Summary
Oral probiotic administration has been advocated for treatment and prevention of a diverse range of disorders. This study was undertaken to evaluate the effect of probiotic supplementation on outcome of pediatric post-burn patients. Forty thermally-injured pediatric patients with total body surface burns between 20-50% and depth between 5-10% were randomized in a prospective, double-blind, controlled clinical trial into two even groups: probiotic group (n=20), who received probiotic preparations, and placebo control group (n=20). Clinical outcomes, including GIT tolerance, incidence of infection, need for grafting, length of hospital stay and mortality were recorded. Laboratory measurements of serum CRP, serum albumin, serum IgA and total lymphocyte count were done upon admission and on days 4, 7 and 14. There were no significant differences between the groups regarding age (3.67 ± 0.67 vs. 3.56 ± 0.73), sex, %BSA (34.5 ± 1.96 vs. 33.9 ± 1.82) and %deep burns (6.95 ± 0.34 vs. 7.25 ± 0.39). Frequency of diarrhea (3 vs. 9), need for grafting (2 vs. 8) and length of hospital stay (17.25 ± 0.5 days vs. 21.9 ± 2.2 days) were significantly lower in the probiotic group (p=0.038, p=0.028 and p=0.044, respectively). A trend towards a decrease in incidence of infections (7 vs. 12) was noted in the probiotic group (p=0.113). There was no mortality in our series. There was improvement in the patients’ overall outcome related to wound healing and length of hospital stay following the use of probiotics. However, their effects on infectious morbidity and mortality remain unclear and require further investigation.
Keywords: probiotics, thermal burn, pediatric patients
Abstract
L’administration orale de probiotiques a été évoquée pour la prévention et le traitement de pathologies variées. Cette étude a été réalisée pour évaluer l’effet d’une supplémentation probiotique sur l’évolution d’enfants brûlés. Quarante enfants brûlés sur une surface de 20 à 50% (5 à 10% de profond) ont été inclus dans une étude prospective randomisée et répartis en 2 groupes de 20, l’un recevant une préparation probiotique (C), l’autre un placebo (T). Le devenir, la tolérance digestive, l’incidence des infections, la nécessité de greffes, la mortalité et la durée d’hospitalisation ont été colligés. Une mesure de la CRP, de l’albumine sérique, des IgA et un compte lymphocytaire étaient réalisés à l’admission et à J4, 7 et 14. Les groupes étaient comparables en termes d’âge (3,67+/-0,67 ans VS 3,56+/-0,73), de sexe, de surface brûlée (34,5+/-1,96% VS 33,9+/-1,82%) et de pourcentage profondément atteint (6,95+/-0,34% VS 7,25+/-0,39). L’occurrence de diarrhée (3 VS 9), la nécessité de greffe (2 VS 8), la durée de séjour (17,25+/-0,5 jours VS 21,9+/-2,2) étaient significativement moindres dans le groupe C (p=0,038 ; 0,028 et 0,044 respectivement). Dans ce même groupe C, on notait une tendance (p=0,013) vers une diminution des infections (7 VS 12). Aucun patient n’est décédé. Nous avons observé une amélioration de la cicatrisation et une réduction de la durée d’hospitalisation en cas d’utilisation de probiotiques. Toutefois, leurs effets sur la morbidité infectieuse et la mortalité restent flous et nécessitent d’autres études.
Introduction
Burn injuries are the 3rd leading cause of death resulting from trauma in children, following road traffic accidents and drowning.1 These injuries produce extensive skin barrier disruption, which creates novel sites for bacterial colonization2 and contributes to an immunosuppressive state, making the burn patients vulnerable to infectious complications.3 Moreover, the dramatic increase of gut permeability with disturbance of the intestinal flora and translocation of the microorganisms and/or their products from gastrointestinal tract to extra-intestinal sites seems to contribute to systemic sepsis and associated multiple organ failure after severe burns.4 Various treatment modalities were investigated to prevent the occurrence of bacterial translocation and enhance immune function after thermal injury.5 Administration of an enteral diet enriched with immunomodulatory compounds, such as glutamine and omega-3 fatty acids, has been shown to reduce wound infection rates and length of hospital stay in critically ill patients.6,7 One such treatment involves a per os supplement of probiotics. Probiotics are defined according to the World Health Organization (WHO) as “live micro-organisms which, when administered in adequate amounts, confer a health benefit on the host”.8 Major strains of probiotics include Lactobacillus and Bifidobacterium species.2
These bacteria can maintain gut equilibrium and prevent bacterial translocation by several mechanisms, including: i. maintenance of the gut barrier function; ii. protection of the sites of bacterial invasion from colonization by pathogenic agents; iii. competition with pathogenic micro-organisms for nutritional requirements; iv. increase of intestinal acidity, motility and mucin; v. inhibition of the growth of pathogenic bacteria through production of organic acids and bacteriocin-like substances.9 Lactic acid bacteria (LAB) were reported to have a direct stimulatory effect on the cells of the innate immune system that exert adjuvant activity at the intestinal mucosal surface and improve phagocytosis by increasing the proportion of natural killer (NK) cells, macrophages and lymphocytes.10
Oral probiotic administration has been advocated for the treatment and prevention of a diverse range of disorders, such as antibiotic-associated diarrhea, acute infantile diarrhea, necrotizing entercolitis in preterm infants and inflammatory bowel disease (IBD).11Moreover, a benefit was shown after liver transplantation, acute pancreatitis and major abdominal surgery.12 Augmentation of the gut barrier with the use of probiotics has been observed in burn rat models.4,9,13 Nevertheless, the probiotic effect has not been thoroughly examined in the clinical burn setting. Therefore, this study was undertaken to evaluate the effect of probiotic supplementation on the outcome of pediatric patients after thermal burn.
Materials and methods
This prospective study included 40 thermally-injured pediatric patients, of both sexes, aged between 1-14 years, who were admitted to the Burn Unit of the Plastic Surgery Department, Tanta University Hospital between May 2014 and May 2016, with acute burns affecting 20-50% of total body surface area (TBSA), including deep areas between 5 and 10% of TBSA. Patients admitted after the first 24 hours following their injury, those with previous or current gastrointestinal diseases, or with chronic diseases such as diabetes, as well as those with burns of the upper respiratory tract or inhalation injury, were excluded from the study.
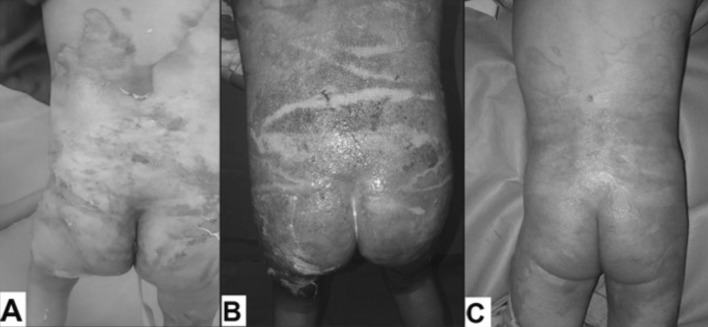
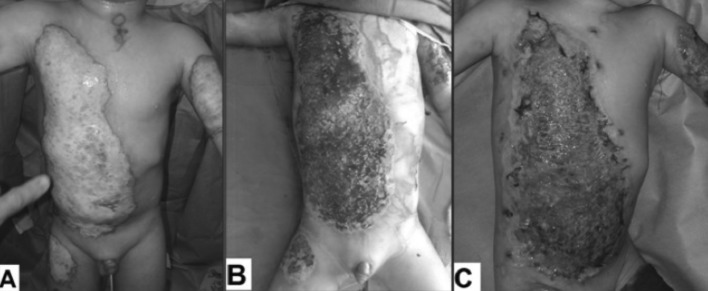
After approval from the University Ethical Committee and upon completion of informed consent by the patients’ parents or their relatives, all subjects underwent detailed clinical evaluation as well as immediate resuscitation according to the Parkland formula. Early enteral feeding was adapted by us, with a caloric goal of 35 kcal/kg/day and 20% of the calories were given as protein; the feeding regimen contained no fibre or fermented foodstuffs. All burn wounds were dressed daily with topical silver sulphadiazine (SSD) cream, which was covered with low adherent dressing and tubular net bandage over the dressing for support. After two weeks, the wounds were reevaluated and those with early signs of healing, including macroscopic epithelialization, advancing margin, decreased bleeding and exudates and less pain continued to be dressed (Fig. 1) and the others were prepared for grafting (Fig. 2).
Fig. 1. A 5-year-old male with scald burn affecting 40% TBSA, including deep areas 6%. (A) 2nd post-burn day. (B) 14th post-burn day showing acroscopic epithelialization and advancing margin. (C) 22nd post-burn day showing complete burn wound healing.
Fig. 2. A 2.5-year-old male with scald burn affecting 34% TBSA, including deep areas 8%. (A) 3rd post-burn day. (B) 18th post-burn day showing hypertrophic granulation tissue. (C) 24th post-burn day showing complete graft take.
All patients were randomly distributed into two equal groups according to sequence of hospital admission: the probiotic group (n=20) received probiotic preparation twice daily (Lacteol Forte; Rameda, Egypt) as sachets containing powder with 10 billion colony forming units (CFU) of Lactobacillus fermentum and Lactobacillus delbruekii. The formulas were disintegrated in 50 mL of fresh water, and the 20 patients in the control group received starch as the formulation. Clinical outcomes, including gastrointestinal tract tolerance (incidence of vomiting, flatulence, diarrhea and constipation), incidence of infection (burn-related or systemic sepsis), need for grafting, length of hospital stay and mortality were recorded and compared between the groups. Wound and blood cultures were obtained based on clinical judgment. Laboratory monitoring of serum C-reactive protein, serum albumin, serum IgA and total lymphocyte count were carried out on admission and on days 4, 7 and 14 afterwards. The data gathered for statistical analysis were presented as means and standard error of the means. Chi-square test and Student’s t-test were used for comparative analysis. The level of statistical significance was set at a P value of <0.05. A statistical trend was distinguished as a p≤ 0.30
Results
In terms of demographics, no differences were noted between the treatment and control groups. As shown in Table I, both groups were similar in age, sex, type of burns, total burn size and burn depth.
Table I. Demographic data (Mean ± SEM).
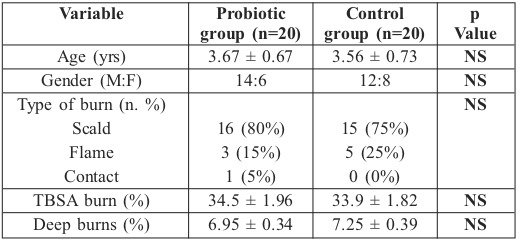
Table II. Demographic data (Mean ± SEM).
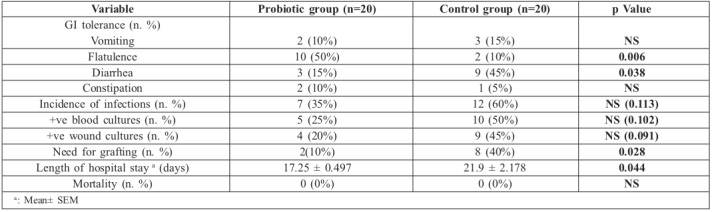
Table III. Demographic data (Mean ± SEM).
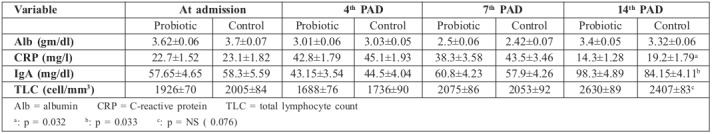
As regards clinical evaluation, frequency of vomiting and constipation was similar between the two groups; however, a significant increase in the frequency of flatulence (p= 0.006) and a significant decrease in the frequency of diarrhea (p=0.038) were observed in the probiotic group. Furthermore, a trend towards diminished incidence of infections and the number of positive blood and swab cultures was noted in the probiotic group compared to the control group (p=0.113, p=0.102 and p=0.091 respectively). There was improvement in overall wound healing in the probiotic group as evidenced by the significant decrease in the need for grafting in this group (p= 0.028). There was complete graft take in the probiotic group compared to partial graft loss in one case in the control group (Fig. 3). Moreover, when grafting was not done, there was a significant decrease in the time needed for complete burn wound healing in the probiotic group (16.5 ± 0.23 days versus 20.7 ± 0.51 days) (p=0.048) and the outcomes were satisfactory in all patients except in 3 cases in the probiotic group and 2 cases in the control group that developed hypertrophic scars and were treated conservatively with pressure garments. Length of hospital stay was significantly lower in the probiotic group compared to the control group (p=0.044), as shown in Table II. Importantly, there was no mortality in our series.
Fig. 3. Grafting phase outcome.
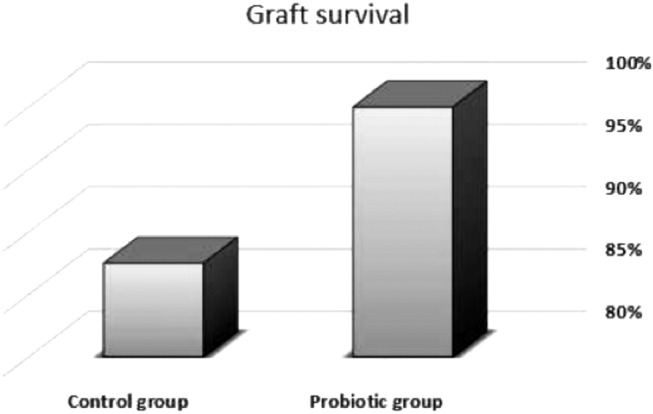
Table III shows that none of the measured laboratory variables differed at admission and at days 4 and 7 post-admission. At day 14 post-admission, serum albumin levels were similar in both groups, serum C-reactive protein (CRP) decreased significantly in the probiotic group (p=0.032), serum IgA increased significantly in the probiotic group (p=0.033) and a trend towards an increase in total lymphocyte counts was observed in the treatment group (p= 0.076).
Discussion
Multiple studies have evaluated probiotic consumption in humans and concluded that probiotics are safe and can confer health benefits, such as disease treatment and prevention; however, there is a lack of evidence regarding the benefits of probiotics for patients with critical illness, such as burns. In our study, we aimed to evaluate the effect of probiotic supplementation on the outcome of pediatric patients after thermal burn.
Forty acutely-burned pediatric patients were included in this study. Subjects were randomly allocated into two equal groups. Demographically, there was no significant difference between the two groups, but we noticed that scalds were the most frequent type of pediatric burn and that males under the age of two were most likely to be scalded. This could be attributed to their limited motor and cognitive development at this age. Our findings are consistent with other studies.14,15
We adopted the enteral route of feeding, which is recommended by Rousseau et al.16 for burn patients as it decreases the acute phase response (APR), enhances gut barrier function and reduces the incidence of septic complications.
This study demonstrated a significant increase in the frequency of flatulence in the probiotic group (P= 0.006), which was expected as the package insert warns of more flatulence during the early days of treatment. Importantly, we observed that the frequency of diarrhea decreased significantly in the probiotic group (p =0.038), which is consistent with the results of Schlotterer et al.,17 who studied the effect of oral probiotics on burn patients, and Frohmader et al.,18 who described the effectiveness of probiotics in reducing liquid stool frequency in critically ill patients. Similar benefits of probiotic therapy were reported in a meta-analysis that evaluated 25 randomized controlled trials and concluded that probiotics can reduce the frequency of diarrhea and the incidence of Colostridium difficile infection associated with antibiotic use.19 However, another recent report on the impact of probiotics in critically ill patients showed they had no effect on incidence of diarrhea.20 One hypothesis for this could be that probiotic effect depends on the type of strain and the site of action; for example, strains acting primarily at the small intestine confer different effects than those which act primarily on the large intestine, with the greatest impact on diarrhea expected to occur from the colonic strains.
In our study, there was a trend towards a reduction in the incidence of infections in the probiotic group (p=0.113) and there was no mortality in either group. Our data confirmed the findings of Morrow et al.21 who evaluated the use of probiotics in ICU patients, and Kotzampassi et al.,22 who investigated the effect of synbiotics on post-traumatic critically ill patients. They found that there was a significant reduction in rate of infection, incidence of ventilator associated pneumonia (VAP) and mortality. In another study Jebur et al.2 found that Lactobacillus acidophilus at a concentration of 1×108 cells/ml (in vitro) can inhibit the growth of all gram +ve and gram –ve pathogenic bacteria that can infect burn wounds. Contrary to our results, Koren et al.23 in a retrospective cohort study to evaluate the effect of Lactobacillus supplements on sepsis in acute burn patients found that morbidity parameters were higher in the probiotic group; however, mortality was lower. This inverse trend could be attributed to the fact that the control group had a higher mortality rate, with fewer patients surviving long enough to develop septic complications. Also, Olguin et al.24 reported that prebiotic ingestion had no effect on the incidence of burn wound infection and on gut barrier function. This lack of effect could be explained by the use of large doses of antibiotics that inhibit the growth of probiotics, even after stimulation with prebiotics. Therefore, supplements of probiotics or synbiotics may be an ideal solution for these patients.
We noticed that there was a significant decrease in need for grafting and duration of hospital stay in the probiotic group compared to the control group (p=0.028 and p=0.044 respectively). Similar to our study and findings, Mayes et al.25 examined the safety and efficacy of probiotics in pediatric post-burn patients. They observed that there was a trend toward lower requirement for graft procedures in the treatment group, and wound length of stay was shortened with probiotics. In an experimental study, Valdez et al.26 tested the activity of Lactobacillus plantarum on burn wounds infected with Pseudomonas aeruginosa and noted that there was an improvement in the wound repair process and enhancement of phagocytosis of Pseudomonas aeruginosa by the tissue phagocytes at 10 days. Also Rahimzadeh et al.27 recorded enhancement of the burn wound healing process after using probiotic gel. In this study, there was a significant decrease in serum Creactive protein (CRP) at day 14 post-admission in the probiotic group (p=0.032). This finding is similar to Lu et al.28 who studied the effect of early enteral feeding supplemented with synbiotics in severely burned patients, and Sanaie et al.29 who evaluated the effect of probiotics on inflammatory markers in ICU patients. They concluded that probiotics can possibly reduce inflammation and inflammatory stress response by decreasing the release of pro-inflammatory cytokines. Unlike our results, McNaught et al.30 found no significant difference in CRP levels, septic morbidity or days in the ICU. Our study witnessed a significant rise in serum IgA and a trend towards increased total lymphocyte counts at day 14 post admission in the probiotic group (p=0.033 and p=0.076 respectively). Our data are consistent with those of Alberda et al.31 who studied the effect of synbiotics on ICU patients and demonstrated a significant rise in IgA levels in the treatment group. They are also consistent with the findings of Wang et al.13 and Zhang et al.,32 who found that probiotics enhance the expression and the excretion of secretory IgA from the intestinal mucosa after burn. In another study Tahir et al.33 noticed that probiotics can improve systemic immune functions and provide healthy granulation tissues for better graft survival, in spite of the insignificant difference in septic complications between both groups.
Petrof et al.34 performed a meta-analysis of 23 randomized controlled trials of probiotics in critically ill patients. Fifteen were ICU patients, 4 had acute pancreatitis, 1 was a poly-traumatized patient, 1 had a head injury and 2 were burn patients. They concluded that probiotics can reduce overall infection rates, including ventilator associated pneumonia (VAP), and may affect ICU mortality. However, clinical heterogeneity preclude strong clinical recommendations, and further research is needed to determine if some strains and/or doses may have a better effect than others.
Conclusion
We can conclude that probiotic administration is safe to use in pediatric post burn patients. Probiotics can effectively enhance immune function, protect GIT mucosal integrity, improve wound healing and reduce length of hospital stay. However, their effects on infectious morbidity and mortality remain unclear and require further investigations and larger clinical trials to better understand the efficacy of probiotics.
References
- 1.National Vital Statistics System. Deaths. Final data for 1997. Centers for Disease Control and Prevention. 1999;47(19):1–105. [PubMed] [Google Scholar]
- 2.Jebur MS. Therapeutic efficacy of Lactobacillus acidophilus against some bacterial isolates burn wound cases. North Am J Med Sci. 2010;2:586–591. doi: 10.4297/najms.2010.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atiyeh BS, Al-Amm CA. Immunology of burn injury. An overview. Ann Burns Fire Disasters. 2001;14(2):78–84. [Google Scholar]
- 4.Gun F, Salman T, Gurler N. Effect of probiotic supplementation on bacterial translocation in thermal injury. Surg Today. 2005;35:760–764. doi: 10.1007/s00595-005-3023-6. [DOI] [PubMed] [Google Scholar]
- 5.Kurmis R, Parker A, Greenwood J. The use of immunonutrition in burn injury care: Where are we? J Burn Care Res. 2010;31:677–691. doi: 10.1097/BCR.0b013e3181eebf01. [DOI] [PubMed] [Google Scholar]
- 6.Marik PE, Zaloga GP. Immunonutrition in high-risk surgical patients: A systematic review and analysis of the literature. J Parenter Enteral Nutr. 2010;34:378–386. doi: 10.1177/0148607110362692. [DOI] [PubMed] [Google Scholar]
- 7.Mahmoud WH, Mostafa W, Abdel-Khalek AH. Effect of immuneenhancing diets on the outcomes of patients after major burns. Ann Burns Fire Disasters. 2014;27(4):192–196. [PMC free article] [PubMed] [Google Scholar]
- 8.Food and Health Agriculture Organization of United Nations and World Health Organization. Guidelines for the evaluation of probiotics in food. [August 31, 2009];Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. 2002 Available at: fttp://www.fao.org/es/esn/food/wgreport2.pdf . [Google Scholar]
- 9.Zhongtang Y, Guangxia X, Yongming Y. The role of bifidobacteria in gut barrier function after thermal injury in rats. J Trauma. 2006;61:650–657. doi: 10.1097/01.ta.0000196574.70614.27. [DOI] [PubMed] [Google Scholar]
- 10.Borchers AT, Selmi C, Meyers FJ. Probiotics and immunity. J Gastroenterol. 2009;44:26–46. doi: 10.1007/s00535-008-2296-0. [DOI] [PubMed] [Google Scholar]
- 11.Romeo J, Nova E, Warnberg J. Immunomodulatory effect of fibres, probiotics and synbiotics in different life-stages. Nutr Hosp. 2010;25(3):341–349. [PubMed] [Google Scholar]
- 12.Rayes N, Hansen S, Seehofer D. Early enteral supply of fiber and Lactobacilli versus conventional nutrition: a controlled trial in patients with major abdominal surgery. Nutrition. 2002;18(7-8):609–615. doi: 10.1016/s0899-9007(02)00811-0. [DOI] [PubMed] [Google Scholar]
- 13.Wang ZT, Yao YM, Xiao GX. Bifidobacterial supplement enhances the expression and excretion of intestinal sIgA in severely burned rats. Zhonghua Wai Ke Za Zhi. 2003;41:385–388. [PubMed] [Google Scholar]
- 14.Lin TM, Wang KH, Lai CS. Epidemiology of pediatric burn in southern Taiwan. Burns. 2005;31:182–187. doi: 10.1016/j.burns.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Drago DA. Kitchen scalds and thermal burns in children five years and younger. Pediatrics, 2005;155:10–16. doi: 10.1542/peds.2004-0249. [DOI] [PubMed] [Google Scholar]
- 16.Rousseau A, Losser M, Ichai C. ESPEN endorsed recommendations: Nutritional therapy in major burns. Clinical Nutrition. 2013;32:497–502. doi: 10.1016/j.clnu.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Schlotterer M, Bernasconi P, Lebreton F. Value of Saccharomyces boulardii in the digestive acceptability of continuous-flow enteral nutrition in burnt patients. Nutr Clin Metabol. 1987;1987:31–34. [Google Scholar]
- 18.Frohmader TJ, Chaboyer WP, Robertson IK. Decrease in frequency of liquid stool in enterally fed critically ill patients given the multispecies probiotic VSL#3: a pilot trial. Am J Crit Care. 2010;19:e1–e11. doi: 10.4037/ajcc2010976. [DOI] [PubMed] [Google Scholar]
- 19.McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101:812–822. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 20.Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: A metaanalysis of randomized controlled trials. Crit Care Med. 2010;38:954–962. doi: 10.1097/CCM.0b013e3181c8fe4b. [DOI] [PubMed] [Google Scholar]
- 21.Morrow LE, Kollef MH, Casale TB. Probiotic Prophylaxis of Ventilator associated Pneumonia: A Blinded, Randomized, Controlled Trial. Am J Respir Crit Care Med. 2010;182(8):1058–1064. doi: 10.1164/rccm.200912-1853OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A. Benefits of a synbiotic formula (Synbiotic 2000 Forte) in critically ill trauma patients: early results of a randomized controlled trial. World J Surg. 2006;30:1848–1855. doi: 10.1007/s00268-005-0653-1. [DOI] [PubMed] [Google Scholar]
- 23.Koren L, Gurfinkel R, Glezinger R. The effect of Lactobacillus bacteria supplement on sepsis and its complications in patients with acute burns. Burns. 2007;33:594–598. doi: 10.1016/j.burns.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Olguin F, Araya M, Hirsch S. Prebiotic ingestion does not improve gastrointestinal barrier function in burn patients. Burns. 2005;31(4):482–484. doi: 10.1016/j.burns.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 25.Mayes T, Gottschlich MM, James LE. Clinical safety and efficacy of probiotic administration following burn injury. J Burn Care Res. 2015;36(1):92–99. doi: 10.1097/BCR.0000000000000139. [DOI] [PubMed] [Google Scholar]
- 26.Valdéz JC, Peral MC, Rachid M. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: the potential use of probiotics in wound treatment. Clin Microbiol Infect. 2005;11(6):472–479. doi: 10.1111/j.1469-0691.2005.01142.x. [DOI] [PubMed] [Google Scholar]
- 27.Rahimzadeh g, Dolatabad ss, Rostami ff. Comparison of Two Types of Gels in Improving Burn Wound. Crescent J Med & Biol Sci. 2014;1(1):28–32. [Google Scholar]
- 28.Lu X, Han CM, Yu JX. Preliminary comparative study on the effects of early enteral supplementation of synbiotics on severely burned patients. Zhonghua Shao Shang Za Zhi. 2004;20:198–201. [PubMed] [Google Scholar]
- 29.Sanaie S, Ebrahimi-Mameghani M, Hamishehkar H. Effect of a multispecies probiotic on inflammatory markers in critically ill patients: A randomized, double-blind, placebo-controlled trial. J Res Med Sci. 2014;19(9):827–833. [PMC free article] [PubMed] [Google Scholar]
- 30.McNaught CE, Woodcock NP, Anderson AD. A prospective randomised trial of probiotics in critically ill patients. Clin Nutr. 2005;24:211–219. doi: 10.1016/j.clnu.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Alberda C, Gramlich L, Meddings J. Effects of probiotic therapy in critically ill patients: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2007;85:816–823. doi: 10.1093/ajcn/85.3.816. [DOI] [PubMed] [Google Scholar]
- 32.Zhang YP, Shi ZR. Effects of probiotics on the bacterial groups of intestinal and sIgA in severely burned rats. Chinese Journal of Microecology. 2004;16(5):257–259. [Google Scholar]
- 33.Tahir SM, Makhdoom A, Awan S. Role of Probiotics in the Management of Burns Patients. World J Med Sci. 2014;11(3):417–421. [Google Scholar]
- 34.Petrof EO, Dhaliwal R, Manzanares W. Probiotics in the critically ill: a systematic review of the randomized trial evidence. Crit Care Med. 2012;40(12):3290–3302. doi: 10.1097/CCM.0b013e318260cc33. [DOI] [PubMed] [Google Scholar]