Abstract
BACKGROUND
Severe asthma in children is a heterogeneous disorder associated with variable responses to corticosteroid treatment. Criterion standards for corticosteroid responsiveness assessment in children are lacking.
OBJECTIVE
This study sought to characterize systemic corticosteroid responses in children with severe asthma after treatment with intramuscular triamcinolone and to identify phenotypic and molecular predictors of an intramuscular triamcinolone response.
METHODS
Asthma-related quality of life, exhaled nitric oxide, blood eosinophils, lung function, and inflammatory cytokine and chemokine mRNA gene expression in peripheral blood mononuclear cells were assessed in 56 children with severe asthma at baseline and 14 days after intramuscular triamcinolone injection. The Asthma Control Questionnaire was used to classify children with severe asthma into corticosteroid response groups.
RESULTS
Three groups of children with severe asthma were identified: controlled severe asthma, children who achieved control after triamcinolone, and children who did not achieve control. At baseline, these groups were phenotypically similar. After triamcinolone, discordance between symptoms, lung function, exhaled nitric oxide, and blood eosinophils was noted. Clinical phenotypic predictors were of limited utility in predicting the triamcinolone response, whereas systemic mRNA expression of inflammatory cytokines and chemokines related to IL-2, IL-10, and TNF signaling pathways, namely, AIMP1, CCR2, IL10RB, and IL5, strongly differentiated children who failed to achieve control with triamcinolone administration.
CONCLUSIONS
Systemic corticosteroid responsiveness in children with severe asthma is heterogeneous. Alternative prediction models that include molecular endotypic as well as clinical phenotypic features are needed to identify which children derive the most clinical benefit from systemic corticosteroid step-up therapy given the potential side effects.
Keywords: Childhood asthma, Phenotype, Refractory asthma, Severe asthma, Gene expression, Corticosteroid
Severe refractory asthma in children is a complicated disorder that is often difficult to evaluate in the clinical setting. A recent Task Force Report1 defined “severe asthma” as the requirement for high doses of inhaled corticosteroids (ICSs) plus additional controller medications to maintain asthma control and/or prevent future exacerbations, implying that corticosteroid insensitivity is a fundamental feature underlying the disorder. Indeed, ex vivo studies in populations with severe asthma have noted improvements in cellular function and inflammation with inhibition of signaling pathways that are normally suppressed by glucocorticoid receptor activation.2,3 However, in clinical practice, variable responses to corticosteroid treatment have been observed,4,5 highlighting the phenotypic and potentially endotypic heterogeneity among children with severe asthma.
Because the biological mechanisms associated with corticosteroid sensitivity in children with severe asthma are likely numerous and complex,6–9 clinical assessment of corticosteroid responses are difficult and can further be confounded by poor medication adherence and delivery. Moreover, the lack of clinically applicable definitions of corticosteroid “responsiveness” has limited research in this field. Although studies in adults have relied on changes in lung function as an indicator of corticosteroid responsiveness,10,11 children with severe asthma often have less airflow limitation than do adults12,13 and do not always have concordance between lung function measures and symptoms.14 Furthermore, lung function is only one component of asthma control and is best assessed in combination with current symptoms.15
Given the lack of a criterion standard for the assessment of corticosteroid responsiveness in children, the purpose of this study was to (1) characterize systemic corticosteroid responses in children with severe asthma after treatment with intramuscular triamcinolone and (2) identify phenotypic and molecular predictors of a response to intramuscular triamcinolone administration. Using a clinically available and validated questionnaire of asthma control, we identified 3 groups of children with severe asthma with similar baseline phenotypic features but differing systemic mRNA expression of inflammatory cytokines and chemokines related to IL-2, IL-10, and TNF signaling pathways. The findings highlight the heterogeneity of severe asthma in children as defined by current guidelines and further demonstrate the complicated nature of corticosteroid responsiveness assessment in these children.
METHODS
Children aged 6 to 17 years with physician-diagnosed asthma treated with high-dose ICS and a second controller medication were recruited from an outpatient severe asthma clinic in Atlanta, Georgia. All children met published criteria for severe asthma1 including adherence to ICS evidenced by 10 or more monthly electronic prescription refills over the previous 12 months. Each participant had a history of either 12% or more reversibility in FEV1 after bronchodilator administration or airway hyperresponsiveness to methacholine, evidenced by a provocative concentration of methacholine of 16 mg/mL or less. Exclusion criteria included premature birth before 35 weeks’ gestation, aspiration disorders, vocal cord dysfunction, avascular necrosis, diabetes mellitus, historical or current bronchopulmonary aspergillosis, treatment with nonsteroidal anti-inflammatory drugs or omalizumab, chronic bone disorders, or bone fractures within the previous 6 months. All participants were stable at the time of baseline characterization with no signs of acute respiratory illnesses. If a recent exacerbation was reported, the first visit was conducted 4 weeks after completion of an oral or injectable systemic corticosteroid burst. Permission to proceed with this study was granted by the Emory University Institutional Review Board. Informed written consent was obtained from the legally authorized representatives of eligible children and assent was also obtained from participants aged 6 years and older.
Study design and group classification
Certified study personnel conducted the study under a standardized protocol and manual of procedures. After consent was obtained, participants completed 2 research characterization visits separated by 14 days. Intramuscular triamcinolone (1 mg/kg, 60 mg maximum dose) was administered in the gluteal muscle at the completion of the first visit to all participating children. Children were telephoned 24 to 48 hours after the injection to assess for adverse events. Daily short-acting beta agonist (SABA) use for asthma symptoms (excluding pretreatment before exercise) and corresponding symptoms were recorded in paper diaries between visits.
At the baseline visit, children were considered “controlled” if their Asthma Control Questionnaire (ACQ) score16 was less than 0.75, which corresponds to “well-controlled asthma” with a positive predictive value of 0.73 and a negative predictive value of 0.85.17 Children with uncontrolled asthma were classified as not achieving asthma control if their ACQ score was 1.5 or more (which corresponds to a positive predictive value of 0.8817) at the second visit after triamcinolone receipt.
Characterization procedures
Allergy skin prick testing with 12 extracts was performed at the first visit after a 3-day antihistamine withhold: tree mix (Quercus alba, Ulmus americana, Platanus acerifolia, Salix caprea, Populus deltoides), grass mix (Cynodon dactylon, Lolium perenne, Phleum pratense, Poa pratensis, Sorghum halepense, Paspalum notatum), weed mix (Artemisia vulgaris, Chrysanthemum leucanthemum, Taraxacum vulgare, Solidago virgaurea), common ragweed (Ambrosia artemisiifolia), Alternaria alternata, Aspergillus fumagatis, Cladosporidium herbarum, dog dander, cat dander, German cockroach (Blatella germanica), Dermatophagoides farinae, and Dermatophagoides pteronyssinus (Greer Laboratories, Lenoir, NC). Histamine and saline served as positive and negative controls, respectively. Tests were considered positive if a wheal of 3 mm diameter or greater and flare 10 mm or more was present 15 minutes after application. Up to 10 mL of blood was obtained by venipuncture for total serum IgE (Children’s Healthcare of Atlanta, Atlanta, Ga) and peripheral blood mononuclear cell (PBMC) isolation from whole blood through a density gradient.
Clinical outcomes
Clinical outcomes of interest included asthma quality of life as assessed by the Asthma Quality of Life Questionnaire (AQLQ), blood eosinophils, exhaled nitric oxide concentrations, and FEV1 values. The AQLQ was completed with technical assistance as previously recommended.18,19 Blood eosinophils were quantified by a local laboratory (Children’s Healthcare of Atlanta, Atlanta, Ga) and exhaled nitric oxide concentrations were determined using online methods (NIOX MINO, Aerocrine, Morrisville, NC).20 Spirometry (KoKo PDS, Ferraris, Louisville, Colo) was performed at baseline and the best of 3 vital capacity maneuvers was interpreted.21 Participants were asked to withhold bronchodilator medication before the study visits (ie, ≥4 hours for SABAs and ≥12 hours for long-acting beta agonists).
mRNA gene expression analyses
PBMC mRNA gene expression was determined with a real-time PCR array (Human Inflammatory Cytokines and Receptors RT2 Profiler; SABiosciences, Frederick, Md) after isolation of RNA with a commercial kit (RNeasy Mini; Qiagen, Valencia, Calif). Equal amounts of total RNA per participant were used for first-strand cDNA synthesis and the reaction was preamplified (RT2 PreAMP PCR Master Mix and RT2 PreAMP Human Inflammatory Cytokines and Receptors Primer Mix, SABiosciences). The equivalent of 1.0 μL of cDNA was added to each well of the array plate. Genes of interest are listed in Table E1 in this article’s Online Repository at www.jaci-inpractice.org. The cycle threshold values of the target cDNAs for each subject were normalized to the average cycle threshold of 5 housekeeping genes (ACTB, B2M, GAPDH, HPRT1, RPLP0).
Statistical analyses
Statistical analyses were performed with IBM SPSS Statistics software (Version 23; SPSS, Chicago, Ill). Skewed variables were logarithmically transformed before analysis. Chi-square tests, ANOVA, and t tests were used to compare baseline features and outcomes between groups. Diary card SABA use was plotted and best-fit lines were generated using fourth-order polynomial regression. Goodness of fit was determined by R2 values. Pathway analysis of mRNA data was performed with GNCPro software (SABiosciences). Predictor analyses were performed with logistic regression. Receiver-operating characteristic analyses were also performed for continuous predictor variables. A 2-tailed probability of .05 or less was the threshold of significance for all comparisons.
RESULTS
Enrollment for the study occurred year-round between March 2010 and March 2014. Sixty-six children with severe asthma were enrolled and 56 children completed the study (see Figure E1 in this article’s Online Repository at www.jaci-inpractice.org). The features of children who were lost to follow-up were not significantly different (age, 12 ± 5 years; 64% males; 86% black). Participants were treated for 12 months or more before enrollment with high-dose ICS (827 ± 295 μg/d inhaled fluticasone equivalent) and additional controller medications, most commonly long-acting beta agonists. Their features are presented in Table I. Season of enrollment did not differ between groups (P = .850).
TABLE I.
Features of the participants
| Baseline feature | Controlled severe asthma (N = 15) | Achieved control with triamcinolone (N = 24) | Did not achieve control with triamcinolone (N = 17) |
|---|---|---|---|
| Age (y) | 12 (10–16) | 12 (10–15) | 13 (10–14) |
| Males | 11 (73) | 20 (83) | 10 (59) |
| Race | |||
| White | 1 (7) | 1 (4) | 0 |
| Black | 10 (67) | 18 (75) | 16 (94) |
| More than 1 race | 4 (27) | 5 (21) | 1 (6) |
| Body mass index | |||
| Normal weight (<85%) | 9 (60) | 10 (42) | 4 (24) |
| Overweight (85%–95%) | 3 (20) | 8 (33) | 4 (24) |
| Obese (>95%) | 3 (20) | 6 (25) | 9 (53) |
| Parent with asthma | 8 (53) | 14 (58) | 12 (71) |
| Comorbid conditions | |||
| Atopic dermatitis | 8 (53) | 15 (63) | 13 (77) |
| Recurrent pneumonia | 6 (40) | 14 (58) | 11 (65) |
| Chronic sinusitis | 8 (53) | 13 (54) | 7 (41) |
| Add-on controller medications | |||
| Long-acting beta agonists | 11 (73) | 23 (96) | 12 (71) |
| Montelukast | 10 (67) | 20 (83) | 11 (65) |
| Oral corticosteroids | 0 | 2 (8) | 4 (24) |
| Exposures | |||
| Tobacco smoke | 2 (13) | 8 (35) | 1 (6) |
| Cat inside home | 2 (13) | 0 | 1 (4) |
| Dog inside home | 7 (47) | 12 (50) | 6 (35) |
| Asthma triggers | |||
| Upper respiratory tract infections | 13 (87) | 24 (100) | 16 (94) |
| Furry pets (cats or dogs) | 10 (67) | 15 (63) | 13 (77) |
| Daily activities | 3 (20) | 8 (33) | 9 (53) |
| Vigorous activities | 10 (67) | 20 (83) | 14 (82) |
| Total serum IgE (kU/L)* | 299 (150–594) | 439 (171–792) | 340 (158–1399) |
Data are from participants who completed the study. Values represent the number of participants (%) or the median (interquartile range). Groups are not significantly different.
Statistical analyses were performed after logarithmic transformation.
At the initial study visit, 15 children (27%) had ACQ scores of less than 0.75 (termed “controlled severe asthma”), while 41 (73%) had ACQ scores of more than 0.75. After triamcinolone administration, 24 of these children (43%) achieved asthma control (mean ACQ score, 0.62 ± 0.33) while 17 children (30%) did not achieve asthma control (mean ACQ score, 2.08 ± 0.92) (Figure 1, A). Likewise, self-reported asthma symptoms at the study visits were also more frequent in children who did not achieve asthma control with triamcinolone (Figure 1, B). Daily diary cards similarly revealed less frequent SABA use for asthma symptoms in children with controlled severe asthma (Figure 1, C) and in children who achieved control with triamcinolone (Figure 1, D), in contrast to children who did not achieve control (Figure 1, E).
FIGURE 1.
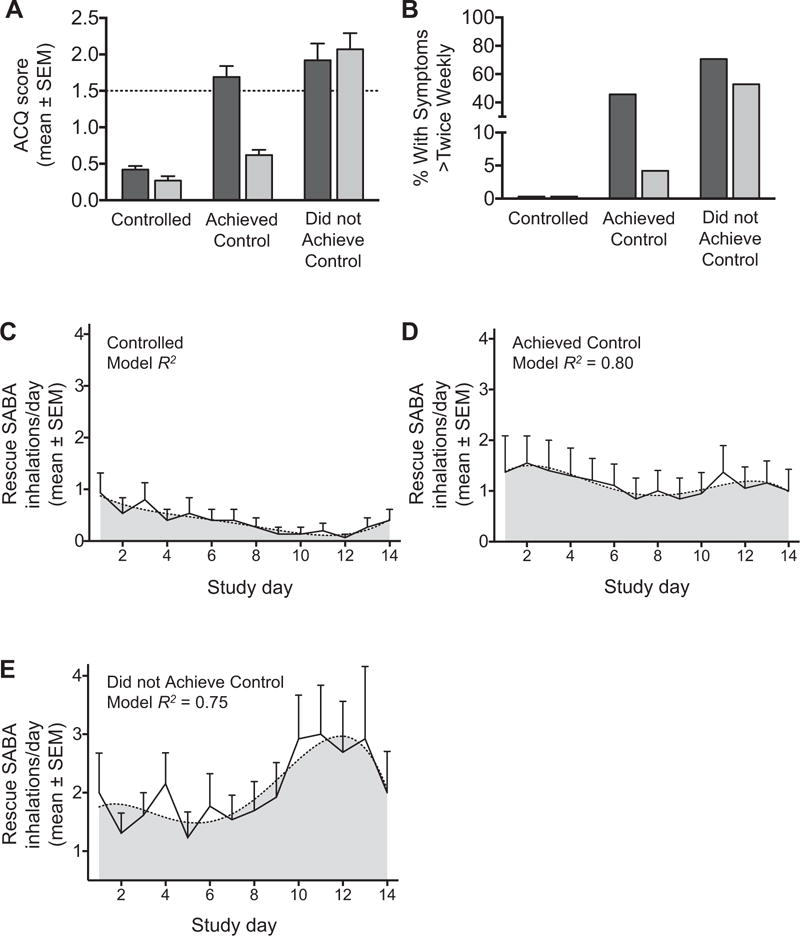
Baseline (dark bars) and posttriamcinolone (light bars) (A) ACQ scores and (B) self-reported asthma symptoms. SABA use in controlled severe asthma (C), in children who achieved control (D), and in children who did not (E). Dashed lines in panels A and C to E reflect the cut-point and best-fit lines, respectively.
Clinical outcomes
Clinical outcome variables are presented in Table II. Despite having minimal symptoms and airflow limitation, children with controlled severe asthma did have numerical improvement in asthma quality of life as reflected by the AQLQ score after triamcinolone administration. Children with controlled severe asthma also had significant reductions in blood eosinophils and exhaled nitric oxide concentrations, as well as improvement in FEV1 values. Similarly, children who achieved control after triamcinolone also had improvement in AQLQ scores, a reduction in blood eosinophils and exhaled nitric oxide, and improvement in FEV1. In contrast, children who did not achieve control after triamcinolone had no improvement in AQLQ scores or FEV1 despite reductions in blood eosinophils and exhaled nitric oxide.
TABLE II.
Clinical outcomes
| Outcome | Controlled severe asthma (N = 15)
|
Achieved control with triamcinolone (N = 24)
|
Did not achieve control with triamcinolone (N = 17)
|
|||
|---|---|---|---|---|---|---|
| Baseline | After triamcinolone | Baseline | After triamcinolone | Baseline | After triamcinolone | |
| AQLQ score* | 6.4 (5.9–6.6) | 6.6† (6.5–6.9) | 5.2 (4.1–5.9) | 6.3‡ (5.4–6.6) | 4.9 (3.9–5.5) | 5.0 (4.1–5.8) |
| Blood eosinophils (%)§ | 7.9 (5.5–10.3) | 1.7* (1.0–3.3) | 5.4 (3.8–8.9) | 2.1‡ (1.0–2.2) | 5.8 (3.9–10.0) | 2.6* (1.2–3.3) |
| Exhaled nitric oxide (ppb)§ | 28 (11–58) | 13‡ (8–19) | 25 (19–77) | 16‡ (11–25) | 36 (21–46) | 26‖ (15–42) |
| FEV1 (% predicted) | 95 (84–105) | 99‖ (86–129) | 89 (71–97) | 92‖ (86–97) | 86 (74–103) | 79 (75–89) |
IQR, Interquartile range.
Data are from participants who completed the study. Values represent the median (IQR).
Maximum score on the AQLQ is 7, with higher scores representing greater asthma-related quality of life.
P < .01 vs baseline.
P < .001 vs baseline.
Data were logarithmically transformed before analysis.
P < .05 vs baseline.
The changes in the primary outcomes, as opposed to median values before and after triamcinolone, are shown in Figure 2. Seventy-eight percent (n = 18) of children who achieved control had an AQLQ minimal important difference of 0.519 after triamcinolone, compared with only 22% (n = 5) of children who did not achieve control (Figure 2, A). There were no significant differences in the magnitude of change in blood eosinophils and exhaled nitric oxide concentrations between children who achieved control versus those who did not (Figure 2, B–D). There was also a nonsignificant trend toward a greater magnitude of improvement in FEV1 in children who achieved control versus those who did not (Figure 2, E and F). Associations between raw ACQ scores, AQLQ scores, exhaled nitric oxide concentrations, and blood eosinophils in all participants after triamcinolone injection are presented in Table E2 in this article’s Online Repository at www.jaci-inpractice.org.
FIGURE 2.
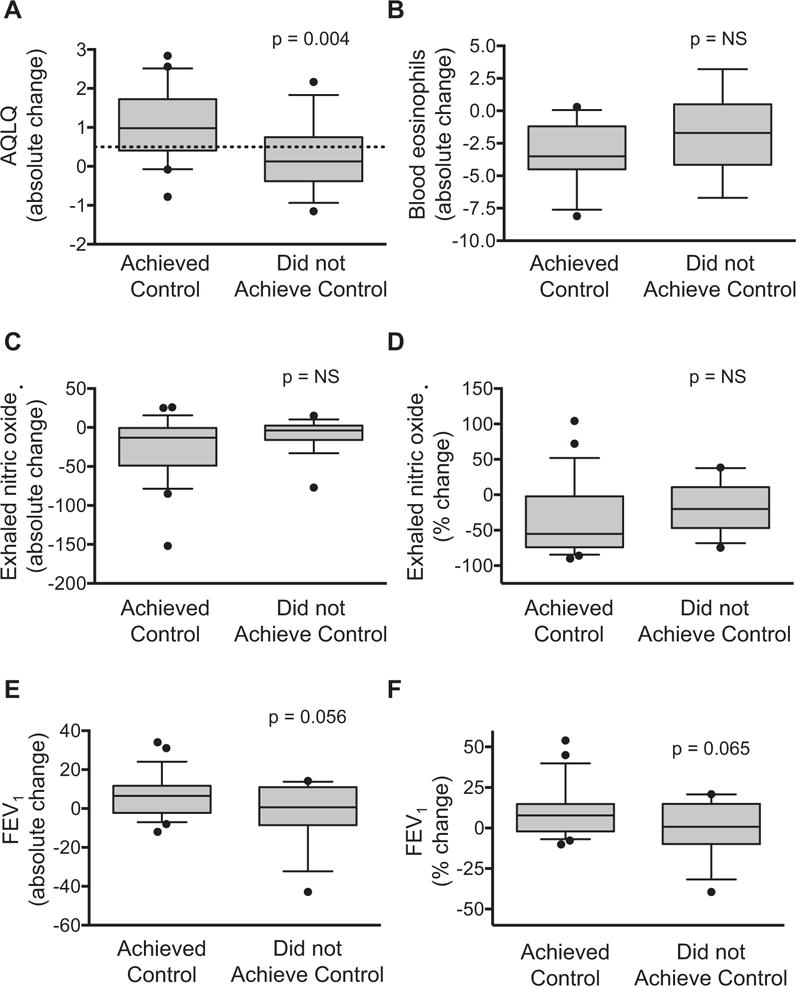
Absolute change in (A) AQLQ scores, (B) blood eosinophils, (C) exhaled nitric oxide, and (D) FEV1 values in children who achieved control versus children who did not. Panels (E) and (F) depict percent change relative to baseline values. Whiskers reflect the 10th to 90th percentile. The dashed line reflects the AQLQ minimally important difference. NS, Not significant.
mRNA gene expression
Using a liberal (P ≤ .05) threshold of significance, PBMC mRNA gene array of 84 inflammatory cytokines and chemokines (Table E1) identified 14 genes that differed between children who achieved control after triamcinolone versus those who did not (Table III; Figure 3, A and B). Pathway analysis suggested that the differences in gene expression might be related to disturbances in IL-2, IL-10, and TNF pathways (Figure 3, C). With a more stringent (P < .01) significance threshold, only 4 genes (AIMP1, CCR2, IL10RB, and IL5) differed between groups. The sensitivity and specificity of these genes for discriminating uncontrolled asthma after triamcinolone injection, compared with exhaled nitric oxide concentrations, blood eosinophils, and FEV1 values, is shown in Figure 4 (area under the curve, AIMP1: 0.846, P = .001; CCR2: 0.863, P = .001; IL10RB: 0.892, P < .001; IL5: 0.808, P = .004; exhaled nitric oxide: 0.508, P = .934; eosinophils: 0.507, P = .945; FEV1: 0.542, P = .659). Correlations between identified genes, exhaled nitric oxide concentrations, blood eosinophils, and FEV1 values are also presented in Table E3 in this article’s Online Repository at www.jaci-inpractice.org.
TABLE III.
Mean fold-change (95% CI) mRNA gene expression values in children with severe asthma who did not achieve control with triamcinolone, vs those who did achieve control
| Gene | Fold change (95% CI) | P value |
|---|---|---|
| AIMP1 | 0.66 (0.50–0.82) | .006 |
| C5 | 0.44 (0.20–0.69) | .020 |
| CCL3 | 0.63 (0.38–0.87) | .021 |
| CCR2 | 0.55 (0.38–0.72) | .002 |
| CCR5 | 0.56 (0.33–0.78) | .022 |
| CCR6 | 0.28 (<0.01–0.74) | .025 |
| IFNG | 0.51 (0.23–0.79) | .028 |
| IL10RB | 0.59 (0.44–0.74) | .001 |
| IL17C | 0.51 (0.12–0.89) | .041 |
| IL1A | 0.36 (0.11–0.61) | .032 |
| IL1RN | 0.55 (0.31–0.79) | .028 |
| IL5 | 0.13 (<0.01–0.27) | .008 |
| TNFSF13B | 0.57 (0.33–0.82) | .038 |
| VEGFA | 0.43 (0.17–0.68) | .031 |
FIGURE 3.
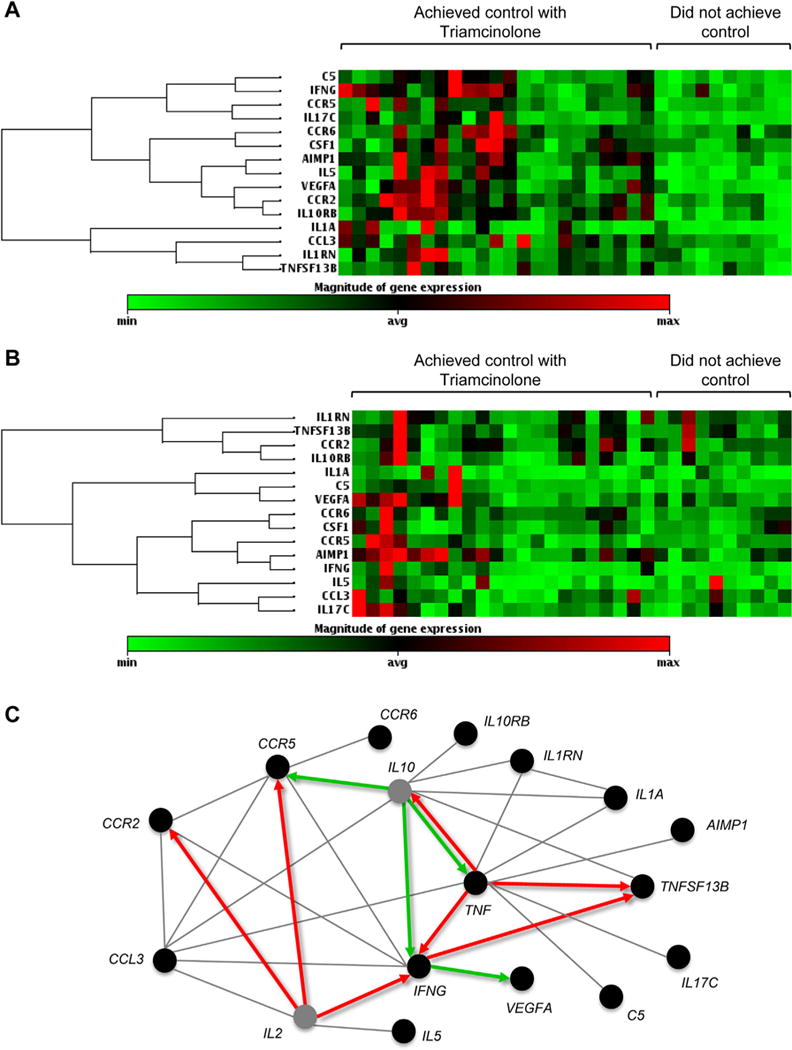
PBMC mRNA gene expression (A) at baseline and (B) after triamcinolone. (C) Pathway analysis of significant genes (P <.05) in children who did not achieve control with triamcinolone (note: TNF, P > .05). Red and green arrows depict upregulation and downregulation, respectively. Gray lines depict reported or probable associations. Gray circles represent genes that were not tested.
FIGURE 4.
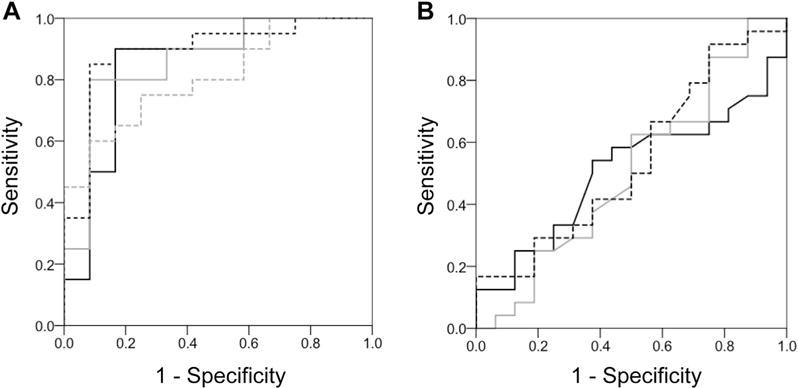
Receiver-operating characteristic curves depicting the performance of baseline (A) AIMP1 (black line), CCR2 (gray line), IL10RB (dashed black line), and IL5 (dashed gray line) mRNA delta cycle threshold values versus (B) exhaled nitric oxide (black line), blood eosinophil percentages (gray line), and FEV1 (dashed black line) in discriminating uncontrolled asthma after triamcinolone, defined by an ACQ score of 1.5 or more.
Predictor analyses
Predictor analyses were undertaken to determine whether baseline clinical features could identify children who failed to achieve asthma control after systemic triamcinolone administration. Of the clinical features tested, only cockroach sensitization was associated with uncontrolled asthma after triamcinolone receipt, although lack of precision in the point estimate was noted (odds ratio vs children who achieved control, 15.40; 95% CI, 2.50–95.05) (see Table E4 in this article’s Online Repository at www.jaci-inpractice.org).
Safety of the triamcinolone injection
The triamcinolone injection was well tolerated and no unexpected adverse events were reported. Expected adverse events within 24 to 48 hours of triamcinolone receipt included tenderness (n = 15 [25%]), warmth (n = 2 [3%]), and mild itching (n = 1 [2%]) at the injection site. No activity limitation was reported. Other unrelated adverse events included dizziness (n = 1), atopic dermatitis (n = 1), facial rash (n = 1), headache (n = 1), nausea and vomiting (n = 1), pharyngitis (n = 1), and cough (n = 1). There were no reports of subcutaneous atrophy following the injection. The distribution of adverse events did not differ between children with controlled severe asthma, children who achieved control after triamcinolone, and children who did not achieve control after triamcinolone. Each event resolved before the 14-day follow-up visit.
DISCUSSION
High doses of corticosteroids are the cornerstone of therapy for children with severe asthma, but responses to corticosteroids are variable and difficult to assess in the clinical setting. Using a clinically accessible questionnaire of asthma control, we identified 3 groups of children with severe asthma, including 1 group of children who failed to achieve asthma control after receipt of intramuscular triamcinolone. These children had overall reductions in blood eosinophil percentages and exhaled nitric oxide concentrations after triamcinolone administration, but their symptoms, asthma-related quality of life, and lung function values were largely unchanged. Although children who failed to achieve control with triamcinolone were phenotypically similar to other children with severe asthma, they had decreased systemic mRNA expression of inflammatory cytokines and chemokines associated with IL-2, IL-10, and TNF pathways. These findings highlight the heterogeneity among children with severe asthma and argue for consideration of endotypic as well as phenotypic features in the evaluation of systemic corticosteroid responses.
Bossley et al4 previously described features of children with difficult-to-treat asthma after a 2-week course of prednisolone (40 mg daily) or a single dose of intramuscular triamcinolone (80 mg). In their retrospective report, “complete” corticosteroid responsiveness assessed by selected cut-points of symptoms, FEV1, bronchodilator reversibility, and exhaled nitric oxide was identified in only 11% of children.4 Moreover, 9% of participating children were “nonresponders” to each of the parameters, while 80% were “partial” responders.4 A more recent report of 82 children with severe asthma that also included sputum eosinophils similarly noted 13% with a complete response, 15% with nonresponse, and 72% with a partial response to intramuscular triamcinolone with no reliable clinical phenotypic predictors of response pattern.5 Our findings also highlight the discordance between asthma symptoms, airway obstruction, exhaled nitric oxide, and eosinophils in children with severe asthma and similarly demonstrate no striking phenotypic dissimilarities between children who achieved control and children who did not achieve control after triamcinolone administration. Alternatively, our findings might also support the hypothesis of a single corticosteroid responsiveness endophenotype with various clinical presentations.22
In nonsmoking adults, airway neutrophils may contribute to impaired corticosteroid responsiveness because neutrophilic and mixed granulocytic patterns of airway inflammation have been associated with more significant airflow limitation and higher rates of health care utilization in patients with asthma.23,24 It is possible that the lack of response to triamcinolone that we observed may be due to concomitant infection and airway neutrophil infiltration in the absence of fever or purulent sputum or nasal discharge given the prevalence of historical pneumonia and sinusitis in enrolled participants. However, the role of the neutrophil in asthma is somewhat controversial because high doses of corticosteroids may promote neutrophil survival in the absence of hypoxemia.25 Although the pattern of airway inflammation in children with severe asthma can be heterogeneous,26 bronchoscopy studies have failed to detect higher percentages of neutrophils in the bronchoalveolar lavage or airway mucosa of children with severe asthma compared with children with nonsevere asthma and healthy controls.27,28 Instead, limited numbers of studies in children with severe asthma suggest that the airway inflammation is predominantly eosinophilic28 and persists in a subset of children even after treatment with oral prednisolone,29 perhaps due to ongoing allergen exposures. In keeping with this hypothesis, most children enrolled in this study had atopic features including multiple aeroallergen sensitization and increased blood eosinophils. Cockroach sensitization, which was more prevalent in children who did not achieve control with triamcinolone, has also been associated with higher blood eosinophil concentrations30 and more frequent asthma exacerbations necessitating urgent care.31,32 Although blood eosinophil percentages decreased after triamcinolone in children who failed to achieve control, whether eosinophils persisted in the airways of these children is unclear because blood eosinophil counts do not accurately reflect airway eosinophilia in children.33
Studies have previously compared inflammatory cytokine and chemokine expression between children with severe asthma (as a whole) and children with milder asthma27 or healthy controls,28 whereas this study was one of the first to compare inflammatory gene expression within severe asthma groups. Although the use of PBMCs for this purpose can be criticized, we34 and others35 have previously shown similarities between PBMCs and airway mononuclear cells in patients with severe asthma, although some functional differences are apparent. The sample size was also relatively small and prohibited analysis of subendotypes within groups. However, our finding of decreased expression of genes associated with IL-2, IL-10, and TNF pathways, namely, AIMP1, CCR2, IL10RB, and IL5, does have biologic plausibility because IL-2, along with IL-4, inhibits the ligand-binding affinity of the glucocorticoid receptor and the modulation of IL-10 release in corticosteroid-dependent adults with asthma.36 Previous reports in children with severe, therapy-resistant asthma have also noted decreased airway and systemic IL-10 expression37 and variable phenotypes of TNF expression.38 In mice, AIMP1 deficiency promotes airway hyperresponsiveness, recruitment of eosinophils, and TH2 cytokine production in the lung,39 while depletion of the IL-10 receptor, IL10RB, impairs the generation of function of anti-inflammatory macrophages and their ability to secrete IL-10.40 CCR2 inhibition also reduces the hematopoetic functions of IL-33,41 a relatively corticosteroid-resistant inflammatory mediator that has been associated with reticular basement membrane thickening in children with severe asthma.9 Furthermore, IL5 inhibition in adults with severe refractory asthma results in fewer blood eosinophils and decreased eosinophil activation.42 The fact that lower IL5 expression was observed in children with severe asthma who failed to achieve control with triamcinolone is therefore surprising given that no significant differences in baseline blood eosinophils were observed in this group. However, other studies have shown that children with severe refractory asthma, as a group, do not have increased airway expression of the IL-5 protein as compared with children with milder asthma or healthy controls.27,28
The present study does have limitations. Adherence to ICS was assessed indirectly by electronic prescription refills, and this does not rule out other issues related to ICS delivery, including inappropriate inhaler technique or inhaler particle size, that may result in inadequate airway deposition. Triamcinolone acetonide was selected for its injectable nature and depot effect to minimize additional confounding related to systemic drug delivery, but it is possible that the dose of triamcinolone used (1 mg/kg, 60 mg maximum) was not sufficient because previous studies in adults with refractory asthma noted significant improvements in symptoms and sputum eosinophilia with a much higher single dose (120–360 mg).43,44 However, side effects including cushingoid facies, hypertension, and elevated blood glucose levels were reported44 and thus the benefits of a higher single triamcinolone dose were thought to be outweighed by the potential for systemic side effects. Although the exact pharmacokinetic potency of triamcinolone versus oral prednisolone is unclear, triamcinolone likely has greater affinity for the glucocorticoid receptor because early studies in adults with severe refractory asthma have shown greater treatment efficacy with a single 80-mg dose of triamcinolone dose than with a 10-mg dose of prednisolone daily.45,46 The dose of triamcinolone used in this study was based on previous reports of feasibility and efficacy in children with a similar dosing strategy.47,48 The 2-week time point that was used for outcome assessment was based on previous studies in adults with severe refractory asthma that demonstrated no clinically meaningful differences between 2 versus 4 weeks of triamcinolone treatment45 and significant improvement in symptoms, lung function, and airway eosinophils (with almost complete eosinophil disappearance) after only 2 weeks postadministration.43 The lack of participant blinding and the lack of a placebo arm are also potential limitations, but given the limited available literature on severe asthma in children, we felt that this was an important design consideration to increase the benefit/risk ratio of additional corticosteroid exposure in a high-risk group of participants.
It is also important to note that our results may be dependent on the tool that we used for corticosteroid response assignment, in this case the ACQ score. Although this tool has been extensively validated for the assessment of asthma control,16,17 it may have limitations in the determination of corticosteroid response. Because this study was conducted at a single center that serves a predominantly inner-city population, it is also possible that the findings have limited external validity. Racial disparities in asthma and potentially corticosteroid responsiveness have been previously reported,49 but we were underpowered to address racial differences in the present study. Genetic analyses of GLCCI1 (glucocorticoid-induced transcript 1), CRHR1 (corticotrophin releasing hormone receptor 1), and FBXL7 (F-box and leucine-rich repeat protein 7), which have been associated with ICS responsiveness,50–52 were also not possible, although those studies were limited to non-Hispanic white participants with mild-to-moderate asthma and may have limited relevance to our population.
In conclusion, using a clinically accessible measure of asthma control, we identified 3 groups of children with severe asthma with differing asthma control profiles that responded differently to systemic triamcinolone administration. At baseline, these groups were phenotypically similar, but discordance between symptoms, lung function, exhaled nitric oxide, and blood eosinophils was noted after the triamcinolone injection. Clinical phenotypic predictors were of limited utility in the ascertainment of triamcinolone response, whereas systemic mRNA expression of inflammatory cytokines and chemokines related to IL-2, IL-10, and TNF pathways strongly differentiated children who failed to achieve control with triamcinolone administration. These findings highlight the heterogeneity of severe asthma in children and the complicated nature of corticosteroid responsiveness assessment in this population. Further study of molecular profiles of children with severe asthma may help to identify which children will derive the most clinical benefit from systemic corticosteroid step-up therapy given the potential side effects of these medications.
Supplementary Material
What is already known about this topic?
Children with severe asthma are heterogeneous and have variable responses to systemic corticosteroids. Assessment of these responses is challenging due to a lack of a criterion standard and challenges with systemic corticosteroid delivery.
What does this article add to our knowledge?
Clinical phenotypic predictors were of limited utility in discriminating triamcinolone response. Systemic mRNA expression of inflammatory mediators related to IL-2, IL-10, and TNF pathways strongly differentiated children who failed to achieve control after triamcinolone administration.
How does this study impact current management guidelines?
Current definitions of severe asthma in children are associated with varied responses to systemic corticosteroids. Molecular endotypic as well as clinical phenotypic features should be considered in the evaluation of systemic corticosteroid responses.
Acknowledgments
This study was funded by grant number R01 NR012021 and was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health (award no. UL1 TR000454).
Conflicts of interest: A. M. Fitzpatrick has received money for consultancy from Genentech and Boehringer Ingelheim. A. M. Fitzpatrick’s institution, S. T. Stephenson’s institution, M. R. Brown’s institution, K. Nguyen’s institution, S. Douglas’ institution, and L. A. S. Brown’s institution have received a grant from the National Institutes of Health (NIH)/National Institute of Nursing Research (grant no. R01NR012021).
Abbreviations used
- ACQ
Asthma Control Questionnaire
- AQLQ
Asthma Quality of Life Questionnaire
- ICS
inhaled corticosteroid
- PBMC
peripheral blood mononuclear cell
- SABA
short-acting beta agonist
References
- 1.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–73. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 2.Mercado N, Hakim A, Kobayashi Y, Meah S, Usmani OS, Chung KF, et al. Restoration of corticosteroid sensitivity by p38 mitogen activated protein kinase inhibition in peripheral blood mononuclear cells from severe asthma. PLoS One. 2012;7:e41582. doi: 10.1371/journal.pone.0041582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossios C, To Y, Osoata G, Ito M, Barnes PJ, Ito K. Corticosteroid insensitivity is reversed by formoterol via phosphoinositide-3-kinase inhibition. Br J Pharmacol. 2012;167:775–86. doi: 10.1111/j.1476-5381.2012.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bossley CJ, Saglani S, Kavanagh C, Payne DN, Wilson N, Tsartsali L, et al. Corticosteroid responsiveness and clinical characteristics in childhood difficult asthma. Eur Respir J. 2009;34:1052–9. doi: 10.1183/09031936.00186508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bossley CJ, Fleming L, Ullmann N, Gupta A, Adams A, Nagakumar P, et al. Assessment of corticosteroid response in pediatric patients with severe asthma by using a multidomain approach. J Allergy Clin Immunol. 2016;138:413–420.e6. doi: 10.1016/j.jaci.2015.12.1347. [DOI] [PubMed] [Google Scholar]
- 6.Stephenson ST, Brown LA, Helms MN, Qu H, Brown SD, Brown MR, et al. Cysteine oxidation impairs systemic glucocorticoid responsiveness in children with difficult-to-treat asthma. J Allergy Clin Immunol. 2015;136:454–461.e9. doi: 10.1016/j.jaci.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan X, Chu JH, Gomez J, Koenigs M, Holm C, He X, et al. Noninvasive analysis of the sputum transcriptome discriminates clinical phenotypes of asthma. Am J Respir Crit Care Med. 2015;191:1116–25. doi: 10.1164/rccm.201408-1440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Persson H, Kwon AT, Ramilowski JA, Silberberg G, Soderhall C, Orsmark-Pietras C, et al. Transcriptome analysis of controlled and therapy-resistant childhood asthma reveals distinct gene expression profiles. J Allergy Clin Immunol. 2015;136:638–48. doi: 10.1016/j.jaci.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Saglani S, Lui S, Ullmann N, Campbell GA, Sherburn RT, Mathie SA, et al. IL-33 promotes airway remodeling in pediatric patients with severe steroid-resistant asthma. J Allergy Clin Immunol. 2013;132:676–685.e13. doi: 10.1016/j.jaci.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin RJ, Szefler SJ, King TS, Kraft M, Boushey HA, Chinchilli VM, et al. The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial. J Allergy Clin Immunol. 2007;119:73–80. doi: 10.1016/j.jaci.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmichael J, Paterson IC, Diaz P, Crompton GK, Kay AB, Grant IW. Corticosteroid resistance in chronic asthma. Br Med J (Clin Res Ed) 1981;282:1419–22. doi: 10.1136/bmj.282.6274.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore WC, Fitzpatrick AM, Li X, Hastie AT, Li H, Meyers DA, et al. Clinical heterogeneity in the severe asthma research program. Ann Am Thorac Soc. 2013;10:S118–24. doi: 10.1513/AnnalsATS.201309-307AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorkness RL, Teague WG, Penugonda M, Fitzpatrick AM, National Institutes of Health National Heart, Lung, and Blood Institute’s Severe Asthma Research Program Sex dependence of airflow limitation and air trapping in children with severe asthma. J Allergy Clin Immunol. 2011;127:1073–4. doi: 10.1016/j.jaci.2010.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacharier LB, Strunk RC, Mauger D, White D, Lemanske RF, Jr, Sorkness CA. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004;170:426–32. doi: 10.1164/rccm.200308-1178OC. [DOI] [PubMed] [Google Scholar]
- 15.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma—Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 16.Juniper EF, Gruffydd-Jones K, Ward S, Svensson K. Asthma Control Questionnaire in children: validation, measurement properties, interpretation. Eur Respir J. 2010;36:1410–6. doi: 10.1183/09031936.00117509. [DOI] [PubMed] [Google Scholar]
- 17.Juniper EF, Bousquet J, Abetz L, Bateman ED, GOAL Committee Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100:616–21. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Juniper EF, Guyatt GH, Ferrie PJ, Griffith LE. Measuring quality of life in asthma. Am Rev Respir Dis. 1993;147:832–8. doi: 10.1164/ajrccm/147.4.832. [DOI] [PubMed] [Google Scholar]
- 19.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol. 1994;47:81–7. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 20.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 22.Clemmer GL, Wu AC, Rosner B, McGeachie MJ, Litonjua AA, Tantisira KG, et al. Measuring the corticosteroid responsiveness endophenotype in asthmatic patients. J Allergy Clin Immunol. 2015;136:274–281.e8. doi: 10.1016/j.jaci.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010;125:1028–1036.e13. doi: 10.1016/j.jaci.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133:1557–1563.e5. doi: 10.1016/j.jaci.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marwick JA, Dorward DA, Lucas CD, Jones KO, Sheldrake TA, Fox S, et al. Oxygen levels determine the ability of glucocorticoids to influence neutrophil survival in inflammatory environments. J Leukoc Biol. 2013;94:1285–92. doi: 10.1189/jlb.0912462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien CE, Tsirilakis K, Santiago MT, Goldman DL, Vicencio AG. Heterogeneity of lower airway inflammation in children with severe-persistent asthma. Pediatr Pulmonol. 2015;50:1200–4. doi: 10.1002/ppul.23165. [DOI] [PubMed] [Google Scholar]
- 27.Fitzpatrick AM, Higgins M, Holguin F, Brown LA, Teague WG, National Institutes of Health/National Heart, Lung, and Blood Institute The molecular phenotype of severe asthma in children. J Allergy Clin Immunol. 2010;125:851–857.e18. doi: 10.1016/j.jaci.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bossley CJ, Fleming L, Gupta A, Regamey N, Frith J, Oates T, et al. Pediatric severe asthma is characterized by eosinophilia and remodeling without T(H)2 cytokines. J Allergy Clin Immunol. 2012;129:974–982.e13. doi: 10.1016/j.jaci.2012.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Payne DN, Adcock IM, Wilson NM, Oates T, Scallan M, Bush A. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med. 2001;164:1376–81. doi: 10.1164/ajrccm.164.8.2101145. [DOI] [PubMed] [Google Scholar]
- 30.Matsui EC, Sampson HA, Bahnson HT, Gruchalla RS, Pongracic JA, Teach SJ, et al. Allergen-specific IgE as a biomarker of exposure plus sensitization in inner-city adolescents with asthma. Allergy. 2010;65:1414–22. doi: 10.1111/j.1398-9995.2010.02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thorne PS, Mendy A, Metwali N, Salo P, Co C, Jaramillo R, et al. Endotoxin exposure: predictors and prevalence of associated asthma outcomes in the United States. Am J Respir Crit Care Med. 2015;192:1287–97. doi: 10.1164/rccm.201502-0251OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arroyave WD, Rabito FA, Carlson JC. The relationship between a specific IgE level and asthma outcomes: results from the 2005–2006 National Health and Nutrition Examination Survey. J Allergy Clin Immunol Pract. 2013;1:501–8. doi: 10.1016/j.jaip.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Ullmann N, Bossley CJ, Fleming L, Silvestri M, Bush A, Saglani S. Blood eosinophil counts rarely reflect airway eosinophilia in children with severe asthma. Allergy. 2013;68:402–6. doi: 10.1111/all.12101. [DOI] [PubMed] [Google Scholar]
- 34.Fitzpatrick AM, Stephenson ST, Hadley GR, Burwell L, Penugonda M, Simon DM, et al. Thiol redox disturbances in children with severe asthma are associated with posttranslational modification of the transcription factor nuclear factor (erythroid-derived 2)-like 2. J Allergy Clin Immunol. 2011;127:1604–11. doi: 10.1016/j.jaci.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goleva E, Jackson LP, Gleason M, Leung DY. Usefulness of PBMCs to predict clinical response to corticosteroids in asthmatic patients. J Allergy Clin Immunol. 2012;129:687–693.e1. doi: 10.1016/j.jaci.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Irusen E, Matthews JG, Takahashi A, Barnes PJ, Chung KF, Adcock IM. p38 Mitogen-activated protein kinase-induced glucocorticoid receptor phosphorylation reduces its activity: role in steroid-insensitive asthma. J Allergy Clin Immunol. 2002;109:649–57. doi: 10.1067/mai.2002.122465. [DOI] [PubMed] [Google Scholar]
- 37.Gupta A, Dimeloe S, Richards DF, Chambers ES, Black C, Urry Z, et al. Defective IL-10 expression and in vitro steroid-induced IL-17A in paediatric severe therapy-resistant asthma. Thorax. 2014;69:508–15. doi: 10.1136/thoraxjnl-2013-203421. [DOI] [PubMed] [Google Scholar]
- 38.Brown SD, Brown LA, Stephenson S, Dodds JC, Douglas SL, Qu H, et al. Characterization of a high TNF-alpha phenotype in children with moderate-to-severe asthma. J Allergy Clin Immunol. 2015;135:1651–4. doi: 10.1016/j.jaci.2014.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong HJ, Kim E, Jung MY, Kim S, Kim TS. AIMP1 deficiency enhances airway hyperreactivity in mice via increased TH2 immune responses. Clin Immunol. 2012;143:256–65. doi: 10.1016/j.clim.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40:706–19. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J, Kim W, Le HT, Moon UJ, Tran VG, Kim HJ, et al. IL-33-induced hematopoietic stem and progenitor cell mobilization depends upon CCR2. J Immunol. 2014;193:3792–802. doi: 10.4049/jimmunol.1400176. [DOI] [PubMed] [Google Scholar]
- 42.Stein ML, Villanueva JM, Buckmeier BK, Yamada Y, Filipovich AH, Assa’ad AH, et al. Anti-IL-5 (mepolizumab) therapy reduces eosinophil activation ex vivo and increases IL-5 and IL-5 receptor levels. J Allergy Clin Immunol. 2008;121:1473–83. 1483.e1–4. doi: 10.1016/j.jaci.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. “Refractory” eosinophilic airway inflammation in severe asthma: effect of parenteral corticosteroids. Am J Respir Crit Care Med. 2004;170:601–5. doi: 10.1164/rccm.200404-440OC. [DOI] [PubMed] [Google Scholar]
- 44.Ogirala RG, Sturm TM, Aldrich TK, Meller FF, Pacia EB, Keane AM, et al. Single, high-dose intramuscular triamcinolone acetonide versus weekly oral methotrexate in life-threatening asthma: a double-blind study. Am J Respir Crit Care Med. 1995;152:1461–6. doi: 10.1164/ajrccm.152.5.7582277. [DOI] [PubMed] [Google Scholar]
- 45.Willey RF, Fergusson RJ, Godden DJ, Crompton GK, Grant IW. Comparison of oral prednisolone and intramuscular depot triamcinolone in patients with severe chronic asthma. Thorax. 1984;39:340–4. doi: 10.1136/thx.39.5.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McLeod DT, Capewell SJ, Law J, MacLaren W, Seaton A. Intramuscular triamcinolone acetonide in chronic severe asthma. Thorax. 1985;40:840–5. doi: 10.1136/thx.40.11.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panickar JR, Kenia P, Silverman M, Grigg J. Intramuscular triamcinolone for difficult asthma. Pediatr Pulmonol. 2005;39:421–5. doi: 10.1002/ppul.20176. [DOI] [PubMed] [Google Scholar]
- 48.Panickar JR, Bhatnagar N, Grigg J. Exhaled nitric oxide after a single dose of intramuscular triamcinolone in children with difficult to control asthma. Pediatr Pulmonol. 2007;42:573–8. doi: 10.1002/ppul.20583. [DOI] [PubMed] [Google Scholar]
- 49.Wechsler ME, Castro M, Lehman E, Chinchilli VM, Sutherland ER, Denlinger L, et al. Impact of race on asthma treatment failures in the Asthma Clinical Research Network. Am J Respir Crit Care Med. 2011;184:1247–53. doi: 10.1164/rccm.201103-0514OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tantisira KG, Lasky-Su J, Harada M, Murphy A, Litonjua AA, Himes BE, et al. Genomewide association between GLCCI1 and response to glucocorticoid therapy in asthma. N Engl J Med. 2011;365:1173–83. doi: 10.1056/NEJMoa0911353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGeachie MJ, Wu AC, Chang HH, Lima JJ, Peters SP, Tantisira KG. Predicting inhaled corticosteroid response in asthma with two associated SNPs. Pharmacogenomics J. 2013;13:306–11. doi: 10.1038/tpj.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park HW, Dahlin A, Tse S, Duan QL, Schuemann B, Martinez FD, et al. Genetic predictors associated with improvement of asthma symptoms in response to inhaled corticosteroids. J Allergy Clin Immunol. 2014;133:664–669.e5. doi: 10.1016/j.jaci.2013.12.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


