Abstract
Cognitive theories of anxiety psychopathology cite biased attention towards threat as a central vulnerability and maintaining factor. However, many studies have found threat bias indices to have poor reliability and have failed to observe the theorized relationship between threat bias and anxiety symptoms; this may be due to the non-unitary nature of threat bias and the influence of state-level variables on its expression. Accumulating data suggests that state anxious mood is important for the robust expression of threat bias and for relations to emerge between threat bias and symptoms, though this possibility has not been experimentally tested. Eye-tracking was used to assess multiple forms of threat bias (i.e., early vigilance, sustained attention, facilitated engagement, delayed disengagement) thought to be related to anxiety. A non-clinical sample (N = 165) was recruited to test the hypothesis that biased attention towards threat, but not dysphoric or positive emotional stimuli, during an anxious mood induction, but not at a pre-stress baseline, would prospectively predict greater worry symptoms on days in which more naturalistic stressors occurred. Results revealed the hypothesized moderation effect for sustained attention towards threat after the mood induction but not at baseline, though sustained attention towards dysphoric stimuli also moderated the effect of stressors on worry. Worry-relevant sustained attention towards negative emotional stimuli may be a partially mood-context dependent phenomenon.
Keywords: worry, attention bias, mood induction, longitudinal, eye-tracking
Introduction
Anxiety disorders comprise one of the most prevalent classes of psychiatric diagnoses (Kessler, Petukhova, Sampson, Zaslavsky, & Wittchen, 2012; Kessler et al, 2005). Despite improvements in treatment efficacy and utilization, the staggering personal and economic burden of these disorders has persisted (Greenberg et al., 2003; Kessler & Greenberg, 2002; Whiteford et al., 2013). This underscores the urgent need to better understand mechanisms contributing to anxiety pathology that may inform more targeted treatments. Cognitive theories of anxiety disorders posit that information processing abnormalities are central to their etiology and maintenance (e.g., Dalgleish & Watts, 1990; Eysenck, Derakshan, Santos, & Calvo, 2007). One well-researched aberration of information processing that has been tied to anxiety psychopathology is attention bias towards threat (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoom, 2007; Van Bockstaele et al., 2014).
Attention Biases towards Threat in Anxiety
Attention bias towards threat can be defined as selective attentional allocation to threatening relative to neutral stimuli (Cisler & Koster, 2010). Although meta-analytic evidence suggests that manual reaction time (RT) and eye-tracking assessed threat bias is significantly associated with clinical and non-clinical anxiety (Armstrong & Olatunji, 2012; Bar-Haim et al., 2007), attention bias towards threat is not a unitary phenomenon (Cisler, Bacon, & Williams, 2009; Cisler & Koster, 2010). Sustained attention towards/delayed disengagement from threat as well as facilitated engagement with threat have all been linked with anxiety symptomatology (Armstrong & Olatunji, 2012; Cisler et al., 2009; Cisler & Koster, 2010). Sustained attention towards threat and delayed disengagement of attention from threat refers to the degree to which threatening stimuli capture and hold attention, whereas facilitated engagement refers to the speed at which attention orients towards threat (Cisler & Koster, 2010). According to Cisler and Koster’s (2010) review, delayed disengagement from threat has been consistently linked with anxiety across reaction time (RT) and eye-tracking assessment methods, whereas facilitated engagement with threat has been associated with anxiety in some studies but not others. Further, among studies that employed bias assessments capable of distinguishing facilitated engagement with threat from delayed disengagement from threat, anxiety was generally found to be specifically related to delayed disengagement. In contrast, the relationship between anxiety and facilitated engagement with threat is less consistently found and possibly moderated by assessment method (RT vs. eye-tracking), attention bias task (visual search/free-viewing vs. dot-probe/spatial cueing), stimulus threat intensity (high vs. low), and/or stimulus presentation duration (i.e., short/subliminal vs. long/supraliminal) (Cisler & Koster, 2010).
In addition to attention bias towards threat, numerous studies have also documented a relationship between anxiety and attention bias away from threat (see Cisler & Koster, 2010 for a review). Given accumulating data demonstrating a relationship between anxiety and attentional bias towards as well as away from threat, some authors have suggested that these seemingly contradictory findings are indicative of a vigilance-avoidance pattern of attention in anxious individuals (e.g., Mogg, Bradley, Miles, & Dixon, 2004; Wieser, Pauli, Weyers, Alpers, Muhlberger, 2009). Overall, extant data suggest that threat bias is not a unitary phenomenon and may vary over time, underscoring the need to use methods (e.g., eye-tracking) and tasks capable of parsing multiple forms of threat bias across time (e.g., Sanchez, Vazquez, Marker, LeMoult, & Joormann, 2013). However, despite progress in our understanding of the various forms of anxiety-related threat bias, a recent review of the literature highlighted the significant heterogeneity in effect sizes across studies measuring the relationship between manual RT-assessed threat bias and anxiety psychopathology (Van Bockstaele et al., 2014), suggesting that moderator variables may affect the extent to which threat bias indexes anxiety vulnerability. Indeed, a number of studies, including those using eye-tracking indices of threat bias, have failed to find a relationship between threat bias and anxiety (e.g., Mohlman, Price, & Vietri, 2013; Price et al., 2013; Waters, Lipp, & Spence, 2004). Relatedly, accumulating evidence suggests that most manual RT and eye-tracking attention bias assessments demonstrate poor internal and test-retest reliability, limiting their convergent validity (Price et al., 2015; Rodebaugh et al., 2016; Waechter, Nelson, Wright, Hyatt, & Oakman, 2014). Taken together, these data suggest that the role of threat bias in anxiety psychopathology may depend upon the specific form of threat bias (e.g., facilitated engagement vs. delayed disengagement) as well as the manner in which bias is assessed.
In a review of the extant evidence for a causal role of threat bias in anxiety psychopathology, Van Bockstaele and colleagues (2014) propose that the relationship between anxious mood and threat bias is a bidirectional, mutually-maintaining one, suggesting that threat biases relevant to anxiety psychopathology are sensitive to fluctuations in anxious mood state. Indeed, measures of threat bias derived from manual RT and eye-tracking paradigms have demonstrated poor test-retest reliability (e.g., Price et al., 2015), which may partially be attributable to the influence of state-level factors (e.g., acute stress/anxious mood) on the expression of threat bias during a particular assessment session. Thus, as with other forms of learned behavior (Bower, 1981), threat bias may be a partially mood context-dependent as opposed to a trait-like phenomenon. It is conceivable that selectively attending to threatening relative to neutral stimuli under acute stress specifically confers vulnerability to anxiety symptomatology, possibly via maintenance of anxious responding to stressors as suggested by Van Bockstaele and colleagues (2014). In summary, the degree to which biased attention towards threat emerges under conditions of acute stress may be a superior predictor of anxiety symptoms, particularly in response to stressors, relative to threat bias under baseline conditions, which may or may not reflect attentional behavior in an anxious mood state.
Attention Biases towards Threat under Baseline vs. Acute Stress conditions
A number of studies have investigated the effect of mood state on threat bias (Ellenbogen, Schwartzman, Stewart, & Walker, 2002; Ford et al., 2010; Isaacowitz, Toner, Goren, & Wilson, 2008; Nelson, Purdon, Quigley, Carriere, & Smilek, 2015; Quigley, Nelson, Carriere, Smilek, & Purdon, 2012; Sanchez, Vazquez, Gomez, & Joormann, 2014). For instance, Quigley and colleagues (2012) utilized eye-tracking methodology and found that an overall attentional bias towards threat, but not positive stimuli, was observed after an anxious mood induction, but not under baseline conditions. Further, elevated state but not trait anxiety was associated with increased attention to threatening stimuli, consistent with the notion that acute anxious mood affects threat bias. Nelson and colleagues (2015) replicated these findings and demonstrated that the relationship between state anxiety and threat bias is specific to sustained attention towards threat, but unrelated to initial engagement with threat. Isaacowitz and colleagues (2008) subjected participants to neutral, positive, and negative mood inductions prior to an eye-tracking assessment of attention bias and, at least in young adults, generally found mood-congruent effects such that a significant negative attention bias emerged following a negative mood induction and an attention bias towards positive information was found after neutral and positive mood inductions. Likewise, Ford and colleagues (2010) utilized eye-tracking methodology and found that participants displayed greater attention bias towards threat following an anxious mood induction and greater attention bias towards rewarding stimuli following excitement and anger mood inductions.
Although multiple studies have demonstrated mood-congruent effects on attention bias, two studies known to the authors have found mood-incongruent effects (Ellenbogen et al., 2002; Sanchez et al., 2014). Ellenbogen and colleagues (2002) employed a spatial cueing task to examine the effects of a stress induction on attentional biases towards negatively-valenced stimuli. The authors found that participants who underwent the stress induction, but not participants in the control conditions, shifted their attention away more rapidly from negative relative to positive and neutral stimuli. Further, they found that greater increases in negative mood was related to faster shifting of attention away from negative relative to positive and neutral stimuli, suggesting that attentional avoidance of negative stimuli during acute stress may reflect an emotion regulation strategy. In line with this suggestion, Sanchez and colleagues (2014) found that participants who experienced greater reductions in positive mood after a negative mood induction demonstrated greater subsequent attention bias towards positive emotional stimuli (assessed via eye-tracking). Further, greater attention bias towards positive emotional stimuli after the negative mood induction predicted greater improvement in positive mood at the end of the experiment, consistent with the notion that attention bias during acute mood states is partially reflective of emotion regulatory behavior.
To summarize, the experimental literature on acute mood state and attention bias suggests that induced mood can causally affect the expression of attention bias towards emotional stimuli. Although these data are suggestive of the moderating role of acute stress on the expression of threat bias, no studies known to the authors have explicitly tested the differential validity of stress-elicited relative to baseline threat biases with respect to anxiety vulnerability. However, there is some meta-analytic evidence to support the notion that stress-elicited threat bias may be a more valid predictor of anxiety symptoms. Hallion and Ruscio (2011) reviewed the affective disorder-relevant attention bias modification literature and found bias modification to be more robustly linked with symptom reduction when symptoms were assessed following a laboratory stressor (Hallion & Ruscio, 2011), suggesting that threat bias may primarily contribute to anxiety symptoms via alterations in acute stress reactivity/recovery, a possibility consistent with Van Bockstaele and colleagues’ (2014) conclusion that anxious mood and threat bias are bidirectionally involved in the maintenance of anxiety psychopathology. Nevertheless, experimental and prospective research is required before firmer conclusions can be drawn. Studies are needed in which threat bias is assessed before and after an anxious mood induction and its differential, prospective relationship with anxiety symptoms is examined, particularly in the context of naturalistic stressors. Assessing threat bias before and after anxious mood elicitation and examining its differential prospective relationship with daily anxiety symptoms, particularly on days in which stressful events occurred, would allow for a stringent test of the hypothesized mood-context sensitivity of the relationship between threat bias and anxiety symptoms.
Attention Bias towards Threat and Maladaptive Worry
Extant data suggest that excessive, uncontrollable worry is found in multiple anxiety disorders (McEvoy et al., 2013) and may have a specific relationship among anxiety symptomatology with dysregulated emotional responding to stressors (Mennin et al., 2007; Mennin et al., 2009; Turk et al., 2005). Further, greater attention bias towards threat has been consistently found in individuals experiencing clinically significant worry but less so in non-clinical high worry/trait-anxiety samples (Beckwé & Deroost, 2015; Van Bockstaele et al., 2014), which may partially be due to the effect of pervasive anxious mood, which is necessary for a GAD diagnosis (DSM-5; American Psychiatric Association, 2013), on threat bias. Thus, the differential predictive validity of threat bias assessed under baseline and acute stress conditions may be especially relevant to worry symptoms, particularly stressor-elicited worry. However, experimental and prospective research is required to explicitly test the differential validity of attention bias towards threat assessed under baseline and acutely stressful conditions in the prediction of worry symptoms over time, particularly in the context of naturalistic stressors.
The Current Study
The current study is to our knowledge the first to empirically test the influence of acute anxious mood on the prospective relationship between attention bias towards threat and naturalistic stressor-linked worry. The present study utilized eye-tracking methodology and a laboratory anxious mood induction to evaluate the effect of acute anxious mood on attention bias towards threat. Self-reported anxious mood and heart rate were measured throughout the mood induction procedure to ensure that acute stress was successfully elicited. Compared to manual RT methods of measuring threat bias, eye-tracking methods produce more psychometrically reliable indices (Price et al., 2015) and allow for the measurement of multiple forms of attention bias towards threat found to be related to anxiety psychopathology (Armstrong & Olatunji, 2012), including early vigilance (i.e., initial orienting to threat), sustained attention (i.e., prolonged attending to threat), facilitated engagement (i.e., faster engagement with threat), and delayed disengagement (i.e., slower disengagement from threat) (e.g., Sanchez et al., 2013). To test the predictive utility and specificity of multiple forms of attention bias towards threat on naturalistic stressor-elicited worry, this investigation used a prospective design in which participants rated their daily stressors, negative affect, and worry for two weeks following a laboratory assessment of baseline and post-anxiety induction attention bias towards threat, dysphoric, and positive emotional stimuli.
In concert with existing meta-analytic findings on attention bias and anxiety psychopathology (Hallion & Ruscio, 2011; Van Bockstaele et al., 2014) as well as experimental work on the effect of mood inductions on corresponding attention biases assessed with eye-tracking methods (Ford et al., 2010; Isaacowitz et al., 2008; Nelson et al., 2015; Quigley et al., 2012), we hypothesized that an increase in multiple forms of attention bias towards threat (i.e., early vigilance, sustained attention, facilitated engagement, delayed disengagement) from pre- to post-anxiety induction would be observed. Further, we hypothesized that attention bias towards threat measured in the context of the anxious mood induction, but not at baseline, would predict daily worry symptoms, but only in the context of high levels of daily stress. Moreover, given the laboratory mood induction’s specificity to anxiety, we hypothesized that the predicted moderation effect would only be observed for attention bias towards threat, but not for dysphoric or positive stimuli.
Method
Participants
To test the present study’s hypotheses, a non-clinical sample recruited as part of a larger study on transdiagnostic risk factors was utilized. A non-clinical sample was chosen given our concerns that using a clinically anxious sample might reduce the effect of the anxious mood induction on threat bias given the increased chronicity of anxious mood in clinical samples. The sample was recruited from the undergraduate psychology student population at a large southeastern university (N = 165; 77% female; M age = 19.32, SD = 1.96) over three semesters. The study was open to all students who expressed interest in participating, though individuals in the top quartile of scores on a measure of distress intolerance were oversampled as part of the larger study aims (Distress Intolerance Index; McHugh & Otto, 2012). Given that distress intolerance has been robustly linked to worry in multiple studies (Allan, Macatee, Norr, & Schmidt, 2014; Macatee, Capron, Guthrie, Schmidt, & Cougle, 2015; Macatee et al., 2016), we believed that the sample would provide a suitably wide range of negative attention bias and worry scores for testing our hypotheses. Participants earned course credit for completing the study. The sample was predominantly Caucasian (70.9%), although other ethnicities were also represented (Hispanic: 13.3%, African-American: 11.5%, Asian: 1.2%, American Indian or Alaskan Native: 0.6%, Other: 2.4%). Data on income/SES were not collected.
Measures
Questionnaires
State mood
Three visual analog scales (VASs) for happy, anxious, and sad mood states were used to assess state mood at three different times during the experiment. Each scale consisted of an emotion word and a line with 11 anchor points ranging from 0 (not at all) to 10 (very much); participants were asked to indicate the extent to which they were experiencing that particular emotion at that moment. Each mood state was assessed using three emotion words (happy mood: optimistic, joyful, happy; anxious mood: nervous, tense, anxious; sad mood: upset, sad, depressed). Mean internal consistencies were excellent for each mood state (happy: α = .95; anxious: α = .93; sad: α = .92; see Table 1 for descriptives).
Table 1.
Change in Affect across Experiment
| Time 1: Pre-Stress | Time 2: Anticipatory Stress | Time 3: Post-stress | |
|---|---|---|---|
|
| |||
| M (SD) | M (SD) | M (SD) | |
| Self-Report | |||
| Happy | 16.27 (7.11) | 16.69 (7.51) | 16.43 (7.74) |
| Depressed | 4.71 (5.69) | 4.23 (5.42) | 3.11 (4.36)*** |
| Anxious | 7.47 (7.19) | 10.43 (8.49)*** | 6.03 (6.53)*** |
| Psychophysiology | |||
| Mean HR | 75.10 (9.30) | 79.31 (10.33)*** | 76.50 (9.94)*** |
Note. HR = Heart Rate.
Significant difference from previous assessment at p < .001.
Daily stressful events
Participants completed a self-report version of the Daily Inventory of Stressful Events (DISE; Almeida et al., 2002) on six separate days across a two-week period to assess the occurrence of specific types of stressors that had occurred that day. Almeida and colleagues (2002) used seven stem questions reflecting broad categories of stressor types derived from a nationally representative sample. Interrater agreement on stressor classification was high (κ = .66 – .95) and daily stressors demonstrated small associations with daily distress (rs = .02 – .31), suggesting that stressor occurrence using these categories is not redundant with negative affect. Participants responded to seven yes/no questions based on Almeida and colleague’s (2002) classifications regarding different sorts of stressors that may have occurred throughout the day, including arguments, potential arguments that were let go to avoid disagreement, work/school stress, home stress, discrimination events, friend/relative stress, and stressors not captured by the other categories. The number of ‘yes’ responses for a given day were summed to form the daily stressors variable used in the present study. Greater endorsement of daily stressors has been significantly associated with established, validated measures of stressful events in prior work (Macatee et al., 2015), suggestive of convergent validity. In the current study, endorsed stressors averaged across all seven categories were rated as moderately stressful based on a 1 (‘Not at all Stressful’) to 4 (‘Very Stressful’) scale (M = 2.57, SD = 0.88), providing evidence that the DISE events in the current study were perceived as stressful. Amongst all of the daily diary entries completed in the current study (n = 554), 55.1% indicated that they had experienced at least one stressful event that day and 31.6% indicated that they had experienced multiple stressors that day (M = 1.12, SD = 1.33).
Daily worry
A three-item version of the Penn State Worry Questionnaire (PSWQ; Berle et al., 2011) was employed on six separate days across a two-week period to assess their daily worry on a 1 (‘Not at all typical of me today’) to 5 (‘Very typical of me today’) scale for that particular day (M = 4.90, SD = 2.96). Berle and colleagues (2011) showed that the brief version of the PSWQ had convergent/discriminant validity and internal consistency (α = .85) comparable to the standard PSWQ. In the current sample, the brief PSWQ demonstrated excellent internal consistency (α = .92).
Daily negative affect
The NA subscale of the short form of the PANAS was used to measure daily NA on six separate days across a two week period in the present study (PANAS-NA; Mackinnon et al., 1999). The PANAS has been used to index emotional reactivity in prior investigations (Sloan, 2004) and the short form of the PANAS has been shown to have good internal consistency (α = .87; Mackinnon et al., 1999) and validity (Gyollai, Simor, Koteles, & Demetrovics, 2011). The NA subscale is composed of five negative emotion words and the participant is asked to indicate the extent to which he or she experienced each negative emotion that day (M = 7.85, SD = 3.52). In the current sample, the NA subscale of the short form of the PANAS demonstrated good internal consistency (α = .89).
Attention Task
Stimuli materials
Stimuli were pairs of face images consisting of an emotional and neutral facial expression of the same person. The same image set used by Sanchez and colleagues (2013) was used in the present study. The images were derived from the Karolinska Directed Emotional Faces (KDEF) database (Lundqvist, Flykt, & Ohman, 1998). Images were modified to fit into an oval window, and the hair, neck, and surrounding parts of the pictures were darkened to remove irrelevant aspects of the faces. KDEF images were chosen based upon emotional intensity and prototypicality of the corresponding facial emotion (see Sanchez & Vazquez, 2013 for validation data). The final stimuli set consisted of 36 happy, angry, and sad facial expressions (18 men and 18 women for each emotion), together with a corresponding neutral expression by the same person.
Experimental set-up and attention indices
The attention task consisted of six practice trials and 108 experimental trials in which an emotional and neutral face were presented on opposite sides of the screen (36 happy – neutral, 36 sad – neutral, 36 angry – neutral). Trial order was randomized and emotional and neutral faces were presented equally often on the left and right sides of the screen.
Stimuli were presented on a 41 cm (width) x 30.5 cm (height) screen. Each face was 9 cm (width) x 12.7 cm (height). Facial stimuli were centered on the screen, 18.1 cm apart (measured from their centers). Participants were seated approximately 90 cm from the screen’s center, resulting in a visual angle of approximately 5.7 degrees between each picture’s center and the screen’s center. These dimensions replicate the stimuli size/position ratios used by Sanchez and colleagues (2013).
The attention task design used in the present study was developed by Sanchez and colleagues (2013). Each trial started with a blank black screen for 500 ms, followed by a centered white fixation cross for 500 ms. A random number then appeared on the center of the screen for 1,000 ms. Participants were instructed to fixate on the number and silently name it as quickly as possible; note that participants did not say the number out loud because of concerns that vocalization would affect the positioning of the participant’s head in the mount. This procedure has been used in prior studies to ensure the participant’s gaze is focused on the center of the screen prior to facial stimuli presentation (Calvo & Avero, 2005). After the offset of the random number, an emotion-neutral face pair (happy – neutral, sad – neutral, angry – neutral) was presented for 3,000 ms during which time participants were instructed to look at the screen freely. Free viewing of the facial stimuli was implemented to derive indices of naturalistic attention bias (Isaacowitz, 2005). Fixation data recorded during the 3,000 ms period were used to calculate two indices of naturalistic attention bias (e.g., Kellough, Beevers, Ellis, & Wells, 2008) for each emotion-neutral category: 1) Early vigilance - the probability of initially fixating on an emotional face after onset of the facial pair – was derived by dividing the number of trials in which the participant initially fixated upon the emotional face by the total number of trials for that emotion-neutral category; and 2) Sustained attention – the proportion of fixations the participant made on the emotion face relative to the neutral face across the entire trial – was derived by computing the average proportion of emotion face fixations for that emotion-neutral category.
The engagement-disengagement portion of the attention task began after the 3,000 ms naturalistic processing period and allowed for an assessment of both the ability to disengage from and engage with emotional stimuli. The engagement-disengagement task consisted of three conditions: 1) On one-third of the trials in each emotion-neutral category, attentional engagement with the emotional face was assessed. After 3,000 ms of naturalistic processing, the ‘wait for fixation period’ began during which the task awaited a fixation of at least 100 ms on the neutral face. As soon as the 100 ms fixation was registered, the ‘wait for fixation period’ ended and a frame (square or circle) appeared surrounding the emotional face. Participants were instructed to move their gaze as quickly as possible toward the framed face and to identify the frame shape on the keyboard using one of two response keys. Thus, engagement trials allowed for an assessment of how long it took participants to disengage from the neutral face and fixate on the emotional face. 2) On another one-third of the trials in each emotion-neutral category, attentional disengagement from the emotional face was assessed. After 3,000 ms of naturalistic processing, the ‘wait for fixation period’ began during which the task awaited a fixation of at least 100 ms on the emotional face. As soon as the 100 ms fixation was registered, the ‘wait for fixation period’ ended and a frame (square or circle) appeared surrounding the neutral face. As with the engagement condition trials, participants were instructed to move their gaze as quickly as possible toward the framed face and to identify the frame shape on the keyboard using one of two response keys. Thus, disengagement trials allowed for an assessment of how long it took participants to disengage from the emotional face and fixate on the neutral face. 3) The remaining one-third of trials in each emotion-neutral category consisted of control trials in which a new trial began immediately after the 3,000 ms naturalistic processing period. Disengagement, engagement, and control condition trials for each emotion-neutral category were randomly presented for each participant, with both types of frame shapes equally likely to appear on both sides of the screen for each condition.
In line with Sanchez and colleagues’ (2013) procedure, fixation data collected after the end of the ‘wait for fixation period’ were used to compute engagement and disengagement latencies for each emotion-neutral category. Engagement latency was computed by adding 100 ms (i.e., the neutral face fixation duration required to end the ‘wait for fixation period’) to the amount of time that elapsed from the end of the ‘wait for fixation period’ to the first 100 ms fixation on the framed, emotional face. Disengagement latency was computed by adding 100 ms (i.e., the emotional face fixation duration required to end the ‘wait for fixation period’) to the amount of time that elapsed from the end of the ‘wait for fixation period’ to the first 100 ms fixation on the framed, neutral face.
Eye-tracking device
The desktop mounted EyeLink 1000 (SR Research Ltd, Ottawa, Ontario, Canada) was used to record participants’ eye movements. The EyeLink 1000 is a video camera-based infrared eye-tracking system that uses a velocity-based event-detection algorithm to filter raw gaze samples into saccade, fixation, and blink events (Stampe, 1993). Velocity thresholds for saccade detection were set to the recommended values for cognitive research (30 degrees/second; EyeLink 1000 User Manual, Version 1.5.2). Gaze data was acquired from the right eye at a sampling rate of 1000 Hz. A 9-point calibration and validation procedure was conducted prior to starting the attention task to configure the system such that the spatial accuracy error was below 0.5 degrees on average. Further, calibration accuracy was re-checked after every trial. Participants were required to fixate on a central fixation point; if error was greater than 1 degree, the calibration validation procedure was conducted again to obtain a spatial accuracy of less than 0.5 degrees of average error (number of required calibrations; baseline: M = 0.87, SD = 1.36; post-stress: M = 0.89, SD = 1.48). OpenSesame was used to control stimuli presentation and its synchronization with the eye-tracking system (Mathot, Schreij, & Theeuwes, 2012). The participant’s head was kept stable using an adjustable head mount, with a distance between the participant’s eyes and the camera of approximately 60 cm. All participants had normal to corrected-to-normal vision and were allowed to wear contact lenses or glasses during the attention tasks if necessary; further, participants were required to remove eye make-up before completing the attention tasks.
Stress Induction
Speech Anticipation Task
To assess attention bias in an anxious mood, a speech anticipation procedure developed by Waugh, Panage, Mendes, and Gotlib (2010) was utilized. After participants completed the baseline attention task, they rested for five minutes (i.e., the pre-stress period) and then rated their current mood. The experimenter then placed a camera in the room and told participants that they would have two minutes to prepare a five-minute speech during which they would be recorded and judged by evaluators on their clarity, coherence, and persuasiveness. The speech topic was “Why are you a good friend?”, a topic that has been used successfully in prior studies to elicit anxiety responses (Sanchez et al., 2013; Waugh et al., 2010). Participants were told that there would be two coin flips that would determine if and when the participants gave the speech. They were informed that the first coin would be flipped immediately after the two minute speech preparation period and would determine if they gave the speech immediately, or waited for the second coin flip after the second attention task, which would determine whether they gave the speech at that time or not at all. The experimenter then informed the participant to remain still and silent so as not to disturb the heart rate recording and left the participant alone for two minutes to prepare their speech (i.e., the anticipatory-stress period). After the two minute speech preparation period, the first coin was flipped and participants were told that a computer randomizer would determine if the result of the coin flip indicated that they would give the speech now or wait until the second attention task was completed. All participants were told that they would not give the speech immediately and that the second coin flip, taking place after the second attention task, would determine whether they gave the speech. After participants rated their mood, they completed the second attention task and then the second coin flip was conducted. All participants were told that they would not be giving the speech, and they rested for five minutes (i.e., the post-stress recovery period) before completing a final mood rating.
Psychophysiology Recording
Polar RS800CX
Participants wore heart rate monitors (i.e., Polar Electro RS800CX wristwatch monitors; Anderson & Hope, 2009) to record heart rate at a sampling rate of 1000 Hz from the pre-stress relaxation period through the end of the post-stress recovery period. The heart rate monitor consisted of a wristwatch and a dampened two-lead elastic band worn underneath the clothing around the sternum, and has been used in prior work on cardiovascular stress reactivity (Gouin, Deschenes, & Dugas, 2014). Timestamps were recorded to mark the beginning of the five minute pre-stress period, the two minute anticipatory-stress period, and the five minute post-stress period.
Procedure
After providing consent, participants completed questionnaires not relevant to the present study followed by the baseline attention task. Next, the heart rate monitor was attached and participants sat quietly for a five minute pre-stress relaxation period, after which they rated their current mood. Participants then completed the speech-anticipation period, rated their current mood, and completed the attention task again. After completing the post-stress attention task, participants sat quietly for a five minute post-stress recovery period, after which they rated their current mood and were informed about the daily diary portion of the study. Participants were told that they would be receiving e-mailed links to a daily diary questionnaire battery three days per week (i.e., Monday, Tuesday, Thursday) at 6:00PM for the next two weeks. Further, participants were instructed to complete each daily diary questionnaire within 24 hours (i.e., before 6:00PM the following day). All study procedures were IRB-approved and in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Results
Data Preprocessing
Naturalistic Attention Bias
Of the 165 participants consented, 137 completed the baseline attention task and 126 completed the post-stress attention task. The consented participants who did not complete either attention task were unable to achieve adequate calibration parameters (i.e., < 0.5 degrees avg. error; n = 21) and thus did not complete the experiment. These excluded participants were generally unable to achieve adequate calibration parameters due to factors known to decrease eye-tracking accuracy and precision including thick glasses/contact lenses, sleepiness and “droopy” eye-lids, and long eye-lashes (Nystrom, Andersson, Holmqvist, & van de Weijer, 2013). The remaining seven participants who did not complete either attention task were excluded due to reasons unrelated to eye-tracker calibration (e.g., computer errors). Of the 137 participants who completed the baseline attention task, two participants’ data was lost due to technical errors and one participant’s data was excluded because they misunderstood task instructions, leaving a total of 134 participants with baseline data. Of the 11 participants who completed the baseline but not the post-stress attention task, four participants were dismissed due to inadequate calibration during the post-stress task, whereas the remaining seven participants withdrew voluntarily (n = 5) or were dismissed for other reasons (e.g., excessive sleepiness; n = 2). Scripts were developed to parse the event data generated by the EyeLink system. The EyeLink generates a paired saccade-blink event to demarcate recording periods during which the pupil could not be found (e.g., due to a blink or technical error). Paired saccade-blink events that occurred during the free-viewing portion of a trial were identified and removed by parsing scripts (% of total free-viewing samples removed for baseline and post-stress tasks, respectively; M = 8.0, SD = 6.6, range: 0.3–33.4; M = 9.8, SD = 6.6, range: 0.4–31.5). Prior work has used a 40% criterion for participant exclusion due to excessive signal loss (Graham, Hoover, Ceballos, & Komogortsev, 2011); thus, no participants were excluded for this reason. Next, individual trials were removed if the participant made a saccade away from the center of the screen before facial stimuli onset or if a fixation was not made on either facial stimulus during the 3,000 ms free-viewing period (1.6% and 2.2% of all trials in the baseline and stress tasks, respectively). One outlier had 26.9% and 64.8% of their trials removed on the baseline and post-stress attention tasks, respectively; this participant was excluded from all analyses utilizing the naturalistic attention bias indices. Finally, fixations on either facial stimulus during the 3,000 ms free-viewing period with durations >= 100 ms were extracted and used in the computation of the attentional bias indices. A 100ms minimum fixation duration was utilized for two reasons: 1) Prior work on free-viewing paradigms has identified 100 ms as an optimal threshold for discriminating genuine fixations from other oculomotor activity (Manor & Gordon, 2003), and 2) Fixations of less than 100ms are more likely to be artifacts (e.g., due to partial pupil occlusion during a blink) (EyeLink 1000 User Manual, version 1.5.2, SR Research Ltd, Mississauga, Ontario, Canada, 2005–2010).
In line with recent recommendations (Gibb et al., 2016), split-half reliabilities were computed for attention bias across the three emotion-neutral categories for the baseline and post-stress attention tasks for early vigilance and sustained attention bias indices (see Table 2).
Table 2.
Descriptives and Internal Reliabilities for Attention Bias Indices
| Early Vigilance | Baseline | Stress | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| M | SD | Split-Half Reliability | M | SD | Split-Half Reliability | |
|
| ||||||
| Happy | 0.58 | 0.08 | −.03(n=133,31 trials) – .17(n=94,36 trials) | 0.60 | 0.10 | .30(n=125,31 trials) – .11(n=85,36 trials) |
| Angry | 0.50 | 0.07 | −.49(n=133,32 trials) – −.46(n=93,36 trials) | 0.50 | 0.07 | .01(n=125,30 trials) – −.31(n=76,36 trials) |
| Sad | 0.49 | 0.06 | −.63(n=133,30 trials) – −1.07(n=95,36 trials) | 0.50 | 0.08 | −.27(n=125, 28 trials) – −.03(n=91,36 trials) |
| Sustained Attention | ||||||
| Happy | 0.57 | 0.06 | .70(n=133,31 trials) – .76(n=94,36 trials) | 0.57 | 0.07 | .76(n=125,31 trials) – .79(n=85,36 trials) |
| Angry | 0.53 | 0.05 | .56(n=133,32 trials) – .58(n=93,36 trials) | 0.52 | 0.05 | .67(n=125,30 trials) – .61(n=76,36 trials) |
| Sad | 0.51 | 0.05 | .44(n=133,30 trials) – .54(n=95,36 trials) | 0.52 | 0.05 | .51(n=125, 28 trials) – .66(n=91,36 trials) |
| Engagement | ||||||
| Happy | 469.84 | 55.97 | .66(n=131,5 trials) – .79(n=42,12 trials) | 469.80 | 57.20 | .43(n=123,5 trials) – .86(n=38,12 trials) |
| Angry | 480.50 | 64.31 | .62(n=132,5 trials) – .75(n=48,12 trials) | 476.60 | 66.24 | .52(n=123,5 trials) – .81(n=43,12 trials) |
| Sad | 478.64 | 63.49 | .67(n=129,5 trials) – .68(n=43,12 trials) | 467.93 | 55.41 | .67(n=123,5 trials) – .82(n=44,12 trials) |
| Disengagement | ||||||
| Happy | 469.98 | 60.95 | .75(n=131,5 trials) – .71(n=44,12 trials) | 466.81 | 53.23 | .77(n=123,6 trials) – .88(n=39,12 trials) |
| Angry | 465.03 | 52.95 | .58(n=132,5 trials) – .76(n=46,12 trials) | 465.45 | 51.70 | .57(n=121,5 trials) – .69(n=51,12 trials) |
| Sad | 471.18 | 63.53 | .68(n=131,5 trials) – .56(n=41,12 trials) | 467.71 | 54.10 | .61(n=124,6 trials) – .89(n=42,12 trials) |
Note. Early Vigilance is quantified as the proportion of trials in which participants initially oriented towards the emotional relative to the neutral stimulus. Sustained attention is quantified as the average proportion of fixations on the emotional relative to the neutral stimulus during the naturalistic viewing period. Engagement/Disengagement means are latencies in milliseconds. For early vigilance/sustained attention, split-half reliabilities are computed using the entire sample as well as a subsample with all 36 valid trials for each index of attention bias; trials were grouped temporally such that the first half of trials presented was compared to the second half of trials presented. For engagement/disengagement, participants’ data was excluded if they had less than three SD valid trials available for computation of the mean latency. Split-half reliabilities are computed using the entire sample as well as the subsample with all 12 valid trials for each index of attention bias; trials were grouped temporally such that the first half of trials presented was compared to the second half of trials presented.
Engagement/Disengagement Attention Bias
Scripts were developed to derive engagement and disengagement latencies according to Sanchez and colleague’s (2013) procedure (see Table 2 for descriptives and reliabilities). The following criteria were used to ensure that the engagement/disengagement latencies reflected valid shifts in gaze elicited by the appearance of the shape (86.3% and 87.1% of baseline and post-stress trials, respectively): 1) Gaze was on the non-framed facial stimulus for at least 100 additional ms after the end of the ‘wait for fixation period’ and appearance of the frame; 2) Fixation on the framed facial stimulus lasted for at least 100 ms; 3) Time elapsed from the end of the ‘wait for fixation period’ to the first 100ms fixation on the framed face was < 1000 ms. Further, trials were considered invalid if the participant incorrectly identified the shape of the frame.
Of the invalid trials during the baseline task, 55.2% were invalid due to a <100 ms or absent fixation on the framed face and 19.3% were invalid due to <100ms of additional gaze time on the non-framed facial stimulus after the end of the ‘wait for fixation’ period. Similarly, among the invalid trials during the post-stress task, 59.4% were invalid due to a <100 ms or absent fixation on the framed face and 22.0% contained <100ms of additional gaze time on the non-framed facial stimulus after the end of the ‘wait for fixation’ period Seven outliers (i.e., defined as < 3 SD valid trials for at least one emotion category) were identified in the baseline task data (valid trials: M = 10.55; SD = 1.59) and five outliers were identified in the post-stress task data (valid trials: M = 10.65; SD = 1.45); outliers in a particular emotion category were excluded from those analyses.
Heart Rate
Of the 126 participants who completed the experiment, 92, 95, and 109 had physiological data for the pre-stress, anticipatory-stress, and post-stress periods, respectively; data loss occurred primarily because of technical errors and transmission signal loss during the experiment. Heart rate data were cleaned and extracted using a five step process. First, we used the Polar Pro Trainer 5TM software to export the collected IBI data to a text file for preprocessing before analysis. Second, the IBI series was loaded into HRV analysis software (HRVAS; Ramshur, 2010) and visually inspected for ectopic intervals. Third, an automated filter was utilized to confirm visually identified ectopic intervals by marking IBIs that differed more than 20% from the previous interval; these intervals were then corrected using cubic spline interpolation (% of IBIs interpolated; pre-stress: M = 2.0, SD = 2.7, range: 0–14.9; anticipatory-stress: M = 2.0, SD = 3.6, range: 0–30; post-stress: M = 2.8, SD = 3.9, range: 0–24.2). Fourth, the corrected IBI series was detrended using the discrete wavelet packet transform (Shafqat, Pal, & Kyriacou, 2007). Lastly, HRVAS was used to compute mean heart rate for the pre-stress, anticipatory-stress, and post-stress periods.
Daily Diary
Of the 137 participants who had data for at least one of the attention tasks, 93.4% (n = 128) completed at least one of their six diary entries (completed entries: M = 4.29, SD = 1.51), whereas 74.2% (n = 95) completed at least four entries and 23.4% (n = 30) completed all six entries. Entries were considered invalid and excluded if they were completed more than 24 hours after they were sent or if the control item to ensure the participant was paying attention (e.g., ‘Please select ‘Strongly agree’ for this item’) was answered incorrectly. 25.1% of all submitted entries were excluded. After exclusion of invalid entries, 554 valid daily diaries were available for analysis.
Data Analysis
Anxious Mood Manipulation Check
To ensure that the uncertain speech threat procedure elicited anxious mood specifically, a repeated measures ANOVA predicting change in self-reported mood across the experiment was conducted. Mood was entered as a three-level within-subject factor (Happy, Anxious, Depressed) and Time was also entered as a three-level within-subject factor (Pre-Stress, Anticipatory Stress, Post-Stress). Mauchly’s test indicated that the assumption of sphericity was violated for the main effect of Mood, χ2(2) = 75.49, p < .001, Time, χ2(2) = 12.13, p = .002, and the Mood*Time interaction, χ2(9) = 87.65, p < .001 ; thus, degrees of freedom were corrected using Greenhouse Geisser estimates of sphericity for all tests involving the main effect of Mood (ε = 0.67), Time (ε = 0.91), and the Mood*Time interaction (ε = 0.68) (see the Supplemental material for main effect results). As predicted, a significant Mood*Time interaction emerged, F(2.72, 301.35) = 15.90, p < .001, pη2 = .13.
To probe the Mood*Time interaction, a repeated measures ANOVA with Time as a three-level within-subject factor was conducted for each mood type. For happy mood, Mauchly’s test indicated that the assumption of sphericity was met, χ2(2) = 3.06, p = .22. However, the within-subject effect of Time was non-significant, F(2, 248) = .77, p = .46, pη2 = .01. For depressed mood, Mauchly’s test indicated that the assumption of sphericity was met, χ2(2) = 5.90, p = .052. In contrast to the happy mood analysis, the within-subject effect of Time for depressed mood was significant, F(2, 248) = 16.04, p < .001, pη2 = .12. Two paired-sample t-tests were conducted to assess depressed mood reactivity (i.e., change in depressed mood from the pre-stress to the anticipatory-stress period) and depressed mood recovery (i.e., change in depressed mood from the anticipatory-stress to the post-stress period); a p-value of .025 was utilized to adjust for multiple comparisons. Results revealed a non-significant change in depressed mood from pre-stress (M = 4.71, SD = 5.69) to the anticipatory-stress period (M = 4.23, SD = 5.42), t(129) = 1.76, p = .08, but a significant decrease in depressed mood from the anticipatory-stress period to the post-stress period (M = 3.11, SD = 4.36), t(126) = 3.85, p < .001. For anxious mood, Mauchly’s test indicated that the assumption of sphericity was violated, χ2(2) = 30.64, p < .001; thus, degrees of freedom were corrected using Greenhouse Geisser estimates of sphericity (ε = 0.82). As with depressed mood, the within-subject effect of Time for anxious mood was significant, F(1.63, 195.60) = 36.47, p < .001, pη2 = .23. Paired-sample t-test results revealed a significant increase in anxious mood from pre-stress (M = 7.47, SD = 7.19) to the anticipatory-stress period (M = 10.43, SD = 8.49), t(125) = −5.07, p < .001, and a significant decrease in anxious mood from the anticipatory-stress period to the post-stress period (M = 6.03, SD = 6.53), t(123) = 7.17, p < .001.
To examine a physiological index of stressor reactivity and recovery, a repeated measures ANOVA predicting change in mean HR across the experiment was conducted. Time was entered as a three-level within-subject factor (mean HR at Pre-Stress, Anticipatory Stress, and Post-Stress). Mauchly’s test indicated that the assumption of sphericity was not violated, χ2(2) = 4.61, p = .10. As predicted, the within-subject effect of Time was significant, F(2, 172) = 22.88, p < .001, pη2 = .21. Paired sample t–test results revealed a significant increase in mean HR from pre-stress (M = 75.10, SD = 9.30) to the anticipatory-stress period (M = 79.31, SD = 10.33), t(90) = −6.52, p < .001, and a significant decrease in mean HR from the anticipatory-stress period to the post-stress period (M = 76.50, SD = 9.94), t(89) = 4.38, p < .001.
The results demonstrate that the uncertain speech threat manipulation successfully elicited subjective anxious mood and a physiological stress response (see Table 1). Further, the self-report and physiological recovery data demonstrate stress response deactivation upon offset of the uncertain speech threat, suggesting that participants’ second completion of the attention task (i.e., the post-stress attention task) occurred within a stressful context.
Effect of the Anxiety Induction on Attention Bias Towards Threat
To test the hypothesis that the anxious mood induction would significantly increase attention bias towards threat, four repeated measures ANOVAs predicting change in each form of threat bias (i.e., early vigilance, sustained attention, facilitated engagement, delayed disengagement) across the experiment were conducted. Emotion was entered as a three-level within-subject factor (Positive, Threat, Dysphoric) and Time was entered as a two-level within-subject factor (Baseline, Post-Stress). For early vigilance, Mauchly’s test indicated that the assumption of sphericity was violated for the main effect of Emotion, χ2(2) = 10.62, p = .005, but was met for the Emotion*Time interaction, χ2(2) = 1.07, p = .59; thus, degrees of freedom were corrected using Greenhouse Geisser estimates of sphericity for all tests involving the main effect of Emotion (ε = .92) (see the Supplemental material for main effect results). Contrary to predictions, the Emotion*Time interaction was non-significant, F(2, 242) = 0.64, p = .53, pη2 = .01.
For sustained attention, Mauchly’s test indicated that the assumption of sphericity was violated for the main effect of Emotion, χ2(2) = 35.85, p < .001, and the Emotion*Time interaction, χ2(2) = 6.63, p = .04; thus, degrees of freedom were corrected using Greenhouse Geisser estimates of sphericity for all tests involving the main effect of Emotion (ε = 0.80) and the Emotion*Time interaction (ε = 0.95) (see the Supplemental material for main effect results). As predicted, the Emotion*Time interaction was significant, F(1.90, 229.65) = 3.28, p = .04, pη2 = .03. Three paired sample t-tests were conducted to test the hypothesis that the anxiety induction would significantly increase attention bias towards threat; a p-value of .017 was utilized to adjust for multiple comparisons. Paired sample t-tests indicated that sustained attention towards threat significantly decreased from pre (M = .53, SD = 0.05) to post anxiety induction (M = .52, SD = 0.05), t(121) = 2.45, p = .016, whereas no significant change was observed for sustained attention towards dysphoric, t(121) = −0.10, p = .92, or positive emotional stimuli, t(121) = −1.03, p = .31.
For facilitated engagement, Mauchly’s test indicated that the assumption of sphericity was met for the main effect of Emotion, χ2(2) = 5.91, p = .052, and the Emotion*Time interaction, χ2(2) = 4.05, p = .13 (see the Supplemental material for main effect results). Contrary to predictions, the Emotion*Time interaction was non-significant, F(2, 230) = 0.66, p = .52, pη2 = .01.
For delayed disengagement, Mauchly’s test indicated that the assumption of sphericity was met for the main effect of Emotion, χ2(2) = 0.89, p = .64, and the Emotion*Time interaction, χ2(2) = 4.44, p = .11 (see the Supplemental material for main effect results). Contrary to predictions, the Emotion*Time interaction was non-significant, F(2, 232) = 0.02, p = .98, pη2 = .000.
Due to the unexpected decrease in sustained attention bias towards threat from pre- to post-stressor, exploratory analyses were conducted to determine if the observed decrease in threat bias reflected an emotion regulation strategy as has been observed in prior work on the effect of mood inductions on attention bias (Ellenbogen et al., 2002; Sanchez et al., 2013) (see the Supplemental material for results).
Statistical Analyses
To examine the associations between threat bias, daily stressors, daily NA, and daily ratings of worry symptoms, separate hierarchical linear models were constructed (HLM 7.0; Raudenbush & Bryk, 2002) for early vigilance, sustained attention, engagement, and disengagement indices of threat bias. Hierarchical linear modeling (HLM) was chosen because this framework takes into account the lack of independence among repeated, within-subject measurements and easily handles variations in number of within-subject measurements by assuming that the observed data points are representative of the population of all possible time points. All equations were constructed such that Level 1 included repeated, within-subject variables (i.e., daily negative affect, stressors, and worry) and Level 2 included between-subjects variables (i.e., attention bias). An unconditional, random ANOVA model was examined first in order to partition variance in daily ratings of worry symptoms into Level 1 and Level 2. Substantial variability between subjects was found for daily worry symptoms, as the ICC was .58, indicating that hierarchical modeling of the data was necessary, χ2 = 986.15, p < .001 (Garson, 2012).
Next, the time variable was added in order to determine whether daily worry symptoms significantly changed throughout the course of the study and whether time should be consequently included in the final model. A Kolmogorov-Smirnov test indicated that the daily worry symptoms variable was not normally distributed, D = .28, p < .001, and so robust standard errors were used in all subsequent analyses (Garson, 2012). Results indicated that the fixed effect of time was non-significant, t(473) = −1.68, p = .10. Thus, time was not included as a fixed effect in the final model.
To evaluate the hypothesized prospective, moderating role of threat bias on daily-level associations between stressors and worry, Level 1 and Level 2 variables were added as fixed effects in order to examine predictors of daily worry. Daily stressors and daily negative affect were entered as Level 1 predictors. Daily negative affect was included as a covariate to test threat bias’s incremental validity in the prediction of daily worry symptoms. The threat bias index was grand mean-centered and entered as a Level 2 variable, reflecting threat bias’s main effect on daily worry. Finally, a cross-level interaction term between the Level 2 threat bias index and the Level 1 daily stressors variable was also entered, reflecting the impact of threat bias on worry in the context of naturalistic stressors. Both Level 1 variables were group mean-centered.
Relationships Between Early Vigilance Towards Threat, Daily Stressors, Daily NA, and Daily Worry Symptoms
First, the model utilizing the post-stress early vigilance index of threat bias was run (see Table S1). In contrast with hypotheses, the cross-level interaction term between early vigilance towards threat after anxious mood induction and daily stressors did not significantly predict daily worry symptoms, B = 1.99, SE = 1.34, t(392) = 1.48, p = .14, nor was there a significant main effect of early vigilance towards threat, B = 0.81, SE = 3.00, t(115) = 0.27, p = .79. Second, the model utilizing the baseline early vigilance index of threat bias was run (see Table S2); both the cross-level interaction term, B = 1.12, SE = 1.01, t(407) = 1.10, p = .27, and main effect, B = 0.42, SE = 2.75, t(122) = 0.15, p = .88, were non-significant.
Exploratory analyses were conducted to determine if early vigilance towards positive or dysphoric emotional stimuli predicted daily worry; results revealed non-significant cross-level interactions and main effects for biases assessed at baseline (ps > .10) and after anxious mood elicitation (ps > .23).
Relationships Between Sustained Attention Towards Threat, Daily Stressors, Daily NA, and Daily Worry Symptoms
First, the model utilizing the post-stress sustained attention index of threat bias was run (see Table 3). Consistent with hypotheses, the cross-level interaction term between Level 2 sustained attention threat bias and daily stressors was significant, B = 4.62, SE = 1.85, t(392) = 2.50, p = .01, as was the main effect of threat bias, B = 8.10, SE = 3.65, t(115) = 2.22, p = .03. Second, to examine specificity to post-anxiety induction threat bias but not baseline threat bias, the same model was run using the baseline sustained attention index of threat bias (see Table S3). In contrast to the post-anxiety induction threat bias results and consistent with hypotheses, Level 2 baseline sustained attention threat bias did not significantly interact with daily stressors to predict daily worry, B = 1.65, SE = 1.29, t(407) = 1.28, p = .20, though there was a marginal main effect of baseline threat bias, B = 7.27, SE = 4.23, t(122) = 1.72, p = .09.
Table 3.
The Effects of Post-Stress Sustained Attention Towards Threat on Daily Worry
| Coefficient | SE | t-ratio | Approximate df | p value | |
|---|---|---|---|---|---|
| Fixed effects | |||||
| Intercept of Daily Worry | 4.80 | 0.21 | 23.08 | 115 | <.001 |
| Level 2 variables | |||||
| Sustained Threat Bias | 8.10 | 3.65 | 2.22 | 115 | .03 |
| Level 1 variables | |||||
| NA | 0.34 | 0.06 | 5.67 | 392 | <.001 |
| Stressors | 0.34 | 0.12 | 2.99 | 392 | .003 |
| Interaction effects | |||||
|
|
|||||
| Stressors*Sustained Threat Bias | 4.62 | 1.85 | 2.50 | 392 | .01 |
|
|
|||||
| SD | Variance component | df | Chi-square | p value | |
| Random effects | |||||
| Between-subjectss residual | 2.10 | 4.40 | 115.00 | 980.26 | <.001 |
| Within-subject residual | 1.63 | 2.65 | |||
Note. NA = Negative Affect.
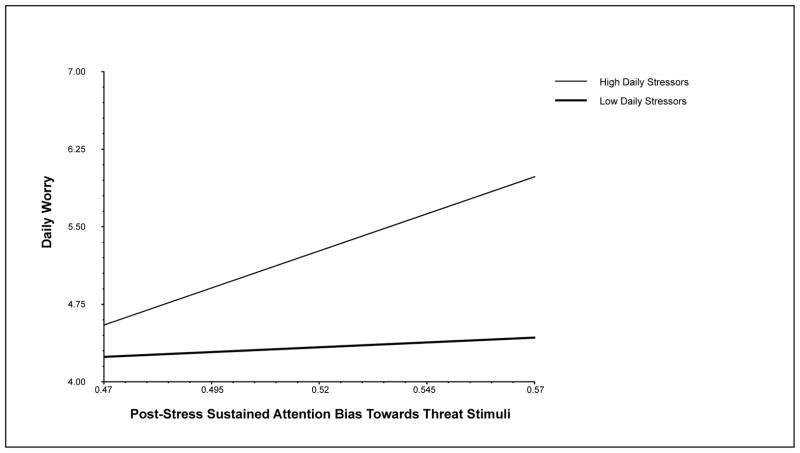
To test the effect of post-anxiety induction threat bias on daily worry symptoms at varying levels of daily stressors, regression coefficients, coefficient variances, and covariances were entered into an online calculator to compute simple slopes of the relationship between post-stress threat bias and daily worry symptoms at low (−1 SD) and high (+1 SD) levels of daily stressors (Preacher et al., 2006). A significant positive relationship between post-stress threat bias and daily worry symptoms was found at high (+1 SD) levels of daily stressors, B = 14.33, SE = 4.91, z = 2.92, p = .004, but not low (−1 SD) levels of daily stressors, B = 1.87, SE = 3.87, z = 0.48, p = .63 (see Figure 1).
Figure 1.
The moderating effect of daily stressors on the relationship between sustained attention bias towards threat stimuli in the context of acute stress and daily worry.
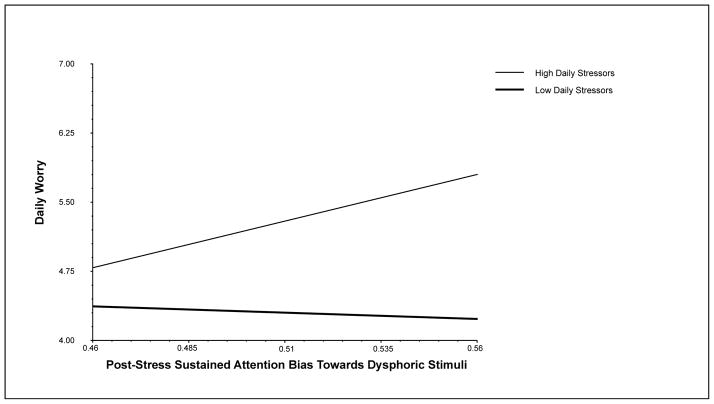
To determine if the observed results were specific to threat bias, the same models were run using the sustained attention bias indices of post-anxiety induction positive bias and dysphoric bias. As expected, the cross-level interaction term for positive bias was non-significant, B = −1.83, SE = 1.49, t(392) = −1.23, p = .22, though the main effect was significant, B = −7.27, SE = 2.85, t(115) = −2.55, p = .01 (see Table S4). Contrary to predictions, the cross-level interaction term for dysphoric bias was significant, B = 4.26, SE = 1.92, t(392) = 2.22, p = .03, though the main effect was not, B = 4.38, SE = 3.72, t(115) = 1.18, p = .24 (see Table 4). As with post-anxiety induction threat bias, a significant positive relationship between post-stress dysphoric bias and daily worry symptoms was found at high (+1 SD) levels of daily stressors, B = 10.12, SE = 5.16, z = 1.96, p = .0496, but not low (−1 SD) levels of daily stressors, B = −1.37, SE = 3.81, z = −0.36, p = 0.72 (see Figure 2).
Table 4.
The Effects of Post-Stress Sustained Attention Towards Dysphoric Stimuli on Daily Worry
| Coefficient | SE | t-ratio | Approximate df | p value | |
|---|---|---|---|---|---|
| Fixed effects | |||||
| Intercept of Daily Worry | 4.80 | 0.21 | 22.84 | 115 | <.001 |
| Level 2 variables | |||||
| Sustained Dysphoric Bias | 4.38 | 3.72 | 1.18 | 115 | .24 |
| Level 1 variables | |||||
| NA | 0.33 | 0.06 | 5.50 | 392 | <.001 |
| Stressors | 0.37 | 0.13 | 2.85 | 392 | .005 |
| Interaction effects | |||||
|
|
|||||
| Stressors*Sustained Dysphoric Bias | 4.26 | 1.92 | 2.22 | 392 | .03 |
|
|
|||||
| SD | Variance component | df | Chi-square | p value | |
| Random effects | |||||
| Between-subjectss residual | 2.13 | 4.53 | 115.00 | 1014.37 | <.001 |
| Within-subject residual | 1.63 | 2.66 | |||
Note. NA = Negative Affect.
Figure 2.
The moderating effect of daily stressors on the relationship between sustained attention bias towards dysphoric stimuli in the context of acute stress and daily worry.
An exploratory analysis was conducted to determine if the same specificity to post-stress, but not baseline bias, observed for threat would also emerge for dysphoric bias. Specificity was found such that the cross-level interaction term, B = −0.40, SE = 2.02, t(407) = −0.20, p = .84, as well as the main effect, B = −3.23, SE = 3.96, t(122) = −0.82, p = .42, of baseline dysphoric bias were non-significant (see Table S5). Given the significant main effect of post-stress sustained attention bias towards positive stimuli, an exploratory analysis was conducted to determine if baseline positive bias significantly predicted daily worry symptoms (see Table S6). Results revealed a non-significant main effect of baseline positive bias, B = −1.94, SE = 3.71, z = −0.52, p = .60, as well as a non-significant interaction with daily stressors, B = −2.38, SE = 1.59, t(407) = −1.50, p = .13.
Relationships Between Facilitated Engagement with Threat, Daily Stressors, Daily NA, and Daily Worry Symptoms
First, the model utilizing the post-stress engagement index of threat bias was run (see Table S7). In contrast with hypotheses, the cross-level interaction term between facilitated engagement with threat after the anxious mood induction and daily stressors did not significantly predict daily worry symptoms, B = −0.001, SE = 0.00, t(384) = −0.84, p = .40, nor was there a significant main effect of facilitated engagement with threat, B = −0.002, SE = 0.00, t(113) = −0.94, p = .35. Second, the model utilizing the baseline engagement index of threat bias was run (see Table S8); both the cross-level interaction term, B = −0.001, SE = 0.002, t(403) = −0.41, p = .69, and main effect, B = −0.005, SE = 0.003, t(121) = −1.57, p = .12, were non-significant.
Exploratory analyses were conducted to determine if engagement with positive or dysphoric emotional stimuli predicted daily worry. For engagement with positive stimuli, there was a significant main effect of bias at baseline, B = −0.009, SE = 0.003, t(120) = −3.00, p = .003, but not after the anxious mood induction, B = −0.005, SE = 0.003, t(113) = −1.56, p = .12; in contrast, the cross-level interaction terms were non-significant, ps > .79 (see Tables S9 and S10). For engagement with dysphoric stimuli, there was a trend-level main effect of bias at baseline, B = −0.005, SE = 0.003, t(118) = −1.90, p = .06, and after the anxious mood induction, B = −0.005, SE = 0.003, t(113) = −1.84, p = .07; in contrast, the cross-level interaction terms were non-significant, ps > .10 (see Tables S11 and S12).
Relationships Between Delayed Disengagement from Threat, Daily Stressors, Daily NA, and Daily Worry Symptoms
First, the model utilizing the post-stress disengagement index of threat bias was run (see Table S13). In contrast with hypotheses, the cross-level interaction term between delayed engagement from threat after the anxious mood induction and daily stressors did not significantly predict daily worry symptoms, B = −0.0008, SE = 0.002, t(378) = −0.38, p = .70, nor was there a significant main effect of delayed disengagement from threat, B = −0.002, SE = 0.00, t(111) = −0.64, p = .53. Second, the model utilizing the baseline disengagement index of threat bias was run (see Table S14); both the cross-level interaction term, B = −0.0009, SE = 0.00, t(402) = −0.43, p = .67, and main effect, B = −0.002, SE = 0.003, t(121) = −0.47, p = .64, were non-significant.
Exploratory analyses were conducted to determine if disengagement from positive or dysphoric emotional stimuli predicted daily worry. All main effects, ps > .16, and cross-level interaction terms were non-significant, ps > .21.
Discussion
The central aim of the present study was to test the hypothesis that attention bias towards threat, but not dysphoric or positive emotional information, assessed in the context of acute anxious mood, but not at baseline, would predict greater worry symptoms in the context of real-world stressors. Results provided partial support for this hypothesis. As expected, a significant effect of the anxious mood induction was observed on attention bias towards threat, but not positive or dysphoric emotional information. However, effects were found for sustained attention but not other threat bias indices (i.e., early vigilance, facilitated engagement, delayed disengagement) and the observed change was opposite the direction expected such that threat bias decreased from the baseline to stress context. Despite this unexpected finding, as hypothesized, sustained attention towards threat assessed after anxious mood elicitation, but not at baseline, predicted daily worry symptoms on days with more naturalistic stressors. Further, a significant main effect was also found such that greater sustained attention towards threat in the context of acute stress predicted greater daily worry independently of daily stressors. However, the hypothesized specificity to threat bias was not observed. Instead, significant moderation effects were observed for both threat and dysphoric biases, but not positive bias, suggesting that greater sustained processing of negative emotional information in the context of a laboratory stressor is predictive of greater worry in response to naturalistic stressors. In addition, though the moderation effect was non-significant, the main effect of post-stress, but not baseline, positive bias was significant such that greater sustained attention towards positive stimuli under acute stress predicted decreased daily worry symptoms independent of daily stressors. Finally, exploratory analyses revealed a significant main effect of baseline facilitated engagement with positive stimuli on daily worry as well as marginal baseline and post-stress main effects of facilitated engagement with dysphoric stimuli on daily worry, suggesting that faster engagement with emotional information in general may be a trait-like form of attentional bias relevant to excessive worry. Overall, the pattern of effects was consistent with our hypothesis that acute stress is an important moderator of the relationship between attention bias and worry symptoms, though this effect appears to be specific to sustained attention bias.
Interestingly, whereas greater sustained attention towards threat in the context of a laboratory stressor predicted more naturalistic stressor-elicited worry, we found evidence for an overall stress-elicited decrease in sustained attention towards threat across the entire sample. Although greater decreases in sustained attention towards threat was associated with greater anxious mood reactivity to and recovery from the stressor, this effect was not specific to threat but was found for dysphoric and positive attention biases as well. The decreased threat bias finding is inconsistent with prior studies demonstrating greater attention towards threat relative to neutral stimuli following an anxious mood induction (Ford et al., 2010; Nelson et al., 2015; Quigley et al., 2012), though these studies used autobiographical recall and emotional music to induce anxious mood. In contrast, our study used a speech stressor to elicit anticipation of uncertain threat that was not resolved until the end of the attention task. Indeed, other work utilizing a speech stress induction has also found a stress-elicited decrease in attention bias towards threat measured with the emotional Stroop (Amir et al., 1996), though other studies that used eye-tracking and dot-probe assessments in conjunction with social-evaluative threat inductions have produced mixed results (Garner, Mogg, & Bradley, 2006; Mansell, Ehlers, Clark, & Chen, 2002). It is possible that the presence of an impending, uncertain social-evaluative threat decreased the salience of irrelevant threat stimuli (i.e., angry faces), which suggests that the observed positive association between processing of these stimuli in the presence of impending, uncertain threat and daily worry may reflect the latter’s cognitive avoidance function (Borkovec, Alcaine, & Behar, 2004) and/or fear overgeneralization (Lissek et al., 2014). It is also possible that decreasing threat processing is normative and adaptive when the threat is uncertain and uncontrollable. Future research is needed to better understand the sample (e.g., non-clinical vs. clinical), assessment method/paradigm (e.g., eye-tracking, emotional stroop), and mood induction (e.g., certain vs. uncertain threat) characteristics likely to increase as well as decrease attention bias towards threat.
The moderating impact of daily stressors on the relationship between post-stress threat bias and worry was specific to sustained attention towards threat. The null results for early vigilance suggest that late rather than early threat bias is predictive of worry symptoms, which is inconsistent with some prior work (e.g., Mogg, Millar, & Bradley, 2000). However, though the moderating effect of early vigilance was in the expected direction and became stronger after anxious mood elicitation, split-half reliabilities of the early vigilance indices were much lower than those of the sustained attention indices, which likely limited our ability to find significant effects. Indeed, a prior study utilizing eye-tracking methods also found poor internal reliabilities for early vigilance indices (Waechter et al., 2014), suggesting that failure to find a relationship between early attentional vigilance and worry should be interpreted with caution. The observed null associations between threat disengagement biases and worry indicate that impaired attentional disengagement from threat may not contribute to excessive worry. In the task used in the current study, efficiency of overt visual disengagement from threat was isolated whereas prior studies reporting significant associations used visual search and manual RT-based assessments of delayed disengagement (Armstrong & Olatunji, 2012; Cisler & Koster, 2010). Given that these assessments produce disengagement indices influenced by overt as well as covert attentional processes, delayed covert disengagement from threat may be primarily relevant to worry, though this hypothesis is speculative and more research is needed. Null associations were also found between threat engagement biases and worry, but the observed pattern of effects for dysphoric/positive engagement biases suggests that faster engagement with positive and dysphoric emotional stimuli may, in contrast to sustained attention bias’s context-sensitivity, reflect a more stable, trait-like vulnerability to excessive worry. Indeed, greater vigilance to negative and positive facial stimuli in anxious individuals has been found in prior work (e.g., Garner, Mogg, & Bradley, 2006). However, given the exploratory and marginal nature of the engagement bias findings, this interpretation is tentative and replication is needed. Overall, these data support the notion that attention bias is not a unitary phenomenon and should be decomposed into distinct components when methodologically feasible.
The observed interaction between sustained attention towards dysphoric stimuli and daily stressors suggests that increased sustained processing of dysphoric as well as threat information under stress is predictive of greater naturalistic stressor-elicited worry symptoms. Although the hypothesized specificity to threat bias was not observed, the significant dysphoric bias interaction effect may reflect the high rates of co-occurrence among anxiety and depressive symptoms (Moffitt et al., 2007). Specifically, the high covariation between depressive rumination and worry is thought to reflect a common underlying vulnerability to negative mood-elicited perseverative thought (McEvoy et al., 2013). Given that depressive rumination has been linked to a dwell time index of dysphoric bias in a prior study (Duque, Sanchez, & Vazquez, 2014), it may be that both sustained dysphoric and threat biases in the context of a laboratory stressor were predictive of worry in the present study because they are both behavioral correlates of vulnerability to negative mood-elicited perseverative thought. Future research should explicitly test this hypothesis by directly manipulating worry/rumination in a negative mood state and observing the effects on sustained negative attention bias. Although contrary to predictions, the significant inverse main effect of sustained attention towards positive stimuli after the anxious mood induction on daily worry is consistent with a recent experimental study demonstrating an effect of attention training to positive stimuli on worry symptoms (Sass, Evans, Xiong, Mirghassemi, & Tran, in press). However, the relationship between greater decreases in attention towards negative as well as positive emotional stimuli and improved anxious mood recovery from the laboratory stressor suggests that sustained attention towards positive emotional stimuli under acute stress may not always be adaptive; given the exploratory nature of these findings, replication is required before firmer conclusions can be drawn. Future research on attention bias and worry should consider assessing positive as well as negative attention biases.
The present findings have theoretical implications. The moderating role of acute stress on the relationship between attention bias and worry symptoms observed in the present study may partially account for the modest test-retest reliability previously found in eye-tracking studies of sustained attention biases towards threat (ICC = .32; Price et al., 2015). Poor test-retest reliability suggests that single assessments of threat bias poorly reflect the underlying construct, which might be due to state factors (e.g., anxious mood) affecting the expression of threat bias in an individual assessment session. Our experimental data provides support for this hypothesis given that split-half reliabilities of the sustained attention bias indices improved after the anxious mood induction relative to those obtained at baseline, which may indicate that the current motivational relevance of task stimuli is important for the reliability of attention bias assessments. Indeed, some studies have found that utilization of personally-relevant stimuli improves the internal reliability of attention bias assessments (Christiansen, Mansfield, Duckworth, Field, & Jones, 2015; Field & Christiansen, 2012). Thus, measurements of attention bias might demonstrate greater internal and test-retest reliability if biases are assessed in a clinically-relevant context (e.g., anticipatory threat/anxiety; sad mood/depression; craving/substance dependence). Future research could test this hypothesis for attention bias towards threat by comparing test-retest reliability between repeated baseline assessments of bias and repeated assessments of bias after an anxious mood induction. Relatedly, the differential effect of mood context on the prospective relationship between attention bias and daily stressor-elicited worry suggests that attention bias may also demonstrate greater convergent validity when task stimuli are emotionally salient. Variability in the current motivational relevance of valenced task stimuli (e.g., due to state mood, emotion regulation goals) may influence the degree to which a given measure of attention bias is valid. Future research should measure the motivational salience of the valenced stimuli utilized in attention bias tasks (e.g., with EEG methods; Hajcak, MacNamara, & Olvet, 2010) to determine its relationship with the reliability and validity of attention bias towards emotional information.
In line with recent theoretical reviews of attention bias (Rodebaugh et al., 2016; Van Bockstaele et al., 2014), the present results suggest that the conceptualization of attention bias towards threat as a static, trait-like construct may not be entirely accurate. Instead, the degree to which an individual selectively deploys attention towards threat may, like other forms of learned behavior (Bower, 1981), depend largely upon the context. Individual differences in reinforcement histories may contribute to learned patterns of attentional behavior that emerge in the contexts in which these patterns of attentional behavior have historically been reinforced. For instance, the present study’s results suggest that greater selective attention towards threat cues in a context characterized by an uncertain, future stressor is prospectively associated with worry symptoms, which may reflect an attentional pattern that has been repeatedly negatively reinforced in similar contexts, possibly due to the relationship between threat hypervigilance, cognitive avoidance, and worry (Borkovec et al., 2004). In contrast, some studies have found a relationship between greater selective attention away from threat cues in a context of imminent, life-threatening danger and greater anxiety symptoms (Bar-Haim et al., 2010; Wald et al., 2011a), which may similarly reflect a negatively reinforced attentional pattern that minimizes stress exposure in the short-term but contributes to anxiety psychopathology in the long-term (e.g., Wald et al., 2011b). A context-dependent, learned behavior conceptualization of attention bias towards emotional stimuli may also account for mood induction-incongruent patterns of attentional behavior observed in some laboratory studies (Ellenbogen et al., 2002; Sanchez et al., 2013). Individuals who demonstrate negative mood-elicited attention biases away from negative and towards positive stimuli may be employing attentional emotion regulation strategies that have been reinforced via negative affect reduction in similar contexts in their daily lives, a possibility consistent with data on attention bias towards appetitive stimuli in individuals who report high levels of coping-motivated eating/alcohol use behavior (Field & Powell, 2007; Field & Quigley, 2009; Frayn, Sears, & von Ranson, 2016). Future studies should consider the theoretical role of external (e.g., proximity and severity of threat) and internal (e.g., emotion regulation goals, state mood) contexts with respect to the expression of attention biases towards emotional stimuli and their relationship with psychopathology.
The present study has a number of limitations. First, despite our best efforts to prevent it, enhanced peripheral processing of the frame’s shape in the engagement/disengagement paradigm produced substantially more trials that could not be analyzed compared to other studies that have used this paradigm (Sanchez et al., 2013; Sanchez et al., 2016). Thus, the validity of the engagement/disengagement indices were compromised and could not be confidently used in analyses. Future studies using the engagement/disengagement paradigm should ensure that participants are only able to sufficiently process the frame’s shape if they disengage completely from the target facial stimulus and fixate on the framed face. Second, reliabilities for early vigilance indices were poor, which has also been reported in other eye-tracking studies (Waechter et al., 2014). Although our measure of early vigilance is commonly used (Kellough et al., 2008; Sanchez et al., 2013) and has been linked to theoretically-consistent phenomena (Kimble, Fleming, Bandy, Kim, & Zambetti, 2010), our reliability data suggest that early vigilance on a trial by trial basis does not index a unitary construct. Future studies may want to measure early attentional vigilance using a different approach (e.g., Wieser, Pauli, Weyers, Alpers, & Muhlberger, 2009). Third, although participants with greater vulnerability to affective psychopathology were oversampled, the sample was recruited from a non-clinical population. It is unclear if the mood manipulation used in the present study would similarly affect expression of negative attention bias in a clinical sample given that individuals with anxiety disorders would likely have higher baseline levels of negative affect. Future studies should attempt to replicate the observed findings in a clinical sample to address this question. Fourth, although the stress induction utilized in the present study elicited physiological arousal and subjective feelings of anxiety, it is unclear if similar attention biases would emerge in the context of other commonly used laboratory mood inductions less directly relevant to worry. Future studies should investigate the relative efficacy of more disorder-relevant (e.g., anticipation of uncertain threat for anxiety) vs. general (e.g., uncontrollable white noise blasts) mood inductions for eliciting negative attention biases. Fifth, the daily diary period was relatively short and the rate of non-compliance (i.e., missing/late entries, missed control items) could be improved. Future studies should consider providing stronger incentives for participation and using longer follow-up periods to ensure the relationships observed in the present study are stable.
The present findings have potential clinical implications. Few studies have compared the efficacy of standard bias modification to modification completed subsequent to a relevant mood induction, though one study found the latter to be more efficacious than the former in reducing social anxiety symptoms (Kuckertz et al., 2014). Indeed, recent bias modification studies have delivered intervention sessions during critical time points in which the task stimuli are expected to be maximally salient, with promising results (Milkins, Notebaert, MacLeod, & Clarke, in press; Wald et al., in press). Further research systematically evaluating the relative efficacy of bias modification with and without manipulation of a symptom-relevant mood context is needed. Further, given that multiple meta-analyses have revealed stronger effects of bias modification on symptoms assessed following a stressor (Hallion & Ruscio, 2011; Mogoase et al., 2014), future intervention studies should include outcome measures sensitive to changes in responding to acute stress in addition to trait-level symptom measures.
Supplementary Material
Acknowledgments
We have no acknowledgements to declare for the present manuscript.
Funding Sources
This research was supported in part by National Institute on Drug Abuse (NIDA) grant F31DA039644 (Richard J. Macatee) and the National Institute of Mental Health (NIMH) grant T32MH093311 (Brian J. Albanese). NIDA/NIMH had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
References
- Allan NP, Macatee RJ, Norr AM, Schmidt NB. Direct and interactive effects of distress tolerance and anxiety sensitivity on generalized anxiety and depression. Cognitive Therapy and Research. 2014;38(5):530–540. [Google Scholar]
- Almeida DM, Wethington E, Kessler RC. The daily inventory of stressful events an interview-based approach for measuring daily stressors. Assessment. 2002;9(1):41–55. doi: 10.1177/1073191102091006. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. DSM 5. American Psychiatric Association; 2013. [Google Scholar]
- Amir N, McNally RJ, Riemann BC, Burns J, Lorenz M, Mullen JT. Suppression of the emotional Stroop effect by increased anxiety in patients with social phobia. Behaviour Research and Therapy. 1996;34(11):945–948. doi: 10.1016/s0005-7967(96)00054-x. [DOI] [PubMed] [Google Scholar]
- Anderson ER, Hope DA. The relationship among social phobia, objective and perceived physiological reactivity, and anxiety sensitivity in an adolescent population. Journal Of Anxiety Disorders. 2009;23(1):18–26. doi: 10.1016/j.janxdis.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong T, Olatunji BO. Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clinical psychology review. 2012;32(8):704–723. doi: 10.1016/j.cpr.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Holoshitz Y, Eldar S, Frenkel TI, Muller D, Charney DS, … Wald I. Life-threatening danger and suppression of attention bias to threat. American Journal of Psychiatry. 2010;167:694–698. doi: 10.1176/appi.ajp.2009.09070956. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, Van Ijzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta- analytic study. Psychological bulletin. 2007;133(1):1. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beckwé M, Deroost N. Attentional biases in ruminators and worriers. Psychological Research. 2015:1–11. doi: 10.1007/s00426-015-0703-8. [DOI] [PubMed] [Google Scholar]
- Berle D, Starcevic V, Moses K, Hannan A, Milicevic D, Sammut P. Preliminary validation of an ultra-brief version of the Penn State Worry Questionnaire. Clinical psychology & psychotherapy. 2011;18(4):339–346. doi: 10.1002/cpp.724. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Alcaine OM, Behar E. Avoidance theory of worry and generalized anxiety disorder. In: Heimberg R, Turk C, Mennin D, editors. Generalized anxiety disorder: advances in research and practice. New York, NY, US: Guilford Press; 2004. pp. 77–108. [Google Scholar]
- Bower GH. Mood and memory. American psychologist. 1981;36(2):129–148. doi: 10.1037//0003-066x.36.2.129. [DOI] [PubMed] [Google Scholar]
- Calvo MG, Avero P. Time course of attentional bias to emotional scenes in anxiety: Gaze direction and duration. Cognition & Emotion. 2005;19(3):433–451. doi: 10.1080/02699930441000157. [DOI] [PubMed] [Google Scholar]
- Christiansen P, Mansfield R, Duckworth J, Field M, Jones A. Internal reliability of the alcohol-related visual probe task is increased by utilising personalised stimuli and eye-tracking. Drug and alcohol dependence. 2015;155:170–174. doi: 10.1016/j.drugalcdep.2015.07.672. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Bacon AK, Williams NL. Phenomenological characteristics of attentional biases towards threat: A critical review. Cognitive therapy and research. 2009;33(2):221–234. doi: 10.1007/s10608-007-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Koster EH. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical psychology review. 2010;30(2):203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish T, Watts FN. Biases of attention and memory in disorders of anxiety and depression. Clinical Psychology Review. 1990;10(5):589–604. [Google Scholar]
- Duque A, Sanchez A, Vazquez C. Gaze-fixation and pupil dilation in the processing of emotional faces: The role of rumination. Cognition & Emotion. 2014;28(8):1347–66. doi: 10.1080/02699931.2014.881327. [DOI] [PubMed] [Google Scholar]
- Ellenbogen MA, Schwartzman AE, Stewart J, Walker CD. Stress and selective attention: The interplay of mood, cortisol levels, and emotional information processing. Psychophysiology. 2002;39(6):723–732. doi: 10.1111/1469-8986.3960723. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7(2):336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Field M, Christiansen P. Commentary on, ‘Internal reliability of measures of substance-related cognitive bias’. Drug and alcohol dependence. 2012;124(3):189–190. doi: 10.1016/j.drugalcdep.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Field M, Powell H. Stress increases attentional bias for alcohol cues in social drinkers who drink to cope. Alcohol and Alcoholism. 2007;42(6):560–566. doi: 10.1093/alcalc/agm064. [DOI] [PubMed] [Google Scholar]
- Field M, Quigley M. Mild stress increases attentional bias in social drinkers who drink to cope: a replication and extension. Experimental and clinical psychopharmacology. 2009;17(5):312. doi: 10.1037/a0017090. [DOI] [PubMed] [Google Scholar]
- Ford BQ, Tamir M, Brunyé TT, Shirer WR, Mahoney CR, Taylor HA. Keeping your eyes on the prize anger and visual attention to threats and rewards. Psychological Science. 2010;21(8):1098–1105. doi: 10.1177/0956797610375450. [DOI] [PubMed] [Google Scholar]
- Frayn M, Sears CR, von Ranson KM. A sad mood increases attention to unhealthy food images in women with food addiction. Appetite. 2016;100:55–63. doi: 10.1016/j.appet.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Garner M, Mogg K, Bradley BP. Orienting and maintenance of gaze to facial expressions in social anxiety. Journal of Abnormal Psychology. 2006;115(4):760. doi: 10.1037/0021-843X.115.4.760. [DOI] [PubMed] [Google Scholar]
- Garson GD. Hierarchical Linear Modeling: Guide and Applications. Thousand Oaks, CA: Sage Publications, Inc; 2012. [Google Scholar]
- Gibb BE, McGeary JE, Beevers CG. Attentional biases to emotional stimuli: Key components of the RDoC constructs of sustained threat and loss. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2016;171(1):65–80. doi: 10.1002/ajmg.b.32383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin J, Deschênes SS, Dugas MJ. Respiratory sinus arrhythmia during worry forecasts stress-related increases in psychological distress. Stress: The International Journal On The Biology Of Stress. 2014;17(5):416–422. doi: 10.3109/10253890.2014.949666. [DOI] [PubMed] [Google Scholar]
- Graham R, Hoover A, Ceballos NA, Komogortsev O. Body mass index moderates gaze orienting biases and pupil diameter to high and low calorie food images. Appetite. 2011;56(3):577–586. doi: 10.1016/j.appet.2011.01.029. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the United States: how did it change between 1990 and 2000? Journal of clinical psychiatry. 2003;64(12):1465– 1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- Gyollai ÁG, Simor PÉ, Köteles FE, Demetrovics ZS. Psychometric properties of the Hungarian version of the original and the short form of the Positive and Negative Affect Schedule (PANAS) Neuropsychopharmacologia Hungarica. 2011;13(2):73–79. [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Olvet DM. Event-Related Potentials, Emotion, and Emotion Regulation: An Integrative Review. Developmental Neuropsychology. 2010;35(2):129–155. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological bulletin. 2011;137(6):940. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Toner K, Goren D, Wilson HR. Looking while unhappy mood- congruent gaze in young adults, positive gaze in older adults. Psychological Science. 2008;19(9):848–853. doi: 10.1111/j.1467-9280.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellough JL, Beevers CG, Ellis AJ, Wells TT. Time course of selective attention in clinically depressed young adults: An eye tracking study. Behaviour research and therapy. 2008;46(11):1238–1243. doi: 10.1016/j.brat.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International journal of methods in psychiatric research. 2012;21(3):169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Greenberg PE. The economic burden of anxiety and stress disorders. Neuropsychopharmacology: The fifth generation of progress. 2002;67:982–992. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kimble MO, Fleming K, Bandy C, Kim J, Zambetti A. Eye tracking and visual attention to threatening stimuli in veterans of the Iraq war. Journal Of Anxiety Disorders. 2010;24(3):293–299. doi: 10.1016/j.janxdis.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuckertz JM, Gildebrant E, Liliequist B, Karlstrom P, Vappling C, Bodlund O, Stenlund T, Hofmann SG, Andersson G, Amir N, Carlbring P. Moderation and mediation of the effect of attention training in social anxiety disorder. Behaviour Research and Therapy. 2014;53:30–40. doi: 10.1016/j.brat.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Lissek Shmuel, Kaczkurkin Antonia N, Rabin Stephanie, Geraci Marilla, Pine Daniel S, Grillon Christian. Generalized Anxiety Disorder is associated with overgeneralization of classically conditioned fear. Biological Psychiatry. 2014;75(11):909– 915. doi: 10.1016/j.biopsych.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. Psychology section. Karolinska Institutet; 1998. The Karolinska Directed Emotional Faces - KDEF, CD ROM from Department of Clinical Neuroscience. [Google Scholar]
- Macatee RJ, Albanese BJ, Allan NP, Schmidt NB, Cougle JR. Distress intolerance as a moderator of the relationship between daily stressors and affective symptoms: Tests of incremental and prospective relationships. Journal of Affective Disorders. 2016;206:125–132. doi: 10.1016/j.jad.2016.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatee RJ, Capron DW, Guthrie W, Schmidt NB, Cougle JR. Distress Tolerance and Pathological Worry: Tests of Incremental and Prospective Relationships. Behavior therapy. 2015;46(4):449–462. doi: 10.1016/j.beth.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Mackinnon A, Jorm AF, Christensen H, Korten AE, Jacomb PA, Rodgers B. A short form of the Positive and Negative Affect Schedule: Evaluation of factorial validity and invariance across demographic variables in a community sample. Personality and Individual Differences. 1999;27:405–416. [Google Scholar]
- Manor BR, Gordon E. Defining the temporal threshold for ocular fixation in free-viewing visuocognitive tasks. Journal of neuroscience methods. 2003;128(1):85–93. doi: 10.1016/s0165-0270(03)00151-1. [DOI] [PubMed] [Google Scholar]
- Mansell W, Ehlers A, Clark D, Chen YP. Attention to positive and negative social-evaluative words: Investigating the effects of social anxiety, trait anxiety and social threat. Anxiety, Stress & Coping. 2002;15(1):19–29. [Google Scholar]
- Mathôt S, Schreij D, Theeuwes J. OpenSesame: An open-source, graphical experiment builder for the social sciences. Behavior research methods. 2012;44(2):314–324. doi: 10.3758/s13428-011-0168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy PM, Watson H, Watkins ER, Nathan P. The relationship between worry, rumination, and comorbidity: Evidence for repetitive negative thinking as a transdiagnostic construct. Journal of Affective Disorders. 2013;151(1):313–320. doi: 10.1016/j.jad.2013.06.014. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Otto MW. Refining the measurement of distress intolerance. Behavior therapy. 2012;43(3):641–651. doi: 10.1016/j.beth.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennin DS, Holaway RM, Fresco DM, Moore MT, Heimberg RG. Delineating components of emotion and its dysregulation in anxiety and mood psychopathology. Behavior Therapy. 2007;38(3):284–302. doi: 10.1016/j.beth.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Mennin DS, McLaughlin KA, Flanagan TJ. Emotion regulation deficits in generalized anxiety disorder, social anxiety disorder, and their co-occurrence. Journal of anxiety disorders. 2009;23(7):866–871. doi: 10.1016/j.janxdis.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkins B, Notebaert L, MacLeod C, Clarke PJF. The Potential Benefits of Targeted Attentional Bias Modification on Cognitive Arousal and Sleep Quality in Worry-Related Sleep Disturbance. Clinical Psychological Science in press. [Google Scholar]
- Moffitt Terrie E, Capsi Avshalom, Harrington Honalee, Milne Barry J, Melchior Maria, Goldberg David, Poulton Richie. Generalized anxiety disorder and depression: childhood risk factors in a birth cohort followed to age 32. Psychological Medicine. 2007;37:441–452. doi: 10.1017/S0033291706009640. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley B, Miles F, Dixon Time course of attentional bias for threat scenes: Testing the vigilance-avoidance hypothesis. Cognition and Emotion. 2004;18(5):689–700. [Google Scholar]
- Mogg K, Millar N, Bradley BP. Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. Journal of abnormal psychology. 2000;109(4):695–704. doi: 10.1037//0021-843x.109.4.695. [DOI] [PubMed] [Google Scholar]
- Mogoase C, David D, Koster EHW. Clinical Efficacy of Attentional Bias Modification Procedures: An Updated Meta-Analysis. Journal of Clinical Psychology. 2014;70(12):1133–57. doi: 10.1002/jclp.22081. [DOI] [PubMed] [Google Scholar]
- Mohlman J, Price RB, Vietri J. Attentional bias in older adults: effects of generalized anxiety disorder and cognitive behavior therapy. Journal of anxiety disorders. 2013;27(6):585–591. doi: 10.1016/j.janxdis.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AL, Purdon C, Quigley L, Carriere J, Smilek D. Distinguishing the roles of trait and state anxiety on the nature of anxiety-related attentional biases to threat using a free viewing eye movement paradigm. Cognition and Emotion. 2015;29(3):504–526. doi: 10.1080/02699931.2014.922460. [DOI] [PubMed] [Google Scholar]
- Nyström M, Andersson R, Holmqvist K, Van De Weijer J. The influence of calibration method and eye physiology on eyetracking data quality. Behavior research methods. 2013;45(1):272–288. doi: 10.3758/s13428-012-0247-4. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;32:437–448. [Google Scholar]
- Price RB, Kuckertz JM, Siegle GJ, Ladouceur CD, Silk JS, Ryan ND, Dahl RE, Amir N. Empirical recommendations for improving the stability of the dot- probe task in clinical research. Psychological Assessment. 2015;27(2):365–76. doi: 10.1037/pas0000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Siegle GJ, Silk JS, Ladouceur C, McFarland A, Dahl RE, Ryan ND. Sustained neural alterations in anxious youth performing an attentional bias task: a pupilometry study. Depression and anxiety. 2013;30(1):22–30. doi: 10.1002/da.21966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley L, Nelson AL, Carriere J, Smilek D, Purdon C. The effects of trait and state anxiety on attention to emotional images: An eye-tracking study. Cognition & emotion. 2012;26(8):1390–1411. doi: 10.1080/02699931.2012.662892. [DOI] [PubMed] [Google Scholar]
- Ramshur JT. Doctoral dissertation, Master’s thesis. The University of Memphis; Memphis, TN: 2010. Design, evaluation, and application of heart rate variability software (HRVAS) Master’s thesis. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models. 2. Thousand Oaks: Sage Publications; 2002. [Google Scholar]
- Rodebaugh TL, Scullin RB, Langer JK, Dixon DJ, Huppert JD, Bernstein A, … Lenze EJ. Unreliability as a threat to understanding psychopathology: The cautionary tale of attentional bias. Journal of Abnormal Psychology. 2016;125(6):840. doi: 10.1037/abn0000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Vanderhasselt MA, Baeken C, De Raedt R. Effects of tDCS over the right DLPFC on attentional disengagement from positive and negative faces: An eye-tracking study. Cognitive, Affective, & Behavioral Neuroscience. 2016:1–12. doi: 10.3758/s13415-016-0450-3. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Vazquez C. Prototypicality and intensity of emotional faces using an anchor-point method. Spanish Journal of Psychology. 2013;16:E7, 1–11. doi: 10.1017/sjp.2013.9. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Vazquez C, Gomez D, Joormann J. Gaze-fixation to happy faces predicts mood repair after a negative mood induction. Emotion. 2014;14(1):85. doi: 10.1037/a0034500. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Vazquez C, Marker C, LeMoult J, Joormann J. Attentional disengagement predicts stress recovery in depression: An eye-tracking study. Journal Of Abnormal Psychology. 2013;122(2):303–313. doi: 10.1037/a0031529. [DOI] [PubMed] [Google Scholar]
- Sass SM, Evans TC, Xiong K, Mirghassemi F, Tran H. Attention training to pleasant stimuli in anxiety. Biological psychology. doi: 10.1016/j.biopsycho.2016.03.003. in press. [DOI] [PubMed] [Google Scholar]
- Shafqat K, Pal SK, Kyriacou PA. Evaluation of two detrending techniques for application in heart rate variability. Proceedings of the 29th Annual International Conference of the IEEE EMBS, Cite Internationale; Lyon, France. 2007. [DOI] [PubMed] [Google Scholar]
- SR Research Ltd. EyeLink 1000 User Manual (version 1.5.2) 2005–2010. [Google Scholar]
- Turk CL, Heimberg RG, Luterek JA, Mennin DS, Fresco DM. Emotion dysregulation in generalized anxiety disorder: A comparison with social anxiety disorder. Cognitive Therapy and Research. 2005;29(1):89–106. [Google Scholar]
- Van Bockstaele B, Verschuere B, Tibboel H, De Houwer J, Crombez G, Koster EH. A review of current evidence for the causal impact of attentional bias on fear and anxiety. Psychological Bulletin. 2014;140(3):682. doi: 10.1037/a0034834. [DOI] [PubMed] [Google Scholar]
- Waechter S, Nelson AL, Wright C, Hyatt A, Oakman J. Measuring attentional bias to threat: Reliability of dot probe and eye movement indices. Cognitive Therapy and Research. 2014;38(3):313–333. [Google Scholar]
- Wald I, Bitton S, Levi O, Zusmanovich S, Fruchter E, Ginat K, Bar-Haim Y. Acute Delivery of Attention Bias Modification Training (ABMT) Moderates the Association between Combat Exposure and Posttraumatic Symptoms: A Feasibility Study. Biological psychology. doi: 10.1016/j.biopsycho.2016.01.005. in press. [DOI] [PubMed] [Google Scholar]
- Wald I, Lubin G, Holoshitz Y, Muller D, Fruchter E, Pine DS, … Bar-Haim Y. Battlefield-like stress following simulated combat and suppression of attention bias to threat. Psychological medicine. 2011b;41(04):699–707. doi: 10.1017/S0033291710002308. [DOI] [PubMed] [Google Scholar]
- Wald I, Shechner T, Bitton S, Holoshitz Y, Charney DS, Muller D, … Bar-Haim Y. Attention bias away from threat during life threatening danger predicts PTSD symptoms at one-year follow-up. Depression and anxiety. 2011a;28(5):406–411. doi: 10.1002/da.20808. [DOI] [PubMed] [Google Scholar]
- Waters AM, Lipp OV, Spence SH. Attentional bias toward fear-related stimuli: An investigation with nonselected children and adults and children with anxiety disorders. Journal of experimental child psychology. 2004;89(4):320–337. doi: 10.1016/j.jecp.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Stuart S. Hierarchical structures of affect and psychopathology and their implications for the classification of emotional disorders. Depression and Anxiety. 2008;25(4):282–288. doi: 10.1002/da.20496. [DOI] [PubMed] [Google Scholar]
- Waugh CE, Panage S, Mendes WB, Gotlib IH. Cardiovascular and affective recovery from anticipatory threat. Biological Psychology. 2010;84(2):169–175. doi: 10.1016/j.biopsycho.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, … Burstein R. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. The Lancet. 2013;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Wieser MJ, Pauli P, Weyers P, Alpers GW, Mühlberger A. Fear of negative evaluation and the hypervigilance-avoidance hypothesis: an eye-tracking study. Journal Of Neural Transmission. 2009;116(6):717–723. doi: 10.1007/s00702-008-0101-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.