Abstract
Background:
Human Cripto-1, a member of the EGF-CFC family, is involved in embryonic development, embryonic stem cell maintenance, and tumor progression. It also participates in multiple cell signaling pathways including Wnt, Notch, and TGF-β. Remarkably, it is expressed in cancer stem cell (CSC) compartments, boosting tumor cell migration, invasion, and angiogenesis. Although Cripto-1 is overexpressed in a variety of human malignant tumors, its expression in esophageal squamous cell carcinoma (ESCC) remains unclear. Our aim in this study was to evaluate the possible oncogenic role of Cripto-1 in ESCC progression and elucidate its association with clinicopathological parameters in patients.
Methods:
In this study, Cripto-1 expression in 50 ESCC tissue samples was analyzed and compared to corresponding margin-normal esophageal tissues using quantitative real-time PCR.
Results:
Cripto-1 was overexpressed in nearly 40% of ESCC samples compared with normal tissue samples. Significant correlations were observed between Cripto-1 expression and tumor differentiation grade, progression stage, and location (p < 0.05).
Conclusions:
Our results indicate that overexpression of Cripto-1 is involved in the development of ESCC. Further assessment will be necessary to determine the role of Cripto-1 cross talk in ESCC tumorigenesis.
Key Words: Cancer stem cell, Cripto-1, ESCC, Expressional analysis, Real-time PCR
Introduction
Esophageal squamous cell carcinoma (ESCC) is the most common cancer in the "Central Asian Esophageal Cancer Belt,” which reaches from China to northeast Iran. Because various molecular mechanisms can influence the initiation, progression, and invasion of ESCC (1), better understanding of signaling pathways utilized during ESCC development may improve prognoses, diagnoses, and treatment strategies. Therefore, identifying molecular markers involved in various ESCC signaling pathways seems critical (2). Cripto-1 (CR-1) or teratocarcinoma-derived growth factor-1 (TDGF-1), a member of the EGF-CFC gene family (epidermal growth factor- Cripto/FRL1/Cryptic as a cysteine- rich region), plays a critical role in self-renewal and pluripotency capabilities of embryonic stem cells (ESC) (3). CR-1, an identified oncogene, is overexpressed in a wide spectrum of human malignant solid tumors, introducing a potential therapeutic target for human cancers (4, 5). Increased CR-1 expression is significantly associated with initiation and aggressiveness of human cancers. Intriguingly, CR-1 shows an embryonic pattern of gene expression, commonly expressed in ESCs but not in normal adult tissues or normal cell lines (6, 7). Overexpression of CR-1 in human tumors may confirm the linkage between stem cell biology and tumor development (8, 9), and identify effective markers to predict cancer initiation and progression (3). Along with cellular transformational and angiogenic activities, CR-1 can function through major cell signaling pathways such as the Nodal/ALK4/Smad-2 and the Glypican-1/c-Src/MAPK/AKT pathways (10, 11). CR-1, as a co-receptor for Nodal, a member of the transforming growth factor β superfamily, binds to the activin-like kinase type I (ALK4) and type II receptor complex (ActRII), triggering Smad-2/Smad-3 phosphorylation and Smad-4 accumulation to mediate transcription of target genes. Consequently, CR-1/Nodal signaling participates in early embryonic development and is involved in formation of the primitive streak and patterning of the anterior/posterior axis. (12). In addition, the interaction of CR-1 with glypican-1, a heparin sulfate proteoglycan, can boost the tyrosine kinase c-Src, enhancing activation of mitogen-activated protein kinase (MAPK) and AKT in a Nodal-independent manner. Activation of the MAPK and AKT intracellular signaling pathways affects cell growth, differentiation, motility, and survival (13, 14). CR-1 can also prevent signaling by other members of the TGF-β family, such as TGF-β1 and activin A/B, leading to tumor cell growth (15). Wnt and Notch signaling target genes are directly involved in ESCC progression and development, and their deregulation plays a fundamental role in tumorigenesis (1, 16). CR-1 interacts with several components of various signaling pathways, including Wnt, Notch, TGF-β, Oct-4, and NANOG (17). CR-1 is a downstream target gene of the Wnt/β-catenin signaling pathway, and its interaction with Wnts can regulate different biological activities including embryo development, stimulation of cell migration, invasion, and epithelial-to-mesenchymal transition (EMT) (12). Remarkably, the Wnt pathway is activated in both normal and cancer stem cells, playing roles in cell cycle regulation and self-renewal (18). Moreover, cross talk occurs between Notch and the Nodal/CR-1 signaling pathway, suggesting Notch directly regulates the expression of Nodal, and CR-1 binds to all four Notch receptors (19). Notch, as an upstream gene of Nodal, is essential for the induction of Nodal expression (20). Notably, Notch signaling affects embryogenesis, cell differentiation, proliferation, organogenesis, and apoptosis, and its deregulation may induce carcinogenesis (16, 21). CR-1 is a downstream target of the transcription factors Oct-4 and NANOG, which regulate CR-1 expression through binding to the CR-1 promoter. In addition, these are stem cell-related genes. This suggests that CR-1 expression can mediate NANOG and Oct-4 function by initiating and maintaining their expression (22, 23). It is to be mentioned that the master transcriptional complex containing Oct-4, Sox2, and NANOG can deregulate differentiated cells to the pluripotent stem cell phenotype (24).
Considering the important role of this embryonic cancer stem cell marker in different cell signaling pathways, evaluation of its clinical relevance in cancer may be crucial for cancer therapy. The impact of CR-1 mRNA expression on ESCC progression and development has not been investigated, although its overexpression was reported in various malignancies. Therefore, our aim in this study was to analyze CR-1 expression and determine its clinicopathological relevance in ESCC.
Materials and Methods
Patients and Tissue Samples
Fresh tumor and distant tumor-free tissue samples were collected from 50 ESCC patients who underwent esophagectomy at the Omid, Qaem, and Imamreza Hospital of Mashhad University of Medical Sciences (MUMS), Iran, and transferred to RNAlater solution (Qiagen, Hilden, Germany). The recruited patients had not received chemo- or radio-therapeutic treatments before their operations. All tumor samples were histologically confirmed to contain at least 70% tumor cells. The study was performed based on the ethical guidelines of the MUMS. The patients declared their written informed consent.
RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR
RNA was isolated and complementary DNA (cDNA) synthesized from the tumoral and tumor-free samples as previously described (25, 26). Comparative real-time PCR analyses of CR-1 mRNA expression was performed in duplicate reactions using the primer sets listed in Table 1 in a Stratagene Mx-3000P thermo cycler (Stratagene, La Jolla, CA) applying the SYBR Green (Ampliqune, Denmark) method. The housekeeping glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was applied as an internal control to normalize real-time PCR data through the comparative cycle threshold (Ct) method (27-29). The following thermal profile was applied: 5 min at 95 °C as the initial denaturation step, followed by 40 cycles at 95 °C for 30 s, 58 °C for 30 s, and finally 72 °C for 30 s. Fold-change measurement of CR-1 mRNA expression in tumors relative to normal tissue was assessed as described before (30, 31).
Table 1.
Primer sequences used in real-time PCR.
| Forward primer | Reverse primer | |
|---|---|---|
| Cripto-1 | GGGATACAGCACAGTAAGGAG | ACGGTGGTAGTTCTGGAGTC |
| GAPDH | GGAAGGTGAAGGTCGGAGTCA | GTCATTGATGGCAACAATATCCACT |
Statistical analysis
Data was analyzed using the SPSS 19.9 (SPSS, Chicago, IL, USA). The χ2 or Fisher’s exact tests were applied to evaluate correlations between categorical variables and CR-1 mRNA expression. Comparisons of expression levels with lymph node metastasis, tumor depth of invasion, tumor location, grade, and tumor stage were performed via the independent sample t test and ANOVA. Results were considered significant with p values < 0.05.
Results
Study Population
CR-1 expression was assessed in ESCC samples by comparative real-time PCR. The ESCC patients' clinicopathological features are shown in Table 2. The 50 ESCC patients recruited in this study included 26 (52%) females and 24 (48%) males with a mean age ± standard deviation (SD) of 61.94 ± 11.76 years. Patient ages ranged from 30 to 87 years. The tumor specimens’ mean size ± SD was 4.2 ± 1.86 cm. Tumor sizes ranged from 1.5 to 12 cm. Twenty-six (52%) tumor samples were cut from the middle and 23 (46%) from lower regions of the esophagus. The entire esophagus was involved in one sample. Thirty-three (66%) samples were moderately differentiated, while 8 (16%) and 9 (18%) were poorly and well differentiated, respectively. One tumor sample (2%) was in stage I of tumor progression, while 29 (58%) and 20 (40%) were in stages II and III, respectively. In addition, 42 (84%) of tumor tissues showed advanced depth of tumor invasion categorizing as T3/T4, and 23 (46%) tumors had metastasized to lymph nodes.
Up-regulation of Cripto-1 in ESCC patients
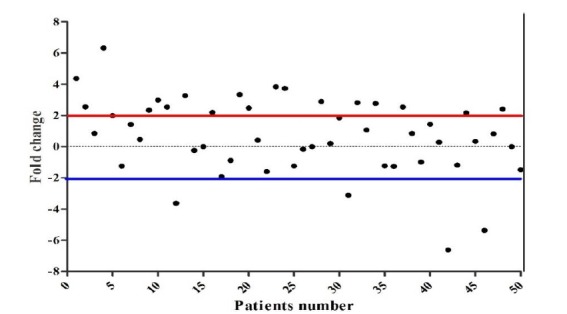
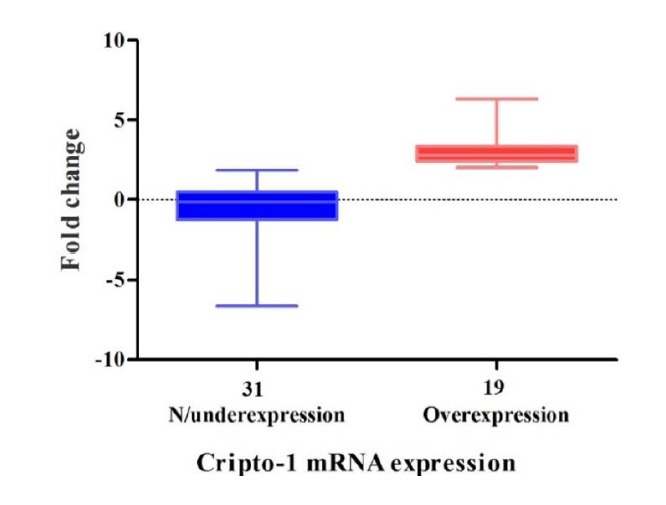
We evaluated CR-1 mRNA expression in 50 tumors and their paired normal specimens by real-time RT-PCR. The expression pattern of all patients is illustrated as a scatter plot in Fig. 1. Nineteen (38%) of the ESCC tumors overexpressed CR-1. The fold-changes ranged from -6.62 to 6.33 (mean ± SD = 0.58 ± 2.48). The expression levels in underexpressed/unchanged vs. overexpressed samples are presented in Fig. 2 as a box plot. Nineteen (38%) and six (12%) samples showed normal and underexpression of CR-1 mRNA, respectively.
Fig. 2.
Box plot representation of relative Cripto-1 mRNA expression in ESCC patients. The Y-axis indicates the fold change in expression, and the X-axis represents the patient groups. This plot represents the lowest, median, and highest observations of fold changes in patients either with normal, underexpressed, or overexpressed Cripto-1.
Association of patients’ clinicopathological variables with Cripto-1 mRNA expression
To evaluate the effect of CR1 gene expression on ESCC progression and development, we investigated the associations between CR-1 expression and patients’ clinicopathological features. The results are listed in Table 2. In the tumor samples that overexpressed CR1, expression was significantly correlated with tumor cell progression stage, differentiation grade, and tumor mass location (p < 0.05). Of the 19 patients with CR-1 overexpression, the tumors of the middle esophagus showed higher levels of CR-1 expression than tumors of the lower esophagus (mean fold change ± SD = 0.683 ± 0.407 vs. 0.495 ± 0.617, p = 0.05). Furthermore, higher CR-1 expression correlated with higher grade of tumor differentiation (p = 0.05). Moreover, the stage III CR-1 overexpressers had higher CR-1 expression than the stage II overexpressers (mean fold change ± SD = 0.756 ± 0.595 vs. 0.55 ± 0.432, p = 0.016). Nine of the 19 (47.4%) tumor sample CR-1 overexpressers had metastases to lymph nodes. The tumors with lymph node metastasis had higher levels of CR-1 expression than those without (mean fold change ± SD = 0.681 ± 0.477 vs. 0.499 ± 0.514).
Table 2.
Correlations between Cripto-1 expression and clinicopathological characteristics of the ESCC patients
| Criteria | Total | Cripto-1 overexpression | p value |
|---|---|---|---|
| Patients | 50 | 19 | |
| Age (mean± SD) | 61.94 ± 11.76 years | ||
| Size (mean ± SD) | 4.2 ± 1.86 cm | ||
| Sex | |||
| Male | 24 (48%) | 0 (52.6%) | |
| Female | 26 (52%) | 9 (47.4%) | |
| Location | |||
| Lower | 23 (48%) | 11 (57.9%) | 0.05 |
| Middle | 26 (52%) | 8 (42.1%) | |
| Middle and lower | 1 (2%) | - | |
| Grade | |||
| P.D. | 8 (16%) | 2 (10.5%) | 0.05 |
| M.D. | 33 (66%) | 12 (63.2%) | |
| W.D. | 9 (18%) | 5 (26.3%) | |
| Lymph node | |||
| Yes | 23 (46%) | 9 (47.4%) | |
| No | 27 (54%) | 10 (52.6%) | |
| Stage | |||
| I | 1 (2%) | - | 0.016 |
| II | 29 (58%) | 11 (57.9%) | |
| III | 20 (40%) | 8 (42.1%) | |
| Tumor invasion (T) | |||
| T1/T2 | 8 (16%) | 5 (26.4%) | |
| T3/T4 | 42 (84%) | 14 (73.7%) | |
WD: Well differentiated; MD: Moderately differentiated; PD: Poorly differentiated
N0: No regional lymph node metastasis; N1: Metastasis in 1 to 2 regional lymph nodes;
N2: Metastasis in 3 to 6 regional lymph nodes; N3: Metastasis in 7 or more regional lymph nodes
Fig. 1.
Scatter plot of Cripto-1 expression in ESCC patients. The Y-axis indicates the fold change of gene expression, and the X-axis represents the patients. A two-fold increase in gene expression in tumor tissue was considered overexpression, whereas a two-fold decrease was considered as underexpression. Expression levels between the two were defined as normal.
Discussion
Cancer cells and embryonic stem cells regulate cell signaling pathways in similar ways (32). A small subpopulation of either adult or progenitor stem cells can contribute cancer stem cells (CSCs), which support the growth and maintenance of tumor cells in most types of cancers, including colon, breast, prostate, and ESCC (24). Therefore, investigation of stem cell regulatory genes in cancers may help to identify potential biological markers for early diagnosis.
Cancer cells proliferate from CSCs, and recent findings have demonstrated that CR-1 is overexpressed in human embryonal carcinoma cells, which have cancer stem cell-like features (33). CR-1 has a key regulatory role in early embryogenesis, oncogenic proliferation, and transformation via its activity in multiple signal transduction pathways, which include TGF-β, Notch, and Wnt signaling cascades. CR-1 overexpression can induce cell proliferation, epithelial-to-mesenchymal transition (EMT), and tumor angiogenesis (34, 35). CR-1 induces invasion, tumor cell proliferation, cellular migration, and cell motility in many different cancers (10).
Previous studies reported that CR-1 is significantly up-regulated in various cancers (36). Furthermore, a correlation between CR1 expression and cancer cell proliferation, both in vitro and in vivo, has been demonstrated. CR-1 mRNA overexpression has been demonstrated in the early stages of colon and breast cancers (5, 37, 38), and CR-1 protein overexpression has been shown in breast and cervical carcinomas, gastric and pancreatic adenocarcinomas, and oral squamous cell carcinoma (10). Previous studies of several cancers have shown that CR-1 overexpression may be an early event in tumorigenesis (10). Accordingly, the high CR-1 expression in most malignant tumors, but its absence or low expression in normal tissues, as well as its role in various signaling pathways, indicate CR-1 may be a potential marker for cancer prognosis and target for treatment (11).
In this study CR-1 mRNA expression was elucidated in human ESCC using real-time RT-PCR. CR-1 overexpression was detected in 38% of our ESCC samples. CR-1 expression also correlated with ESCC linicopathological characteristics including tumor grade, stage, and location. These results demonstrate the contribution of CR-1 in ESCC tumorigenesis. CR-1 was overexpressed in moderately- and well-differentiated ESCC samples. Previous studies have demonstrated the correlation between CR-1 overexpression with high grade, moderately- and well-differentiated breast and cervical carcinomas, gastric adenocarcinomas, oral squamous cell carcinoma, and bladder cancer (3, 5, 10, 39), although no correlation was reported between CR-1 expression and the tumor grade in pancreatic neoplasms (40). Furthermore, CR-1 expression was significantly associated with the tumor stage, with overexpression found in the advanced stage (III). In gastric, cervical, and nasopharyngeal carcinomas, CR-1 overexpression was significantly associated with advanced clinical stages (40-42). The significant correlation of CR-1 mRNA expression with high grade and stage indicates a critical role for CR-1 in ESCC proliferation and metastasis.
Other clinicopathological characteristics, including lymph node metastasis, tumor size, and depth of tumor invasion have been demonstrated to associate with CR-1 expression in gastric, cervical, and nasopharyngeal carcinomas (40-42); however, no significant correlation was found between these clinical parameters and CR-1 expression in ESCC. It has been illustrated that CR-1 affects tumor cell formation, invasion, migration, and metastasis, as well as tumor angiogenesis. Our findings indicate CR-1 involvement in ESCC invasion and metastasis. Although the precise role of CR-1 in ESCC progression is not yet clear, it can activate various signaling pathways leading to ESCC development.
The molecular oncogenic mechanisms of CR-1 remain to be elucidated; however, various functions for CR-1 protein can be described. First, it is a vital factor during normal early embryonic development and acts as a co-receptor for its ligand; Nodal. Second, CR-1 controls two important stem cell properties, including the self-renewal and generation of all differentiated cell types in a specific tissue through interaction with other embryonic stem cell genes. And finally, CR-1, as a multifunctional signaling protein, is re-expressed in various human cancers and contributes to malignancy formation and progression. In conclusion, in this study we showed that CR-1 is overexpressed in ESCC and introduced its oncogenic role ESCC progression and development. CR1 overexpression correlated significantly with tumor stage, grade, and location. Effective therapies are desperately needed for ESCC, and our results suggest that CR-1 may be a suitable target for therapeutic intervention in ESCC patients.
Acknowledgments
The authors gratefully acknowledge the colleagues at Division of Human Genetics and Immunology Department in Mashhad University of Medical Sciences for their technical assistance. The author(s) declare that this article content has no conflict of interest. This study was supported by a grant from Vice chancellor of Mashhad University of Medical Sciences (#950677).
References
- 1.Moghbeli M, Abbaszadegan MR, Farshchian M, Montazer M, Raeisossadati R, Abdollahi A, et al. Association of PYGO2 and EGFR in esophageal squamous cell carcinoma. Medical Oncology. 2013;30(2):1–9. doi: 10.1007/s12032-013-0516-9. [DOI] [PubMed] [Google Scholar]
- 2.Moghbeli M, Moghbeli F, Forghanifard MM, Garayali A, Abbaszadegan MR. Cancer stem cell markers in esophageal cancer. American Journal of Cancer Science. 2013;2(2):37–50. [Google Scholar]
- 3.Wei B, Jin W, Ruan J, Xu Z, Zhou Y, Liang J, et al. Cripto-1 expression and its prognostic value in human bladder cancer patients. Tumor Biology. 2015;36(2):1105–13. doi: 10.1007/s13277-014-2695-1. [DOI] [PubMed] [Google Scholar]
- 4.Bianco C, Rangel MC, Castro NP, Nagaoka T, Rollman K, Gonzales M, et al. Role of Cripto-1 in stem cell maintenance and malignant progression. The American journal of pathology. 2010;177(2):532–40. doi: 10.2353/ajpath.2010.100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J-G, Zhao J, Xin Y. Significance and relationship between Cripto-1 and p-STAT3 expression in gastric cancer and precancerous lesions. World journal of gastroenterology: WJG. 2010;16(5):571. doi: 10.3748/wjg.v16.i5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francescangeli F, Contavalli P, De Angelis M, Baiocchi M, Gambara G, Pagliuca A, et al. Dynamic regulation of the cancer stem cell compartment by Cripto-1 in colorectal cancer. Cell Death & Differentiation. 2015 doi: 10.1038/cdd.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianco C, Strizzi L, Ebert A, Chang C, Rehman A, Normanno N, et al. Interaction between osteoblast and osteoclast: impact in bone disease. Journal of the National Cancer Institute. 2005;97(2):132–41. doi: 10.1093/jnci/dji011. [DOI] [PubMed] [Google Scholar]
- 8.Bianco C, Adkins HB, Wechselberger C, Seno M, Normanno N, De Luca A, et al. Cripto-1 activates nodal-and ALK4-dependent and-independent signaling pathways in mammary epithelial cells. Molecular and cellular biology. 2002;22(8):2586–97. doi: 10.1128/MCB.22.8.2586-2597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schier AF. Nodal signaling in vertebrate development. Annual review of cell and developmental biology. 2003;19(1):589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- 10.Yoon H-J, Hong J-S, Shin W-J, Lee Y-J, Hong K-O, Lee J-I, et al. The role of Cripto-1 in the tumorigenesis and progression of oral squamous cell carcinoma. Oral oncology. 2011;47(11):1023–31. doi: 10.1016/j.oraloncology.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Bianco C, Strizzi L, Normanno N, Khan N, Salomon DS. 3-Cripto-1: An Oncofetal Gene with Many Faces. Current topics in developmental biology. 2005;67:85–133. doi: 10.1016/S0070-2153(05)67003-2. [DOI] [PubMed] [Google Scholar]
- 12.Strizzi L, Bianco C, Normanno N, Salomon D. Cripto-1: a multifunctional modulator during embryogenesis and oncogenesis. Oncogene. 2005;24(37):5731–41. doi: 10.1038/sj.onc.1208918. [DOI] [PubMed] [Google Scholar]
- 13.Bianco C, Mysliwiec M, Watanabe K, Mancino M, Nagaoka T, Gonzales M, et al. Activation of a Nodal-independent signaling pathway by Cripto-1 mutants with impaired activation of a Nodal-dependent signaling pathway. FEBS letters. 2008;582(29):3997–4002. doi: 10.1016/j.febslet.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianco C, Rehman A, Normanno N, Wechselberger C, Sun Y, et al. Nodal-and ALK4-independent signaling pathway activated by Cripto-1 through Glypican-1 and c-Src. Cancer research. 2003;63(6):1192–7. [PubMed] [Google Scholar]
- 15.Rangel MC, Karasawa H, Castro NP, Nagaoka T, Salomon DS, Bianco C. Role of Cripto-1 during epithelial-to-mesenchymal transition in development and cancer. The American journal of pathology. 2012;180(6):2188–200. doi: 10.1016/j.ajpath.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forghanifard MM, Taleb S, Abbaszadegan MR. Notch Signaling Target Genes are Directly Correlated to Esophageal Squamous Cell Carcinoma Tumorigenesis. Pathology & Oncology Research. 2015;21(2):463–7. doi: 10.1007/s12253-014-9849-8. [DOI] [PubMed] [Google Scholar]
- 17.Klauzinska M, Castro NP, Rangel MC, Spike BT, Gray PC, Bertolette D, et al., editors. The multifaceted role of the embryonic gene Cripto-1 in cancer, stem cells and epithelial-mesenchymal transition. Elsevier; 2014. Seminars in cancer biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moghbeli M, Forghanifard MM, Sadrizadeh A, Mozaffari HM, Golmakani E, Abbaszadegan MR. Role of Msi1 and MAML1 in Regulation of Notch Signaling Pathway in Patients with Esophageal Squamous Cell Carcinoma. Journal of gastrointestinal cancer. 2015;46(4):365–9. doi: 10.1007/s12029-015-9753-9. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe K, Nagaoka T, Lee JM, Bianco C, Gonzales M, Castro NP, et al. Enhancement of Notch receptor maturation and signaling sensitivity by Cripto-1. The Journal of cell biology. 2009;187(3):343–53. doi: 10.1083/jcb.200905105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raya A, Kawakami Y, Rodríguez-Esteban C, Büscher D, Koth CM, Itoh T, et al. Notch activity induces Nodal expression and mediates the establishment of left–right asymmetry in vertebrate embryos. Genes & development. 2003;17(10):1213–8. doi: 10.1101/gad.1084403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taleb S, Abbaszadegan MR, Moghbeli M, Roudbari NH, Forghanifard MM. HES1 as an Independent Prognostic Marker in Esophageal Squamous Cell Carcinoma. Journal of gastrointestinal cancer. 2014;45(4):466–71. doi: 10.1007/s12029-014-9648-1. [DOI] [PubMed] [Google Scholar]
- 22.Strizzi L, Postovit L-M, Margaryan NV, Seftor EA, Abbott DE, Seftor RE, et al. Emerging roles of nodal and Cripto-1: from embryogenesis to breast cancer progression. Breast disease. 2008;29:91. doi: 10.3233/bd-2008-29110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vallier L, Mendjan S, Brown S, Chng Z, Teo A, Smithers LE, et al. Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. . Development. 2009;136(8):1339–49. doi: 10.1242/dev.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forghanifard MM, Khales SA, Javdani-Mallak A, Rad A, Farshchian M, Abbaszadegan MR. Stemness state regulators SALL4 and SOX2 are involved in progression and invasiveness of esophageal squamous cell carcinoma. Medical Oncology. 2014;31(4):1–8. doi: 10.1007/s12032-014-0922-7. [DOI] [PubMed] [Google Scholar]
- 25.Kahkhaie KR, Moaven O, Abbaszadegan MR, Montazer M, Gholamin M. Specific MUC1 splice variants are correlated with tumor progression in esophageal cancer. World journal of surgery. 2014;38(8):2052–7. doi: 10.1007/s00268-014-2523-1. [DOI] [PubMed] [Google Scholar]
- 26.Barooei R, Mahmoudian RA, Abbaszadegan MR, Mansouri A, Gholamin M. Evaluation of thymic stromal lymphopoietin (TSLP) and its correlation with lymphatic metastasis in human gastric cancer. Medical Oncology. 2015;32(8):1–8. doi: 10.1007/s12032-015-0653-4. [DOI] [PubMed] [Google Scholar]
- 27.Mansouri A, Foroughmand AM, Abbaszadegan MR, Memar B, Mahmoudian RA, Gholamin M. Iranian journal of basic medical sciences. Journal of Bone and Mineral Research. 2015;18(4):380. [PMC free article] [PubMed] [Google Scholar]
- 28.Rubie C, Kempf K, Hans J, Su T, Tilton B, Georg T, et al. Housekeeping gene variability in normal and cancerous colorectal, pancreatic, esophageal, gastric and hepatic tissues. Molecular and cellular probes. 2005;19(2):101–9. doi: 10.1016/j.mcp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 29.alsadat Mahmoudian R, Abbaszadegan MR, Mansouri A, Gholamin M. Amplification of Tumor Transcripts from Limited Quantity of Esophageal Squamous Cell Carcinoma Tissue Samples. American Journal of Cancer Science. 2015;4(1):54–62. [Google Scholar]
- 30.Gholamin M, Moaven O, Memar B, Farshchian M, Naseh H, Malekzadeh R, et al. Overexpression and interactions of interleukin-10, transforming growth factor β, and vascular endothelial growth factor in esophageal squamous cell carcinoma. World journal of surgery. 2009;33(7):1439–45. doi: 10.1007/s00268-009-0070-y. [DOI] [PubMed] [Google Scholar]
- 31.Forghanifard MM, Gholamin M, Farshchian M, Moaven O, Memar B, Abbaszadegan MR. Cancer-testis gene expression profiling in esophageal squamous cell carcinoma. Cancer biology & therapy. 12(3):191–7. doi: 10.4161/cbt.12.3.15949. [DOI] [PubMed] [Google Scholar]
- 32.Dreesen O, Brivanlou AH. Signaling pathways in cancer and embryonic stem cells. Stem cell reviews. 2007;3(1):7–17. doi: 10.1007/s12015-007-0004-8. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe K, Meyer MJ, Strizzi L, Lee JM, Gonzales M, Bianco C, et al. Cripto-1 is a cell surface marker for a tumorigenic, undifferentiated subpopulation in human embryonal carcinoma cells. Stem Cells. 2010;28(8):1303–14. doi: 10.1002/stem.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wechselberger C, Ebert AD, Bianco C, Khan NI, Sun Y, et al. Cripto-1 enhances migration and Wallace-Jones B branching morphogenesis of mouse mammary epithelial cells. Experimental cell research. 2001;266(1):95–105. doi: 10.1006/excr.2001.5195. [DOI] [PubMed] [Google Scholar]
- 35.Strizzi L, Bianco C, Normanno N, Seno M, Wechselberger C, Wallace-Jones B, et al. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary gland from MMTV‐Cripto-1 transgenic mice. Journal of cellular physiology. 2004;201(2):266–76. doi: 10.1002/jcp.20062. [DOI] [PubMed] [Google Scholar]
- 36.Pilgaard L, Mortensen JH, Henriksen M, Olesen P, Sørensen P, Laursen R, et al. Cripto-1 Expression in Glioblastoma Multiforme. Brain Pathology. 2014;24(4):360–70. doi: 10.1111/bpa.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saeki T, Stromberg K, Qi C-F, Gullick WJ, Tahara E, Normanno N, et al. Differential immunohistochemical detection of amphiregulin and cripto in human normal colon and colorectal tumors. Cancer research. 1992;52(12):3467–73. [PubMed] [Google Scholar]
- 38.Panico L, D'Antonio A, Salvatore G, Mezza E, Tortora G, De Laurentiis M, et al. Differential immunohistochemical detection of transforming growth factor α, amphiregulin and CRIPTO in human normal and malignant breast tissues. International journal of cancer. 1996;65(1):51–6. doi: 10.1002/(SICI)1097-0215(19960103)65:1<51::AID-IJC9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 39.Gong Y, Yarrow P, Carmalt H, Kwun S, Kennedy C, Lin B, et al. Overexpression of Cripto and its prognostic significance in breast cancer: a study with long-term survival. European Journal of Surgical Oncology (EJSO) 2000;26(4):455–9. doi: 10.1016/j.ejso.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 40.Wu Z, Li G, Wu L, Weng D, Li X, Yao K. Cripto-1 overexpression is involved in the tumorigenesis of nasopharyngeal carcinoma. BMC cancer. 2009;9(1):315. doi: 10.1186/1471-2407-9-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ertoy D, Ayhan A, Sarac E, Karaaǧaoǧlu E, Yasui W, Tahara E, et al. Clinicopathological implication of cripto expression in early stage invasive cervical carcinomas. European Journal of Cancer. 2000;36(8):1002–7. doi: 10.1016/s0959-8049(00)00033-2. [DOI] [PubMed] [Google Scholar]
- 42.Zhong XY, Zhang LH, Jia SQ, Shi T, Niu ZJ, Du H, et al. Positive association of up-regulated Cripto-1 and down-regulated E-cadherin with tumour progression and poor prognosis in gastric cancer. Histopathology. 2008;52(5):560–8. doi: 10.1111/j.1365-2559.2008.02971.x. [DOI] [PubMed] [Google Scholar]


