Abstract
Preterm birth remains the major cause of neonatal morbidity and mortality throughout the world. This is due, in part, to our incomplete understanding of the mechanisms that underlie the maintenance of pregnancy and the initiation of parturition at term. In this article, we review our current knowledge of the complex, interrelated and concerted mechanisms whereby progesterone maintains myometrial quiescence throughout most of pregnancy, as well as those that mediate the upregulation of the inflammatory response and decline in progesterone receptor function leading to parturition. Herein, we review findings that demonstrate a role of the fetus in the timing of birth. Specifically, we focus on our own studies indicating that maturation of the fetal lung and enhanced secretion of the surfactant components, surfactant protein A (SP-A), and the potent inflammatory glycerophospholipid, platelet-activating factor (PAF) initiate a signaling cascade culminating in parturition. Our studies suggest an essential role of steroid receptor coactivators, SRC-1 and SRC-2, which activate expression of genes encoding SP-A and LPCAT1. LPCAT1 is a key enzyme in the synthesis of PAF, as well as DPPC, a highly surface-active glycerophospholipid component of surfactant. Thus, we describe a novel pathway through which the fetus contributes to the initiation of labor by signaling the mother when its lungs have achieved sufficient maturity for survival in an aerobic environment.
Keywords: Pregnancy, parturition, progesterone, NF-κB, fetal lung, surfactant protein A, platelet-activating factor
1. Introduction
Preterm birth (<37 weeks gestation) is the leading cause of infant mortality during the first four weeks of life throughout the world [1]. The highest rates of preterm birth are found in parts of Africa and in North America. In the United States, the incidence of preterm birth has increased steadily over the past several decades and has recently plateaued at ~11.5% of all live births. Moreover, there remain significant and persistent racial disparities in preterm birth rates. While the prematurity rate among white infants is ~11%, close to 18.0% of births among black infants are preterm. The underlying factors contributing to this racial disparity remain unclear [2]. Whereas, infection with associated inflammation of the fetal membranes likely contributes an important stimulus for preterm labor [3,4], signaling molecules that promote the inflammatory response leading to labor at term are less certain. There is increasing evidence to suggest that the fetus generates signals that contribute to the initiation of labor. In this mini-review, we will consider evidence to suggest that the fetus produces signals leading to labor at term. We first will consider the fetal tissues and signaling molecules proposed to serve a role in the initiation of parturition at term and then focus on our own studies which strongly suggest that these signaling molecules arise from the fetal lung.
2. Progesterone (P4) acting through nuclear progesterone receptor (PR) maintains myometrial quiescence throughout most of pregnancy
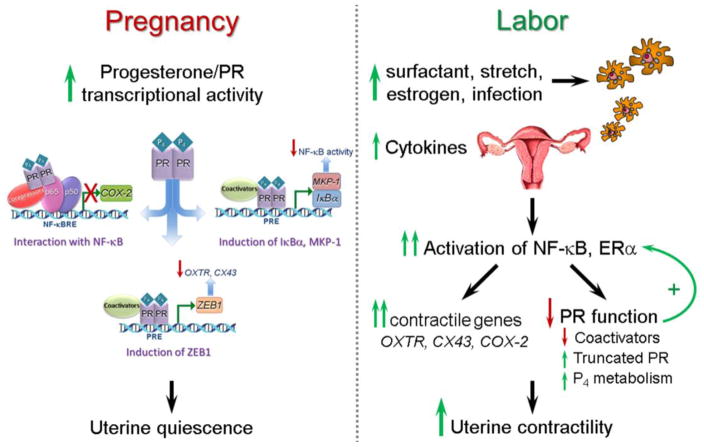
Myometrial quiescence throughout most of pregnancy is mediated by increased circulating levels of P4, produced by placenta and/or the ovarian corpus luteum, depending upon the species. Progesterone blocks myometrial contractility through its binding to two nuclear receptor isoforms, PR-A (94 kDa) and PR-B (114 kDa), which are the products of a single gene [5,6]. As shown in Figure 1 (left panel), the mechanisms whereby P4/PR maintains uterine quiescence are multiple and overlapping. For example, P4/PR can exert a pronounced anti-inflammatory effect, by inhibiting activation/DNA binding of the pro-inflammatory transcription factors, nuclear factor κB (NF-κB) [7] and activating protein 1 (AP-1) [8]. P4/PR also can block induction of the proinflammatory response by upregulating expression of the NF-κB inhibitor, IκBα [7], and the mitogen-activated protein kinase (MAPK) inhibitor, MAPK phosphatase-1/dual specificity phosphatase 1 (MKP-1/DUSP1) [9]. Moreover, P4/PR also maintains myometrial quiescence by increasing expression of the transcriptional inhibitor, ZEB1, which binds to the promoters of the contractile genes, gap junction protein connexin-43 (CX43/GJA1) and oxytocin receptor (OXTR), to suppress their expression throughout most of pregnancy [10,11].
Fig. 1.
Mechanisms for P4/PR maintenance of myometrial quiescence throughout most of pregnancy (left panel) and for the initiation of term and preterm parturition (right panel). During pregnancy, increased P4 acting through PR can maintain myometrial quiescence via several mechanisms, including: interaction with NF-κB p65 and recruitment of co-repressors to block p65 transcriptional activation; transcriptional induction of the NF-κB inhibitor, IκBα, and/or the MAPK inhibitor, MKP-1/DUSP1, and; transcriptional induction of ZEB1, which suppresses transcription of myometrial contractile genes. The initiation of labor at term (by increased secretion of surfactant components from the fetal lung, increased uterine stretch and increased circulating E2) and preterm (by infection with chorioamnionitis) is accompanied by activation of macrophages that may be of fetal origin, their migration to the maternal uterus and release of proinflammatory cytokines. This results in an increase in myometrial NF-κB and ERα transcriptional activity and upregulation of contractile and proinflammatory genes, as well as a decline in PR function, which further accelerates the inflammatory process leading to labor.
3. Inflammatory signaling and the initiation of term and preterm labor
Labor, both term and preterm, is accompanied by an inflammatory response. Increased levels of proinflammatory cytokines are found in amniotic fluid [12] and the myometrium, cervix, and fetal membranes are infiltrated by immune cells [13–15], which secrete proinflammatory cytokines and chemokines [3] (Fig. 1, right panel). This promotes an inflammatory signaling cascade that results in activation of NF-κB and other proinflammatory transcription factors (e.g. AP-1) in myometrium [14,16], cervix and fetal membranes [17–19]. Activated NF-κB and AP-1 increase expression of genes that promote myometrial contractility, including the prostaglandin F2α receptor [20], CX43 [21], OXTR [22] and cyclooxygenase-2 (PGHS2, COX-2) [23], which catalyzes the production of contractile prostaglandins [24–26]. In preterm labor, intra-amniotic infection associated with chorioamnionitis may provide the stimulus for increased amniotic fluid interleukins and inflammatory cell migration [27]. On the other hand, the inflammatory response leading to labor at term is triggered by increased mechanical stretch [28,29] imposed by the growing conceptus, as well as by fetal signaling molecules produced in increasing amounts toward term [14,30–35] (Fig. 1), which are described in Section 4.
The increased inflammatory response that occurs toward term also is caused by a relative increase in E2/ERα signaling and decline in P4/PR function (Fig. 1, right panel). The increase in circulating estradiol-17β (E2) [36,37] and/or increased estrogen receptor α (ERα) activity [38,39] induce immune cell migration into the uterus and antagonize the anti-inflammatory actions of P4/PR [39,40]. Furthermore, ERα activation directly enhances transcription of OXTR [41], CX43 [42] and COX-2 [39]. Importantly, the enhanced inflammatory response further impairs PR function in myometrium through upregulation of NF-κB and AP-1, which directly interact with PR to impair its transcriptional activity, causes a decline in PR coactivators [43,44], and increases the expression of truncated PR-A [39,45] and PR-C [16] isoforms relative to full-length PR-B (Fig. 1). The relative increase in E2/ERα activity and decline in P4/PR function near term also cause downregulation of myometrial ZEB1, resulting in upregulation of the miR-200 family [10], which directly targets STAT5B, causing the induction of 20α-hydroxysteroid dehydrogenase, which increases the local metabolism of P4 to inactive products [46].
4. Fetal signals leading to the initiation of parturition
There is accumulating evidence that the fetus secretes hormonal signals that contribute to the initiation of labor at term. Described below are several of the signaling molecules and the fetal tissues from which they arise in a number of different animal models.
4.1 Fetal adrenal cortisol production
During late gestation in sheep, maturation of the hypothalamic-pituitary-adrenal axis promotes increased cortisol production by the fetal adrenal [47]. The increased fetal cortisol can enhance production of surfactant components by the fetal lung [48], which as discussed below, may serve as important fetal signals for the initiation of labor. The increased cortisol also stimulates expression of placental COX-2 and production of prostaglandins, which enhance 17α-hydroxylase/17,20 lyase (CYP17) and synthesis of C19-steroids, which are metabolized to estrogens by placental aromatase P450 (CYP19). The increased estrogens induce immune cell migration into the uterus, antagonize anti-inflammatory actions of P4/PR [39,40] and turn on proinflammatory and contractile genes [24–26,39,41,42], as described above.
4.2 Placental corticotropin-releasing hormone
CRH uniquely produced by the human placenta near term, has been implicated as a fetal signal for labor [49–51]. Unlike sheep, the human placenta does not express CYP17 and therefore cannot convert progestins to C19-steroids, which instead, are produced in exceedingly high amounts by the fetal adrenals [52]. Increased placental CRH is suggested to stimulate adrenocorticotrophin (ACTH) secretion by the fetal pituitary, which enhances production of cortisol and C19-steroids by the fetal adrenals. C19-steroids can be metabolized to estrogens in the placenta, which enhance the myometrial inflammatory response and contractile gene expression, as described above. Notably, CRH mRNA also is developmentally upregulated in fetal mouse lung bronchiolar epithelium between 13.5 and 17.5 dpc and is then is dimished at 18.5 dpc [53]. This increase in lung CRH precedes the developmental induction of fetal lung SPA expression. Accordingly, in CRH null mouse fetuses, lung maturation and induction of SP-A expression were impaired [54]. This could be due to the lack of local actions of CRH within the fetal lung, or to the effects of diminished fetal adrenal cortisol production on lung maturation and the production of surfactant components.
4.3 Surfactant lipids and proteins produced by the fetal lung serve as signals for the initiation of parturition
Maturation of the fetal lung and increased production of pulmonary surfactant components has been proposed to provide signals for the initiation of parturition [14,33,35,55,56]. Surfactant synthesis by alveolar type II cells is initiated in the fetal lung after ~80% of gestation is complete and is secreted into amniotic fluid. After birth, surfactant functions to reduce alveolar surface tension and is essential for air breathing. Dipalmitoylphosphatidylcholine (DPPC), the major glycerophospholipid surfactant component, is highly surface-active. Proteins comprise ~10% of surfactant composition. There are four surfactant proteins, SP-A, SP-B, SP-C and SP-D [57,58]. SP-B and SP-C are highly lipophilic proteins that act with surfactant glycerophospholipids to reduce alveolar surface tension [57,59]; SP-A and SP-D are collectins that function as part of the innate immune system [60]. In that capacity, they function within the alveolus to enhance uptake of variety of microbial pathogens by macrophages [58,60,61].
The first evidence that fetal lung surfactant could promote an inflammatory response within fetal membranes came from studies of Lopez-Bernal and colleagues [55] who found that surfactant from human amniotic fluid stimulated prostaglandin synthesis in human amnion discs. They proposed that surfactant phospholipids within amniotic fluid provided a source of arachidonic acid for prostaglandin synthesis. In studies of human amnion cells, others reported that a substance in amniotic fluid enhanced PGE2 production [32]. Neither the identity nor the source of this substance was determined, although it was postulated to be produced by the fetal kidney and secreted into amniotic fluid via the urine. Subsequently, it was discovered that platelet-activating factor (PAF), a potent proinflammatory phospholipid known to be involved in hypersensitivity reactions, was secreted into amniotic fluid with fetal lung surfactant near term [62]. It was suggested that PAF of fetal lung origin may promote myometrial contractility.
4.4 Surfactant protein A produced by the fetal lung near term serves as an important signal for the initiation of parturition
As mentioned, SP-A is a C-type lectin (collectin) that acts postnatally within the lung alveolus to enhance macrophage function to engulf microorganisms [60,61,63,64]. During late gestation, SP-A produced by the fetal lung in concert with surfactant glycerophospholipids serves as an excellent marker of lung maturation and type II cell differentiation. In the fetal mouse, lung SP-A expression and secretion into amniotic fluid are induced at 17.5 dpc and continue to increase toward term (19 dpc) [14,65,66]. This was found to be associated with increased IL-1β expression in amniotic fluid macrophages, their migration to the maternal uterus and activation of uterine NF-κB [14].
To directly assess the capacity of SP-A to initiate labor in vivo, parallel groups of mice were injected intraamniotically within the right uterine horn with purified SP-A or with an SP-A-depleted preparation, as control [14]. The majority of mice injected with SP-A at 15.5 dpc delivered prematurely at 16.5 –17.5 dpc from the injected horn only. Migration of fetal macrophages and activation of NF-κB within the SP-A-injected uterine horn were detected within 4.5 h. Moreover, intraamniotic injection of an SP-A antibody resulted in a 24 h delay in labor (20 dpc) [14].
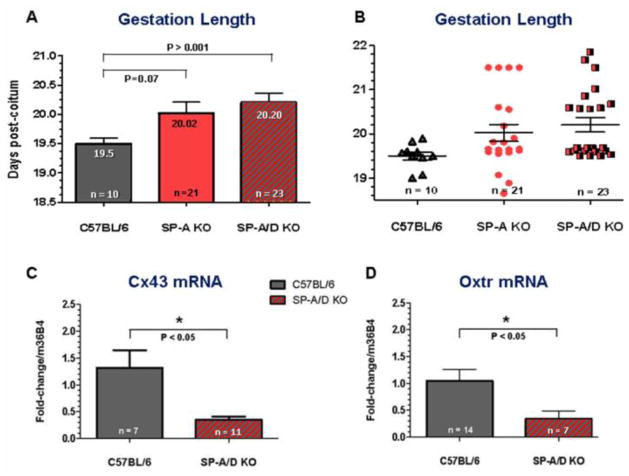
In light of the potential role of SP-A in the initiation of parturition, its structural and functional relatedness to SP-D, and the known SP-A and SP-D interactions with Toll-like receptors (TLR) [67–72], it was of interest to define the functional roles of these endogenous surfactant proteins and their putative receptors using gene targeted mice. The timing of parturition was assessed during first and second pregnancies in C57BL/6 WT mice and in mice homozygous null for SP-A, SP-D and doubly deficient in SP-A and SP-D. SP-A−/− and SP-A−/−/SP-D−/− female mice bred to genetically-like males delivered normally at term during first pregnancies. However, during second pregnancies, SP-A and SP-A/SP-D null females bred to genetically-like males manifested a ~12 h delay in parturition [35] (Fig. 2A, B). While the delay did not reach statistical significance for the SP-A gene targeted mice (p = 0.07), a similar time delay in parturition in the SP-A/SP-D dKO mice was highly significant. We reasoned that the normal timing of parturition observed in the SP-A deficient and the SP-A/D doubly deficient mice in first pregnancies was potentially due to multifactorial regulation of parturition timing and the dominant role of uterine stretch as a signal for parturition [29,73,74] in the non-adapted uterus. However, in subsequent pregnancies, the prior mechanical adaptation of the uterus to stretch, resulting in increased elasticity [75] may allow other signals (e.g. surfactant proteins) to play a more significant signaling role. Myometrium of SP-A/D−/− females expressed significantly lower levels of IL-1β, IL-6, Cx43 and Oxtr at 18.5 dpc compared to WT [35] (Fig. 2C, D). TLR2−/− females manifested a significant (~12 h) (P < 0.001) delay in timing of parturition compared to WT, as well as reduced expression of Cx43 and Mϕ marker, F4/80, at 18.5 dpc during first pregnancies [35]. F4/80+ AF Mϕs from TLR2−/− and SP-A/D−/− mice expressed significantly lower levels of both pro-inflammatory and anti-inflammatory activation markers (e.g. IL-1β, IL-6, Arg 1, Ym1) compared to gestation-matched WT AF Mϕs [35]. These findings suggest that the pulmonary collectins acting via TLR2 serve a modulatory role in the timing of labor; their relative impact may be dependent upon parity.
Fig. 2.
Deficiency of surfactant proteins SP-A and SP-D in mice causes a delay and dysregulation of the timing of parturition (A, B), as well as impaired upregulation of contractile genes (Cx43 and Oxtr) in the maternal myometrium at term (C, D). Reproduced from [35].
4.5 Steroid receptor coactivator (SRC)-1 and SRC-2 serve key roles in fetal-to-maternal signaling leading to parturition
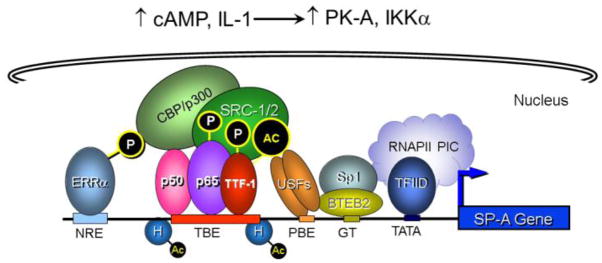
In studies using transgenic mice and transfected type II cells, we observed that a 300-bp region upstream of the rabbit [76–79] and human [80–86] SP-A genes is critical for lung cell-specific, developmental and cAMP/IL-1β-induced expression (Fig. 3). Steroid receptor coactivators, SRC-1 and SRC-2, serve important roles in transcriptional upregulation of SP-A gene expression in fetal lung type II cells [85,87]. Endogenous SRC-1 in human fetal lung (HFL) type II cells was recruited to thyroid transcription factor-1 (TTF-1/Nkx2.1) and NF-κB bound to the hSP-A promoter upon cAMP and interleukin-1 (IL-1) stimulation of SP-A expression [87,88]. cAMP also enhanced estrogen-related receptor α (ERRα) transcriptional activity at the SP-A promoter by increasing its interaction with protein kinase A and SRC-2 [85]. Recruitment of the histone acetylases, CREB-binding protein (CBP) and p300, by SRC-1/SRC-2 resulted in the local acetylation of histones and an opening of chromatin structure [87] (Fig. 3).
Fig. 3.
A 300-bp region upstream of the SP-A gene assembles an enhanceosome that mediates developmental, type II cell-specific and hormonal regulation of expression in fetal lung. Studies in transgenic mice and transfected type II cells indicate that a 300-bp region upstream of the SP-A gene mediates its developmental, tissue-specific and hormonal regulation of expression. Endogenous and exogenous signals (cAMP, IL-1) induce phosphorylation, activation and DNA-binding of TTF-1, NF-κB and ERRα. Phosphorylated TTF-1, NF-κB p65 and ERRα bound to response elements recruit SRC-1 and SRC-2 coactivators and the histone acetylase, CBP/p300. The opening of chromatin structure permits binding of transcription factor IID (TFIID) and stable assembly of components of the RNA polymerase II preinitiation complex (RNAPII PIC).
SRCs are members of the p160 coactivator family, comprised of: SRC-1 (NCoA-1, ERAP160, ERAP140), SRC-2 (NCoA-2, GRIP-1, TIF-2) and SRC-3 (AIB1, TRAM-1, RAC3, p/CIP, ACTR) [89]. Whereas SRCs do not bind to DNA directly, they upregulate transcriptional activities of nuclear receptors and other transcription factors by facilitating their interactions, and by recruiting coregulators with histone modifying activities [89,90]. In this manner they can alter chromatin structure and cause transcriptional activation or repression [91].
In studies using Src-1, Src-2 and Src-3 gene-targeted mice, different reproductive phenotypes were observed [90]. Whereas Src-1−/− mice were fertile, females exhibited reduced stimulation of uterine growth in response to estradiol-17β, and a decreased uterine decidual response to P4 [92]. In males, Src-2 deficiency resulted in infertility due to impaired spermatogenesis [93], while females manifested placental growth defects and sub-fertility [94]. Remarkably, mice that were double KO (dKO) for Src-1 and Src-2 died at birth of atelectasis [95], suggesting a deficiency in lung surfactant production. This was intriguing in consideration of our previous findings concerning the roles of SRC-1 and SRC-2 in SP-A expression.
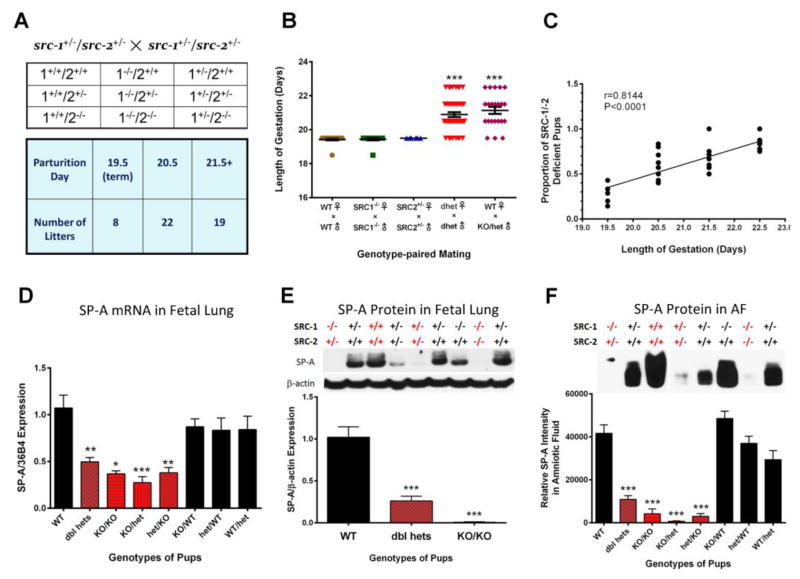
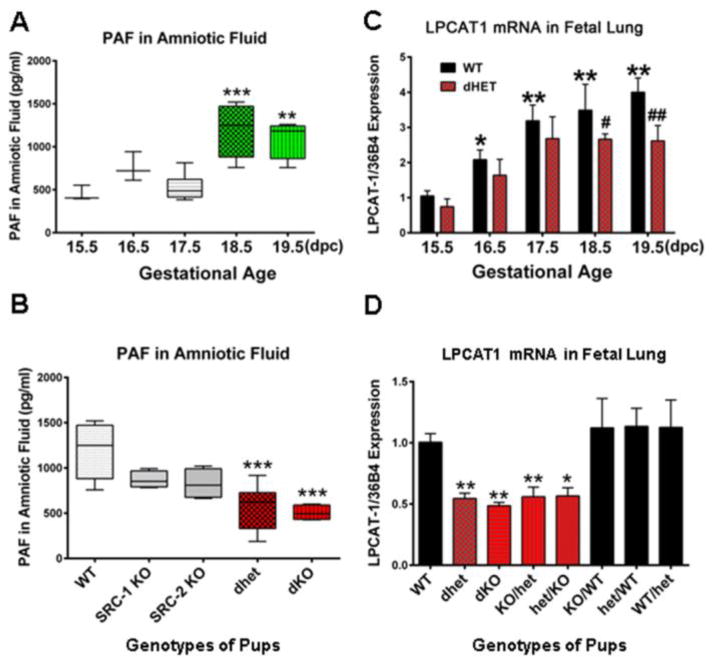
To further study these mice, we bred Src-1/Src-2 doubly heterozygous females (Src-1+/−/Src-2+/−, Src-1/2 dhet) with genetically like males. To our surprise, they manifested a pronounced delay in parturition timing (~38 h delay) (Fig. 4A, B) [33]. Parturition timing was normal in Src-1-KO males bred to Src-1-KO females and in Src-2+/− (Src-2-het) females bred to Src-2-het males, suggesting that Src-1/Src-2 double-deficiency mediated the delay in parturition. Remarkably, when we crossed wild-type (WT) females with Src-1/Src-2 doubly deficient males (Src-1/2 dhet or Src-1-KO/Src-2 het) an equivalent (~38 h) delay in parturition timing also was evident [33] (Fig. 4B). This demonstrates that the Src-1/2 deficiency of the fetuses delays the timing of birth. Moreover, the delay in parturition was highly correlated with the proportion of fetuses that were doubly-deficient in Src-1 and Src-2 (Fig. 4C). In light of our prior work on SPA, we analyzed SP-A in lungs and amniotic fluid surrounding fetuses from Src-1/2 dhet females bred to Src-1/2 dhet males and discovered that SP-A expression was significantly decreased in the lungs and amniotic fluid of all doubly deficient fetuses, compared to WT. By contrast, SP-A in lungs and amniotic fluid of fetuses that were singly deficient in Src-1 or Src-2 were no different from WT (Fig. 4D–F). In addition, myometrial NF-κB activation, expression of Oxtr, Cx43, PGF2α synthase/Akr1b3 were significantly reduced in myometrial tissues of Src-1/2 dhet females bred to Src-1/2 dhet males, and from WT females bred to Src-1-KO/Src-2-het males, whereas, expression of the prostaglandin degrading enzyme, prostaglandin dehydrogenase (PGDH) was significantly increased. This also was associated with a significant decrease in levels of the contractile prostaglandin, PGF2α, in myometrium of Src-1/2-dhet or WT females crossed with Src-1/2-doubly deficient males [33]. Since increased uterine PGF2α is known to circulate to the ovary and promote luteolysis resulting in a decline in P4 production in most sub-primate species [96], we analyzed ovarian tissues from these genetic crosses for expression of steroidogenic acute regulatory protein (StAR), a key regulator of cholesterol metabolism to progestins [97]. We observed that StAR protein and mRNA failed to decline in corpora lutea of Src-1/2-dhet females bred to genetically-like males and from WT females bred to Src-1/2 double-deficient males at term; consequently circulating P4 remained elevated [33].
Fig. 4.
The marked parturition delay in Src-1/-2 dhet female mice crossed with Src-1/-2 dhet males is associated with reduced SP-A expression in lungs of Src-1/-2 double-deficient fetuses. (A) Expected fetal genotypes from Src-1/-2dhet crosses and gestation length. (B) Gestation length is plotted according to genotypes of males and females. (C) Gestation length of SRC-1/-2dhet females bred to Src-1/-2 dhet males is positively correlated with proportion of Src-1/Src-2 double-deficient pups. (D) SP-A mRNA levels according to genotype at 18.5 dpc from matings of SRC-1/-2dhet females×SRC-1/-2dhet males. (E) Levels of SP-A protein in 18.5 dpc fetal lungs from Src-1/-2dhet females bred to Src-1/-2dhet males. Upper panel: representative immunoblot of SP-A protein according to genotype. Lower panel: data from scans of SP-A immunoblots normalized to β-actin according to fetal genotype. (F) SP-A protein levels in AF surrounding WT and Src-1/Src-2 deficient fetuses. Data are the mean ± SEM. *P< 0.05, **P<0.01, ***P<0.001 (ANOVA). Reproduced from [33]. Please see original source for details regarding numbers of mice studied.
Clearly, the parturition delay in mice carrying Src-1/Src-2 doubly deficient fetuses (~38 h) was markedly greater than that observed in SP-A-KO and in SP-A/SP-D-dKO mice (~12 h) (Fig. 2). This suggests that other fetal factors are impacted by Src-1/2 double deficiency. The finding that Src-1/Src-2 dKO mice die at birth of respiratory distress [95] indicated that surfactant lipid components also were affected. In this regard, we found that levels of the highly surface-active glycerophospholipid, DPPC, were significantly decreased in amniotic fluid from Src-1/Src-2 double-deficient fetuses as compared to WT [33].
As mentioned, PAF, an inflammatory glycerophospholipid produced by the developing fetal lung and secreted into amniotic fluid was previously suggested to contribute to the initiation of term and preterm labor [34,56,98–100]. PAF levels were increased in amniotic fluid of women in preterm labor [98,101]. Studies with PAF receptor KO mice suggested that PAF acts on polymorphonuclear leukocytes to stimulate their activation [102]. Moreover, PAF enhanced cytokine production and migration of PMNs into the cervix at time of parturition and directly stimulated contraction of myometrial strips [103–106]. Accordingly, we found that PAF levels failed to increase toward term and were significantly reduced in fetal lungs and amniotic fluid of SRC-1/-2 double-deficient mice, compared to WT or Src-1 or Src-2 singly-deficient fetuses at 18.5 dpc (Fig. 5A, B).
Fig. 5.
PAF secretion and Lpcat1 expression are reduced in lungs of SRC-1/-2 double-deficient fetuses. (A) PAF levels increase in amniotic fluid surrounding WT fetuses during late gestation. (B) PAF levels fail to increase in amniotic fluid of Src-1/-2 double-deficient fetuses at 18.5 dpc compared to Src-1 or Src-2 single KO and WT. (C) Lpcat1 mRNA levels in lungs of Src-1/-2 dhet fetuses are significantly decreased compared to WT at 18.5 and 19.5 dpc. (D) Lpcat1 mRNA levels are significantly decreased in lungs of Src-1/-2 double-deficient fetuses compared to WT and to lungs of fetuses with deficiency in either Src-1 or Src-2. Data are mean ± SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001; #P<0.05, ##P<0.01 compared to WT at same dpc (ANOVA). Adapted from [33]. Please see original source for details regarding numbers of mice studied.
In considering the glycerophospholipid metabolizing enzymes that might coordinately regulate both DPPC and PAF synthesis in fetal lung, we discovered that expression of lysophosphatidylcholine acyltransferase 1 (Lpcat1), a key enzyme in the remodeling/acylation of both DPPC and PAF [107–109], was decreased in lungs of Src-1/-2 dKO fetuses compared to WT. Lpcat1 previously was reported to be specifically expressed in mouse lung type II cells, upregulated in fetal lung toward term, and stimulated by glucocorticoids [108]. We observed that the developmental upregulation of Lpcat1 was prevented in lungs of Src-1/-2 double-deficient fetuses (Fig. 5C). Moreover, a significant decrease in Lpcat1 mRNA was evident only in lungs of fetuses that were doubly-deficient for Src-1/-2, compared to WT (Fig. 5D). The finding that mice homozygous for a hypomorphic allele of Lpcat1 manifested respiratory distress at birth, with an associated deficiency in surfactant DPPC [107], underscores the importance of LPCAT1 in fetal lung surfactant synthesis.
In studies using cultured human fetal lung type II cells, we observed that the synthetic glucocorticoid dexamethasone induced LPCAT1 expression in a GR/SRC-1/SRC-2-dependent manner. Glucocorticoid upregulation of LPCAT1 promoter activity was mediated by two consensus GRE repeats that bound endogenous GR, SRC-1 and SRC-2 in a cooperative manner [33]. Previous findings that Src-1-KO and Src-2-KO mice manifested hyposensitivity to glucocorticoids [110] further suggest that compromised action of glucocorticoids in Src-1/Src-2-deficient fetal mice may contribute to decreased LPCAT1 expression and reduced production of DPPC and PAF.
Because both PAF and SP-A were decreased in fetal lungs and amniotic fluid of Src-1/-2 double-deficient fetuses, we determined whether administration of either of these factors could prevent the parturition defect. Importantly, intraamniotic injection of either PAF or SP-A at 17.5 dpc corrected the parturition delay in Src-1/-2 deficient mice and restored increased NF-κB activation, Cx43 and Oxtr expression in the maternal myometrium, StAR expression in the ovary and circulating P4 levels to WT values at 18.5 dpc [33].
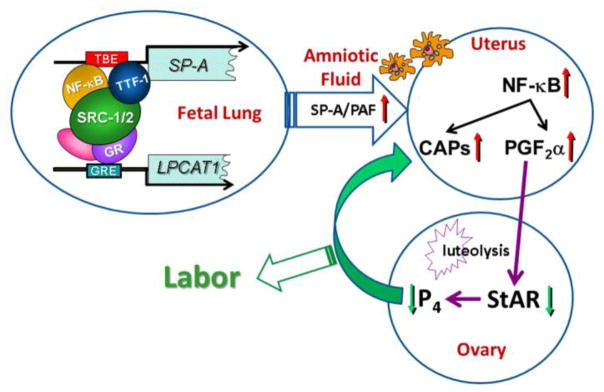
These collective findings suggest that SP-A and PAF produced by the maturing fetal lung comprise important signals for the initiation of parturition (Fig. 6). SRC-1 and SRC-2, serve critical roles through transcriptional upregulation of genes encoding SP-A and LPCAT1, a key regulatory enzyme in the synthesis of surfactant DPPC and PAF, a potent proinflammatory glycerophospholipid. The surfactant components SP-A and PAF activate immune cells, which infiltrate the maternal uterus to cause an inflammatory response leading to a decline in PR function. This promotes the increased expression of myometrial contractile genes and enhanced production of PGF2α, which can circulate to the ovary and cause luteolysis, resulting in decreased P4, which further enhances the myometrial inflammatory response, culminating in the initiation of labor. Thus, the fetus contributes to the timing of its birth by signaling the mother when its lungs can produce sufficient surfactant DPPC to transition from an aqueous to an aerobic environment.
Fig. 6.
Model: SRC-1/2 regulation of SP-A and LPCAT1 in fetal lung promote a signaling cascade leading to the initiation of labor. During the last trimester of gestation, enhanced binding of TTF-1 and NF-κB to the TTF-1-binding element (TBE) upstream of the SP-A gene and of glucocorticoid receptor (GR) to glucocorticoid-response elements (GREs) upstream of the LPCAT1 gene recruit SRC-1 and SRC-2, resulting in activation of SP-A and LPCAT1 expression. Increased SP-A and PAF secretion into amniotic fluid activates fetal macrophages which migrate to the maternal myometrium, resulting in an inflammatory response with activation of NF-κB and increased transcription of the contraction-associated protein (CAP) genes, OXTR, CX43 and PDGF2α synthase. Increased PGF2α is transported to the ovary where it promotes luteolysis and decreased StAR expression, resulting in a decline in circulating P4, which further enhances the myometrial inflammatory response, leading to labor. Reproduced from [33].
Highlights.
Progesterone acting via PR maintains myometrial quiescence during pregnancy.
This occurs via inhibition of inflammatory and contractile genes.
Myometrial contractility is induced by increased inflammatory signaling.
The fetal lung secretes signals that contribute to the initiation of labor at term.
Signals include surfactant protein-A (SP-A) and platelet-activating factor (PAF).
Acknowledgments
Studies from our laboratory reviewed in this article were supported, in part, by NIH 5-P01-HD011149 (C.R.M.), NIH 5-R01-HL050022 (C.R.M.), Prematurity Research Grants #21-FY11-30 and #21-FY14-146 from the March of Dimes Foundation (C.R.M.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera GC, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Peltier MR, Drobek CO, Bhat G, Saade G, Fortunato SJ, Menon R. Amniotic fluid and maternal race influence responsiveness of fetal membranes to bacteria. J Reprod Immunol. 2012;96:68–78. doi: 10.1016/j.jri.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kastner P, Bocquel MT, Turcotte B, Garnier JM, Horwitz KB, Chambon P, Gronemeyer H. Transient expression of human and chicken progesterone receptors does not support alternative translational initiation from a single mRNA as the mechanism generating two receptor isoforms. J Biol Chem. 1990;265:12163–12167. [PubMed] [Google Scholar]
- 6.Conneely OM, Maxwell BL, Toft DO, Schrader WT, O’Malley BW. The A and B forms of the chicken progesterone receptor arise by alternate initiation of translation of a unique mRNA. Biochem Biophys Res Commun. 1987;149:493–501. doi: 10.1016/0006-291x(87)90395-0. [DOI] [PubMed] [Google Scholar]
- 7.Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor (PR) plays a major anti-inflammatory role in human myometrial cells by antagonism of NF-κB activation of cyclooxygenase 2 (COX-2) expression. Mol Endocrinol. 2006;20:2724–2733. doi: 10.1210/me.2006-0112. [DOI] [PubMed] [Google Scholar]
- 8.Dong X, Yu C, Shynlova O, Challis JR, Rennie PS, Lye SJ. p54nrb is a transcriptional corepressor of the progesterone receptor that modulates transcription of the labor-associated gene, connexin 43 (Gja1) Mol Endocrinol. 2009;23:1147–1160. doi: 10.1210/me.2008-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CC, Hardy DB, Mendelson CR. Progesterone receptor inhibits proliferation of human breast cancer cells via induction of MAPK phosphatase 1 (MKP-1/DUSP1) J Biol Chem. 2011;286:43091–43102. doi: 10.1074/jbc.M111.295865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renthal NE, Chen CC, Williams KC, Gerard RD, Prange-Kiel J, Mendelson CR. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc Natl Acad Sci U S A. 2010;107:20828–20833. doi: 10.1073/pnas.1008301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renthal NE, Williams KC, Mendelson CR. MicroRNAs-mediators of myometrial contractility during pregnancy and labour. Nat Rev Endocrinol. 2013;9:391–401. doi: 10.1038/nrendo.2013.96. [DOI] [PubMed] [Google Scholar]
- 12.Cox SM, Casey ML, MacDonald PC. Accumulation of interleukin-1beta and interleukin-6 in amniotic fluid: a sequela of labour at term and preterm. Hum Reprod Update. 1997;3:517–527. doi: 10.1093/humupd/3.5.517. [DOI] [PubMed] [Google Scholar]
- 13.Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–236. [PubMed] [Google Scholar]
- 14.Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A. 2004;101:4978–4983. doi: 10.1073/pnas.0401124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9:41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 16.Condon JC, Hardy DB, Kovaric K, Mendelson CR. Upregulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of NF-κB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20:764–775. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- 17.Allport VC, Pieber D, Slater DM, Newton R, White JO, Bennett PR. Human labour is associated with nuclear factor-κB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal’. Mol Hum Reprod. 2001;7:581–586. doi: 10.1093/molehr/7.6.581. [DOI] [PubMed] [Google Scholar]
- 18.Lee YS, Terzidou V, Lindstrom T, Johnson M, Bennett PR. The role of CCAAT/enhancer-binding protein beta in the transcriptional regulation of COX-2 in human amnion. Mol Hum Reprod. 2005;11:853–858. doi: 10.1093/molehr/gah194. [DOI] [PubMed] [Google Scholar]
- 19.Elliott CL, Allport VC, Loudon JA, Wu GD, Bennett PR. Nuclear factor-kappa B is essential for up-regulation of interleukin-8 expression in human amnion and cervical epithelial cells. Mol Hum Reprod. 2001;7:787–790. doi: 10.1093/molehr/7.8.787. [DOI] [PubMed] [Google Scholar]
- 20.Olson DM. The role of prostaglandins in the initiation of parturition. Best Pract Res Clin Obstet Gynaecol. 2003;17:717–730. doi: 10.1016/s1521-6934(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 21.Chow L, Lye SJ. Expression of the gap junction protein connexin-43 is increased in the human myometrium toward term and with the onset of labor. Am J Obstet Gynecol. 1994;170:788–795. doi: 10.1016/s0002-9378(94)70284-5. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150:734–741. doi: 10.1016/0002-9378(84)90677-x. [DOI] [PubMed] [Google Scholar]
- 23.Soloff MS, Cook DL, Jr, Jeng YJ, Anderson GD. In situ analysis of interleukin-1-induced transcription of cox-2 and IL-8 in cultured human myometrial cells. Endocrinology. 2004;145:1248–1254. doi: 10.1210/en.2003-1310. [DOI] [PubMed] [Google Scholar]
- 24.Tsuboi K, Sugimoto Y, Iwane A, Yamamoto K, Yamamoto S, Ichikawa A. Uterine expression of prostaglandin H2 synthase in late pregnancy and during parturition in prostaglandin F receptor-deficient mice. Endocrinology. 2000;141:315–324. doi: 10.1210/endo.141.1.7236. [DOI] [PubMed] [Google Scholar]
- 25.Engstrom T. The regulation by ovarian steroids of prostaglandin synthesis and prostaglandin-induced contractility in non-pregnant rat myometrium. Modulating effects of isoproterenol. J Endocrinol. 2001;169:33–41. doi: 10.1677/joe.0.1690033. [DOI] [PubMed] [Google Scholar]
- 26.Gibb W. The role of prostaglandins in human parturition. Ann Med. 1998;30:235–241. doi: 10.3109/07853899809005850. [DOI] [PubMed] [Google Scholar]
- 27.Rauk PN, Chiao JP. Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am J Reprod Immunol. 2000;43:152–159. doi: 10.1111/j.8755-8920.2000.430304.x. [DOI] [PubMed] [Google Scholar]
- 28.Shynlova O, Tsui P, Dorogin A, Lye SJ. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol. 2008;181:1470–1479. doi: 10.4049/jimmunol.181.2.1470. [DOI] [PubMed] [Google Scholar]
- 29.Sooranna SR, Lee Y, Kim LU, Mohan AR, Bennett PR, Johnson MR. Mechanical stretch activates type 2 cyclooxygenase via activator protein-1 transcription factor in human myometrial cells. Mol Hum Reprod. 2004;10:109–113. doi: 10.1093/molehr/gah021. [DOI] [PubMed] [Google Scholar]
- 30.Shaw G, Renfree MB. Fetal control of parturition in marsupials. Reprod Fertil Dev. 2001;13:653–659. doi: 10.1071/rd01095. [DOI] [PubMed] [Google Scholar]
- 31.Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell MD, MacDonald PC, Casey ML. Stimulation of prostaglandin E2 synthesis in human amnion cells maintained in monolayer culture by a substance(s) in amniotic fluid. Prostaglandins Leukot Med. 1984;15:399–407. doi: 10.1016/0262-1746(84)90138-0. [DOI] [PubMed] [Google Scholar]
- 33.Gao L, Rabbitt EH, Condon JC, Renthal NE, Johnston JM, Mitsche MA, Chambon P, Xu J, O’Malley BW, Mendelson CR. Steroid receptor coactivators 1 and 2 mediate fetal-to-maternal signaling that initiates parturition. J Clin Invest. 2015;125:2808–2824. doi: 10.1172/JCI78544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toyoshima K, Narahara H, Furukawa M, Frenkel RA, Johnston JM. Platelet-activating factor. Role in fetal lung development and relationship to normal and premature labor. Clin Perinatol. 1995;22:263–280. [PubMed] [Google Scholar]
- 35.Montalbano AP, Hawgood S, Mendelson CR. Mice deficient in surfactant protein A (SP-A) and SP-D or in TLR2 manifest delayed parturition and decreased expression of inflammatory and contractile genes. Endocrinology. 2013;154:483–498. doi: 10.1210/en.2012-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Challis JR. Sharp increase in free circulating oestrogens immediately before parturition in sheep. Nature. 1971;229:208. doi: 10.1038/229208a0. [DOI] [PubMed] [Google Scholar]
- 37.Buster JE, Chang RJ, Preston DL, Elashoff RM, Cousins LM, Abraham GE, Hobel CJ, Marshall JR. Interrelationships of circulating maternal steroid concentrations in third trimester pregnancies. II. C18 and C19 steroids: estradiol, estriol, dehydroepiandrosterone, dehydroepiandrosterone sulfate, Δ5-androstenediol, Δ4-androstenedione, testosterone, and dihydrotestosterone. J Clin Endocrinol Metab. 1979;48:139–142. doi: 10.1210/jcem-48-1-139. [DOI] [PubMed] [Google Scholar]
- 38.Wu WX, Myers DA, Nathanielsz PW. Changes in estrogen receptor messenger ribonucleic acid in sheep fetal and maternal tissues during late gestation and labor. Am J Obstet Gynecol. 1995;172:844–850. doi: 10.1016/0002-9378(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 39.Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab. 2002;87:2924–2930. doi: 10.1210/jcem.87.6.8609. [DOI] [PubMed] [Google Scholar]
- 40.Tibbetts TA, Conneely OM, O’Malley BW. Progesterone via its receptor antagonizes the pro-inflammatory activity of estrogen in the mouse uterus. Biol Reprod. 1999;60:1158–1165. doi: 10.1095/biolreprod60.5.1158. [DOI] [PubMed] [Google Scholar]
- 41.Murata T, Narita K, Honda K, Matsukawa S, Higuchi T. Differential regulation of estrogen receptor α and β mRNAs in the rat uterus during pregnancy and labor: possible involvement of estrogen receptors in oxytocin receptor regulation. Endocr J. 2003;50:579–587. doi: 10.1507/endocrj.50.579. [DOI] [PubMed] [Google Scholar]
- 42.Piersanti M, Lye SJ. Increase in messenger ribonucleic acid encoding the myometrial gap junction protein, connexin-43, requires protein synthesis and is associated with increased expression of the activator protein-1, c-fos. Endocrinology. 1995;136:3571–3578. doi: 10.1210/endo.136.8.7628395. [DOI] [PubMed] [Google Scholar]
- 43.Condon JC, Jeyasuria P, Faust JM, Wilson JM, Mendelson CR. A decline in progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of labor. Proc Natl Acad Sci U S A. 2003;100:9518–9523. doi: 10.1073/pnas.1633616100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leite RS, Brown AG, Strauss JF., III Tumor necrosis factor-alpha suppresses the expression of steroid receptor coactivator-1 and -2: a possible mechanism contributing to changes in steroid hormone responsiveness. FASEB J. 2004;18:1418–1420. doi: 10.1096/fj.04-1684fje. [DOI] [PubMed] [Google Scholar]
- 45.Merlino AA, Welsh TN, Tan H, Yi LJ, Cannon V, Mercer BM, Mesiano S. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab. 2007;92:1927–1933. doi: 10.1210/jc.2007-0077. [DOI] [PubMed] [Google Scholar]
- 46.Williams KC, Renthal NE, Condon JC, Gerard RD, Mendelson CR. MicroRNA-200a serves a key role in the decline of progesterone receptor function leading to term and preterm labor. Proc Natl Acad Sci U S A. 2012;109:7529–7534. doi: 10.1073/pnas.1200650109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liggins GC, Fairclough RJ, Grieves SA, Kendall JZ, Knox BS. The mechanism of initiation of parturition in the ewe. Recent Prog Horm Res. 1973;29:111–159. doi: 10.1016/b978-0-12-571129-6.50007-5. [DOI] [PubMed] [Google Scholar]
- 48.Liggins GC. Premature delivery of foetal lambs infused with glucocorticoids. J Endocrinol. 1969;45:515–523. doi: 10.1677/joe.0.0450515. [DOI] [PubMed] [Google Scholar]
- 49.Florio P, Cobellis L, Woodman J, Severi FM, Linton EA, Petraglia F. Levels of maternal plasma corticotropin-releasing factor and urocortin during labor. J Soc Gynecol Investig. 2002;9:233–237. [PubMed] [Google Scholar]
- 50.Torricelli M, Giovannelli A, Leucci E, De Falco G, Reis FM, Imperatore A, Florio P, Petraglia F. Labor (term and preterm) is associated with changes in the placental mRNA expression of corticotrophin-releasing factor. Reprod Sci. 2007;14:241–245. doi: 10.1177/1933719107300971. [DOI] [PubMed] [Google Scholar]
- 51.Robinson BG, Arbiser JL, Emanuel RL, Majzoub JA. Species-specific placental corticotropin releasing hormone messenger RNA and peptide expression. Mol Cell Endocrinol. 1989;62:337–341. doi: 10.1016/0303-7207(89)90022-1. [DOI] [PubMed] [Google Scholar]
- 52.Rainey WE, Rehman KS, Carr BR. The human fetal adrenal: making adrenal androgens for placental estrogens. Semin Reprod Med. 2004;22:327–336. doi: 10.1055/s-2004-861549. [DOI] [PubMed] [Google Scholar]
- 53.Keegan CE, Herman JP, Karolyi IJ, O’Shea KS, Camper SA, Seasholtz AF. Differential expression of corticotropin-releasing hormone in developing mouse embryos and adult brain. Endocrinology. 1994;134:2547–2555. doi: 10.1210/endo.134.6.8194481. [DOI] [PubMed] [Google Scholar]
- 54.Muglia LJ, Bae DS, Brown TT, Vogt SK, Alvarez JG, Sunday ME, Majzoub JA. Proliferation and differentiation defects during lung development in corticotropin-releasing hormone-deficient mice. Am J Respir Cell Mol Biol. 1999;20:181–188. doi: 10.1165/ajrcmb.20.2.3381. [DOI] [PubMed] [Google Scholar]
- 55.Lopez BA, Newman GE, Phizackerley PJ, Turnbull AC. Surfactant stimulates prostaglandin E production in human amnion. Br J Obstet Gynaecol. 1988;95:1013–1017. doi: 10.1111/j.1471-0528.1988.tb06506.x. [DOI] [PubMed] [Google Scholar]
- 56.Frenkel RA, Muguruma K, Johnston JM. The biochemical role of platelet-activating factor in reproduction. Prog Lipid Res. 1996;35:155–168. doi: 10.1016/0163-7827(96)00002-1. [DOI] [PubMed] [Google Scholar]
- 57.Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med. 2002;347:2141–2148. doi: 10.1056/NEJMra022387. [DOI] [PubMed] [Google Scholar]
- 58.Kuroki Y, Takahashi M, Nishitani C. Pulmonary collectins in innate immunity of the lung. Cell Microbiol. 2007;9:1871–1879. doi: 10.1111/j.1462-5822.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 59.Hawgood S, Shiffer K. Structures and properties of the surfactant-associated proteins. Annu Rev Physiol. 1991;53:375–394. doi: 10.1146/annurev.ph.53.030191.002111. [DOI] [PubMed] [Google Scholar]
- 60.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 61.Crouch E, Wright JR. Surfactant proteins A and D and pulmonary host defense. Annu Rev Physiol. 2001;63:521–554. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- 62.Toyoshima K, Narahara H, Furukawa M, Frenkel RA, Johnston JM. Platelet-activating factor. Role in fetal lung development and relationship to normal and premature labor. Clin Perinatol. 1995;22:263–280. [PubMed] [Google Scholar]
- 63.Kremlev SG, Phelps DS. Surfactant protein A stimulation of inflammatory cytokine and immunoglobulin production. Am J Physiol Lung Cell Mol Physiol. 1994;267:L712–L719. doi: 10.1152/ajplung.1994.267.6.L712. [DOI] [PubMed] [Google Scholar]
- 64.Phelps DS. Surfactant regulation of host defense function in the lung: a question of balance. Pediatr Pathol Mol Med. 2001;20:269–292. [PubMed] [Google Scholar]
- 65.Korfhagen TR, Bruno MD, Glasser SW, Ciraolo PJ, Whitsett JA, Lattier DL, Wikenheiser KA, Clark JC. Murine pulmonary surfactant SP-A gene: cloning, sequence, and transcriptional activity. Am J Physiol Lung Cell Mol Physiol. 1992;263:L546–L554. doi: 10.1152/ajplung.1992.263.5.L546. [DOI] [PubMed] [Google Scholar]
- 66.Alcorn JL, Hammer RE, Graves KR, Smith ME, Maika SD, Michael LF, Gao E, Wang Y, Mendelson CR. Analysis of genomic regions involved in regulation of the rabbit surfactant protein A gene in transgenic mice. Am J Physiol Lung Cell Mol Physiol. 1999;277:L349–L361. doi: 10.1152/ajplung.1999.277.2.L349. [DOI] [PubMed] [Google Scholar]
- 67.Murakami S, Iwaki D, Mitsuzawa H, Sano H, Takahashi H, Voelker DR, Akino T, Kuroki Y. Surfactant protein A inhibits peptidoglycan-induced tumor necrosis factor-alpha secretion in U937 cells and alveolar macrophages by direct interaction with toll-like receptor 2. J Biol Chem. 2002;277:6830–6837. doi: 10.1074/jbc.M106671200. [DOI] [PubMed] [Google Scholar]
- 68.Sato M, Sano H, Iwaki D, Kudo K, Konishi M, Takahashi H, Takahashi T, Imaizumi H, Asai Y, Kuroki Y. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-κB Activation and TNF-α secretion are down-regulated by lung collectin surfactant protein A. J Immunol. 2003;171:417–425. doi: 10.4049/jimmunol.171.1.417. [DOI] [PubMed] [Google Scholar]
- 69.Ohya M, Nishitani C, Sano H, Yamada C, Mitsuzawa H, Shimizu T, Saito T, Smith K, Crouch E, Kuroki Y. Human pulmonary surfactant protein D binds the extracellular domains of Toll-like receptors 2 and 4 through the carbohydrate recognition domain by a mechanism different from its binding to phosphatidylinositol and lipopolysaccharide. Biochemistry. 2006;45:8657–8664. doi: 10.1021/bi060176z. [DOI] [PubMed] [Google Scholar]
- 70.Guillot L, Balloy V, McCormack FX, Golenbock DT, Chignard M, Si-Tahar M. Cutting edge: the immunostimulatory activity of the lung surfactant protein-A involves Toll-like receptor 4. J Immunol. 2002;168:5989–5992. doi: 10.4049/jimmunol.168.12.5989. [DOI] [PubMed] [Google Scholar]
- 71.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 72.Yamada C, Sano H, Shimizu T, Mitsuzawa H, Nishitani C, Himi T, Kuroki Y. Surfactant protein A directly interacts with TLR4 and MD-2 and regulates inflammatory cellular response. Importance of supratrimeric oligomerization. J Biol Chem. 2006;281:21771–21780. doi: 10.1074/jbc.M513041200. [DOI] [PubMed] [Google Scholar]
- 73.Shynlova O, Tsui P, Dorogin A, Lye SJ. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol. 2008;181:1470–1479. doi: 10.4049/jimmunol.181.2.1470. [DOI] [PubMed] [Google Scholar]
- 74.Shynlova O, Tsui P, Jaffer S, Lye SJ. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S2–10. doi: 10.1016/j.ejogrb.2009.02.044. [DOI] [PubMed] [Google Scholar]
- 75.Wu X, Morgan KG, Jones CJ, Tribe RM, Taggart MJ. Myometrial mechanoadaptation during pregnancy: implications for smooth muscle plasticity and remodelling. J Cell Mol Med. 2008;12:1360–1373. doi: 10.1111/j.1582-4934.2008.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alcorn JL, Gao E, Chen Q, Smith ME, Gerard RD, Mendelson CR. Genomic elements involved in transcriptional regulation of the rabbit surfactant protein-A gene. Mol Endocrinol. 1993;7:1072–1085. doi: 10.1210/mend.7.8.8232306. [DOI] [PubMed] [Google Scholar]
- 77.Michael LF, Alcorn JL, Gao E, Mendelson CR. Characterization of the cyclic adenosine 3′,5′-monophosphate response element of the rabbit surfactant protein-A gene: evidence for transactivators distinct from CREB/ATF family members. Mol Endocrinol. 1996;10:159–170. doi: 10.1210/mend.10.2.8825556. [DOI] [PubMed] [Google Scholar]
- 78.Gao E, Alcorn JL, Mendelson CR. Identification of enhancers in the 5′-flanking region of the rabbit surfactant protein A (SP-A) gene and characterization of their binding proteins. J Biol Chem. 1993;268:19697–19709. [PubMed] [Google Scholar]
- 79.Gao E, Wang Y, Alcorn JL, Mendelson CR. Transcription factor USF2 is developmentally regulated in fetal lung and acts together with USF1 to induce SP-A gene expression. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1027–L1036. doi: 10.1152/ajplung.00219.2002. [DOI] [PubMed] [Google Scholar]
- 80.Young PP, Mendelson CR. A CRE-like element plays an essential role in cAMP regulation of human SP-A2 gene in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 1996;271:L287–L299. doi: 10.1152/ajplung.1996.271.2.L287. [DOI] [PubMed] [Google Scholar]
- 81.Liu D, Hinshelwood MM, Giguere V, Mendelson CR. Estrogen related receptor-α enhances surfactant protein-A gene expression in fetal lung type II cells. Endocrinology. 2006;147:5187–5195. doi: 10.1210/en.2006-0664. [DOI] [PubMed] [Google Scholar]
- 82.Li J, Gao E, Mendelson CR. Cyclic AMP-responsive expression of the surfactant protein-A gene is mediated by increased DNA binding and transcriptional activity of thyroid transcription factor-1. J Biol Chem. 1998;273:4592–4600. doi: 10.1074/jbc.273.8.4592. [DOI] [PubMed] [Google Scholar]
- 83.Islam KN, Mendelson CR. Potential role of nuclear factor κB and reactive oxygen species in cAMP and cytokine regulation of surfactant protein-A gene expression in lung type II cells. Mol Endocrinol. 2002;16:1428–1440. doi: 10.1210/mend.16.6.0856. [DOI] [PubMed] [Google Scholar]
- 84.Young PP, Mendelson CR. A GT box element is essential for basal and cyclic adenosine 3′,5′-monophosphate regulation of the human surfactant protein A2 gene in alveolar type II cells: evidence for the binding of lung nuclear factors distinct from Sp1. Mol Endocrinol. 1997;11:1082–1093. doi: 10.1210/mend.11.8.9950. [DOI] [PubMed] [Google Scholar]
- 85.Liu D, Benlhabib H, Mendelson CR. cAMP enhances estrogen-related receptor α (ERRα) transcriptional activity at the SP-A promoter by increasing its interaction with protein kinase A and steroid receptor coactivator 2 (SRC-2) Mol Endocrinol. 2009;23:772–783. doi: 10.1210/me.2008-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu D, Yi M, Smith ME, Mendelson CR. Critical role of the TTF-1 response element in temporal, spatial and hormonal regulation of human surfactant protein-A2 promoter activity in transgenic mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:L264–L271. doi: 10.1152/ajplung.00069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yi M, Tong GX, Murry B, Mendelson CR. Role of CBP/p300 and SRC-1 in transcriptional regulation of the pulmonary surfactant protein-A (SP-A) gene by thyroid transcription factor-1 (TTF-1) J Biol Chem. 2001;277:2997–3005. doi: 10.1074/jbc.M109793200. [DOI] [PubMed] [Google Scholar]
- 88.Islam KN, Mendelson CR. Permissive effects of oxygen on cyclic AMP and interleukin-1 stimulation of surfactant protein A gene expression are mediated by epigenetic mechanisms. Mol Cell Biol. 2006;26:2901–2912. doi: 10.1128/MCB.26.8.2901-2912.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lonard DM, O’Malley BW. The expanding cosmos of nuclear receptor coactivators. Cell. 2006;125:411–414. doi: 10.1016/j.cell.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 90.Xu J, Wu RC, O’Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9:615–630. doi: 10.1038/nrc2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnson AB, O’Malley BW. Steroid receptor coactivators 1, 2, and 3: critical regulators of nuclear receptor activity and steroid receptor modulator (SRM)-based cancer therapy. Mol Cell Endocrinol. 2012;348:430–439. doi: 10.1016/j.mce.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O’Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 93.Ye X, Han SJ, Tsai SY, DeMayo FJ, Xu J, Tsai MJ, O’Malley BW. Roles of steroid receptor coactivator (SRC)-1 and transcriptional intermediary factor (TIF) 2 in androgen receptor activity in mice. Proc Natl Acad Sci U S A. 2005;102:9487–9492. doi: 10.1073/pnas.0503577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gehin M, Mark M, Dennefeld C, Dierich A, Gronemeyer H, Chambon P. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol Cell Biol. 2002;22:5923–5937. doi: 10.1128/MCB.22.16.5923-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mark M, Yoshida-Komiya H, Gehin M, Liao L, Tsai MJ, O’Malley BW, Chambon P, Xu J. Partially redundant functions of SRC-1 and TIF2 in postnatal survival and male reproduction. Proc Natl Acad Sci U S A. 2004;101:4453–4458. doi: 10.1073/pnas.0400234101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev. 2000;80:1–29. doi: 10.1152/physrev.2000.80.1.1. [DOI] [PubMed] [Google Scholar]
- 97.Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR) J Biol Chem. 1994;269:28314–28322. [PubMed] [Google Scholar]
- 98.Hoffman DR, Romero R, Johnston JM. Detection of platelet-activating factor in amniotic fluid of complicated pregnancies. Am J Obstet Gynecol. 1990;162:525–528. doi: 10.1016/0002-9378(90)90423-5. [DOI] [PubMed] [Google Scholar]
- 99.Yasuda K, Furukawa M, Johnston JM. Effect of estrogens on plasma platelet-activating factor acetylhydrolase and the timing of parturition in the rat. Biol Reprod. 1996;54:224–229. doi: 10.1095/biolreprod54.1.224. [DOI] [PubMed] [Google Scholar]
- 100.Zhu YP, Hoffman DR, Hwang SB, Miyaura S, Johnston JM. Prolongation of parturition in the pregnant rat following treatment with a platelet activating factor receptor antagonist. Biol Reprod. 1991;44:39–42. doi: 10.1095/biolreprod44.1.39. [DOI] [PubMed] [Google Scholar]
- 101.Silver RK, Caplan MS, Kelly AM. Amniotic fluid platelet-activating factor (PAF) is elevated in patients with tocolytic failure and preterm delivery. Prostaglandins. 1992;43:181–187. doi: 10.1016/0090-6980(92)90085-8. [DOI] [PubMed] [Google Scholar]
- 102.Ishii S, Kuwaki T, Nagase T, Maki K, Tashiro F, Sunaga S, Cao WH, Kume K, Fukuchi Y, Ikuta K, Miyazaki J, Kumada M, Shimizu T. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998;187:1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jeanneton O, Delvaux M, Botella A, Frexinos J, Bueno L. Platelet-activating factor (PAF) induces a contraction of isolated smooth muscle cells from guinea pig ileum: intracellular pathway involved. J Pharmacol Exp Ther. 1993;267:31–37. [PubMed] [Google Scholar]
- 104.Kim BK, Ozaki H, Lee SM, Karaki H. Increased sensitivity of rat myometrium to the contractile effect of platelet activating factor before delivery. Br J Pharmacol. 1995;115:1211–1214. doi: 10.1111/j.1476-5381.1995.tb15027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Montrucchio G, Alloatti G, Tetta C, Roffinello C, Emanuelli G, Camussi G. In vitro contractile effect of platelet-activating factor on guinea-pig myometrium. Prostaglandins. 1986;32:539–554. doi: 10.1016/0090-6980(86)90036-5. [DOI] [PubMed] [Google Scholar]
- 106.Tetta C, Montrucchio G, Alloatti G, Roffinello C, Emanuelli G, Benedetto C, Camussi G, Massobrio M. Platelet-activating factor contracts human myometrium in vitro. Proc Soc Exp Biol Med. 1986;183:376–381. doi: 10.3181/00379727-183-42435. [DOI] [PubMed] [Google Scholar]
- 107.Bridges JP, Ikegami M, Brilli LL, Chen X, Mason RJ, Shannon JM. LPCAT1 regulates surfactant phospholipid synthesis and is required for transitioning to air breathing in mice. J Clin Invest. 2010;120:1736–1748. doi: 10.1172/JCI38061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen X, Hyatt BA, Mucenski ML, Mason RJ, Shannon JM. Identification and characterization of a lysophosphatidylcholine acyltransferase in alveolar type II cells. Proc Natl Acad Sci U S A. 2006;103:11724–11729. doi: 10.1073/pnas.0604946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nakanishi H, Shindou H, Hishikawa D, Harayama T, Ogasawara R, Suwabe A, Taguchi R, Shimizu T. Cloning and characterization of mouse lung-type acyl-CoA:lysophosphatidylcholine acyltransferase 1 (LPCAT1). Expression in alveolar type II cells and possible involvement in surfactant production. J Biol Chem. 2006;281:20140–20147. doi: 10.1074/jbc.M600225200. [DOI] [PubMed] [Google Scholar]
- 110.Winnay JN, Xu J, O’Malley BW, Hammer GD. Steroid receptor coactivator-1-deficient mice exhibit altered hypothalamic-pituitary-adrenal axis function. Endocrinology. 2006;147:1322–1332. doi: 10.1210/en.2005-0751. [DOI] [PubMed] [Google Scholar]