Abstract
Background
Variation in the CYP2A6 gene alters the rate of nicotine metabolic inactivation and is associated with smoking behaviors and cessation success rates. The underlying neurobiological mechanisms of this genetic influence remain unknown.
Methods
Intrinsic functional connectivity strength (FCS), a whole brain, data driven, graph theory based method, was applied to resting state fMRI data in 66 smokers and 92 nonsmokers. A subset of subjects (n=23/20; smokers/nonsmokers) performed the Monetary Incentive Delay (MID) task, probing reward anticipation and a Go/NoGo task, probing response inhibition, on two occasions, in the presence and absence of a nicotine patch.
Results
A significant CYP2A6 genotype × Smoking effect was found in the dorsal anterior cingulate cortex (dACC) and ventral striatum (VS), such that the normal (vs. slow) genotype individuals showed greater FCS in smokers but not nonsmokers. FCS was negatively associated with nicotine dependence severity in slow metabolizers. Both hubs were biased by inputs from the insula identified from seed-based connectivity. Similar Gene × Environment interactions were seen in VS during smoking abstinence when subjects performed the MID task and in dACC when they performed the Go/NoGo task; both reductions were “normalized” in smokers (and increased in nonsmokers) following acute nicotine administration.
Conclusions
Since the CYP2A6 effect was seen only in smokers, these data suggest that the rate of nicotine metabolism, and thus the concentration of nicotine presented to brain over the course of nicotine addiction, shapes brain circuits that, among other functions, compute reward and impulsivity processes.
Keywords: CYP2A6 genotype, smoking, nicotine, functional connectivity strength, striatal-cingulate-insula circuits, neuroadaptations
Introduction
Nicotine is the principal addictive component in tobacco that maintains smoking behavior. Nicotine (and other drug) dependence induces neuroplastic alterations in brain circuits (1), which in turn contribute to the altered behavioral and cognitive processes seen in dependent individuals that impact their ability to stop smoking (2). However, identifying the neuroanatomical loci and then mechanistically linking these dependence-induced neuroadaptations and genetic influences with their behavioral consequences remains very poorly understood. With the consistently poor outcomes following treatment for nicotine dependence (3), empirically based neurobiological mechanisms have the potential to improve treatment efficacy via individualized treatment optimization along with the discovery of novel drug targets.
The hepatic enzyme cytochrome P450 (CYP2A6) is the main metabolic pathway responsible for nicotine inactivation and clearance (4); CYP2A6 is expressed at very low levels in the human brain, or not at all (5, 6). Variation in the CYP2A6 gene alters the rate of nicotine metabolism, with the slow genotype reducing nicotine metabolism more than 50% (7). Smokers with the reduced (slow and intermediate) CYP2A6 genotype start smoking earlier in life (8–10), become dependent quicker (11, 12), but smoke fewer cigarettes (10, 13) and have a higher likelihood of quitting smoking (14, 15). Slow metabolizers have higher quit success rates when using either placebo or transdermal nicotine replacement therapy (NRT, i.e. nicotine patch) vs. varenicline (Chantix®) usage, but have greater adverse effects from varenicline, while normal genotype individuals respond better to varenicline than to NRT (16–18). Thus these CYP2A6 genetic effects on cessation persist months after being nicotine free, suggesting that genetic variations in this metabolic pathway likely created long-lasting brain changes that result in these differential smoking phenotypes and treatment response differences.
The underlying neurobiological mechanisms of the impact of this genetically variable pharmacokinetic (PK) enzyme on smoking-related behaviors and neuroplasticity are largely unknown. Only a single study has been published, which reported that slow metabolizing smokers have reduced limbic responses to smoking cues (19). However, both human and preclinical data indicate the importance of PK influences on drug addiction – both the rate of drug onset (primarily influenced by route of delivery) and offset (primarily influenced by biotransformation and distribution) (20). Smoking (relative to nicotine patch) delivers nicotine to the brain in seconds, while genetically variable rates of nicotine inactivation by CYP2A6 leads to variable offset time, both of which may result in rapid and long lasting neuroplastic effects and differential abuse potential. Indeed, the rapid onset of action for both nicotine and cocaine preferentially engage mesocorticolimbic (MCL) circuits to promote neurobehavioral plasticity (21). In contrast, variation in the ‘off-time’ can alter the abuse and dependence of drugs from a number of classes, including cocaine, benzodiazepines and nicotine (22). For example, while both cocaine and methylphenidate have high affinity for the dopamine transporter and reach the brain rapidly after intravenous administration, cocaine’s shorter duration of action, due to rapid removal from brain, both promotes and enables its frequent use, while methylphenidate has a much longer duration of action due to its slower brain clearance and has a much lower abuse profile (23).
Since smokers with the slow metabolism CYP2A6 genotype have relatively constant nicotine plasma levels throughout the day, while those with faster metabolism likely experience a greater surge in plasma nicotine with each cigarette (24), and as changes in PK can change fundamental neurobiological mechanisms, we reasoned that CYP2A6-induced modifications in brain nicotine concentration differentially alters nicotinic receptor function and in turn, modifies brain circuits that contribute to the development and/or maintenance of nicotine addiction and its plasticity-related behavioral and cognitive profile. We also reasoned that this would occur only among smokers, while those not exposed to nicotine would have no genotype-induced neuroplastic differences, leading to significant and meaningful Gene × Environment (CYP2A6-smoking) interactions.
However, as smokers present with a spectrum of altered behaviors and neuronal circuits (25), we could not a priori predict those circuits that might be most sensitive to variable nicotine exposure. We thus interrogated the effects of CYP2A6 genotype on whole brain network topological organization using resting state functional connectivity (rsFC) (26) and a data driven analytical graph theory metric, functional connectivity strength (FCS). High FCS of a region is thought to reflect high nodal activity, thought to reflect regions of high neuronal integration (27–29).
To complement this data driven network approach, we also considered smoking status (smoker vs. nonsmoker), current level of smoking (cigarettes per day, CPD) and nicotine dependence severity (Fagerstrom Test for Nicotine Dependence, FTND (30)). We hypothesized that the CYP2A6 genotype would, among smokers but not among nonsmokers, 1) influence brain FCS; 2) alter FCS within nodes of the MCL dopamine system; and 3) modulate neuronal computations and cognitive processes, specifically reward processing and inhibitory control.
To test these hypotheses, we genotyped smokers and nonsmokers for the CYP2A6 polymorphism and subsequently classified them as normal, intermediate or slow nicotine metabolizers (16, 19, 31). In addition to acquiring resting state BOLD data, a subset of smokers and nonsmokers also performed the MID reward task and a Go/NoGo response inhibition task on two sessions-once while wearing a nicotine patch and once after 12 hours of smoking abstinence.
Methods and Materials
Participants
Resting state BOLD fMRI data were acquired from non-treatment seeking smokers (n=66) and nonsmokers (n=92), matched by age, sex, IQ and race (Table 1). Subjects were selected from a large cohort of individuals who gave written informed consent under protocols approved by the NIDA-IRP IRB. IRB approval for CYP2A6 genotyping was granted at the University of Toronto. A subset of smokers (n=23; 15 normal and 8 reduced metabolizers) and nonsmokers (n=20; 9 normal and 11 reduced) also performed two fMRI tasks following resting BOLD data acquisition: the Monetary Incentive Delay (MID) task, which assesses neuronal activity during anticipation of and outcome for monetary rewards (32), and a Go/NoGo task assessing response inhibition (33). See SI for details of fMRI data acquisition and preprocessing and voxel-based morphometry (VBM) data analyses and results.
Table 1.
Demographic characteristics (entire cohort)
| NONSMOKERS (n=92) |
SMOKERS (n=66) |
Statistical Analysis |
|||||
|---|---|---|---|---|---|---|---|
| CYP2A6 | Normal | Intermediate | Slow | Normal | Intermediate | Slow | P |
| Number of subjects | 28 | 21 | 43 | 25 | 18 | 23 | 0.36 |
| Age (years) | 34.2±9.6 | 29.8±7.9 | 32.4±8.5 | 36.5±11.0 | 30.7±10.4 | 34.5±8.4 | all Ps > 0.09 |
| Gender (M:F) | 13:15 | 12:9 | 24:19 | 11:14 | 10:8 | 15:8 | all Ps > 0.29 |
| Race | |||||||
| Black/African American |
13 | 10 | 35 | 7 | 4 | 17 | |
| Caucasian | 12 | 8 | 5 | 18 | 14 | 5 | |
| Others | 3 | 3 | 3 | -- | -- | 1 | |
| Years of educationa | 14.1±1.8 | 14.5±1.2 | 14.0±2.1 | 13.2±2.3 | 13.1±1.6 | 13.1±1.7 | all Ps > 0.88 |
| IQ (Verbal)b | 59.8±8.2 | 59.9±9.0 | 58.6±10.0 | 57.6±8.6 | 53.9±7.5 | 54.0±6.8 | all Ps > 0.36 |
| FTND | 5.4±2.1 | 5.3±2.0 | 4.6±1.9 | 0.33 | |||
| CPDc | 20.7+7.4 | 17.6+8.5 | 14.5+3.7 | 0.04 | |||
| CO | 22.4+14.6 | 14.6+9.2 | 17.4+8.7 | 0.12 | |||
| Interval (hours) | 5.3+5.0 | 3.6+1.2 | 5.0+4.8 | 0.62 | |||
Pearson’s χ2-test was used to compare the frequency/gender distribution of the three CYP2A6 genotype groups between smokers and nonsmokers. Two-way ANOVAs were conducted to compare the six groups in age, years of education, and IQ. One-way ANOVA was used to compare differences in FTND, CPD, CO, and interval among the three genotype smokers. Tukey’s t-test was used for multiple comparisons. Data represents mean + SD. FTND, Fagerström Test for Nicotine Dependence; CPD, cigarettes per day; M, male; F, female; CO, exhaled carbon monoxide level (ppm); Interval, time from last cigarette smoked to data acquisition beginning.
Significant difference in education years between smokers and nonsmokers (P = 0.004).
Significant difference in IQ score between smokers and nonsmokers (P = 0.002).
Significant difference in CPD between normal and slow metabolizers (P = 0.003).
Genotyping
Genomic DNA was extracted from blood samples and genotyped for CYP2A6 as described (16, 19, 31). Based on the impact of enzymatic activity (7, 31), subjects were grouped into normal, intermediate and slow metabolizers (16, 19, 31). Due to the more limited cohort size available for task data, we combined the slow and intermediate genotype groups into a single reduced genotype group (19) for task analysis.
FCS analysis
FCS, defined as the sum of functional connectivity between any one voxel and all other brain voxels (27, 28), was computed for each subject from 6 min of BOLD fMRI acquired with subjects’ eyes open. Functional connectivity between voxels was measured using Pearson correlation in MATLAB (The MathWorks Inc, Natick, Massachusetts). FCS was computed after thresholding the correlation map at r > 0.25 (28). A voxel-wise threshold of P < 0.01 and a spatial-extent of 30 contiguous voxels were determined via Monte Carlo simulation to provide a corrected threshold of P < 0.05.
MID and Go/NoGo tasks
Subjects performed two tasks on two occasions, separated by 1–7 days. A nicotine or placebo patch was placed, in random order, 2–9 hours prior to scanning. Trials in the MID task included four sequential stimuli: PRIME-1 (gain, loss or neutral), PRIME-2 (win/loss magnitude), TARGET, and FEEDBACK; a button press was required to the TARGET. For the Go/NoGo task, subjects viewed a serial stream of alternating ‘X’ and ‘Y’ letters and were to respond to each target while the stimulus was present on the screen. NoGo stimuli, in which the target stimuli did not alternate (e.g. X, X), required response inhibition (34–36).
Since we had strong a priori interest in the ventral striatum (VS) for the MID task and dorsal anterior cingulate cortex (dACC) for the Go/NoGo task, we did region of interest analysis and small volume correction (Pcorrected < 0.05) using respectively, a striatal atlas (http://fsl.fmrib.ox.ac.uk/fsl) and dACC mask (37).
Statistical analysis
A 3 (Genotype) × 2 (Smokers, Nonsmokers) ANCOVA was performed on whole brain FCS maps, using age, gender, IQ, race and years of education as covariates. Post-hoc one-way ANOVA and two-tailed independent t-tests identified differences between groups. MID and Go/NoGo tasks were analyzed using GLM (35). Associations between FCS in regions showing significant GENOTYPE × SMOKING interactions and FTND/CPD were tested using Pearson’s correlation analyses. The mean FCS in these regions was regressed against age, IQ and years of education. One and two way ANOVAs followed by Tukey’s t-test for multiple comparisons and frequency analyses (Pearson’s χ2-test) were conducted (SPSS 20, IBM, Armonk, New York) to compare demographic and behavioral characteristics among groups.
Results
Demographics, genotypes and behavioral characteristics
For the entire sample, there was no significant difference in the frequency distribution of the three CYP2A6 groups between smokers and nonsmokers (P = 0.36) and the CYP2A6 genotype frequency distributions were in Hardy-Weinberg equilibrium for both groups. The samples were well matched, although nonsmokers had higher verbal IQ (P = 0.002) and years of education (P = 0.004) than smokers, which did not differ between the three genotype groups in either cohort. As expected (9–11, 13), there was a significant difference in CPD (P = 0.04) between the three genotype smoker groups, such that slow (vs. normal) genotype smokers smoked fewer cigarettes (P = 0.003) (See Table 1 for details). Groups were generally well matched in the task subgroup as well except that smokers and reduced genotype individuals both had lower IQ and fewer years of education than nonsmokers (see Table 2). Additionally, there was no difference in the frequency distribution of the four groups (P = 0.18) with the CYP2A6 genotype frequency distributions were in Hardy-Weinberg equilibrium.
Table 2.
Demographic characteristics (MID task and Go/NoGo cohort)
| NONSMOKERS | SMOKERS | Statistical Analysis | |||
|---|---|---|---|---|---|
| P | |||||
| (n=20) | (n=23) | ||||
| CYP2A6 | Normal | Reduced | Normal | Reduced | |
| Number of subjects | 9 | 11 | 15 | 8 | 0.18 |
| Age | 30.7±7.8 | 30.2±6.9 | 35.5±10.5 | 35.6±9.4 | all Ps > 0.08 |
| Race | |||||
| Black or African American | 1 | 5 | 2 | 4 | |
| Caucasian | 7 | 3 | 10 | 3 | |
| Others | 1 | 3 | 3 | 1 | |
| Gender (M:F) | 5:4 | 5:6 | 6:9 | 5:3 | 0.23 |
| IQ (Verbal)a | 64.9±8.9 | 58.6±3.3 | 58.3±10.8 | 51.4±5.9 | all Ps > 0.01 |
| Years of educationb | 15.6±0.9 | 14.4±1.6 | 13.8±1.8 | 13.2±2.0 | all Ps > 0.007 |
| FTND | 4.8±1.9 | 5.0±2.2 | 0.82 | ||
| CPD | 16.8±7.0 | 13.1±9.0 | 0.94 | ||
| CO | 6.8±2.8 | 7.5±4.9 | 0.68 | ||
Pearson’s χ2-test was used to compare the frequency/gender distribution of the two CYP2A6 genotype groups between smokers and nonsmokers. Two-way ANOVAs were conducted to compare the four groups in age, years of education, and IQ. Tukey’s t-tests were used to compare differences in FTND, CPD, and CO between the two genotype smokers, and also were used for multiple comparisons. Reduced: slow and intermediate. Data represents mean ± SD; FTND, Fagerström Test for Nicotine Dependence; CPD, cigarettes per day; M, male; F, female; CO, exhaled carbon monoxide level (ppm).
Smokers had lower IQ than nonsmokers (P = 0.011), and reduced genotype individuals had lower IQ score than those with normal genotype (P = 0.015).
Smokers had fewer years of education than nonsmokers (P = 0.007).
FCS and correlation with smoking behaviors
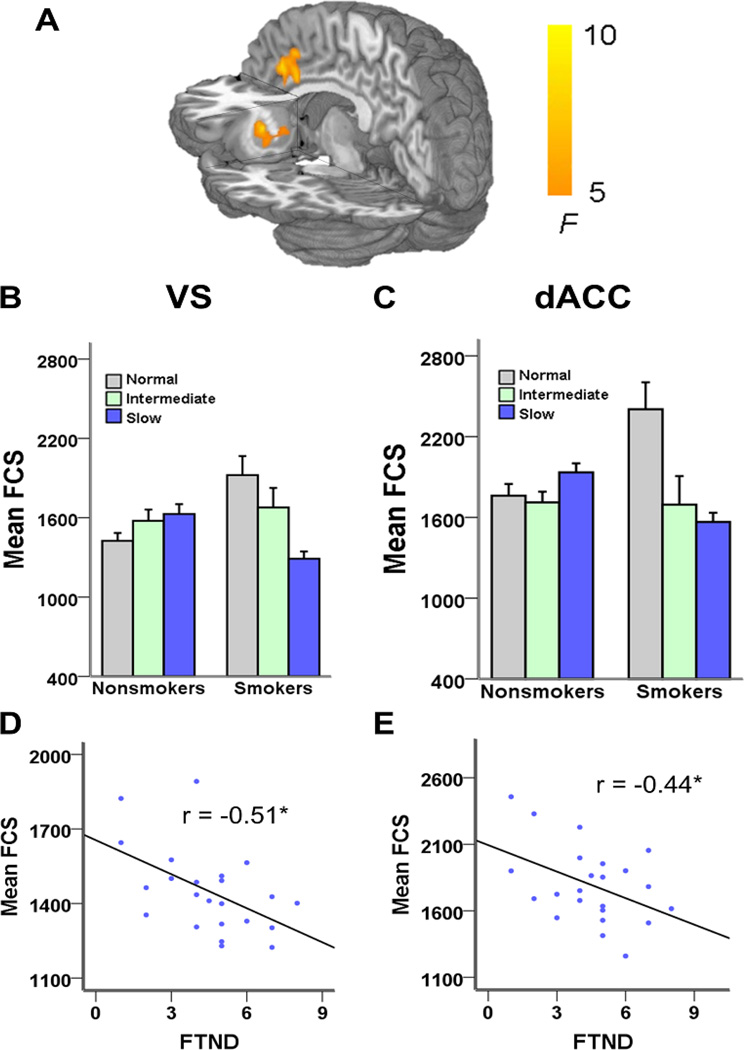
FCS was utilized as a metric of regional connectivity strength (27, 28). A 3 × 2 ANCOVA on whole brain FCS revealed a significant CYP2A6 GENOTYPE × SMOKING status interaction in only two brain regions, the dACC and left VS (Pcorrected < 0.05) (Fig. 1A and Supplementary Table 1). Based on their anatomical overlap with the FCS analysis, post-hoc analyses (whole brain, voxel-wise, one-way ANCOVA, followed by two sample t-test) suggest that in both brain regions smokers with the normal metabolizer genotype had higher FCS than a) all nonsmoker groups and b) smokers with the slow metabolizing genotype (Fig. 1 B–C). Importantly, there was no effect of the CYP2A6 genotype on FCS among nonsmokers.
Fig. 1. Interaction between CYP2A6 genotype and smoking status.
A) FCS network analysis illustrating the interaction between the CYP2A6 genotype and smoking status in the dorsal anterior cingulate (dACC) and left ventral striatum (VS). B–C) Mean FCS values in the dACC and VS in each of the six groups. In both brain regions smokers with the normal metabolizing genotype had higher FCS than all nonsmoker groups and smokers with the slow metabolizing genotype. There was no effect of the CYP2A6 genotype on FCS among nonsmokers. D–E) There was a negative correlation in both hubs in the slow but not in the normal or intermediate genotype groups, i.e. as smoking dependence increased, FCS decreased in both VS (r = −0.51, P = 0.01) and dACC (r = −0.44, P = 0.04). *P < 0.05.
Exploratory correlation analysis between FCS and dependence severity (FTND) revealed a negative correlation in both hub regions in the slow but not in the normal or intermediate genotype groups, i.e. as smoking dependence increased, FCS decreased in VS (r = −0.51, P = 0.01) and dACC (r = −0.44, P = 0.04) (Fig. 1 D–E). These data recapitulate negative correlations in these addiction-related regions previously identified using different analytical strategies and samples (37, 38). Finally, there was no relationship between FCS and CPD across the three genotype groups.
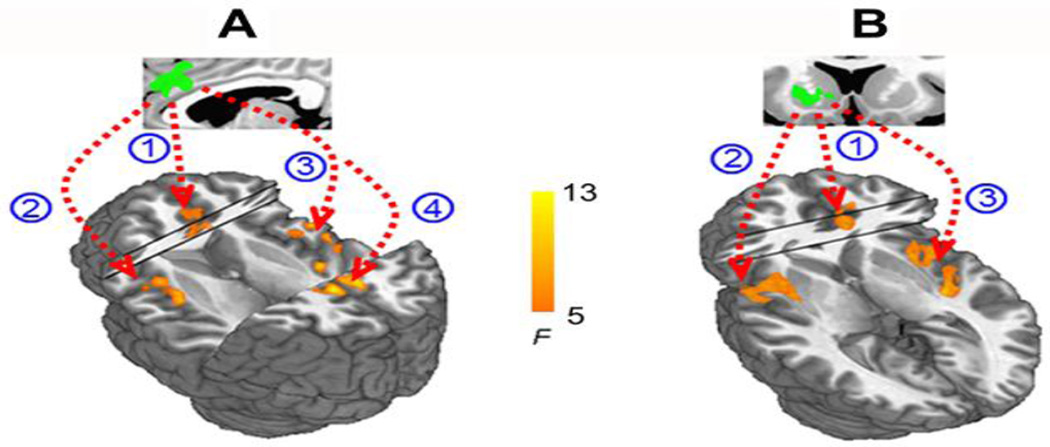
To determine those brain circuits contributing to the above GENOTYPE × SMOKING interaction on FCS, we next used the identified dACC and VS regions as seeds in a functional connectivity analysis. We identified a functional circuit between the dACC and bilateral insula and posterior cingulate cortex that contributed to the dACC FCS hub finding (Fig. 2A), while a functional circuit between the VS and bilateral insula and dACC contributed to the VS interaction (Fig. 2B) (Pcorrected < 0.05)
Fig. 2. Seed-based resting state functional connectivity.
A) The dACC seed based on the CYP2A6 × smoking interaction from Fig. 1 (green) was significantly connected to (1) dACC (2, 3) bilateral insula and (4) posterior cingulate cortex. B) The VS seed based on the CYP2A6 × smoking interaction from Fig. 1 (green) was significantly connected with (1) dACC and (2, 3) bilateral insula.
Task-induced striatal and dACC activity
To addressed the functional consequences of the above GENOTYPE × SMOKING alterations relevant to nicotine dependence, a subset of smokers and nonsmokers (n=43) performed the MID reward task and the Go/NoGo response inhibition task on two separate occasions, in the presence and absence of an acute nicotine patch. A standard general linear model (GLM) approach was used in the analysis.
MID
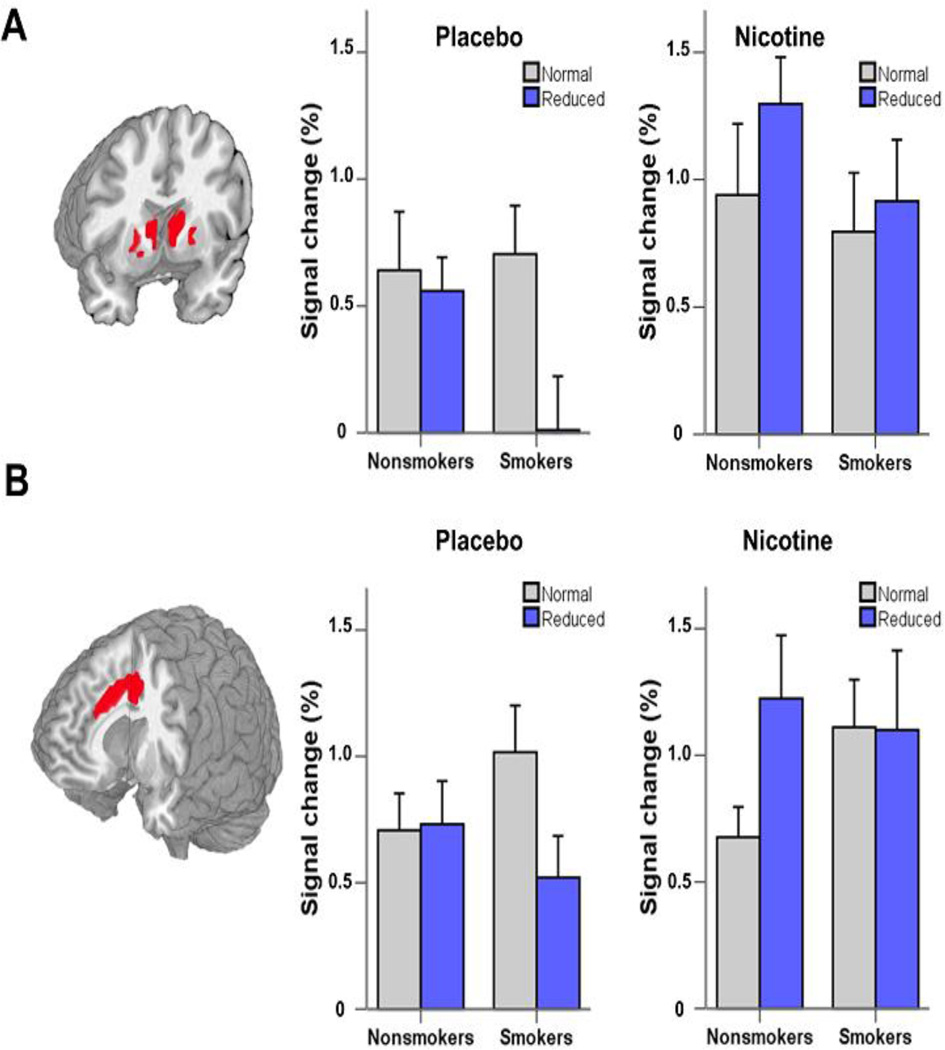
Consistent with previous reports in both healthy individuals (32) and smokers (34, 36), subjects showed greater VS activation in the anticipatory gain compared with the neutral reward expectation condition (there was no effect in the [loss-neutral] condition). Consistent with the pattern seen in the FCS analysis (Fig. 1), there was a significant GENOTYPE × SMOKING interaction in VS activation in the placebo patch (abstinent) condition such that there was less activation in smokers with the reduced (slow and intermediate) versus normal metabolizing genotype, which was, once again, not seen in nonsmokers (Fig. 3A). In contrast, NRT administration appeared to ‘reverse’ the reduced VS response in smokers such that there was now no longer a genotype specific signal difference when anticipating gains.
Fig. 3. CYP2A6 genotype alters neural activation during the MID-R and Go/NoGo task.
A) Coronal section illustrating the task map from all subjects when performing the MID task with significant activation in bilateral VS when anticipating a gain (vs. neutral) condition. Smokers (but not nonsmokers) with the reduced (slow and intermediate) genotype showed less BOLD signal change compared with those with the normal genotype under the placebo patch condition (i.e. acute abstinence). Acute nicotine patch administration normalized the genotype effect in smokers and appeared to also enhance activity in nonsmokers with the reduced genotype. B) Similarly, dACC activation was smaller in reduced metabolizing smokers (but not nonsmokers) following unsuccessful inhibitions in the Go/NoGo task under the placebo patch condition. Once again, nicotine patch administration appeared to normalize this effect in reduced metabolizing smokers, while also increasing dACC activity in reduced metabolizing nonsmokers.
Go/NoGo
Consistent with that seen in the MID task, smokers with the reduced genotype showed reduced dACC activity during unsuccessful inhibitions (Fig. 3B) when they were in the abstinent state than normal metabolizers. Acute NRT again appeared to enhance activation in this group up to that of the normal genotype group. Consistent with the metabolic role of the CYP enzyme, task activity was enhanced by NRT in nonsmokers, but only in the reduced metabolizers.
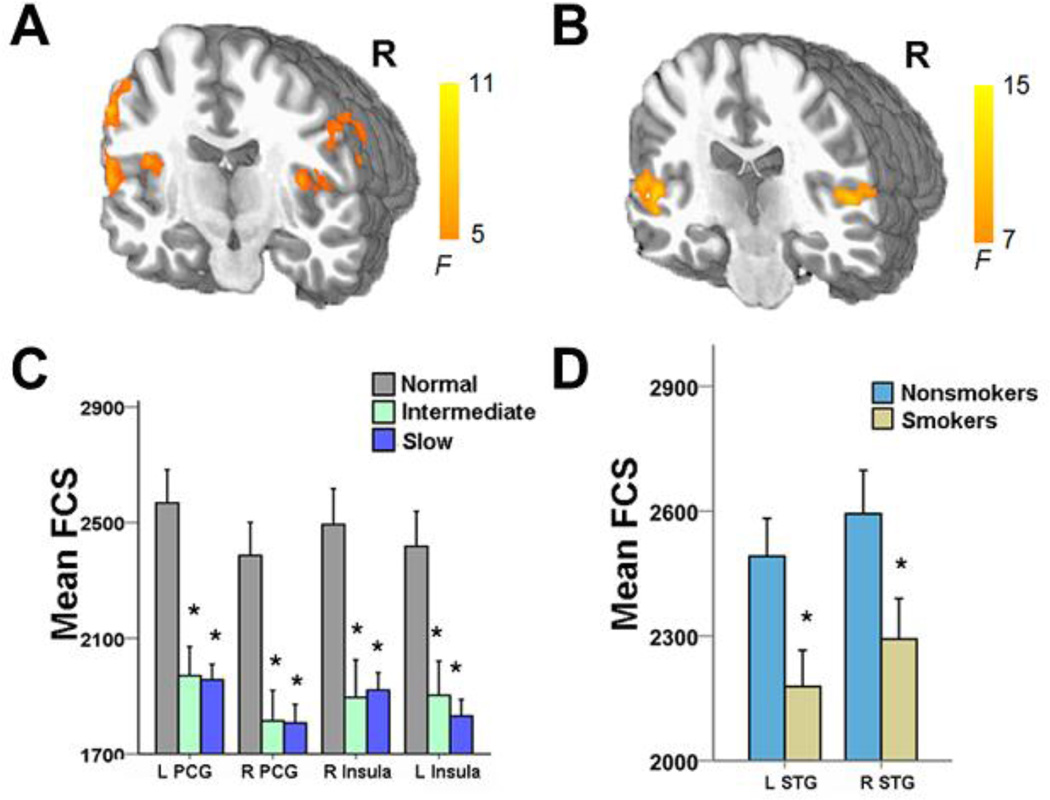
Finally, there was a main effect of CYP2A6 GENOTYPE in bilateral precentral gyrus and bilateral anterior insula, such that the normal genotype group had higher FCS than those with the slow and intermediate genotypes, regardless of smoking status (Fig. 4 A, C). There was also a main effect of SMOKING in the bilateral superior temporal gyrus, with smokers showing decreased FCS across the three genotypes (Fig. 4 B, D and Supplemental Table 1).
Fig. 4. ANCOVA results demonstrating main effect of CYP2A6 genotype and smoking status.
A–B) Main effect of CYP2A6 genotype, demonstrating that both the slow and intermediate genotype groups showed significantly smaller FCS in bilateral precentral gyrus and bilateral insula than the normal genotype group, regardless of smoking status. C–D) Main effect of smoking status, demonstrating that smokers showed significantly less FCS in the bilateral superior temporal gyrus than nonsmokers, regardless of genotype. L, left; R, right; PCG, Precentral gyrus; STG, superior temporal gyrus; *, Pcorrected < 0.05.
Discussion
Alterations in the environment can modulate the influence of genetics upon behavioral outcomes, including nicotine addiction (39). Individuals with the slow CYP2A6 genotype, which leads to higher levels and/or longer durations of plasma and brain nicotine after smoking (7, 15, 24), display distinct smoking behaviors compared to those with the normal genotype, including levels of dependence and quit rates (9, 10, 40). To understand the neurobiological mechanisms underlying this altered smoking phenotype and in service of potential clinical implications, we examined the effect of CYP2A6, smoking status and their interaction on brain intrinsic functional connectivity and task-induced activity. We found that in smokers CYP2A6 modified neuronal circuits involved in computations during the processing of both reward and response inhibition, and critically, does so consistent with the underlying resting network strength, supporting a behaviorally relevant and phenotype specific consequence of this GENOTYPE × SMOKING network interaction.
Using FCS, a data driven, graph theory network metric coupled with a whole brain search, we found significant GENOTYPE × SMOKING interactions localized within two key striatal-cingulate circuit components, the VS and dACC, such that FCS in both regions was highest in the normal metabolizing smokers. Further, FCS in both regions was negatively correlated with dependence severity, but only in the slow metabolizing group. Both of these findings are remarkably coincident with those reported previously by our group. Using independent data sets and, critically, independent analysis strategies, we previously identified a resting state functional circuit between the dACC and VS that was negatively correlated to FTND and was driven, in part, by the nicotinic alpha 5 subunit polymorphism (37, 38).
The current VS and dACC neuroanatomical findings, and their consistent identification across studies (41–43) implicate their prominent role in drug dependence. Notably, the VS receives dopaminergic input from the mesencephalic VTA, while also getting descending glutamatergic afferents from multiple frontal cortical regions, including the cingulate (44). The dopaminergic afferents impart a learning signal that identifies a mismatch between an expected and actual reward (45), while top-down cortical modulation is thought to provide context to these teaching signals (45, 46).
Since the VS is activated during reward processing (32, 47, 48) and is differentially activated in smokers vs. nonsmokers during reward (34, 36) or to the presentation of smoking-related cues (49, 50), we next addressed the functional significance of the resting state G × E FCS VS findings. We identified an identical G × E pattern of neuronal activity when anticipating a gain during the MID reward task, i.e. genotype had no effect in nonsmokers, while abstinent smokers with the reduced (vs. normal) CYP2A6 genotype demonstrated less VS activation (Fig 1B & Fig 3A). These data suggest that enhanced brain nicotine concentration as a function of genotype modulated striatal circuit plasticity that is manifest both at rest and during dopamine related neuronal computations of reward anticipation. Since FCS in the VS is negatively correlated with FTND in slow metabolizers (Fig 1D), these data suggest that such smokers not only have a smaller reward attribution signal during nicotine absence, perhaps speaking to their more rapid development of nicotine dependence, but also have relatively greater success when in treatment (9, 15, 16, 18, 40). Notably, our VBM analysis did not demonstrate any G × E differences in GMV, suggesting that the functional alterations seen here were not influenced by underlying gray matter anatomy differences as previously reported in smokers (51). The coherence of both the resting and task data is consistent with the well-known linkage between resting connectivity strength, task activity and behavioral performance (52, 53).
Reduced inhibitory control with diminished action monitoring, which is most often localized to the ACC (54, 55), and diminished responsivity to one’s errors, represent an executive function profile of addiction (56) that may, at least in part, serve to speed the initiation and perhaps prolong the maintenance of dependence. Reduced metabolizing smokers, but critically not nonsmokers, demonstrated smaller dACC activation when making errors during a response inhibition task, similar to that previously reported in cocaine users (35). That this profile was only seen in reduced metabolizers suggests that such dysregulated neuronal processing does not predate the development of nicotine addiction but may rather be the consequence of chronic nicotine use. That acute nicotine administration (in our case, NRT) appears to reverse or ‘normalize’ this reduction in processing in both the dACC and the VS suggests a mechanism wherein acute nicotine intake serves to reduce cognitive deficits and potentiate the addiction. That said, since slow metabolizers also have higher quit rates when in treatment, our data encourage aggressive treatment intervention.
The differences in nicotine PK between slow and normal metabolizers may be directly responsible for differences between genotype smoking patterns (CPD and depth of inhalation) (10, 57), and presumably result in differential receptor signaling and the neuroplasticity observed herein. In contrast, during the initiation of smoking, the metabolically induced altered PK may have resulted in differential receptor signaling and neuroplasticity, which in turn altered smoking behaviors. Though not easy to disambiguate, these alternatives from the current design, extant data support the latter model. There is a 3.2 hazard ratio increased risk for conversion to tobacco dependence in adolescent slow relative to normal metabolizers, and as there is no evidence of titration of cigarette consumption before dependence (9), this suggests a change in receptor activation among nicotine smokers, resulting in differential response and neurotransmitter release leading to elevated risk for converting to dependence. As the rates of nicotine inactivation are the same among adolescence and adult smokers (58), the very low levels of smoking in slow smokers (4.2 versus 1.8 CPD) (9) are not easily compatible with models of nicotine titration based on PK differences alone. Examination in those smokers who are not yet dependent might tease apart the PK causal relationship between neurochemical changes and smoking behavioral differences among CYP2A6 genotypes.
The FCS method identifies voxels in the brain that have the greatest connectivity to other brain regions, which can be conceptualized as forming neurocircuit hubs that integrate diverse informational sources (27). Aberrant connections to and from or within such hubs may indicate dysregulation across multiple cognitive domains that underlie neuropsychiatric diseases. Using the FCS identified hubs as seeds in independent functional connectivity analyzes, we identified circuit linkages between bilateral insula, dACC, and posterior cingulate cortex that underlie the dACC alteration, while insula and dACC constituted circuit linkage alterations between the VS hub. Together the dACC, insula and VS form the key components of the salience network (SN) (59), which is thought to link the current homeostatic state of the organism with other brain networks to facilitate appropriate, adaptive responding. Sutherland et al (1) proposed a model linking the SN, default-mode network (DMN) and executive control network (ECN) for such adaptive responding in smokers such that the strength of the internetwork linkage varied as a function of smoking status, with acute abstinence strengthening the SN – DMN linkage, to the detriment of the SN-ECN. Recent empirical evidence supports this model, with SN - DMN strength correlated with enhanced craving and reduced working memory performance in abstinence vs. sated smoking (60). Consistently, NRT administration in the current study enhanced task induced VS and dACC activation in both genotype groups of smokers.
The current findings have clear implications for smoking cessation. Since the nicotine half-life in slow metabolizers is about 50% greater than in normal metabolizers (169 vs 113 minutes) (7), within a day or so of cessation, all of the nicotine in both genotype groups is cleared from the body. If there is no nicotine present for the CYP2A6 to metabolize, how is variation in CYP2A6 mediating these long-term differences in smoking cessation?
Our data for the first time suggests that smoking-induced and genetically modulated nicotine PK differences can alter specific brain circuits and network hubs critically related to nicotine abstinence and recidivism. Slow metabolizers show reduced neural reactivity to smoking related cues (19). Reactivity to smoking cues predicts smoking cessation success (61, 62), while greater insula-dACC coupling at rest predicts enhanced smoking cue-reactivity (50). That smokers with stroke lesions limited to the insula spontaneously quit smoking because their bodies ‘forgot they were smokers’ (63), suggests that the insula (and presumably its regional interconnectivity) process the body sensations and/or perceptions of nicotine withdrawal (64, 65). Thus, the current finding of reduced insula connectivity strength between the VS and dACC (key SN hubs) in the slow metabolizers mechanistically support both the reported reduction in cue responding in this CYP2A6 genotype and helps explain the epidemiological and experimental observations of enhanced cessation success in slow metabolizers.
There is growing evidence that the CYP2A6 genotype differentially contributes to smoking cessation treatments. Slow metabolizers respond better to NRT when it is administered for an extended period of time, while the additional treatment time provides no incremental benefit to normal metabolizers (16, 18), even after controlling for genotype differences in baseline CPD, dependence level, and patch-derived plasma nicotine levels (18). These observations suggest that the PK of NRT may more closely resemble the smoking-induced nicotine PK in slow metabolizers that originally shaped the neural circuits, replacing downstream nicotine signaling from cigarettes more effectively than among normal metabolizers. In contrast, normal metabolizers respond more favorably to varenicline than NRT, report fewer drug related side effects and greater cognitive improvement (17). This metabolism group-specific responding is supported in part by the knowledge that varenicline is not a substrate for CYP2A6 (66); thus one might have anticipated identical responses to varenicline among slow and normal metabolizers if not for genotype differences in neurobiological adaptation as reported herein that must have occurred before cessation treatment.
Taken together, this genetic imaging study demonstrated a strong Gene × Environment interaction in cingulate and ventral striatal network hubs supported by circuits that prominently implicate the insula. The ability of these network hubs to process reward signals and stimulus response inhibition are also compromised (although due to the limited sized cohort that performed the MID and Go/NoGo tasks, replication with larger samples is encouraged). Nevertheless, the genetic influence on both task activation and network hubs was seen only in smokers, suggesting that variable brain nicotine levels established during smoking can significantly and differentially modify brain circuits linked to, and perhaps enhance, subsequent nicotine dependence. Our data further suggest that these circuit alterations are a result of, rather than predisposing to, nicotine dependence. This conjecture is further supported by the enhanced BOLD signal seen following acute nicotine administration in nonsmokers. If indeed the identified striatal and cingulate hubs and circuits are altered by chronic nicotine use, they may serve as reversible biomarkers and novel targets for the development of medications to differentially treat nicotine addiction based upon the CYP2A6 genotype.
Supplementary Material
Acknowledgments
We thank Hong Gu and Xia Liang for data analysis assistance. This work was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health; PGRN grant DA020830, CIHR grant TMH109787, an Endowed Chair in Addiction for the Department of Psychiatry, Campbell Family Mental Health Research Institute of CAMH and the CAMH foundation, the Canada Foundation for Innovation (#20289 and #16014) and the Ontario Ministry of Research and Innovation.
This study utilized the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health, Bethesda, Md. (http://biowulf.nih.gov).
Rachel F. Tyndale has previously consulted for Apotex on non-smoking related issues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wise RA. Addiction becomes a brain disease. Neuron. 2000;26:27–33. doi: 10.1016/s0896-6273(00)81134-4. [DOI] [PubMed] [Google Scholar]
- 3.Hughes JR, Stead LF, Hartmann-Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;1:CD000031. doi: 10.1002/14651858.CD000031.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messina ES, Tyndale RF, Sellers EM. A major role for CYP2A6 in nicotine C-oxidation by human liver microsomes. J Pharmacol Exp Ther. 1997;282:1608–1614. [PubMed] [Google Scholar]
- 5.Dutheil F, Dauchy S, Diry M, Sazdovitch V, Cloarec O, Mellottée L, et al. Xenobiotic-metabolizing enzymes and transporters in the normal human brain: regional and cellular mapping as a basis for putative roles in cerebral function. Drug Metab Dispos. 2009;37:1528–1538. doi: 10.1124/dmd.109.027011. [DOI] [PubMed] [Google Scholar]
- 6.Miksys S, Tyndale RF. The unique regulation of brain cytochrome P450 2 (CYP2) family enzymes by drugs and genetics. Drug Metab Rev. 2004;36:313–333. doi: 10.1081/dmr-120034149. [DOI] [PubMed] [Google Scholar]
- 7.Benowitz NL, Swan GE, Jacob P, Lessov-Schlaggar CN, Tyndale RF. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin Pharmacol Ther. 2006;80:457–467. doi: 10.1016/j.clpt.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Gu DF, Hinks LJ, Morton NE, Day IN. The use of long PCR to confirm three common alleles at the CYP2A6 locus and the relationship between genotype and smoking habit. Ann Hum Genet. 2000;64:383–390. doi: 10.1046/j.1469-1809.2000.6450383.x. [DOI] [PubMed] [Google Scholar]
- 9.O'Loughlin J, Paradis G, Kim W, DiFranza J, Meshefedjian G, McMillan-Davey E, et al. Genetically decreased CYP2A6 and the risk of tobacco dependence: a prospective study of novice smokers. Tob Control. 2004;13:422–428. doi: 10.1136/tc.2003.007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Liu T, David SP, Tyndale RF, Wang H, Zhou Q, Ding P, et al. Associations of CYP2A6 genotype with smoking behaviors in southern China. Addiction. 2011;106:985–994. doi: 10.1111/j.1360-0443.2010.03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Styn MA, Nukui T, Romkes M, Perkins KA, Land SR, Weissfeld JL. CYP2A6 genotype and smoking behavior in current smokers screened for lung cancer. Subst Use Misuse. 2013;48:490–494. doi: 10.3109/10826084.2013.778280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malaiyandi V, Lerman C, Benowitz NL, Jepson C, Patterson F, Tyndale RF. Impact of CYP2A6 genotype on pretreatment smoking behaviour and nicotine levels from and usage of nicotine replacement therapy. Mol Psychiatry. 2006;11:400–409. doi: 10.1038/sj.mp.4001794. [DOI] [PubMed] [Google Scholar]
- 14.Chenoweth MJ, O'Loughlin J, Sylvestre MP, Tyndale RF. CYP2A6 slow nicotine metabolism is associated with increased quitting by adolescent smokers. Pharmacogenet Genomics. 2013;23:232–235. doi: 10.1097/FPC.0b013e32835f834d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein LH, Shields PG, et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin Pharmacol Ther. 2008;84:320–325. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- 16.Lerman C, Jepson C, Wileyto EP, Patterson F, Schnoll R, Mroziewicz M, et al. Genetic variation in nicotine metabolism predicts the efficacy of extended-duration transdermal nicotine therapy. Clin Pharmacol Ther. 2010;87:553–557. doi: 10.1038/clpt.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lerman C, Schnoll RA, Hawk LW, Cinciripini P, George TP, Wileyto EP, et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3:131–138. doi: 10.1016/S2213-2600(14)70294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79:600–608. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Tang DW, Hello B, Mroziewicz M, Fellows LK, Tyndale RF, Dagher A. Genetic variation in CYP2A6 predicts neural reactivity to smoking cues as measured using fMRI. Neuroimage. 2012;60:2136–2143. doi: 10.1016/j.neuroimage.2012.01.119. [DOI] [PubMed] [Google Scholar]
- 20.Allain F, Minogianis EA, Roberts DC, Samaha AN. How fast and how often: The pharmacokinetics of drug use are decisive in addiction. Neurosci Biobehav Rev. 2015;56:166–179. doi: 10.1016/j.neubiorev.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Samaha AN, Robinson TE. Why does the rapid delivery of drugs to the brain promote addiction? Trends Pharmacol Sci. 2005;26:82–87. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 22.O'brien CP. Benzodiazepine use, abuse, and dependence. J Clin Psychiatry. 2005;66(Suppl 2):28–33. [PubMed] [Google Scholar]
- 23.Volkow ND, Ding YS, Fowler JS, Wang GJ. Cocaine addiction: hypothesis derived from imaging studies with PET. J Addict Dis. 1996;15:55–71. doi: 10.1300/J069v15n04_04. [DOI] [PubMed] [Google Scholar]
- 24.Dempsey D, Tutka P, Jacob P, Allen F, Schoedel K, Tyndale RF, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- 26.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 27.Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang X, Zou Q, He Y, Yang Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci U S A. 2013;110:1929–1934. doi: 10.1073/pnas.1214900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomasi D, Wang GJ, Volkow ND. Energetic cost of brain functional connectivity. Proc Natl Acad Sci U S A. 2013;110:13642–13647. doi: 10.1073/pnas.1303346110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 31.Piliguian M, Zhu AZ, Zhou Q, Benowitz NL, Ahluwalia JS, Sanderson Cox L, et al. Novel CYP2A6 variants identified in African Americans are associated with slow nicotine metabolism in vitro and in vivo. Pharmacogenet Genomics. 2014;24:118–128. doi: 10.1097/FPC.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- 33.Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rose EJ, Ross TJ, Salmeron BJ, Lee M, Shakleya DM, Huestis MA, et al. Acute nicotine differentially impacts anticipatory valence- and magnitude-related striatal activity. Biol Psychiatry. 2013;73:280–288. doi: 10.1016/j.biopsych.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fedota JR, Sutherland MT, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Reward Anticipation Is Differentially Modulated by Varenicline and Nicotine in Smokers. Neuropsychopharmacology. 2015;40:2038–2046. doi: 10.1038/npp.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, et al. Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci U S A. 2010;107:13509–13514. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.True WR, Heath AC, Scherrer JF, Waterman B, Goldberg J, Lin N, et al. Genetic and environmental contributions to smoking. Addiction. 1997;92:1277–1287. [PubMed] [Google Scholar]
- 40.Audrain-McGovern J, Al Koudsi N, Rodriguez D, Wileyto EP, Shields PG, Tyndale RF. The role of CYP2A6 in the emergence of nicotine dependence in adolescents. Pediatrics. 2007;119:e264–e274. doi: 10.1542/peds.2006-1583. [DOI] [PubMed] [Google Scholar]
- 41.Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry. 2015;72:584–592. doi: 10.1001/jamapsychiatry.2015.1. [DOI] [PubMed] [Google Scholar]
- 42.Kravitz AV, Tomasi D, LeBlanc KH, Baler R, Volkow ND, Bonci A, et al. Cortico-striatal circuits: Novel therapeutic targets for substance use disorders. Brain Res. 2015;1628:186–198. doi: 10.1016/j.brainres.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morein-Zamir S, Robbins TW. Fronto-striatal circuits in response-inhibition: Relevance to addiction. Brain Res. 2015;1628:117–129. doi: 10.1016/j.brainres.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultz W, Tremblay L, Hollerman JR. Reward prediction in primate basal ganglia and frontal cortex. Neuropharmacology. 1998;37:421–429. doi: 10.1016/s0028-3908(98)00071-9. [DOI] [PubMed] [Google Scholar]
- 46.Tobler PN, Christopoulos GI, O'Doherty JP, Dolan RJ, Schultz W. Risk-dependent reward value signal in human prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106:7185–7190. doi: 10.1073/pnas.0809599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 48.Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, et al. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- 49.David SP, Munafò MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, et al. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janes AC, Farmer S, Peechatka AL, Frederick BB, Lukas SE. Insula-Dorsal Anterior Cingulate Cortex Coupling is Associated with Enhanced Brain Reactivity to Smoking Cues. Neuropsychopharmacology. 2015;40:1561–1568. doi: 10.1038/npp.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage. 2011;54:42–48. doi: 10.1016/j.neuroimage.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou Q, Ross TJ, Gu H, Geng X, Zuo XN, Hong LE, et al. Intrinsic resting-state activity predicts working memory brain activation and behavioral performance. Hum Brain Mapp. 2013;34:3204–3215. doi: 10.1002/hbm.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 55.Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- 56.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strasser AA, Malaiyandi V, Hoffmann E, Tyndale RF, Lerman C. An association of CYP2A6 genotype and smoking topography. Nicotine Tob Res. 2007;9:511–518. doi: 10.1080/14622200701239605. [DOI] [PubMed] [Google Scholar]
- 58.Al Koudsi N, Hoffmann EB, Assadzadeh A, Tyndale RF. Hepatic CYP2A6 levels and nicotine metabolism: impact of genetic, physiological, environmental, and epigenetic factors. Eur J Clin Pharmacol. 2010;66:239–251. doi: 10.1007/s00228-009-0762-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 2014;71:523–530. doi: 10.1001/jamapsychiatry.2013.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janes AC, Pizzagalli DA, Richardt S, deB Frederick B, Chuzi S, Pachas G, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Payne TJ, Smith PO, Adams SG, Diefenbach L. Pretreatment cue reactivity predicts end-of-treatment smoking. Addict Behav. 2006;31:702–710. doi: 10.1016/j.addbeh.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 63.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- 65.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 66.Obach RS, Reed-Hagen AE, Krueger SS, Obach BJ, O'Connell TN, Zandi KS, et al. Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab Dispos. 2006;34:121–130. doi: 10.1124/dmd.105.006767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.