Abstract
Diabetes in whites of European descent with hemochromatosis was first attributed to pancreatic siderosis. Later observations revealed that the pathogenesis of diabetes in HFE hemochromatosis is multifactorial and its clinical manifestations are heterogeneous. Increased type 2 diabetes risk in HFE hemochromatosis is associated with one or more factors, including abnormal iron homeostasis and iron overload, decreased insulin secretion, cirrhosis, diabetes in first-degree relatives, increased body mass index, insulin resistance, and metabolic syndrome. In p.C282Y homozygotes, serum ferritin, usually elevated at hemochromatosis diagnosis, largely reflects body iron stores but not diabetes risk. In persons with diabetes type 2 without hemochromatosis diagnoses, serum ferritin levels are higher than those of persons without diabetes, but most values are within the reference range. Phlebotomy therapy to achieve iron depletion does not improve diabetes control in all persons with HFE hemochromatosis. The prevalence of type 2 diabetes diagnosed today in whites of European descent with and without HFE hemochromatosis is similar. Routine iron phenotyping or HFE genotyping of patients with type 2 diabetes is not recommended. Herein, we review diabetes in HFE hemochromatosis and the role of iron in diabetes pathogenesis in whites of European descent with and without HFE hemochromatosis.
1. Decreasing Prevalence of Diabetes and Cirrhosis in Hemochromatosis
The prevalence of diabetes decreased among hemochromatosis case series published in the interval 1935–1998 (Figure 1). Earlier diagnosis of hemochromatosis due to iron phenotyping in probands and family members and subsequent phlebotomy therapy could partly explain the decrease. In two nonscreening hemochromatosis case series published in 2006 and 2008, respectively [1, 2], the prevalence of diabetes was lower than reported in the 20th C. The widespread adoption of HFE genotyping to confirm and enhance early hemochromatosis diagnoses after 1996 could explain much of the further decline in diabetes prevalence (Figure 1). The prevalence of diabetes in p.C282Y homozygotes identified in population screening programs is relatively low (Figure 2).
Figure 1.
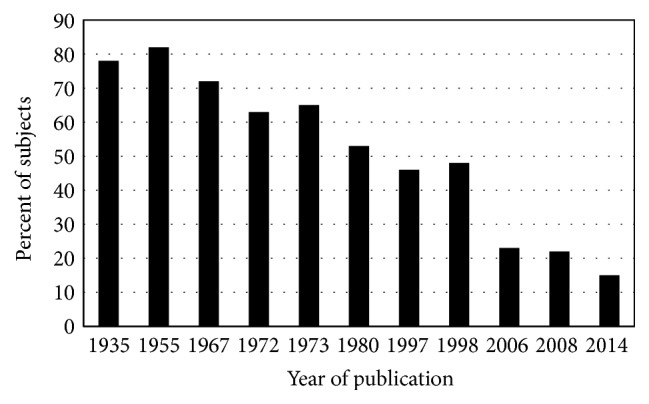
Diabetes in nonscreening hemochromatosis. Percentages of patients diagnosed to have hemochromatosis phenotypes in nonscreening settings who also had diabetes [1, 2, 8–10, 42, 131, 167–169]. HFE mutation genotyping was a diagnostic adjunct in three studies [1, 2, 10].
Figure 2.
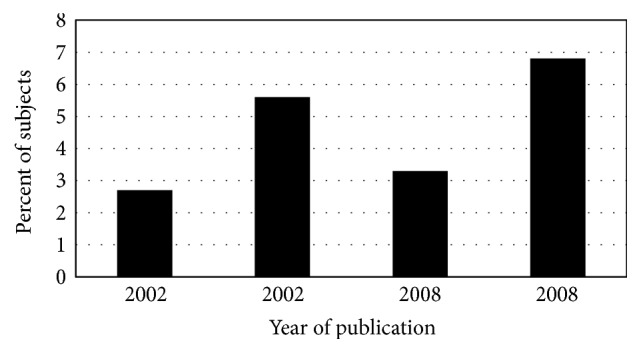
Diabetes in screening hemochromatosis. Percentages of participants in population-based screening studies discovered to have HFE p.C282Y homozygosity who reported that they had previous diagnoses of diabetes [115, 170–172]. There were two such reports from 2002 and two others from 2008. Hemochromatosis was also evaluated with iron phenotyping. In the respective populations, the prevalence of diabetes in p.C282Y homozygotes and control subjects did not differ significantly.
The prevalence of cirrhosis in hemochromatosis case series also decreased in the interval 1935–1996 (Figure 3). Earlier diagnosis of hemochromatosis due to iron phenotyping of probands and family members and their subsequent phlebotomy therapy to achieve iron depletion could partly explain this decrease. The widespread adoption of HFE genotyping to enhance hemochromatosis diagnosis after 1996 could partly explain the further decline in cirrhosis prevalence in nonscreening hemochromatosis index patients reported in 2000 [3] (Figure 3). The proportion of p.C282Y homozygotes identified in population screening who had biopsy-proven cirrhosis was lower than that observed in nonscreening settings but is typically higher than in control subjects (Figure 4).
Figure 3.
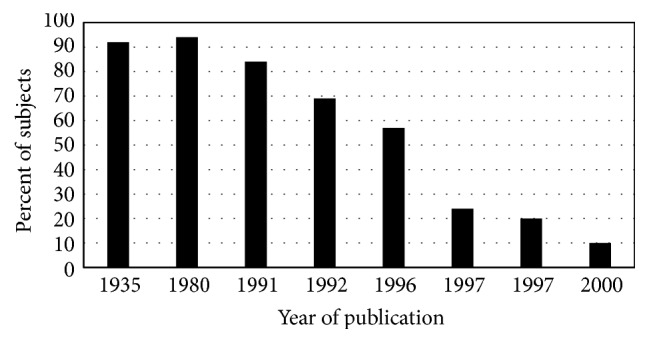
Cirrhosis in nonscreening hemochromatosis. Percentages of patients diagnosed to have hemochromatosis phenotypes who also had cirrhosis [3, 8, 169, 173–177]. There were two such reports from 1997. HFE mutation genotyping was a diagnostic adjunct in the more recent study [3]. Modified from [25, 178]. Greater proportions of men than women had cirrhosis. See cirrhosis prevalence in screening hemochromatosis cases in Figure 4.
Figure 4.
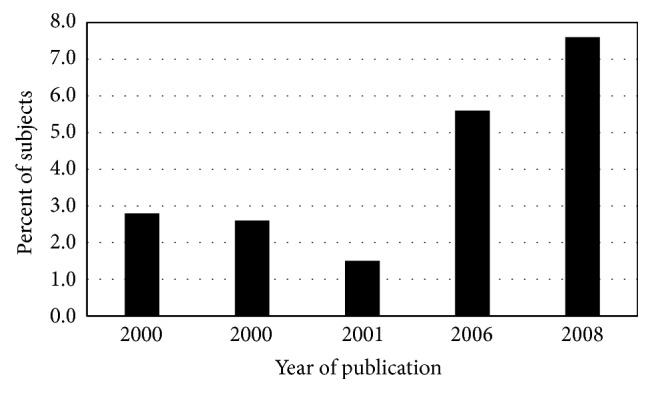
Cirrhosis in screening hemochromatosis. Percentages of participants in population-based studies [3, 172, 179, 180] and in an archived liver biopsy collection (second 2000 publication) [181] discovered to have HFE p.C282Y homozygosity who were previously diagnosed or were subsequently demonstrated to have advanced hepatic fibrosis or cirrhosis by biopsy [3, 170, 172, 180, 181]. Greater proportions of men than women had cirrhosis. See cirrhosis prevalence in nonscreening hemochromatosis cases in Figure 3.
2. History of Hemochromatosis and Diabetes
In 1865, Trousseau described the syndrome of hepatic cirrhosis, pancreatic fibrosis, and cutaneous hyperpigmentation [4]. Troisier's confirmatory 1871 report of diabète bronze et cirrhose pigmentaire described iron deposition in various tissues [5]. In 1889, von Recklinghausen described staining excess iron and its tissue distribution at autopsy of persons with hämochromatose [6]. Clinicians in Europe and derivative countries reported the association of hemochromatosis and diabetes mellitus in whites with increasing frequency in the remaining 19th C [7]. Diabetes, a sine qua non of most hemochromatosis diagnoses through the first two-thirds of the 20th century, was usually observed in persons who also had severe iron loading and cirrhosis [8, 9].
During the latter third of the 20th century, the development of methods to measure serum iron, transferrin saturation (TS), and serum ferritin (SF) and the increased use of liver biopsy facilitated diagnosis of hemochromatosis phenotypes. After the discovery of HFE in 1996, hemochromatosis diagnosis shifted toward a genetic criterion. Most persons who were ascertained to have hemochromatosis using HFE genotyping had milder iron overload phenotypes and fewer complications, including diabetes, than patients with the diagnostic triad [10].
3. HFE Hemochromatosis
HFE hemochromatosis occurs as an autosomal recessive trait [11, 12] in 0.3–0.6% of whites of European descent [13–15] that is due to homozygosity for p.C282Y of the HFE gene (chromosome 6p21.3) [13]. HFE is linked to the major histocompatibility complex (MHC) [13]. p.C282Y homozygosity accounts for ~90% of whites of European descent with “classical” hemochromatosis iron phenotypes [13–15]. Severe iron overload in p.C282Y homozygotes may cause cirrhosis, primary liver cancer, diabetes, other endocrinopathies, and cardiomyopathy [15, 16].
p.C282Y allele frequencies in whites who reside in different regions of Europe range from 13% in Ireland to less than 2% in Italy, Greece, and Spain [17–19]. p.C282Y allele frequencies in non-Hispanic whites who reside in North America are 6-7% [19]. Mean serum iron, TS, and SF levels of adults with p.C282Y homozygosity are higher than those of adults with other common HFE genotypes [20]. In clinical practice, persons with common HFE genotypes other than p.C282Y homozygosity cannot be distinguished by serum iron, TS, and SF measurements [19].
The membrane protein HFE has a structure similar to that of MHC class I proteins and also binds beta-2 microglobulin [13]. Transferrin receptor binds to the HFE extracellular α1-α2 domain [21, 22]. HFE contributes to regulation of hepatic synthesis of hepcidin, the main controller of iron metabolism [23].
Ferroportin, a transmembrane iron-binding protein and the hepcidin receptor, exports iron from absorptive enterocytes, macrophages, and hepatocytes [23]. Hepcidin regulates small intestinal iron absorption, plasma iron concentrations, and tissue iron distribution by inducing inactivation and ubiquitination of ferroportin [23, 24].
Hepcidin synthesis by the liver is regulated by extracellular and intracellular iron concentrations and the iron requirements of erythroid precursors via complex mechanisms that are incompletely understood [23]. In HFE hemochromatosis, excessive iron absorption and increased iron stores are due to lack of hepcidin upregulation, although mutations in non-HFE genes may act as positive “modifiers” of iron absorption in some p.C282Y homozygotes [19, 25, 26].
4. Measuring Iron Stores
Quantitative phlebotomy, the standard reference method for assessing body iron stores, permits measurement of the amount of iron mobilizable for hemoglobin synthesis [15, 27, 28] (Table 1). Measuring liver iron by atomic absorption spectrometry is also widely used for clinical assessments of iron overload [15, 29]. SF is the most widely used surrogate indicator of body iron stores (Table 1). Elevated SF levels in most patients without p.C282Y homozygosity are caused by noniron liver disease and other conditions [28, 30, 31] (Table 1). Histologic grading of Prussian blue positivity in bone marrow aspirates and liver biopsy specimens is not quantitative.
Table 1.
Measures of body iron stores.
| Measures | Specimen | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Iron removed to achieve iron depletion | Blood | Standard reference method; therapeutic; minimally invasive; quantitative; whole body; widely available | Lengthy; inconvenient; moderate cost | [25, 27, 30, 182, 183] |
|
| ||||
| Hepatic iron content | Biopsy | Invasive; quantitative; widely available; strong correlation with quantitative phlebotomy; permits evaluation of liver histology | Possible inadequate specimen; risks of pain, bleeding, pneumothorax, bile leak; single organ; moderate cost | [25, 183, 184] |
|
| ||||
| Iron in liver | SQUID | Noninvasive; quantitative | Few devices exist; not routinely available; single organ; expensive | [183, 185–187] |
|
| ||||
| Iron in liver, heart, pancreas | Magnetic resonance scan | Noninvasive; quantitative; detects iron overload over wide range of concentrations | Equipment expensive; all MRI devices not calibrated to measure iron | [183, 188] |
|
| ||||
| Serum ferritin | Blood | Widely available; semiquantitative; inexpensive | Elevated in many subjects with excess alcohol consumption, inflammation, infection, chronic disease, malignancy; fair correlation with measured iron stores | [25, 28, 30, 80, 182, 183] |
|
| ||||
| Serum transferrin receptor/serum ferritin (sTfR/SF) | Blood | Widely available; semiquantitative; inexpensive | Unsuitable for subjects with inflammation, infection, chronic disease, malignancy; not validated for iron overload study | [189, 190] |
5. Diabetes Pathogenesis in Hemochromatosis
5.1. Pancreatic Siderosis
Through the mid-20th C, diabetes was observed in ~80% of patients with hemochromatosis (Figure 1). Most of them also had heavy liver iron loading and cirrhosis [8, 9]. Postmortem evaluations revealed severe hemosiderin deposition and iron-induced fibrosis of the islets of Langerhans and pancreatic acini [8, 9]. Specificity of iron deposition for the pancreatic beta cells was described in 1956 [32] and confirmed in 1987 [33].
5.2. Iron Entry into Pancreatic Islets
Transferrin receptors in normal human pancreas are expressed predominantly in the islets [34] and are presumed to be a physiologic means by which transferrin-bound iron enters islet cells. Divalent metal transporter 1 (DMT1) is also localized primarily to pancreatic islet cells [35]. The abundant expression of DMT1 in islet cells suggests that DMT1 also plays an important role in iron uptake by beta cells [36]. In a study of mice with global or tissue-specific inactivation of the Slc11a2 gene that encodes DMT1, the investigators concluded that hepatocytes and most cells (other than placenta, small intestinal mucosa, and erythroid cells) “must have an alternative, as-yet-unknown, iron uptake mechanism,” although iron uptake by pancreas was not reported [37].
In HFE hemochromatosis, iron loading of parenchymal cells is partly due to uptake of non-transferrin-bound iron (NTBI) from plasma. In one study, an anti-DMT1 antibody significantly decreased the uptake of NTBI into human hepatocytes and hepatocellular carcinoma cells (HLF), although pancreas cells were not evaluated [38].
Mouse solute carrier Slc39a14 mediates NTBI uptake into cells in vitro [39]. Slc39a14 deficiency in mice with hemochromatosis induced by double homozygosity for Slc39a14−/− and either Hfe−/− or Hfe2−/− greatly diminished liver iron loading and prevented iron deposition in hepatocytes and pancreatic acinar cells [40].
5.3. Hepcidin Expression in Beta Cells
Immunohistochemical studies in humans and rats localized hepcidin exclusively to pancreatic beta cells [41]. Immunoelectron microscopy analyses demonstrated that hepcidin is limited to the beta-cell secretory granules that store insulin [41]. Hepcidin expression in beta cells is regulated by iron in vitro [41]. Thus, beta cells, in addition to hepatocytes, are sources of hepcidin and may contribute to iron homeostasis and blood glucose regulation [41].
5.4. Decreased Insulin Secretion in HFE Hemochromatosis
In an early study, some patients with hemochromatosis had subnormal fasting plasma insulin levels and suboptimal increments in plasma insulin levels after intravenous glucose infusions [42]. In a subsequent report, loss of insulin secretory capacity was the primary event leading to hemochromatosis-related diabetes in thirty nonscreening patients with hemochromatosis (26 p.C282Y homozygotes) and mean SF 1501 ± 287 (standard deviation) ng/mL [1].
5.5. Iron, Islet Cells, and Insulin Secretion in Hfe−/− Mice
Iron metabolism characteristics of Hfe knockout (Hfe−/−) mice are inherited as autosomal recessive traits and are typical of HFE hemochromatosis [19, 43], but iron metabolism characteristics of different Hfe−/− mouse strains vary [44]. Hepatic gene expressions in Hfe−/− mice profiles also differ by strain and age [45]. The inheritance of hepatic iron loading in Hfe−/− mice is polygenic [46].
In Hfe−/− mice (C57BL/6J genetic background) 10 weeks of age, Perls' Prussian blue-stained sections of liver, spleen, and small intestine, but not pancreas, revealed iron deposition [43]. At 10–12 months of age, Hfe−/− mice (C57BL/6J background) had decreased glucose tolerance caused by inadequate increments of insulin levels [47]. Hfe−/− mice (129/SvEvTac background) had islet cell iron content that was 72% higher than that of wild-type controls by age 5 weeks [47]. Similar results were obtained in mice with homozygosity for a Hfe mutation knockin orthologous to human p.C282Y homozygosity [47]. Cooksey et al. concluded that excess iron in mice induces beta-cell oxidant stress and decreases insulin secretion due to desensitization and apoptosis [47]. Regardless, Hfe−/− mice of both the C57BL/6J and 129/SvEvTac strains usually do not develop diabetes [47]. These observations suggest that diabetogenesis in Hfe−/− mice requires decreased insulin secretion and other factor(s).
5.6. Diabetes and Liver Disease
Diabetes is a frequent complication of cirrhosis [25, 48–50]. Cirrhosis due to both iron overload and nonhemochromatosis causes occurs in HFE hemochromatosis [51]. Some persons with cirrhosis also have insulin resistance (IR) [52] and hyperglucagonemia [53] that may contribute to diabetogenesis. Kushner informally reported that “of 104 clinically affected male [hemochromatosis] probands, 32 (31%) had diabetes, and of these, 23 had biopsy-proven cirrhosis, five had moderate fibrosis, and only four had normal liver architecture” [47]. In hemochromatosis probands homozygous for p.C282Y diagnosed in medical care, neither biopsy-proven cirrhosis nor an aggregate variable “liver disorders” was significantly associated with diabetes [10]. In another study, the prevalence of diabetes in men with p.C282Y homozygosity and markedly increased iron stores (14% diabetes; 40% cirrhosis) did not differ significantly from that of men with p. C282Y homozygosity and normal or mildly increased iron stores (15% diabetes; no cirrhosis) [26].
5.7. Glucagon Secretion
Patients with hemochromatosis and impaired glucose tolerance or diabetes have enhanced glucagon responses after arginine infusion [54–59]. In one study, glucagon immunoreactivity in plasma was higher in patients with hemochromatosis than in control subjects, regardless of glucose tolerance [57]. When nonspecific reactivity was deducted, the resulting values for true glucagon concentrations were similar in hemochromatosis and control subjects [57]. It can be inferred from these reports that alpha-cell function is preserved in typical patients with hemochromatosis and diabetes [58].
5.8. Chromium
Retention of radiochromium administered intravenously to persons with hemochromatosis was reduced [60, 61]. Chromium potentiates the action of insulin in vivo and in vitro [62, 63] and may alleviate IR [64]. Chromium deficiency is common in persons with prediabetes [65]. Persons with type 2 diabetes have lower blood levels of chromium than those without diabetes [66]. DMT1 preferentially transports ferrous iron from the intestinal lumen into absorptive cells by a H+-dependent process. In HFE hemochromatosis, DMT1 mRNA levels are increased [67]. Expression of DMT1 in Xenopus oocytes did not stimulate the transport of Cr(2+) or Cr(3+) [68]. Chromium, like iron, binds plasma transferrin [69, 70]. Mechanisms and kinetics of chromium absorption, intermediate metabolism, and excretion in hemochromatosis are unreported.
6. Iron Phenotypes and Diabetes
6.1. Hemochromatosis
The prevalence of previously undiagnosed hemochromatosis in patients attending a diabetes clinic in Australia was 2.4-fold higher than that of the general population [71]. The prevalence of hemochromatosis phenotypes was significantly greater in Italian patients with diabetes (117 type 1; 777 type 2) than control subjects (odds ratio 6.3) [72]. In the two aforementioned studies, hemochromatosis was diagnosed using iron phenotyping; HFE genotyping was not performed.
In the multiracial, multiethnic Hemochromatosis and Iron Overload Screening (HEIRS) Study of 97,470 participants in North America, 2.0% of participants who reported that they had diabetes also had hemochromatosis or iron overload [73].
6.2. Transferrin Saturation
TS was not a significant predictor of diabetes in non-Hispanic whites with p.C282Y homozygosity detected in the HEIRS Study [51]. In contrast, there was a significant negative trend of TS across increasing homeostasis model assessment-insulin resistance (HOMA-IR) quartiles in a postscreening cohort of p.C282Y homozygotes and HFE wild-type homozygotes [74].
In cohorts unselected for hemochromatosis, TS was inversely related to prediabetes [75] and diabetes [73, 76, 77]. In the HEIRS Study, mean TS was lower in non-Hispanic whites with diabetes [73]. To the contrary, TS was not a risk factor for diabetes in Australian adults in a large cross-sectional analysis [78]. In a meta-analysis, TS was a risk factor for type 2 diabetes [79].
6.3. Serum Ferritin in Diabetes with Hemochromatosis
SF levels were not significantly associated with diabetes in p.C282Y homozygotes identified in screening [51]. Neither SF nor quantities of iron removed to achieve iron depletion was significantly associated with type 2 diabetes in hemochromatosis probands with p.C282Y homozygosity diagnosed in medical practice [10]. In screening and nonscreening p.C282Y homozygotes, correlations of SF with iron burdens were positive and significant [51, 80]. These results indicate that increased storage iron in p.C282Y homozygotes, not diabetes, is the major determinant of SF levels [51].
6.4. Serum Ferritin in Diabetes without Hemochromatosis
SF levels were positively associated with fasting glucose [81]; impaired glucose metabolism [77]; insulin levels [81]; prediabetes [75]; and diabetes [73, 76–78, 82–85] in cross-sectional studies of participants unselected for hemochromatosis diagnoses. In a longitudinal study, SF levels were positively associated with glucose intolerance and IR [86]. In overall HEIRS Study analyses of observations of 97,470 participants, SF levels were significantly associated with diabetes in a regression model that included HFE genotype [73]. In 769 postscreening HEIRS Study participants (including 188 p.C282Y homozygotes), log SF was significantly associated with diabetes in a regression model that included HFE genotype [74]. In meta-analyses, there were positive associations of SF with type 2 diabetes [79, 87]. The ratio of serum transferrin receptor (sTfR) to SF (sTfR/SF ratio) was inversely associated with diabetes in case-control studies [82, 85, 88] and in a case-cohort study [84]. Lower ratios of sTfR/SF were independent predictors of incident type 2 diabetes [89]. Evidence from the EPIC-InterAct Study suggests that the relationship between type 2 diabetes and iron stores in persons unselected for hemochromatosis diagnoses is more complex than the association with SF levels alone [90].
6.5. Serum Ferritin in Diabetes: Hemochromatosis versus Nonhemochromatosis
Mean SF levels in subjects with untreated hemochromatosis and p.C282Y homozygosity [20, 91] were much higher than SF levels in subjects with or without diabetes who did not have HFE hemochromatosis genotypes [78, 92]. SF levels predict type 2 diabetes in persons without hemochromatosis diagnoses but the SF levels are typically below the concentration indicative of iron overload (7, 10, 13–22). Some authors mistakenly interpret or report higher mean SF levels in subjects with diabetes than controls or upward trends of SF levels across HOMA quartiles as evidence of increased body iron stores or iron overload. Elevated iron stores are not typical of patients with type 2 diabetes [88, 93]. A persistently elevated SF criterion has a low positive predictive value in screening patients with diabetes for hemochromatosis [94].
Hyperferritinemia is not significantly associated with diabetes in untreated p.C282Y homozygotes [10, 51]. In p.C282Y homozygotes, prephlebotomy plasma levels of C-reactive protein (CRP) and interleukin- (IL-) 6 did not differ significantly between those with high iron stores and those with low iron stores [95].
Ferritin is an iron storage protein. SF consists of iron-rich ferritin and iron-poor apoferritin [96, 97]. Body iron stores are in equilibrium with iron-rich SF [98, 99]. Levels and iron content of SF are increased in hemochromatosis and other iron overload disorders [30, 96]. The iron content of SF in noniron liver disorders associated with hepatocyte injury is also increased due to release of iron-rich hepatocyte ferritin into the blood [30, 96, 97]. Ferritin released from diverse tissues into the blood due to inflammation, anemia of chronic disease, or malignancy is typically apoferritin [30, 96, 97, 100–103]. Apoferritin synthesis and secretion are enhanced by interleukin-1 and chronic ethanol consumption [104, 105].
7. HFE Genotypes and Diabetes
In patients with type 2 diabetes, the prevalence of p.C282Y homozygosity did not differ significantly from that of control subjects [106–109]. The prevalence of undiagnosed diabetes or impaired fasting glucose in p.C282Y homozygotes identified in population screening was similar to that in control subjects with HFE wild-type genotypes [110]. Diabetes also occurs in some persons with the common HFE genotypes p.C282Y/p.H63D and p.H63D homozygosity [111, 112] and in other persons with hemochromatosis phenotypes and novel HFE genotypes [113, 114]. Regardless, clinical and screening studies of persons with hemochromatosis phenotypes did not detect significantly increased diabetes prevalence associated with common HFE genotypes, including p.C282Y homozygosity [2, 73, 115].
8. Morbidity and Mortality of Diabetes in HFE Hemochromatosis
8.1. Inflammation
Higher SF levels, lower TS levels, and higher blood neutrophil counts in patients with hemochromatosis and diabetes [51] may signify inflammation related to underlying processes that ultimately result in diabetes, rather than representing diabetogenic factors. Common inflammatory disorders in persons with diabetes, with or without hemochromatosis, include obesity, arthropathy, atherosclerosis, dyslipidemia, microvascular disease, and fatty liver. In persons with type 2 diabetes, CRP levels are elevated in ~50% of those with [51] and in ~40% of those without [116] hemochromatosis. Elevated CRP and IL-6 concentrations are significantly associated with increased type 2 diabetes risks in populations unselected for hemochromatosis diagnoses [117]. Subclinical inflammation is associated with hyperglycemia and IR in type 2 diabetes unassociated with hemochromatosis [118]. Single-nucleotide polymorphisms (SNPs) of three genes were associated with markers of islet cell inflammation [119].
8.2. Diabetes Risk
Decreased insulin secretion increases diabetes risk in persons with hemochromatosis [1, 42]. Obesity or increased body mass index (BMI) in persons with HFE hemochromatosis also increases diabetes risk [10, 51, 74, 120]. IR and metabolic syndrome (MetS) are common in patients with hemochromatosis [42, 121–123]. In non-Hispanic white adults without diabetes (including 188 p.C282Y homozygotes), IR as determined by HOMA-IR was a significant predictor of MetS but p.C282Y homozygosity was not [74]. In screening p.C282Y homozygotes, SF was significantly associated with HOMA-IR 4th quartile, MetS, and diabetes [51]. In addition, age, male sex, and BMI were significantly associated with HOMA-IR fourth quartile [51]. Only HOMA-IR fourth quartile was significantly associated with MetS [51]. Diabetes in first-degree family members was significantly associated with type 2 diabetes in hemochromatosis probands with p.C282Y homozygosity diagnosed in medical care (odds ratio 8.5 [95% confidence interval 2.9–24.8]) [10].
The general population prevalence of type 1 diabetes defined as autoimmune beta-cell destruction and absolute insulin deficiency is approximately the same as that of hemochromatosis [124]. Genes within the MHC are major risk factors for type 1 diabetes [125, 126], although diverse autoimmune conditions in 236 nonscreening hemochromatosis probands with p.C282Y homozygosity did not include type 1 diabetes [127]. In a population study of hemochromatosis and iron overload, it was unclear whether participants with “late-onset type 1 diabetes” had beta-cell autoimmunity [128]. Genome-wide association studies have not identified a consistent association of human leukocyte antigen (HLA) region genes with type 2 diabetes although many other associated genes occur on chromosomes other than 6p [129, 130]. Type 2 diabetes risk in nonscreening p.C282Y homozygotes was not associated with common HLA types and haplotypes [10].
9. Complications of Diabetes
9.1. Typical Complications
Many complications of diabetes in patients with hemochromatosis are typical of those that occur in patients without hemochromatosis. These include obesity; fat atrophy; proteinuria/albuminuria; retinopathy; peripheral neuropathy; and coronary artery and peripheral vascular disease [42, 131].
9.2. Diabetes, Arthropathy, Cirrhosis, and Pancreatic Cancer
The prevalence of second and third metacarpophalangeal arthropathy, a proxy for hemochromatosis hand arthropathy, was significantly associated with diabetes in p.C282Y homozygotes [51]. Erosive hand osteoarthritis in persons with type 2 diabetes unselected for hemochromatosis was also associated with hand pain [132]. Serum levels of the cellular adhesion molecule VCAM-1 were significantly associated with hemochromatosis arthropathy, independent of diabetes, BMI, and age [133]. Elevated VCAM-1 is also a significant predictor of incident diabetes [134]. Phlebotomy therapy reverses cirrhosis due to iron overload and hemochromatosis in some patients [135], although it is unreported whether cirrhosis reversal also reduces IR or diabetes manifestations. In a meta-analysis, the risk of cancer of the pancreas in persons with diabetes was increased (odds ratio 1.8) [136]. In contrast, pancreatic adenocarcinoma risk is not increased in hemochromatosis [137, 138], although adenocarcinoma of the pancreas has been described in hemochromatosis case series [10, 137, 138].
9.3. Diabetes, Survival, and Causes of Death in Hemochromatosis
Survival of German subjects after hemochromatosis diagnosis between 1959 and 1983 was decreased in those who had either cirrhosis or diabetes [139]. There was a 7-fold increased risk of death due to diabetes in patients with hemochromatosis [139]. The common feature of subjects with cirrhosis and diabetes was heavy iron overload [124, 139]. In a 1991 study of Canadian patients with hemochromatosis, diabetes did not increase the risk of death after data were controlled for the presence of cirrhosis [140]. In a large study of Danes reported in 2014, the mortality risk in individuals with diabetes was more than threefold greater in those with HFE p.C282Y/p.C282Y than in those with HFE wt/wt genotypes [141].
In the US, hemochromatosis was more likely to have been diagnosed in subjects who died with liver disease, liver neoplasms, cardiomyopathy, diabetes, or viral hepatitis [142]. The proportionate mortality ratios were even higher when liver neoplasms or liver disease was combined with diabetes [142]. Liver disease, liver neoplasms, cardiomyopathy, diabetes, and viral hepatitis were more likely to occur among hemochromatosis-associated deaths than among all deaths [142]. Men were more likely to have liver disease (excluding neoplasms), cardiac disorders, nonhepatic neoplasms, diabetes, liver neoplasms, and infectious diseases [142].
10. Iron in Nonhemochromatosis Diabetes
Most persons diagnosed to have type 2 diabetes do not have iron overload [88, 93]. Favorable effects of phlebotomy on diabetes manifestations suggest that abnormal distribution of normal quantities of body iron contribute to diabetogenesis in some persons without hemochromatosis [143–145]. Iron-related dietary, cellular, and molecular mechanisms may contribute to the development or expression of type 2 diabetes [146] (Table 2). These and other mechanisms may also be associated with or cause impaired glucose metabolism, IR, and MetS. It is plausible but unproven that the same mechanisms would be applicable to persons with hemochromatosis. Detailed review of these mechanisms is beyond the scope of this review.
Table 2.
Proposed roles of iron in type 2 diabetes.
| Variable | Mechanism | Reference |
|---|---|---|
| Body iron status | Modulates transcription, membrane expression/affinity of insulin receptor expression in hepatocytes, influences insulin-dependent gene expression | [191] |
|
| ||
| Dietary iron | Controls circadian hepatic glucose metabolism through heme synthesis | [192] |
|
| ||
| Intake of processed meat, red meat | Higher risk of type 2 diabetes | [161, 193, 194] |
|
| ||
| Dietary iron restriction, iron chelation | Increased insulin sensitivity, beta-cell function (ob/ob lep−/− mice) | [195] |
|
| ||
| Iron chelation | Ameliorates adipocyte hypertrophy via suppression of oxidative stress, inflammatory cytokines, and macrophage infiltration | [196] |
|
| ||
| Starvation | Increased liver Pck1 transcription, hepcidin expression, and degradation of ferroportin; hypoferremia, hepatic iron retention (C57BL/6Crl, 129S2/SvPas, BALB/c, and Creb3l3−/− null mice) | [197] |
|
| ||
| High fat diet | Increased hepatic iron regulatory protein-1, increased transferrin receptor 1 expression, increased hepcidin, decreased ferroportin (Hfe−/− mice); increased fatty acid oxidation, hypermetabolism, elevated hepatic glucose production (Hfe−/− mice) | [198, 199] |
|
| ||
| Cellular iron uptake | Stimulated by insulin | [200] |
|
| ||
| Excess hepatic iron | Hyperinsulinemia due to decreased insulin extraction, impaired insulin secretion | [121] |
|
| ||
| Iron-related proteins in adipose tissue | Expression modulated by insulin resistance | [201] |
|
| ||
| Adipocyte iron | Regulates leptin and food intake | [202] |
|
| ||
| Adiponectin | Transcription negatively regulated by iron | [203, 204] |
|
| ||
| Visfatin | Positive association with serum prohepcidin, negative correlation with serum soluble transferrin receptor in men with altered glucose tolerance | [205] |
|
| ||
| Heme oxygenase-1 promoter microsatellite polymorphism | Higher ferritin with short (GT)(n) repeats | [206] |
|
| ||
| Antioxidants | Lower levels partially explained by iron alterations | [207] |
11. Management of Diabetes in HFE Hemochromatosis
11.1. General Management
Treatment of type 2 diabetes in persons with or without hemochromatosis is similar [15, 147]. Reducing inflammation of diverse sources may have a positive effect on potentially injurious iron-related mechanisms, although this is unproven. In hemochromatosis probands with p.C282Y homozygosity, probands with diabetes had greater mean BMI [10, 120].
Physicians should recommend appropriate weight reduction via diet modifications and increased activity to all patients. Modifiable risks for patients with elevated CRP include suboptimal physical activity (men) and central obesity and lack of statin use (women) [116]. Some patients with diabetes would benefit from reduced consumption of red meat and alcohol [15]. Persons with hemochromatosis, diabetes, or chronic liver disease have increased risks to develop septicemia or wound infections due to Vibrio vulnificus, a cosmopolitan halophilic bacterium [148–152]. These persons should not consume uncooked shellfish or expose wounds to warm coastal waters [15, 148–152].
11.2. Phlebotomy
Persons with HFE hemochromatosis who present with elevated SF levels (men > 300 µg/L, women > 200 µg/L) should undergo phlebotomy therapy to achieve iron depletion [15, 147]. The goal of phlebotomy thereafter is to maintain nonelevated SF values [15]. Elevated TS in p.C282Y homozygotes is due to decreased hepcidin available to bind ferroportin and consequent increased storage iron release from macrophages and hepatocytes, not iron overload. Thus, elevated TS levels persist after iron depletion is achieved. Elevated TS is not a target of treatment in HFE hemochromatosis [15, 147].
In patients with hemochromatosis and diabetes who are presumed or known to have pancreatic siderosis, phlebotomy therapy is likely to improve insulin secretion only when hemochromatosis diagnosis and iron depletion are early [15, 153, 154]. Hemochromatosis patients with neither diabetes nor cirrhosis had normal insulin sensitivity but their acute insulin responses to glucose were decreased [122]. Phlebotomy treatment normalized their SF levels, increased their acute insulin responses, and normalized their glucose tolerance [122]. In five referred adults with hemochromatosis and iron overload, insulin secretory capacity improved after normalization of iron stores [155].
The efficacy of iron depletion in decreasing IR in persons with hemochromatosis or p.C282Y homozygosity is variable [122, 154, 156]. Phlebotomy therapy did not improve diabetes control in the majority of 44 patients with hemochromatosis (25 insulin-dependent, 19 noninsulin-dependent) [154]. In 15 men with hemochromatosis, phlebotomy therapy lowered insulin requirements in those with insulin dependency and improved diabetes control in about half of those without insulin dependency [154]. In another study, IR in patients with hemochromatosis and either cirrhosis or diabetes was unaffected by phlebotomy treatment [15]. Impaired glucose tolerance resulting from IR in hemochromatosis subjects with cirrhosis or diabetes is not affected by phlebotomy treatment [122].
Treating type 2 diabetes with phlebotomy is not routine. In type 2 diabetes without hemochromatosis, participants randomized to phlebotomy therapy achieved decreased hemoglobin A1c levels and favorable changes in insulin secretion and IR [143]. As expected, phlebotomy also decreased SF, TS, and hemoglobin levels [143]. Iron depletion improved control of poorly controlled type 2 diabetes in patients with elevated SF levels who did not have common HFE alleles [145]. Repeated phlebotomies of patients with type 2 diabetes significantly decreased serum glucose levels [144]. Blood donation or phlebotomy was associated with more favorable or improved metabolic indices associated with diabetes risk in subjects without diagnosed diabetes [144, 157–160]. In another study, blood donations did not influence diabetes risk [161].
12. Problems That Have Been Resolved
Pathogenesis of diabetes in HFE hemochromatosis is multifactorial and the clinical manifestations of diabetes are heterogeneous (Table 3). Increased type 2 diabetes risk in HFE hemochromatosis is associated with one or more factors, including iron overload, decreased insulin secretion, increased BMI, IR, MetS, diabetes in first-degree relatives, and cirrhosis. Iron overload alone is insufficient to cause type 2 diabetes in most p.C282Y homozygotes. Iron removed by phlebotomy is not significantly associated with diabetes in p.C282Y homozygotes in multivariate analyses. Phlebotomy therapy to achieve iron depletion does not improve control of diagnosed diabetes in all persons with HFE hemochromatosis. SF levels do not predict diabetes in p.C282Y homozygotes. No consistent association of chromosome 6p or HLA region genes (including HFE) that increase type 2 diabetes risk has been demonstrated. Prevalence of type 2 diabetes in persons with and without HFE hemochromatosis diagnosed today is similar. Routine iron phenotyping or HFE genotyping of patients with type 2 diabetes is not recommended. Persons with newly diagnosed type 2 diabetes who have arthropathy involving the second and third metacarpophalangeal joints are candidates for iron phenotyping or HFE genotyping because this manifestation is associated with increased diabetes risk. Iron overload alone is insufficient to cause type 2 diabetes in most HFE p.C282Y homozygotes.
Table 3.
Diabetes risk in HFE hemochromatosis.
| Risk factors | Proposed mechanisms and pathophysiology |
|---|---|
| Increased iron entry into beta cells of islets | Increased transferrin saturation and transport via transferrin receptors Elevated nontransferrin bound iron in plasma and entry by incompletely described mechanisms Increased iron transport by divalent metal transporter 1 |
|
| |
| Decreased insulin secretion | Islet inflammation Beta-cell injury Pancreatic islet fibrosis |
|
| |
| Cirrhosis | Associated with severe iron overload, pancreatic fibrosis Hyperglucagonemia PCSK7 rs236918 allele C |
|
| |
| History of diabetes in first-degree relatives | Multiple genetic and acquired factors |
|
| |
| Genetic markers | Multiple loci for type 2 diabetes Chromosome 6p loci for type 1 (autoimmune) diabetes |
13. Problems That Remain to Be Resolved
It is unknown whether phlebotomy of all p.C282Y homozygotes will increase insulin secretion if diagnosis of hemochromatosis and induction phlebotomy therapy are early. It is unknown whether early identification and treatment of p.C282Y homozygotes who also have common putative genetic “modifiers” that increase severity of iron phenotypes would decrease diabetes prevalence. Bone morphogenetic proteins have been implicated in glucose metabolism [162] and it has been proposed that BMP2 rs235756 is associated with SF levels in p.C282Y homozygotes [163], although we found no documentation of the relationship of BMP2 rs235756 to diabetes risk. It is unknown whether maintaining lower SF levels in persons with HFE hemochromatosis and diabetes than presently recommended for “maintenance” therapy could maintain or improve insulin secretion and diabetes control or decrease diabetes risk. The proportion of patients with HFE hemochromatosis who develop diabetes after diagnosis and treatment of hemochromatosis is unknown. The role of tumor necrosis factor (TNF; chromosome 6p21.33) in hemochromatosis-associated diabetes is unknown. The prevalence of HFE alleles and genotypes in cohorts of patients with autoimmune diabetes is unknown.
14. Directions for Future Research
Longitudinal studies that compare the incidence rates of diabetes in p.C282Y homozygotes and control subjects matched for age, sex, and race would provide greater insights into the burden of diabetes in HFE hemochromatosis, especially diabetes risk after hemochromatosis diagnosis. Effects of early diagnosis and phlebotomy on diabetes incidence in hemochromatosis could also be determined in longitudinal studies.
Genome-wide association or whole-genome sequencing studies of cohorts of p.C282Y homozygotes with and without diabetes could identify alleles associated with increased diabetes risk. It is anticipated that such studies would identify novel loci in p.C282Y homozygotes not previously identified in studies of participants with type 2 diabetes who were unselected for hemochromatosis diagnoses. Comparing frequencies of SNPs associated with DMT1, SLC39A14, F13A1, RIPK2, STEAP4, and BMP2 in p.C282Y homozygotes with and without diabetes would provide information about the role of these genes and their corresponding proteins in iron uptake in and inflammatory injury to beta cells. TNF-308G→A was significantly associated with TS but not SF levels measured in population screening [164]. Comparing frequencies of TNF-308G→A and other TNF promoter variants in p.C282Y homozygotes with and without diabetes would provide insights into the role of tumor necrosis factor in hemochromatosis-associated diabetes. PCSK7 rs236918 genotyping in p.C282Y homozygotes may reveal relationships of cirrhosis risk [165] and changes in insulin sensitivity with dietary carbohydrate intake [166]. Analysis of HFE allele and genotype frequencies in patients with autoimmune diabetes and an appropriate comparator group would identify a significant relationship of common HFE alleles to autoimmune diabetes, if it exists.
Acknowledgments
This work was supported in part by Southern Iron Disorders Center.
Abbreviations
- BMI:
Body mass index
- CRP:
C-reactive protein
- DMT1:
Divalent metal transporter 1
- HLA:
Human leukocyte antigen
- HEIRS Study:
Hemochromatosis and Iron Overload Screening Study
- HOMA:
Homeostasis model assessment
- IL-6:
Interleukin-6
- IR:
Insulin resistance
- MetS:
Metabolic syndrome
- MHC:
Major histocompatibility complex
- NTBI:
Non-transferrin-bound iron
- SF:
Serum ferritin
- SNP:
Single-nucleotide polymorphism
- sTfR:
Serum transferrin receptor
- TS:
Transferrin saturation.
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contributions
Both authors contributed equally to conception and design of this review, literature review and analysis, drafting, critical revision, and editing, and approval of the final version.
References
- 1.McClain D. A., Abraham D., Rogers J., et al. High prevalence of abnormal glucose homeostasis secondary to decreased insulin secretion in individuals with hereditary haemochromatosis. Diabetologia. 2006;49(7):1661–1669. doi: 10.1007/s00125-006-0200-0. [DOI] [PubMed] [Google Scholar]
- 2.O'Sullivan E. P., McDermott J. H., Murphy M. S., Sen S., Walsh C. H. Declining prevalence of diabetes mellitus in hereditary haemochromatosis—the result of earlier diagnosis. Diabetes Research and Clinical Practice. 2008;81(3):316–320. doi: 10.1016/j.diabres.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Bulaj Z. J., Ajioka R. S., Phillips J. D., et al. Disease-related conditions in relatives of patients with hemochromatosis. The New England Journal of Medicine. 2000;343(21):1529–1535. doi: 10.1056/nejm200011233432104. [DOI] [PubMed] [Google Scholar]
- 4.Trousseau A. Glycosurie, diabète sucre. Clinique Médicale de l'Hôtel-Dieu de Paris. 1865;2:663–698. [Google Scholar]
- 5.Troisier M. Diabète sucre. Bulletins de la Société Anatomique de Paris. 1871;44:231–235. [Google Scholar]
- 6.von Recklinghausen F. D. Über hämochromatose. Tagebl Versamml Natur Ärtze Heidelberg. 1889;62:324–325. [Google Scholar]
- 7.Barton J. C., Edwards C. Q., Phatak P. D., Britton R. S., Bacon B. R. History of iron overload disorders. In: Barton J. C., Edwards C. Q., Phatak P. D., Britton R. S., Bacon B. R., editors. Handbook of Iron Overload Disorders. Chapter 1. Cambridge, UK: Cambridge University Press; 2010. pp. 1–9. [Google Scholar]
- 8.Sheldon J. H. Haemochromatosis. Oxford University Press; 1935. [Google Scholar]
- 9.Finch S. C., Finch C. A. Idiopathic hemochromatosis, an iron storage disease. A. Iron metabolism in hemochromatosis. Medicine. 1955;34(4):381–430. doi: 10.1097/00005792-195512000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Barton J. C., Barton J. C., Acton R. T. Diabetes in first-degree family members: a predictor of type 2 diabetes in 159 nonscreening Alabama hemochromatosis probands with HFE C282Y homozygosity. Diabetes Care. 2014;37(1):259–266. doi: 10.2337/dc13-0713. [DOI] [PubMed] [Google Scholar]
- 11.Saddi R., Feingold J. Idiopathic haemochromatosis: an autosomal recessive disease. Clinical Genetics. 1974;5(3):234–241. doi: 10.1111/j.1399-0004.1974.tb01688.x. [DOI] [PubMed] [Google Scholar]
- 12.Edwards C. Q., Griffin L. M., Goldgar D., Drummond C., Skolnick M. H., Kushner J. P. Prevalence of hemochromatosis among 11,065 presumably healthy blood donors. New England Journal of Medicine. 1988;318(21):1355–1362. doi: 10.1056/NEJM198805263182103. [DOI] [PubMed] [Google Scholar]
- 13.Feder J. N., Gnirke A., Thomas W., et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nature Genetics. 1996;13(4):399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 14.Barton J. C., Acton R. T., Dawkins F. W., et al. Initial screening transferrin saturation values, serum ferritin concentrations, and HFE genotypes in whites and blacks in the Hemochromatosis and Iron Overload Screening Study. Genetic Testing. 2005;9(3):231–241. doi: 10.1089/gte.2005.9.231. [DOI] [PubMed] [Google Scholar]
- 15.Adams P. C., Barton J. C. How I treat hemochromatosis. Blood. 2010;116(3):317–325. doi: 10.1182/blood-2010-01-261875. [DOI] [PubMed] [Google Scholar]
- 16.Barton J. C., Edwards C. Q., Phatak P. D., Britton R. S., Bacon B. R. Complications of hemochromatosis and iron overload. In: Barton J. C., Edwards C. Q., Phatak P. D., Britton R. S., Bacon B. R., editors. Handbook of Iron Overload Disorders. chapter 5. Cambridge, UK: Cambridge University Press; 2010. pp. 65–107. [Google Scholar]
- 17.Merryweather-Clarke A. T., Pointon J. J., Shearman J. D., Robson K. J. H. Global prevalence of putative haemochromatosis mutations. Journal of Medical Genetics. 1997;34(4):275–278. doi: 10.1136/jmg.34.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Association for the Study of the Liver. EASL clinical practice guidelines for HFE hemochromatosis. Journal of Hepatology. 2010;53(1):3–22. doi: 10.1016/j.jhep.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Barton J. C., Edwards C. Q., Acton R. T. HFE gene: structure, function, mutations, and associated iron abnormalities. Gene. 2015;574(2):179–192. doi: 10.1016/j.gene.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams P. C., Reboussin D. M., Barton J. C., et al. Hemochromatosis and iron-overload screening in a racially diverse population. New England Journal of Medicine. 2005;352(17):1769–1778. doi: 10.1056/NEJMoa041534. [DOI] [PubMed] [Google Scholar]
- 21.Lebrón J. A., West A. P., Jr., Bjorkman P. J. The hemochromatosis protein HFE competes with transferrin for binding to the transferrin receptor. Journal of Molecular Biology. 1999;294(1):239–245. doi: 10.1006/jmbi.1999.3252. [DOI] [PubMed] [Google Scholar]
- 22.Lebrón J. A., Bjorkman P. J. The transferrin receptor binding site on HFE, the class I MHC-related protein mutated in hereditary hemochromatosis. Journal of Molecular Biology. 1999;289(4):1109–1118. doi: 10.1006/jmbi.1999.2842. [DOI] [PubMed] [Google Scholar]
- 23.Ganz T., Nemeth E. Hepcidin and iron homeostasis. Biochimica et Biophysica Acta (BBA)—Molecular Cell Research. 2012;1823(9):1434–1443. doi: 10.1016/j.bbamcr.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao B., Sugianto P., Fung E., et al. Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metabolism. 2012;15(6):918–924. doi: 10.1016/j.cmet.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards C. Q., Barton J. C. Hemochromatosis. In: Greer J. P., Arber D. A., Glader B., et al., editors. Wintrobe's Clinical Hematology. 13th. chapter 25. Philadelphia, Pa, USA: Wolters Kluwer/Lippincott Williams & Wilkins; 2014. pp. 662–681. [Google Scholar]
- 26.Mclaren C. E., Emond M. J., Subramaniam V. N., et al. Exome sequencing in HFE C282Y homozygous men with extreme phenotypes identifies a GNPAT variant associated with severe iron overload. Hepatology. 2015;62(2):429–439. doi: 10.1002/hep.27711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haskins D., Stevens A. R., Jr., Finch S., Finch C. A. Iron metabolism; iron stores in man as measured by phlebotomy. The Journal of Clinical Investigation. 1952;31(6):543–547. doi: 10.1172/jci102639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Worwood M. Assessing the iron status of populations: report of a joint World Health Organization/Centers for Disease Control and Prevention technical consultation on the assessment of iron status at the population level. Geneva, Switzerland: WHO, CDC; 2007. Indicators of the iron status of populations: ferritin; pp. 35–73. [Google Scholar]
- 29.Weinfeld A., Lundin P., Lundvall O. Significance for the diagnosis of iron overload of histochemical and chemical iron in the liver of control subjects. Journal of Clinical Pathology. 1968;21(1):35–40. doi: 10.1136/jcp.21.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipschitz D. A., Cook J. D., Finch C. A. A clinical evaluation of serum ferritin as an index of iron stores. New England Journal of Medicine. 1974;290(22):1213–1216. doi: 10.1056/nejm197405302902201. [DOI] [PubMed] [Google Scholar]
- 31.Adams P. C., Barton J. C. A diagnostic approach to hyperferritinemia with a non-elevated transferrin saturation. Journal of Hepatology. 2011;55(2):453–458. doi: 10.1016/j.jhep.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Hartroft W. S. Islet pathology in diabetes. Diabetes. 1956;5(2):98–104. doi: 10.2337/diab.5.2.98. [DOI] [PubMed] [Google Scholar]
- 33.Rahier J., Loozen S., Goebbels R. M., Abrahem M. The haemochromatotic human pancreas: a quantitative immunohistochemical and ultrastructural study. Diabetologia. 1987;30(1):5–12. doi: 10.1007/BF01788899. [DOI] [PubMed] [Google Scholar]
- 34.Gatter K. C., Brown G., Trowbridge I. S., Woolston R. E., Mason D. Y. Transferrin receptors in human tissues: their distribution and possible clinical relevance. Journal of Clinical Pathology. 1983;36(5):539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koch R. O., Zoller H., Theurl I., et al. Distribution of DMT1 within the human glandular system. Histology and Histopathology. 2003;18(4):1095–1101. doi: 10.14670/HH-18.1095. [DOI] [PubMed] [Google Scholar]
- 36.Nam H., Wang C.-Y., Zhang L., et al. ZIP14 and DMT1 in the liver, pancreas, and heart are differentially regulated by iron deficiency and overload: implications for tissue iron uptake in iron-related disorders. Haematologica. 2013;98(7):1049–1057. doi: 10.3324/haematol.2012.072314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunshin H., Fujiwara Y., Custodio A. O., DiRenzo C., Robine S., Andrews N. C. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. Journal of Clinical Investigation. 2005;115(5):1258–1266. doi: 10.1172/JCI200524356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shindo M., Torimoto Y., Saito H., et al. Functional role of DMT1 in transferrin-independent iron uptake by human hepatocyte and hepatocellular carcinoma cell, HLF. Hepatology Research. 2006;35(3):152–162. doi: 10.1016/j.hepres.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Liuzzi J. P., Aydemir F., Nam H., Knutson M. D., Cousins R. J. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(37):13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jenkitkasemwong S., Wang C.-Y., Coffey R., et al. SLC39A14 is required for the development of hepatocellular iron overload in murine models of hereditary hemochromatosis. Cell Metabolism. 2015;22(1):138–150. doi: 10.1016/j.cmet.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kulaksiz H., Fein E., Redecker P., Stremmel W., Adler G., Cetin Y. Pancreatic β-cells express hepcidin, an iron-uptake regulatory peptide. Journal of Endocrinology. 2008;197(2):241–249. doi: 10.1677/JOE-07-0528. [DOI] [PubMed] [Google Scholar]
- 42.Dymock I. W., Cassar J., Pyke D. A., Oakley W. G., Williams R. Observations on the pathogenesis, complications and treatment of diabetes in 115 cases of haemochromatosis. The American Journal of Medicine. 1972;52(2):203–210. doi: 10.1016/0002-9343(72)90070-8. [DOI] [PubMed] [Google Scholar]
- 43.Zhou X. Y., Tomatsu S., Fleming R. E., et al. HFE gene knockout produces mouse model of hereditary hemochromatosis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(5):2492–2497. doi: 10.1073/pnas.95.5.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleming R. E., Holden C. C., Tomatsu S., et al. Mouse strain differences determine severity of iron accumulation in Hfe knockout model of hereditary hemochromatosis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(5):2707–2711. doi: 10.1073/pnas.051630898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee S.-M., Loguinov A., Fleming R. E., Vulpe C. D. Effects of strain and age on hepatic gene expression profiles in murine models of HFE-associated hereditary hemochromatosis. Genes and Nutrition. 2015;10(1):1–9. doi: 10.1007/s12263-014-0443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bensaid M., Fruchon S., Mazères C., Bahram S., Roth M.-P., Coppin H. Multigenic control of hepatic iron loading in a murine model of hemochromatosis. Gastroenterology. 2004;126(5):1400–1408. doi: 10.1053/j.gastro.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 47.Cooksey R. C., Jouihan H. A., Ajioka R. S., et al. Oxidative stress, β-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology. 2004;145(11):5305–5312. doi: 10.1210/en.2004-0392. [DOI] [PubMed] [Google Scholar]
- 48.Hed R. Clinical studies in chronic alcoholism. II: carbohydrate metabolism in chronic alcoholism with particular reference to glucose and insulin tolerances. Acta Medica Scandinavica. 1958;162(3):195–202. doi: 10.1111/j.0954-6820.1958.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 49.Guillon J., Charbonnel B. Diabetes mellitus secondary to liver diseases. A review (author's transl) Diabete et Metabolisme. 1975;1(3):191–199. [PubMed] [Google Scholar]
- 50.Caronia S., Taylor K., Pagliaro L., et al. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30(4):1059–1063. doi: 10.1002/hep.510300416. [DOI] [PubMed] [Google Scholar]
- 51.Barton J. C., Barton J. C., Adams P. C., Acton R. T. Risk factors for insulin resistance, metabolic syndrome, and diabetes in 248 HFE C282Y homozygotes identified by population screening in the HEIRS Study. Metabolic Syndrome and Related Disorders. 2016;14(2):94–101. doi: 10.1089/met.2015.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Megyesi C., Samols E., Marks V. Glucose tolerance and diabetes in chronic liver disease. Lancet. 1967;2(7525):1051–1056. doi: 10.1016/s0140-6736(67)90334-0. [DOI] [PubMed] [Google Scholar]
- 53.Sherwin R., Joshi P., Hendler R., Felig P., Conn H. O. Hyperglucagonemia in Laennec's cirrhosis. The role of portal-systemic shunting. New England Journal of Medicine. 1974;290(5):239–242. doi: 10.1056/nejm197401312900502. [DOI] [PubMed] [Google Scholar]
- 54.Gay J., Tchobroutsky G., Rosselin G., et al. Study of 8 cases of primary hemochromatosis, including in particular the plasma radio-immunologic determination of somatotropic and follicle-stimulating hormones and glucagon. Pathologie Biologie. 1968;16(1):53–60. [PubMed] [Google Scholar]
- 55.Gonvers J. J., Henchoz L., Hofstetter J. R., Muller W. A. Study of glucagon secretion in patients with hemochromatosis. Schweizerische Medizinische Wochenschrift. 1977;107(49):1841–1842. [PubMed] [Google Scholar]
- 56.Passa P., Luyckx A. S., Carpentier J. L., Lefebvre P. J., Canivet J. Glucagon secretion in diabetic patients with idiopathic haemochromatosis. Diabetologia. 1977;13(5):509–513. doi: 10.1007/BF01234504. [DOI] [PubMed] [Google Scholar]
- 57.Muller W. A., Berger M., Cuppers H. J., et al. Plasma glucagon in diabetes of haemochromatosis: too low or too high? Gut. 1979;20(3):200–204. doi: 10.1136/gut.20.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nelson R. L., Baldus W. P., Rubenstein A. H., Go V. L., Service F. J. Pancreatic α-cell function in diabetic hemochromatotic subjects. The Journal of Clinical Endocrinology & Metabolism. 1979;49(3):412–416. doi: 10.1210/jcem-49-3-412. [DOI] [PubMed] [Google Scholar]
- 59.Rumbak M. J., Joffe B. I., Seftel H. C., Kalk W. J., Bezwoda W. R., Bothwell T. H. Plasma C-peptide and insulin responses to intravenous glucagon stimulation in idiopathic haemochromatosis. South African Medical Journal. 1987;71(6):351–353. [PubMed] [Google Scholar]
- 60.Sargent T., III, Lim T. H., Jenson R. L. Reduced chromium retention in patients with hemochromatosis, a possible basis of hemochromatotic diabetes. Metabolism. 1979;28(1):70–79. doi: 10.1016/0026-0495(79)90171-9. [DOI] [PubMed] [Google Scholar]
- 61.Lim T. H., Sargent T., Kusubov N. Kinetics of trace element chromium(III) in the human body. The American Journal of Physiology. 1983;244(4):R445–454. doi: 10.1152/ajpregu.1983.244.4.R445. [DOI] [PubMed] [Google Scholar]
- 62.Schwarz K., Mertz W. Chromium(III) and the glucose tolerance factor. Archives of Biochemistry and Biophysics. 1959;85(1):292–295. doi: 10.1016/0003-9861(59)90479-5. [DOI] [PubMed] [Google Scholar]
- 63.Institute of Medicine Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. chapter 6. Washington, DC, USA: National Academy Press; 2001. Chromium; pp. 197–223. [PubMed] [Google Scholar]
- 64.Hua Y., Clark S., Ren J., Sreejayan N. Molecular mechanisms of chromium in alleviating insulin resistance. Journal of Nutritional Biochemistry. 2012;23(4):313–319. doi: 10.1016/j.jnutbio.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rafiei R., Habyby Z., Fouladi L., Najafi S., Asgary S., Torabi Z. Chromium level in prediction of diabetes in pre-diabetic patients. Advanced Biomedical Research. 2014;3, article 235 doi: 10.4103/2277-9175.145737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Forte G., Bocca B., Peruzzu A., et al. Blood metals concentration in type 1 and type 2 diabetics. Biological Trace Element Research. 2013;156(1-3):79–90. doi: 10.1007/s12011-013-9858-6. [DOI] [PubMed] [Google Scholar]
- 67.Zoller H., Koch R. O., Theurl I., et al. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology. 2001;120(6):1412–1419. doi: 10.1053/gast.2001.24033. [DOI] [PubMed] [Google Scholar]
- 68.Illing A. C., Shawki A., Cunningham C. L., Mackenzie B. Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. The Journal of Biological Chemistry. 2012;287(36):30485–30496. doi: 10.1074/jbc.m112.364208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aisen P., Aasa R., Redfield A. G. The chromium, manganese, and cobalt complexes of transferrin. Journal of Biological Chemistry. 1969;244(17):4628–4633. [PubMed] [Google Scholar]
- 70.Harris D. C. Different metal-binding properties of the two sites of human transferrin. Biochemistry. 1977;16(3):560–564. doi: 10.1021/bi00622a033. [DOI] [PubMed] [Google Scholar]
- 71.Phelps G., Hall P., Chapman I., Braund W., Mackinnon M. Prevalence of genetic haemochromatosis among diabetic patients. The Lancet. 1989;334(8657):233–234. doi: 10.1016/s0140-6736(89)90426-1. [DOI] [PubMed] [Google Scholar]
- 72.Conte D., Manachino D., Colli A., et al. Prevalence of genetic hemochromatosis in a cohort of Italian patients with diabetes mellitus. Annals of Internal Medicine. 1998;128(5):370–373. doi: 10.7326/0003-4819-128-5-199803010-00005. [DOI] [PubMed] [Google Scholar]
- 73.Acton R. T., Barton J. C., Passmore L. V., et al. Relationships of serum ferritin, transferrin saturation, and HFE mutations and self-reported diabetes in the Hemochromatosis and Iron Overload Screening (HEIRS) Study. Diabetes Care. 2006;29(9):2084–2089. doi: 10.2337/dc05-1592. [DOI] [PubMed] [Google Scholar]
- 74.Acton R. T., Clayborn Barton J., Barton J. C. Serum ferritin, insulin resistance, and metabolic syndrome: clinical and laboratory associations in 769 non-hispanic whites without diabetes mellitus in the HEIRS Study. Metabolic Syndrome and Related Disorders. 2015;13(2):57–63. doi: 10.1089/met.2014.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheung C.-L., Cheung T. T., Lam K. S. L., Cheung B. M. Y. High ferritin and low transferrin saturation are associated with pre-diabetes among a national representative sample of U.S. adults. Clinical Nutrition. 2013;32(6):1055–1060. doi: 10.1016/j.clnu.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 76.Ford E. S., Cogswell M. E. Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care. 1999;22(12):1978–1983. doi: 10.2337/diacare.22.12.1978. [DOI] [PubMed] [Google Scholar]
- 77.Huth C., Beuerle S., Zierer A., et al. Biomarkers of iron metabolism are independently associated with impaired glucose metabolism and type 2 diabetes: the KORA F4 study. European Journal of Endocrinology. 2015;173(5):643–653. doi: 10.1530/eje-15-0631. [DOI] [PubMed] [Google Scholar]
- 78.Yeap B. B., Divitini M. L., Gunton J. E., et al. Higher ferritin levels, but not serum iron or transferrin saturation, are associated with Type 2 diabetes mellitus in adult men and women free of genetic haemochromatosis. Clinical Endocrinology. 2015;82(4):525–532. doi: 10.1111/cen.12529. [DOI] [PubMed] [Google Scholar]
- 79.Orban E., Schwab S., Thorand B., Huth C. Association of iron indices and type 2 diabetes: a meta-analysis of observational studies. Diabetes/Metabolism Research and Reviews. 2014;30(5):372–394. doi: 10.1002/dmrr.2506. [DOI] [PubMed] [Google Scholar]
- 80.Beutler E., Felitti V., Ho N. J., Gelbart T. Relationship of body iron stores to levels of serum ferritin, serum iron, unsaturated iron binding capacity and transferrin saturation in patients with iron storage disease. Acta Haematologica. 2002;107(3):145–149. doi: 10.1159/000057632. [DOI] [PubMed] [Google Scholar]
- 81.Tuomainen T.-P., Nyyssönen K., Salonen R., et al. Body iron stores are associated with serum insulin and blood glucose concentrations. Population study in 1,013 eastern Finnish men. Diabetes Care. 1997;20(3):426–428. doi: 10.2337/diacare.20.3.426. [DOI] [PubMed] [Google Scholar]
- 82.Jiang R., Manson J. E., Meigs J. B., Ma J., Rifai N., Hu F. B. Body iron stores in relation to risk of Type 2 diabetes in apparently healthy women. JAMA. 2004;291(6):711–717. doi: 10.1001/jama.291.6.711. [DOI] [PubMed] [Google Scholar]
- 83.Rajpathak S. N., Wylie-Rosett J., Gunter M. J., et al. Biomarkers of body iron stores and risk of developing type 2 diabetes. Diabetes, Obesity and Metabolism. 2009;11(5):472–479. doi: 10.1111/j.1463-1326.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Montonen J., Boeing H., Steffen A., et al. Body iron stores and risk of type 2 diabetes: results from the European prospective investigation into cancer and nutrition (EPIC)-Potsdam Study. Diabetologia. 2012;55(10):2613–2621. doi: 10.1007/s00125-012-2633-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arija V., Fernández-Cao J. C., Basora J., et al. Excess body iron and the risk of type 2 diabetes mellitus: a nested case-control in the PREDIMED (PREvention with MEDiterranean Diet) Study. British Journal of Nutrition. 2014;112(11):1896–1904. doi: 10.1017/s0007114514002852. [DOI] [PubMed] [Google Scholar]
- 86.Wlazlo N., van Greevenbroek M. M. J., Ferreira I., et al. Iron metabolism is prospectively associated with insulin resistance and glucose intolerance over a 7-year follow-up period: the CODAM study. Acta Diabetologica. 2015;52(2):337–348. doi: 10.1007/s00592-014-0646-3. [DOI] [PubMed] [Google Scholar]
- 87.Kunutsor S. K., Apekey T. A., Walley J., Kain K. Ferritin levels and risk of type 2 diabetes mellitus: an updated systematic review and meta-analysis of prospective evidence. Diabetes/Metabolism Research and Reviews. 2013;29(4):308–318. doi: 10.1002/dmrr.2394. [DOI] [PubMed] [Google Scholar]
- 88.Salonen J. T., Tuomainen T.-P., Nyyssönen K., Lakka H.-M., Punnonen K. Relation between iron stores and non-insulin dependent diabetes in men: case-control study. British Medical Journal. 1998;317(7160):p. 727. doi: 10.1136/bmj.317.7160.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pechlaner R., Weiss G., Bansal S., et al. Inadequate hepcidin serum concentrations predict incident type 2 diabetes mellitus. Diabetes/Metabolism Research and Reviews. 2016;32(2):187–192. doi: 10.1002/dmrr.2711. [DOI] [PubMed] [Google Scholar]
- 90.Podmore C., Meidtner K., Schulze M. B., et al. Association of multiple biomarkers of iron metabolism and type 2 diabetes: The EPIC-Inter Act Study. Diabetes Care. 2016;39(4):572–581. doi: 10.2337/dc15-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barton J. C., Barton J. C., Acton R. T., So J., Chan S., Adams P. C. Increased risk of death from iron overload among 422 treated probands with HFE hemochromatosis and serum levels of ferritin greater than 1000 μg/L at diagnosis. Clinical Gastroenterology and Hepatology. 2012;10(4):412–416. doi: 10.1016/j.cgh.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 92.Bozzini C., Girelli D., Olivieri O., et al. Prevalence of body iron excess in the metabolic syndrome. Diabetes Care. 2005;28(8):2061–2063. doi: 10.2337/diacare.28.8.2061. [DOI] [PubMed] [Google Scholar]
- 93.Eshed I., Elis A., Lishner M. Plasma ferritin and type 2 diabetes mellitus: a critical review. Endocrine Research. 2001;27(1-2):91–97. doi: 10.1081/erc-100107172. [DOI] [PubMed] [Google Scholar]
- 94.O'Brien T., Barrett B., Murray D. M., Dinneen S., O'Sullivan D. J. Usefulness of biochemical screening of diabetic patients for hemochromatosis. Diabetes Care. 1990;13(5):532–534. doi: 10.2337/diacare.13.5.532. [DOI] [PubMed] [Google Scholar]
- 95.Beutler E., Waalen J., Gelbart T. Chronic inflammation does not appear to modify the homozygous hereditary hemochromatosis phenotype. Blood Cells, Molecules, and Diseases. 2005;35(3):326–327. doi: 10.1016/j.bcmd.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 96.Herbert V., Jayatilleke E., Shaw S., et al. Serum ferritin iron, a new test, measures human body iron stores unconfounded by inflammation. Stem Cells. 1997;15(4):291–296. doi: 10.1002/stem.150291. [DOI] [PubMed] [Google Scholar]
- 97.Nielsen P., Günther U., Dürken M., Fischer R., Düllmann J. Serum ferritin iron in iron overload and liver damage: correlation to body iron stores and diagnostic relevance. The Journal of Laboratory and Clinical Medicine. 2000;135(5):413–418. doi: 10.1067/mlc.2000.106456. [DOI] [PubMed] [Google Scholar]
- 98.Cook J. D., Finch C. A., Smith N. J. Evaluation of the iron status of a population. Blood. 1976;48(3):449–455. [PubMed] [Google Scholar]
- 99.Alfrey C. P. Serum ferritin assay. Critical Reviews in Clinical Laboratory Sciences. 1978;9(3):179–208. doi: 10.3109/10408367809150919. [DOI] [PubMed] [Google Scholar]
- 100.Reissman K. R., Dietrich M. R. On the presence of ferritin in the peripheral blood of patients with hepatocellular disease. The Journal of Laboratory and Clinical Medicine. 1956;35:588–595. doi: 10.1172/JCI103312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matzner Y., Konijn A. M., Hershko C. Serum ferritin in hematologic malignancies. American Journal of Hematology. 1980;9(1):13–22. doi: 10.1002/ajh.2830090103. [DOI] [PubMed] [Google Scholar]
- 102.Rothwell R. S., Davis P. Relationship between serum ferritin, anemia, and disease activity in acute and chronic rheumatoid arthritis. Rheumatology International. 1981;1(2):65–67. doi: 10.1007/BF00541155. [DOI] [PubMed] [Google Scholar]
- 103.Jacobs A. Serum ferritin and malignant tumours. Medical Oncology and Tumor Pharmacotherapy. 1984;1(3):149–156. doi: 10.1007/BF02934136. [DOI] [PubMed] [Google Scholar]
- 104.Piñero D. J., Hu J., Cook B. M., Scaduto R. C., Jr., Connor J. R. Interleukin-1β increases binding of the iron regulatory protein and the synthesis of ferritin by increasing the labile iron pool. Biochimica et Biophysica Acta. 2000;1497(3):279–288. doi: 10.1016/s0167-4889(00)00066-5. [DOI] [PubMed] [Google Scholar]
- 105.Moirand R., Lescoat G., Delamaire D., et al. Increase in glycosylated and nonglycosylated serum ferritin in chronic alcoholism and their evolution during alcohol withdrawal. Alcoholism: Clinical and Experimental Research. 1991;15(6):963–969. doi: 10.1111/j.1530-0277.1991.tb05196.x. [DOI] [PubMed] [Google Scholar]
- 106.Frayling T., Ellard S., Grove J., Walker M., Hattersley A. T. C282Y mutation in HFE (haemochromatosis) gene and type 2 diabetes. Lancet. 1998;351(9120):1933–1934. doi: 10.1016/S0140-6736(05)78618-9. [DOI] [PubMed] [Google Scholar]
- 107.Fernandez-Real J.-M., Vendrell J., Baiget M., Gimferrer E., Ricart W. C282Y and H63D mutations of the hemochromatosis candidate gene in type 2 diabetes. Diabetes Care. 1999;22(3):525–526. doi: 10.2337/diacare.22.3.525. [DOI] [PubMed] [Google Scholar]
- 108.Sampson M. J., Williams T., Heyburn P. J., et al. Prevalence of HFE (hemochromatosis gene) mutations in unselected male patients with type 2 diabetes. Journal of Laboratory and Clinical Medicine. 2000;135(2):170–173. doi: 10.1067/mlc.2000.104464. [DOI] [PubMed] [Google Scholar]
- 109.Moczulski D. K., Grzeszczak W., Gawlik B. Role of hemochromatosis C282Y and H63D mutations in HFE gene in development of type 2 diabetes and diabetic nephropathy. Diabetes Care. 2001;24(7):1187–1191. doi: 10.2337/diacare.24.7.1187. [DOI] [PubMed] [Google Scholar]
- 110.Barton J. C., Adams P. C., Acton R. T. Undiagnosed diabetes and impaired fasting glucose in HFE C282Y homozygotes and HFE wild-type controls in the HEIRS Study. BMJ Open Diabetes Research & Care. 2016;4 doi: 10.1136/bmjdrc-2016-000278.e000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qi L., Meigs J., Manson J. E., et al. HFE genetic variability, body iron stores, and the risk of type 2 diabetes in U.S. women. Diabetes. 2005;54(12):3567–3572. doi: 10.2337/diabetes.54.12.3567. [DOI] [PubMed] [Google Scholar]
- 112.Walsh A., Dixon J. L., Ramm G. A., et al. The clinical relevance of compound heterozygosity for the C282Y and H63D substitutions in hemochromatosis. Clinical Gastroenterology and Hepatology. 2006;4(11):1403–1410. doi: 10.1016/j.cgh.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 113.Barton J. C., Sawada-Hirai R., Rothenberg B. E., Acton R. T. Two novel missense mutations of the HFE gene (I105T and G93R) and identification of the S65C mutation in Alabama hemochromatosis probands. Blood Cells, Molecules, and Diseases. 1999;25(3-4):147–155. doi: 10.1006/bcmd.1999.0240. [DOI] [PubMed] [Google Scholar]
- 114.Wallace D. F., Dooley J. S., Walker A. P. A novel mutation of HFE explains the classical phenotype of genetic hemochromatosis in a C282Y heterozygote. Gastroenterology. 1999;116(6):1409–1412. doi: 10.1016/s0016-5085(99)70505-6. [DOI] [PubMed] [Google Scholar]
- 115.McLaren G. D., McLaren C. E., Adams P. C., et al. Clinical manifestations of hemochromatosis in HFE C282Y homozygotes identified by screening. Canadian Journal of Gastroenterology. 2008;22(11):923–930. doi: 10.1155/2008/907356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Svensson E., Mor A., Rungby J., et al. Lifestyle and clinical factors associated with elevated C-reactive protein among newly diagnosed Type 2 diabetes mellitus patients: a cross-sectional study from the nationwide DD2 cohort. BMC Endocrine Disorders. 2014;14(1, article 74) doi: 10.1186/1472-6823-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang X., Bao W., Liu J., et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36(1):166–175. doi: 10.2337/dc12-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Daniele G., Guardado Mendoza R., Winnier D., et al. The inflammatory status score including IL-6, TNF-α, osteopontin, fractalkine, MCP-1 and adiponectin underlies whole-body insulin resistance and hyperglycemia in type 2 diabetes mellitus. Acta Diabetologica. 2014;51(1):123–131. doi: 10.1007/s00592-013-0543-1. [DOI] [PubMed] [Google Scholar]
- 119.Sharma P. R., Mackey A. J., Dejene E. A., et al. An islet-targeted genome-wide association scan identifies novel genes implicated in cytokine-mediated islet stress in type 2 diabetes. Endocrinology. 2015;156(9):3147–3156. doi: 10.1210/en.2015-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Al Abbas M., Abraham D., Kushner J. P., McClain D. A. Anti-obesity and pro-diabetic effects of hemochromatosis. Obesity. 2014;22(10):2120–2122. doi: 10.1002/oby.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Niederau C., Berger M., Stremmel W., et al. Hyperinsulinaemia in non-cirrhotic haemochromatosis: impaired hepatic insulin degradation? Diabetologia. 1984;26(6):441–444. doi: 10.1007/BF00262217. [DOI] [PubMed] [Google Scholar]
- 122.Hramiak I. M., Finegood D. T., Adams P. C. Factors affecting glucose tolerance in hereditary hemochromatosis. Clinical and Investigative Medicine. 1997;20(2):110–118. [PubMed] [Google Scholar]
- 123.Adams L. A., Angulo P., Abraham S. C., Torgerson H., Brandhagen D. The effect of the metabolic syndrome, hepatic steatosis and steatohepatitis on liver fibrosis in hereditary hemochromatosis. Liver International. 2006;26(3):298–304. doi: 10.1111/j.1478-3231.2005.01238.x. [DOI] [PubMed] [Google Scholar]
- 124.American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(supplement 1):S18–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 125.Acton R. T., Barger B. O., Boshell B. R., et al. Epidemiology and genetics of insulin dependent diabetes mellitus in the white and black population from the southeastern United States. In: Hilderman W. H., editor. Frontiers in Immunogenetics. New York, NY, USA: Elsevier-North Holland; 1981. pp. 239–255. [Google Scholar]
- 126.Barrett J. C., Clayton D. G., Concannon P., et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nature Genetics. 2009;41(6):703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barton J. C., Barton J. C. Autoimmune conditions in 235 hemochromatosis probands with HFE C282Y homozygosity and their first-degree relatives. Journal of Immunology Research. 2015;2015:11. doi: 10.1155/2015/453046.453046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ellervik C., Mandrup-Poulsen T., Nordestgaard B. G., et al. Prevalence of hereditary haemochromatosis in late-onset type 1 diabetes mellitus: a retrospective study. Lancet. 2001;358(9291):1405–1409. doi: 10.1016/s0140-6736(01)06526-6. [DOI] [PubMed] [Google Scholar]
- 129.Morris A. P., Voight B. F., Teslovich T. M., et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nature Genetics. 2012;44(9):981–990. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saxena R., Elbers C. C., Guo Y., et al. Large-scale gene-centric meta-analysis across 39 studies identifies type 2 diabetes loci. American Journal of Human Genetics. 2012;90(3):410–425. doi: 10.1016/j.ajhg.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mirouze J., Schmouker Y. Physionomie actuelle du diabète sucré au cours de l'hémochromatose idiopathique. Presse Médicale. 1967;45:2245–2250. [PubMed] [Google Scholar]
- 132.Magnusson K., Hagen K. B., Osteras N., Nordsletten L., Natvig B., Haugen I. K. Diabetes is associated with increased hand pain in erosive hand osteoarthritis: data from a population-based study. Arthritis Care and Research. 2015;67(2):187–195. doi: 10.1002/acr.22460. [DOI] [PubMed] [Google Scholar]
- 133.Nell-Duxneuner V., Axmann R., Husar-Memmer E., et al. VCAM-1 serum levels are associated with arthropathy in hereditary haemochromatosis. Annals of the Rheumatic Diseases. 2013;72(12):2006–2010. doi: 10.1136/annrheumdis-2012-202800. [DOI] [PubMed] [Google Scholar]
- 134.Pankow J. S., Decker P. A., Berardi C., et al. Circulating cellular adhesion molecules and risk of diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetic Medicine. 2016;33(7):985–991. doi: 10.1111/dme.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Falize L., Guillygomarc'h A., Perrin M., et al. Reversibility of hepatic fibrosis in treated genetic hemochromatosis: a study of 36 cases. Hepatology. 2006;44(2):472–477. doi: 10.1002/hep.21260. [DOI] [PubMed] [Google Scholar]
- 136.Huxley R., Ansary-Moghaddam A., Berrington De González A., Barzi F., Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. British Journal of Cancer. 2005;92(11):2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fracanzani A. L., Conte D., Fraquelli M., et al. Increased cancer risk in a cohort of 230 patients with hereditary hemochromatosis in comparison to matched control patients with non-iron-related chronic liver disease. Hepatology. 2001;33(3):647–651. doi: 10.1053/jhep.2001.22506. [DOI] [PubMed] [Google Scholar]
- 138.Elmberg M., Hultcrantz R., Ekbom A., et al. Cancer risk in patients with hereditary hemochromatosis and in their first-degree relatives. Gastroenterology. 2003;125(6):1733–1741. doi: 10.1053/j.gastro.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 139.Niederau C., Fischer R., Sonnenberg A., Stremmel W., Trampisch H. J., Strohmeyer G. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. New England Journal of Medicine. 1985;313(20):1256–1262. doi: 10.1056/nejm198511143132004. [DOI] [PubMed] [Google Scholar]
- 140.Adams P. C., Speechley M., Kertesz A. E. Long-term survival analysis in hereditary hemochromatosis. Gastroenterology. 1991;101(2):368–372. doi: 10.1016/0016-5085(91)90013-B. [DOI] [PubMed] [Google Scholar]
- 141.Ellervik C., Mandrup-Poulsen T., Tybjaerg-Hansen A., Nordestgaard B. G. Total and cause-specific mortality by elevated transferrin saturation and hemochromatosis genotype in individuals with diabetes: two general population studies. Diabetes Care. 2014;37(2):444–452. doi: 10.2337/dc13-1198. [DOI] [PubMed] [Google Scholar]
- 142.Yang Q., McDonnell S. M., Khoury M. J., Cono J., Parrish R. G. Hemochromatosis-associated mortality in the United States from 1979 to 1992: an analysis of multiple-cause mortality data. Annals of Internal Medicine. 1998;129(11):946–953. doi: 10.7326/0003-4819-129-11_part_2-199812011-00005. [DOI] [PubMed] [Google Scholar]
- 143.Fernández-Real J. M., Peñarroja G., Castro A., García-Bragado F., Hernández-Aguado I., Ricart W. Blood letting in high-ferritin type 2 diabetes: effects on insulin sensitivity and β-cell function. Diabetes. 2002;51(4):1000–1004. doi: 10.2337/diabetes.51.4.1000. [DOI] [PubMed] [Google Scholar]
- 144.Bofill C., Joven J., Bages J., et al. Response to repeated phlebotomies in patients with non-insulin-dependent diabetes mellitus. Metabolism. 1994;43(5):614–620. doi: 10.1016/0026-0495(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 145.Mahmoud A. A., Elged A. A., Elgamal R. A., Hamada A. M. Iron depletion improves glycemic control in poorly controlled type 2 diabetic patients with iron overload and negative main HFE-gene mutations. Journal of Diabetes Mellitus. 2015;5(3):164–172. doi: 10.4236/jdm.2015.53020. [DOI] [Google Scholar]
- 146.Ferńandez-Real J. M., Mcclain D., Manco M. Mechanisms linking glucose homeostasis and iron metabolism toward the onset and progression of type 2 diabetes. Diabetes Care. 2015;38(11):2169–2176. doi: 10.2337/dc14-3082. [DOI] [PubMed] [Google Scholar]
- 147.Barton J. C., McDonnell S. M., Adams P. C., et al. Management of hemochromatosis. Hemochromatosis Management Working Group. Annals of Internal Medicine. 1998;129(11):932–939. doi: 10.7326/0003-4819-129-11_part_2-199812011-00003. [DOI] [PubMed] [Google Scholar]
- 148.Muench K. H. Hemochromatosis and infection: alcohol and iron, oysters and sepsis. American Journal of Medicine. 1989;87(3):40N–43N. [PubMed] [Google Scholar]
- 149.Hlady W. G., Klontz K. C. The epidemiology of Vibrio infections in Florida, 1981–1993. The Journal of Infectious Diseases. 1996;173(5):1176–1183. doi: 10.1093/infdis/173.5.1176. [DOI] [PubMed] [Google Scholar]
- 150.Hor L.-I., Chang T.-T., Wang S.-T. Survival of Vibrio vulnificus in whole blood from patients with chronic liver diseases: association with phagocytosis by neutrophils and serum ferritin levels. The Journal of Infectious Diseases. 1999;179(1):275–278. doi: 10.1086/314554. [DOI] [PubMed] [Google Scholar]
- 151.Barton J. C., Acton R. T. Hemochromatosis and Vibrio vulnificus wound infections. Journal of Clinical Gastroenterology. 2009;43(9):890–893. doi: 10.1097/mcg.0b013e31819069c1. [DOI] [PubMed] [Google Scholar]
- 152.Chen S.-C., Chan K.-S., Chao W.-N., et al. Clinical outcomes and prognostic factors for patients with Vibrio vulnificus infections requiring intensive care: A 10-yr Retrospective Study. Critical Care Medicine. 2010;38(10):1984–1990. doi: 10.1097/ccm.0b013e3181eeda2c. [DOI] [PubMed] [Google Scholar]
- 153.Stremmel W., Niederau C., Berger M., Kley H.-K., Kruskemper H.-L., Strohmeyer G. Abnormalities in estrogen, androgen, and insulin metabolism in idiopathic hemochromatosis. Annals of the New York Academy of Sciences. 1988;526:209–223. doi: 10.1111/j.1749-6632.1988.tb55507.x. [DOI] [PubMed] [Google Scholar]
- 154.Strohmeyer G., Niederau C. Diabetes mellitus and hemochromatosis. In: Barton J. C., Edwards C. Q., editors. Hemochromatosis. Genetics, Pathophysiology, Diagnosis and Treatment. chapter 25. Cambridge, UK: Cambridge University Press; 2000. pp. 268–277. [Google Scholar]
- 155.Abraham D., Rogers J., Gault P., Kushner J. P., McClain D. A. Increased insulin secretory capacity but decreased insulin sensitivity after correction of iron overload by phlebotomy in hereditary haemochromatosis. Diabetologia. 2006;49(11):2546–2551. doi: 10.1007/s00125-006-0445-7. [DOI] [PubMed] [Google Scholar]
- 156.Hatunic M., Finucane F. M., Norris S., Pacini G., Nolan J. J. Glucose metabolism after normalization of markers of iron overload by venesection in subjects with hereditary hemochromatosis. Metabolism: Clinical and Experimental. 2010;59(12):1811–1815. doi: 10.1016/j.metabol.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 157.Facchini F. S. Effect of phlebotomy on plasma glucose and insulin concentrations. Diabetes Care. 1998;21(12) [PubMed] [Google Scholar]
- 158.Fernández-Real J. M., López-Bermejo A., Ricart W. Iron stores, blood donation, and insulin sensitivity and secretion. Clinical Chemistry. 2005;51(7):1201–1205. doi: 10.1373/clinchem.2004.046847. [DOI] [PubMed] [Google Scholar]
- 159.Houschyar K. S., Lüdtke R., Dobos G. J., et al. Effects of phlebotomy-induced reduction of body iron stores on metabolic syndrome: results from a randomized clinical trial. BMC Medicine. 2012;10, article 54 doi: 10.1186/1741-7015-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Borai A., Livingstone C., Farzal A., et al. Changes in metabolic indices in response to whole blood donation in male subjects with normal glucose tolerance. Clinical Biochemistry. 2016;49(1):51–56. doi: 10.1016/j.clinbiochem.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 161.Jiang R., Ma J., Ascherio A., Stampfer M. J., Willett W. C., Hu F. B. Dietary iron intake and blood donations in relation to risk of type 2 diabetes in men: a prospective cohort study. American Journal of Clinical Nutrition. 2004;79(1):70–75. doi: 10.1093/ajcn/79.1.70. [DOI] [PubMed] [Google Scholar]
- 162.Grgurevic L., Christensen G. L., Schulz T. J., Vukicevic S. Bone morphogenetic proteins in inflammation, glucose homeostasis and adipose tissue energy metabolism. Cytokine and Growth Factor Reviews. 2016;27:105–118. doi: 10.1016/j.cytogfr.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 163.Milet J., Déhais V., Bourgain C., et al. Common variants in the BMP2, BMP4, and HJV genes of the hepcidin regulation pathway modulate HFE hemochromatosis penetrance. American Journal of Human Genetics. 2007;81(4):799–807. doi: 10.1086/520001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Acton R. T., Barton J. C., Leiendecker-Foster C., Zaun C., McLaren C. E., Eckfeldt J. H. Tumor necrosis factor-alpha promoter variants and iron phenotypes in 785 Hemochromatosis and Iron Overload Screening (HEIRS) Study participants. Blood Cells, Molecules, and Diseases. 2010;44(4):252–256. doi: 10.1016/j.bcmd.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stickel F., Buch S., Zoller H., et al. Evaluation of genome-wide loci of iron metabolism in hereditary hemochromatosis identifies PCSK7 as a host risk factor of liver cirrhosis. Human Molecular Genetics. 2014;23(14):3883–3890. doi: 10.1093/hmg/ddu076.ddu076 [DOI] [PubMed] [Google Scholar]
- 166.Huang T., Huang J., Qi Q., et al. PCSK7 genotype modifies effect of a weight-loss diet on 2-year changes of insulin resistance: the pounds lost trial. Diabetes Care. 2015;38(3):439–444. doi: 10.2337/dc14-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Stocks A. E., Powell L. W. Carbohydrate intolerance in idiopathic haemochromatosis and cirrhosis of the liver. Quarterly Journal of Medicine. 1973;42(168):733–749. [PubMed] [Google Scholar]
- 168.Niederau C., Strohmeyer G., Stremmel W. Epidemiology, clinical spectrum and prognosis of hemochromatosis. Advances in Experimental Medicine and Biology. 1994;356:293–302. doi: 10.1007/978-1-4615-2554-7_31. [DOI] [PubMed] [Google Scholar]
- 169.Milder M. S., Cook J. D., Stray S., Finch C. A. Idiopathic hemochromatosis, an interim report. Medicine (United States) 1980;59(1):34–49. doi: 10.1097/00005792-198001000-00002. [DOI] [PubMed] [Google Scholar]
- 170.Åsberg A., Hveem K., Krüger Ø., Bjerve K. S. Persons with screening-detected haemochromatosis: as healthy as the general population? Scandinavian Journal of Gastroenterology. 2002;37(6):719–724. doi: 10.1080/00365520212510. [DOI] [PubMed] [Google Scholar]
- 171.Beutler E., Felitti V. J., Koziol J. A., Ho N. J., Gelbart T. Penetrance of 845G → A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet. 2002;359(9302):211–218. doi: 10.1016/s0140-6736(02)07447-0. [DOI] [PubMed] [Google Scholar]
- 172.Allen K. J., Gurrin L. C., Constantine C. C., et al. Iron-overload-related disease in HFE hereditary hemochromatosis. New England Journal of Medicine. 2008;358(3):221–230. doi: 10.1056/nejmoa073286. [DOI] [PubMed] [Google Scholar]
- 173.Milman N. Hereditary haemochromatosis in Denmark 1950-1985. Clinical, biochemical and histological features in 179 patients and 13 preclinical cases. Danish Medical Bulletin. 1991;38(4):385–393. [PubMed] [Google Scholar]
- 174.Fargion S., Mandelli C., Piperno A., et al. Survival and prognostic factors in 212 Italian patients with genetic hemochromatosis. Hepatology. 1992;15(4):655–659. doi: 10.1002/hep.1840150417. [DOI] [PubMed] [Google Scholar]
- 175.Niederau C., Fischer R., Porschel A., Stremmel W., Haussinger D., Strohmeyer G. Long-term survival in patients with hereditary hemochromatosis. Gastroenterology. 1996;110(4):1107–1119. doi: 10.1053/gast.1996.v110.pm8613000. [DOI] [PubMed] [Google Scholar]
- 176.Moirand R., Adams P. C., Bicheler V., Brissot P., Deugnier Y. Clinical features of genetic hemochromatosis in women compared with men. Annals of Internal Medicine. 1997;127(2):105–110. doi: 10.7326/0003-4819-127-2-199707150-00002. [DOI] [PubMed] [Google Scholar]
- 177.Barton J. C., Barton N. H., Alford T. J. Diagnosis of hemochromatosis probands in a community hospital. American Journal of Medicine. 1997;103(6):498–503. doi: 10.1016/S0002-9343(97)00276-3. [DOI] [PubMed] [Google Scholar]
- 178.Edwards C. Q., Griffen L. M., Bulaj Z. P., Ajioka R. S., Kushner J. P. Estimate of the frequency of morbid complications of hemochromatosis. In: Barton J. C., Edwards C. Q., editors. Hemochromatosis. Genetics, Pathophysiology, Diagnosis and Treatment. chapter 29. Cambridge, UK: Cambridge University Press; 2000. pp. 315–317. [Google Scholar]
- 179.Åsberg A., Hveem K., Thorstensen K., et al. Screening for hemochromatosis: high prevalence and low morbidity in an unselected population of 65,238 persons. Scandinavian Journal of Gastroenterology. 2001;36(10):1108–1115. doi: 10.1080/003655201750422747. [DOI] [PubMed] [Google Scholar]
- 180.Powell L. W., Dixon J. L., Ramm G. A., et al. Screening for hemochromatosis in asymptomatic subjects with or without a family history. Archives of Internal Medicine. 2006;166(3):294–301. doi: 10.1001/archinte.166.3.294. [DOI] [PubMed] [Google Scholar]
- 181.Willis G., Wimperis J. Z., Lonsdale R., et al. Incidence of liver disease in people with HFE mutations. Gut. 2000;46(3):401–404. doi: 10.1136/gut.46.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Walters G. O., Miller F. M., Worwood M. Serum ferritin concentration and iron stores in normal subjects. Journal of Clinical Pathology. 1973;26(10):770–772. doi: 10.1136/jcp.26.10.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Barton J. C., Edwards C. Q., Phatak P. D., Britton R. S., Bacon B. R. Handbook of Iron Overload Disorders. chapter 5. Cambridge, UK: Cambridge University Press; 2010. Tests for hemochromatosis and iron overload; pp. 34–63. [DOI] [Google Scholar]
- 184.Phatak P. D., Barton J. C. Phlebotomy-mobilized iron as a surrogate for liver iron content in hemochromatosis patients. Hematology. 2003;8(6):429–432. doi: 10.1080/1024533032000158832. [DOI] [PubMed] [Google Scholar]
- 185.Brittenham G. M., Farrell D. E., Harris J. W., et al. Magnetic-susceptibility measurement of human iron stores. New England Journal of Medicine. 1982;307(27):1671–1675. doi: 10.1056/nejm198212303072703. [DOI] [PubMed] [Google Scholar]
- 186.Nielsen P., Engelhardt R., Düllmann J., Fischer R. Non-invasive liver iron quantification by SQUID-biosusceptometry and serum ferritin iron as new diagnostic parameters in hereditary hemochromatosis. Blood Cells, Molecules & Diseases. 2002;29(3):451–458. doi: 10.1006/bcmd.2002.0583. [DOI] [PubMed] [Google Scholar]
- 187.Fung E. B., Fischer R., Pakbaz Z., et al. The new SQUID biosusceptometer at Oakland: first year of experience. Neurology & Clinical Neurophysiology. 2004;2004:p. 5. [PubMed] [Google Scholar]
- 188.Bonkovsky H. L., Rubin R. B., Cable E. E., Davidoff A., Pels Rijcken T. H., Stark D. D. Hepatic iron concentration: noninvasive estimation by means of MR imaging techniques. Radiology. 1999;212(1):227–234. doi: 10.1148/radiology.212.1.r99jl35227. [DOI] [PubMed] [Google Scholar]
- 189.Skikne B. S., Flowers C. H., Cook J. D. Serum transferrin receptor: a quantitative measure of tissue iron deficiency. Blood. 1990;75(9):1870–1876. [PubMed] [Google Scholar]
- 190.Cook J. D., Flowers C. H., Skikne B. S. The quantitative assessment of body iron. Blood. 2003;101(9):3359–3364. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- 191.Fargion S., Dongiovanni P., Guzzo A., Colombo S., Valenti L., Fracanzani A. L. Iron and insulin resistance. Alimentary Pharmacology and Therapeutics, Supplement. 2005;22(2):61–63. doi: 10.1111/j.1365-2036.2005.02599.x. [DOI] [PubMed] [Google Scholar]
- 192.Simcox J. A., Mitchell T. C., Gao Y., et al. Dietary iron controls circadian hepatic glucose metabolism through heme synthesis. Diabetes. 2015;64(4):1108–1119. doi: 10.2337/db14-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Van Woudenbergh G. J., Kuijsten A., Tigcheler B., et al. Meat consumption and its association with C-reactive protein and incident type 2 diabetes: The Rotterdam Study. Diabetes Care. 2012;35(7):1499–1505. doi: 10.2337/dc11-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Feskens E. J. M., Sluik D., Van Woudenbergh G. J. Meat consumption, diabetes, and its complications. Current Diabetes Reports. 2013;13(2):298–306. doi: 10.1007/s11892-013-0365-0. [DOI] [PubMed] [Google Scholar]
- 195.Cooksey R. C., Jones D., Gabrielsen S., et al. Dietary iron restriction or iron chelation protects from diabetes and loss of β-cell function in the obese (ob/ob lep−/−) mouse. American Journal of Physiology—Endocrinology and Metabolism. 2010;298(6):E1236–E1243. doi: 10.1152/ajpendo.00022.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tajima S., Ikeda Y., Sawada K., et al. Iron reduction by deferoxamine leads to amelioration of adiposity via the regulation of oxidative stress and inflammation in obese and type 2 diabetes KKAy mice. American Journal of Physiology—Endocrinology and Metabolism. 2012;302(1):E77–E86. doi: 10.1152/ajpendo.00033.2011. [DOI] [PubMed] [Google Scholar]
- 197.Vecchi C., Montosi G., Garuti C., et al. Gluconeogenic signals regulate iron homeostasis via hepcidin in mice. Gastroenterology. 2014;146(4):1060–1069. doi: 10.1053/j.gastro.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Huang J., Jones D., Luo B., et al. Iron overload and diabetes risk: a shift from glucose to fatty acid oxidation and increased hepatic glucose production in a mouse model of hereditary hemochromatosis. Diabetes. 2011;60(1):80–87. doi: 10.2337/db10-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Meli R., Raso G. M., Irace C., et al. High fat diet induces liver steatosis and early dysregulation of iron metabolism in rats. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0066570.e66570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Davis R. J., Corvera S., Czech M. P. Insulin stimulates cellular iron uptake and causes the redistribution of intracellular transferrin receptors to the plasma membrane. Journal of Biological Chemistry. 1986;261(19):8708–8711. [PubMed] [Google Scholar]
- 201.Moreno-Navarrete J. M., Novelle M. G., Catalán V., et al. Insulin resistance modulates iron-related proteins in adipose tissue. Diabetes Care. 2014;37(4):1092–1100. doi: 10.2337/dc13-1602. [DOI] [PubMed] [Google Scholar]
- 202.Gao Y., Li Z., Scott Gabrielsen J., et al. Adipocyte iron regulates leptin and food intake. Journal of Clinical Investigation. 2015;125(9):3681–3691. doi: 10.1172/JCI81860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gabrielsen J. S., Gao Y., Simcox J. A., et al. Adipocyte iron regulates adiponectin and insulin sensitivity. Journal of Clinical Investigation. 2012;122(10):3529–3540. doi: 10.1172/JCI44421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wlazlo N., Van Greevenbroek M. M. J., Ferreira I., et al. Iron metabolism is associated with adipocyte insulin resistance and plasma adiponectin: the Cohort on Diabetes and Atherosclerosis Maastricht (CODAM) study. Diabetes Care. 2013;36(2):309–315. doi: 10.2337/dc12-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fernández-Real J. M., Moreno J. M., Chico B., López-Bermejo A., Ricart W. Circulating visfatin is associated with parameters of iron metabolism in subjects with altered glucose tolerance. Diabetes Care. 2007;30(3):616–621. doi: 10.2337/dc06-1581. [DOI] [PubMed] [Google Scholar]
- 206.Arredondo M., Jorquera D., Carrasco E., Albala C., Hertrampf E. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with iron status in persons with type 2 diabetes mellitus. American Journal of Clinical Nutrition. 2007;86(5):1347–1353. doi: 10.1093/ajcn/86.5.1347. [DOI] [PubMed] [Google Scholar]
- 207.Van Campenhout A., Van Campenhout C., Lagrou A. R., et al. Impact of diabetes mellitus on the relationships between iron-, inflammatory- and oxidative stress status. Diabetes/Metabolism Research and Reviews. 2006;22(6):444–454. doi: 10.1002/dmrr.635. [DOI] [PubMed] [Google Scholar]


