Abstract
The efficiency of drug research and development has paradoxically declined over the last decades despite major scientific and technological advances, promoting new cost-effective strategies such as drug repositioning by systematic screening for new actions of known drugs. Here, we performed a screening for positive allosteric modulators (PAMs) at melanocortin (MC) receptors. The non-steroidal anti-inflammatory drug fenoprofen, but not the similar compound ibuprofen, presented PAM activity at MC3, MC4, and MC5 receptors. In a model of inflammatory arthritis, fenoprofen afforded potent inhibition while ibuprofen was nearly inactive. Fenoprofen presented anti-arthritic actions on cartilage integrity and synovitis, effects markedly attenuated in Mc3r−/− mice. Fenoprofen displayed pro-resolving properties promoting macrophage phagocytosis and efferocytosis, independently of cyclooxygenase inhibition. In conclusion, combining repositioning with advances in G-protein coupled receptor biology (allosterism) may lead to potential new therapeutics. In addition, MC3 PAMs emerged as a viable approach to the development of innovative therapeutics for joint diseases.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-016-2419-3) contains supplementary material, which is available to authorized users.
Keywords: Inflammation, Arthritis, GPCR, Drug repositioning, Resolution of inflammation
Introduction
In recent years, we have witnessed a steady and progressive decline in productivity of the pharmaceutical industry despite increased investments in R&D, leading to absence of medicines active against the diseases of modern societies [38]. To obviate to this financial and medical problem, drug repositioning has emerged as a new business model to reduce developing timeframe, costs, and improve success rates. Identification of new indications for old drugs (typically generics or drugs that failed approval for reasons others than safety) has proven to be a feasible approach as demonstrated, for instance, by the recent successful repositioning of thalidomide and sildenafil [31]. Compound libraries containing approved drugs offer an opportunity for systematic screening for potential new uses of existing drugs, while simultaneously identifying novel candidates, as it is accepted that drug repositioning by itself will not be sustainable as sole development model for the medicines required by western societies. However, merely by including known molecules in drug screenings might not represent a substantial improvement in success rate as the complexity of disease mechanisms and still limited understanding of whole biological systems (compared to one-cell-at-a-time assays) are behind the unexpected lack of efficacy of promising drugs when tested in phase II and III human trials.
The field of G-protein coupled receptors (GPCR), considered one of the largest classes for drug discovery, has experienced a significant progress in recent years leading to innovative approaches for the pharmacological exploitation of these receptors including identification of biased ligands and allosteric modulators. One of these promising targets in the area of inflammation research is the melanocortin (MC) receptors. Pharmacological strategies for targeting the MC system are emerging as viable therapeutic approaches for a number of diseases, including obesity, cachexia, vitiligo, sexual dysfunction, dermatitis, and gouty arthritis [26]. Establishing receptor predominance in a given condition, out of the five receptors identified (MC1–MC5), together with development of selective agonists, is of paramount importance to avoid off-target side effects. However, two decades of extensive search for selective molecules using classical orthosteric ligand approaches did not deliver any successful drug. Contrasting with this lack of lead compounds, there is a renewed interest in targeting the MC system derived from the appreciation of a non-cortisol-mediated properties of the MC peptide ACTH which involve activation of peripheral MC receptors, including MC3 [12, 26]. Activation of MC3 by its endogenous ligands, ACTH and melanocyte-stimulating hormone (MSH), attenuates inflammatory joint diseases in models mimicking rheumatoid arthritis (RA) and gout. Treatment of arthritic mice with the synthetic peptides DTrp8-γMSH and AP214 reduces disease severity in the K/BxN serum transfer model [29, 30, 34], while the natural peptides αMSH, γ2MSH, and MT-II (a stable derivative of αMSH) inhibit urate crystal inflammation [4, 11]. Notwithstanding this, the difficulty in achieving selective agonism has been hampering full harnessing of MC biology for innovative drug discovery programmes until now.
A promising strategy to attain selective activation of closely related G-protein coupled receptors (GPCR) is based on the development of allosteric ligands [1, 25, 33]. In contrast to orthosteric sites, where the natural ligands bind, allosteric sites (i.e., sites spatially distinct from endogenous ligands binding sites) display a higher degree of sequence divergence, providing new opportunities for the identification of selective compounds [9, 42]. Positive allosteric modulators (PAMs) or allosteric enhancers, potentiate receptor signaling in the presence of the endogenous ligand thus maintaining both temporal and spatial control, and may be burdened by fewer side effects in view of their likely higher degree of selectivity [9, 42]. Recently, Pantel et al. performed a screening on potential PAMs at MC4 to identify novel drugs for obesity with reduced side-effects profile, yielding 62 positive modulators [33].
We screened, here, a library of known small molecules for PAM activity at MC3, identifying the marketed drug fenoprofen (Fenopron®, UK, Nalfon®, US) as a positive hit. Long known for its anti-arthritic activities and efficacy in RA and osteoarthritis (OA) [17] fenoprofen is a non-steroidal anti-inflammatory drug (NSAID) and inhibits cyclooxygenase (COX) but as stated in the label, “its exact mode of action is unknown”. Here, we identify a new skill of fenoprofen (fundamentally distinct from its known COX-related mode of action) that may lead to further functional studies as a unique tool compound, and perhaps reconsider its use in conditions where the MC system has a substantial role. In addition, our study reveals an important contribution of MC3 in the therapeutic actions of fenoprofen in arthritis, providing proof of concept support to the allosteric enhancing mode of targeting the MC system for the treatment of inflammatory diseases.
Results
Fenoprofen is a positive allosteric modulator at the MC3 receptor
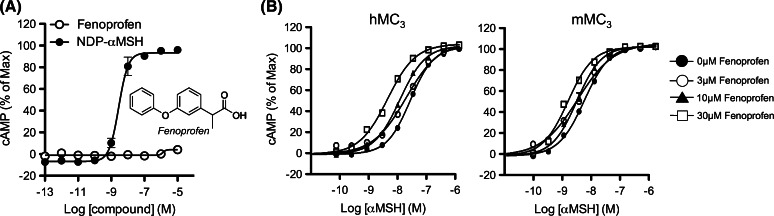
Screening on a commercial library of known pharmacologically active molecules (>1400 compounds) using CHO cells stably expressing MC3 was run, using both an “agonist mode” (cAMP production) and a “PAM mode” (enhancement of cAMP production in the presence of a suboptimal (~EC20) concentration of αMSH). Among the most potent positive hits, the marketed NSAID fenoprofen emerged as a PAM at human MC3. Fenoprofen did not induce cAMP production when tested alone (Fig. 1a) but induced a leftward-shift of the αMSH concentration response in both human and mouse receptors (Fig. 1b). Fenoprofen was not agonist at other MCRs (Supplementary Figure S1) but displayed PAM activity at human MC3, MC4, and MC5 as well as mouse MC3, when αMSH, ACTH, and Lys-γ3MSH were used as endogenous ligands (Table 1), indicating that the PAM activity of fenoprofen was in general terms non-receptor and non-probe selective.
Fig. 1.
Allosteric modulatory activity of fenoprofen at the human MC3 receptor. cAMP production was studied in human MC3 transfected CHO-K1 cells (GeneBLAzer® beta-lactamase Reporter Technology). Fenoprofen was tested for agonistic activity (a, agonist mode) or in the presence of the agonist αMSH to address PAM activity in human and mouse receptors (b, PAM mode)
Table 1.
Positive allosteric modulator activity of fenoprofen at melanocortin receptors
| Drug | Receptor | |||||||
|---|---|---|---|---|---|---|---|---|
| mMC3 | hMC(3*) | hMC4 | hMC5 | |||||
| NDP-αMSH | pEC50 | Log shift | pEC50 | Log shift | pEC50 | Log shift | pEC50 | Log shift |
| +0 μM Fen | 9.78 | 9.40 | 10.49 | 8.98 | ||||
| +3 μM Fen | 9.86 | 0.08 | 9.51 | 0.11 | – | 8.97 | −0.01 | |
| +10 μM Fen | 9.76 | −0.02 | 9.58 | 0.19 | 10.47 | −0.02 | 9.06 | 0.08 |
| +30 μM Fen | 9.71 | −0.07 | 9.66 | 0.26 | 10.38 | −0.11 | 8.99 | 0.01 |
| Drug | Receptor | |||||||
|---|---|---|---|---|---|---|---|---|
| mMC(3*) | hMC(3*) | hMC(4*) | hMC(5*) | |||||
| αMSH | pEC50 | Log shift | pEC50 | Log shift | pEC50 | Log shift | pEC50 | Log shift |
| +0 μM Fen | 8.33 | 7.58 | 8.38 | 6.64 | ||||
| +3 μM Fen | 8.42 | 0.10 | 7.67 | 0.09 | – | 6.75 | 0.11 | |
| +10 μM Fen | 8.58 | 0.25 | 7.92 | 0.34 | 8.80 | 0.41 | 6.89 | 0.25 |
| +30 μM Fen | 8.86 | 0.53 | 8.30 | 0.71 | 9.11 | 0.73 | 7.22 | 0.58 |
| Drug | Receptor | |||||||
|---|---|---|---|---|---|---|---|---|
| mMC(3*) | hMC(3*) | hMC(4*) | hMC(5*) | |||||
| ACTH | pEC50 | Log shift | pEC50 | Log shift | pEC50 | Log shift | pEC50 | Log shift |
| +0 μM Fen | 8.69 | 8.19 | 8.76 | 6.78 | ||||
| +3 μM Fen | 8.72 | 0.04 | 8.29 | 0.10 | – | 6.84 | 0.06 | |
| +10 μM Fen | 8.85 | 0.16 | 8.57 | 0.38 | 9.09 | 0.33 | 7.03 | 0.25 |
| +30 μM Fen | 9.03 | 0.34 | 8.87 | 0.68 | 9.25 | 0.48 | 7.29 | 0.51 |
| Drug | Receptor | |||||||
|---|---|---|---|---|---|---|---|---|
| mMC3 | hMC(3*) | hMC(4*) | hMC(5*) | |||||
| Lys-γ3MSH | pEC50 | Log shift | pEC50 | Log shift | pEC50 | Log shift | pEC50 | Log shift |
| +0 μM Fen | 9.19 | 8.80 | 7.33 | 6.13 | ||||
| +3 μM Fen | 9.24 | 0.05 | 8.89 | 0.09 | – | 6.31 | 0.18 | |
| +10 μM Fen | 9.30 | 0.11 | 9.09 | 0.29 | 7.48 | 0.14 | 6.61 | 0.48 |
| +30 μM Fen | 9.27 | 0.08 | 9.35 | 0.55 | 7.72 | 0.39 | 6.80 | 0.67 |
Leftward log shift of the peptide ligand and EC50 values are reported at murine MC3 and human MC3,4,5 receptors (*indicates PAM activity)
Next, we addressed if MC receptor PAM activity was a common feature of other COX inhibitors. We focused on the NSAID family of propionic acid derivatives to which fenoprofen belongs [17]. Of the seven COX inhibitors tested, including ibuprofen, none potentiated melanocortin-induced cAMP production (Supplementary Table S1 and Supplementary Figure S2), indicating that the structural and physicochemical requirements for COX inhibition do not translate to MC3 PAM activity, with fenoprofen being unique in its class for this bioaction.
MC3 mediates the anti-arthritic actions of fenoprofen
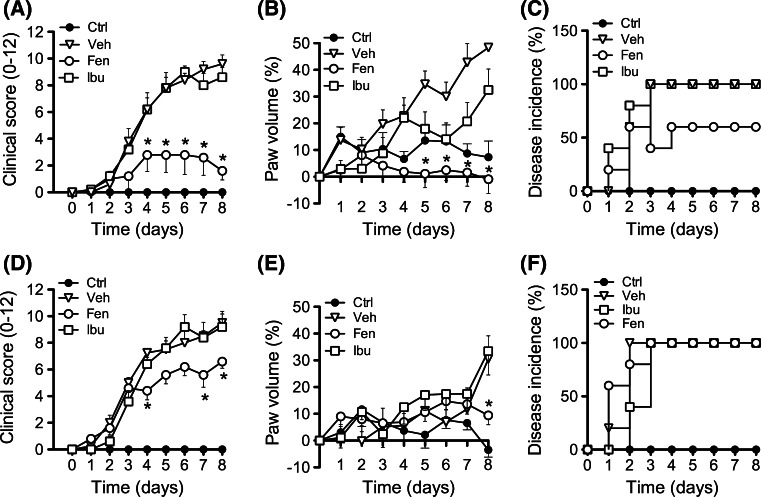
Since MC3 is emerging as a crucial receptor in controlling the severity of inflammatory arthritis, we next studied its potential involvement in the anti-arthritic actions of fenoprofen. The K/BxN serum transfer model of arthritis was induced in both wild type (WT) and MC3 deficient mice (Mc3r−/−). Arthritis developed normally in both strains acquiring moderate to severe disease at days 4–5 (Fig. 2). Pharmacological treatment of mice with fenoprofen or ibuprofen (10 mg/kg twice per day from day 2; dose selected from a pilot peritonitis—see Supplementary Figure S3) revealed distinct modulation of the disease. While fenoprofen had a significant impact on clinical score, with 80% reduction at day 8 (Fig. 2a) and paw volume (~100% reduction; Fig. 2b), ibuprofen was much less effective affording only a moderate response on edema with no effect on clinical score. Fenoprofen, but not ibuprofen, also reduced disease incidence (Fig. 2c). A large majority of the inhibitory properties of fenoprofen was lost in Mc3r−/− mice (Fig. 2d, e), with residual effectiveness of ~30% both for clinical score and paw volume. All Mc3r−/− mice treated with fenoprofen developed arthritis (Fig. 2f). Identical dosing with Ibuprofen was not particularly effective in this model, resulting in effects ranging from 5 to 30% inhibition for the majority of parameters tested. Therefore, absence of Mc3r brings fenoprofen efficacy comparable to that evoked by ibuprofen.
Fig. 2.
Antiarthritic effect of fenoprofen in the K/BxN serum transfer model. Arthritis was induced on wild type (a–c) and Mc3r−/− (d–f) mice by two i.p. injections of arthritogenic serum on day 0 and 2. Pharmacological treatments (fenoprofen, Fen, and ibuprofen, Ibu: 10 mg/kg; vehicle: PBS) were administered i.p. twice daily from day 2. Non-arthritic mice were used as controls (Ctrl). Clinical score (a, d), paw volume (b,e) and disease incidence (c,f) were recorded over 8 days. Data are mean ± SEM of n = 5; *p < 0.05 ANOVA followed by Bonferroni multiple comparison test
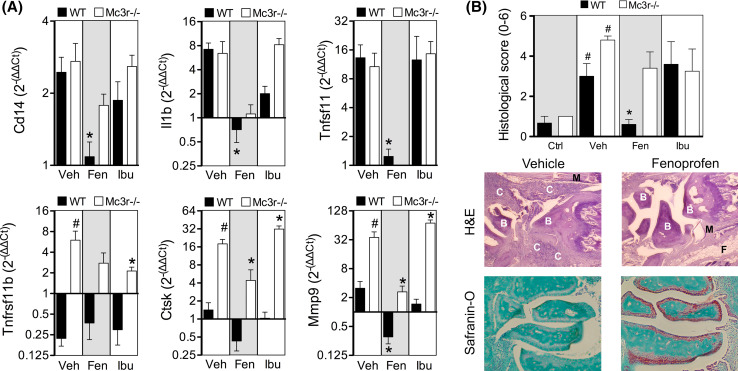
Gene expression analyses demonstrated a selective anti-inflammatory action of fenoprofen with marked reduction on monocyte marker Cd14, interleukin 1β (Il1b), RANK ligand (Tnfsf11), cathepsin K (Ctsk), and metalloproteinase 9 (Mmp9) (Fig. 3a). Ibuprofen mildly reduced Il1b expression albeit not significantly. Consistent with the known effect of MC3 agonism on cell recruitment [14, 30], the effect on Cd14 was absent in Mc3r−/− mice suggesting that endogenous MC3 mediated this particular action of fenoprofen. Interestingly, elevated levels of osteoprotegerin (Tnfrsf11b), Ctsk, and Mmp9 in Mc3r−/− vehicle-treated mice compared to WT suggest a defective bone metabolism in mice lacking this receptor. Histological analyses accounting for cell infiltration (H&E) and cartilage integrity (safranin-O) (see Supplementary Figure S4 for scoring criteria) reiterated the potent inhibitory actions of fenoprofen in the joints of WT but not in Mc3r−/− mice (Fig. 3b). Ibuprofen did not exert tissue protective actions.
Fig. 3.
Gene expression and histological changes in arthritic joints. a Gene expression was analyzed in ankles from wild type (WT, black bars) and Mc3r−/− (white bars) mice as collected at day 8, and expressed as fold change with respect to non-arthritic control mouse joints. Groups correspond to vehicle (Veh), fenoprofen 10 mg/kg (Fen), and ibuprofen 10 mg/kg (Ibu). Data are mean ± SEM of n = 5; *p < 0.05 two-way ANOVA followed by Bonferroni multiple comparison test of veh vs. drugs, # p < 0.05 WT vs. Mc3r−/−. b Ankle sections (4 µm) were stained with hematoxylin and eosin (H&E) and fast green and safranin-O. Sections were graded from 0 (no disease) to 3 (severe) based on the degree of synovitis (purple staining in the H&E sections) and cartilage erosion (loss of red coloration in the safranin-O sections). C cell infiltrate, B bone, M muscle, F fat. The sum of H&E and safranin-O scores are represented in the left graph. Data are mean ± SEM of n = 5; p < 0.05 drug vs. Veh (*), or Veh vs. Ctrl (#) ANOVA followed by Bonferroni multiple comparison test
These results indicate an important contribution of MC3 to the anti-arthritic actions of fenoprofen in vivo.
Local increase of endogenous melanocortin peptides at inflammatory sites
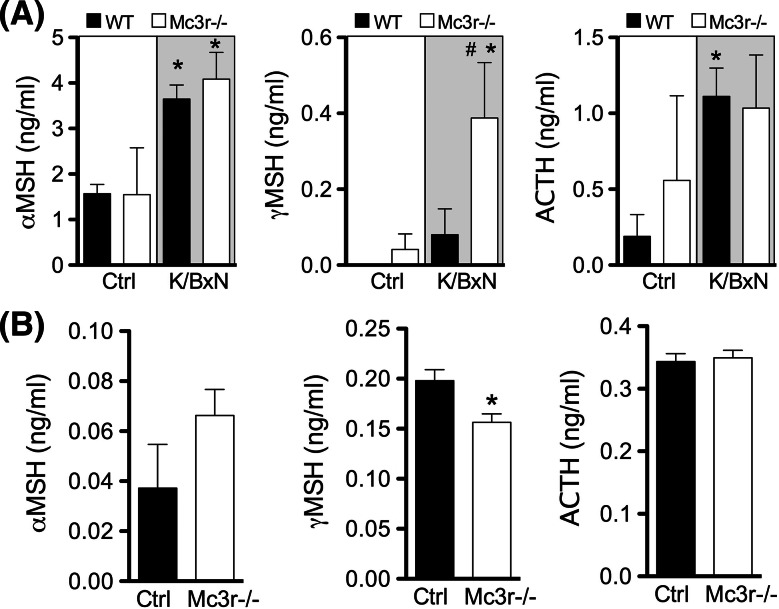
Allosteric enhancers exert their biological effects only in the presence of the endogenous agonist. Thus, we measured levels of melanocortin peptides in arthritic (day 8) and naïve joints. Interestingly, αMSH and ACTH were significantly increased in the inflamed joints of arthritic mice compared to non-arthritic mice (Fig. 4a), while plasma levels remained stable with values within the 3–5 ng/ml range (Supplementary Figure S5). γMSH levels were very low or not detectable in control joints. The elevated levels of melanocortin peptides measured in arthritic joints provide mechanistic support to the PAM efficacy of fenoprofen. Moreover, measurements in Mc3r−/− tissues demonstrated elevated values for γMSH suggesting (1) the existence of a negative loop between receptor expression and its high-affinity natural ligand and (2) that the lack of effect of fenoprofen in this genotype was not secondary to inadequate production of the endogenous ligands.
Fig. 4.
Production of endogenous melanocortin peptides in arthritic mouse joints and primary macrophages. a Wrists joints from K/BxN arthritic mice (wild type and Mc3r−/−) were collected at day 8 and protein extracts prepared on RIPA buffer. The melanocortin peptides αMSH, γMSH, and ACTH were determined by enzyme immunoassay following manufacturers’ protocol. b Biogel-elicited macrophages were collected from mice (wild type and Mc3r−/−) 4 days after an i.p. injection with 1 ml of 2% biogel. Cells were cultured in vitro during 24 h in 10% FCS-RPMI. Supernatants were analyzed for αMSH, γMSH, and ACTH levels by enzyme immunoassay. Data are mean ± SEM of n = 5; p < 0.05 ANOVA followed by Bonferroni multiple comparison test K/BxN vs. Ctrl (*) or WT vs. Mc3r−/− (#)
Fenoprofen exerts pro-resolving actions via MC3
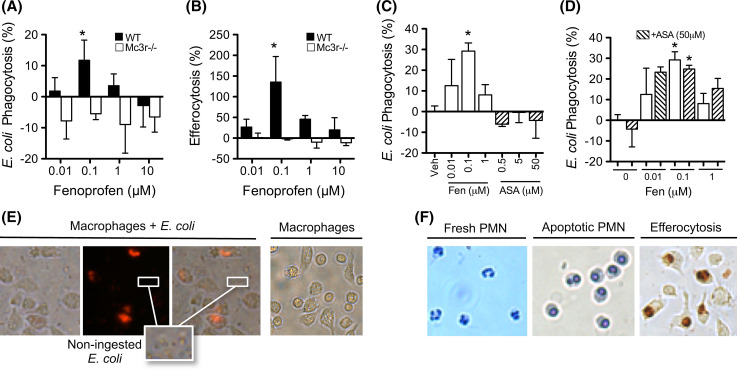
We next studied if fenoprofen could produce known MC3-mediated effects, such as increased phagocytosis by murine peritoneal macrophages [30]. The latter cell type provides a good model system for studying the effects of MC3 PAMs since these cells produce endogenous peptides (Fig. 4b) and express the melanocortin receptors MC1,3,5 [30]. As reported in Fig. 4, fenoprofen promoted phagocytosis of bacteria (Fig. 5a, e) and apoptotic neutrophils (Fig. 5b, f), the latter process often termed efferocytosis. The potentiation of phagocytosis by fenoprofen was marked and followed a bell-shaped curve, commonly observed for melanocortin activation [13, 14], with 0.1 μM being the most active concentration. Of interest fenoprofen was inactive when Mc3r−/− cells were used (Fig. 5a, b), although Mc3r−/− cells in vitro produced similar levels of endogenous agonists (Fig. 4b).
Fig. 5.
Pro-phagocytic actions of fenoprofen. Biogel-elicited peritoneal macrophages from wild type (WT) and Mc3r−/− mice were incubated with: a pHrodo™ Red E. coli for 20 min and analyzed by flow cytometry, or b apoptotic human neutrophils for 1 h and analyzed by myeloperoxidase (MPO) staining and cell counting. c Fenoprofen (Fen) and acetylsalicylic acid (ASA) were added 30 min prior addition of E. coli. d ASA was added 20 min before fenoprofen, and E. coli was added after 30 min incubation with fenoprofen. Images in e show that fluorescence develops specifically on ingested bacteria. Images in f show neutrophils (dark brown MPO staining) ingested by macrophages (efferocytosis). Data are mean ± SEM of n = 3; *p < 0.05 ANOVA followed by Bonferroni multiple comparison test
To exclude COX inhibition as part of the pro-phagocytic actions of fenoprofen, aspirin (ASA) was chosen as a control for the next study as it inhibits COX irreversibly and it is chemically unrelated to fenoprofen. While the pro-phagocytic and pro-efferocytic effect of fenoprofen was confirmed, ASA did not have any effect on phagocytosis (Fig. 5c). In a subsequent set of experiments, the pro-phagocytic effects of fenoprofen were assayed 20 min after pre-incubation with ASA (50 μM) to irreversibly block COX enzymes. As shown in Fig. 5d, the pro-phagocytic effect of fenoprofen was retained even in experimental settings with cells pre-treated with ASA.
These results, together with the experiment conducted with Mc3r−/− cells (Fig. 5a, b), indicate that this receptor genuinely mediates specific pro-resolving actions of fenoprofen.
Discussion
The so-called Eroom’s law [38] refers to the decline in pharmaceutical R&D efficiency despite marked improvements in technology, where the cost of developing a new drug roughly doubles every 9 years. However, some estimates suggests the cost of repositioning a drug may drop from $2–3 billion to $300 million, leading to an increase in new companies entirely focused on this strategy [31]. Drug repositioning is proven attainable as several drugs have already been repurposed—e.g., the antihypertensive minoxidil for hair loss, or the antimalarial hydroxychloroquine for arthritis—although in many cases these novel indications are discovered by serendipity. However, the advance strategy resides in incorporating repositioning as an active systematic approach to drug discovery, for instance, by including known drugs in compound screening programmes.
Targeting the melanocortin system is emerging as a potential therapeutic approach to treat a variety of inflammatory conditions. This approach is corroborated not only by the large amount of scientific literature [5, 15, 32, 40], but also by the clinical evidence obtained from the use of ACTH in clinical practice since its approval in 1952. This natural melanocortin peptide has been used to treat gouty arthritis for more than 60 years and it is currently included in the ACR guidelines for the treatment of acute attacks of this disease of the joints [22, 36]. Acthar® Gel and Synacthen Depot® are the formulations currently available and conditions such as RA, multiple sclerosis, inflammatory bowel disease, and dermatitis fall within their FDA- and EMA-approved indications [26]. With the exception of gouty arthritis and/or specific regions using clinical evidence-based practice, ACTH is otherwise seldom used. The main reason for its limited use in patients is related to the persistent activation of MC2, which leads to the excessive production of cortisol with the instauration, over time, of substantial side effects.
However, the development of new MC drugs as an anti-inflammatory strategy is experiencing a renewed interest due to the discovery of MC3 activation as a crucial pathway mediating the anti-arthritic actions of ACTH. This “ACTH renaissance” applies to conditions like multiple sclerosis [2, 37], proteinuric nephropathies [16], and lupus [24], where the anti-inflammatory properties of ACTH might also be mediated through the peripheral, or non-adrenal, MC system. On the other hand, lack of receptor selectivity achieved by currently developed MC molecules may limit their clinical development.
While challenging, the complexity of GPCR biology represents a source of innovation in drug discovery. For example, we recently characterized a small molecule that activated only a subset of signaling cascades associated with MC3 (a process known as GPCR-biased signaling) with the functional consequences of retaining full anti-inflammatory effect while preventing the unwanted melanogenic effects [28]. In the present work, we exploit the existence of GPCR-allosterism as an opportunity to achieve selectivity among highly conserved receptor families, as it is the case within the MC system. Two reports have already identified positive and negative allosteric modulators to MC4 and MC5, respectively [18, 33]. In more general terms, the utility of PAMs as a therapeutic strategy in the clinic is exemplified by diazepam (Valium®), which potentiates the activity of GABA.
In our study, we performed a compound screening on a commercially available library aiming to identify novel agonists and/or PAMs of the human MC3. In this way, we integrated a drug repositioning approach (using a library containing known drugs) with the exploitation of novel capabilities of GPCRs like allosteric enhancing. Intriguingly, the anti-inflammatory drug fenoprofen, a COX inhibitor with high efficacy in RA, was identified as a potent hit. This PAM activity was, however, not specific for MC3, as the potency of αMSH, ACTH, and Lys-γ3MSH in MC4 and MC5 transfected cells was also increased. Although fenoprofen did not display receptor selectivity, as a PAMs it could offer a second wave of functional selectivity by acting only when endogenous ligands are expressed. From our perspective, the novel aspect of our findings resides in the fact that a drug with indication for RA and OA might be engaging the melanocortin system, providing proof-of-concept for MC3 targeting as a treatment of joint diseases.
A growing body of evidence supports the role of melanocortins and MC3 in joint inflammation. Melanocortin drugs can reduce cytokine levels, prevent immune cell infiltration, reduce joint inflammation, modulate nitric oxide production, and promote pro-resolving actions like efferocytosis [11, 30, 34, 41]. Many of these parameters were also affected by fenoprofen when tested in experimental arthritis. The target cells of MC agonists, relevant in the context of inflammation, are immune cells such as macrophages [4] and neutrophils [6] as well as non-immune cells in the joint. For example, MC3 is particularly important for osteoclast biology, as these cells show increased resorptive activity when derived from Mc3r−/− macrophages compared to WT cells [34]. Osteoblasts can also be targeted by melanocortins as shown for ACTH [43], while chondrocyte anabolic activity can be stimulated by MC agonists [20, 21], counteracting the detrimental effects of pro-inflammatory cytokines.
We provide evidence for this fundamental role of MC3 with further in vivo and in vitro experiments. In the model of K/BxN serum transfer arthritis [10], at 10 mg/kg daily, fenoprofen reduced clinical score by 80% in WT mice, while this effect was markedly attenuated in animals lacking MC3. Under these conditions, fenoprofen reduced disease incidence to 60% compared to 100% in vehicle-treated mice, an effect only observed in WT mice. These results suggest an important contribution of the receptor MC3 in the anti-arthritic actions of fenoprofen. We note here that, among the propionic acid COX inhibitors, fenoprofen is the only one associated with body weight loss, at least upon prolonged administration [17]. We reason this effect can be secondary to the MC4 activation we describe here: this compound—likely to cross the blood brain barrier—might activate MC4 in multiple brain regions to reduce appetite [3]. Another adverse reaction reported for fenoprofen is increased sweating (~5% incidence), which would be consistent with MC5 activation on exocrine glands [19].
Presence of endogenous MC peptides in the arthritic joints of K/BxN serum-treated mice is remarkable since fenoprofen allosteric enhancing activity would manifest only in their presence. At peak of arthritis, it was important to quantify a selective increase in ACTH and αMSH levels in the joints, with no concomitant changes in blood levels. Of note, it has been documented that endogenous MC peptides levels are increased in human synovial fluid from RA patients [7].
It was then crucial to compare the contribution of COX inhibition to the PAM activity of fenoprofen; hence, we tested the ability of a panel of propionic acid derivative NSAIDs to left-shift the cAMP concentration response curve. None of the drugs tested, apart from fenoprofen and its calcium salt, displayed PAM activity. This suggests on one hand, the existence of specific structural and conformational requirements for a small molecule MC3 PAM and, on the other hand, no associated requirement for COX inhibition to achieve this specific enhancing property.
In the final part of the study, we determined if fenoprofen could evoke MC3-dependent pro-resolving functions [35, 39], properties described for the melanocortin pan-agonist AP214 [30]. The pro-phagocytic and pro-efferocytic actions of fenoprofen were absent when tested with macrophages from Mc3r−/− mice. In these controlled settings aspirin and ibuprofen were inactive providing strong indication that these biological effects are unrelated to COX inhibition.
An important control that could not be run was the assessment of the contribution of COX inhibition to the anti-arthritic effects of fenoprofen because in the K/BxN serum-induced model of arthritis, mice lacking the COX-1 isoform do not develop disease [8], whereas selective COX-2 inhibitors are inactive. However, the in vitro phagocytosis assays allowed us to address this issue, since aspirin was ineffective on its own and did not affect the pro-phagocytic properties of fenoprofen.
In conclusion, MC receptors represent novel targets for the development of innovative therapies for RA and other inflammatory diseases: targeting MC3 using PAMs constitutes a viable and biologically effective means to reduce synovial inflammation. This work also provides the first link between MC3 targeting and anti-arthritic efficacy in man: fenoprofen, in contrast to other propionic acid derivatives, is particularly effective in RA and OA and here we show that activity at MC receptors, only achieved by this molecule, might explain this therapeutic advantage over other NSAIDs. Our approach also reflects the potential of repositioning screening strategies, to rediscover new mechanisms and actions for old drugs. These results may pave the way for further identification and development of new MC3 allosteric enhancers, and possibly also for MC1 and/or MC5, as discussed above, to modulate host inflammatory responses in chronic pathologies.
Materials and methods
Compound library screening
The compound screening was performed on the commercially available library NINDS (National Institute of Neurological Disorders and Stroke) containing >1400 drugs using a single concentration of test compound (10 μM). The HTRF® cAMP assay (Cisbio Bioassays, Codolet, France) was performed on CHO cells stably expressing the human MC3 (GeneBLAzer® beta-lactamase Reporter Technology, Invitrogen, Paisley, UK) according to manufacturer’s protocol. Human MC1, MC4, MC5 (SNAP-tag Taglite® Technology, HEK293 cells; Cisbio Bioassays), human MC2 (GeneBLAzer®, CHO cells) and mouse MC3 (FLAG-tag, HEK293 cells; Genecopoeia, Source BioScience, Nottingham, UK) were used to study selectivity and ortholog activation. Drugs were tested for agonistic activity, in which the HTRF cAMP accumulation assay was performed with 10-point 0.5 log serial dilutions (0.316–10 μM) and for their ability to potentiate the effect of the agonist Lys-γ3MSH (Cambridge Biosciences, Cambridge, UK) used at EC20. Leftwad-shifts assays were then conducted on positive hits using a concentration response curve of Lys-γ3MSH (0.01–1 μM) in the absence or presence (0.1–100 μM) of test compound.
Animals
7–8 weeks old, male, C57BL/6J wild-type (WT) mice were purchased from Charles River (Kent, UK). Mc3r−/− mice were a generous gift of Dr Chen (Merck Laboratories). All animal studies were approved and performed under the guidelines of the Ethical Committee for the Use of Animals, Barts and The London School of Medicine and Home Office Regulations (Guidance on the Operation of Animals, Scientific Procedures Act, 1986).
Zymosan-induced peritonitis
Peritonitis was induced by the injection of 1 mg zymosan A (Sigma-Aldrich, Poole, UK) i.p. in 0.5 ml sterile PBS. Animals (4 per group) were pre-treated with fenoprofen or vehicle (PBS) administered i.p. 30 min before zymosan injection. Four hours later, mice were killed by CO2 exposure and peritoneal cavities washed with 4 ml of ice cold PBS containing 3 mM EDTA and 25U/ml heparin. 100 µl aliquots of lavage fluids were stained with Turk’s solution (0.01% crystal violet in 3% acetic acid) and cells counted on a Neubauer haemocytometer or stained with conjugated antibodies for Ly-6G (FITC) and F4/80 (APC) used in 1:100 dilution. Corresponding isotype controls and blocking antibody anti-mouse CD16/32 were used. All antibodies were purchased from eBioscience (Hatfield, UK). Flow cytometry was performed in the BD FACSCalibur™.
K/BxN serum transfer arthritis model
Arthritis was induced with two i.p. injections of 100 μl of K/BxN serum on days 0 and 2. Disease was monitored daily until day 8 by assessing the paw volume using a plethysmometer (Ugo Basile, Comerio, Italy), disease incidence (mice showing any signs of arthritis) and clinical score (score per paw: 0 = no signs of inflammation; 1 = subtle inflammation, localized; 2 = easily identified inflammation but localized; 3 = evident inflammation, not localized; max score = 12 per mouse) [23]. Pharmacological treatments were administered i.p. twice daily from day 2 until the end of the experiment. Fenoprofen calcium salt hydrate and ibuprofen sodium salt were obtained from Sigma-Aldrich (Poole, UK).
RNA extraction, cDNA synthesis, and real time-PCR
Total RNA was extracted using TRIzol (Invitrogen, Paisley, UK) following manufacturer’s instructions. cDNA was synthesized using 1ug of RNA with the SuperScript III Reverse Transcriptase (Invitrogen, Paisley, UK). Real time-PCR was performed in duplicates, with 2 μl cDNA diluted 1/5, 1 μl primers and Power SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK), using the ABI Prism 7900HT Sequence Detection System. Quantitect primers (QIAGEN, Crawley, UK) used are the following: Hprt (QT00166768), Cd14 (QT00246190), Il1b (QT01048355), Tnfsf11 (QT00147385), Tnfrsf11b (QT00106757), Ctsk (QT00150703) and Mmp9 (QT00108815). Dissociation step was always included to confirm the absence of un-specific products. Fold change was calculated as 2−ΔΔCt using Hprt as reference gene.
Enzyme immunoassay
Melanocortin peptide levels were determined by enzyme immunoassay (EIA) in plasma and proteins extracts prepared from the right upper limbs homogenized in RIPA buffer using the Precellys®24 tissue homogenizer (Stretton Scientific, Derbyshire, UK) and hard tissue grinding beads. Alpha-MSH, gamma2-MSH, and ATCH EIA kits (Phoenix Pharmaceuticals, Karlsruhe, Germany) were used following manufacturers protocols.
Histological analyses
Joints were fixed with 4% neutral buffered formalin, decalcified with 10% formic acid and paraffin embedded. Sections (4 μm) were stained with hematoxylin and eosin (H&E) and fast green and safranin-O. Sections were graded from 0 (no disease) to 3 (severe) based on the degree of synovitis and cartilage erosion [34].
Escherichia coli phagocytosis by macrophages
Biogel-elicited peritoneal macrophages were obtained from wild type and Mc3r−/− mice 4 days after an i.p. injection with 1 ml of 2% biogel (BioRad, Hemel Hempstead, UK) [27]. 1 × 106 cells were plated in 24-well plates and pre-treated with corresponding drugs for 30 min before addition of bacteria (pHrodo™ Red E. coli Bioparticles® Conjugate, Invitrogen, Paisley, UK). After 20 min, non-ingested bacteria were washed and cells incubated for further 30 min to allow fluorescence to develop. Cells were trypsinized and analyzed by flow cytometry (BD FACSCalibur™, FL-2).
Phagocytosis of apoptotic neutrophils by macrophages
Experiments using healthy volunteers were approved by the local research ethics committee (P/00/029 East London and The City Local Research Ethics Committee 1). Informed written consent was provided according to the Declaration of Helsinki. Neutrophils were isolated from human blood using the double-density gradient with Histopaque 10771 and 11191 following manufacturer’s protocols [27]. Cells were incubated overnight in 10% FCS to let neutrophils undergo spontaneous apoptosis. 0.5 × 106 biogel-elicited macrophages were plated in 24-well plates and pre-treated with corresponding drugs for 30 min before addition of apoptotic neutrophils. After 1 h, macrophages were washed and fixed with 2.5% glutaraldehyde. The myeloperoxidase (MPO) assay was performed by adding 0.1 mg/ml of o-dianisidine dihydrochloride (Sigma-Aldrich, Poole, UK) and 0.03% (v/v) hydrogen peroxide. After 1 h, cells were washed and analyzed by light microscopy, with three random fields being acquired per well.
Statistics
Data are reported as mean ± SEM of n animals or tissue samples or, for in vitro experiments, performed in duplicate or triplicate from at least three distinct experiments. A p value <0.05 was taken as significant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (TIFF 3790 kb) Supplementary Figure S1. Agonistic activity of fenoprofen. cAMP production upon melanocortin receptor activation was studied in MC1, MC4 and MC5 transfected HEK293 cells (SNAP-tag Taglite® Technology, Cisbio bioassays, Codolet, France) and MC2 transfected CHO-K1 cells (GeneBLAzer® beta-lactamase Reporter Technology, Invitrogen, Paisley, UK). Fenoprofen was tested to explore its potential agonistic activity against MC1 (A), MC2 (B), MC4 (C) or MC5 (D). Control cells (transfected with empty vector DNA) were used to confirm lack of activity of NDP-αMSH and fenoprofen in HEK293 cells in the absence of melanocortin receptors (E). The effect on MC1 could not be accurately addressed due to the known high constitutive activity of this receptor
Supplementary material 2 (TIFF 1584 kb) Supplementary Figure S2. Ibuprofen does not modulate human and mouse MC3 receptor. cAMP production upon ibuprofen treatment was studied in: (A) human MC3 transfected CHO-K1 cells (GeneBLAzer® beta-lactamase Reporter Technology, Invitrogen, Paisley, UK) and (B) mouse MC3 transfected HEK293 cells (FLAG-tag, Genecopeia, Source BioScience, Nottingham, UK) in the presence of αMSH
Supplementary material 3 (TIFF 3102 kb) Supplementary Figure S3. Dose response effect of fenoprofen in the zymosan-induced peritonitis model. Peritonitis was induced with 1 mg zymosan injected i.p., 30 min after drug administration. Mice (C57BL/6) were sacrificed 4 h later and peritoneal cells analyzed by cell counting and flow cytometry. (A) Total cells per mouse; (B) neutrophils (Ly6Ghi/F4/80-); (C) inflammatory monocytes (Ly6Glow/F4/80 +); (D) effective dose (ED50) calculated on neutrophil counts. Data are mean ± SEM of n = 5; *p < 0.05 ANOVA followed by Bonferroni multiple comparison test
Supplementary material 4 (TIFF 9798 kb) Supplementary Figure S4. Histologial scoring criteria using H&E and safranin-O staining. Tissue Sects. (4 µm) were stained with hematoxylin and eosin (H&E) and fast green and safranin-O. Sections were graded from 0 (no disease) to 3 (severe) based on the degree of synovitis (purple staining in the H&E sections, C) and cartilage erosion (loss of red coloration in the safranin-O sections)
Supplementary material 5 (TIFF 974 kb) Supplementary Figure S5. Plasma levels of endogenous melanocortin peptides. αMSH (A), γMSH (B) and ACTH (C) were determined by EIA in plasma collected at day 8 from arthritic (K/BxN) and control mice. Data are mean ± SEM of n = 5, analyzed by t test (no significant changes were detected)
Acknowledgements
This project was supported by Queen Mary Innovation Ltd Proof of Concept Fund (2012/13) and The William Harvey Research Foundation.
Footnotes
T. Montero-Melendez and R. A. E. Forfar share first authorship.
D. L. Taylor and M. Perretti share senior authorship.
References
- 1.Bartfai T, Wang MW. Positive allosteric modulators to peptide GPCRs: a promising class of drugs. Acta Pharmacol Sin. 2013 doi: 10.1038/aps.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkovich R. Treatment of acute relapses in multiple sclerosis. Neurother: J Am Soc Exp Neurother. 2013;10(1):97–105. doi: 10.1007/s13311-012-0160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci. 2001;4(6):605–611. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- 4.Capsoni F, Ongari AM, Reali E, Catania A. Melanocortin peptides inhibit urate crystal-induced activation of phagocytic cells. Arthritis Res Ther. 2009;11(5):R151. doi: 10.1186/ar2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catania A, Lonati C, Sordi A, Carlin A, Leonardi P, Gatti S. The melanocortin system in control of inflammation. Sci World J. 2010;10:1840–1853. doi: 10.1100/tsw.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catania A, Rajora N, Capsoni F, Minonzio F, Star RA, Lipton JM. The neuropeptide alpha-MSH has specific receptors on neutrophils and reduces chemotaxis in vitro. Peptides. 1996;17(4):675–679. doi: 10.1016/0196-9781(96)00037-X. [DOI] [PubMed] [Google Scholar]
- 7.Ceriani G, Diaz J, Murphree S, Catania A, Lipton JM. The neuropeptide alpha-melanocyte-stimulating hormone inhibits experimental arthritis in rats. Neuroimmunomodulation. 1994;1(1):28–32. doi: 10.1159/000097087. [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Boilard E, Nigrovic PA, Clark P, Xu D, Fitzgerald GA, Audoly LP, Lee DM. Predominance of cyclooxygenase 1 over cyclooxygenase 2 in the generation of proinflammatory prostaglandins in autoantibody-driven K/BxN serum-transfer arthritis. Arthritis Rheum. 2008;58(5):1354–1365. doi: 10.1002/art.23453. [DOI] [PubMed] [Google Scholar]
- 9.Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov. 2002;1(3):198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- 10.Ditzel HJ. The K/BxN mouse: a model of human inflammatory arthritis. Trends Mol Med. 2004;10(1):40–45. doi: 10.1016/j.molmed.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Getting SJ, Allcock GH, Flower R, Perretti M. Natural and synthetic agonists of the melanocortin receptor type 3 possess anti-inflammatory properties. J Leukoc Biol. 2001;69(1):98–104. [PubMed] [Google Scholar]
- 12.Getting SJ, Christian HC, Flower RJ, Perretti M. Activation of melanocortin type 3 receptor as a molecular mechanism for adrenocorticotropic hormone efficacy in gouty arthritis. Arthritis Rheum. 2002;46(10):2765–2775. doi: 10.1002/art.10526. [DOI] [PubMed] [Google Scholar]
- 13.Getting SJ, Gibbs L, Clark AJ, Flower RJ, Perretti M. POMC gene-derived peptides activate melanocortin type 3 receptor on murine macrophages, suppress cytokine release, and inhibit neutrophil migration in acute experimental inflammation. J Immunol. 1999;162(12):7446–7453. [PubMed] [Google Scholar]
- 14.Getting SJ, Lam CW, Chen AS, Grieco P, Perretti M. Melanocortin 3 receptors control crystal-induced inflammation. FASEB J: Off Publ Fed Am Soc Exp Biol. 2006;20(13):2234–2241. doi: 10.1096/fj.06-6339com. [DOI] [PubMed] [Google Scholar]
- 15.Getting SJ, Riffo-Vasquez Y, Pitchford S, Kaneva M, Grieco P, Page CP, Perretti M, Spina D. A role for MC3R in modulating lung inflammation. Pulm Pharmacol Ther. 2008;21(6):866–873. doi: 10.1016/j.pupt.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Gong R. The renaissance of corticotropin therapy in proteinuric nephropathies. Nat Rev Nephrol. 2012;8(2):122–128. doi: 10.1038/nrneph.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman G, Gilman AG, Ruddon RW, Molinoff PB, Limbird L. Goodman and Gilman’s the pharmacological basis of therapeutics. 9. New York: McGraw-Hill; 1996. [Google Scholar]
- 18.Grieco P, Cai M, Liu L, Mayorov A, Chandler K, Trivedi D, Lin G, Campiglia P, Novellino E, Hruby VJ. Design and microwave-assisted synthesis of novel macrocyclic peptides active at melanocortin receptors: discovery of potent and selective hMC5R receptor antagonists. J Med Chem. 2008;51(9):2701–2707. doi: 10.1021/jm701181n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatta N, Dixon C, Ray AJ, Phillips SR, Cunliffe WJ, Dale M, Todd C, Meggit S, Birch-MacHin MA, Rees JL. Expression, candidate gene, and population studies of the melanocortin 5 receptor. J Investig Dermatol. 2001;116(4):564–570. doi: 10.1046/j.0022-202x.2001.01286.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaneva MK, Kerrigan MJ, Grieco P, Curley GP, Locke IC, Getting SJ. Chondroprotective and anti-inflammatory role of melanocortin peptides in TNF-alpha activated human C-20/A4 chondrocytes. Br J Pharmacol. 2012;167(1):67–79. doi: 10.1111/j.1476-5381.2012.01968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneva MK, Kerrigan MJ, Grieco P, Curley GP, Locke IC, Getting SJ. Melanocortin peptides protect chondrocytes from mechanically induced cartilage injury. Biochem Pharmacol. 2014;92(2):336–347. doi: 10.1016/j.bcp.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Khanna D, Khanna PP, Fitzgerald JD, Singh MK, Bae S, Neogi T, Pillinger MH, Merill J, Lee S, Prakash S, Kaldas M, Gogia M, Perez-Ruiz F, Taylor W, Liote F, Choi H, Singh JA, Dalbeth N, Kaplan S, Niyyar V, Jones D, Yarows SA, Roessler B, Kerr G, King C, Levy G, Furst DE, Edwards NL, Mandell B, Schumacher HR, Robbins M, Wenger N, Terkeltaub R, American College of R 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64(10):1447–1461. doi: 10.1002/acr.21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297(5587):1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 24.Loram LC, Culp ME, Connolly-Strong EC, Sturgill-Koszycki S. Melanocortin peptides: potential targets in systemic lupus erythematosus. Inflammation. 2014 doi: 10.1007/s10753-014-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melancon BJ, Hopkins CR, Wood MR, Emmitte KA, Niswender CM, Christopoulos A, Conn PJ, Lindsley CW. Allosteric modulation of seven transmembrane spanning receptors: theory, practice, and opportunities for central nervous system drug discovery. J Med Chem. 2012;55(4):1445–1464. doi: 10.1021/jm201139r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montero-Melendez T. ACTH: the forgotten therapy. Semin Immunol. 2015;27(3):216–226. doi: 10.1016/j.smim.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Montero-Melendez T, Dalli J, Perretti M. Gene expression signature-based approach identifies a pro-resolving mechanism of action for histone deacetylase inhibitors. Cell Death Differ. 2013;20(4):567–575. doi: 10.1038/cdd.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montero-Melendez T, Gobbetti T, Cooray SN, Jonassen TE, Perretti M. Biased agonism as a novel strategy to harness the proresolving properties of melanocortin receptors without eliciting melanogenic effects. J Immunol. 2015;194(7):3381–3388. doi: 10.4049/jimmunol.1402645. [DOI] [PubMed] [Google Scholar]
- 29.Montero-Melendez T, Madeira MF, Norling LV, Alsam A, Curtis MA, da Silva TA, Perretti M. Association between periodontal disease and inflammatory arthritis reveals modulatory functions by melanocortin receptor type 3. Am J Pathol. 2014;184(8):2333–2341. doi: 10.1016/j.ajpath.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montero-Melendez T, Patel HB, Seed M, Nielsen S, Jonassen TE, Perretti M. The melanocortin agonist AP214 exerts anti-inflammatory and proresolving properties. Am J Pathol. 2011;179(1):259–269. doi: 10.1016/j.ajpath.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nosengo N. Can you teach old drugs new tricks? Nature. 2016;534(7607):314–316. doi: 10.1038/534314a. [DOI] [PubMed] [Google Scholar]
- 32.Ottani A, Giuliani D, Galantucci M, Spaccapelo L, Novellino E, Grieco P, Jochem J, Guarini S. Melanocortins counteract inflammatory and apoptotic responses to prolonged myocardial ischemia/reperfusion through a vagus nerve-mediated mechanism. Eur J Pharmacol. 2010;637(1–3):124–130. doi: 10.1016/j.ejphar.2010.03.052. [DOI] [PubMed] [Google Scholar]
- 33.Pantel J, Williams SY, Mi D, Sebag J, Corbin JD, Weaver CD, Cone RD. Development of a high throughput screen for allosteric modulators of melanocortin-4 receptor signaling using a real time cAMP assay. Eur J Pharmacol. 2011;660(1):139–147. doi: 10.1016/j.ejphar.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel HB, Bombardieri M, Sampaio AL, D’Acquisto F, Gray M, Grieco P, Getting SJ, Pitzalis C, Perretti M. Anti-inflammatory and antiosteoclastogenesis properties of endogenous melanocortin receptor type 3 in experimental arthritis. FASEB J: Off Publ Fed Am Soc Exp Biol. 2010;24(12):4835–4843. doi: 10.1096/fj.10-167759. [DOI] [PubMed] [Google Scholar]
- 35.Perretti M, Leroy X, Bland EJ, Montero-Melendez T. Resolution pharmacology: opportunities for therapeutic innovation in inflammation. Trends Pharmacol Sci. 2015;36(11):737–755. doi: 10.1016/j.tips.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Ritter J, Kerr LD, Valeriano-Marcet J, Spiera H. ACTH revisited: effective treatment for acute crystal induced synovitis in patients with multiple medical problems. J Rheumatol. 1994;21(4):696–699. [PubMed] [Google Scholar]
- 37.Ross AP, Ben-Zacharia A, Harris C, Smrtka J. Multiple sclerosis, relapses, and the mechanism of action of adrenocorticotropic hormone. Front Neurol. 2013;4:21. doi: 10.3389/fneur.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov. 2012;11(3):191–200. doi: 10.1038/nrd3681. [DOI] [PubMed] [Google Scholar]
- 39.Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB J Off Publ Fed Am Soc Exp Biol. 2011;25(5):1441–1448. doi: 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spaccapelo L, Bitto A, Galantucci M, Ottani A, Irrera N, Minutoli L, Altavilla D, Novellino E, Grieco P, Zaffe D, Squadrito F, Giuliani D, Guarini S. Melanocortin MC(4) receptor agonists counteract late inflammatory and apoptotic responses and improve neuronal functionality after cerebral ischemia. Eur J Pharmacol. 2011;670(2–3):479–486. doi: 10.1016/j.ejphar.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Star RA, Rajora N, Huang J, Stock RC, Catania A, Lipton JM. Evidence of autocrine modulation of macrophage nitric oxide synthase by alpha-melanocyte-stimulating hormone. Proc Natl Acad Sci USA. 1995;92(17):8016–8020. doi: 10.1073/pnas.92.17.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wootten D, Christopoulos A, Sexton PM. Emerging paradigms in GPCR allostery: implications for drug discovery. Nat Rev Drug Discov. 2013;12(8):630–644. doi: 10.1038/nrd4052. [DOI] [PubMed] [Google Scholar]
- 43.Zaidi M, Sun L, Robinson LJ, Tourkova IL, Liu L, Wang Y, Zhu LL, Liu X, Li J, Peng Y, Yang G, Shi X, Levine A, Iqbal J, Yaroslavskiy BB, Isales C, Blair HC. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad Sci USA. 2010;107(19):8782–8787. doi: 10.1073/pnas.0912176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (TIFF 3790 kb) Supplementary Figure S1. Agonistic activity of fenoprofen. cAMP production upon melanocortin receptor activation was studied in MC1, MC4 and MC5 transfected HEK293 cells (SNAP-tag Taglite® Technology, Cisbio bioassays, Codolet, France) and MC2 transfected CHO-K1 cells (GeneBLAzer® beta-lactamase Reporter Technology, Invitrogen, Paisley, UK). Fenoprofen was tested to explore its potential agonistic activity against MC1 (A), MC2 (B), MC4 (C) or MC5 (D). Control cells (transfected with empty vector DNA) were used to confirm lack of activity of NDP-αMSH and fenoprofen in HEK293 cells in the absence of melanocortin receptors (E). The effect on MC1 could not be accurately addressed due to the known high constitutive activity of this receptor
Supplementary material 2 (TIFF 1584 kb) Supplementary Figure S2. Ibuprofen does not modulate human and mouse MC3 receptor. cAMP production upon ibuprofen treatment was studied in: (A) human MC3 transfected CHO-K1 cells (GeneBLAzer® beta-lactamase Reporter Technology, Invitrogen, Paisley, UK) and (B) mouse MC3 transfected HEK293 cells (FLAG-tag, Genecopeia, Source BioScience, Nottingham, UK) in the presence of αMSH
Supplementary material 3 (TIFF 3102 kb) Supplementary Figure S3. Dose response effect of fenoprofen in the zymosan-induced peritonitis model. Peritonitis was induced with 1 mg zymosan injected i.p., 30 min after drug administration. Mice (C57BL/6) were sacrificed 4 h later and peritoneal cells analyzed by cell counting and flow cytometry. (A) Total cells per mouse; (B) neutrophils (Ly6Ghi/F4/80-); (C) inflammatory monocytes (Ly6Glow/F4/80 +); (D) effective dose (ED50) calculated on neutrophil counts. Data are mean ± SEM of n = 5; *p < 0.05 ANOVA followed by Bonferroni multiple comparison test
Supplementary material 4 (TIFF 9798 kb) Supplementary Figure S4. Histologial scoring criteria using H&E and safranin-O staining. Tissue Sects. (4 µm) were stained with hematoxylin and eosin (H&E) and fast green and safranin-O. Sections were graded from 0 (no disease) to 3 (severe) based on the degree of synovitis (purple staining in the H&E sections, C) and cartilage erosion (loss of red coloration in the safranin-O sections)
Supplementary material 5 (TIFF 974 kb) Supplementary Figure S5. Plasma levels of endogenous melanocortin peptides. αMSH (A), γMSH (B) and ACTH (C) were determined by EIA in plasma collected at day 8 from arthritic (K/BxN) and control mice. Data are mean ± SEM of n = 5, analyzed by t test (no significant changes were detected)