Abstract
Parasitic diseases remain an unarguable public health problem worldwide. Liver fluke Clonorchis sinensis is a high risk pathogenic parasitic helminth which is endemic predominantly in Asian countries, including Korea, China, Taiwan, Vietnam, and the far eastern parts of Russia, and is still actively transmitted. According to the earlier 8th National Survey on the Prevalence of Intestinal Parasitic Infections in 2012, C. sinensis was revealed as the parasite with highest prevalence of 1.86% in general population among all parasite species surveyed in Korea. This fluke is now classified under one of the definite Group 1 human biological agents (carcinogens) by International Agency of Research on Cancer (IARC) along with two other parasites, Opisthorchis viverrini and Schistosoma haematobium. C. sinensis infestation is mainly linked to liver and biliary disorders, especially cholangiocarcinoma (CCA). For the purposes of this mini-review, we will only focus on C. sinensis and review pathogenesis and carcinogenesis of clonorchiasis, disease condition by C. sinensis infestation, and association between C. sinensis infestation and CCA. In this presentation, we briefly consider the current scientific status for progression of CCA by heavy C. sinensis infestation from the food-borne trematode and development of CCA.
Keywords: Cholangiocarcinoma, Clonorchiasis, Clonorchis sinensis, Liver cancer, Liver flukes
INTRODUCTION
Cholangiocarcinoma (CCA) with features of cholangiocyte differentiation is one of the main histological types of malignant tumors of biliary tract epithelia and is a relatively rare type of liver cancer (1). The only therapy for CCA is surgical operation or liver transplantation. Usually, CCA is diagnosed at advanced stages and is considered as an incurable and lethal cancer with poor survival rate of < 24 months (2). This intimidatory cancer develops in the epithelial cells which line the bile ducts and occur in the bile ducts within the liver (intrahepatic), the bile ducts just outside the liver (perihilar) and distal bile ducts. However, this rare tumor is exceptional in regions within Asia, including northeastern Thailand and many areas of southeastern Asia, where infestation with two liver flukes, Opisthorchis viverrini and Clonorchis sinensis is widespread, respectively (3–5). Due to higher prevalence of liver flukes (a common parasitic infestation) in these areas, there is a higher incidence of CCA (6–8). Therefore, infestations with the two liver flukes are now classified under definite Group 1 biological agents (carcinogens) by the International Agency of Research on Cancer (IARC) based on sufficient evidences in humans (3, 9). Nowadays, three helminth infestations by two food-borne liver flukes, O. viverrini and C. sinensis and Schistosoma haematobium associated with urinary bladder cancer, have been classified under definite group 1 carcinogens. Disease conditions caused by O. viverrini and C. sinensis infestations are referred to as opisthorchiasis and clonorchiasis, respectively. Although opisthorchiasis and clonorchiasis are the well-known main risk factors of CCA, chronic infection with hepatitis B and C viruses, liver cirrhosis, chronic non-alcoholic liver disease, obesity and hepatolithiasis (gallstones) are also the other minor known risk factors (10). In fact, the connection between CCA and these liver flukes has been the subject of clinical attention for more than 60 years (11, 12). As experimental and epidemiological evidences accumulated, C. sinensis infestation strongly implicated the detrimental etiology of CCA with pooled odds ratios between 4.5 and 6.1 (6, 10, 13–15). In this mini-review, we will limit our focus on the association between C. sinensis infestation and CCA by briefly summarizing the recent significant scientific progresses (for comprehensive review on O. viverrini please refer to work by Sripa B et al.).
LIFE CYCLE OF C. sinensis, SYMPTOMS, DIAGNOSIS AND EPIDEMIOLOGY
C. sinensis is a leaf-shaped slender digenetic trematode, measuring 15–20 mm in length and 3–4 mm in width belonging to class Trematoda, phylum Platyhelminthes. This oriental or Chinese liver fluke is the most pivotal species of food-borne zoonotic parasite in East Asia including Korea, China (except for northwestern regions), Taiwan, northern Vietnam and the far eastern part of Russia, where it is still actively transmitted (17). The life cycle of C. sinensis is characterized by an alternation of sexual and asexual reproductions in three different hosts, such as snails, fish and mammals (18, 19). Embryonated eggs laid by hermaphroditical adult worms are discharged in the biliary ducts and stool of a definite human host. The discharged eggs ingested by a suitable snail intermediate host release miracidia, which go through some developmental stages, such as sporocysts, rediae and cercariae in a regular sequence. The finally developed cercariae in the infected snail are shed into water. These larval stages in the snails reproduce asexually and this reproduction allows for an exponential multiplication of cercariae from a miracidium. After a short period of free-swimming time in water, the shed cercariae meet the 2nd intermediate cyprinid fish, invade the mucous skin and become encysted metacercariae in the subcutaneous tissues or muscles. When a definite mammal host including humans, cats, mink, badgers, rats and dogs eats insufficient cooked, salted, pickled, dried or smoked infested fish, metacercariae separates from the flesh through gastric juice digestion and excyst in the duodenum by a combined action of trypsin and cysteine proteases. Subsequently, the excysted flukes migrate to intrahepatic bile duct through the ampulla of Vater, develop into adult flukes and can dwell for up to 30 years. One worm in human host produces approximately 4000 eggs a day by sexual reproduction (20).
Despite several pathological changes, most of the patients with clonorchiasis in a manner similar to most of the human parasitic infestations have asymptomatic or mild non-specific symptoms except for increased frequency of palpable liver, such as asthenia, nausea, indigestion, headache, dizziness, vertigo, abdominal discomfort, diarrhea, or abdominal pain. Asymptomatic or mild non-specific symptoms may be reflected in the host-parasite relationship, which evolves intimately and progresses in a less harmful way to its host. However, based on few case reports, clinical manifestations caused by clonorchiasis have been mainly related to worm burden (20). Typical physical symptoms of C. sinensis infestation are jaundice, hepatomegaly and liver tenderness (19). Heavy and chronic C. sinensis infestation results in various complications in the liver and biliary systems, primarily cholelithiasis, cholangitis and cholecystitis (21). Growth retardation has been reported in children with heavy infestation. In addition, it is now widely acknowledged that C. sinensis infestation may be associated with CCA. Beyond pathogenesis induced by helminth, hygiene hypothesis and considerable investigations demonstrate sudden rise in epidemic in allergic diseases, such as asthma, anaphylaxis, allergic rhinitis and atopic dermatitis in developed countries; furthermore, this phenomenon has been reported to be lower in developing countries that show a high rate of helminth infestation (22–24). As shown in human and experimental animal models, helminthes are potent immune modulators and induce down T-cell responsiveness, which is partially due to modulation of dendritic cells (DCs) and macrophages (Mφ), and dampens allergic TH2 immune responses through CD4+CD25+Foxp3+ Treg cells. The suppression of airway inflammation in murine asthma model through treatment of C. sinensis-derived total protein is characterized by induction of CD4+CD25+Foxp3+ Treg cell development and modulation of DC functions (25), and a specific C. sinensis-derived antigen demonstrates suppressive skin inflammation through effective mast cell inhibition in allergic and inflammatory diseases (26). It is noteworthy that parasitic helminthes stimulate some regulatory mechanisms associated with suppression of development of allergies in human and animal models and helminthes are candidates for broader therapeutic application through immune modulation by helminthes (27), although no universal mechanism has yet been elucidated.
The standard diagnosis of clonorchiasis is usually established by microscopic examination of the stool for eggs. The formalin-ether sedimentation technique is known to be more reliable than the direct-smear method for detecting the eggs in stool (28). Although some serological ELISA screening methods for adult C. sinensis antigens are currently available for detection of antibodies, they are not reliably used due to their considerable cross-reactivity and low specificity (29, 30). Application of recombinant proteins for excretory-secretory products (ESPs) of C. sinensis has enhanced the specificity for diagnosis of clonorchiasis (31). Various DNA-based techniques have been developed for the specific detection of C. sinensis (32). Recently, clonorchiasis was commonly diagnosed incidentally during radiological screening, especially by ultrasonography of the abdomen for other purpose, in view of the fact that symptoms of C. sinensis infestation are nonspecific in most of the case (33). Praziquantel is a powerful and effective Clonorchis-cidal drug of choice. Recently, tribendimidine, a derivative of amidantel and a broad-spectrum anthelmintic agent, has been acknowledged as an effective and safe agent (34).
As mentioned above, liver fluke infestations occur in some Asian countries where people eat raw (salted, pickled, dried or smoked) or undercooked fish that are infested with these tiny parasite worms. In humans, these flukes dwell in the bile ducts and can cause bile duct cancer. The parasites closely related to risk of developing bile duct cancer are C. sinensis and O. viverrini. In case of C. sinensis, approximately 700 million people are at the risk of infestation and an estimated 35 million are infested with C. sinensis (35). In Korea, according to the most recent 8th National Survey on the Prevalence of Intestinal Parasitic Infections in 2012, C. sinensis was revealed as the parasite with highest prevalence of 1.86% in general population as compared to overall prevalence of 2.42% in 2004 (36, 37). In addition, the known endemic regions for C. sinensis, especially southern areas along Nakdong and Seomjin rivers, showed high incidence rates of CCA (10, 15, 38). According to 2012 survey data, 0.93 million people were estimated to be infested with clonorchiasis on Korea. However, C. sinensis infestation causes one fourth of CCA cases in the endemic area, approximately 10% of CCA cases are estimated to be due to infestation with C. sinensis, and estimated CCA relative risk has been continuously rising particularly in areas hyper-endemic for C. sinensis infestation. In China, where food-borne parasitic infestations are on a rapid rise, C. sinensis infestations have been reported in 27 of 34 provinces and currently the national average prevalence has increased by 75% when compared to the results from the first national survey in 2003 (12.49 million people estimated to be infested with C. sinensis with 0.58% prevalence) (39). From a comparative point of view, an enhanced vulnerability to CCA in patients with O. viverrini infestation has been reported in Thailand (4).
PATHOGENESIS AND CARCINOGENESIS
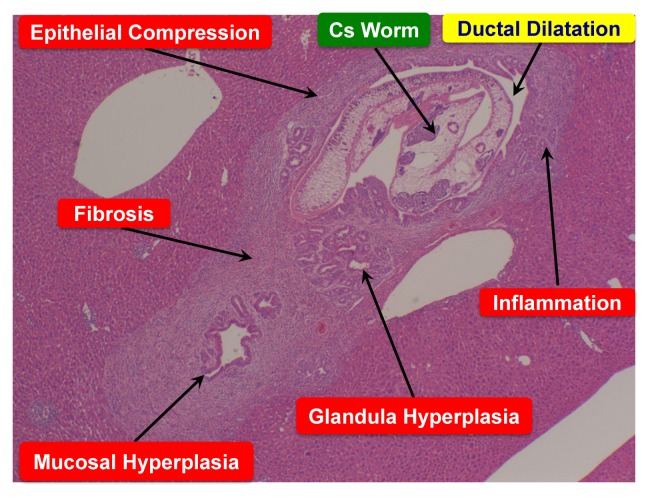
C. sinensis causes mechanical injury and inflammation at the environs of biliary tree due to fluke activities, metaplasia of mucin-producing cells in the mucosa, progressive periductal fibrosis and hyperplasia of epithelial cells (40, Fig. 1). The severity of these changes exhibits a tendency to correlate with the duration of fluke infestation, the worm burden, and the susceptibility of the host (41). The pathological changes and the adult flukes might be conducive as a nidus for bacterial infection and intrahepatic stone formation. In addition, the liver flukes secrete or excrete some metabolic products (so-called ESPs), which are highly immunogenic and may be toxic to or interact with the biliary epithelia to stimulate inflammation, promote proliferation and suppress apoptosis (42, 43). Thus, the histopathological changes originate from a combination of mechanical irritation caused by physical contact with infested worms and chemical irritation by their ESPs. Recently analyzed gene expression profiles of three developmental stages of C. sinensis might reflect the pathogenesis and carcinogenesis provoked by this liver fluke infestation (44).
Fig. 1.
Histopathological liver-section image of clonorchiasis (hematoxylin and eosin staining) at 4th week of C. sinensis post-infestation.
Although the molecular mechanism involved in the development of CCA are poorly elucidated in detail, it might be simply proposed as a multistep process: normal cholangiocytes → pathogen recognition → chronic inflammation → cell damage → reactive cell proliferation → genetic/epigenetic mutations → malignant cholangiocytes in regular sequence (45). Until date, C. sinensis-induced CCA is widely acceptable to be closely linked to chronic inflammation and oxidative stress pathways for creating feasible microenvironment conducive for initiation and promotion of CCA, involving a complex process of several separate mechanisms (4, 46, 47). For pathogen recognition, Toll-like receptors (TLRs) encompass distinctive capacity to sense the initial infection and are the most potent initiators of the inflammatory responses (48). However, prolonged inflammation through excessive production of inflammatory cytokines and chemokines via TLR-mediated signaling could be detrimental because it may cause host toxicity and tissue damage. In the mouse model of clonorchiasis, the expressions of TLR2 and TLR4 were upregulated during the infestation of C. sinensis, indicating probable participation of TLR2 and TLR4 in the stimulation of innate immune response during C. sinensis infestation (49). The TH1-based inflammatory consequences instructed by TLRs are not only involved in eliminating pathogenic infections but can also induce fatal pathogenic consequences (50). Similarly, the TH2-based pathogen-modulated TLR-mediated signaling event leads to development of immune response beneficial for the pathogen i.e. disease progression. During the chronic C. sinensis infestation, clonorchiasis is associated with predominant TH2 cytokine production as well as suppression of TH1 cytokine production (51, 52). Substantial evidences support the concept that chronic inflammation as a key feature of helminth infestation is linked to various processes involved in carcinogenesis, including cellular transformation, promotion, survival, proliferation, etc (14). In general, inflammation of the bile duct walls is only inconsiderable in regular cases. Sucking onto the biliary epithelium by the fluke results in mechanical tissue damage even at early stages of infestation and, as the fluke matures; the lesion becomes more pronounced and starts to ulcerate (53). Metaplasia of the biliary epithelial cells into mucin-producing cells occurs during very early C. sinensis infestation. The mucin-producing cells may proliferate to produce ESPs in the mucosa, leading to persistent and excessive mucus content in the bile (54). This event is initiated by several factors, such as mechanical obstruction of the bile ducts, mechanical injury from the physical activities of feeding and migrating worms, infestation-related inflammation including secondary infection, especially Escherichia coli, and toxic effects of ESPs (42, 43, 55–59). Several reports demonstrated that ESPs from adult C. sinensis provoke profile changes in transcriptome, proteome and microRNA expression in human HuCCT1 CCA cells and in mouse liver (56, 58–60). Moreover, ESPs from C. sinensis may lead the hyperplasia of normal biliary cells to adenomatous cells with subsequent transformation into CCA by alteration of the transcriptional modification of carcinogenic target genes, such as Mcm7, through histone modifications (16, 57). However, the exact mechanism by which carcinogenesis occur remains to be elusive; it is hypothesized that many processes could be implicated. The following possible mechanisms of cholangiocarcinogenesis due to C. sinensis infestations have been postulated (16, 61): First, chronic irritation and chronic inflammation caused by the infested C. sinensis results in pathologic hyperplasia as a sign of abnormal or precancerous changes and adenomatous changes of bile duct epithelia. The pathologically hyperplastic cells induced by host-parasite interactions due to worm’s physical activities are fragile to carcinogen because the biological agent could easily induce DNA damage during active cell proliferation. Second, endogenous oxidative and nitrative DNA damage caused by C. sinensis infestation has been studied in both humans and animals (54, 62, 63). It is probable that oxidative lesion products, such as 8-nitroguanine and 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-OxodG), accumulate in chronic inflammation site around the bile ducts via local nitric oxide production induced by nitric oxide synthase (iNOS) (Fig. 2). Therefore, bile duct epithelial cells are exposed continuously to high concentrations of oxidative lesion contributing to CCA initiation and/or promotion (52, 64). Third, C. sinensis-induced redox imbalance is due to the enzymatic trigger for production of drug metabolizing enzymes and free-radical generating enzymes (65): For example, experimentally cytochrome P-450 in C. sinensis is responsible for metabolism in the worms and for detoxification contributed to worm survival and drug resistance. Also, free radicals generated by C. sinensis infestation play a critical role in triggering NF-κB-mediated inflammation (57). ESPs of C. sinensis can induce recruitment of histone acetyltransferases (HAT) and regulation of mini-chromosome maintenance (Mcm) proteins for the physiological hyperplasia (57). Fourth, recent evidences have shown modulation of carcinogenesis prevention processes as one of the multiple cholangiocarcinogenic pathways, for example, involvement of small non-protein-coding RNAs (microRNA). Indeed, it is now generally accepted that microRNAs serve as a negative gene regulator by participating in the modulation of a variety of physiological pathways and have the potential to control various gene targets (66). Recent finding indicates that, during C. sinensis-associated cholangiocarcinogenesis in animal model and humans, microRNAs function as both tumor suppressors and oncogenes (67). In addition, IL-6 overexpressing malignant cholangiocytes have been reported to modulate the expression of DNA methyltransferase 1 in a microRNA-dependent manner (68). In the case of carcinogenetic pathway induced by C. sinensis infestation, exposure of human HuCCT1 CCA cells to ESPs for different periods of time as compared to normal H69 cholangiocyte cells has shown differentially altered microRNA profile changes revealing the involvement of microRNA in cell proliferation, inflammation, oncogene activation/suppression, migration/invasion/metastasis, and DNA methylation (59).
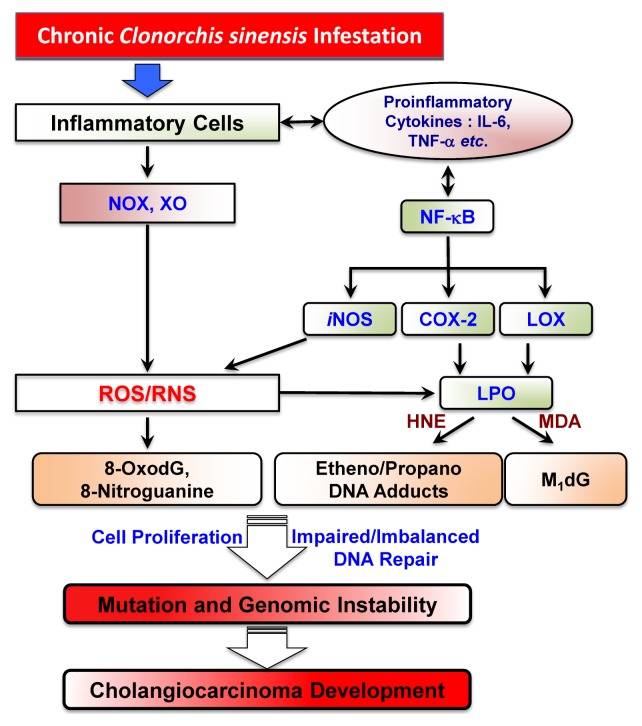
Fig. 2.
Possible link of liver fluke C. sinensis-induced redox imbalance with CCA development.
Inflammation drives generation of free radicals (reactive oxygen species (ROS) and reactive nitrogen species (RNS)), which leads to lipid peroxidation (LPO), and promotes the acquisition of considerable oxidative DNA damage and dysregulation of cell homeostasis (Fig. 2). Considerable reports have demonstrated that ROS are involved in the link between chronic inflammation and cancer (69, 70). For example, exposure of human HuCCT1 CCA cells to C. sinensis ESPs revealed enhanced generation of free radicals by activation of NADPH oxidase (NOX), xanthine oxidase (XO), lipoxygenase (LO), cyclooxygenase (COX) and iNOS (59). In the mouse infectious model for C. sinensis, liver fluke infestation differentially elevated secretion of proinflammatory cytokines such as TNF-α, IL-1β and IL-6, indicating that, under the chronic inflammation states, persistent and dysregulated expressions of these pleiotropic cytokines are promutagenic for malignant cell transformation (4, 47). Chronic and elevated signaling events by TNF-α and IL-1β transactivation of NF-κB, which in turn induces the proinflammatory mediating genes including iNOS, IL-6, etc, results in amplification of inflammation (71). Moreover, substantial evidences have demonstrated that nitric oxide (NO) is not only cytotoxic but may also be genotoxic leading to DNA damage. The main role of nitric oxide (synthesized by iNOS after challenge by immunological and inflammatory stimuli) during inflammation involves triggering of carcinogenesis through accumulation of DNA damage by inhibiting DNA repair system and stimulation of COX-2 expression (72, 73). Furthermore, LPO products, such as trans-4-hydroxy-2-nonenal (HNE), malondialdehyde (MDA) and crotonaldehyde, can modulate the 2nd messenger systems involved in inflammation and carcinogenesis for increasing cell proliferation and decreasing apoptosis in the initiated cell population (74, 75). Additional critical connection between chronic inflammation and cancer development is cyclooxygenase (COX)- and lipoxygenase (LOX)-catalyzed arachidonic and linoleic acid metabolism (76, 77). Based on the experiment in C. sinensis-infested mouse liver tissues, expressions of COX-2 and 5-LOX with increased 8-OxodG accumulation in the nucleus of the cells with inflammation were intensively detected in the inflammatory nidus (47). COX-2, an inducible form of COX, is stimulated by cytokines and lipopolysaccharide and mainly expressed during the inflammation responsible for stimulating cell growth (78). In case of RNS, N-nitrosodimethylamine (NDMA), one of the products of endogenous nitrosation, is significantly metabolized by cytochrome P-450. At intracellular level, exposure of HEK293T to ESPs of C. sinensis with NDMA is responsible for proliferation in the G2/M phase and expression of cell cycle related proteins, such as E2F1, phosphorylated RB and cyclin B (42, 79). In a study on Syrian golden hamster (experimental model), the mechanical and chemical irritation caused by C. sinensis worm and NDMA was considered as a probable cause for genetic alterations leading to neoplastic transformation by producing aberrant proteins including a novel oncogene PSMD10, cyclin-dependent kinase 4 gene CDK4, tumor suppressor gene p53 and protein retinoblastoma (RB) and leading to enhanced survival of the transformed bile duct cells through BAX and caspase 9 (80). The researchers provided the evidence on coordination between changes in the levels of gene and protein expression profile and histopathological changes in C. sinensis and NDMA-induced CCA model.
CONCLUDING REMARKS
It is undeniable that DNA damage caused by C. sinensis infestation is provoked in biliary epithelia, while proper homeostatic mechanisms are dysregulated, resulting in genetic alterations that might be indigenous to the biliary tract, thus leading to malignant transformation. The implicated mechanisms in promotion of malignancy from a parasite infestation discussed in the present review includes mechanical and chemical irritation, chronic inflammation, genomic instability, transcriptiomic, proteomic and microRNA profile alterations by ESPs, and dysregulation of immune response. However, it seems that carcinogenesis associated with C. sinensis can be provoked by various mechanisms and may still be a colossal subject to be elucidated. Moreover, low incidence of CCA in some areas showing a high prevalence of O. viverrini and C. sinensis indicates that other factors are pivotally involved in cholangiocarcinogenesis. Animal studies demonstrate that, in the absence of other carcinogens, CCA is unlikely to develop into liver fluke infestation. Consequently, it is proposed that all the described possible mechanisms could be apprehended in a concert during the development of CCA. So these liver flukes are mainly promoters and not initiators of CCA. It is also necessary that, for the discovery of biomarkers for early diagnosis and discrimination of disease from HBV infection, which is highly prevalent in many clonorchiasis-endemic areas, morbidity due to C. sinensis infestation and drivers of carcinogenesis by chronic infestation should be assessed. Furthermore, for control and elimination of clonorchiasis, rapid immunological tools based on the mathematical modeling need to be developed. In conclusion, this brief review provides tiny aspects on current knowledge on the association of C. sinensis infestations with CCA formation, and further elucidation in future experimental and clinical based researches is necessitated.
ACKNOWLEDGEMENTS
This work was partially supported by the fund from National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) (NO. 2012R1A2A2A01014237) (JHP).
REFERENCES
- 1.Gibson JB, Sobin LH. Histological typing of tumors of the liver biliary tract and pancreas. Geneva: World Health Organization; 1978. [Google Scholar]
- 2.Blechacz B, Gores GJ. Cholangiocarcinoma: Advances on pathogenesis, diagnosis, and treatment. Hepatol. 2008;48:308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens - Part B: Biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/S1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 4.Sripa B, Kaewkes S, Sithithaworn P, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh J-K, Weiderpass E. Infection and cancer: Global distribution and burden of diseases. Ann Glob Health. 2014;80:384–392. doi: 10.1016/j.aogh.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Choi D, Lim JH, Lee KT, et al. Cholangiocarcinoma and Clonorchis sinensis infection: A care-control study in Korea. J Hepatol. 2006;44:1066–1073. doi: 10.1016/j.jhep.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 7.Lim MK, Ju YH, Franceschi S, et al. Clonorchis sinensis infection and increasing risk of cholangiocarcinoma in the Republic of Korea. Am J Trop Med Hyg. 2006;75:93–96. [PubMed] [Google Scholar]
- 8.Honjo S, Srivatanakul P, Sriplung H, et al. Genetic and environmental determinants of risk for cholangiocarcinoma via Opisthorchis viverrini in a densely infested area in Nakhon Phanom, northeast Thailand. Int J Cancer. 2005;117:854–860. doi: 10.1002/ijc.21146. [DOI] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer. A review of carcinogen - Part B: Biological Agents. Lyon, France: 2011. Monographs on the evaluation of carcinogenic risks to humans. [Google Scholar]
- 10.Shin H-R, Oh J-K, Lim MK, et al. Descriptive epidemiology of cholangiocarcinoma and clonorchiasis in Korea. J Korean Med Sci. 2010;25:1011–1016. doi: 10.3346/jkms.2010.25.7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viranuvatti V, Kshemsant D, Bhamarapravati N. Retention cyst of liver caused by opisthorchiasis associated with carcinoma; Case report. Am J Gastroenterol. 1955;23:442–446. [PubMed] [Google Scholar]
- 12.Hou PC. Relationship between primary carcinoma of the liver and infestation with Clonorchis sinensis. J Pathol Bacteriol. 1956;72:239–246. doi: 10.1002/path.1700720130. [DOI] [PubMed] [Google Scholar]
- 13.Huang S-Y, Zhao G-H, Fu B-Q, et al. Genomics and molecular genetics of Clonorchis sinensis: Current status and perspectives. Parasitol Int. 2012;61:71–76. doi: 10.1016/j.parint.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Vennervald BJ, Polman K. Helminths and malignancy. Parasite Immunol. 2009;31:686–696. doi: 10.1111/j.1365-3024.2009.01163.x. [DOI] [PubMed] [Google Scholar]
- 15.Lim MK, Ju Y-H, Franceschi S, et al. Clonorchis sinensis infection and increasing risk of cholangiocarcinoma in the Republic of Korea. Am J Trop Med Hyg. 2006;75:93–96. [PubMed] [Google Scholar]
- 16.Sripa B, Brindley PJ, Mulvenna J, et al. The tumorigenic liver fluke Opisthorchis viverrini – Multiple pathways to cancer. Trends Parasitol. 2012;28:395–407. doi: 10.1016/j.pt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chai JY, Murrell KD, Lymbery AJ. Fish-borne parasitic zoonoses: Status and issues. Inter J Parasitol. 2005;35:1233–1254. doi: 10.1016/j.ijpara.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Hong S-T, Fang Y. Clonorchis sinensis and clonorchiasis, an update. Parasitol Int. 2012;61:17–24. doi: 10.1016/j.parint.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Qian M-B, Utzinger J, Keiser J, Zhou X-N. Clonorchiasis. Lancet. 2016;387:800–810. doi: 10.1016/S0140-6736(15)60313-0. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Choi MH, Bae YM, et al. Correlation between discharged worms and fecal egg counts in human clonorchiasis. PLoS Negl Trop Dis. 2011;5:e1339. doi: 10.1371/journal.pntd.0001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiao T, Ma RH, Luo XB, et al. Cholecystollithiasis is associated with Clonorchis sinensis infection. PLoS One. 2012;7:e42471. doi: 10.1371/journal.pone.0042471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: Revisiting the hygiene hypothesis. Nat Rev Immunol. 2001;1:69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 23.Okada H, Kuhn C, Feillet H, Bach J-F. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: An update. Clin Exper Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fallon PG, Mangan NE. Suppression of TH2-type allergic reactions by helminth infection. Nat Rev Immunol. 2007;7:220–230. doi: 10.1038/nri2039. [DOI] [PubMed] [Google Scholar]
- 25.Jeong Y-I, Kim SH, Ju JW, et al. Clonorchis sinensis-derived total protein attenuates airway inflammation in murine asthma model by inducing regulatory T cells and modulating dendritic cell functions. Biochem Biophys Res Comm. 2011;407:793–800. doi: 10.1016/j.bbrc.2011.03.102. [DOI] [PubMed] [Google Scholar]
- 26.Jeong Y-I, Kim YJ, Ju JW, et al. Identification of anti-allergic effect of Clonorchis-derived protein venom allergen-like proteins (Cs VAL) Biochem Biophys Res Comm. 2014;445:549–555. doi: 10.1016/j.bbrc.2014.01.189. [DOI] [PubMed] [Google Scholar]
- 27.Dunne DW, Cooke A. A worm’s eye view of the immune system: Consequences for evolution of human autoimmune diseases. Nat Rev Immunol. 2005;5:420–426. doi: 10.1038/nri1601. [DOI] [PubMed] [Google Scholar]
- 28.Rim HJ, Lyu KS, Lee JS, et al. Clinical evaluation of the therapeutic efficacy of paraziquantel (Embay 8440) against Clonorchis sinensis infection in man. Ann Trop Med Parasitol. 1981;75:27–33. doi: 10.1080/00034983.1981.11687405. [DOI] [PubMed] [Google Scholar]
- 29.Ambroise-Thomas P, Goullier A. Parasitological examinations and immunodiagnostic advances in fluke infection. Arzneimittelforschung. 1984;34:1129–1132. [PubMed] [Google Scholar]
- 30.Kang J-M, Ju H-L, Lee J, et al. Mapping the putative epitope domain of Clonorchis sinensis paramyosin (Cs Pmy) recognized by Cs Pmy-specific immunoglobulin G in sera of human clonorchiasis. Mol Biochem Parasitol. 2015;201:66–71. doi: 10.1016/j.molbiopara.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Kim TI, Na BK, Hong SJ. Functional genes and proteins of Clonorchis sinensis. Korean J Parasitol. 2009;47(Suppl):S59–68. doi: 10.3347/kjp.2009.47.S.S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park GM. Genetic comparison of liver flukes, Clonorchis sinensis and Opisthorchis viverrini based on rDNA and mtDNA gene sequences. Parasitol Res. 2007;100:351–357. doi: 10.1007/s00436-006-0269-x. [DOI] [PubMed] [Google Scholar]
- 33.Lim JH. Radiologic findings of clonorchiasis. Am J Roentgenol. 1990;155:1001–1008. doi: 10.2214/ajr.155.5.2120925. [DOI] [PubMed] [Google Scholar]
- 34.Xiao SH, Hui-Ming W, Tanner M, et al. Tribendimidine: A promising, safe and broad-spectrum anthelmintic agent from China. Acta Trop. 2005;94:1–14. doi: 10.1016/j.actatropica.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Echaubard P, Sripa B, Mallory FF, Wilcox BA. The role of evolutionary biology in research and control of liver flukes in southeast. Asia Infect Genet Evol. 2016 doi: 10.1016/j.meegid.2016.05.019. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korean National Research Institute of Health, Centers for Disease Control & Prevention. Prevalence of intestinal parasitic infections in Korea - The 8th report. 2013. [Google Scholar]
- 37.Ministry of Health and Welfare, Korea Association of Health Promotion. Prevalence of intestinal parasitic infection in Korea - The 7th report. 2004. [Google Scholar]
- 38.Jeong Y-I, Shin H-E, Lee S-E, et al. Prevalence of Clonorchis sinensis infection among residents along 5 major rivers in the Republic of Korea. Korean J Parasitol. 2016;54:215–221. doi: 10.3347/kjp.2016.54.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li T, He S, Zhao G, et al. Major trends in human parasitic diseases in China. Trends Parasitol. 2010;26:264–270. doi: 10.1016/j.pt.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Hong ST, Huh S, Kho WG, et al. Changes of histopathological and serological findings of the liver after treatment in rabbit clonorchiasis. Seoul J Med. 1990;31:117–127. [Google Scholar]
- 41.Min HK. Clonorchis sinensis: Pathogenesis and clinical features of infection. Arzneimittelforschung. 1984;34:1151–1153. [PubMed] [Google Scholar]
- 42.Kim YJ, Choi MH, Hong ST, et al. Proliferative effects of excretory/secretory products from Clonorchis sinensis on the human epithelial cell line HEK293 via regulation of the transcription factor E2F1. Parasitol Res. 2008;102:411–417. doi: 10.1007/s00436-007-0778-2. [DOI] [PubMed] [Google Scholar]
- 43.Kim YJ, Choi MH, Hong ST, et al. Resistance of cholangiocarcinoma cells to parthenolide-induced apoptosis by the excretory-secretory products of Clonorchis sinensis. Parasitol Res. 2009;104:1011–1016. doi: 10.1007/s00436-008-1283-y. [DOI] [PubMed] [Google Scholar]
- 44.Yoo WG, Kim D-W, Ju J-W, et al. Developmental transcriptomic features of the carcinogenic liver fluke, Clonorchis sinensis. PLoS Neg Trop Dis. 2011;5:e1208. doi: 10.1371/journal.pntd.0001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fava G, Lorenzini I. Molecular pathogenesis of cholangiocarcinoma. Int J Hepatol Article. 2012;2012;630543 doi: 10.1155/2012/630543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawanishi S, Hiraku Y. Oxidative and nitrative DNA damage as biomarker for carcinogenesis with special reference to inflammation. Antioxid Redox Signal. 2006;8:1047–1058. doi: 10.1089/ars.2006.8.1047. [DOI] [PubMed] [Google Scholar]
- 47.Maeng A, Lee HW, Bashir Q, et al. Oxidative stress-mediated mouse liver lesions caused by Clonorchis sinensis infection. Int J Parasitol. 2016;46:195–204. doi: 10.1016/j.ijpara.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Yan C, Li X-Y, Li B, et al. Expression of Toll-like receptor (TLR) 2 and TLR4 in the livers of mice infected by Clonorchis sinensis. J Infect Dev Ctries. 2015;9:1147–1155. doi: 10.3855/jidc.6698. [DOI] [PubMed] [Google Scholar]
- 50.Liu Q, Ding JL. The molecular mechanisms of TLR-signaling cooperation in cytokine regulation. Immunol Cell Biol. 2016 doi: 10.1038/icb.2016.18. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.Choi YK, Yoon BI, Won YS, et al. Cytokine responses in mice infected with Clonorchis sinensis. Parasitol Res. 2003;91:87–93. doi: 10.1007/s00436-003-0934-2. [DOI] [PubMed] [Google Scholar]
- 52.Kim EM, Bae YM, Choi MH, Hong ST. Cyst formation, increased anti-inflammatory cytokines and expression of chemokines support for Clonorchis sinensis infection in FVB mice. Parasitol Int. 2012;61:124–129. doi: 10.1016/j.parint.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Bhamarapravati N, Thammavit W, Vajrasthira S. Liver changes in hamsters infected with a liver fluke of man, Opisthorchis viverrini. Am J Trop Med Hyg. 1978;38:356–362. doi: 10.4269/ajtmh.1978.27.787. [DOI] [PubMed] [Google Scholar]
- 54.Choi BI, Han JK, Hong ST, Lee KH. Clonorchiasis and cholangiocarcinoma: Etiologic relationship and imaging diagnosis. Clin Microbiol Rev. 2004;17:540–552. doi: 10.1128/CMR.17.3.540-552.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi M-H, Park IC, Li S, Hong S-T. Excretory-secretory antigen is better than crude antigen for the serodiagnosis of clonorchiasis by ELISA. Korean J Parasitol. 2003;41:35–38. doi: 10.3347/kjp.2003.41.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pak JH, Kim D-W, Moon JH, et al. Differential gene expression profiling in human cholangiocarcinoma cells treated with Clonorchis sinensis excretory-secretory products. Parasitol Res. 2009;104:1011–1016. doi: 10.1007/s00436-008-1286-8. [DOI] [PubMed] [Google Scholar]
- 57.Kim D-W, Kim J-Y, Moon JH, et al. Transcriptional induction of minichromosome maintenance protein 7 (Mcm7) in human cholangiocarcinoma cells treated with Clonorchis sinensis excretory-secretory products. Mol Biochem Parasitol. 2010;173:10–16. doi: 10.1016/j.molbiopara.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Pak JH, Moon JH, Hwang SJ, et al. Proteomic analysis of differentially expressed proteins in human cholangiocarcinoma cells treated with Clonorchis sinensis excretory-secretory products. J Cell Biochem. 2009;108:1376–1388. doi: 10.1002/jcb.22368. [DOI] [PubMed] [Google Scholar]
- 59.Pak JH, Kim IK, Kim SM, et al. Induction of cancer-related microRNA expression profiling using excretory-secretory products of Clonorchis sinensis. Parasitol Res. 2014;113:4447–4455. doi: 10.1007/s00436-014-4127-y. [DOI] [PubMed] [Google Scholar]
- 60.Ju JW, Joo HN, Lee MR, et al. Identification of a serodiagnostic antigen, legumain, by immunoproteomic analysis of excretory-secretory products of Clonorchis sinensis adult worms. Proteomics. 2009;9:3066–3078. doi: 10.1002/pmic.200700613. [DOI] [PubMed] [Google Scholar]
- 61.Khurana S, Dubey ML, Malla N. Association of parasitic infections and cancers. Indian J Med Microbiol. 2005;23:74–79. doi: 10.4103/0255-0857.16044. [DOI] [PubMed] [Google Scholar]
- 62.Oshima H, Bandaletova TY, Brouet I, et al. Increased nitrosamine and nitrate biosynthesis mediated by nitric oxide synthase induced in hamsters infected with liver fluke (Opisthorchis viverrini) Carcinogenesis. 1994;15:271–275. doi: 10.1093/carcin/15.2.271. [DOI] [PubMed] [Google Scholar]
- 63.Yang Q-L, Shen J-Q, Xue Y, et al. Pathological lesions and inducible nitric oxide synthase expressions in the liver of mice experimentally infected with Clonorchis sinensis. Korean J Parasitol. 2015;53:777–783. doi: 10.3347/kjp.2015.53.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pinlaor S, Ma N, Hiraku Y, et al. Repeated infection with Opisthorchis viverrini induces accumulation of 8-nitroguanine and 8-oxo-7,8-dihydro-2′-deoxyguanine in the bile duct of hamsters via inducible nitric oxide synthase. Carcinogenesis. 2004;25:1535–1542. doi: 10.1093/carcin/bgh157. [DOI] [PubMed] [Google Scholar]
- 65.Nam J-H, Moon JH, Kim IK, et al. Free radicals enzymatically triggered by Clonorchis sinensis excretory-secretory products cause NF-κB-mediated inflammation in human cholangiocarcinoma cells. Int J Parasitol. 2012;42:103–113. doi: 10.1016/j.ijpara.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 66.Esquela-Kerscher A, Slack FJ. Oncomirs – MicroRNAs with a role in cancer. Nat Rev. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 67.Namwat N, Chusorn P, Loilome W, et al. Expression profiles of oncomir miR-21 and tumor suppressor let-7a in the progression of opisthorchiasis-associated cholangiocarcinoma. Asian Pac J Cancer Prev. 2012;13:65–69. [PubMed] [Google Scholar]
- 68.Braconi C, Huang N, Patel T. MicroRNA-dependent regulation of DNA methyltransferase-1 and tumor suppressor gene expression by interleukin-6 in human malignant cholangiocyte. Hepathol. 2010;51:881–890. doi: 10.1002/hep.23381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oshima H, Bartsch H. Chronic infectious and inflammation processes as cancer risk factors: Possible role of nitric oxide in carcinogenesis. Mutat Res. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 70.Weitzman SA, Gordon LI. Inflammation and cancer: Role of phagocyte-generated oxidants in carcinogenesis. Blood. 1990;76:655–663. [PubMed] [Google Scholar]
- 71.Apte RN, Voronov E. Interleukin-1 – A major pleiotropic cytokine in tumor host interactions. Cancer Biol. 2002;12:277–290. doi: 10.1016/S1044-579X(02)00014-7. [DOI] [PubMed] [Google Scholar]
- 72.Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase upregulates cyclooxygenase-2 in mouse cholangiocytes promoting cell growth. Gastrointest Liver Physiol. 2004;287:G88–G95. doi: 10.1152/ajpgi.00539.2003. [DOI] [PubMed] [Google Scholar]
- 73.Lowenstein CJ, Padalko E. iNOS (NOS2) at a glance. J Cell Sci. 2004;117:2865–2867. doi: 10.1242/jcs.01166. [DOI] [PubMed] [Google Scholar]
- 74.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: Role of lipid peroxidation, DNA damage and Repair. Langenbeck’s Arch Surg. 2006;391:499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 75.Reuter S, Gupta SC, Chaturveri MM, Aggarwal BB. Oxidative stress, inflammation and cancer: How are they linked? Free Radical Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fitzpatrick FA. Inflammation, carcinogenesis and cancer. Int Immunopharmacol. 2001;1:1651–1667. doi: 10.1016/S1567-5769(01)00102-3. [DOI] [PubMed] [Google Scholar]
- 77.Furstenberger G, Krieg P, Muller-Decker K, Habenicht AJR. What are cyclooxygenases and lipoxygenases doing in the driver’s seat of carcinogenesis? Int J Cancer. 2006;119:2247–2254. doi: 10.1002/ijc.22153. [DOI] [PubMed] [Google Scholar]
- 78.Brown JR, DuBois RN. COX-2: A molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840–2855. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 79.Kim EM, Kim JS, Choi MH, et al. Effects of excretory/secretory products from Clonorchis sinensis and the carcinogen dimethylnitrosamine on the proliferation and cell cycle modulation of human epithelial HEK293T cells. Korean J Parasitol. 2008;46:127–132. doi: 10.3347/kjp.2008.46.3.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uddin MH, Choi MH, Kim WH, et al. Involvement of PSMD10, CDK4 and tumor suppressors in development of intrahepatic cholangiocarcinoma of Syrian golden hamsters induced by Clonorchis sinensis and N-nitrosodimethylamine. PLoS Negl Trop Dis. 2015;9:e0004008. doi: 10.1371/journal.pntd.0004008. [DOI] [PMC free article] [PubMed] [Google Scholar]