Abstract
BACKGROUND
Relapse is a two-component process consisting of a highly motivated drug-seeking phase that, if successful, is followed by a drug-using phase resulting in temporary satiation. In rodents, cue-induced drug-seeking requires transient synaptic potentiation (t-SP) of cortical glutamatergic synapses on nucleus accumbens core medium spiny neurons, but it is unknown how achieving drug use affects this plasticity. We modeled the two phases of relapse after extinction from cocaine self-administration to assess how cocaine use affects t-SP associated with cue-induced drug seeking.
METHODS
Rats were trained to self-administer cocaine (n=96) or used as yoked-saline controls (n=21). After extinction, reinstatement was initiated by 10 min of cue-induced drug seeking, followed by 45 min with contingent cocaine access, after which cocaine was discontinued and unreinforced lever pressing ensued. Three measures of t-SP were assayed during reinstatement: dendritic spine morphology, AMPA:NMDA ratios, and matrix metalloproteinase activity.
RESULTS
We found that cocaine use for 10 min collapsed all three measures of cue-potentiated t-SP back to baseline. Moreover, when cocaine use was discontinued 45 min later, dendritic spine morphology and AMPA:NMDA were restored as animals became motivated to engage unrewarded lever pressing. Non-reinforced drug seeking was positively correlated with changes in spine morphology and cocaine access reversed this relationship.
CONCLUSIONS
Using a novel modification of the reinstatement paradigm we show that achieving cocaine use reversed the synaptic plasticity underpinning the motivation to seek drug.
Keywords: drug abuse, synaptic plasticity, motivation, nucleus accumbens, cocaine, reinstatement
INTRODUCTION
Drug addiction is a chronic illness defined by enduring vulnerability to relapse to drug use after extended abstinence (1). A relapse consists of two sequential phases: a highly motivated drug-seeking state, and if seeking is successful, subsequent drug use (2, 3). The circuit and cellular substrates underpinning the high motivation associated with the drug-seeking phase of relapse is studied in the rodent self-administration/reinstatement model using contingent presentation of Pavlovian cues previously associated with drug infusions to recapitulate the powerful motivation to seek produced by drug-associated cues in addicts (4). In this model, motivation is operationally defined as non-reinforced lever pressing in pursuit of drug reward. Cued reinstatement of cocaine-, nicotine- and heroin-seeking requires inducing rapid, transient synaptic potentiation (t-SP) at glutamatergic synapses on nucleus accumbens core (NAcore) medium spiny neurons (MSNs) (5–8). Transient-SP is quantified using standard electrophysiological and morphological measures consistent with increasing synaptic strength, including increases in the ratio of AMPA to NMDA currents (AMPA:NMDA) and dendritic spine head diameter (dh) or density (9–11). Recently, we found that t-SP requires catalytic cleavage of the extracellular matrix surrounding cortico-accumbens synapses by activated matrix metalloproteinase-2 and -9 (MMP-2,9), and increased MMP-2,9 activity constitutes a third t-SP measure (6). The importance of t-SP in cue-induced drug seeking is revealed in studies where interfering with glutamatergic transmission from prelimbic afferents to NAcore (5, 12) or blocking MMP-9 activity (6) prevents t-SP induction and drug seeking. Correspondingly, the intensity of drug seeking is positively correlated with the extent of t-SP (5).
Recent studies show that cued drug-seeking depends on t-SP at glutamate synapses in NAcore (5, 6), but it is unknown what happens when the highly motivated state of drug-seeking culminates in drug use that produces temporary satiation (defined as the process by which drug consumption causes a transient decrease in motivation for drug seeking) (2, 3). Understanding the mechanism whereby drug use temporarily reduces drug seeking could reveal cellular mechanisms of satiation as useful targets for inhibiting the excessive desire to relapse. We postulated that if drug seeking successfully culminates in drug use, switching from the drug-seeking to drug-using phase of relapse would reverse t-SP. Moreover, when drug availability is discontinued, rats re-enter a robust drug-seeking phase (13, 14), and we hypothesized that the return to highly motivated drug seeking would renew t-SP. Thus, we adapted a reinstatement paradigm to model distinct phases of relapse and measure t-SP: 1) cued reinstatement of lever pressing (non-reinforced drug seeking), 2) cue-induced reinstatement leading to cocaine use (drug using), and 3) discontinuing cocaine availability (return to non-reinforced drug seeking). We found that t-SP underpinning cocaine seeking was collapsed by cocaine use and when drug seeking was renewed, inputs to NAcore MSN synapses re-potentiated.
METHODS AND MATERIALS
Subjects and surgery
Male Sprague–Dawley rats (250–300 g, Charles River Laboratories) were maintained on a 12–12 hr reverse light-dark cycle with ad-libitum food and water. After 1 week of vivarium acclimation, rats were implanted with jugular catheters. Rats used for in vivo zymography additionally underwent stereotaxic surgery delivering 23-gauge cannula to NAcore (SI Experimental Procedures). Food was temporarily restricted to 25 g/day standard chow prior to food training. After one food training session, rats were restored to ad lib feeding. Experimental procedures were approved by the Animal Care and Use Committee of the Medical University of South Carolina and performed in accordance with National Institutes of Health guidelines.
Self-administration
Rats were trained to acquire operant responding for food in a single 2-hour session prior to beginning 2-hour daily self-administration sessions or were yoked saline controls receiving noncontingent saline infusions paired with light/tone cues according to a pre-programmed pattern of average cocaine responding (Fig 1A). Rats self-administered cocaine (0.2 mg/0.05 ml infusion, ~0.5–0.67 mg/kg/infusion; National Institute of Drug Abuse) until reaching criterion of ≥10 days with >10 infusions, followed by 1–2 weeks extinction (15). Training was performed in standard operant chambers containing house and cue lights, tone, and two retractable levers (Med Associates, St. Albans, VT). Subjects were trained on a fixed-ratio 1 schedule of reinforcement paired with light and tone (78 dB, 4.5 kHz) and followed by a 20-s timeout period signaled by absence of house light. During extinction, active lever presses no longer resulted in cocaine or cues. Extinction criteria were met when animals averaged <25 active lever presses for 2 days prior to reinstatement testing.
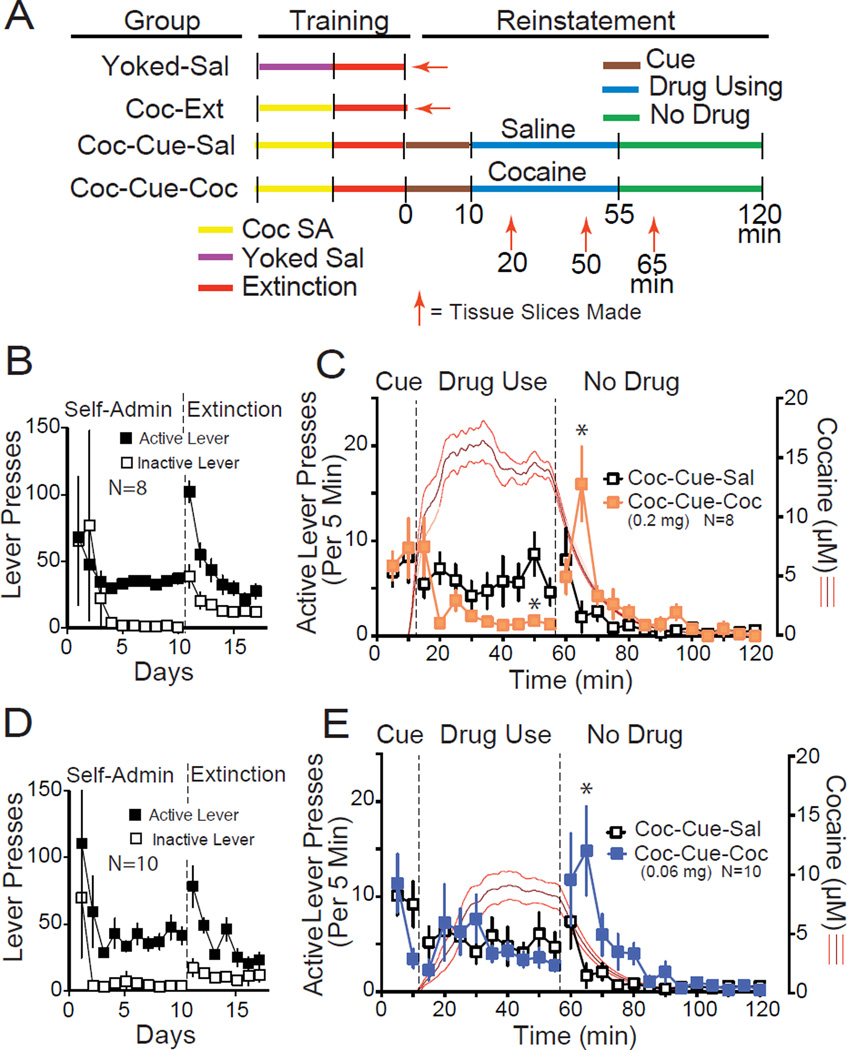
Figure 1. Adapting the cue-induced reinstatement model to assess relapse that includes using cocaine.
(A) Diagram of the experimental time line and all comparison groups used throughout this study. Schematic outlines the modified reinstatement protocol and time points for obtaining slices of NAcore tissue and making measures of t-SP (red arrows). Two control groups did not enter the reinstatement protocol (Yoked-Sal and Coc-Ext), and tissue was obtained 24 h after the last extinction session. Two groups entered the reinstatement protocol. After 10 min of standard cue-induced reinstatement of lever pressing in both groups, either cocaine (Coc-Cue-Coc) or saline (Coc-Cue-Sal) was restored to the active lever, and 45 min later cues and cocaine/saline infusions were discontinued in both groups. SA=period of self-administration. (B) Active and inactive lever pressing during cocaine self-administration and extinction for rats assigned to receive the higher concentration of cocaine (0.2 mg/0.05 ml) during reinstatement. (C) Active lever pressing in 5 min bins over the time course of the protocol in Figure 1A using the 0.2 mg/infusion dose of cocaine (rats were reinstated in a randomized crossover design such that each rat was reinstated with restoration of saline and cocaine on alternate days with ≥2 days of extinction between tests). The red line indicates modeled mean ± SEM concentration of brain cocaine using an algorithm based on animal weight and infusions of cocaine self-administered (16). (D) Active and inactive lever pressing during cocaine self-administration and extinction for rats assigned to receive the lower concentration of cocaine (0.06 mg/0.05 ml) during reinstatement. (E) Active lever pressing in 5 min bins over the time course of the protocol in Fig 1A using the 0.06 mg/infusion dose of cocaine. The red line indicates the modeled mean ± SEM concentration of cocaine.
* p< 0.05 comparing the active lever presses between the Coc-Cue-Sal versus Coc-Cue-Coc, using a Tukey’s post hoc test.
Reinstatement and obtaining brain tissue
The protocol and timeline for obtaining tissue is illustrated in Fig 1A. Two groups (Yoked-Sal; Coc-Ext) were killed 24 hr after the last extinction session and did not enter reinstatement. Two groups entered a reinstatement session (Coc-Cue-Sal; Coc-Cue-Coc). During the first 10 minutes of all reinstatement sessions, responses on the active lever resulted in contingent delivery of previously drug-paired light/tone cues, akin to standard cue-induced reinstatement (0–10 min). Between 11–55 min saline infusions accompanied cues with each lever press in the Coc-Cue-Sal group, while cocaine plus cues was delivered in the Coc-Cue-Coc group using self-administration conditions outlined above. Cocaine was delivered at 0.2 mg/0.05 ml infusion during this phase of the reinstatement session, except for in a control experiment where 0.06 mg/0.05 ml infusion was used. Brain cocaine concentrations were estimated in accordance with the method described by Pan et al (16) (SI Experimental Procedures). During the last 65 minutes of the trial (56–120 min), infusions and cue delivery ceased for both the Coc-Cue-Sal and Coc-Cue-Coc groups. Reinstatement groups were killed at various times after initiating the session (20, 50 or 65 min) to capture the effect of cocaine delivery on cue-induced t-SP (20 min), the effect of continued saline versus cocaine delivery (50 min), and the effect of removing cocaine and saline delivery (65 min).
Dendritic spine quantification
A confocal microscope (LEICA) was used to image DiI-labeled sections (SI Experimental procedures) using the Helium/ Neon 543-nm laser line. DiI-labeled neurons and dendrites (Figure 2A,B) were imaged via optical sectioning by a 63× oil immersion objective (numerical aperture =1.4) with pixel size 0.01 µm in the XY plane and 0.13-µm intervals along the z-axis. Images were deconvolved by Autoquant (Media Cybernetics), and a 3D perspective was rendered by the Surpass module of the Imaris software (Bitplane, Concord, MA). Spines on dendrites beginning at >75 µm and ending at ≤200 µm distal to the soma and after the first branch point were quantified from NAcore MSNs. Three to twelve segments (45–55 µm each) were analyzed per animal (mean = 8 segments/rat). Minimum dh was set at ≥0.143 µm to reflect the Nyquist frequency resolution limits of the microscope.
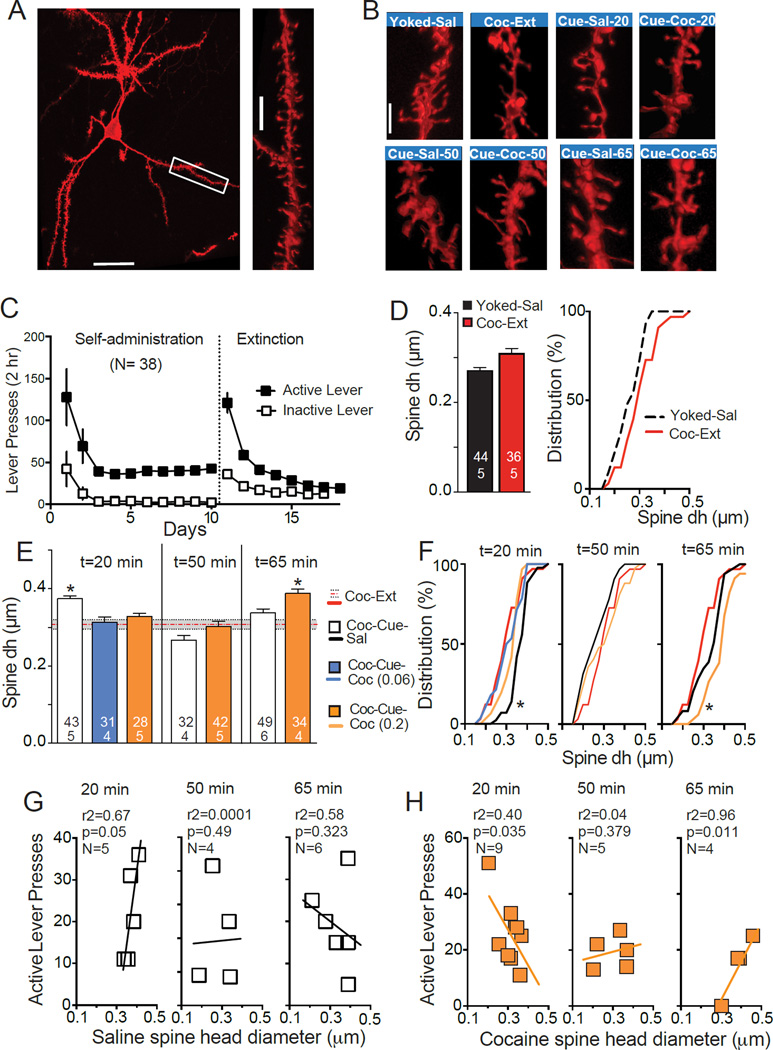
Figure 2. Cocaine access reverses cue-induced increases in dendritic spine dh during cue-induced cocaine-seeking.
(A) Representative diolistically-labeled neuron from NAcore. Bar= 50 µm. Box indicates source of the high magnification dendrite in the right panel. Bar= 5 µm. (B) Representative segments of dendrite from each condition. Sal-20, -50, -65 and Coc-20, -50, -65 = rats examined at time= 20, 50 or 65 min (Fig 1B) that were allowed to self-administer saline (Coc-Cue-Sal) or cocaine (Coc-Cue-Coc). Bar= 3 µm. (C) Active and inactive lever pressing during self-administration and extinction for rats used to quantify spine morphology. (D) There was no difference in dh between Yoked-Sal and Coc-Ext rats when the data were compared using a Students t-test (left panel) or regression analysis of a frequency distribution plot (right panel). Numbers in each bar refer to the number of neurons quantified over number of animals. (E) Spine dh that was increased with contingent presentation of drug cues in Coc-Cue-Sal rats was reduced back to extinction levels by access to cocaine in Coc-Cue-Coc animals (t= 20 min), and enlarged again when drug-seeking was renewed by removing cocaine access in the Coc-Cue-Coc group (t=65 min). (F) Frequency distribution plots for each experimental group. (G) Correlations between spine dh and active lever presses in animals self-administering saline averaged over the 15 and 20 min bin for t=20 min, 45 and 50 bins for t=50 min, and 60 and 65 min for t=65 min. Line was drawn via linear regression analysis. (H) Correlations between spine dh and active lever presses in animals self-administering cocaine averaged over 5 min time bins as in Figure 2G. Note that at t=20, both the 0.2 mg and 0.06 mg dose cocaine groups were used in regression analysis since both doses significantly collapsed dh back to extinction baseline (Figure 1E).
*p < 0.05, panel E compared to Coc-Ext dh using a 1-way ANOVA over each time epoch followed by a Dunnett’s post hoc comparison; panel F comparing line elevations of the Coc-Ext group to all other groups in each panel.
Slice preparation and whole-cell recordings
Coronal slices were collected into a vial containing artificial cerebrospinal fluid. Recordings were collected at 32°C in dorsomedial NAcore. Inhibitory synaptic transmission was blocked with picrotoxin (100 µM). AMPA and NMDA currents were recorded in whole-cell patch-clamp configuration. Glass microelectrodes (1.5–2.5 MΩ) were filled with cesium-based internal solution. To evoke postsynaptic currents, we placed a bipolar stimulating electrode (FHC, Bowdoin, Maine) ~300 µm dorsomedial of the recorded cell to maximize chances of stimulating PL afferents. Stimulation intensity was chosen to evoke EPSCs of 200–400 pA while the cell was voltage clamped at −80 mV, which typically represented 30–70% of maximum eEPSC amplitude. Recordings were collected every 20 s and begun >10 min after rupturing the cell membrane to allow diffusion of internal solution. AMPA currents were first measured at −80 mV to ensure response stability. Membrane potential was gradually increased until +40 mV. Recording was resumed 5 min after reaching +40 mV to allow stabilization of cell parameters. Currents composed of both AMPA and NMDA components were obtained. D-AP5 was bath applied (50 µM) to block NMDA currents and recording of AMPA currents at +40 mV was started after 2 min. NMDA currents were obtained by subtracting AMPA from total current at +40 mV.
In vivo zymography measurement of MMP-2,9 activity
Rats were microinjected with intramolecularly dye-quenched fluorescein-conjugated gelatin (Life Technologies) reconstituted in PBS (1 mg/ml) in NAcore 20 min prior to obtaining brain tissue. For the yoked saline or cocaine extinction groups the microinjection was made 24 h after the last extinction session and rats were placed back into the home cage after gelatin microinjection before sacrifice 20 min later. Alternatively, groups undergoing reinstatement (Coc-Cue-Sal and Coc-Cue-Coc) were placed into the operant box immediately after microinjection for 20 min to quantify lever pressing and evaluate the effect of access to cocaine on MMP-2,9 activity. Rats were transcardially perfused with phosphate buffer (PB) followed by 4.0% paraformaldehyde in PB. Coronal sections (50 µm) were mounted in Prolong Gold (Life Technologies). Images were obtained on a confocal microscope (LEICA) using a 488 nm Argon laser line, emissions filtered to 515–535 nm through a 10× objective (numerical aperture=0.3). Slices in which the injection site and anterior commissure were visualized in the same frame were used for quantification. The anterior commissure was masked-out of quantification, as was the injection tract since MMP activity is induced by mechanical tissue damage. Fluorescence was quantified (ImageJ, NIH) as integrated density from 4–6 sections per rat averaged and reported as arbitrary units (AU) normalized to the values obtained from cocaine-extinguished rats.
Statistics
Statistics were performed using Prism (GraphPad Software, La Jolla, CA). Behavioral data were analyzed by one- or two-way ANOVAs as appropriate followed by Tukey post-hoc tests for multiple comparisons. Spine dh was analyzed with a two-level nested ANOVA with a Satterthwaite approximation (17). A nested ANOVA was used because we have one measurement variable (dh) and multiple nominal variables or groups (treatment conditions). The nominal variables are nested to form subgroups within groups (individual rats). In this way, we test for significant variation in means among groups, among subgroups and within groups. The Satterthwaite approximation corrects for unequal sample sizes providing a more accurate P value by using modified mean squares at each level (17). Post-hoc evaluations were made over each experiment (time point of analysis) using a one-way ANOVA followed by Dunnett’s post-hoc for comparison to extinction values. Spine density data were analyzed both using the nested ANOVA, but also according to convention by collapsing data within animals and using a one-way ANOVA followed by Dunnett’s post-hoc for multiple comparisons. Electrophysiological data were analyzed as individual cells from 3–5 animals per group using a one-way ANOVA with Dunnett’s post-hoc for multiple comparisons. Zymography measurements were analyzed by one-way ANOVA. Significance was set at p ≤ 0.05 and data are presented as mean ± SEM.
RESULTS
Modeling the phases of a relapse in rats extinguished from cocaine self-administration
First, we examined behavioral responses in the reinstatement protocol shown in Figure 1A. Rats were trained to self-administer cocaine and extinguished (Figure 1B). After extinction, rats underwent the modified reinstatement protocol (Figure 1A) with trials separated by 2–6 days of extinction using a random crossover design such that all rats had a session with saline (Coc-Cue-Sal) and cocaine (Coc-Cue-Coc). The time course of responding graphed in 5 minute bins (Figure 1C) shows that active lever pressing was affected by whether rats self-administered cocaine (Coc-Cue-Coc) or saline (Coc-Cue-Sal) during the Drug Using epoch (t=11–55 min) using a 2-way ANOVA with repeated measures over treatment and time (time F(23,161)=7.07, p<0.001; treatment F(1,7)=0.37, p=0.562; interaction F(23,161)=4.32, p<0.001). Notably, when cocaine was discontinued there was a significant rebound in lever pressing compared to discontinuing saline. Also, during the Drug Use epoch cocaine significantly reduced active lever pressing compared to saline. This reduction in lever pressing by cocaine left open the possibility that the amount of lever pressing, not cocaine exposure might modulate t-SP in subsequent experiments. Therefore, we added a control group that received a lower dose of cocaine (0.06 mg/infusion) during the reinstatement trial. These animals were trained using the same dose of cocaine as all other experimental groups in this study (Figure 1D). During the reinstatement session, when the lower dose of cocaine was restored to active lever pressing the Coc-Cue-Coc animals maintained lever pressing in the range of the Coc-Cue-Sal group, but also demonstrated rebound pressing when this lower dose of cocaine was discontinued (Figure 1E; time F(23,207)=7.15, p<0.001; treatment F(1,9)=0.62, p=0.452; interaction F(23,207)=2.19, p=0.002). We modeled brain cocaine concentrations in both Coc-Cue-Coc groups receiving during the reinstatement session and found that cocaine levels were reduced in rats using the low dose compared with the high dose of cocaine, although stable levels were reached in both cases demonstrating differential titration by dose (mean ± SEM of the last 15 min of cocaine: 0.06 mg cocaine= 9.01±1.21 µM, N=10; 0.2 mg cocaine= 14.75±0.93 µM, N=8; t(16)= 3.68, p= 0.002) (e.g. compare Figs 1C and 1E).
Cocaine access reverses cue-induced increases in dendritic spine head diameter (dh)
We assessed dynamic changes in dendritic spine morphology of NAcore MSNs that might parallel switching between drug-seeking and drug-using phases of relapse. Spine dh and density were quantified from three-dimensionally reconstructed images of DiI-labeled NAcore MSNs (Figure 2A,B). Rats were trained to self-administer cocaine and lever pressing was extinguished (Figure 2C). There was an overall significant difference between all groups for spine dh (nested ANOVA Fs(8,34)= 2.28, p= 0.046). Rats extinguished from daily cocaine self-administration (Coc-Ext) showed a non-significant trend towards larger mean spine dh than yoked-saline controls (Figure 2D). Ten minutes of cued reinstatement plus 10 min of saline self-administration revealed that cues alone (i.e. Coc-Cue-Sal group) increased mean spine dh compared with Coc-Ext animals (Figure 2B,E). In contrast, spine dh collapsed to extinction baseline in rats allowed to self-administer either dose of cocaine for 10 min (Coc-Cue-Coc groups; F(3,131)= 10.40, p< 0.001). The difference in spine dh can be seen in the cumulative distribution where the Coc-Cue-Sal group is shifted to the right of other groups (comparing elevations F(3,67)= 3.55, p= 0.019), (Figure 2F). The shift was largely due to fewer thin spines in the Coc-Cue-Sal group relative to the other treatment groups. Thus, only 7% of the neurons averaged dh<0.3 µm in the Coc-Cue-Sal group, while the other groups had 57% (Coc-Ext), 29% (Coc-Cue-Coc, 0.2 mg dose) and 50% (Coc-Cue-Coc, 0.06 mg dose).
A separate set of rats was examined after 40 min of cocaine or saline use (i.e. 50 min in Figure 1A). Spine dh returned to extinction baseline in Coc-Cue-Sal animals and Coc-Cue-Coc rats remained at extinction baseline values (Figure 2E). Finally, rats were examined 10 min after saline or cocaine use was discontinued (65 min, Figure 1A), and the Coc-Cue-Coc group showed increased spine dh, while the Coc-Cue-Sal animals remained at extinction baseline (Figure 2E) (F(2,113)= 11.31, p< 0.001). The increased dh is reflected in a shift to the left in the Coc-Cue-Coc group compared with Coc-Ext or Coc-Cue-Sal groups in the cumulative distribution plot (elevations F(2,50)= 9.63, p< 0.001), and this resulted largely from a reduction in thin spines in the Coc-Cue-Coc group. Thus, the percent of neurons with an average dh<0.3 µm was 15% in the Coc-Cue-Coc group, but 35% in Coc-Cue-Sal.
The possibility that the changes in spine dh were related to motivation to seek drug is indicated by a positive correlation between lever pressing and dh after 20 min of drug seeking in the Coc-Cue-Sal group, and the lack of correlation during saline use (t=50 min) and after discontinuing saline availability (t=65 min) when lever pressing reduced to extinction levels (Figure 2G). In contrast, when cocaine was made available in the Coc-Cue-Coc group, a negative correlation between lever pressing and spine dh was found at t=20 min (Figure 2H). When cocaine-using rats were examined at 50 min, the negative correlation between spine dh and active lever pressing was lost, but when cocaine use was discontinued, a positive correlation returned at t=65 (Figure 2H). We also analyzed dendritic spine density, but it did not show an overall significant difference between groups (One-way ANOVA F(7,30)= 0.864, p= 0.053; Nested ANOVA F(7,29)= 1.844, p= 0.117) (Figure S1).
Cocaine access reversed cue-induced increases in AMPA:NMDA ratio
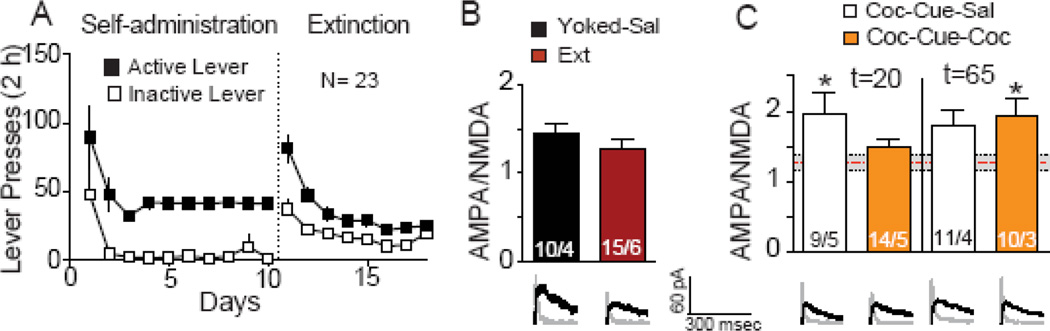
Increased spine dh can be associated with potentiated synaptic function (10). Therefore we conducted experiments using whole-cell patch clamp recordings to measure AMPA:NMDA ratios in NAcore MSNs. Rats were trained to self-administer cocaine and extinguished (Figure 3A). Similar to spine dh, there was no difference in AMPA:NMDA between the Coc-Ext versus Yoked-Sal groups (Fig 3B). Cue-reinstated animals that accessed saline infusions for 10 min (Coc-Cue-Sal) had a significant increase in AMPA:NMDA, and this was reduced to extinction baseline values in Coc-Cue-Coc animals that accessed cocaine self-administration for 10 min (Figure 3C) (F(3,45) =3.05, p = 0.038). Another groups of rats were examined for AMPA:NMDA at the 65 min time point. As observed with dendritic spine dh, there was a rapid repotentiation of AMPA:NMDA upon removal of cocaine access in Coc-Cue-Coc rats (Fig 3C) (F(5,64)= 2.72, p=0.027). There was a trend for AMPA:NMDA to remain elevated in the Coc-Cue-Sal group at t=65. This is consistent with previous work showing that cue-induced increase in AMPA:NMDA remains elevated at 45 min and did not return to extinction baseline until 120 min after initiating reinstatement (5). In contrast with dh, there was no correlation measured between active lever pressing and AMPA:NMDA in either group at either time point (t=20: saline- r2=0.52, p=0.085, N=5; cocaine- r2=0.42, p= 0.171, N=5; t=65: saline- r2=0.57, p= 0.243, N=4; cocaine- r2= 0.95, p= 0.135, N=3).
Fig 3. Cocaine access reverses cue-induced increases in AMPA:NMDA ratio.
(A) Active and inactive lever pressing during self-administration and extinction for rats used to quantify AMPA:NMDA. (B) There was no difference in AMPA:NMDA between Yoked-Sal and Coc-Ext rats when the data were compared using a Students t-test. (C) Increase in AMPA:NMDA (mean±SEM ratio) after cue-induced reinstatement in animals self-administering saline (Coc-Cue-Sal) was reversed by 10 min cocaine access (Coc-Cue-Coc). When cocaine access was removed at t=65 AMPA:NMDA was increased. Dashed red line indicates extinction baseline. Numbers in bars represent number of cells recorded over number of animals in each condition. Representative traces are shown for each treatment beneath the corresponding bar in Panels B and C. Black line= NMDA current, Gray line= AMPA current.
*p< 0.05, comparing all groups to extinction baseline (Ext) using a Dunnett’s post hoc test.
Cocaine access reversed cue-induced increases in MMP-2,9 activity
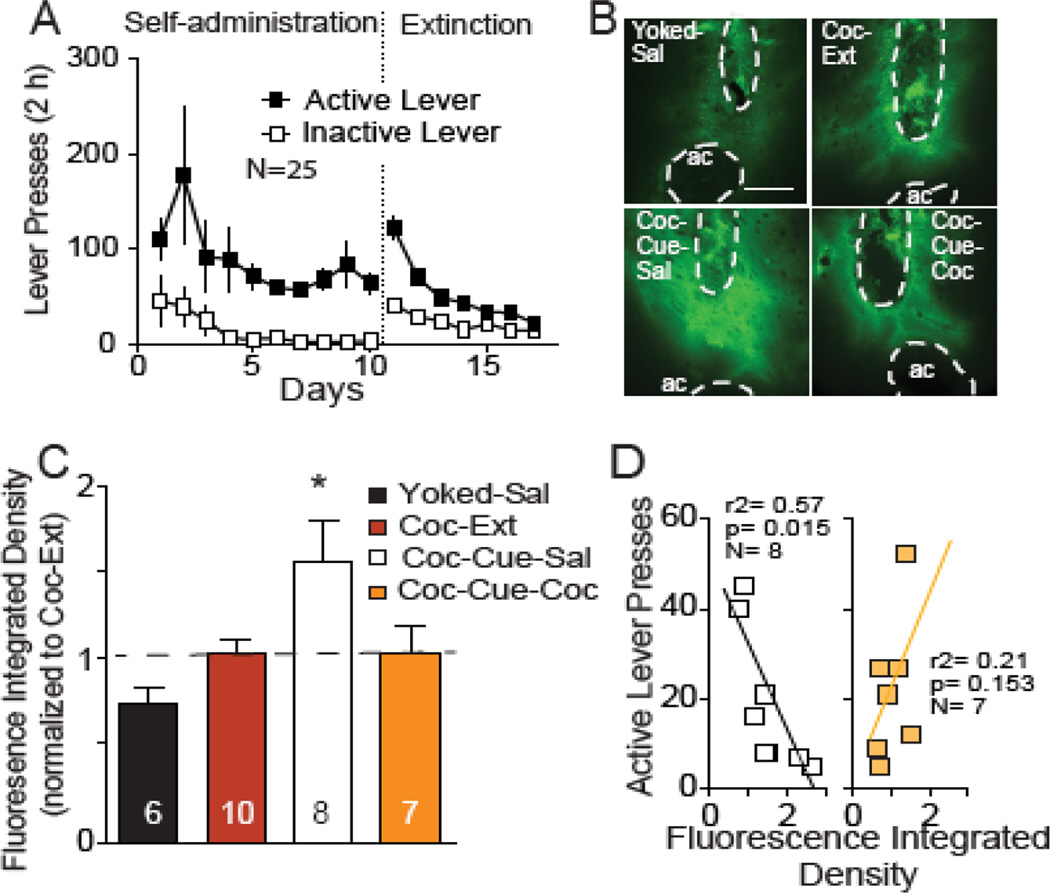
Matrix metalloproteinases are endopeptidases that degrade the extracellular matrix, and catalytic activation of the extracellular matrix by the gelatinase family of MMPs (MMP-2,9) is necessary for inducing hippocampal synaptic plasticity and facilitates hippocampus-dependent memory (18, 19). Activity of MMP-2,9 is also required for increasing AMPA:NMDA and spine dh during cue-induced t-SP in cocaine-trained rats (6). We hypothesized that gelatinase activation during cued-reinstatement would be reversed by access to cocaine self-administration. To test this, a FITC-coupled dye-quenched gelatin was infused into NAcore. This gelatin substrate is cleaved by MMP-2,9 liberating fluorescence that increases linearly over time (6). Animals were trained to self-administer cocaine and extinguished (Figure 4A). MMP-2,9 activity did not differ between Yoked-Sal and Coc-Ext animals (Figure 4B,C), but cue-induced reinstatement produced a 50% increase in MMP-2,9 activity in NAcore of Coc-Cue-Sal rats (Figure 4C)(F(3,27) =4.46, p = 0.011). In contrast, restoration of cocaine infusions in Coc-Cue-Coc rats reversed the cue-induced increase in MMP-2,9 activity and did not differ from Coc-Ext animals. Coc-Cue-Sal rats showed a significant negative correlation between active lever pressing and MMP-2,9 activity, while no correlation was found in Coc-Cue-Coc rats (Figure 4D). The paradoxical negative correlation in Coc-Cue-Sal animals may arise because MMP-2,9 activity is proximal in the sequence of events leading to increased spine dh and AMPA:NMDA, and we previously observed a rapid (within 15 min) induction of tissue inhibitor of metalloproteinase-2 (TIMP-2, inhibitory regulator of MMP-2,9 activity) by cue-induced reinstatement (6). Accordingly, while the motivation to seek cocaine and the increase in spine dh and AMPA:NMDA is initiated by MMP-2,9 activity, this activity may come under inhibitory regulation by TIMP-2 within 15 min after reinstating cocaine-seeking. The 65 min time point was not examined for MMP-2,9 activity because making a microinjection of gelatin substrate during the reinstatement session (i.e. at t=45, 20 min prior to obtaining tissue) disrupted drug-seeking behavior (see Methods).
Fig 4. Cocaine access reverses cue-induced increases in MMP-2,9 activity.
(A) Active and inactive lever pressing during self-administration and extinction for rats used to quantify MMP-2,9 activity. (B) Representative examples of FITC-gelatin fluorescence corresponding to MMP-2,9 activity in NAcore from each treatment group. Dashed line outlines the anterior commissure (ac) and injection site that were masked-out for quantification. Bar= 0.5 mm. (C) Increase in MMP-2,9 (mean ± SEM normalized integrated density of fluorescence reported in arbitrary units) after cue-induced reinstatement in animals self-administering saline (Coc-Cue-Sal) was reversed by 10 min cocaine access (Coc-Cue-Coc). Data from 4–6 coronal slices were averaged per rat, and the number in the bar refers to number of rats in each group. (D) Negative correlation between active lever pressing and the integrated density of fluorescence indicative of MMP-2,9 activity in Coc-Cue-Sal animals, but no correlation was found in the Coc-Cue-Coc rats. These data are derived from the data shown in figure 4C and behavior was quantified as described in Figure 2 legend.
*p< 0.05, comparing all groups to extinction baseline (Ext) using a Dunnett’s post hoc test.
DISCUSSION
Cued reinstatement of drug seeking markedly potentiates (t-SP) glutamatergic synapses on MSNs in NAcore. The degree of potentiation is correlated with the intensity of reinstated lever pressing (5–7), indicating that t-SP is a positive correlate of the motivation to seek drug. Here we show that gaining access to cocaine for 10 min completely reversed all three measures of t-SP. In doing so, lever pressing became inversely correlated with spine dh. Moreover, when cocaine use was discontinued, the motivation to seek drug accelerated (increased non-reinforced lever presses) and the positive correlation between dh and active lever pressing was restored. The sequence of changes in dh and AMPA:NMDA over the cycle of drug-seeking, drug use and return to drug-seeking after drug removal indicates that t-SP is strongly associated with the motivation to seek drug. Thus, it follows that the reduction of t-SP by cocaine use may also be a correlate of the temporarily blunted motivation (or satiation) that occurs in cocaine users when they first achieve use after a period of highly motivated drug-seeking (2, 3). Moreover, the linkage between cue-induced t-SP and motivation is supported by the fact that both reinstated cocaine seeking and t-SP are abolished by inactivating prelimbic cortex (5, 12) or inhibiting MMP-9 activity (6).
Does cocaine-induced dopamine release decrease motivation?
The neurochemical and circuit mechanisms whereby cocaine use reversed t-SP are not clarified in this study. However, numerous studies point to potential involvement of the capacity for cocaine to increase extracellular dopamine (20). In contrast to cued reinstatement, when non-reinforced lever pressing is reinstated by noncontingent cocaine injection, t-SP is delayed to 45 min post-injection. This parallels the time-course of reinstated lever pressing, whereas cue-induced reinstatement peaks within the first 15 min of the session (21). Not only does this support the correlation between t-SP and motivated lever pressing, but also raises the intriguing possibility that the cocaine-induced increase in extracellular dopamine may suppress t-SP. Thus, as brain cocaine levels decline following an acute noncontingent injection, the motivation to seek drug and t-SP increases. Indeed, this would also explain the increase in t-SP and motivated lever pressing produced in the present study when cocaine self-administration was discontinued. Finally, pharmacological inactivation of VTA or systemic antagonism of dopamine receptors blocks cocaine-reinstated lever pressing and prevents t-SP (21), consequently it is reasonable to postulate that the ability of self-administered cocaine to reverse cue-induced t-SP may likewise depend on a dopaminergic mechanism. Consistent with this postulate, animals are known to titrate their cocaine intake around stable brain concentrations of cocaine and extracellular dopamine (22, 23)(also see modeled brain cocaine levels in Figure 1C,E). Moreover, the satiating effects of cocaine are regulated by dopaminergic mechanisms (24). Taken together, these data seem consistent with the notion that increases in dopamine transmission could trigger the collapse of t-SP by cocaine use.
Does cocaine-induced glutamate release decrease motivation?
Our results are also consistent with literature demonstrating that in cocaine-experienced animals a cocaine challenge elicits synaptic depression at constitutively potentiated NAc glutamatergic synapses (25). After extended withdrawal from either contingent or noncontingent cocaine, glutamatergic synapses are potentiated as measured by dh or spine density (5, 10, 26), AMPA currents and receptor surface expression (11, 25, 27, 28), and MMP-2 activity (6). Boudreau and colleagues show that acute cocaine injections cause internalization of accumbens AMPA receptors, and postulate that cocaine-induced AMPA receptor internalization is produced by elevated glutamate release (27), since this is known to occur in vitro (29, 30). This is consistent with the increase in extracellular glutamate produced in NAcore during cocaine-induced reinstatement (31). Surprisingly, in the Boudreau et al. study, a saline challenge induced the same level of AMPA receptor internalization in cocaine-sensitized rats suggesting that the drug associated cue (i.e. the injection procedure) fully substituted for an acute cocaine injection. These results contrast with the present findings in that the presence of drug-associated cues alone (animals given saline access) produced t-SP and contingent cocaine depressed accumbens synapses. This difference may be attributed to differences between noncontingent cocaine-induced sensitization and contingent cocaine self-administration, since the latter produces stronger conditioned associations (32).
Conclusions
The bidirectional change in t-SP according to whether an animal is motivated to seek cocaine by conditioned cues or temporarily satiated by using cocaine indicates that t-SP is potentially a useful index of the state of motivated drug-seeking behavior. Importantly, t-SP is produced in NAcore by drug-associated cues across multiple classes of addictive drug, but not by cue-induced motivation to seek sucrose, a biological reward (5). Together, our data argue that the cellular and circuit mechanisms mediating the bidirectional regulation of t-SP by drug seeking and drug use are important mediators of relapse and highlight the potential value of studying t-SP produced in NAcore by a relapse event towards understanding addiction, a chronic relapsing disorder. Moreover, this study highlights the importance of investigating the neurobiology underpinning the entire relapse event, including both the highly motivated drug-seeking phase, as well as the culmination of drug seeking in drug use.
Supplementary Material
Acknowledgments
We thank Drs. Michael D. Scofield and Peter J. Vento as well as Ben Siemsen, Charles Thomas, and Madhura Athreya for advice and technical assistance. This research was supported by USPHS grants DA003906, DA012513, DA015369 (PWK) and DA037722, Burroughs Wellcome Fund (1012607, SS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures. The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iannaccone LR. Addiction and satiation. Economics Letters. 1986;21:95–99. [Google Scholar]
- 3.Kostowski W. Drug addiction as drive satisfaction ("antidrive") dysfunction. Acta neurobiologiae experimentalis. 2002;62:111–117. doi: 10.55782/ane-2002-1427. [DOI] [PubMed] [Google Scholar]
- 4.Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology. 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith AC, Kupchik YM, Scofield MD, Gipson CD, Wiggins A, Thomas CA, et al. Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci. 2014;17:1655–1657. doi: 10.1038/nn.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, et al. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A. 2013;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci U S A. 2011;108:19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosokawa T, Rusakov DA, Bliss TV, Fine A. Repeated confocal imaging of individual dendritic spines in the living hippocampal slice: evidence for changes in length and orientation associated with chemically induced LTP. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:5560–5573. doi: 10.1523/JNEUROSCI.15-08-05560.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends in Neurosciences. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neuroscience & Biobehavioral Reviews. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stefanik MT, Kupchik YM, Kalivas PW. Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Structure and Function. 2015:1–9. doi: 10.1007/s00429-015-0997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. The Journal of pharmacology and experimental therapeutics. 1968;161:122–129. [PubMed] [Google Scholar]
- 14.Pushparaj A, Pryslawsky Y, Forget B, Yan Y, Foll BL. Extinction bursts in rats trained to self-administer nicotine or food in 1-h daily sessions. American Journal of Translational Research. 2012;4:422–431. [PMC free article] [PubMed] [Google Scholar]
- 15.Spencer S, Brown RM, Quintero GC, Kupchik YM, Thomas CA, Reissner KJ, et al. α2δ-1 Signaling in Nucleus Accumbens Is Necessary for Cocaine-Induced Relapse. The Journal of Neuroscience. 2014;34:8605–8611. doi: 10.1523/JNEUROSCI.1204-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan H-T, Menacherry S, Justice JB. Differences in the Pharmacokinetics of Cocaine in Naive and Cocaine-Experienced Rats. Journal of Neurochemistry. 1991;56:1299–1306. doi: 10.1111/j.1471-4159.1991.tb11425.x. [DOI] [PubMed] [Google Scholar]
- 17.McDonald JH. Handbook of biological statistics. Baltimore, MD: Sparky House Publishing; 2009. [Google Scholar]
- 18.Wojtowicz T, Mozrzymas JW. Late phase of long-term potentiation in the mossy fiber-CA3 hippocampal pathway is critically dependent on metalloproteinases activity. Hippocampus. 2010;20:917–921. doi: 10.1002/hipo.20787. [DOI] [PubMed] [Google Scholar]
- 19.Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, et al. Matrix Metalloproteinase-9 Is Required for Hippocampal Late-Phase Long-Term Potentiation and Memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willuhn I, Wanat MJ, Clark JJ, Phillips PEM. Dopamine Signaling in the Nucleus Accumbens of Animals Self-Administering Drugs of Abuse. Current topics in behavioral neurosciences. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen HW, Gipson CD, Huits M, Kalivas PW. Prelimbic cortex and ventral tegmental area modulate synaptic plasticity differentially in nucleus accumbens during cocaine-reinstated drug seeking. Neuropsychopharmacology. 2014;39:1169–1177. doi: 10.1038/npp.2013.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pettit HO, Justice JB., Jr Effect of dose on cocaine self-administration behavior and dopamine levels in the nucleus accumbens. Brain Res. 1991;539:94–102. doi: 10.1016/0006-8993(91)90690-w. [DOI] [PubMed] [Google Scholar]
- 23.Zimmer BA, Dobrin CV, Roberts DCS. Brain-Cocaine Concentrations Determine the Dose Self-Administered by Rats on a Novel Behaviorally Dependent Dosing Schedule. Neuropsychopharmacology. 2011;36:2741–2749. doi: 10.1038/npp.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suto N, Wise RA. Satiating effects of cocaine are controlled by dopamine actions in the nucleus accumbens core. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:17917–17922. doi: 10.1523/JNEUROSCI.1903-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- 30.Xiao M-Y, Zhou Q, Nicoll RA. Metabotropic glutamate receptor activation causes a rapid redistribution of AMPA receptors. Neuropharmacology. 2001;41:664–671. doi: 10.1016/s0028-3908(01)00134-4. [DOI] [PubMed] [Google Scholar]
- 31.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heidbreder C. Advances in Animal Models of Drug Addiction. In: Hagan JJ, editor. Molecular and Functional Models in Neuropsychiatry. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. pp. 213–250. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.