Abstract
Purpose
Prospectively assess relationships between dosimetric parameters and histopathologic/clinical duodenal toxicities in patients on a phase I trial for pancreatic cancer.
Methods
Forty-six borderline resectable/unresectable patients were enrolled on a prospective trial testing neoadjuvant gemcitabine/5-fluorouracil followed by SBRT (5 daily fractions of 5–8 Gy) and concurrent nelfinavir. Post-SBRT surgery was performed in 13 resectable patients, which constituted the patient population herein. Pathologic duodenal damage was assessed using predetermined criteria: 1, no/minimal; 2, moderate; and 3, marked damage. Clinical toxicities were assessed per CTCAE. Duodenal dosimetric parameters included V5–V40 and mean/maximum doses. Spearman correlation and linear regression evaluated associations between dosimetric parameters and clinical/pathologic duodenal toxicity.
Results
The median duodenal mean and maximum doses were 20 and 37 Gy. Median duodenal V5–V40 were 64, 62, 52, 39, 27, 14, 5 and 0 cc, respectively. The median duodenal damage score was 2 (four 1, eight 2, and one 3). Higher duodenal damage scores correlated with higher duodenal mean doses (r = 0.75, p = 0.003), V35 (r = 0.61, p = 0.03), V30 (r =0.67, p = 0.01), V25 (r =0.68, p = 0.01), V20 (r = 0.56, p = 0.05), and the planning target volume (PTV) mean (r =0.59, p = 0.03) and maximum (r = 0.61, p = 0.03) doses. Clinical toxicities did not correlate with dosimetric parameters or duodenal pathologic damage.
Conclusions
Duodenal histologic damage correlates with mean duodenal dose, V20–V35, and PTV mean/−maximum doses.
Keywords: Pancreatic cancer, Stereotactic body radiotherapy, Duodenum, Toxicity
Pancreatic cancer, most commonly adenocarcinoma, is the fourth-leading cause of cancer death in the U.S., with an estimated 41,780 deaths in 2016 [1]. Though resection is traditionally the cornerstone of therapy, there are many rationales for utilizing neoadjuvant therapy (commonly fluoropyrimidine- and/or gemcitabine-based chemotherapy or chemoradiotherapy). These include possibly downstaging borderline resectable (BR) and unresectable patients, potentially making them resectable candidates [2]. This also theoretically allows for R0 (negative margins) resection. Specifically, radiotherapy in the neoadjuvant setting provides several advantages over postoperative circumstances. Owing to a lack of tissue/architectural/vascular deformation, radiation may be better tolerated with a smaller treated volume, potentially causing reduced toxicity. Additionally, surgical trauma can increase tissue hypoxia, which is a well-known cause of radioresistance, and in other cancers correlates with poor prognosis [3].
Stereotactic body radiation therapy (SBRT) allows for improved conformality and shortened treatment times as compared to conventionally-fractionated radiotherapy. Initial studies demonstrated feasibility and effectiveness of SBRT for pancreatic cancer including high local control [4–5]. However, despite superior conformality and in part due to single-fraction treatment regimens, there were substantial and serious acute and late gastroduodenal/bowel toxicities observed, secondary to the close anatomical relationship with the pancreas [6–10].
With further elucidation of dosimetric variables associated with clinical toxicities, as well as modestly fractionating treatments, later trials have shown improvements in clinical toxicities even with the use of concurrent chemotherapy [11]. However, dosimetric associations with histopathologic duodenal damage have heretofore never been performed. Histopathologic findings may predate clinical symptoms and may delineate higher-risk populations in whom to provide more aggressive supportive therapies (e.g. proton pump inhibitors (PPIs) for bowel toxicity) [12–14]. We are performing a prospective phase I study of chemotherapy followed by SBRT and concurrent administration of the human immunodeficiency virus (HIV) protease inhibitor nelfinavir, which has been shown to have tumoricidal and radiosensitizing effects both in preclinical studies [15] and phase I trials [16]. In a prospectively planned manner using patients who had pancreaticoduodenectomy from this trial, we perform a secondary dosimetric analysis correlating dosimetric parameters with histologic damage to the duodenum.
Materials and methods
Patient selection
This is an Institutional Review Board-approved prospective dosimetric analysis of patients enrolled in a phase I trial of hypofractionated stereotactic body radiation therapy concurrent with nelfinavir following gemcitabine and 5FU in locally advanced pancreatic adenocarcinoma patients. Specifically, this analysis was a pre-defined secondary analysis of the trial. Forty-six patients with biopsy-proven BR/unresectable (as assessed by abdominopelvic computed tomography (CT)/magnetic resonance imaging (MRI) and defined by the National Comprehensive Cancer Network [17]) ductal adenocarcinoma of the pancreatic head were enrolled in this trial. Complete inclusion criteria of the trial are described elsewhere [18] and briefly included no history of significant bowel pathology (including gastroduodenal ulcers), no prior bowel surgery, medically “fit” to receive staging laparoscopy and fiducial marker implantation, and no chemotherapy and/or RT within 5 years of enrollment. In the phase I trial, the primary outcome was dose-limiting toxicities and maximum tolerated SBRT dose. Dosimetric data and histology of duodenal specimens in 13 patients that underwent pancreaticoduodenectomy following chemoradiotherapy were analyzed for this report.
Chemotherapy
The neoadjuvant chemotherapy regimen [19] consisted of three 3-week cycles of intravenous gemcitabine (750 mg/m2 females, 900mg/m2 males), 5-fluorouracil (2700 mg/m2), and leucovorin (50 mg/m2) over 30 min on days 1 and 8. Patients that underwent surgical resection received three additional courses of chemotherapy postoperatively using the above regimen. No chemotherapy was administered between SBRT and surgery.
Radiation therapy
All patients were prescribed a PPI at time of consultation, which they continued indefinitely thereafter. Prior to simulation, two fiducial markers were implanted into the pancreatic head approximately two centimeters apart. Simulation with a free-breathing CT and four-dimensional CT (4DCT), occurring at a minimum of 7 days after fiducial placement, was carried out using body fixation and immobilization devices (Medical Intelligence, Schwabmunchen, Germany), with supine positioning and arms over head. Abdominal compression was not utilized. Oral and intravenous contrast was given unless renal function precluded administration.
The gross tumor volume (GTV) was defined as all gross disease as determined on free-breathing CT together with assistance of MRI fusion on all patients. The internal target volume (ITV) was constructed using 4DCT information. The planning target volume (PTV) consisted of a 6 mm expansion of the ITV, with tighter margins adjacent to the duodenum and stomach. There was no prophylactic lymph nodal irradiation, consistent with other reports [8,10–11]. Normal tissues were contoured per guidelines including the duodenum, which was defined as the duodenal bulb to the point the transverse duodenum crossed the left lateral border of the aorta [20–22]. The prescribed dose was required to cover 95% of the planning target volume at minimum. Dose constraints for the stomach and small bowel included the maximum point dose not exceeding 80% of the prescription dose. Regardless of fractionation scheme, it was always initially attempted to keep the duodenal maximum dose ≤32 Gy, but was considered tolerable if ≤38 Gy. Though the aforementioned duodenum constraints were always prioritized above PTV coverage, both were allowed to be compromised in case of technically challenging cases, especially with large PTV volumes, dose-escalation on protocol, and/or particularly unfavorable anatomy. Thus, very much like several of the aforementioned cited studies, there was no absolute “hard constraint” for the duodenum; the balance of target coverage and duodenal doses was assessed on a case-by-case basis so as to ensure adequate individualized care for each patient based on individualized factors.
Once the treatment plan was finalized, duodenal dosimetric parameters were prospectively collected and tabulated, including V5 (volume (cc) of the duodenum receiving 5 Gy or more), V10, V15, V20, V25, V30, V35, V40, duodenal mean/maximum doses, PTV minimum/mean/maximum doses as well as PTV volume, and total duodenal volume.
The Novalis linear accelerator with an M3 multileaf collimator (BrainLAB AG, Heimstetten, Germany) was used to deliver respiratory-gated and ExacTrac image-guided SBRT. With daily ExacTrac images, 3-dimensional couch shifts were made by matching corresponding fiducial markers to the digitally reconstructed radiograph from the simulation CT scan within a gating window of ±15% centered around complete exhalation. As this was a phase I study, the SBRT and nelfinavir dose-escalation levels were as follows. Level I: 5 Gy × 5 plus nelfinavir 625 mg twice daily for three weeks; level II: 5 Gy × 5 plus nelfinavir 1250 mg twice daily for three weeks; level III: 6 Gy × 5 plus nelfinavir 1250 mg twice daily for three weeks; level IV: 7 Gy × 5 plus nelfinavir 1250 mg twice daily for three weeks; level V: 7 Gy × 5 plus nelfinavir 1250 mg twice daily for five weeks; level VI: 8 Gy × 5 plus nelfinavir 1250 mg twice daily for five weeks. SBRT was delivered in five consecutive daily fractions. Nelfinavir was started two weeks prior to commencing radiation therapy.
Toxicities were assessed prospectively during and after treatment, using the Common Terminology Criteria for Adverse Events (CTCAE). After completion of SBRT and nelfinavir, patients underwent restaging thoracic and abdominopelvic CT scans for consideration of surgical resection. If the disease was resectable at this time without distant metastases, pancreaticoduodenectomy was performed 4–6 weeks after the completion of SBRT.
Pathology
All pathologic analysis of surgical specimens, including duodenal pathology, was performed by a single board-certified gastrointestinal surgical pathologist. Histopathologic duodenal damage was classified using the following predetermined criteria [23]. A score of 1 corresponded to no/minimal signs of mucosal damage: villi remained long and slender, epithelial cells had abundant eosinophilic cytoplasm with few mitotic figures, and the lamina propria had normal amounts of inflammatory cells including few or no neutrophils. A score of 2 corresponded to moderate damage: villi were blunted or absent, epithelial cells had reactive/reparative changes with basophilic cytoplasm, increase mitotic figures, and/or small erosions or focal ulcerations; the lamina propria showed increased inflammation including eosinophils and neutrophils. Severe damage was quantified with a score of 3: epithelial damage was so great that it was at many places absent with or without extensive ulcerations and the residual surviving epithelium displaying marked reactive/reparative changes; the lamina propria was replaced with granulation tissue and/or overlying fibrinoinflammatory exudates with numerous neutrophils. Examples of each type of histologic duodenal damage are provided in Fig. 1.
Fig. 1.
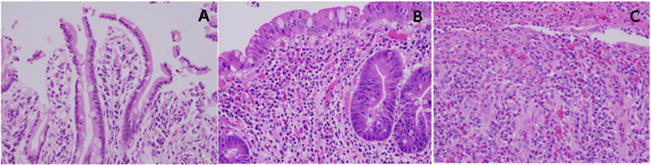
Histologic examples of grade 1 (Panel A), grade 2 (Panel B), and grade 3 (Panel C) duodenal damage.
Statistical analysis
Descriptive statistics were used to describe the baseline patient characteristics, RT dose, and duodenal damage score. Spearman correlation and linear regression were used to determine associations between dosimetric variables and clinical/pathologic toxicities. Statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC).
Results
Table 1 displays clinicopathologic and treatment characteristics of the thirteen patients in the prospective trial that underwent surgical resection. Of note, four patients had a histologic duodenal damage score of 1 (no/minimal damage), eight patients with a score of 2 (moderate damage), and one patient with 3 (marked damage). Table 2 shows the histopathologic characteristics and grading of each patient.
Table 1.
Clinicopathologic characteristics of the study population.
| Characteristic | Number of Patients (Percentage) |
|---|---|
| Age, years | |
| Median (range) | 63 (47–76) |
| Gender | |
| Male | 7/13 (54%) |
| Female | 6/13 (46%) |
| Initial resectability status | |
| Borderline resectable | 13/13 (100%) |
| Unresectable | 0/13 (0%) |
| Pathologic T stage | |
| pT0 | 1/13 (8%) |
| pT1 | 1/13 (8%) |
| pT2 | 1/13 (8%) |
| pT3 | 10/13 (76%) |
| Pathologic N stage | |
| pN0 | 6/13 (46%) |
| pN1 | 7/13 (54%) |
| Largest pre-treatment dimension of tumor, cm | |
| Median (range) | 3 (2–6.2) |
| SBRT dose per fraction | |
| 5 Gy | 1/13 (8%) |
| 6 Gy | 1/13 (8%) |
| 7 Gy | 5/13 (38%) |
| 8 Gy | 6/13 (46%) |
| Duodenal histologic damage score | |
| 1 (no/minimal damage) | 4/13 (31%) |
| 2 (moderate damage) | 8/13 (62%) |
| 3 (marked damage) | 1/13 (8%) |
SBRT, stereotactic body radiation therapy; Gy, Gray.
Table 2.
Histopathologic description of the study population.
| Patient No. | Total Dose (Gy) | Gross Duodenal Appearance | Histology of Duodenal Margin | Other Duodenal Histology | Duodenal Damage Score |
|---|---|---|---|---|---|
| 1 | 25 | Not described | Congestion | Normal (scanty) mucosa over tumor | 1 |
| 2 | 30 | Normal | Normal | Unremarkable | 1 |
| 3 | 35 | Normal | Normal | Duodenum over tumor normal | 1 |
| 4 | 35 | Normal | Congestion | Focal ulceration at duodenal ampulla | 2 |
| 5 | 35 | Normal | Normal | Villous mucosal blunting over tumor, lamina propria with scarring, increased eosinophils, reactive epithelium | 2 |
| 6 | 35 | Normal | Normal | Villous mucosal blunting at ampulla, reactive epithelium, lamina propria scarring, increased eosinophils | 2 |
| 7 | 35 | Normal | Congestion | Villous mucosal blunting over tumor, reactive epithelial changes, increased eosinophils | 2 |
| 8 | 40 | Not described | Unspecified | Extensively ulcerated mucosa over tumor | 3 |
| 9 | 40 | Not described | Not available | Congestion at ampulla | 1 |
| 10 | 40 | Normal | Congestion | Focal erosion at ampulla, villous blunting, reactive epithelium, increased eosinophils | 2 |
| 11 | 40 | Distal end mucosal discoloration, otherwise normal | Congestion | Villous mucosal blunting over tumor with focal erosion | 2 |
| 12 | 40 | Distal 12 cm dusky, otherwise normal | Congestion | Villous mucosal blunting over tumor, reactive epithelial changes. Ampullary villous blunting, reactive epithelial changes, increased eosinophils in lamina propria | 2 |
| 13 | 40 | Major duodenal papilla edematous, distal duodenum “ischemic”-appearing | Congestion | Villous blunting of major duodenal papilla with focal erosions, reactive epithelium, increased eosinophils, and peptic changes | 2 |
Gy, Gray.
Clinical GI toxicities did not correlate with any level of pathologic duodenal damage (Table 3). Tabulation of acute duodenal toxicities of the 13 patients is given in Table 3. Treatment was tolerated well with one grade 3 GI toxicity, four grade 2 toxicities (nausea, vomiting, anorexia and hemoglobin decrease) and twenty-five grade 1 toxicities. Overall, nausea (7/13) and abdominal discomfort (7/13) were most common side effects. Five patients also had grade 1 hemoglobin decrease and one had a grade two hemoglobin decrease, both uncertain to be directly attributed to SBRT versus pre-SBRT chemotherapy.
Table 3.
Comparison of duodenal damage score and clinical toxicities (per CTCAE) potentially attributable to SBRT within 3 months of completion.
| Duodenal Damage Score | Grade 1 Clinical Toxicity | Grade 2 Clinical Toxicity | Grade 3 Clinical Toxicity |
|---|---|---|---|
| 1 |
|
– | – |
| 2 |
|
|
|
| 3 |
|
– | – |
CTCAE, Common Terminology Criteria for Adverse Events; SBRT, stereotactic body radiation therapy; AbD, abdominal discomfort; HbD, hemoglobin decrease; GI, gastrointestinal.
Table 4 and Supplementary Table 1 list dosimetric parameters as well as Spearman correlation coefficients associated with duodenal histopathologic damage scores. Higher duodenal mean doses (p = 0.003), PTV mean dose (p = 0.03), PTV maximum dose (p = 0.03), V20 (p = 0.05), V25 (p = 0.01), V30 (p = 0.01), and V35 (p = 0.03) were found to correlate with greater histologic damage. There was a trend toward significance for duodenal volume (p = 0.06). Duodenal V5, V10, V15, and V40, as well as PTV volume were not statistically correlative. Selected fit plots that correlate duodenal pathologic damage with duodenal V35, V30, V25, and duodenal mean dose are presented in Fig. 2.
Table 4.
Dosimetric parameters analyzed and correlation statistics with pathologic duodenal damage.
| Parameter | Median (range) | Spearman correlation coefficient (r) | P-value |
|---|---|---|---|
| Duodenal volume, cc | 90 (54–107) | −0.53 | 0.06 |
| Mean duodenal dose, Gy | 20 (14–27) | 0.75 | 0.003 |
| Maximum duodenal dose, Gy | 37 (28–44) | 0.47 | 0.11 |
| PTV volume, cc | 113 (71–249) | 0.12 | 0.69 |
| Mean PTV dose, Gy | 37 (27–43) | 0.59 | 0.03 |
| Maximum PTV dose, Gy | 39 (28–44) | 0.61 | 0.03 |
| Minimum PTV dose, Gy | 33 (24–38) | 0.52 | 0.07 |
| Duodenal V5, cc | 64 (45–87) | −0.46 | 0.12 |
| Duodenal V10, cc | 62 (42–84) | −0.47 | 0.10 |
| Duodenal V15, cc | 52 (40–79) | −0.30 | 0.32 |
| Duodenal V20, cc | 39 (26–81) | 0.56 | 0.05 |
| Duodenal V25, cc | 27 (10–50) | 0.68 | 0.01 |
| Duodenal V30, cc | 14 (0–29) | 0.67 | 0.01 |
| Duodenal V35, cc | 5 (0–20) | 0.61 | 0.03 |
| Duodenal V40, cc | 0 (0–11) | 0.28 | 0.35 |
Statistically significant p-values are in bold.
Gy, Gray; PTV, planning target volume.
Fig. 2.
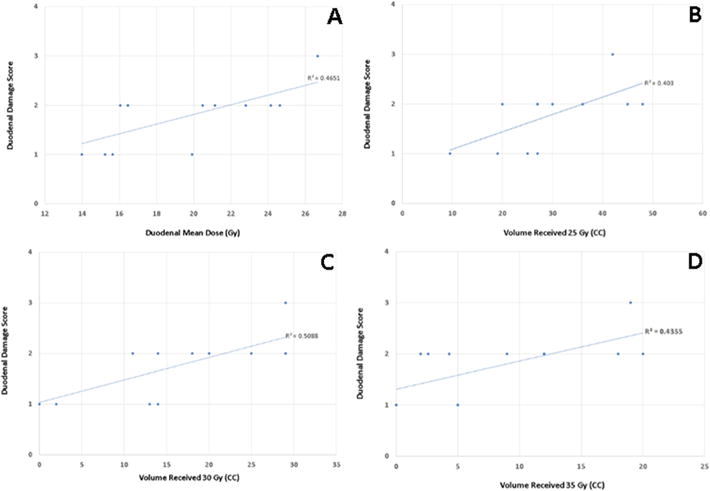
Fit plots with best-fit lines of duodenal histopathologic damage score (y-axis) as a function of mean duodenal dose (Gy) (Panel A), duodenal V25 (cc) (Panel B), duodenal V30 (cc) (Panel C), and duodenal V35 (cc) (Panel D).
Discussion
In this predefined analysis of prospectively collected dosimetric data on a phase I clinical trial, we provide the first known correlation between duodenal dosimetry and histopathologic damage. The importance of this work is that dosimetric delineation of higher-risk patients, correlating with subclinical histopathologic damage, may necessitate changes in medical management.
Although involving extrapolation to nonoperative cases and not specifically assessed herein (routine upper endoscopy was not performed after treatment), aggressive treatment of subclinical histologic damage (e.g. more aggressive PPI therapy) is very much warranted owing to the potential risk of future toxicities/complications (along with low adverse effects with PPIs). Moreover, many patients also note “vague gastrointestinal symptoms” during and following SBRT which do not fit into traditional CTCAE categories, which could be related to subclinical mucosal damage. Furthermore, aggressive treatment of subclinical damage may also help to promote mucosal healing and a return to normal gastrointestinal physiology. Hence, based on dosimetric parameters in this study, we propose that patients that have not undergone resection after SBRT that are “dosimetrically high-risk” be more aggressively treated with supportive medications (e.g. PPIs). The role of using non-steroidal anti-inflammatory drugs in this setting is questioned, owing to gut mucosal damage as a known side effect.
Our dosimetric findings as correlating with histologic damage have not been reported before, and as a result of such, we have now adopted customized dose constraints at our institution, based on median values of various dose-volume parameters found in our prospective series. These include duodenal mean dose ≤20 Gy, V20 < 39 cc, V25 < 27 cc, V30 < 14 cc, and V35 < 5 cc, among others. However, it should be noted that existing data have shown substantial heterogeneity in finding a dosimetric parameter predictive of duodenal/gastrointestinal toxicity. Murphy et al. determined V10–V25 and duodenal maximum dose as predictors in their work with single-fraction 25 Gy SBRT [24]. A study from William Beaumont Hospital demonstrated V20–V35 (univariate) as well as V25 and V35 (multivariate) as predictive factors of gastrointestinal toxicity in patients receiving concurrent fractionated RT and gemcitabine [25]. Another study that examined 3-fraction SBRT found duodenal maximum dose to be the most robust parameter, although correlation with chemotherapy was not mentioned [26]; these results are similar to studies of conventionally-fractionated radiotherapy [27]. Taken together, not only is it true that many dosimetric factors must be accounted for when planning SBRT for pancreatic head lesions, but also that further work is necessary to decrease the inconsistencies between these previous studies. Duodenal doses are also known to depend on phases of the respiratory cycle, which could have substantial impacts on gated treatments and improving techniques to minimize toxicity [28].
There are limitations to our work. We first recognize that there is an overall low sample size, likely resulting in the inability to find dosimetric correlates to clinical GI toxicity; though histopathologic toxicity is not a surrogate for clinical toxicity, it is neither guaranteed nor proven that cellular/architectural duodenal damage predicts for later consequences in this specific setting. Next, though it is acknowledged that the magnitude of correlation coefficients may be small, these were indeed statistically significant. Additionally, our low incidences of toxicity using a 5-fraction regimen as well as sequential chemotherapy-SBRT could also contribute. Given that these patients all underwent duodenum removal, we could not assess duodenal toxicities beyond a few months in this report. Due to the lethality of pancreatic cancer (especially unresected disease), there is a dearth of longer-term late toxicities reported in the literature [29]; to generalize, clinically consequential late effects are relatively rare in radiation oncology. Another factor limiting generalizability is present in nearly all such studies, regarding particular nuances of treatment planning utilized herein, which may not be representative of other institutions. It is important to note that previous dosimetric studies, which have also been less than consistent in terms of most reliable dosimetric parameters, have either studied dosimetry of SBRT alone or concurrent chemoradiotherapy; it is possible that sequential chemotherapy and SBRT could have differing dosimetric profiles due to full-course, dual-agent chemotherapy having been received prior to SBRT in this series. The addition of nelfinavir also cannot be discounted, and a complete account of clinical toxicities (especially in similar settings) has not been described aside from two reports [30–31]. However, nelfinavir has no known data associated with radioprotection; though any of the gastrointestinal side effects observed herein are possible with nelfinavir, they are all quite uncommon [30–31]. Whereas many previous dosimetric studies have been retrospective in nature, a strength of this work is decreasing retrospective biases owing to prospectively collecting these data, having prespecified this secondary analysis when this clinical trial commenced accrual. Regardless, further research, especially with greater sample size, will be needed to verify these data.
SBRT is an attractive option to treat certain cases of pancreatic cancer. However, dosimetric correlates for duodenal toxicity need further validation. Other strategies include ascertaining the impact of treatment technique [32], daily re-planning/optimization [33], and oral fluid intake to distend the duodenum. Additionally, though proton radiotherapy has shown encouraging toxicity profiles [34], in the absence of clinical trials between protons and SBRT, there remain questions of whether dosimetric and clinical differences exist between the two [35].
Conclusions
As part of a prospective dosimetric analysis of a phase I clinical trial, we demonstrate that duodenal histologic damage, but not clinical symptoms (albeit the recognized low sample size), is correlated with duodenal mean dose, V35, V30, V25, V20 and mean/−maximum PTV dose. Whether patients that are more “dosimetrically at-risk” should be treated with more aggressive supportive therapies must be studied more directly.
Supplementary Material
Acknowledgments
This study was presented in part at the 55th Annual Meeting of the American Society for Radiation Oncology, 22–25 September 2013.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.radonc.2016.12.030.
Footnotes
Conflicts of interest
The authors all declare that conflicts of interest do not exist. There were no funding sources for this study.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Verma V, Li J, Lin C. Neoadjuvant therapy for pancreatic cancer: systematic review of postoperative morbidity, mortality, and complications. Am J Clin Oncol. 2016;39:302–13. doi: 10.1097/COC.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 3.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–9. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 4.Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–21. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Koong AC, Christofferson E, Le QT, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320–3. doi: 10.1016/j.ijrobp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Hoyer M, Roed H, Sengelov L, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol. 2005;76:48–53. doi: 10.1016/j.radonc.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678–86. doi: 10.1016/j.ijrobp.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 8.Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–72. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 9.Didolkar MS, Coleman CW, Brenner MJ, et al. Image-guided stereotactic radiosurgery for locally advanced pancreatic adenocarcinoma: results of first 85 patients. J Gastrointest Surg. 2010;14:1547–9. doi: 10.1007/s11605-010-1323-7. [DOI] [PubMed] [Google Scholar]
- 10.Schellenberg D, Kim J, Christman-Skieller C, et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81:181–8. doi: 10.1016/j.ijrobp.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Gurka MK, Collins SP, Slack R, et al. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: a pilot trial demonstrating safety. Radiat Oncol. 2013;8:44. doi: 10.1186/1748-717X-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49. [PubMed] [Google Scholar]
- 13.Carpenter HA, Talley NJ. The importance of clinicopathological correlation in the diagnosis of inflammatory conditions of the colon: histological patterns with clinical implications. Am J Gastroenterol. 2000;95:878–96. doi: 10.1111/j.1572-0241.2000.01924.x. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson CS, Marshall LA, Morgan DW, editors. In vivo models of inflammation. Basel, Switzerland: Birkhauser Verlag; 2006. [Google Scholar]
- 15.Gupta AK, Cerniglia GJ, Mick R, et al. HIV protease inhibitors block Akt signaling and radiosensitize tumor cells both in vitro and in vivo. Cancer Res. 2005;65:8256–65. doi: 10.1158/0008-5472.CAN-05-1220. [DOI] [PubMed] [Google Scholar]
- 16.Brunner TB, Geiger M, Grabenbauer GG, et al. Phase I trial of the human immunodeficiency virus protease inhibitor nelfinavir and chemoradiation for locally advanced pancreatic cancer. J Clin Oncol. 2008;26:2699–706. doi: 10.1200/JCO.2007.15.2355. [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network. Clinical Practice Guidelines for Pancreatic Cancer. http://www.nccn.org/professionals/physician_gls/PDF/pancreatic.pdf [accessed July 31, 2016]
- 18.Clinical Trials, US National Institutes of Health. Clinical Trial NCT01068327. https://clinicaltrials.gov/ct2/show/NCT01068327 [accessed December 23, 2016]
- 19.Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270–5. doi: 10.1200/JCO.2002.11.149. [DOI] [PubMed] [Google Scholar]
- 20.Radiation Therapy Oncology Group. Upper abdominal normal organ contouring consensus guidelines. doi: 10.1016/j.prro.2013.06.004. https://www.rtog.org/CoreLab/ContouringAtlases/UpperAbdominalNormalOrganContouringConsensusGuidelines.aspx [accessed August 8, 2015] [DOI] [PMC free article] [PubMed]
- 21.Grimm J, LaCouture T, Croce R, et al. Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys. 2011;12:267–92. doi: 10.1120/jacmp.v12i2.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radiation Therapy Oncology Group. RTOG 0631 Protocol Information. https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0631 [accessed August 8, 2015]
- 23.Rosai J. Rosai and Ackerman’s surgical pathology. 10th. Vol. 1. St Louis, Missouri: Mosby Elsevier; 2011. [Google Scholar]
- 24.Murphy JD, Christman-Skieller C, Kim J, et al. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:1420–6. doi: 10.1016/j.ijrobp.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Robertson JM, Ye H, et al. Dose-volume analysis of predictors for gastrointestinal toxicity after concurrent full-dose gemcitabine and radiotherapy for locally advanced pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2012;83:1120–5. doi: 10.1016/j.ijrobp.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Bae SH, Kim MS, Cho CK, et al. Predictor of severe gastroduodenal toxicity after stereotactic body radiotherapy for abdominopelvic malignancies. Int J Radiat Oncol Biol Phys. 2012;84:469–74. doi: 10.1016/j.ijrobp.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Kelly P, Das P, Pinnix CC, et al. Duodenal toxicity after fractionated chemoradiation for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2013;85:143–9. doi: 10.1016/j.ijrobp.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi CM, Murphy JD, Eclov N, et al. Dosimetric analysis of organs at risk during expiratory gating in stereotactic body radiation therapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2013;85:1090–5. doi: 10.1016/j.ijrobp.2012.07.2366. [DOI] [PubMed] [Google Scholar]
- 29.Elhammali A, Patel M, Weinberg B, et al. Late gastrointestinal tissue effects after hypofractionated radiation therapy of the pancreas. Radiat Oncol. 2015;10:186. doi: 10.1186/s13014-015-0489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunner TB, Geiger M, Grabenbauer GG, et al. Phase I trial of the human immunodeficiency virus protease inhibitor nelfinavir and chemoradiation for locally advanced pancreatic cancer. J Clin Oncol. 2008;26:2699–1706. doi: 10.1200/JCO.2007.15.2355. [DOI] [PubMed] [Google Scholar]
- 31.Wilson JM, Fokas E, Dutton SJ, et al. ARCII: a phase II trial of the HIV protease inhibitor Nelfinavir in combination with chemoradiation for locally advanced inoperable pancreatic cancer. Radiother Oncol. 2016;119:306–11. doi: 10.1016/j.radonc.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar R, Wild AT, Ziegler MA, et al. Stereotactic body radiation therapy planning with duodenal sparing using volumetric-modulated arc therapy vs intensity-modulated radiation therapy in locally advanced pancreatic cancer: a dosimetric analysis. Med Dosim. 2013;38:243–50. doi: 10.1016/j.meddos.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Hoisak JD, Li N, et al. Dosimetric benefit of adaptive re-planning in pancreatic cancer stereotactic body radiotherapy. Med Dosim. 2015;40:318–24. doi: 10.1016/j.meddos.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Nichols RC, Jr, George TJ, Zaiden RA, Jr, et al. Proton therapy with concomitant capecitabine for pancreatic and ampullary cancers is associated with a low incidence of gastrointestinal toxicity. Acta Oncol. 2013;52:498–505. doi: 10.3109/0284186X.2012.762997. [DOI] [PubMed] [Google Scholar]
- 35.Thompson RF, Mayekar SU, Zhai H, et al. A dosimetric comparison of proton and photon therapy in unresectable cancers of the head of pancreas. Med Phys. 2014;41:081711. doi: 10.1118/1.4887797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


