Abstract
Background
The locus coeruleus (LC) signals salience to sensory stimuli and these responses can modulate the experience of pain stimuli. The pupil dilation response (PDR) to noxious stimuli is thought to be a surrogate for LC responses, but PDR response to Peltier-controlled noxious heat stimuli, the most commonly used method in experimental pain research, has not been described.
New Method
Healthy volunteers were presented with randomly presented heat stimuli of 5 sec duration and provided pain intensity ratings to each stimulus. Pupillometry was performed and a method developed to quantify the PDR relevant to these stimuli. The stimulus response, reliability, and effect of commonly used manipulations on pain experience were explored.
Results
A method of artifact removal and adjusting for lag from stimulus initiation to PDR response was developed, resulting in a close correlation between pain intensity rating and PDR across a large range of heat stimuli. A reliable assessment of PDR within an individual was achieved with fewer presentations as heat stimulus intensity increased. The correlation between pain rating and PDR was disrupted when cognitive load is increased by manipulating expectations or presenting a second pain stimulus.
Comparison with Existing Methods
The PDR began later after skin heating than electrical stimuli and this is the first examination of the PDR using standard nociceptive testing and manipulations of expectations and competing noxious stimulation.
Conclusions
A method is described applying PDR to standard heat nociceptive testing, demonstrating stimulus response, reliability, and disruption by cognitive manipulation.
Keywords: Locus coeruleus, pupillometry, experimental pain testing, human study
1. Introduction
Interactions between pain and noradrenergic system activity in the central nervous system are clinically relevant, but practical barriers have limited our progress in understanding and manipulating these interactions. The locus coeruleus (LC), which provides exclusive or near-exclusive noradrenergic innervation to the cortex, spinal cord, and several midbrain regions, is activated by nociceptive stimuli and can, under some circumstances, profoundly modulate pain neurotransmission and experience (Pertovaara, 2013). Yet, the circumstances and mechanisms by which LC activation by noxious stimuli modulates pain perception in humans are largely unexplored. This is the first in a series of studies designed to elucidate these circumstances and manipulate these mechanisms in humans.
Recent evidence confirms the utility of pupil diameter in humans as a measure of LC activity. There is a tight correlation between pupil diameter and BOLD activity in a dorsal pontine cluster overlapping with an established LC atlas (Keren et al., 2009) in individuals at rest and during a two stimulus oddball task (Murphy et al., 2014) or during systematically manipulated cognitive load (Alnaes et al., 2014). These clinical data are supported by invasive studies in monkeys which show a tight correlation between directly measured LC neuronal activity and fluctuations in pupil diameter at rest and dilation response to auditory stimuli, and consistent pupil dilation within 250–700 msec direct stimulation of the LC (Joshi et al., 2015).
Although noxious stimuli have long been known to result in a phasic pupil dilation response (PDR) in anesthetized animals (Loewenfeld, 1958) and humans (Cullen et al., 1972), less work has been done in conscious humans. PDR has been demonstrated in volunteers receiving noxious pressure (Ellermeier and Westphal, 1995), cold pressor (Tassorelli et al., 1995), or electrical stimuli (Chapman et al., 1999), although only in the latter case was a stimulus-response relationship described. With each modality there is a close relationship between pain intensity rating and PDR, and these are reduced in parallel in a concentration-dependent manner by the analgesic, nitrous oxide (Oka et al., 2007). The current studies aim to add to this understanding by examining the stimulus-response and temporal relationship between noxious phasic heat stimuli, commonly used in pain research, and the PDR.
LC activation, as assessed by pupil dilation, modulates the efficacy of preferred to non-preferred cognitive styles to learning (Eldar et al., 2013), and in signaling of surprise or unexpected uncertainty (Payzan-Lenestour et al., 2013; Preuschoff et al., 2011). As such, we explored in the current study the potential moderation of PDR on changes in pain report during cued learning of pain intensity and in potential surprise when a signal tied to pain intensity is false. Finally, conditioned pain modulation (CPM), wherein a continuous noxious stimulus produces heterotopic analgesia to another noxious test stimulus, is due in part to activation of LC projections to the spinal cord in rodents (Peters et al., 2015), and we explored whether changes in pupil diameter and PDR correlated with the strength of CPM.
2. Materials and Methods
The study was approved by the Wake Forest School of Medicine Institutional Review Board and written informed consent was obtained from all subjects. We recruited a convenience sample of 28 adult volunteers. Excluded were pregnant women and those within 2 years postpartum, those with history of surgery on the iris or taking eye drops which affect pupillary responses (α1-adrenergic receptor antagonists, muscarinic antagonists) or systemic medications with known actions on the LC (α2-adrenergic agonists, modafinil).
2.1 Cognitive and psychologic assessment
The following questionnaires were completed during the first visit: Catastrophizing Scale of the Coping Strategies Questionnaire (CATS), Center for Epidemiological Studies Scale of Depression (CESD), State-Trait Anxiety Inventory (STAI), Pain Anxiety Symptom Scale (PASS), Pain Locus of Control Scale (PLOC), and Anxiety Sensitivity Index (ASI).
2.2 Pain testing
Acute noxious heat stimuli were delivered via a commercially available, FDA approved device (TSA II, Medoc, Ramat Yishai, Israel). Thermal stimuli from 39 to 50°C were applied via a 16×16mm Peltier controlled thermode strapped to the volar aspect of the forearm or calf. The surface of the thermode was maintained at 35°C between stimuli. The sequence of stimuli was pre-programmed into blocks of 5–8 and the probe moved to a different skin area after each block to avoid sensitization. For each stimulus the probe temperature was increased at a rate of 6°C/sec to the target temperature, maintained at this temperature for 5 sec, then decreased to 35°C at 6°C/sec. Stimuli were separated by 30 sec. Within 5 sec after each stimulus the subject was asked to report the intensity of pain on a 0–100 verbal scale, with 0 representing no pain at all and 100 representing the worst imaginable pain. Every subject received the same two initial blocks of stimuli. In the first, stimuli of 39, 41, 43, 45, 47, 49, and 50°C were presented in ascending order to expose the subjects to the range of intensities and to get them used to rating pain intensity. Neither pain scores nor pupillometry data were used for analysis from this block. Next, each subject received these same 7 temperature stimuli, but in random order. These data were used for analysis.
2.3 Pupillometry
During pain testing subjects sat in a comfortable chair in a low-ambient light room with their head positioned on a chinrest and their forehead lightly against a positioning bar for continuous video recording of pupil diameter using a near infrared recording system (Eyestart®, Applied Science Laboratories, Bedford, MA). They were asked to fix their gaze on a gray crosshair on a dark background in the center of a 19 inch LED monitor positioned 22 inches from the chinrest. Gaze was not measured and pupil diameter was not corrected for gaze location. Pupil diameter was recorded at 60 Hz. Probe temperature from the Peltier controlled thermode was passed through an analog to digital converter and input to the EYE-TRAC® 6000 control unit and integrated into simultaneous pupil diameter and temperature file for later analysis.
Following completion of the first two blocks of heat stimuli, subjects received one of two sequences of testing (Figure 1), either for reliability and continuous visual analog scale (COVAS) pain assessment (n=11) or for expectations (n=17).
Figure 1.
Flow of subjects through the study. Boxed numbers in the periphery are the number of subjects in each study measure. CPM=conditioned pain modulation; COVAS=continuous mechanical visual analog scale measure of pain
2.4 Reliability sequence
These subjects received 5 blocks of 8 random, balanced presentations of 41, 47, 49, and 50°C stimuli applied to the calf, with Peltier-controlled thermode moved between each block. Pupillometry and verbal pain report were performed in blocks 1, 2, 4, and 5. During block 3 subjects moved a mechanical tab fixed within a 15 cm long slot at one end representing no pain and at the other representing the worst imaginable pain. The tab was connected to a potentiometer that provided continuous VAS (COVAS) pain intensity, and these data were time matched with probe temperature.
2.5 Expectations sequence
These subjects experienced noxious stimuli during a conditioned expectation paradigm while pupillary responses are assessed. They received 10 blocks of 8 random, balanced presentations of 47 and 50°C stimuli applied to the calf, with Peltier-controlled thermode moved between each block. Pupillometry and verbal pain report were performed for all stimuli. In the first two blocks an auditory cue (one tone for 47°C and two tones for 50°C) was provided 6 s prior to stimulus onset. Subjects were informed that there would be tones, but not that they would cue the intensity of the stimulus. These first two blocks yielded 5 stimuli of 47°C and 5 of 50°C, all correctly cued. In blocks 3–10, 25% of stimuli were incorrectly cued, yielding 5 incorrectly cued stimuli of 47°C and of 50°C.
2.6 Conditioned Pain Modulation
Following completion of the noxious heat protocols, subjects in the expectations sequence were tested for CPM. First, verbal pain report and pupillometry were recorded from a 5 sec 49°C stimulus to the volar forearm (without cue). Approximately one min later, the subject placed their foot in a container of circulating water maintained at 4°C for 90 sec. One minute after immersing their foot in the cold water verbal pain report and pupillometry were recorded during a 5 sec 49°C stimulus to the volar forearm. The difference in pain report to the noxious heat stimulus during the conditioning stimulus (foot in cold water) and that before the conditioning stimulus is a measure of CPM. After 90 sec the subject withdrew their foot from the cold water and it was warmed with warm blankets.
To examine the effect of the conditioning stimulus alone on pupil diameter, a different group of subjects, those in the reliability/COVAS sequence arm, experienced the conditioning stimulus for 90 sec without noxious heat testing (Figure 1).
2.7 Statistics
The goals of these exploratory studies were to estimate effects, effect sizes, and reliability of brief noxious heat stimuli on PDR, and to estimate the direction and influence of resting pupil diameter, expectations, and the conditioning stimulus on the PDR with brief noxious heat. We anticipated the following observations and applied the following exploratory analyses:
That intensity of noxious heat stimuli would be associated with the magnitude of PDR and that verbal pain report would correlate with PDR across the noxious stimulus intensity range and could serve as a biomarker for pain experience. Outliers were considered to be individual pupil diameter measurements less than 20 or greater than 90 mm. These individual measurements identified as outliers were replaced with mean pupil diameter for each noxious heat session within subject. Next, Loess smoothing was performed on the PDR series. To determine the lag of PDR to noxious heat stimuli, the maximum correlation between the two variables were determined using cross correlation of the noxious heat series to the smoothed PDR series. A lag of 4.25 sec after onset of noxious heat stimuli was considered to be optimal. The percentage change in baseline PDR diameter was calculated using a mean percentage change in baseline pupil diameter during the period between 4.25 and 17 sec after onset of noxious heat stimuli. Baseline pupil diameter was calculated at the onset of each individual noxious heat stimuli when the stimulus reached 37°C. Correlation of intensity of stimulus and pupil diameter change was denoted by Pearson’s r and analyzed using simple linear regression.
That verbal pain report and PDR in response to noxious heat would vary among individuals and that a reliable estimate for each individual could be obtained after a modest number of random presentations of noxious stimuli. Cronbach’s alpha was used as the measure of individual internal consistency of pupil diameter change in the reliability analysis.
That, as subjects learn that the intensity of stimuli can be predicted by auditory cues, pain report and PDR to noxious stimuli would be reduced in tandem and that this effect will resolve in tandem with repeated presentations of miscues. Generalized estimating equations were utilized to model pain and pupil responses over 5 learning and 5 unlearning trials.
That the first miscue would decrease pain report to a high intensity stimulus when a lower intensity is expected and increase pain report to a low intensity stimulus when a higher intensity is expected, but that in both cases, due to surprise, PDR will be increased in comparison to the delivered stimulus. The analysis consisted of a paired t-test for average of the last 2 correctly cued pain and pupil responses to 47° and 50°C stimuli to the first miscued stimuli.
That the strength of CPM (% change in pain intensity rating to the test stimulus during compared to before the conditioning stimulus) would be directly associated with tonic activation of the LC induced by the conditioning stimulus, thereby releasing more norepinephrine as a pain inhibitory neurotransmitter in the spinal cord. Thus we anticipated a direct relationship between the change in pupil diameter during the conditioning stimulus, just prior to the noxious heat test stimulus, and the strength of CPM and an indirect relationship between the PDR to noxious heat during the conditioning stimulus and the strength of CPM. Correlation of change in pain report and change in resting and stimulated pupil diameter was denoted by Pearson’s r.
All exploratory analyses were conducted using SAS 9.4, SAS Institute Inc., Cary, NC, USA; IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY, USA, R: A language and environment for statistical computing, R Foundation for Statistical Computing, Version 3.3.1, Vienna, Austria and RStudio: Integrated Development for R., Version 0.99.902, RStudio, Inc., Boston, MA, USA.
3. Results
Seventeen women and 11 men, aged 35 ± 11 years (25 White, 2 Black, 1 Asian) were recruited. Psychological questionnaire responses were consistent with a normal population (CATS 8.9 ± 8.4, CESD 4.7 ± 3.8, STAI 45 ± 3.9, PASS 55 ±24, ASI Total 12 ± 10, PLOC Total 101 ± 14). Of the 28 subjects recruited, 27 provided complete datasets; data during the cueing/miscueing experiment were not available for one subject due to equipment malfunction (Figure 1). All subjects tolerated all testing procedures and <1% of pupil response data were removed due to looking away or excessive blinking prior to or during the stimulus.
3.1 Stimulus-dependent effects on pain report and pupil diameter
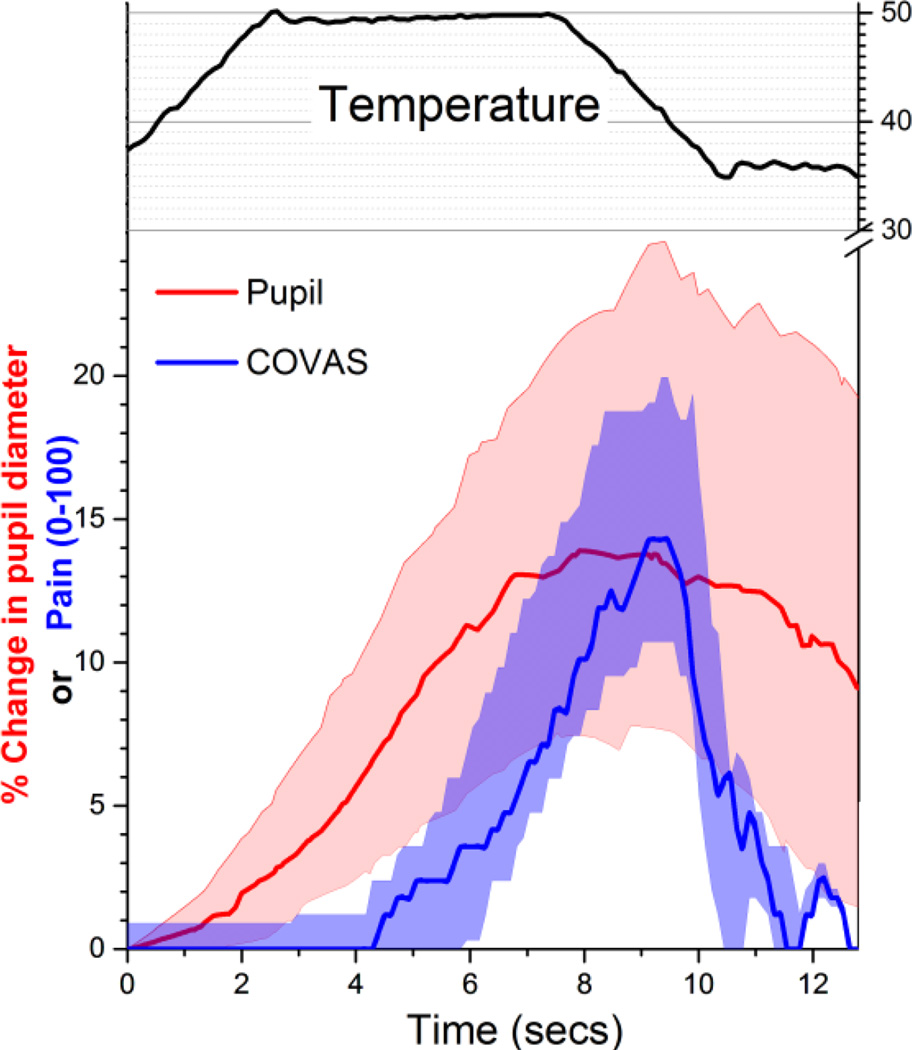
Thermal stimuli resulted in reporting of pain and in increased pupil diameter, although the time courses of these responses differed. As exemplified in the 11 subjects who continuous rated pain using the COVAS and, in separate trials, were assessed using pupillometry, pupil diameter increased earlier and was sustained longer than pain report to a 5 second plateau stimulus of 50°C (Figure 2). There was a lag of several seconds from the time the probe reached 50°C and peak responses to pupil diameter and to pain report. Pupil diameter began to increase nearly co-incident with the start of skin heating.
Figure 2.
Time course of temperature stimulus (°C in upper black trace) and, in separate trials, % change in pupil diameter (red) and continuous visual analog pain scale (COVAS, blue). Values are median (solid line) and 95% confidence intervals of 11 subjects.
Thermal stimuli resulted in a temperature dependent increase in pain intensity report and change in pupil diameter (Figure 3A and B, respectively). Although there was a strong association between pain report and PDR (P < 10−6), the correlation was only modest (Pearson’s r =0.47, adjusted R2=0.21) indicating that, in the conscious human, PDR was not an accurate biomarker for intensity of pain to acute phasic heat.
Figure 3.
Stimulus response for A) pain intensity and B) % change in pupil diameter with 5 sec noxious heat stimuli. Values are mean + SD of 28 subjects.
3.2 Changes in response with repeated stimulation
Since, under some conditions, habituation to noxious heat can be demonstrated (Hashmi and Davis, 2010), we examined the responses to 9 repeated exposures to 41, 47, 49, and 50°C in random order. There was no evidence for habituation in VAS pain (Figure 4A) or in PDR (Figure 4B; average of first and third tertiles shown for clarity) as assessed by GEE using temperature and presentation order as factors. The number of measurements required within an individual to reach 80% reliability as assessed by Cronbach’s alpha for PDR varied with intensity of stimulus: 6 measurements for the 50°C stimulus and 12, 23, and 43 for the 49°C, 47°C, and 41° stimuli, respectively. The relationship between pain report and PDR remained strong over this period of repeated exposure (Figure 4C; Pearson’s r=0.65 [p=1.4×10−6] for the first tertile and R=0.50 [p=5×10−5] for the third tertile). Modeling to account for variance in the outcome measures was consistent with a stable response. Individual subject, temperature, and their interaction accounted for a large portion of the variance in pain report (69%) and in PDR (41%), whereas presentation order accounted for only 0.1% and 2.7% of the variance in these outcomes, respectively.
Figure 4.
Responses to A) visual analog scale (VAS) pain and B) change in pupil diameter during the first 3 of 9 presentations (first tertile) and the last 3 presentations (last tertile) of randomly presented heat stimuli, shown in different colors. C) Relationship between change in pupil diameter and VAS pain for these 4 heat stimuli (using the same color scheme as the first two panels) during the first and last tertile of presentations. Values are mean ± SD of 11 subjects.
3.3 Changes in response while manipulating expectations
With correct verbal cueing prior to 47°C and 50°C stimuli, generalized estimating equations revealed a modest reduction in pain, particularly to the 47°C stimulus, resulting in greater ability of the subject to distinguish the two temperatures by pain report (Figure 5A, left panel). These changes disappeared with repeated, randomly presented stimuli which were miscued (Figure 5A, right panel). Generalized estimating equations modeling PDR with repeated cued and miscued stimuli also showed a greater distinction in this response with repeated correct cueing and loss of this distinction with repeated miscueing (Figure 5B), although responses did not decrease, but rather increased in response to the 50°C during correct cueing (P=0.042).
Figure 5.
Modeled A) pain report and B) change in pupil diameter during presentation of 50°C (red) and 47°C (blue) stimuli during sequential correct cueing (left panels) and sequential incorrect cueing (right panels). Lines are medians and areas are 95% confidence intervals of 16 subjects.
We separately assessed the first miscue in both positive and negative expectations. There was support for a mild effect of negative expectations when the subject received, for this first time, a miscue for 47°C but received 50°C in that verbal pain intensity report on this occasion (33±11) was lower than the average of the previous last correctly cued 50°C stimulus (41±26, P=0.007). We failed to observe a positive expectation response, in that the first miscue for 50°C when 47°C was actually delivered was similar to the average of the previous last correctly cued 47°C stimuli (8±12 vs 10±11, respectively, P=0.16).
PDRs in response to the first miscued stimuli paralleled those of pain report, with PDR being less to the 50°C stimulus when the subject anticipated a 47°C stimulus than during correct cueing of 50°C (4.5±7.9% vs 11±16%, respectively, P=0.039), whereas the PDR to the 47°C stimulus was the same whether it was proceeded by a correct or incorrect cue (1.8±5.2% with correct vs 0.9± 7.9% with incorrect, P=0.33). The relationship between pain report and PDR was stronger during the last two correctly cued (Pearson’s r =0.39; P=0.026) than during the first miscued stimulus (Pearson’s r=0.31; P=0.091).
3.4 Changes in response to Conditioned Pain Modulation
In the 11 subjects who only received the conditioning stimulus, moving the foot to a noxiously cool water bath was preceded by a large increase in pupil diameter with a gradual decline in pupil diameter to initial values over the next 60–90 seconds despite the foot remaining in the water bath (Figure 6A). In the 17 other subjects who also received test stimuli prior to and during the conditioning stimulus, there was an analgesic response in the presence of the conditioning stimulus, with pain report from the test stimulus decreasing from 39±28 to 31±25 (P=0.018). Similarly, PDR to the 49°C test stimulus decreased from 10±12% prior to the conditioning stimulus to 1.5±6.7% during the conditioning stimulus (P=0.003). In contrast to studies without CPM, there was not a significant correlation between pain report and PDR in subjects either before (Pearson’s r=0.28; P=0.27) or during (Pearson’s r=0.23; P=0.35) the conditioning stimulus in the CPM paradigm study. Correlations between strength of CPM and resting pupil diameter or PDR before conditioning, or PDR during conditioning, or change in pupil diameter or PDR during conditioning were not significant. However, smaller pupil diameter during the conditioning stimulus was associated with greater reductions in pain report (Pearson’s r =0.52; P=0.041).
Figure 6.
A) Change in pupil diameter normalized at the time the foot was placed in cold water, before and for 90 sec during immersion. Line is mean ± SD of 11 subjects. B) Strength of conditioned pain modulation, depicted as change in pain report from a noxious test stimulus during compared to before the cold conditioning stimulus, as a function of pupil diameter during the conditioning stimulus just prior to the test stimulus. Each point is one subject.
4. Discussion
The key finding in these studies is that PDR in response to phasic noxious heat stimuli in conscious, normal volunteers correlates strongly with rating of pain intensity only under tightly controlled conditions. When validity of expectations is violated (first miscued stimulus in the learning paradigm) or when anticipation or experience of tonic pain elsewhere is present (prior to and during the conditioning stimulus of CPM), the relationship between PDR and pain intensity rating weakens and, in this modest sample size, fails to reach statistical significance. These findings are consistent with the hypothesis that acute changes in pupil diameter reflect changes in LC activity, because LC activity is affected by noxious heat (Khan and Stroman, 2015) but also by internal factors, including level of arousal (Murphy et al., 2011), cognitive difficulty of task (Alnaes et al., 2014), and distraction (Jepma and Nieuwenhuis, 2011). The current exploratory studies provide a basis for future studies using PDR to assess the role of the LC in pain experience to phasic noxious heat under different cognitive conditions.
4.1 PDR in the absence of other manipulations
The strong relationship between probe temperature during stimulation and pain report or PDR as well as the strong relationship between pain report and PDR within and across individuals in the current study parallels finding with electrical stimuli applied to fingertips (Chapman et al., 1999). In addition to the modality of stimulation, differences between these studies largely reflect temporal factors of stimulation (5 msec pulse of electricity versus 5 sec heat stimulus; nearly instantaneous activation of nociceptors with electricity to slow heating of skin over seconds to reach activation temperature) which are paralleled in timing of PDR response (onset in 300 msec and duration of < 3 sec to electricity; onset in seconds and duration of over 15 sec to heat). In contrast, sustained noxious stimulation, such as a 5 minute immersion of the hand in cold water (Tassorelli et al., 1995) or a 90 sec immersion of the foot in cold water (current study) results in a transient (<60 sec) PDR despite sustained or worsening experience of pain. These data suggest that PDR can under controlled conditions reflect the intensity of a brief noxious stimulus, but cannot be used as a continuous monitor of pain when the stimulus is longer lasting.
The reliability of PDR in the current study depended on the intensity of the stimulus. With the highest stimulus intensity, 50°C, only 6 trials were necessary to reliably define an individual response, whereas the number of trials became progressively larger with less intense stimuli. This could reflect increased variability in pain report as one approaches pain threshold and potential increased cognitive load, which would affect the PDR, in rating pain near threshold. These data suggest that studying individual differences with stimuli near threshold would require large number of repeated trials, and that the utility of PDR to gauge pain response to mildly painful stimuli is practically limited. Whether correcting pupil diameter for shifts in gaze in this setting where subjects are instructed not to shift gaze would improve reliability is under current study.
4.2 PDR during learning and its extinction
Pain report to the 47°C stimulus was reduced during correct cueing, consistent with a reduction in pain when a noxious stimulus is more predictable (Yoshida et al., 2013) and this effect disappeared with loss of predictability during miscuing.
We anticipated, but did not observe, that pain report and PDR would change in parallel during learning and extinction. In one sense, there was a parallel effect of learning in that learning resulted in a clearer distinction both in pain rating and in PDR between the two stimulus intensities, and this improved distinction resolved during extinction. On the other hand, learning produced divergent effects on pain report, with reduction in pain report selectively to the 47°C stimulus but augmentation of the PDR selectively to the 50°C stimulus. We speculate that anticipation of a more intense experience (50°C vs 47°C stimulus) enhances LC activation to the stimulus, although further study would be required to test this hypothesis and whether it was unique to noxious stimuli. LC activation and PDR reflect surprise (Preuschoff et al., 2011) and unexpected uncertainty (Payzan-Lenestour et al., 2013) and we anticipated, but did not observe, that PDR would increase after the first miscue as a reflection of surprise.
4.3 PDR during conditioned pain modulation
As previously described (Tassorelli et al., 1995), we observed that immersion of a limb in noxiously cold water resulted in only a transient PDR at the beginning of the immersion. In the current study the PDR occurred largely just prior to foot immersion in the cold water, consistent with either an anticipatory or effect or internal stimuli regarding the motor response. These could have been distinguished by use of a body temperature water bath as a control. In a previous study (Tavernor et al., 2000), there was no pupil dilation with such a control compared to dilation with noxious cold water, but that report provided only average pupil diameter data over the 90 sec of immersion, so whether there was a transient effect is unknown.
We anticipated, but did not observe, that the conditioning stimulus would induce increased LC activity as measured by increased pupil diameter and that this increased activity would result in release of norepinephrine in the spinal cord causing analgesia and correlating with the strength of CPM on pain report (Peters et al., 2015). This was not observed and, in contrast to this hypothesis, the resting pupil diameter just prior to the test stimulus while the foot was in the water correlated negatively with the strength of CPM. Exploratory analyses found no other associations between strength of CPM and PDR or change in resting pupil diameter. Whether this reflects a lack of importance of descending noradrenergic inhibitory signaling in the spinal cord from the LC on CPM, confounding factors which influence overall LC activity in this paradigm, or disconnect between LC activity and pupil diameter in this setting will require further study.
It is important to place these results in context of what is known regarding processes which affect LC activity and LC neuroanatomy. As noted, the LC responds to many types of internal and external stimuli, including noxious sensory stimuli, and projects to many spinal and supraspinal structures. The LC does not directly innervate neural circuits which control pupil diameter and the close association between acute changes in LC activity and pupil diameter are presumed to reflect coincident activity in an area which does regulate pupil diameter, speculated to be the nucleus paragigantocellularis (Costa and Rudebeck, 2016). There are data supporting the presence of single LC neurons which project both to the cord and supraspinally (Howorth et al., 2009) and other data to support topological segregation of cells in the LC projecting uniquely to specific sites (Uematsu et al., 2015). We do not know the projections of LC neurons which correlate most strongly with changes in pupil diameter.
In summary, phasic noxious heat produces an intensity-dependent increase in pain report and in PDR in healthy volunteers who are otherwise undisturbed. The relationship between pain report and PDR is, however, disrupted with unexpected miscuing of the stimulus intensity and in the presence of an ongoing noxious stimulus elsewhere in the CPM paradigm. Learning to predict the intensity of a pain stimulus by cues results in better ability to distinguish heat stimuli of different intensities by pain report and by PDR. PDR was not predictive of the strength of CPM as anticipated. These data are consistent with the complexity of internal and external factors which regulate LC activity in the conscious human and provide the basis for further mechanistic examination of PDR in assessing LC activity in response to pain.
Highlights.
Acute, non-light induced changes in pupil diameter reflect acute changes in locus coeruleus activity
Pupil dilation occurs in response to noxious stimulation, but the effect of Peltier thermode-controlled heat stimuli on pupil response has not been evaluated
In volunteers, the degree of pupil dilation correlates with noxious heat temperature and with pain intensity report
The relationship between pupil dilation and pain intensity report is disrupted when cognitive load is increased by manipulating expectations or presenting a second pain stimulus
Acknowledgments
Dr. Eisenach has received fees for consultation to Adynxx (San Francisco, CA, USA) on topics unrelated to this article.
Supported in part by grant P01 GM113852 from the National Institute of Health, Bethesda, MD.
ABBREVIATIONS
- ASI
Anxiety Sensitivity Index
- CATS
Catastrophizing Scale of the Coping Strategies Questionnaire
- CESD
Center for Epidemiological Studies Scale of Depression
- COVAS
continuous visual analog scale for pain
- CPM
conditioned pain modulation
- LC
locus coeruleus
- PASS
Pain Anxiety Symptom Scale
- PDR
pupil dilation response
- PLOC
Pain Locus of Control Scale
- VAS
visual analog scale for pain
- STAI
State-Trait Anxiety Inventory
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests. The other authors have no competing interests.
References
- Alnaes D, Sneve MH, Espeseth T, Endestad T, van de Pavert SH, Laeng B. Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. J Vis. 2014;14:1–20. doi: 10.1167/14.4.1. [DOI] [PubMed] [Google Scholar]
- Chapman CR, Oka S, Bradshaw DH, Jacobson RC, Donaldson GW. Phasic pupil dilation response to noxious stimulation in normal volunteers: relationship to brain evoked potentials and pain report. Psychophysiology. 1999;36:44–52. doi: 10.1017/s0048577299970373. [DOI] [PubMed] [Google Scholar]
- Costa VD, Rudebeck PH. More than Meets the Eye: the Relationship between Pupil Size and Locus Coeruleus Activity. Neuron. 2016;89:8–10. doi: 10.1016/j.neuron.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen DJ, Eger EI, 2nd, Stevens WC, Smith NT, Cromwell TH, Cullen BF, Gregory GA, Bahlman SH, Dolan WM, Stoelting RK, Fourcade HE. Clinical signs of anesthesia. Anesthesiology. 1972;36:21–36. doi: 10.1097/00000542-197201000-00005. [DOI] [PubMed] [Google Scholar]
- Eldar E, Cohen JD, Niv Y. The effects of neural gain on attention and learning. Nat. Neurosci. 2013;16:1146–1153. doi: 10.1038/nn.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier W, Westphal W. Gender differences in pain ratings and pupil reactions to painful pressure stimuli. Pain. 1995;61:435–439. doi: 10.1016/0304-3959(94)00203-Q. [DOI] [PubMed] [Google Scholar]
- Hashmi JA, Davis KD. Effects of temperature on heat pain adaptation and habituation in men and women. Pain. 2010;151:737–743. doi: 10.1016/j.pain.2010.08.046. [DOI] [PubMed] [Google Scholar]
- Howorth PW, Teschemacher AG, Pickering AE. Retrograde adenoviral vector targeting of nociresponsive pontospinal noradrenergic neurons in the rat in vivo. J. Comp Neurol. 2009;512:141–157. doi: 10.1002/cne.21879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepma M, Nieuwenhuis S. Pupil diameter predicts changes in the exploration-exploitation trade-off: evidence for the adaptive gain theory. J Cogn Neurosci. 2011;23:1587–1596. doi: 10.1162/jocn.2010.21548. [DOI] [PubMed] [Google Scholar]
- Joshi S, Li Y, Kalwani RM, Gold JI. Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron. 2015 doi: 10.1016/j.neuron.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren NI, Lozar CT, Harris KC, Morgan PS, Eckert MA. In vivo mapping of the human locus coeruleus. Neuroimage. 2009;47:1261–1267. doi: 10.1016/j.neuroimage.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan HS, Stroman PW. Inter-individual differences in pain processing investigated by functional magnetic resonance imaging of the brainstem and spinal cord. Neuroscience. 2015;307:231–241. doi: 10.1016/j.neuroscience.2015.08.059. [DOI] [PubMed] [Google Scholar]
- Loewenfeld IE. Mechanisms of reflex dilatation of the pupil; historical review and experimental analysis. Documenta ophthalmologica. Proceedings series. 1958;12:185–448. doi: 10.1007/BF00913471. [DOI] [PubMed] [Google Scholar]
- Murphy PR, O'connell RG, O'Sullivan M, Robertson IH, Balsters JH. Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum. Brain Mapp. 2014;35:4140–4154. doi: 10.1002/hbm.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PR, Robertson IH, Balsters JH, O'connell RG. Pupillometry and P3 index the locus coeruleus-noradrenergic arousal function in humans. Psychophysiology. 2011;48:1532–1543. doi: 10.1111/j.1469-8986.2011.01226.x. [DOI] [PubMed] [Google Scholar]
- Oka S, Chapman CR, Kim B, Nakajima I, Shimizu O, Oi Y. Pupil dilation response to noxious stimulation: effect of varying nitrous oxide concentration. Clin Neurophysiol. 2007;118:2016–2024. doi: 10.1016/j.clinph.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Payzan-Lenestour E, Dunne S, Bossaerts P, O'Doherty JP. The Neural Representation of Unexpected Uncertainty during Value-Based Decision Making. Neuron. 2013;79:191–201. doi: 10.1016/j.neuron.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertovaara A. The noradrenergic pain regulation system: a potential target for pain therapy. Eur J Pharmacol. 2013;716:2–7. doi: 10.1016/j.ejphar.2013.01.067. [DOI] [PubMed] [Google Scholar]
- Peters CM, Hayashida KI, Suto T, Houle TT, Aschenbrenner CA, Martin TJ, Eisenach JC. Individual Differences in Acute Pain-induced Endogenous Analgesia Predict Time to Resolution of Postoperative Pain in the Rat. Anesthesiology. 2015;122:895–907. doi: 10.1097/ALN.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff K, 't Hart BM, Einhauser W. Pupil Dilation Signals Surprise: Evidence for Noradrenaline's Role in Decision Making. Front Neurosci. 2011;5:115. doi: 10.3389/fnins.2011.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassorelli C, Micieli G, Osipova V, Rossi F, Nappi G. Pupillary and cardiovascular responses to the cold-pressor test. J Auton. Nerv. Syst. 1995;55:45–49. doi: 10.1016/0165-1838(95)00026-t. [DOI] [PubMed] [Google Scholar]
- Tavernor SJ, Abduljawad KA, Langley RW, Bradshaw CM, Szabadi E. Effects of pentagastrin and the cold pressor test on the acoustic startle response and pupillary function in man. J Psychopharmacol. 2000;14:387–394. doi: 10.1177/026988110001400407. [DOI] [PubMed] [Google Scholar]
- Uematsu A, Tan BZ, Johansen JP. Projection specificity in heterogeneous locus coeruleus cell populations: implications for learning and memory. Learning & memory (Cold Spring Harbor, N.Y.) 2015;22:444–451. doi: 10.1101/lm.037283.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida W, Seymour B, Koltzenburg M, Dolan RJ. Uncertainty increases pain: evidence for a novel mechanism of pain modulation involving the periaqueductal gray. J Neurosci. 2013;33:5638–5646. doi: 10.1523/JNEUROSCI.4984-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]