composition, we set out to establish a range of serum 25-OH vitamin D concentrations between insufficiency (>10 ng/ml) and above sufficiency (>30 ng/ml) in lean and obese mice. To do so, we provided lean mice vitamin D3 supplementation at low (125 IU/kg chow), standard for most animal facilities (1,000 IU/kg chow) and high (4,000 IU/kg chow) amounts and in obese mice by providing low (162 IU/kg chow), standard (1,282 IU/kg chow) and high (5,169 IU/kg chow) amounts (Table 1). We increased vitamin D3 supplementation for obese groups to account for the lower food consumption rate that occurs during high fat feeding [25, 26]. As expected, we found no statistically significant difference in the amount of vitamin D3 being consumed between lean and obese mouse groups (Table 2). To confirm establishment of vitamin D insufficient and sufficient mice we measured serum 25-OH vitamin D concentrations at baseline and every two weeks thereafter (Fig. 1a). There was no statistically significant difference in serum 25-OH vitamin D between lean and obese mice (lean n = 26, 32.8 ± 4.4 ng/ml versus obese n = 19, 30.9 ± 1.6 ng/ml, p = 0.09). Similarly, Park et al. did not find differences in serum 25-OH vitamin D in obese and lean 22 week old mice, suggesting body composition does not modulate serum vitamin D levels during consistent supplementation [25].
Table 2.
Mean food and vitamin D3 consumption in mice. Lean and obese mice were given ad libitum access to chow containing varying amounts of vitamin D3 and food was weighed weekly to determine mean (± standard deviation) consumption
| Supplementation | Group | Food Consumption (g/mouse/day) | Vitamin D3
Consumption (IU/mouse/day) |
Comparison Lean and Obese |
|---|---|---|---|---|
| Low | Lean | 3.38 ± 0.47 | 0.42 ± 0.06 | p = 1.00 |
| Obese | 2.82 ± 0.19 | 0.46 ± 0.03 | ||
| Standard | Lean | 3.24 ± 0.39 | 3.24 ± 0.39 | p = 0.86 |
| Obese | 2.77 ± 0.19 | 3.55 ± 0.25 | ||
| High | Lean | 4.00 ± 0.46 | 15.97 ± 1.87 | p = 0.057 |
| Obese | 2.94 ± 0.16 | 15.20 ± 0.85 |
Fig. 1.
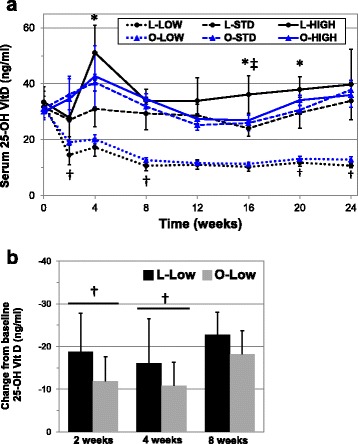
Analysis of serum 25-OH vitamin D3 concentrations of lean and obese mice in response to altered vitamin D3 supplementation. Serum 25-OH vitamin D3 concentration was measured every two weeks using ELISA in lean (L) and obese (O) mice given low (125 IU/kg chow), standard (STD, 1000 IU/kg chow) or high (4000 IU/kg chow) amounts of vitamin D3 a Decline from baseline serum 25-OH vitamin D concentration was further calculated for L-LOW and O-LOW b. Symbols indicate p < 0.05 between L-HIGH and L-STD (*), L-HIGH and O-HIGH (‡), and L-LOW and O-LOW (†)
We then modified cholecalciferol given in chow to elucidate whether body composition affects response to altered supplementation. Mice given low supplementation experienced a rapid decline evident at two weeks, with stabilization between 11–12 ng/ml by 8 weeks, independent of being lean or obese. The rate of decline in serum 25-OH vitamin D for lean mice is consistent with findings in rats, goats and cows [27–29], but more rapid than the reported half-life of approximately 20 days in humans [30]. However, the rate of decline was steeper in lean compared to obese mice, with the decline from baseline over two weeks being 18.8 ± 5.7 ng/ml versus 11.9 ± 1.1 ng/ml (**p = 0.0084), respectively (Fig. 1b). This salient observation supports the notion that fat sequestration may buffer against the loss of serum 25-OH vitamin D during low supplement intake. Furthermore, serum 25-OH vitamin D was significantly greater at several time points in low supplemented obese mice compared to lean (week 8: *p = 0.0247, week 20: *p = 0.0410, and week 24: ***p = 0.0003, Fig. 1a), which would not be consistent with the Drincic et al. dilution model [23]. Yet, the overall magnitude of the differences (~1–2 ng/ml) does not strongly support a role of fat sequestration in controlling serum 25-OH vitamin D.
Interestingly, higher supplementation failed to significantly increase serum 25-OH vitamin D concentrations compared to standard amounts after 24 weeks in both lean and obese mice. Non-linear increases during supplementation to raise serum vitamin D above 30 ng/ml have been observed in rats [31], and similar 25-OH concentrations were detected in lean mice given 1000 IU/kg chow compared to obese mice given just over 2000 IU/kg chow [14]. Taken together, these reports and our data reflecting a non-linear increase in supplementation between 1000–4000 IU/kg chow are inconsistent with those of Fleet et al. [32] demonstrating linear increases from 400 IU/kg chow through 20,000 IU/kg chow. It is important to note that Fleet et al. used weanling mice in and did not specifically interrogate supplementation with 2000 or 4000 IU/kg chow. Furthermore, our data suggest that the obese/lean status of the mouse does not modulate the effects of higher supplementation. These findings run contrary to numerous human clinical trials showing diminished elevations with supplementation due to obesity [20, 21, 33, 34], but not all [19, 35]. A possible explanation for the lack of effect in our study was the failure of higher supplementation to increase 25-OH vitamin D levels above standard supplementation in either lean or obese mice, a ceiling effect that may be masking potential obesity impacts. Our data show that high supplemented lean mice had significantly greater 25-OH vitamin D concentration at week 16 over obese mice (week 16, **p = 0.0040 versus high supplemented obese mice), however, as this difference did not persist we were unable to confidently conclude that obesity modulates the effects of higher supplementation.
Obese mice, independent of serum vitamin D status, exhibit differences in serum 1,25-(OH)2 vitamin D and intact PTH concentration relative to lean mice, with no effect on serum calcium
To further identify the impacts of varied 25-OH vitamin D levels, we analyzed the serum concentration of 1,25-(OH)2 vitamin D, intact parathyroid hormone (PTH) and calcium (Fig. 2a-c) in lean and obese mice after 24 weeks of altered vitamin D3 supplementation. Our findings reveal there were no significant differences in any serum 25-OH vitamin D levels between vitamin D groups in either lean or obese mice. However, obese mice exhibited lower 1,25-(OH)2 vitamin D and higher intact PTH compared to lean mice (****p < 0.0001 for both), and these findings in our mouse model are consistent with data from a study in lean and obese humans [36]. We did not observe statistically significant differences in serum calcium due to obesity (p = 0.27). Our findings for intact PTH and serum calcium in obese and lean 48-week-old mice are in agreement with the reported levels in 22-week-old obese and lean mice [25]. However, we do report a significantly lower, and Park et al. [25] a significantly higher, serum 1,25-(OH)2 vitamin D in obese mice relative to lean. As expected, we detected a positive association between serum levels of 1,25-(OH)2 vitamin D and intact PTH (r2 = 0.24, **p = 0.0014, Fig. 2d). Whether this relationship exists in Park et al. is not reported. The nature of these conflicting data may be due to the greater length of time of exposure to high fat diet (48 weeks in our study versus 18 weeks for Park et al.) that has allowed greater time for an equilibrium between PTH and serum 1,25-(OH)2 vitamin D consistent with observations in human studies [36].
Fig. 2.
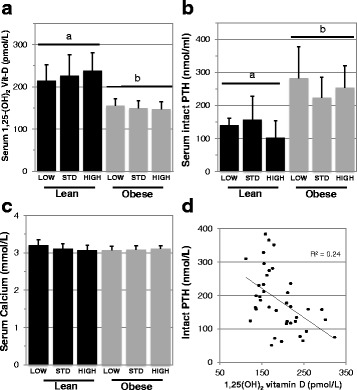
Serum profiles of lean and obese mice following supplementation with varied amounts of Vitamin D 3. Serum was collected following 24 weeks of either low, standard or high vitamin D3 supplementation, and analyzed for serum 1,25-(OH)2 vitamin D3 a, intact PTH b and calcium c concentration using ELISA or colorimetric assay. Correlative analysis was further performed between serum intact PTH and serum 1,25-(OH)2 vitamin D d. Different letters denote a significant difference between lean and obese (****p < 0.0001)
To our surprise, differing supplementation and the subsequent impacts on serum 25-OH vitamin D concentrations, had no significant correlation with 1,25-(OH)2 vitamin D or iPTH serum markers in these mice, a finding that is comparable to observations from a study in rats [31]. Although, this study may have been underpowered to detect effects on 1,25-(OH)2 vitamin D, intact PTH and calcium, this phenomenon may also reflect the narrow dosing range designed to induce insufficiency. Indeed effects on these serum markers were reported in other studies when supplementing outside the normo-physiological range used in our study of roughly 125–5,000 IU/kg chow [32, 37].
Lean mice receiving higher amounts of cholecalciferol have higher caloric intake yet maintain similar body weight
Numerous human studies have identified negative associations between body mass index (BMI) and serum 25-OH vitamin D concentrations [2, 4, 6, 38], leading to speculation that low 25-OH vitamin D may promote weight gain and correction of vitamin D status may promote weight loss in overweight individuals. To investigate the modulating effects of serum vitamin D on body mass index, we followed body weight changes in lean and obese mice supplemented with varying amounts of vitamin D for 24 weeks (Fig. 3a). Our findings suggest vitamin D insufficiency does not potentiate additional weight gain in lean or obese mice compared with sufficiency. Furthermore, no effect on BMI was observed in highly supplemented lean or obese mice, which is consistent with human studies that have attempted to promote weight loss through vitamin D supplementation [7–9, 39]. However, in our study, mice with high supplementation had serum 25-OH and 1,25-(OH)2 vitamin D concentrations similar to mice given standard amounts, which may indicate that these mice did not receive high enough supplementation to affect BMI. Indeed, evidence that greater supplementation might affect BMI was recently reported by Marcotorchino et al. [40], where 15,000 IU/kg chow given to mice prevented weight gain during a high fat diet. Mice in that study were only 6 weeks of age at the start of the experiment and not obese at baseline, yet these findings may indicate a necessary level of supplementation to see an effect.
Fig. 3.
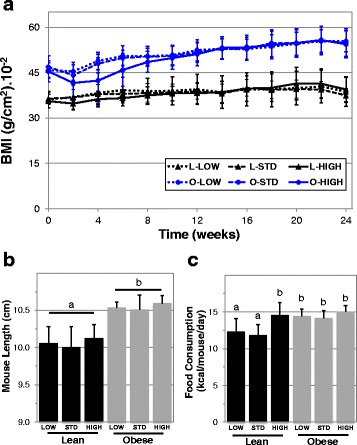
Food consumption and BMI in mice fed high or low fat diet containing varying levels of vitamin D 3. Twenty-four week old male mice were randomly sorted into low, standard (STD) or high vitamin D3 supplementation groups and followed for 24 weeks. Body mass index a for each mouse was determined weekly using mouse length measurements from week 24 b. Mouse length endpoint measurements reflect the distance from nose to the base of the tail. Food consumption was measured weekly and group means were averaged c. Different letters denote significant differences (****p < 0.0001)
Despite the lack of an effect of higher supplementation on BMI or serum vitamin D metabolites, our lean mice supplemented with 4000 IU/kg chow had greater caloric consumption over the length of the study (14.6 ± 1.7 kcal/mouse/day versus 11.8 ± 1.4 for 1000 IU and 12.3 ± 1.7 for 125 IU, ****p < 0.0001 for both, Fig. 3b). The inference from these data is that the greater food consumption was offset by higher metabolism, perhaps suggesting a weight loss strategy involving caloric restriction might be improved by vitamin D supplementation. Interestingly, Shapses et al. were unable to discern an effect on weight loss in humans with a dual vitamin D supplementation and caloric restriction protocol [41]. But it is possible that the 2500 IU vitamin D3/day supplementation strategy was not equivalent to the higher level of supplementation in our mice.
Overall, our findings that vitamin D insufficiency had no effect on BMI in our mice run contrary to findings by Bastie et al. [14] and to a lesser extent Liu et al. [13], where diet induced vitamin D deficient mice were resistant to “western” and high fat diet induced weight gain, respectfully. However these studies induced vitamin D deficiency concurrently with alterations in diet. Whereas in our study the mice had been fed a HFD for four months prior to initiation of the vitamin D intervention. Additionally we believe the age at which mice were made insufficient/deficient may also be an important factor. We observed that the average length of our obese mice at 48 weeks of age was significantly longer than lean mice (105.5 ± 1.3 cm versus 100.6 ± 2.3 cm, ****p < 0.0001, Fig. 3c). We found no length differences between vitamin D groups, which would be expected as mice have likely stopped growing by the time supplementation was adjusted. However, both Bastie et al. and Liu et al. varied vitamin D supplementation before 3–5 weeks of age which may have lead to numerous impacts on the development of the mice not seen in our study since variations in vitamin D supplementation occurred after maturity. Interestingly, although our study is the first report of high fat feeding leading to increased length in mice, several human studies have found trends between higher BMI and height in adolescents [42–44].
Serum 25-OH vitamin D insufficiency does not modulate body fat, lean mass or bone mineral density in either lean or obese mice
Vitamin D is canonically recognized as being important for bone health, and more recently for impacts on body fat and lean mass, yet the impacts of chronic insufficiency on these parameters have not been elucidated. Therefore, we performed dual X-Ray absorptiometry (DEXA) analysis at baseline and every two months thereafter on our mouse cohorts (Figs. 4 and 5). Surprisingly, despite 6 months of vitamin D insufficiency, neither lean nor obese insufficient mice exhibited lower BMD compared to sufficient mice (Fig. 4a). These findings are in general agreement with Fleet et al. [32], with the exception of lower BMD seen in a group receiving 25 IU vitamin D3/kg chow group, for which supplementation was below our lowest tested group. Interestingly, high supplementation failed to increase BMD in mice as well, although we note this may be due to the inability of this level of supplementation to substantially increase serum levels of 25-OH vitamin D or the possibility that we were underpowered to observe this effect. Furthermore, we did not identify association between BMD with either serum 25-OH vitamin D (Fig. 4b) or 1,25-(OH)2 vitamin D (Fig. 4c), but there was a negative correlation between BMD and serum intact PTH (r2 = 0.33, ****p < 0.0001 Fig. 4d). This difference remained significant when controlling for BMI of the mice (**p = 0.0014, data not shown) and reflects the key role of PTH in bone homeostasis [45].
Fig. 4.
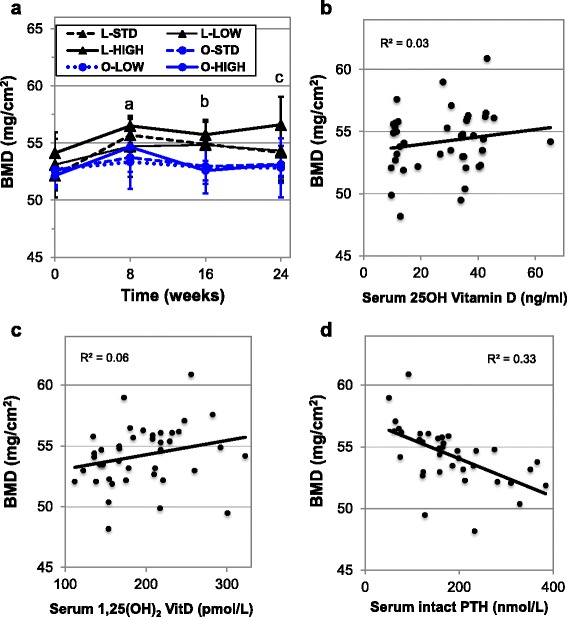
Impacts on bone mineral density and relationship with serum biomarkers. The effects of high fat and low fat diet containing varying levels of vitamin D on bone mineral density were analyzed using DEXA a No statistical differences observed between vitamin D groups. Differences between all lean and obese mice were significant for bone mineral density (a p = 0.004; b p < 0.0001; c p < 0.008). Bone mineral density was not correlated with serum 25-OH b or 1,25-(OH)2 vitamin D c, but was correlated with serum intact PTH (R2 = 0.32, ****p < 0.0001) d
Fig. 5.
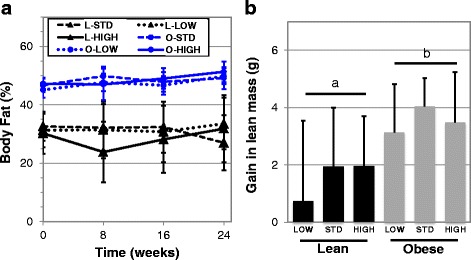
Analysis of body composition of lean and obese mice given low, standard and high vitamin D supplementation. The effects of high fat and low fat diet containing varying levels of vitamin D on body fat % a and the gain in lean body mass over 48 weeks b were analyzed using DEXA. No statistical differences observed between vitamin D groups. Differences between all lean and obese mice were significant for body fat % (at all time points, ****p < 0.0001) and gain in lean body mass (a versus b, **p = 0.002)
However, obese mice exhibited lower BMD at all time points except at baseline (0 weeks: p = 0.17; 8 weeks: **p = 0.004; 16 weeks: ****p < 0.0001; and 24 weeks: **p = 0.008, Fig. 4a). Interestingly, in other mouse studies such differences in total BMD between obese and lean were not observed, yet differences in bone density were observed when utilizing μCT [46–48]. One key distinction between our study and the other reports is that the length of nearly 48 weeks in our study that may have provided enough time for obesity related-effects on BMD to become manifest. It is also noteworthy that higher supplementation was not able to counteract the effects of obesity. However, as higher supplementation did not significantly affect 25-OH or 1,25-(OH)2 vitamin D concentration, the possibility remains that supplementation beyond 4,000 IU/kg chow may rescue obesity related bone deficits.
Our data reveal that vitamin D insufficiency does not modulate body fat percentage (Fig. 5a) or lean tissue mass (Fig. 5b) in lean or obese mice. Yet, we did detect a larger gain of lean mass in obese mice versus lean mice over the 48-week period (**p = 0.002, Fig. 5b). Interestingly, no effects were seen in mice receiving higher amounts of vitamin D3 in chow contrary to observations in several human studies [7, 49, 50], but not all [33]. This discrepancy may be due to a differing range of supplementation levels, or that vitamin D may have roles unique to human physiology.
Conclusions
We have established a model of vitamin D insufficiency in lean and obese mice by reducing vitamin D3 supplementation in chow. Obese mice exhibited a slower rate of decline in serum 25-OH vitamin D and modestly higher levels at equilibrium. No differences in serum 1,25-(OH)2 vitamin D, intact parathyroid hormone (PTH) or serum calcium were observed between lean and obese mice within the same vitamin D supplementation group. Yet obese mice exhibited lower serum calcitriol, higher serum PTH, and lower bone mineral density (BMD) than did lean mice. It also appears that 25-OH vitamin D concentrations between 10 to 40 ng/ml did not correlate with body weight or body fat in mice, although higher supplementation in mice fed a standard diet correlated with increased food consumption weight or lean/fat mass. The increase in caloric consumption without a concurrent increase in energy storage implies a heightened metabolism, which, if coupled to a calorically restricted diet may prove to be an effective strategy for weight loss.
Acknowledgements
The authors thank Guy Howard of the University of Miami and Jessica Reynolds of the University at Buffalo for their suggestions and insights during the preparation of this manuscript. The authors also wish to thank the University at Buffalo, University of Miami, Miami VA, and the research service of the VA Western New York Healthcare System.
Funding
This work was supported by grants from VA RR&D RX001066 and VA BLR&D BX000758 as well as from the Indian Trail Charitable Foundation, Inc.
Availability of data and materials
Please contact author for data requests.
Authors’ contributions
KLS and BRT conceived of the study and drafted the manuscript. KLS and MP performed data analysis. MP and PY performed DEXA analysis of mice. KLS, MP, MRG, MH, PY and ZWS performed longitudinal blood collections, harvested mouse tissues and performed ELISA and colorimetric analyses. KLS, MRG and MH monitored animal weight, chow and health. All authors read and approved the final manuscript.
Competing interest
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- BMI
Body mass index
- DEXA
Dual Emission X-Ray Absorptiometry
- iPTH
Intact parathyroid hormone
- μCT
Micro computed tomography
- VDR
Vitamin D receptor
Contributor Information
Kenneth L. Seldeen, Email: seldeen@buffalo.edu
Manhui Pang, Email: mpang59@gmail.com.
Maria Rodríguez-Gonzalez, Email: rgmaria.a@gmail.com.
Mireya Hernandez, Email: mireyahp67@yahoo.com.
Zachary Sheridan, Email: zws@zacharysheridan.com.
Ping Yu, Email: chinapingyu@hotmail.com.
Bruce R. Troen, Email: troen@buffalo.edu
References
- 1.Wei MY, Giovannucci EL. Vitamin D and multiple health outcomes in the Harvard cohorts. Mol Nutr Food Res. 2010;54(8):1114–26. doi: 10.1002/mnfr.200900574. [DOI] [PubMed] [Google Scholar]
- 2.Dong Y, et al. Low 25-hydroxyvitamin D levels in adolescents: race, season, adiposity, physical activity, and fitness. Pediatrics. 2010;125(6):1104–11. doi: 10.1542/peds.2009-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mithal A, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20(11):1807–20. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 4.Mai XM, et al. Cross-sectional and prospective cohort study of serum 25-hydroxyvitamin D level and obesity in adults: the HUNT study. Am J Epidemiol. 2012;175(10):1029–36. doi: 10.1093/aje/kwr456. [DOI] [PubMed] [Google Scholar]
- 5.Goshayeshi L, et al. Association between metabolic syndrome, BMI, and serum vitamin D concentrations in rheumatoid arthritis. Clin Rheumatol. 2012;31(8):1197–203. doi: 10.1007/s10067-012-1995-3. [DOI] [PubMed] [Google Scholar]
- 6.Farrell SW, Willis BL. Cardiorespiratory fitness, adiposity, and serum 25-dihydroxyvitamin D levels in women: the Cooper Center Longitudinal Study. J Womens Health (Larchmt) 2012;21(1):80–6. doi: 10.1089/jwh.2010.2684. [DOI] [PubMed] [Google Scholar]
- 7.Salehpour A, et al. A 12-week double-blind randomized clinical trial of vitamin D(3) supplementation on body fat mass in healthy overweight and obese women. Nutr J. 2012;11:78. doi: 10.1186/1475-2891-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sneve M, Figenschau Y, Jorde R. Supplementation with cholecalciferol does not result in weight reduction in overweight and obese subjects. Eur J Endocrinol. 2008;159(6):675–84. doi: 10.1530/EJE-08-0339. [DOI] [PubMed] [Google Scholar]
- 9.Zittermann A, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89(5):1321–7. doi: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]
- 10.Kamycheva E, Berg V, Jorde R. Insulin-like growth factor I, growth hormone, and insulin sensitivity: the effects of a one-year cholecalciferol supplementation in middle-aged overweight and obese subjects. Endocrine. 2013;43(2):412–8. doi: 10.1007/s12020-012-9825-6. [DOI] [PubMed] [Google Scholar]
- 11.Narvaez CJ, et al. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology. 2009;150(2):651–61. doi: 10.1210/en.2008-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber K, Erben RG. Differences in triglyceride and cholesterol metabolism and resistance to obesity in male and female vitamin D receptor knockout mice. J Anim Physiol Anim Nutr (Berl) 2013;97(4):675–83. doi: 10.1111/j.1439-0396.2012.01308.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu XJ, et al. Vitamin d deficiency attenuates high-fat diet-induced hyperinsulinemia and hepatic lipid accumulation in male mice. Endocrinology. 2015;156(6):2103–13. doi: 10.1210/en.2014-2037. [DOI] [PubMed] [Google Scholar]
- 14.Bastie CC, et al. Dietary cholecalciferol and calcium levels in a Western-style defined rodent diet alter energy metabolism and inflammatory responses in mice. J Nutr. 2012;142(5):859–65. doi: 10.3945/jn.111.149914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong KE, et al. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J Biol Chem. 2011;286(39):33804–10. doi: 10.1074/jbc.M111.257568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alkharfy KM, et al. Effects of vitamin D treatment on skeletal muscle histology and ultrastructural changes in a rodent model. Molecules. 2012;17(8):9081–9. doi: 10.3390/molecules17089081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin Y, et al. Vitamin D attenuates high fat diet-induced hepatic steatosis in rats by modulating lipid metabolism. Eur J Clin Invest. 2012;42(11):1189–96. doi: 10.1111/j.1365-2362.2012.02706.x. [DOI] [PubMed] [Google Scholar]
- 18.Aguirre Castaneda R, et al. Response to vitamin D3 supplementation in obese and non-obese Caucasian adolescents. Horm Res Paediatr. 2012;78(4):226–31. doi: 10.1159/000343446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canto-Costa MH, Kunii I, Hauache OM. Body fat and cholecalciferol supplementation in elderly homebound individuals. Braz J Med Biol Res. 2006;39(1):91–8. doi: 10.1590/S0100-879X2006000100011. [DOI] [PubMed] [Google Scholar]
- 20.Lee P, et al. Adequacy of vitamin D replacement in severe deficiency is dependent on body mass index. Am J Med. 2009;122(11):1056–60. doi: 10.1016/j.amjmed.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Wortsman J, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 22.Saliba W, Barnett-Griness O, Rennert G. The relationship between obesity and the increase in serum 25(OH)D levels in response to vitamin D supplementation. Osteoporos Int. 2013;24(4):1447–54. doi: 10.1007/s00198-012-2129-0. [DOI] [PubMed] [Google Scholar]
- 23.Drincic AT, et al. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring) 2012;20(7):1444–8. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- 24.Maffei M, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 25.Park JM, Park CY, Han SN. High fat diet-Induced obesity alters vitamin D metabolizing enzyme expression in mice. Biofactors. 2015;41(3):175–82. doi: 10.1002/biof.1211. [DOI] [PubMed] [Google Scholar]
- 26.Fenton PF, Carr CJ. The nutrition of the mouse. XI. Response of four strains to diets differing in fat content. J Nutr. 1951;45(2):225–33. doi: 10.1093/jn/45.2.225. [DOI] [PubMed] [Google Scholar]
- 27.Brouwer DA, et al. Rat adipose tissue rapidly accumulates and slowly releases an orally-administered high vitamin D dose. Br J Nutr. 1998;79(6):527–32. doi: 10.1079/BJN19980091. [DOI] [PubMed] [Google Scholar]
- 28.Hidiroglou M. Kinetics of intravenously administered 25-hydroxyvitamin D3 in sheep and the effect of exposure to ultraviolet radiation. J Anim Sci. 1987;65(3):808–14. doi: 10.2527/jas1987.653808x. [DOI] [PubMed] [Google Scholar]
- 29.Wilkens MR, et al. Is the metabolism of 25-hydroxyvitamin D3 age-dependent in dairy cows? J Steroid Biochem Mol Biol. 2013;136:44–6. doi: 10.1016/j.jsbmb.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Smith JE, Goodman DS. The turnover and transport of vitamin D and of a polar metabolite with the properties of 25-hydroxycholecalciferol in human plasma. J Clin Invest. 1971;50(10):2159–67. doi: 10.1172/JCI106710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson PH, et al. Vitamin D depletion induces RANKL-mediated osteoclastogenesis and bone loss in a rodent model. J Bone Miner Res. 2008;23(11):1789–97. doi: 10.1359/jbmr.080616. [DOI] [PubMed] [Google Scholar]
- 32.Fleet JC, et al. Serum metabolite profiles and target tissue gene expression define the effect of cholecalciferol intake on calcium metabolism in rats and mice. J Nutr. 2008;138(6):1114–20. doi: 10.1093/jn/138.6.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallagher JC, Yalamanchili V, Smith LM. The effect of vitamin D supplementation on serum 25(OH)D in thin and obese women. J Steroid Biochem Mol Biol. 2013;136:195–200. doi: 10.1016/j.jsbmb.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harel Z, et al. Low vitamin D status among obese adolescents: prevalence and response to treatment. J Adolesc Health. 2011;48(5):448–52. doi: 10.1016/j.jadohealth.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Gallagher JC, et al. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med. 2012;156(6):425–37. doi: 10.7326/0003-4819-156-6-201203200-00005. [DOI] [PubMed] [Google Scholar]
- 36.Parikh SJ, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89(3):1196–9. doi: 10.1210/jc.2003-031398. [DOI] [PubMed] [Google Scholar]
- 37.Rowling MJ, et al. High dietary vitamin D prevents hypocalcemia and osteomalacia in CYP27B1 knockout mice. J Nutr. 2007;137(12):2608–15. doi: 10.1093/jn/137.12.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forsythe LK, et al. Effect of adiposity on vitamin D status and the 25-hydroxycholecalciferol response to supplementation in healthy young and older Irish adults. Br J Nutr. 2012;107(1):126–34. doi: 10.1017/S0007114511002662. [DOI] [PubMed] [Google Scholar]
- 39.Belenchia AM, et al. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. Am J Clin Nutr. 2013;97(4):774–81. doi: 10.3945/ajcn.112.050013. [DOI] [PubMed] [Google Scholar]
- 40.Marcotorchino J, et al. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J Nutr Biochem. 2014;25(10):1077–83. doi: 10.1016/j.jnutbio.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Shapses SA, et al. Vitamin D supplementation and calcium absorption during caloric restriction: a randomized double-blind trial. Am J Clin Nutr. 2013;97(3):637–45. doi: 10.3945/ajcn.112.044909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krzyzanowska M, Umlawska W. The relationship of Polish students’ height, weight and BMI with some socioeconomic variables. J Biosoc Sci. 2010;42(5):643–52. doi: 10.1017/S0021932010000180. [DOI] [PubMed] [Google Scholar]
- 43.Stovitz SD, et al. Growing into obesity: patterns of height growth in those who become normal weight, overweight, or obese as young adults. Am J Hum Biol. 2011;23(5):635–41. doi: 10.1002/ajhb.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stovitz SD, et al. The interaction of childhood height and childhood BMI in the prediction of young adult BMI. Obesity (Silver Spring) 2008;16(10):2336–41. doi: 10.1038/oby.2008.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sneve M, et al. The association between serum parathyroid hormone and bone mineral density, and the impact of smoking: the Tromso Study. Eur J Endocrinol. 2008;158(3):401–9. doi: 10.1530/EJE-07-0610. [DOI] [PubMed] [Google Scholar]
- 46.Cao JJ, Sun L, Gao H. Diet-induced obesity alters bone remodeling leading to decreased femoral trabecular bone mass in mice. Ann N Y Acad Sci. 2010;1192:292–7. doi: 10.1111/j.1749-6632.2009.05252.x. [DOI] [PubMed] [Google Scholar]
- 47.Ionova-Martin SS, et al. Changes in cortical bone response to high-fat diet from adolescence to adulthood in mice. Osteoporos Int. 2011;22(8):2283–93. doi: 10.1007/s00198-010-1432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu XM, Zhao H, Wang EH. A high-fat diet induces obesity and impairs bone acquisition in young male mice. Mol Med Rep. 2013;7(4):1203–8. doi: 10.3892/mmr.2013.1297. [DOI] [PubMed] [Google Scholar]
- 49.Caron-Jobin M, et al. Elevated serum 25(OH)D concentrations, vitamin D, and calcium intakes are associated with reduced adipocyte size in women. Obesity (Silver Spring) 2011;19(7):1335–41. doi: 10.1038/oby.2011.90. [DOI] [PubMed] [Google Scholar]
- 50.Carrillo AE, et al. Impact of vitamin D supplementation during a resistance training intervention on body composition, muscle function, and glucose tolerance in overweight and obese adults. Clin Nutr. 2013;32(3):375–81. doi: 10.1016/j.clnu.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact author for data requests.


