Abstract
Background
Opiate abuse and overdose reached epidemic levels in the USA. However, despite significant advances in animal and in vitro models, little knowledge has been directly accrued regarding the neurobiology of the opiate-addicted human brain.
Methods
We used post-mortem human brain specimens from a homogeneous European Caucasian population of heroin users for transcriptional and epigenetic profiling as well as direct assessment of chromatin accessibility in the striatum, a brain region central to reward and emotion. A rat heroin self-administration model was used to obtain translational molecular and behavioral insights.
Results
Our transcriptome approach revealed marked impairments related to glutamatergic neurotransmission and chromatin remodeling in the human striatum. A series of biochemical experiments tracked the specific location of the epigenetic disturbances to hyperacetylation of lysine 27 (H3K27ac) of histone H3, showing dynamic correlations with heroin use history and acute opiate toxicology. Targeted investigation of GRIA1, a glutamatergic gene implicated in drug-seeking behavior, verified the increased enrichment of H3K27ac at discrete loci, accompanied by enhanced chromatin accessibility at hyperacetylated regions in the gene body. Analogous epigenetic impairments were detected in the striatum of heroin self-administering rats. Using this translational model, we showed that bromodomain inhibitor JQ1, which blocks the functional read-out of acetylated lysines, reduced heroin self-administration and cue-induced drug-seeking behavior.
Conclusions
Overall, our data suggest that heroin-related histone H3 hyperacetylation contributes to glutamatergic transcriptional changes that underlie addiction behavior and identify JQ1 as a promising candidate for targeted clinical interventions in heroin use disorder.
Keywords: heroin, addiction, glutamate, epigenetics, histone acetylation, JQ1
INTRODUCTION
With an unprecedented 80% increase in the number of heroin abusing or dependent individuals over the past few years, opiates are currently the second most prevalent class of abused drugs in the United States and opiate overdose is now the leading cause of death among drug users (1–3). The fact that heroin is one of the most addictive drugs, along with increasing availability of both heroin and prescription opioids (4–8), has led to an increasing burden on society (9) and emphasizes the critical need for greater efforts to expand the understanding of neurobiological underpinnings of heroin use disorder in order to develop new medication strategies.
The striatum is a central anatomical hub for the neurobiological actions of heroin since it integrates information from midbrain, cortical and thalamic circuits to regulate reward and cognitive function (10). As with other drugs of abuse, the chronic repeated pattern of heroin seeking and heroin taking behaviors lead to marked alterations of striatal plasticity. Despite a significant body of research in animal and in vitro models demonstrating drug-induced synaptic plasticity, there is still a significant dearth of knowledge accrued about the molecular neurobiology of the human brain in substance use disorders. Shedding light about neural systems directly relevant to the human condition also allows the possibility to provide critical ‘reverse translation’ perspectives to better guide mechanistic studies in animal models.
To that end, we focused on the striatum of human heroin abusers in a series of molecular investigations. Based on converging evidence from post-mortem human molecular studies as well as a translational rodent self-administration model, we identified specific epigenetic impairments related to histone H3 acetylation that contribute to transcriptional changes underlying heroin-induced plasticity at glutamatergic synapses of the striatum. Moreover, we show that by blocking the functional read-out of heroin-related histone H3 hyperacetylation, we were able to reverse heroin self-administration and drug-seeking behavior in vivo. Our findings indicate that targeting heroin-induced histone acetylation in the striatum has a tremendous potential to contribute to the development of novel therapeutic approaches in opiate use disorders.
METHODS AND MATERIALS
Human heroin abuse population
Human brains from apparent heroin overdose and normal control Caucasian subjects (determined by self-report and ancestral informative marker analysis) without head trauma were collected at autopsy within 24 hours at the Department of Forensic and Insurance Medicine, Semmelweis University, Hungary, as described previously (11). Cause of death was determined by the forensic pathologist performing the autopsy. Inclusion criteria for the heroin group were documented history of heroin use and/or positive toxicology at the time of death, physical evidence of i.v. drug use (needle tracks); while multi-drug users (as determined by history and blood/urine toxicology at autopsy), subjects receiving methadone/buprenorphine treatment, and subjects with comorbid psychiatric diagnoses were excluded. For the control group, inclusion criteria were negative toxicology, no history or physical evidence of opiate or other drug use, and no documented psychiatric disorders. Table S1 in Supplement 1 provides detailed demographic information including cause of death for each subject. Table S2 in Supplement 1 shows a summary description of the populations used for each molecular experiment. Univariate correlations for demographic variables (age, pH, etc.) were explored and significant variables were included in the final model. Ventral and dorsal striatal (putamen) tissue punches were obtained at the level of the nucleus accumbens (Figure S1 in Supplement 1).
Rodent heroin self-administration
Adult male Long-Evans rats underwent jugular vein catheterization and trained to self-administer heroin in sound-attenuating boxes (MED Associates Inc., St. Albans, VT) with two levers; depression of one (active lever) delivered 30 μg/kg heroin under a fixed-ratio 1 (FR1) reinforcement schedule, whereas depression of the other (inactive lever) had no programmed consequences (see Methods in Supplement 1 for details). Each Session was 3hrs. Following seven FR1 sessions, animals underwent four additional FR5 sessions. For the JQ1 experiments, only rats that differentiated between active and inactive levers and showed an escalation of correct responses under FR5 schedule were studied. Guide cannulae (C317G-SPC, cut 4 mm below pedestal) for JQ1 administration were inserted into the rostral dorsal striatum (10° angle from midline relative to bregma: AP+1.7mm, ML+3.5mm; DV-2.0mm; Figure S2 in Supplement 1) After establishing a post-surgery baseline (average of two days), 2 μl of 20 μmol/l JQ1 was injected in animals 5 minutes before heroin self-administration and prior to a non-reinforced cue-induced drug-seeking reinstatement session (1hr). All animal testing was carried out using a counter-balanced experimental design at a similar time of the day.
Molecular assays
See Methods in Supplement 1 for a detailed description of our mRNA (microarray, NanoString and qPCR), protein (Western blot) as well as chromatin (chromatin immunoprecipitation and assay for transposase accessible chromatin; ATAC-seq) analyses and statistics.
RESULTS
Chronic heroin use leads to striatal transcriptional impairments related to glutamatergic neurotransmission and histone acetylation
In order to better understand the transcriptional impairments that underlie heroin-induced synaptic plasticity in the striatum, we first examined existing data (12) from transcriptional profiling of the ventral striatum (nucleus accumbens; NAc) of post-mortem human heroin users and matching control subjects (see Tables S1 and S2 in Supplement 1 for demographic information). Using the Affymetrix HG-U133A microarray, we identified 2132 differentially expressed genes (Table S3 in Supplement 1; the raw data has been deposited in GEO). Gene ontology (GO) analysis (DAVIDv6.7; Database for Annotation, Visualization and Integrated Discovery) of this dataset showed enrichment of genes related to synaptic function and glutamatergic neurotransmission (Table S4 in Supplement 1). We next applied weighted gene co-expression network analysis (WGCNA), which clusters genes into modules based on correlations among their expression pattern. The data was normalized using the GeneChip robust multiarray averaging (GC-RMA) method for microarray normalization and corrected for covariates. In line with the GO analysis, the top WGCNA dysregulated module (turquoise; p<0.001 at FDR<0.1%) in heroin subjects corresponded to genes related to synaptic neurotransmission (Figure 1A). Furthermore, an independent network analysis approach using the MetaCore software that is based on connections reported in the literature also showed networks enriched for genes related to glutamatergic neurotransmission (Figure 1B).
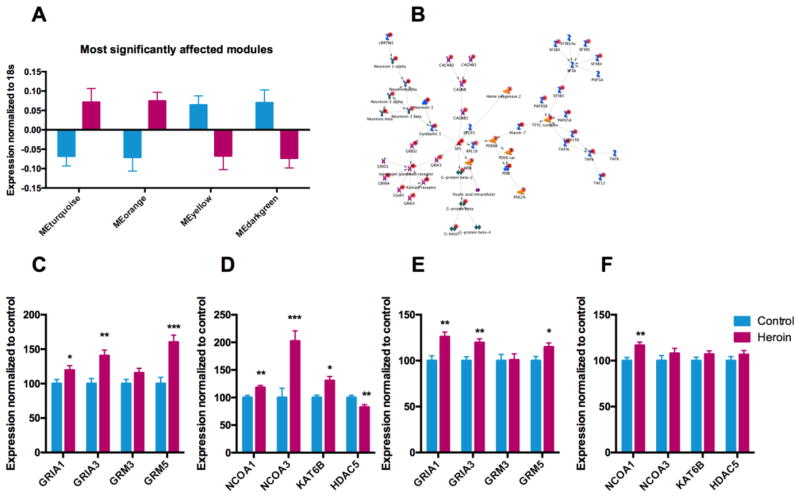
Figure 1.
Chronic heroin use is associated with transcriptional and epigenetic impairments related to glutamatergic neurotransmission and histone acetylation. (a–c) Transcriptional profiling of the ventral striatum of human heroin users and matched controls. (A) The most significantly upregulated and most significantly downregulated WGCNA modules. Turquoise: genes related to neuronal function; orange: genes related to synaptic transmission; yellow: genes related to oxidative phosphorylation; darkgreen: genes related to mitochondrial function. (B) Metacore network analysis of the differentially expressed genes. (C–F) mRNA levels of selected genes related to (C,E) glutamatergic neurotransmission and (D,F) histone acetylation; (C,D) Affymetrix HG-U133A microarray results and (E,F) NanoString results. Bar graphs represent mean ± s.e.m. Multiple regression, *P < 0.05, **P<0.01, ***P<0.001; microarray: n=26 for controls and n=22 for heroin users, NanoString: n=16 for controls and n=20 for heroin users.
Intriguingly, we observed a predominant upregulation of glutamate related genes, with 64 out of the 87 significantly altered glutamatergic genes being expressed at higher levels in heroin abusers (Table S5 in Supplement 1). We validated the microarray findings using NanoString, and confirmed the overexpression of several glutamatergic markers including GRIA1 (glutamate receptor, ionotropic, AMPA 1), GRIA3 (glutamate receptor, ionotropic, AMPA 3), and GRM5 (glutamate receptor, metabotropic 5) (see Figure 1C for microarray and Figure 1E for Nanostring).
In addition to glutamatergic neurotransmission, the microarray analysis revealed significant upregulation of several histone acetyltransferases (HATs) in heroin abusers including NCOA1 (nuclear receptor coactivator 1), NCOA3 (nuclear receptor coactivator 3), and KAT6B (lysine acetyltransferase 6b) (Figure 1D). The increased expression of NCOA1 was also confirmed using NanoString in an additional cohort of heroin users (Figure 1F). Furthermore, we observed significant down-regulation of HDAC5 (histone deacetylase 5) (Figure 1D), which together with the NCOA alterations predicted increased levels of histone H3 acetylation in the striatum of chronic heroin users.
Global perturbations of chromatin remodeling in the dorsal striatum of chronic heroin users relate to drug use history and acute morphine toxicology
To determine whether epigenetic alterations were also evident on the protein level, we focused on biochemical studies of the dorsal striatum (putamen) due to the larger availability of this striatal region for the assays that required significant amounts of tissue and the strong role of this subregion in compulsive habitual behavior that characterizes the addicted state (13). The global state of post-translational modifications of histone H3 proteins was first investigated by Western blotting using antibodies specific to activating marks acetylated histone H3 (pan-AcH3), trimethyl-lysine 4 histone H3 (H3K4me3), trimethyl-lysine 36 histone H3 (H3K36me3), as well as repressive marks trimethyl-lysine 9 histone H3 (H3K9me3), dimethyl-lysine 9 histone H3 (H3K9me2) and trimethyl-lysine 27 histone H3 (H3K27me3). Although no group differences were observed for any of the marks investigated, we found an intriguing and statistically significant positive correlation between pan-AcH3 and years of previous drug use (r=0.57, p=0.0268; Figure 2A). NCOA1 mRNA levels were also positively correlated to years of use (r=0.63, p=0.0163). In addition, pan-AcH3 was negatively correlated with urine morphine levels (r=−0.47, p=0.0144; Figure 2B), whereas H3K27me3 showed a positive correlation (r=0.44, p=0.0182) to urine morphine. Together, these findings suggest that while chronic heroin use leads to histone H3 hyperacetylation, acute exposure to the drug facilitates formation of a more repressed state of chromatin in the dorsal striatum.
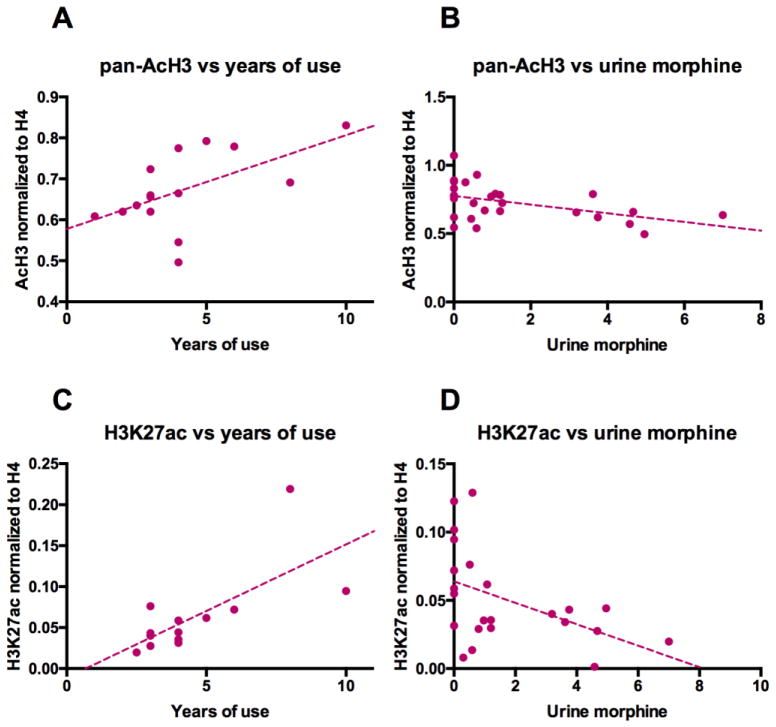
Figure 2.
Heroin-related histone H3 hyperacetylation correlates with drug use history and acute toxicology. Pearson correlations between (A) pan-acetylated histone H3 and years of previous drug use, r=0.57, p=0.0268, n=15, (B) pan-acetylated histone H3 (pan-H3) and urine morphine levels, r=−0.47, p=0.0144, n=27, (C) lysine-27 acetylated histone H3 (H3K27ac) and years of previous drug use, r=0.70, p=0.0381, n=13, and (D) lysine-27 acetylated histone H3 and urine morphine levels, r=−0.47, p=0.0241, n=23.
It is well established that post-translational modifications of histones can have different outcomes based on the lysine residues affected, such that amino acid resolution is necessary in order to reliably interpret epigenetic states. Therefore, we sought to determine which specific lysine residue(s) were hyperacetylated in the dorsal striatum of human heroin users that contributed to the pan-AcH3 observations. To this end, we performed a series of Western blots using antibodies specifically targeting acetylated lysine residues at positions K9, K14, K18, K23, K27, K36 and K56 within the N-terminal tail of histone H3. Interestingly, H3K23 was the only residue that showed differential acetylation in heroin users vs. controls (FC=1.93, p=0.0356; Figure S3A in Supplement 1). H3K23ac, however, did not correlate well with pan-AcH3 and was not affected by drug history and toxicology. In contrast, despite the lack of an overall significant group difference (Figure S3B in Supplement 1), H3K27ac correlated strongly with pan-AcH3 (r=0.62, p=0.0001) and reproduced the correlations with years of heroin use (r=0.70, p=0.0381; Figure 2C) and urine morphine (r=−0.47, p=0.0241; Figure 2D). Taken together, these data suggest that the heroin-induced hyperacetylation occurs in a specific region of histone H3, in the area around lysine residues K23 and K27.
Chronic heroin use leads to histone H3 hyperacetylation and increased chromatin accessibility at glutamatergic genes
The histone H3 hyperacetylation observed in chronic heroin users suggested a more open state of chromatin conducive to increased transcriptional activity, in line with the gene expression impairments described in Figure 1. Thus we hypothesized that histone H3 hyperacetylation might be an underlying mechanism contributing to enhanced glutamatergic gene expression in heroin users. We focused on GRIA1 since this gene encodes the GRIA1 AMPA receptor subunit that is highly implicated in drug addiction behavior and synaptic plasticity (14–17). Interestingly, both pan-AcH3 (r=0.41, p=0.0316; Figure 3A) and H3K27ac (r=0.50, p=0143; Figure 3B) showed a positive correlation with GRIA1 expression in heroin but not in control subjects.
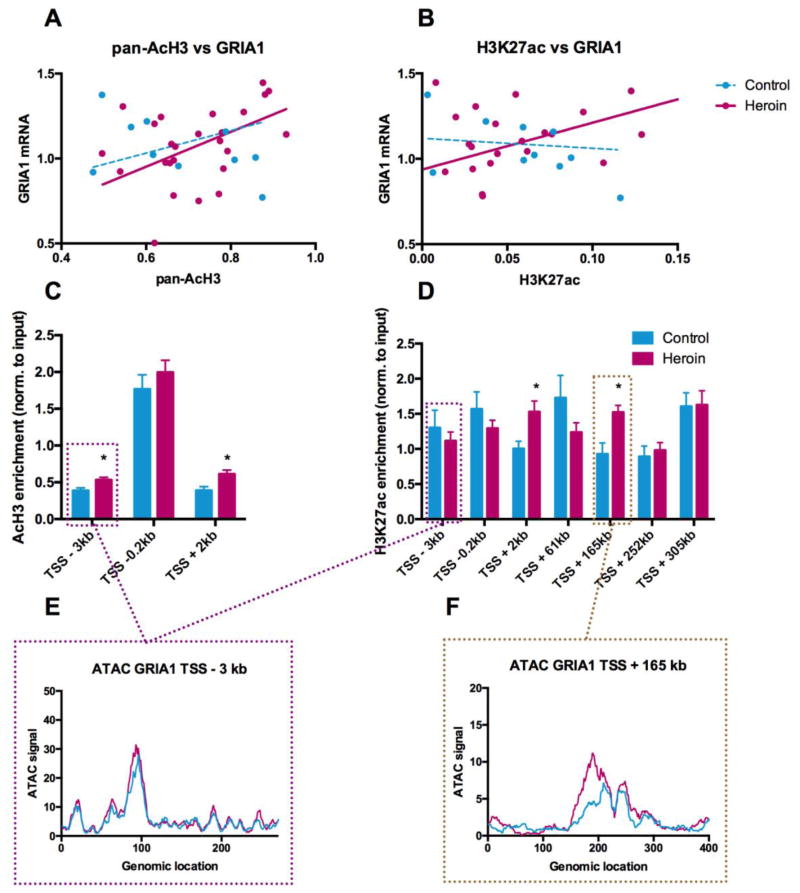
Figure 3.
Heroin users exhibit histone H3 hyperacetylation and enhanced chromatin accessibility at glutamatergic genes in the dorsal striatum of human heroin users. (A,B) Pearson correlations between (A) pan-AcH3 and GRIA1 mRNA levels, r=0.41, p=0.0316, n=12, and (B) H3K27ac and GRIA1 mRNA levels, r=0.50, p=0.0143, n=12. (C,D) Chromatin immunoprecipitation (ChIP) experiments showing enrichment of (C) pan-AcH3 (n=13 in the control group and n=30 in the heroin group) and (D) H3K27ac (n=10 in the control group and n=25 in the heroin group) along the GRIA1 gene. Data are represented as mean ± s.e.m. Independent Student’s t-tests, *P < 0.05. (E,F) ATAC-seq tracks showing chromatin accessibility along the human GRIA1 gene at (e) 3kb upstream and (F) 2kb downstream of the transcription start site. Lines represent means, n=10 in both groups.
To assess heroin-related histone H3 hyperacetylation specifically at glutamate related genes, we performed pan-AcH3 and H3K27ac chromatin immunoprecipitation (ChIP) experiments coupled with real-time PCR targeting the GRIA1 gene. The pan-AcH3 ChIP (Figure 3C) revealed significant hyperacetylation ~3kb upstream and 2kb downstream from the transcription start site (TSS). For H3K27ac, several other conserved regions within the gene body of GRIA1 were assayed to obtain a better resolution of the acetylation landscape. Indeed, H3K27ac enrichment was significantly increased 2kb downstream from the TSS and at another distant intragenic site located 165kb downstream from the TSS (Figure 3D). In addition, gene body acetylation of GRIA1 2kb downstream of TSS was negatively correlated with blood morphine levels (r=−0.42, p=0.0221), in line with the proposed repressive nature of acute morphine toxicology on histone acetylation and gene expression.
To determine whether the observed histone hyperacetylation in fact translates into a more open state of chromatin in chronic heroin users, we next studied chromatin accessibility directly using the assay for transposase accessible chromatin (ATAC) coupled with high-throughput sequencing. ATAC-seq was performed in FACS-sorted neuronal (NeuN positive) cell populations from the dorsal striatum of human heroin users and controls. Emphasizing the strength of this approach, we were able to reliably demonstrate open chromatin in NeuN positive cells at genes expressed specifically in neurons (e.g. CAMK2A, Figure S4A in Supplement 1), whereas glial markers (e.g. OLIG2) were only accessible in the NeuN negative cell population (Figure S4B in Supplement 1). Using this platform, we assayed chromatin state along the GRIA1 gene in regions corresponding to the PCR products of our ChIP PCR primers. Two of the regions investigated with ChIP overlapped with ATAC peaks. Strikingly, chromatin accessibility was significantly increased in heroin users in the same region located 165kb downstream from the TSS (Figure 3F), where H3K27ac enrichment was also significantly higher (Figure 3D). Consistently, chromatin accessibility was not affected by heroin in another genomic location where H3K27ac enrichment was not induced (Figure 3E). Taken together, these results suggest that histone H3 hyperacetylation related to chronic heroin use leads to a more open state of chromatin along genes related to glutamatergic neurotransmission, such as GRIA1, which might be of crucial importance for transcriptional impairments underlying drug-induced striatal synaptic plasticity.
Small molecule bromodomain inhibitor JQ1 decreases heroin self-administration and drug-seeking behavior
In order to establish the basis for follow-up in vivo behavioral studies, we next wanted to determine whether chronic heroin exposure induces similar histone H3 hyperacetylation in a rodent model. Adult male Long-Evans rats were trained to self-administer heroin and sacrificed 1 or 24 hours after the last self-administration session. We first assayed global pan-AcH3 levels in the rat dorsal striatum using Western blot. Total heroin intake 24hrs after the last self-administration session was used to approximate chronic drug-related effects (a proxy for years of previous heroin use in humans) and last session heroin intake 1hr after the last self-administration session to model acute toxicology (a proxy for urine morphine levels in humans). Although not statistically significant, pan-AcH3 tended to be positively correlated with total heroin intake (r=0.52, p=0.0847; Figure S5A in Supplement 1) and, regarding last session heroin intake, was directionally similar to the human findings (r=−0.46, p=0.3013; Figure S5B in Supplement 1), indicating that the opposite relationship of chronic and acute heroin exposure on histone H3 acetylation observed in humans might also to some extent be present in rodents. Next we determined pan-AcH3 and H3K27ac enrichment directly at regions of the rat Gria1 gene corresponding to the ones assayed in humans using ChIP PCR. Strikingly, we found perfectly analogous heroin-related epigenetic impairments in the two species: a significant increase in the enrichment of pan-AcH3 3kb upstream as well as 2kb downstream of the TSS (Figure 4A) and of H3K27ac in the intragenic region (Figure 4B). Importantly, these data together suggest that heroin-related hyperacetylation at glutamatergic genes are conserved between humans and rats.
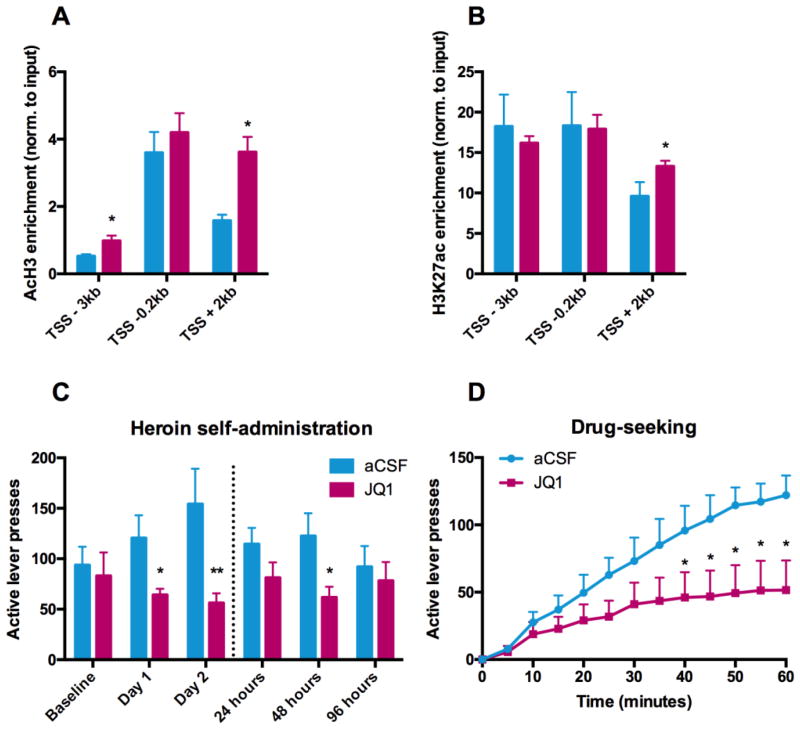
Figure 4.
Small molecule bromodomain inhibitor JQ1 blocks self-administration and drug-seeking behavior in vivo. (A,B) Chromatin immunoprecipitation experiments showing enrichment of (A) pan-AcH3 (n=4 in the control group and n=6 in the heroin group) and (B) H3K27ac (n=3 in the control group and n=15 in the heroin group) along the rat Gria1 gene. Bar graphs represent mean ± s.e.m. Independent Student’s t-tests, *P < 0.05. (C) Active lever presses during 3 hours long heroin self-administration session in JQ1 (n=6) and aCSF (n=5) treated Long-Evans rats. Dashed line represents day of last JQ1 delivery. Bar graphs represent mean ± s.e.m. Independent Student’s t-tests, *P < 0.05. (D) Active lever presses during a 1 hour non-reinforced cue-induced drug-seeking session in JQ1 (n=6) and aCSF (n=5) treated Long-Evans rats. Data is represented as mean ± s.e.m. Two-way ANOVA revealed significant group (F1,117=36.31, p < 0.0001) and time (F12,117=7.811, p < 0.0001) effects. aCSF: artificial cerebrospinal fluid.
To assess the functional behavioral relevance of heroin-induced histone H3 hyperacetylation in vivo, we evaluated in the rodent model the effects of pharmacologically manipulating bromodomains, which specifically recognize acetylated lysine residues and are increasingly being developed as epigenetic therapeutic targets. We implanted guide cannulae bilaterally into the rostral dorsal striatum of adult male Long-Evans rats previously trained to self-administer heroin, and on two consecutive days delivered 20 μmol/l of the small molecule bromodomain inhibitor JQ1 5mins prior to self-administration sessions. We hypothesized that by blocking the functional read-out of heroin-induced histone H3 hyperacetylation, we might be able to inhibit self-administration behavior. Indeed, JQ1 treated animals exhibited a significantly lower number of active lever presses as compared to vehicle-treated rats on both days of testing (53% of control, p=0.0165 and 36% of control, p=0.0083 on the first and second day, respectively; Figure 4C). Strikingly, the effects of JQ1 were long lasting and could still be observed 24hrs (71% of control, p=0.0722) and 48hrs (50% of control, p=0.0179) after the last drug delivery. The effect was no longer evident at 96hrs (85% of control, p=0.4045) (Figure 4C). Importantly, JQ1 did not affect inactive lever pressing or general locomotor behavior, emphasizing the specificity of the JQ1 effects to goal-directed behavior (Figure S6A,B in Supplement 1). We then sought to determine whether JQ1 also affects cue-induced drug-seeking behavior. Following 1 week of abstinence, animals were injected with 20 μmol/l JQ1 through the dorsal striatal guide cannulae 5mins prior to a non-reinforced drug-seeking session (60 minutes). JQ1 treated animals showed significantly decreased active lever pressing (42% of control, p=0.0112) compared to vehicle rats, and, strikingly, exhibited essentially no drug-seeking behavior in the last 20mins of testing (Figure 4D). A two-way ANOVA revealed significant group (F1,117=36.31, p<0.0001) and time (F12,117=7.811, p<0.0001) effects (Figure 4D). Similarly to the previous JQ1 test sessions, inactive lever pressing and general locomotor behavior were not affected by JQ1 (Figure S6C,D in Supplement 1).
Taken together, our in vivo data indicate that blocking the read-out of heroin-related histone H3 hyperacetylation by bromodomain inhibition in self-administering rats reverses addiction-related behavior, and strongly support the functional importance of the observed heroin-related epigenetic and transcriptional impairments.
DISCUSSION
Our translational study revealed marked epigenetic and transcriptional impairments in chronic human heroin users related to abnormal histone acetylation and glutamatergic dysregulation. Using evidence from direct interrogation of the human brain, we show that heroin use is associated with hyperacetylation of histone H3 at specific lysine residues (K27 and K23) at discrete genomic locations (GRIA1 and other genes related to glutamatergic synaptic plasticity). Importantly, these epigenetic impairments were associated with alterations in chromatin accessibility and might play an important role in addiction behavior. Furthermore, our data identify the small molecule bromodomain inhibitor JQ1 as a promising candidate for clinical studies as it potently inhibited heroin self-administration and drug-seeking behaviors in the translational rat model.
Glutamatergic input to the striatum has long been implicated in opiate addiction (15, 18) and shown to play a critical role in context-induced heroin seeking and relapse-related behaviors (19). The AMPA glutamate receptor subunit Gria1 specifically has been implicated in substance use (14, 15, 20, 21) and related neuropsychiatric disorders (17). Our data now provide new insight highlighting the important contribution of specific acetylation disturbances related to transcriptional alterations of striatal GRIA1 in human heroin abusers that is dynamically regulated with repeated use of the drug. Intriguingly, we had previously documented marked impairments of GRIA1 in heroin-related plasticity at glutamatergic synapses in the human amygdala (16). Whether histone H3 hyperacetylation is also involved in the regulation of glutamatergic synaptic plasticity in the amygdala and other brain regions remains to be established.
Disturbances of epigenetic mechanisms including histone H3 acetylation are increasingly being recognized to play a crucial role in the development and maintenance of addictive disorders (22–24). In particular, hyperacetylation of histone H3 was observed in the promoter region of several genes in the NAc that are induced by repeated cocaine exposure (25). In addition, chronic cocaine exposure was shown to disrupt Hdac5 function in the NAc, leading to hyperacetylation and enhanced transcription of Hdac5 target genes (26). Intriguingly, simultaneous acetylation and phosphorylation of histone H3 in the NAc of rats was shown to be critical for heroin-induced conditioned place preference (27). The current study provides the first direct evidence of opiate-related epigenetic impairments in the human brain emphasizing that the acetylation disturbance particularly associated with drug use history is rather restricted to the K27 region of the histone H3 tail. In addition, our analogous findings from heroin self-administering rats strongly suggest the translational nature of drug-related impairments in histone acetylation. Acetylation of H3K27 has been shown to activate transcription (28), inhibit polycomb silencing (29–31), and to be sensitive to stress (32). Nothing, however, is known about its function in the brain or in relation to substance use disorders. In addition, this mark is usually studied in the context of enhancers (33–35) and super-enhancers (36–38), while its functional role in intragenic regions remains elusive. Even less is known about H3K23ac (39, 40) for which a group difference was noted for heroin, but the levels did not relate to drug history or acute use as they did for H3K27ac. As such, H3K27ac appears to be more dynamically sensitive to the heroin experience with acute drug use temporarily reversing the chronic ‘poised’ state of elevated H3K27ac. Interestingly, stress-induced modifications of synaptic plasticity have been shown to be mediated by dynamic epigenetic regulation of glutamatergic genes driven by H3K27ac (32).
A surprising finding in both human addicts and the rat model currently studied was that heroin-related hyperacetylation was most pronounced within the gene body of GRIA1. Acetylation of the gene body is thought to positively regulate transcriptional elongation by achieving an open chromatin structure that facilitates the passage of RNA polymerase II (41–45). In addition, dynamic gene body acetylation plays a critical role in co-transcriptional splicing (46) and regulates the incorporation of histone variants (47, 48). Disease-specific disturbances of gene body acetylation, particularly in the brain, however, have not yet been reported. Importantly, we also provide direct evidence for a more open state of chromatin across the GRIA1 gene specifically in neurons which overlapped the differentially H3K27 acetylated regions by using ATAC (49–51). Our data strongly support a functional role for the heroin-related gene body hyperacetylation with respect to enhanced transcriptional activity of glutamatergic genes in striatal neurons.
An important goal of the study was to evaluate strategies based on our molecular findings that could provide insights for potential clinical treatments. Manipulating acetylation state in various diseases has been attempted mainly with HDAC inhibitors but such strategies have not proven to be clinically effective since, while generally tolerated in cancer patients, the side effect profiles will likely be prohibitive for psychiatric clinical applications (52). Bromodomains (53) specifically recognize acetylated lysine residues and inhibitors (54) of these protein modules can be used to block the functional read-out of acetylated histones and appear to be clinically tolerated by patients (55). Recently, JQ1 has been shown to inhibit transcriptional responses that occur during memory formation (56), which might be important for mechanisms implicated in addiction and extinction learning. In addition, JQ1 attenuated the rewarding effect of cocaine in rats by interfering with drug-induced plasticity in the NAc (57). In the cocaine study, JQ1 was administered during the acquisition phase of conditioned place preference (57). Here, we show that bromodomain inhibitors effectively decrease drug self-administration behavior. Importantly, JQ1 potently reversed addiction behavior during the maintenance phase as well as during reinstatement after abstinence in our model suggesting that this drug might hold clinical promise for the treatment of long-term human heroin users. Importantly, Phase I clinical trials using JQ1 are ongoing in cancer patients and serious side effects have not yet been reported (58, 59) which would set the stage for future clinical addiction studies.
It is important to note that lysine acetylation is not a histone-specific phenomenon and plays an important role in the post-transcriptional regulation of, for example, several transcription factors (e.g. p53, YY1, STAT3), nuclear receptors (e.g. androgen and estrogen receptor), as well as α-tubulin and Hsp90 (60). Off-target effects of JQ1 and other bromodomain inhibitors are thus likely to occur and will need to be addressed. Another important caveat of the current study is that most of our biochemical analyses were restricted to the putamen. While the low abundance of available NAc tissue is prohibitive for many applications, a comparative study of NAc and dorsal striatal epigenetic disturbances is warranted considering the complex roles distinct striatal regions play in regulating addiction behavior as part of a limbic/cognitive/motor interface (13, 61). Similarly, the striatum consists of anatomically, biochemically and functionally distinct cell populations that have different roles in reinstatement (62) and could be differentially affected by heroin. Addressing cell heterogeneity will provide significant further insights into the complex landscape of heroin-induced histone acetylation impairments in the striatum.
Limitations inherent to working with postmortem human subjects clearly apply to our study and are thus important to note. The severity of drug use, the manner of death, suicidal intention of overdose, undocumented drug use and psychiatric history are difficult to address conclusively. Nonetheless, the fact that significant group effects were observed and replicated in the animal model emphasizes that our neurobiological findings relate to heroin use.
Overall, the current study demonstrates that the history of heroin use determines the degree of histone acetylation at specific lysine residues of the histone tail that links directly to impairments of glutamatergic genes involved in the regulation of synaptic plasticity in the striatum of human heroin users, establishing a pathological state that contributes to the continued cycles of drug seeking behavior and relapse. Our behavioral data demonstrate the strong potential of bromodomain inhibitors in the treatment of substance use disorders and provides a foundation for follow-up clinical studies targeting epigenetic modifications.
Supplementary Material
Acknowledgments
The study was supported by NIH DA15446 (YLH and EK), DA008227 (YLH), AG050986 (PR), the Veterans Affairs Merit grant BX002395 (PR). We thank Drs. Michael L. Miller and Stephanie Sillivan for technical assistance.
Footnotes
FINANCIAL DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bryant WK, Galea S, Tracy M, Markham Piper T, Tardiff KJ, Vlahov D. Overdose deaths attributed to methadone and heroin in New York City, 1990–1998. Addiction. 2004;99:846–854. doi: 10.1111/j.1360-0443.2004.00693.x. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease C, Prevention. Unintentional poisoning deaths--United States, 1999–2004. MMWR Morb Mortal Wkly Rep. 2007;56:93–96. [PubMed] [Google Scholar]
- 3.Muazzam S, Swahn MH, Alamgir H, Nasrullah M. Differences in poisoning mortality in the United States, 2003–2007: epidemiology of poisoning deaths classified as unintentional, suicide or homicide. West J Emerg Med. 2012;13:230–238. doi: 10.5811/westjem.2012.3.11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inciardi JA, Surratt HL, Cicero TJ, Beard RA. Prescription opioid abuse and diversion in an urban community: the results of an ultrarapid assessment. Pain Med. 2009;10:537–548. doi: 10.1111/j.1526-4637.2009.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inciardi JA, Surratt HL, Cicero TJ, Kurtz SP, Martin SS, Parrino MW. The “black box” of prescription drug diversion. J Addict Dis. 2009;28:332–347. doi: 10.1080/10550880903182986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peavy KM, Banta-Green CJ, Kingston S, Hanrahan M, Merrill JO, Coffin PO. “Hooked on” prescription-type opiates prior to using heroin: results from a survey of syringe exchange clients. J Psychoactive Drugs. 2012;44:259–265. doi: 10.1080/02791072.2012.704591. [DOI] [PubMed] [Google Scholar]
- 7.Pollini RA, Banta-Green CJ, Cuevas-Mota J, Metzner M, Teshale E, Garfein RS. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Subst Abuse Rehabil. 2011;2:173–180. doi: 10.2147/SAR.S24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider MF, Bailey JE, Cicero TJ, Dart RC, Inciardi JA, Parrino M, et al. Integrating nine prescription opioid analgesics and/or four signal detection systems to summarize statewide prescription drug abuse in the United States in 2007. Pharmacoepidemiol Drug Saf. 2009;18:778–790. doi: 10.1002/pds.1780. [DOI] [PubMed] [Google Scholar]
- 9.Hansen RN, Oster G, Edelsberg J, Woody GE, Sullivan SD. Economic costs of nonmedical use of prescription opioids. Clin J Pain. 2011;27:194–202. doi: 10.1097/AJP.0b013e3181ff04ca. [DOI] [PubMed] [Google Scholar]
- 10.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drakenberg K, Nikoshkov A, Horvath MC, Fagergren P, Gharibyan A, Saarelainen K, et al. Mu opioid receptor A118G polymorphism in association with striatal opioid neuropeptide gene expression in heroin abusers. Proc Natl Acad Sci U S A. 2006;103:7883–7888. doi: 10.1073/pnas.0600871103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sillivan SE, Whittard JD, Jacobs MM, Ren Y, Mazloom AR, Caputi FF, et al. ELK1 transcription factor linked to dysregulated striatal mu opioid receptor signaling network and OPRM1 polymorphism in human heroin abusers. Biol Psychiatry. 2013;74:511–519. doi: 10.1016/j.biopsych.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Hou YY, Cai YQ, Pan ZZ. Persistent pain maintains morphine-seeking behavior after morphine withdrawal through reduced MeCP2 repression of GluA1 in rat central amygdala. J Neurosci. 2015;35:3689–3700. doi: 10.1523/JNEUROSCI.3453-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okvist A, Fagergren P, Whittard J, Garcia-Osta A, Drakenberg K, Horvath MC, et al. Dysregulated postsynaptic density and endocytic zone in the amygdala of human heroin and cocaine abusers. Biol Psychiatry. 2011;69:245–252. doi: 10.1016/j.biopsych.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber T, Vogt MA, Gartside SE, Berger SM, Lujan R, Lau T, et al. Adult AMPA GLUA1 receptor subunit loss in 5-HT neurons results in a specific anxiety-phenotype with evidence for dysregulation of 5-HT neuronal activity. Neuropsychopharmacology. 2015;40:1471–1484. doi: 10.1038/npp.2014.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, et al. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 2012;32:4982–4991. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhardt M, Leixner S, Lujan R, Spanagel R, Bilbao A. Glutamate Receptors within the Mesolimbic Dopamine System Mediate Alcohol Relapse Behavior. J Neurosci. 2015;35:15523–15538. doi: 10.1523/JNEUROSCI.2970-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyers JL, Salling MC, Almli LM, Ratanatharathorn A, Uddin M, Galea S, et al. Frequency of alcohol consumption in humans; the role of metabotropic glutamate receptors and downstream signaling pathways. Transl Psychiatry. 2015;5:e586. doi: 10.1038/tp.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renthal W, Nestler EJ. Chromatin regulation in drug addiction and depression. Dialogues Clin Neurosci. 2009;11:257–268. doi: 10.31887/DCNS.2009.11.3/wrenthal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Renthal W, Maze I, Krishnan V, Covington HE, 3rd, Xiao G, Kumar A, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Sheng J, Lv Z, Wang L, Zhou Y, Hui B. Histone H3 phosphoacetylation is critical for heroin-induced place preference. Neuroreport. 2011;22:575–580. doi: 10.1097/WNR.0b013e328348e6aa. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Zhang MQ. Histone modification profiles are predictive for tissue/cell-type specific expression of both protein-coding and microRNA genes. BMC Bioinformatics. 2011;12:155. doi: 10.1186/1471-2105-12-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau PN, Cheung P. Histone code pathway involving H3 S28 phosphorylation and K27 acetylation activates transcription and antagonizes polycomb silencing. Proc Natl Acad Sci U S A. 2011;108:2801–2806. doi: 10.1073/pnas.1012798108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasini D, Malatesta M, Jung HR, Walfridsson J, Willer A, Olsson L, et al. Characterization of an antagonistic switch between histone H3 lysine 27 methylation and acetylation in the transcriptional regulation of Polycomb group target genes. Nucleic Acids Res. 2010;38:4958–4969. doi: 10.1093/nar/gkq244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tie F, Banerjee R, Saiakhova AR, Howard B, Monteith KE, Scacheri PC, et al. Trithorax monomethylates histone H3K4 and interacts directly with CBP to promote H3K27 acetylation and antagonize Polycomb silencing. Development. 2014;141:1129–1139. doi: 10.1242/dev.102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasca C, Zelli D, Bigio B, Piccinin S, Scaccianoce S, Nistico R, et al. Stress dynamically regulates behavior and glutamatergic gene expression in hippocampus by opening a window of epigenetic plasticity. Proc Natl Acad Sci U S A. 2015;112:14960–14965. doi: 10.1073/pnas.1516016112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charlet J, Duymich CE, Lay FD, Mundbjerg K, Dalsgaard Sorensen K, Liang G, et al. Bivalent Regions of Cytosine Methylation and H3K27 Acetylation Suggest an Active Role for DNA Methylation at Enhancers. Mol Cell. 2016;62:422–431. doi: 10.1016/j.molcel.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emera D, Yin J, Reilly SK, Gockley J, Noonan JP. Origin and evolution of developmental enhancers in the mammalian neocortex. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1603718113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang B, Day DS, Ho JW, Song L, Cao J, Christodoulou D, et al. A dynamic H3K27ac signature identifies VEGFA-stimulated endothelial enhancers and requires EP300 activity. Genome Res. 2013;23:917–927. doi: 10.1101/gr.149674.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ing-Simmons E, Merkenschlager M. Oncometabolite Tinkers with Genome Folding, Boosting Oncogene Expression. Trends Mol Med. 2016;22:185–187. doi: 10.1016/j.molmed.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Mansour MR, Abraham BJ, Anders L, Berezovskaya A, Gutierrez A, Durbin AD, et al. Oncogene regulation. An oncogenic super-enhancer formed through somatic mutation of a noncoding intergenic element. Science. 2014;346:1373–1377. doi: 10.1126/science.1259037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai WW, Wang Z, Yiu TT, Akdemir KC, Xia W, Winter S, et al. TRIM24 links a non-canonical histone signature to breast cancer. Nature. 2010;468:927–932. doi: 10.1038/nature09542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vavouri T, Lehner B. Human genes with CpG island promoters have a distinct transcription-associated chromatin organization. Genome Biol. 2012;13:R110. doi: 10.1186/gb-2012-13-11-r110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Govind CK, Zhang F, Qiu H, Hofmeyer K, Hinnebusch AG. Gcn5 promotes acetylation, eviction, and methylation of nucleosomes in transcribed coding regions. Mol Cell. 2007;25:31–42. doi: 10.1016/j.molcel.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 42.Kurdistani SK, Tavazoie S, Grunstein M. Mapping global histone acetylation patterns to gene expression. Cell. 2004;117:721–733. doi: 10.1016/j.cell.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 43.Orphanides G, LeRoy G, Chang CH, Luse DS, Reinberg D. FACT, a factor that facilitates transcript elongation through nucleosomes. Cell. 1998;92:105–116. doi: 10.1016/s0092-8674(00)80903-4. [DOI] [PubMed] [Google Scholar]
- 44.Vogelauer M, Wu J, Suka N, Grunstein M. Global histone acetylation and deacetylation in yeast. Nature. 2000;408:495–498. doi: 10.1038/35044127. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, et al. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019–1031. doi: 10.1016/j.cell.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gunderson FQ, Merkhofer EC, Johnson TL. Dynamic histone acetylation is critical for cotranscriptional spliceosome assembly and spliceosomal rearrangements. Proc Natl Acad Sci U S A. 2011;108:2004–2009. doi: 10.1073/pnas.1011982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elliott GO, Murphy KJ, Hayes JJ, Thiriet C. Replication-independent nucleosome exchange is enhanced by local and specific acetylation of histone H4. Nucleic Acids Res. 2013;41:2228–2238. doi: 10.1093/nar/gks1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe S, Radman-Livaja M, Rando OJ, Peterson CL. A histone acetylation switch regulates H2A.Z deposition by the SWR-C remodeling enzyme. Science. 2013;340:195–199. doi: 10.1126/science.1229758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10:1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buenrostro JD, Wu B, Chang HY, Greenleaf WJ. ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide. Curr Protoc Mol Biol. 2015;109:21–29. doi: 10.1002/0471142727.mb2129s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buenrostro JD, Wu B, Litzenburger UM, Ruff D, Gonzales ML, Snyder MP, et al. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature. 2015;523:486–490. doi: 10.1038/nature14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slingerland M, Guchelaar HJ, Gelderblom H. Histone deacetylase inhibitors: an overview of the clinical studies in solid tumors. Anticancer Drugs. 2014;25:140–149. doi: 10.1097/CAD.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 53.Horn PJ, Peterson CL. The bromodomain: a regulator of ATP-dependent chromatin remodeling? Front Biosci. 2001;6:D1019–1023. doi: 10.2741/horn. [DOI] [PubMed] [Google Scholar]
- 54.Belkina AC, Denis GV. BET domain co-regulators in obesity, inflammation and cancer. Nat Rev Cancer. 2012;12:465–477. doi: 10.1038/nrc3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stathis A, Zucca E, Bekradda M, Gomez-Roca C, Delord JP, de La Motte Rouge T, et al. Clinical Response of Carcinomas Harboring the BRD4-NUT Oncoprotein to the Targeted Bromodomain Inhibitor OTX015/MK-8628. Cancer Discov. 2016;6:492–500. doi: 10.1158/2159-8290.CD-15-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korb E, Herre M, Zucker-Scharff I, Darnell RB, Allis CD. BET protein Brd4 activates transcription in neurons and BET inhibitor Jq1 blocks memory in mice. Nat Neurosci. 2015;18:1464–1473. doi: 10.1038/nn.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sartor GC, Powell SK, Brothers SP, Wahlestedt C. Epigenetic Readers of Lysine Acetylation Regulate Cocaine-Induced Plasticity. J Neurosci. 2015;35:15062–15072. doi: 10.1523/JNEUROSCI.0826-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amorim S, Stathis A, Gleeson M, Iyengar S, Magarotto V, Leleu X, et al. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol. 2016;3:e196–204. doi: 10.1016/S2352-3026(16)00021-1. [DOI] [PubMed] [Google Scholar]
- 59.Berthon C, Raffoux E, Thomas X, Vey N, Gomez-Roca C, Yee K, et al. Bromodomain inhibitor OTX015 in patients with acute leukaemia: a dose-escalation, phase 1 study. Lancet Haematol. 2016;3:e186–195. doi: 10.1016/S2352-3026(15)00247-1. [DOI] [PubMed] [Google Scholar]
- 60.Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 61.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.