Abstract
Background
Molecules critically involved in cocaine behavioral plasticity are known to regulate and interact with peroxisome proliferator-activated receptor gamma coactivator-1alpha, PGC-1α. Additionally, the PGC-1α promoter has binding sites for early growth response 3 (Egr3), which plays a dynamic role in cocaine action in nucleus accumbens (NAc) medium spiny neurons (MSN) subtypes, those enriched in dopamine receptor D1 vs. D2. However, the role of PGC-1α in NAc in cocaine action is unknown.
Methods
PGC-1α mRNA and protein were examined in NAc after repeated cocaine exposure. Binding of Egr3 to and histone methylation at the PGC-1α promoter was examined in NAc, using chromatin immunoprecipitation (ChIP), after repeated cocaine. PGC-1α ribosome-associated mRNA in MSN subtypes was assessed after repeated cocaine using D1-Cre-RiboTag and D2-Cre-RiboTag lines. Finally, PGC-1α was expressed in NAc D1-MSNs vs. D2-MSNs using a Cre-inducible AAV and Cre lines during cocaine conditioned place preference and cocaine-induced locomotion.
Results
Repeated cocaine increased PGC-1α levels, and increased Egr3 binding and H3K4me3 at the PGC-1α promoter in NAc. Increased PGC-1α occurred in D1-MSNs, while D2-MSNs showed reduced levels. Viral mediated expression of PGC-1α in D1-MSNs enhanced behavioral responses to cocaine, while expression in D2-MSNs blunted these behaviors.
Conclusions
We demonstrate a novel role for PGC-1α in NAc in cocaine action. PGC-1α is enhanced in NAc D1-MSNs specifically after cocaine exposure. These data are consistent with increased active methylation and Egr3 binding at the PGC-1α promoter. Finally, we demonstrate a bidirectional role for PGC-1α in mediating behavioral plasticity to cocaine through D1-MSNs vs. D2-MSNs.
Keywords: Cocaine, Nucleus accumbens, MSN subtype, RiboTag, PGC-1α, Epigenetics
Introduction
Peroxisome proliferator-activated receptor gamma coactivator-1alpha, PGC-1α, is a member of the PGC-1 family of transcriptional coactivators. The PGC-1 coactivators play important roles in gene regulation of molecules critical for metabolic processes throughout the body including the maintenance of glucose, lipid, and energy homeostasis such as mitochondrial biogenesis (1, 2). Through their role as transcriptional coactivators, the PGC1 family is implicated in a number of diseases. This includes diabetes, neurodegeneration, and obesity (1, 2). However, PGC-1α coactivators have not been examined in diseased motivational states, such as drug abuse.
Interestingly, many molecules that interact with or regulate PGC-1α are implicated in cocaine mediated behavioral plasticity and corresponding cellular plasticity in the ventral striatum or nucleus accumbens (NAc), a major brain reward region. For instance, PGC-1α can interact in transcriptional complexes on DNA with the histone deacetylase, Sirtuin 1 (Sirt1) and the histone acetyltransferase, cAMP-response binding protein (CREB) binding protein (CBP) (3–5). Both CBP and Sirt1 are regulated by cocaine in NAc and in turn regulate gene transcription resulting in altered cellular and behavioral adaptations associated with cocaine (6–8). Additionally, PGC-1α is transcriptionally regulated by the transcription factors CREB and myocyte enhancer factor 2 (Mef2) and PGC-1α has been shown to interact with Mef2 to regulate gene transcription (9–11). Both CREB and Mef2 are well established to mediate the molecular, cellular, and behavioral responses to cocaine or other drugs of abuse through their actions in the NAc (12–15).
Along with CREB and Mef2, many other transcription factors are induced by cocaine in the NAc and/or dorsal striatum and associated with cocaine cellular and behavioral plasticity (16–25), have binding sites on the PGC-1α gene promoter (TRANSFAC from AliBaba2.1). This includes AP-1 sites to which FosB and its truncated isoform deltaFosB can bind (18); an NfκB binding site; and early growth response (Egr) binding sites. Further, a previous study implicated an Egr family member in the regulation of PGC-1α mRNA in adipose tissue under dietary obesity conditions (26).
We recently demonstrated that a member of the Egr family, Egr3, is bidirectionally induced in dopamine receptor 1 vs. 2 expressing NAc medium spiny neuron (MSN) subtypes after repeated cocaine (25). We observed Egr3 induction in D1-MSNs after cocaine exposure and a reduction in D2-MSNs. Furthermore, increasing Egr3 levels in D1-MSNs or reducing Egr3 levels in D2-MSNs enhances behavioral responses to cocaine. In contrast the opposite manipulation of Egr3 in MSN subtypes, reduction in D1-MSNs and increase in D2-MSNs, blunts these cocaine-mediated behaviors. This is consistent with studies demonstrating divergent behavioral outcomes to cocaine when altering activity in each MSN subtype (27–30).
Given the association of PGC-1α with molecules critical for cocaine induced plasticity, we investigate PGC-1α mRNA and protein in NAc after repeated cocaine exposure. Since PGC-1α has an Egr3 binding site we then examined Egr3 binding to the PGC-1α gene promoter, using chromatin immunoprecipitation (ChIP), after repeated cocaine. To determine the underlying epigenetic regulation of PGC-1α transcription, we performed ChIP to examine the active and repressive histone methylation marks, H3K4me3 and H3K27me3 respectively (31), at the PGC-1α promoter. Since, PGC-1α has Egr3 binding sites and Egr3 is bidirectionally regulated in MSN subtypes we then examined ribosome-associated mRNA for PGC-1α in D1-MSNs vs. D2-MSNs after repeated cocaine. Finally, we used a Cre-inducible PGC-1α adenoassociated virus (AAV) in D1-Cre and D2-Cre lines to determine if altering PGC-1α levels in MSN subtypes can mediate behavioral outcomes to cocaine.
Methods and Materials
Animals
D1-Cre hemizygote (line FK150) or D2-Cre hemizygote (line ER44) bacterial artificial chromosome (BAC) transgenic mice from GENSAT (32, 33) (www.gensat.org) on a C57BL/6J background were used for behavioral experiments and virus validation. Homozygous RiboTag (RT) mice on a C57BL/6J background, expressing a Cre-inducible HA-Rpl22 (34) were crossed to D1-Cre or D2-Cre mouse lines to generate D1-Cre-RT and D2-Cre-RT mice (25) and used for cell type–specific ribosome-associated mRNA isolation. Male C57BL/6J mice obtained from Jackson Laboratory were used for RT-PCR, western blot, and ChIP assays. The mice were maintained on a 12h light/dark cycle ad libitum food and water. All studies were conducted in accordance with the guidelines set up by the Institutional Animal Care and Use Committee’s at The University of Maryland School of Medicine.
Repeated cocaine treatment
C57BL/6J mice received 7 daily intraperitoneal injections (i.p.) of cocaine (20 mg/kg) or 0.9% saline in the home cage. NAc tissue was collected 24h after the last injection. Cocaine hydrochloride (Sigma) was dissolved in sterile saline. The dose of cocaine was selected based on previous studies (21, 25, 27, 35)
Adeno-Associated Viral Vectors
Recombinant Cre-dependent adeno-associated viruses (AAVs) PGC-1α and EYFP were used in this study. The PGC-1α vectors were provided by Dr. K. Chandrasekharan (University of Maryland, Baltimore, USA). PGC-1α sequences were PCR amplified (Phusion DNA polymerase, New England Biolabs) and cloned into NheI and StuI restriction sites in an AAV-hSyn-DIO-IRES-mCitrine vector backbone gifted from Dr. B.L. Roth (University of North Carolina), to generate the AAV-hSyn-DIO-PGC-1α-mCitrine vector. Finally, vectors were validated in Neuro2a cells by immunohistochemistry before packaging into AAV (serotype 9) as described previously (25). AAV-DIO-EYFP was purchased from the UNC Vector Core Facility.
Mouse stereotaxic surgery
D1-Cre or D2-Cre mice were anesthetized using 3% isoflurane in a small induction chamber. After the initial induction, isoflurane was maintained at 1% for the remainder of the surgery. Animals were placed in a stereotaxic instrument and their skull was exposed. 33 gauge Hamilton syringe needles were used to inject 0.6µl of either AAV-DIO-EYFP or AAV-hSyn-DIO-PGC-1α-mCitrine, bilaterally into the NAc (anterior/posterior, AP+1.6; medial/lateral, ML±1.5; dorsal/ventral, DV-4.4, 10° angle) according to our previous studies (25, 27). Mice were then returned to the vivarium for 2 weeks to allow for recovery and maximal virus expression.
Conditioned place preference
Conditioned place preference (CPP) was conducted as previously described using Topscan tracking software (Clever Sys Inc) (25, 27). Briefly, two weeks after intra-NAc infusions of AAV-DIO-PGC-1α-mCitrine or AAV-DIO-EYFP, D1-Cre or D2-Cre mice were placed into the conditioning boxes. The apparatus consisted of three chambers with two conditioning chambers, consisting of distinct environments with unique wall patterns and floor textures, separated by a middle neutral chamber. On the first day, mice were allowed to freely explore the 3 chambers for 20 min. Groups were then balanced and adjusted for any chamber bias that may have occurred (mice that showed significant preference for one compartment were excluded from the experiment). During the conditioning days 2 and 3, mice received an i.p. injection of saline before noon and were confined for 30 min to one chamber. In the afternoon, mice received an injection of cocaine (7.5mg/kg in saline, i.p.) and were placed in the chamber, opposite to the saline conditioning for 30 min. On day 4, animals were placed in the apparatus for 20 min without any treatment. Time spent in the drug-paired chamber minus time spent in the saline-paired chamber was assessed.
Cocaine-induced locomotion
Cocaine-induced locomotor activity was performed using protocols routinely used in our laboratory (25, 27). Briefly, two weeks after intra-NAc infusions of AAV-DIO-PGC-1α-mCitrine or AAV-DIO-EYFP in D1-Cre or D2-Cre mice were habituated to the apparatus for 30 min. On the next day, mice were habituated for 30 min after one saline injection (i.p). Mice received a daily injection (for five days) of cocaine (10mg/kg, i.p.) in an open field box. Activity was tracked over 30 min with Topscan tracking software on Day 1 and 5 (Clever Sys Inc).
Immunoprecipitation of Polyribosomes and RNA Isolation from MSN subtypes
Immunoprecipitation of polyribosomes was prepared from NAc of D1-Cre-RT and D2-Cre-RT mice according to our previous study (25). In brief, four 14-gauge NAc punches per animal (four animals pooled per sample) were collected and homogenized by douncing in homogenization buffer and 800µl of the supernatant was added directly to the HA coupled beads (Invitrogen: 100.03D; Covance: MMS-101R) for constant rotation overnight at 4°C. The following day, magnetic beads were washed three times in magnet for five minutes in high salt buffer. Finally, RNA was extracted by adding TRK lysis buffer to the pellet provided in MicroElute Total RNA Kit (Omega,) according to manufacturer’s instructions. RNA was quantified with a NanoDrop (Thermo Scientific). For cDNA synthesis and qRT-PCR see below.
RNA extraction and quantitative RT-PCR
AAV-DIO-PGC-1α-mCitrine or AAV-DIO-EYFP infused in D1-Cre or D2-Cre mice or C57BL/6J mice NAc tissue punches were collected 24 h after the last cocaine administration and stored at −80°C. RNA was extracted using Trizol (Invitrogen) and the MicroElute Total RNA Kit (Omega) with a DNase step (Qiagen). All RNA quantity was measured on a Nanodrop. 300–400ng cDNA was then synthesized using reverse transcriptase iScript cDNA synthesis kit (Bio-Rad). mRNA expression changes were measured using quantitative polymerase chain reaction (qPCR) with PerfeCTa SYBR Green FastMix (Quanta). Quantification of mRNA changes was performed using the −ΔΔ CT method, using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a housekeeping gene. The list of primers used in this study is included GAPDH forward: AGGTCGGTGTGAACGGATTTG, GAPDH reverse: TGTAGACCATGTAGTTGAGGTCA, PGC-1α forward: CGACCATGGTGTTGTTCTTG, and PGC-1α reverse ATGGCAGCGACTCCATACTC.
Western blots
Protein samples were prepared as previously described (25) and protein concentrations were determined using DC protein assay (Bio-Rad) and then 20µg samples of total protein were loaded onto Tris–HCl polyacrylamide gel (Bio-Rad). The samples were transferred to a nitrocellulose membrane and blocked for 1h in blocking buffer, 5% non-fat dry milk in Tris buffered saline (pH 7.6) with 0.1% Tween. Blocked membranes were incubated overnight at 4°C in blocking buffer with primary antibodies using either 1:1000 PGC-1α (Santa Cruz Biotech, cat.# SC-13067), 1:5,000 beta-tubulin (Cell Signaling, cat.# 2128S). Membranes were then incubated with goat anti-rabbit peroxidase-labeled secondary antibodies (Vector Laboratories, cat.# PI-1000, 1:20,000) in blocking buffer. The bands were visualized using SuperSignal West Dura Extended Duration substrate (Pierce, cat.#34075). Bands were quantified with Image Lab Software (Bio-Rad) and normalized to beta-tubulin to control for equal loading.
Chromatin immunoprecipitation (ChIP)
NAc collection and fixation for ChIP experiments was performed as previously described (25). In brief, NAc tissue was homogenized in 1ml lysis buffer 1 (50mM Hepes-KOH, pH 7.5, 140mM NaCl, 1mM EDTA, 10% glycerol, 0.5% NP-40, 0.25% Triton x-100 and protease inhibitors) by douncing followed by constant rotation at 4°C for 10 min. Samples were centrifuged at 1,350g for 5 min at 4°C and the pellet was resuspended in 1ml Lysis Buffer 2 (10mM Tris-HCl, pH 8.0, 200mM NaCl, 1mM EDTA, 0.5mM EGTA and protease inhibitors) and incubated gently on shaker at room temperature for 10 min. Pelleted cell nuclei by centrifugation at 1,350g for 5 min at 4°C and resuspended in 300ul Lysis Buffer 3 (10mM Tris-HCl, pH 8.0, 100mM NaCl, 1mM EDTA, 0.5mM EGTA, 0.1% Na-Deoxycholate, 0.5% N-lauroylsarcosine and protease inhibitors). Chromatin was sheared to an average length of 500–700bp by the Diagenode Bioruptor® Pico using 8 cycles of 30 sec on and off. 1/10 volume of 10% Triton X-100 was added to sonicated lysate to dissolve the nuclear membrane. Samples were centrifuge at 20,000g for 10 min at 4°C to pellet the debris. Following the washing and re-suspension of the antibody–bead conjugates, antibody–bead mixtures were added to each chromatin sample (600µl) and incubated for ~16 hour under constant rotation at 4°C. Samples were then washed and reverse cross-linked at 65°C overnight and DNA was purified using a PCR purification kit (QIAGEN). After DNA purification, samples were used for qPCR analysis and normalized to their appropriate input controls and compared to IgG chipped samples as previously described (20, 25, 36). The Egr3 transcription factor binding site on the PGC-1α promoter was predicted from AliBaba2.1 and primers were designed for this site. The primers sequence used for ChIP PCR are PGC-1α forward CAAGGCACTAGGGTTGGAGT and PGC-1α reverse TCCGTGGATCTCATAGGCTC.
Immunohistochemistry
D1-Cre or D2-Cre mice infused with AAV-DIO-PGC-1α-mCitrine were perfused with 0.1 M PBS followed by 4% paraformaldehyde (PFA). Brains were immersed in PFA overnight and then cryopreserved in 30% sucrose. Brains were cryosectioned (Leica) at 35 µm into 0.1 M PBS. Brain sections were blocked in 3% normal donkey serum with 0.3% Triton X-100 for 30 min at room temperature. Sections were then incubated overnight at room temperature in primary antibodies, 1:8000 chicken anti-GFP (Aves) diluted in the above blocking solution. On the second day, tissue sections were rinsed in 0.1 M PBS followed by 1-hour incubation at room temperature in secondary antibodies, 1:1000 donkey anti-chickens Alexa Fluor 488 (Jackson ImmunoResearch). Sections were rinsed in PBS, mounted onto slides, and coverslipped. Immunofluorescence was imaged on an Olympus Bx61 confocal microscope.
Statistical analyses
Statistical analyses were performed using Graphpad Prism software. Statistical analyses for mRNA expression, protein levels, ChIP PCR, and CPP were performed using Student’s t-tests. Statistical analyses for locomotor sensitization experiments were performed using repeated measures two-way ANOVAs followed by Bonferroni post-tests. D-Agostino-Pearson omnibus normality tests were performed to determine that the data met the assumptions of the statistical approach.
Results
To determine if repeated cocaine alters PGC-1α we first examined PGC-1α mRNA in NAc of C57BL/6J mice receiving cocaine (20 mg/kg/day for 7 days) or saline. Tissue was collected 24 hours after the last injection (Figure 1A), a time point shown to induce transcriptional and cellular plasticity in NAc (20, 25, 27, 37). Quantitative RT-PCR demonstrates that the PGC-1a mRNA is increased in NAc after repeated cocaine (Figure 1B). We further observed an increase in PGC-1α protein in NAc after repeated cocaine (20 mg/kg/day for 7 days) compared to saline controls (Figure 1C), which is consistent with the increase in PGC-1α mRNA.
Figure 1.
Cocaine enhances PGC-1α mRNA and protein in NAc. (A) The timeline of cocaine (20mg/kg; i.p.) or saline injections and NAc tissue collection after 24-hour withdrawal. (B) PGC-1α mRNA is increased in NAc after repeated cocaine (20mg/kg, 7 days). Student’s t test t(11)=2.64, *p<0.05, n=6 saline and n=7 cocaine. (C) PGC-1α protein level is higher in NAc after repeated cocaine (20mg/kg, 7 days). Student’s t test t(24)=2.49, *p<0.05, n=13 per group. Error bars, SEM.
Since our previous study demonstrated that the transcription factor, Egr3, is induced in NAc with repeated cocaine and it transcriptionally regulates a number of genes involved with cocaine cellular and behavioral plasticity (25); we next examined if Egr3 transcriptionally regulates PGC-1α transcription after cocaine exposure. We performed chromatin immunoprecipitation (ChIP), using an Egr3 antibody, on NAc tissue in C57BL/6J mice receiving cocaine (20mg/kg for 7 days) or saline followed by 24-hour withdrawal (Figure 2A). We observed significant enrichment of Egr3 on the PGC-1α promoter in NAc of the cocaine treated group compared to saline controls (Figure 2B). We further investigated the active histone methylation mark, H3K4me3, and the repressive histone methylation mark, H3K27me3 on the PGC-1α promoter in same conditions. Using antibodies to H3K4me3 and H3K27me3, demonstrates that the active mark, H3K4me3, but not the repressive mark, H3K27me3, is enriched on the PGC-1α promoter in NAc of the cocaine treated group compared to the saline group (Figure 2C, 2D). This is consistent with increased PGC-1α mRNA after cocaine (Figure 1B).
Figure 2.
Cocaine induced PGC-1α transcriptional regulation in NAc. (A) The timeline of cocaine (20mg/kg, i.p.) or saline injections and NAc tissue collection after 24-hour withdrawal. (B) Egr3 binding is increased on PGC-1α gene promoter in NAc in the cocaine group compared to saline injected mice. Student’s t test, t(16)=2.22, *p<0.05; n=8 saline and n= 10 cocaine. (C, D) H3K4me3 and H2K27me3 ChIP in the NAc demonstrates enrichment of H3K4me3 on the PGC-1α gene promoter in the cocaine group Student’s t test t(10)=2.39 for H3K4me3, t(10)=0.68 for H2K27me3,, *p<0.05; n=6 per group. Error bars, SEM.
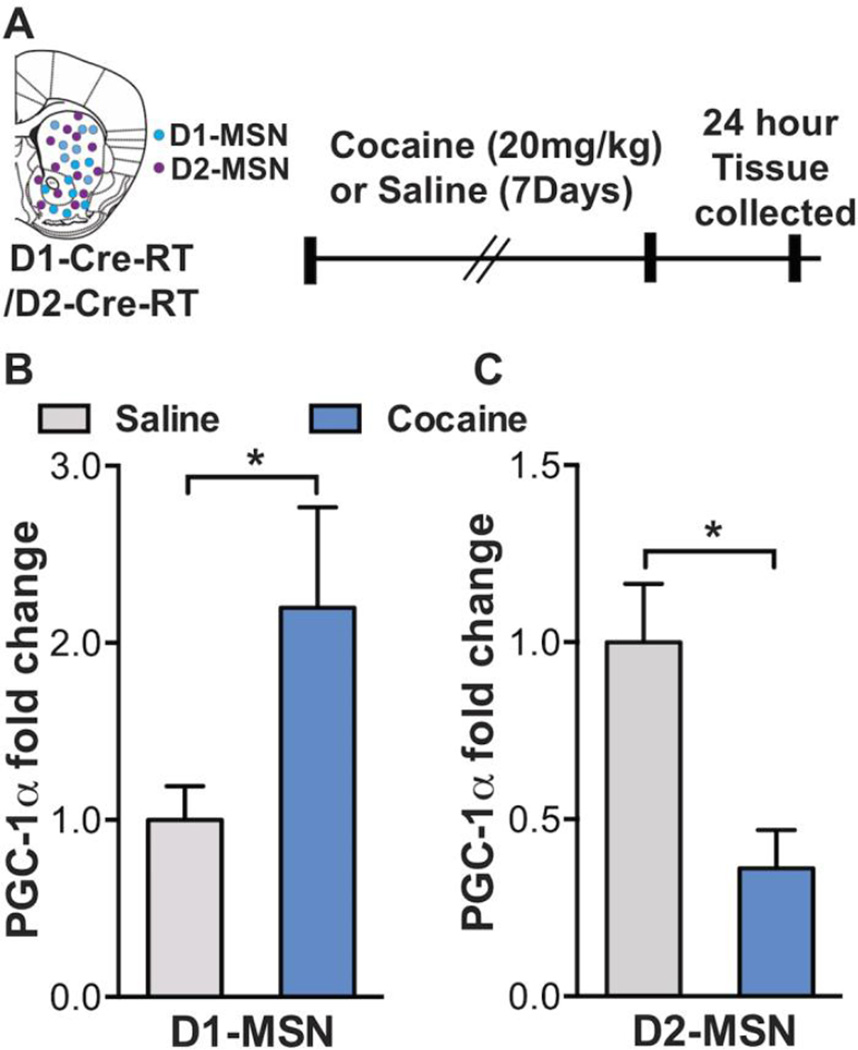
Since Egr3 binding was increased on the PGC-1α promoter and since we previously found Egr3 bidirectionally induced in two NAc projection neuron subtypes, D1-MSNs vs. D2-MSNS, with cocaine exposure (25); we next examined PGC-1α mRNA in each MSN subtype after repeated cocaine. We used a well-established RiboTag approach to examine mRNA from NAc of D1-Cre-RiboTag (RT) or D2-Cre-RT mice receiving repeated cocaine (20mg/kg for 7 days) or saline followed by a 24-hour withdrawal period (Figure 3A). The RiboTag approach allows cell type specific immunoprecipitation of polyribosomes (34, 38), allowing selective examination of ribosome-associated mRNA from D1-MSNs and D2-MSNs (25). qRT-PCR analyses showed an increase in PGC-1α ribosome-associated mRNA in NAc D1-MSNs in the cocaine treated group compared to saline controls (Figure 3B). In contrast PGC-1α ribosome-associated mRNA was decreased in D2-MSNs after cocaine (Figure 3C).
Figure 3.
Cocaine bidirectionally induces PGC-1α in NAc MSN subtypes. (A) Illustration of the heterogeneous mixture of D1-MSN (blue) and D2-MSN (magenta) subtypes in a dorsal striatum and NAc in coronal section. The timeline of cocaine (20mg/kg, i.p.) injections and NAc tissue collection is also displayed. (B, C) PGC-1α ribosome-associated mRNA is increased in D1-MSNs and reduced in D2-MSNs of D1-Cre-RT and D2-Cre-RT respectively, after repeated cocaine (7 days, 20mg/kg). Student’s t test, t(19)=2.08 for D1-MSN, t(9)=3.09 for D2-MSN *p<0.05; n=11 saline, n=10 cocaine in D1-MSNs; n=6 saline group, n=5 cocaine group in D2-MSNs. Error bars, SEM.
To determine if PGC-1α levels in MSN subtypes can directly alter behavioral responses to cocaine we developed a Cre-inducible adeno-associated virus (AAV) to overexpress PGC-1α in D1-MSNs and D2-MSNs during cocaine conditioned place preference (CPP) and cocaine-induced locomotion. The AAV- double inverted open reading frame (DIO)-PGC-1α-mCitrine (Figure 4A) or AAV-DIO-EYFP control was first infused into the NAc of D1-Cre mice (Figure 4B). mCitrine expression was detected in D1-Cre NAc but not in wild-type littermates that received AAV-DIO-PGC-1α-mCitrine (Figure 4C). We also observed a two fold increase in PGC-1α mRNA in NAc of D1-Cre mice receiving AAV-DIO-PGC-1α-mCitrine compared to control conditions which received AAV-DIO-EYFP to NAc (Figure 4D). After two days of cocaine (7.5mg/kg) conditioned place preference, mice with PGC-1α overexpression in NAc D1-MSNs displayed enhanced time in the cocaine-paired chamber compared to D1-Cre mice receiving EYFP control virus (Figure 4E). We observed no difference between the PGC-1α or EYFP groups during time spent in the cocaine-paired chamber in the pre-test (PGC-1α: −17.61 ± 60.70 sec and EYFP: 2.22 ± 74.77 sec). We next examined locomotor responses to cocaine after overexpression of PGC-1α in NAc D1-MSNs. Mice received an injection of cocaine (10mg/kg) over five days and locomotor activity was assessed on day 1 and 5. Mice receiving PGC-1α overexpression in D1-MSNs and EYFP controls both displayed sensitized locomotor responses to cocaine on day 5 compared to day 1. However, the PGC-1α group displayed overall enhanced locomotion to cocaine on day 5 compared to EYFP controls (Figure 4F). Locomotion was unchanged between the PGC-1α group and the EYFP group during the habituation in the novel box (PGC-1α: 100,482 ± 9,407 mm and EYFP: 95,497 ± 3,995 mm) and during a saline injection (PGC-1α: 81,143 ± 6,375 mm and EYFP: 78,183 ± 3,184 mm).
Figure 4.
PGC-1α overexpression in D1-MSN enhances cocaine conditioned place preference and cocaine-induced locomotor activity. (A) Schematic of the double-floxed inverted open reading frame Cre-dependent AAV vector expressing PGC-1α-mCitrine. (B) Illustration of sagittal brain section in transgenic D1-Cre mice, showing the virus delivery site in NAc. (C) PGC-1α-mCitrine is expressed in NAc of D1-Cre mice but no expression is observed in wild type mice. Scale bar is 100µm. (D) AAV-DIO-PGC-1α- mCitrine overexpression D1-Cre NAc results in up-regulation of PGC-1α mRNA as compare to the EYFP control (n=8 per group). Student’s t test, t(14)=3.38 **p<0.01. (E) Cocaine conditioned place preference (7.5mg/kg) is enhanced in D1-Cre mice that received AAV-DIO-PGC-1α-mCitrine into NAc compared to mice receiving AAV-DIO-EYFP controls virus. Student’s t test, t(14)=2.26 *p<0.05; n=8 per group. (F) Locomotor activity was enhanced on day 5 of cocaine (10mg/kg) in D1-Cre mice receiving AAV-DIO-PGC-1α-mCitrine into NAc compared to controls mice expressing AAV-DIO-EYFP. Repeated measures two-way ANOVA; Interaction F(1,16) = 5.586; p=0.031, Bonferroni post test: **p<0.01; n=10 saline and n=8 cocaine. Error bars, SEM.
We next tested whether PGC-1α expression in D2-MSNs can alter behavioral responses to cocaine. We observed mCitrine expression in D2-Cre NAc but not wild-type NAc after AAV-DIO-PGC-1α-mCitrine infusion (Figure 5B). Similar to the D1-Cre mice, we observed an increase in PGC-1α mRNA in NAc of D2-Cre mice receiving AAV-DIO-PGC-1α-mCitrine compared to those receiving AAV-DIO-EYFP (Figure 5C). Overexpression of PGC-1α in D2-MSNs reduced time spent in the cocaine (7.5mg/kg) paired chamber during the post-test of CPP, compared to the EYFP control condition (Figure 5D). PGC-1α overexpression in D2-MSNs did not alter time in the cocaine-paired chamber during the pre-test compared to EYFP controls (PGC-1α: −17.61 ± 60.70 sec and EYFP: 3.02 ± 72.67 sec). PGC-1α overexpression in D2-MSNs did not result in differential locomotor responses on day 1 or day 5 of cocaine when compared to EYFP controls (Figure 5E). Nor did it alter locomotion during the habituation in the novel box (PGC-1α: 112,799 ± 8,167 mm and EYFP: 104,030 ± 8,232 mm) and during a saline injection (PGC-1α: 95,978 ± 8,451 mm and EYFP: 88,681 ± 6,864 mm). However, we did observe a failure to sensitize to cocaine from day 1 to day 5 in the PGC-1α group, whereas EYFP control mice displayed sensitization to cocaine (Figure 5E).
Figure 5.
PGC-1α overexpression in D2-MSNs disrupted cocaine conditioned place preference and sensitization to cocaine. (A) Illustration of sagittal brain section in transgenic D2-Cre mice, showing the virus delivery site in NAc. (B) PGC-1α-mCitrine is expressed in NAc of D2-Cre mice but no expression is observed in wild type mice. Scale bar is 100µm. (C) AAV-DIO-PGC-1α-mCitrine overexpression in D2-Cre NAc results in up-regulation of PGC-1α mRNA (n=4 per group). Student’s t test, t(6)=3.51 *p<0.05. (D) Cocaine conditioned place preference (7.5mg/kg) is decreased in D2-Cre mice that received AAV-DIO-PGC-1α-mCitrine into NAc compared to AAV-DIO-EYFP controls. Student’s t test, t(15)=2.14 *p<0.05; n=8–9 per group. (E) Locomotor activity (10mg/kg) D2-Cre mice receiving AAV-DIO-PGC-1α-mCitrine failed to sensitize to cocaine on day 5 compared to day 1, whereas expressing AAV-DIO-EYFP D2-Cre mice displayed sensitization to cocaine on day 5. Repeated measures two-way ANOVA; Interaction F(1, 14)=10.59; p=0.005, Bonferroni post test: ***p<0.001; n=8 per group. Error bars, SEM.
Discussion
Our data demonstrate for the first time a role for the transcriptional co-activator, PGC-1α, in the NAc in cocaine action. Repeated cocaine exposure increases PGC-1α mRNA and protein in NAc. This increase is specific to NAc D1-MSNs as we observed an increase in PGC-1α ribosome-associated mRNA, which likely represents mRNA undergoing translation to protein, in D1-MSNs after repeated cocaine. The increase in PGC-1α is consistent with enrichment of H3K4me3, a transcriptional activating methylation mark (31), on the PGC-1α promoter in the cocaine condition. Further, the increased binding of the transcription factor Egr3 to the PGC-1α promoter after repeated cocaine, implicates a role for Egr3 in active transcription of PGC-1α. It is plausible that the ChIP data is reflective of increased transcription in D1-MSNs but our current study cannot determine if the Egr3 regulation of and H3K4me3 binding on the PGC-1α promoter is occurring in these MSN subtypes. Nonetheless, the increase in PGC-1α mRNA in D1-MSNs is consistent with our previous study demonstrating enhanced Egr3 in NAc D1-MSNs after repeated cocaine (25).
Consistent with the increase of PGC-1α in D1-MSNs, we observed enhanced place preference for cocaine and enhanced cocaine-induced locomotor activity when PGC-1α was overexpressed in these MSNs. We previously demonstrated a similar behavioral outcome when expressing Egr3 selectively in D1-MSNs (25), thus our data implicate that Egr3 transcriptional regulation of PGC-1α is important for the cocaine behavioral plasticity. Our findings could also reflect enhanced transcription of PGC-1α in D1-MSNs by other factors including the transcription factors FosB and Nf-κB. FosB binding sites are found on the PGC-1α gene (39) and the long lasting isoform of FosB, deltaFosB, is increased in D1-MSNs after repeated cocaine (17, 21). Furthermore, enhancing deltaFosB levels in D1-MSNs potentiates behavioral responses to cocaine (19, 23). Similarly, the NF-κB pathway in the NAc regulates structural and behavioral plasticity to cocaine via increased expression level in NAc after repeated cocaine (24). Studies have shown that PGC-1α expression is regulated by NF- κB promoter binding (40, 41). Overall, our findings are consistent with numerous studies demonstrating enhanced transcriptional adaptations in D1-MSNs after repeated cocaine and that these transcriptional adaptations can underlie the pro-reward and locomotor behavioral responses to cocaine (42). Finally, a study by Chen et al demonstrating PGC-1α is involved in the formation and maintenance of dendritic spines (43) is consistent with numerous studies demonstrating enhanced synaptic and structural plasticity in D1-MSNs after repeated cocaine (44, 45, 46).
In contrast to the D1-MSN results we observed a decrease in PGC-1α in D2-MSNs after repeated cocaine exposure. This potentially reflects desensitization of Egr3 transcription at the PGC-1α promoter after repeated cocaine since Egr3 is reduced in D2-MSNs in this condition (25). However, the Egr3 and histone methylation ChIP data did not reflect the reduced PGC-1α transcription in D2-MSNs. It is interesting that an increase of PGC-1α in D1-MSNs and a decrease in D2-MSNs resulted in an overall net increase in PGC-1α levels in total NAc tissue after repeated cocaine. However, the results are not surprising because previous studies assessing molecular changes in total NAc often demonstrate that the change is specific to D1-MSNs and either opposite or unchanged in D2-MSNs (12, 19, 21, 22, 25). Additionally, the net increase in total NAc tissue could reflect alterations of PGC-1α in interneurons and non-neuronal cells.
Consistent with the reduction of PGC-1α in D2-MSNs we found that enhancing PGC-1α in these MSNs can blunt behavioral responses to cocaine, as observed by reduced place preference to cocaine and a failure to sensitize to cocaine. It was interesting that we observed basal differences in cocaine responses between the D1-Cre and D2-Cre lines in the control virus conditions. This did not appear to affect the behavioral responses to cocaine with PGC-1α overexpression in each MSN subtype, within each mouse line. This difference could reflect potential intrinsic differences between the two genotypes, including the integration of Cre recombinase within different sites of the genome.
Overall our studies demonstrate a novel role of PGC-1α in cocaine action. Additionally, we provide further evidence for bidirectional transcriptional regulation in D1-MSNs vs. D2-MSNs after a repeated cocaine regiment. It will be important to find molecules transcriptionally regulated by PGC-1α in MSN subtypes, with cocaine, as this could uncover molecules that can be directly therapeutically targeted in addiction. Furthermore, pharmacological agents that alter expression levels of PGC-1α or its upstream regulator, Egr3, in MSN subtypes could be potential therapeutic targets for translational studies. Additionally, examination of PGC-1α in clinically relevant behavioral models of drug addiction especially relapse paradigms (i.e. reinstatement to cocaine self-administration) can provide important information on PGC-1α following cocaine priming and further provide evidence for PGC-1α as a clinically relevant molecular target. Finally, given PGC-1α is an important transcriptional coactivator of genes involved in metabolic processes (1, 2), it will be important to examine these metabolic genes and their function in MSN subtypes, as well as examine whether PGC-1α also regulates these metabolic genes after cocaine. This could potentially uncover differential metabolic or energetic states in D1-MSNs vs. D2-MSNs, that underlie differential plasticity in these MSN subtypes and ultimately drive psychostimulant behavioral plasticity.
Acknowledgments
We would like to thank to Dr. K. Chandrasekharan for providing the PGC-1α vector (University of Maryland, Baltimore, USA). This work is supported by NIH R01DA038613. R.C. is supported by the Brain and Behavior Research Foundation (NARSAD Young Investigator, P&S Fund). ME is supported by Mission Interministérielle de Lutte contre les Drogues Et les Conduites Addictives.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures:
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Wallberg AE, Yamamura S, Malik S, Spiegelman BM, Roeder RG. Coordination of p300-mediated chromatin remodeling and TRAP/mediator function through coactivator PGC-1alpha. Mol Cell. 2003;12:1137–1149. doi: 10.1016/s1097-2765(03)00391-5. [DOI] [PubMed] [Google Scholar]
- 4.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 5.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malvaez M, Mhillaj E, Matheos DP, Palmery M, Wood MA. CBP in the nucleus accumbens regulates cocaine-induced histone acetylation and is critical for cocaine-associated behaviors. J Neurosci. 2011;31:16941–16948. doi: 10.1523/JNEUROSCI.2747-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson D, Koo JW, Feng J, Heller E, Rabkin J, Heshmati M, et al. Essential role of SIRT1 signaling in the nucleus accumbens in cocaine and morphine action. J Neurosci. 2013;33:16088–16098. doi: 10.1523/JNEUROSCI.1284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson D, Shao N, Heller E, Feng J, Neve R, Kim HD, et al. SIRT1-FOXO3a regulate cocaine actions in the nucleus accumbens. J Neurosci. 2015;35:3100–3111. doi: 10.1523/JNEUROSCI.4012-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Puigserver P, Spiegelman B, Montminy M, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 10.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 11.Czubryt MP, McAnally J, Fishman GI, Olson EN. Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1 alpha) and mitochondrial function by MEF2 and HDAC5. Proc Natl Acad Sci U S A. 2003;100:1711–1716. doi: 10.1073/pnas.0337639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlezon WAJ, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 13.Dinieri JA, Nemeth CL, Parsegian A, Carle T, Gurevich VV, Gurevich E, et al. Altered sensitivity to rewarding and aversive drugs in mice with inducible disruption of cAMP response element-binding protein function within the nucleus accumbens. J Neurosci. 2009;29:1855–1859. doi: 10.1523/JNEUROSCI.5104-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, et al. CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci. 2006;9:475–477. doi: 10.1038/nn1661. [DOI] [PubMed] [Google Scholar]
- 15.Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moratalla R, Robertson HA, Graybiel AM. Dynamic regulation of NGFI-A (zif268, egr1) gene expression in the striatum. J Neurosci. 1992;12:2609–2622. doi: 10.1523/JNEUROSCI.12-07-02609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moratalla R, Elibol B, Vallejo M, Graybiel AM. Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron. 1996;17:147–156. doi: 10.1016/s0896-6273(00)80288-3. [DOI] [PubMed] [Google Scholar]
- 18.Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, et al. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 19.Kelz MB, Chen J, Carlezon WAJ, Whisler K, Gilden L, Beckmann AM, et al. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- 20.Maze I, Covington HE, Dietz DM, LaPlant Q, Renthal W, Russo SJ, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, et al. DeltaFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci. 2013;33:18381–18395. doi: 10.1523/JNEUROSCI.1875-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robison AJ, Vialou V, Mazei-Robison M, Feng J, Kourrich S, Collins M, et al. Behavioral and structural responses to chronic cocaine require a feedforward loop involving DeltaFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J Neurosci. 2013;33:4295–4307. doi: 10.1523/JNEUROSCI.5192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. FosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci U S A. 2013;110:1923–1928. doi: 10.1073/pnas.1221742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, et al. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R, Francis TC, Konkalmatt P, Amgalan A, Gancarz AM, Dietz DM, et al. Opposing role for Egr3 in nucleus accumbens cell subtypes in cocaine action. J Neurosci. 2015;35:7927–7937. doi: 10.1523/JNEUROSCI.0548-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Zhang Y, Sun T, Guo F, Huang S, Chandalia M, et al. Dietary obesity-induced Egr-1 in adipocytes facilitates energy storage via suppression of FOXC2. Sci Rep. 2013;3:1476. doi: 10.1038/srep01476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16:632–638. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maze I, Nestler EJ. The epigenetic landscape of addiction. Ann N Y Acad Sci. 2011;1216:99–113. doi: 10.1111/j.1749-6632.2010.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJJ, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 34.Sanz E, Yang L, Su T, Morris DR, McKnight GS, et al. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci U S A. 2009;106:13939–13944. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandra R, Lenz JD, Gancarz AM, Chaudhury D, Schroeder GL, Han MH, C, et al. Optogenetic inhibition of D1R containing nucleus accumbens neurons alters cocaine-mediated regulation of Tiam1. Front Mol Neurosci. 2013;6:13. doi: 10.3389/fnmol.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Renthal W, Carle TL, Maze I, Covington HE, Truong HT, Alibhai I, et al. Delta FosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. J Neurosci. 2008;28:7344–7349. doi: 10.1523/JNEUROSCI.1043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng J, Wilkinson M, Liu X, Purushothaman I, Ferguson D, Vialou V, et al. Chronic cocaine-regulated epigenomic changes in mouse nucleus accumbens. Genome Biol. 2014;15:R65. doi: 10.1186/gb-2014-15-4-r65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanz E, Evanoff R, Quintana A, Evans E, Miller JA, Ko C, et al. RiboTag analysis of actively translated mRNAs in Sertoli and Leydig cells in vivo. PLoS One. 2013;8:e66179. doi: 10.1371/journal.pone.0066179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baresic M, Salatino S, Kupr B, van Nimwegen E, Handschin C. Transcriptional network analysis in muscle reveals AP-1 as a partner of PGC-1alpha in the regulation of the hypoxic gene program. Mol Cell Biol. 2014;34:2996–3012. doi: 10.1128/MCB.01710-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarez-Guardia D, Palomer X, Coll T, Davidson MM, Chan TO, Feldman AM, et al. The p65 subunit of NF-kappaB binds to PGC-1alpha, linking inflammation and metabolic disturbances in cardiac cells. Cardiovasc Res. 2010;87:449–458. doi: 10.1093/cvr/cvq080. [DOI] [PubMed] [Google Scholar]
- 41.Eisele PS, Salatino S, Sobek J, Hottiger MO, Handschin C. The peroxisome proliferator-activated receptor gamma coactivator 1alpha/beta (PGC-1) coactivators repress the transcriptional activity of NF-kappaB in skeletal muscle cells. J Biol Chem. 2013;288:2246–2260. doi: 10.1074/jbc.M112.375253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng A, Wan R, Yang JL, Kamimura N, Son TG, Ouyang X, et al. Involvement of PGC-1alpha in the formation and maintenance of neuronal dendritic spines. Nat Commun. 2012;3:1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J, Park BH, Lee JH, Park SK, Kim JH. Cell type-specific alterations in the nucleus accumbens by repeated exposures to cocaine. Biol Psychiatry. 2011;69:1026–1034. doi: 10.1016/j.biopsych.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 45.MacAskill AF, Cassel JM, Carter AG. Cocaine exposure reorganizes cell type- and input-specific connectivity in the nucleus accumbens. Nat Neurosci. 2014;17:1198–1207. doi: 10.1038/nn.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graziane NM, Sun S, Wright WJ, Jang D, Liu Z, Huang YH, et al. Opposing mechanisms mediate morphine- and cocaine-induced generation of silent synapses. Nat Neurosci. 2016;19:915–925. doi: 10.1038/nn.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]