Abstract
Hypoxic-ischemic (HI) brain injury is a major cause of neurological abnormalities in the perinatal period. Inflammation contributes to the evolution of HI brain injury. Inter-alpha inhibitor proteins (IAIPs) are a family of proteins that are part of the innate immune system. We have reported that endogenous IAIPs exhibit developmental changes in ovine brain and that exogenous IAIP treatment reduces neuronal death in HI neonatal rats. However, the effects of HI on endogenous IAIPs in brain have not been previously examined. In this study, we examined the effects of ischemia-reperfusion on endogenous IAIPs levels in fetal sheep brain. Cerebral cortex, cerebellum, cervical spinal cord, choroid plexus, and CSF were snap frozen from sham control fetuses at 127 days gestation and after 30-min of carotid occlusion and 4-, 24-, and 48-hours of reperfusion. IAIP levels were determined by Western immunoblot. IAIP expressions of the 250 kDa Inter-alpha inhibitor (IaI) and 125 kDa Pre-alpha inhibitor (PaI) in cerebral cortex and PaI in cerebellum were reduced (P<0.05) 4-hours after ischemia compared with controls and returned toward control levels 24- and 48-hours after ischemia. CSF PaI and IaI were reduced 48 hours after ischemia. We conclude that IAIPs in cerebral cortex and cerebellum are reduced by brain ischemia, and return toward control levels between 24 and 48 hours after ischemia. However, changes in CSF IAIPs were delayed, exhibiting decreases 48 hours after ischemia. We speculate that the decreases in endogenous IAIPs reflect increased utilization, potentially suggesting that they have endogenous neuroprotective properties.
Keywords: brain, CSF, fetus, ischemia-reperfusion, inter-alpha inhibitor proteins, sheep
Introduction
Hypoxic-ischemic (HI) brain injury is the one of the most common neurological problems occurring in the perinatal period (Vannucci and Hagberg, 2004; Volpe, 2009). Recent studies suggest that fetal inflammation is a strong predictor of perinatal brain injury both in full term and premature infants and that pro-inflammatory cytokines are important in the evolution of ischemic brain injury (Ferriero, 2004; Dammann and Leviton, 2014).
Inter-alpha-inhibitor proteins (IAIPs) are a family of proteins that are part of the innate immune system and demonstrate substantial anti-inflammatory properties (Fries and Kaczmarczyk, 2003). They are a complex of serine protease inhibitor proteins (125 and 250 kDa) synthesized mainly in the liver and are present in plasma at high concentrations in adults and neonates (Opal et al., 2007; Chaaban et al., 2009; Chaaban et al., 2010; Spasova et al., 2014). The two major forms, found in human plasma are Inter-alpha Inhibitor (IaI), consisting of two heavy chains (H1 and H2) and a single light chain termed bikunin (or urinary trypsin inhibitor, 30kDa), and Pre-alpha Inhibitor (PaI), which consists of one heavy (H3) and one light chain (bikunin). IaI and PaI are the most abundant bikunin-containing molecules that are found in circulating blood.
Recently, we have identified the presence of IAIPs in brain, choroid plexus, cerebral spinal fluid (CSF), and in somatic organs over a wide span of ovine development (Spasova et al., 2014). IAIPs exhibited unique ontogenic patterns of expression specific for each major isoform and organ during development (Spasova et al., 2014). In addition, we reported endogenous IAIP genes and proteins in cultured mouse neurons and immunoreactivity in neurons, microglia and astrocytes in isolated cultured neonatal rat cerebral cortical cells. IAIP immunoreactivity was also identified in discrete brain regions including cerebral cortex, hippocampus, ependyma, choroid plexus, and microvessels in neonatal rats and specifically in neurons, microglia cells, and astrocytes in paraffin embedded sections from fetal and neonatal rodent brain (Chen et al., 2016). The ubiquitous nature of the expression of endogenous IAIP genes and proteins in a variety of brain cells and regions in mouse and rat central nervous system (CNS) both in vitro and in vivo supports our contention that endogenous IAIPs represent previously unrecognized important components of normal brain composition and most likely function (Spasova et al., 2014; Chen et al., 2016). These findings are also consistent with previous work suggesting that IAIP related molecules are important in CNS inflammatory disorders such as multiple sclerosis and stroke (Yano et al., 2003; Kashyap et al., 2009; Shu et al., 2011; Shu et al., 2015). A recent report has suggested that CSF urinary trypsin inhibitor (light chain of IAIPs) levels were lower in patients with multiple sclerosis than in control subjects, and that CSF urinary trypsin inhibitor levels predict the severity of demyelination (Shu et al., 2015). Furthermore, systemic inflammatory disorders such as sepsis and necrotizing enterocolitis (NEC) result in decreased IAIP blood levels in premature infants (Chaaban et al., 2009; Chaaban et al., 2010).
Therefore, endogenous IAIPs have been shown to be expressed in the brain over a wide developmental period in sheep, and in fetal and neonatal rodents, are decreased in blood of premature infants after systemic inflammation, and urinary trypsin inhibitor levels are decreased in CSF of adults with inflammatory CNS disorders (Chaaban et al., 2009; Chaaban et al., 2010; Shu et al., 2011; Spasova et al., 2014; Shu et al., 2015). Importantly, inflammation is a critical component in the evolution of HI brain injury in neonates (Ferriero, 2004). Consequently, the potential exists that IAIPs represent important endogenous neuroprotective molecules (Spasova et al., 2014). However, the effects of ischemia and reperfusion on endogenous IAIPs in brain have not been previously examined. In an effort to elucidate, the effects of ischemia-reperfusion on these endogenous proteins in brain, we examined the effects of ischemia and different durations of reperfusion on IAIPs levels in fetal sheep plasma, cerebral cortical, cerebellar, cervical spinal cord, choroid plexus, and CSF samples.
Methods
The present study was performed after approval by the Institutional Animal Care and Use Committees of The Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island and in accordance to the National Institutes of Health Guidelines for the use of experimental animals.
Animal Preparation and Experimental Design
The plasma, cerebral cortical, cerebellar, cervical spinal cord, choroid plexus, and CSF samples for the present study were obtained from fetal sheep studied in our former work (Chen et al., 2012). The surgical and physiological procedures were performed for our previous studies as described in detail (Chen et al., 2012). In brief, surgery was performed under 1–2% isoflurane anesthesia on mixed breed pregnant ewes at 120–122 days of gestation. Catheters were placed in fetal brachial veins and arteries and in the femoral artery of the ewe for blood pressure and heart rate monitoring and to obtain blood samples for our earlier study (Chen et al., 2012). Two inflatable 4-mm vascular occluders (In Vivo Metric, Healdsburg, CA, USA) were placed around each carotid artery along with ultrasonic flow probes (Transonic Systems Inc., Ithaca, NY, USA). Our previous study had been designed to quantify blood-brain barrier permeability under conditions of ischemia with differing durations of reperfusion (Chen et al., 2012). The fetal sheep were studied at 85% of gestation (125–128 days of gestation). Full term gestation in this breed of sheep is 145–147 days. The sheep brain at 85% gestation is generally thought to be similar to those of the term equivalent human infants (Barlow, 1969; Back et al., 2006). Samples were obtained from sham operated control (n=5) and fetal sheep exposed to 30-minutes of carotid occlusion and 4 (n=5), 24 (n=5) and 48 (n=5) hours of reperfusion. At the end of the studies, a CSF sample was obtained from the fetal sheep via a direct puncture of the allantoic membrane. The CSF sample was inspected for blood contamination and discarded if contamination was present. All samples were obtained at the end of the studies immediately before euthanasia (Chen et al., 2012). Regional brain, CSF, and plasma samples were snap frozen in liquid nitrogen and remained at -80° C until analysis.
Competitive Enzyme-Linked Immunosorbent Assay (ELISA) to Measure IAIPs in Ovine Plasma
Plasma samples from the fetal sheep in the control group and in the groups exposed to ischemia and 4, 24, and 48 hours of reperfusion were obtained before and serially after exposure to the ischemic insult. Plasma IAIPs concentrations were measured with a competitive ELISA in sheep plasma using a polyclonal antibody raised against human IAIPs (R-20 pAb) as we previously described (Spasova et al., 2014). The polyclonal antibody was generated by immunizing rabbits with purified human plasma derived IAIPs (Lim et al., 2005). The R-20 pAb cross-reacts with non-human IAIPs including sheep and detects both the 250 kDa IaI and 125 kDa PaI protein moieties in the Western immunoblot analysis. The R-20 pAb also binds to heavy chains as well as light chain of human IAIPs after enzymatic digestion (Lim et al., 2005). Ninety-six-well high-binding microplates (Microlon 600, Greiner Bio-One, Monroe, NC, USA) were coated with purified sheep IAIPs. The sheep IAIPs were purified from sheep serum (Quad Five, Ryegate, MO, USA) by anion-exchange chromatography on a Toyopearl Q-600C-AR column (Tosoh Bioscience, King of Prussia, PA, USA). Bound IAIPs were eluted with a buffer containing 750 mM NaCl. Purified sheep IAIPs were diluted in 100 mM NaPO4 buffer pH 6.5 and immobilized on the microplates (50 ng/per well) for 1 h at room temperature or overnight at 4°C. The microplate was blocked with 200 μL of 5% non-fat dried milk in phosphate buffered saline (PBS) and 0.05% Tween. Known amounts of purified sheep IAIPs were serially diluted in PBS to establish the standard curve for quantitative analysis of IAIPs concentrations in the samples. After 50 μL of samples and serially diluted IAIPs standards were added to the wells, 50 μL of R-20 pAb diluted in 1:1200 in PBS was added to each well. Plates were incubated for 1 hour at room temperature and washed with PBS and 0.05% Tween using automated plate washer (Biotek EL-404, Winooski, VT,USA). The bound R-20 pAb was detected by adding HRP-conjugated goat anti-rabbit IgG (Invitrogen, Carlsbad, CA, USA) for 1 hour at room temperature. After washing, 100 μL Enhanced K-Blue TMB substrate (Neogen Corp, Lexington, KY, USA) was added to the wells and reaction was stopped with 100 μL 1 N HCL solution. The absorbance at 450 nm was measured on SpectraMAX Plus microplate reader (Molecular Devices, Sunnyvale, CA, USA). Each sample was measured in triplicate and assays were repeated at least twice on all samples.
Preparation of Cytosolic Tissue Fractions
Cytosolic cellular fractions of cerebral cortex, cerebellum, spinal cord, and choroid plexus for IAIPs were extracted in buffer A (TRIS 10 mM pH 6.8, sucrose, MgCl2) with 1% complete protease inhibitor cocktail (Sigma, St. Louis, MO, USA). Total protein concentrations of the homogenates were measured with a bicinchoninic acid protein assay (BCA, Pierce, Rockford, IL, USA). Aliquots of extracted samples were stored at –80°C. We determined IAIPs in the cytosolic fraction because the primary antibody R-20 recognized only proteins in the cytosol.
Western Immunoblot Detection and Quantification of IAIPs Proteins
Fifteen μg protein of total protein per well from each brain region were fractionated on SDS-polyacrylamide gels. Five μl of CSF were used. The gels were then transferred on to polyvinylidene diflouride membranes (PVDF, 0.2 μm, Bio-Rad Laboratories, Hercules, CA) using a semi-dry technique. The membranes were incubated with rabbit polyclonal antibody against human IAIPs (R-20 pAb, ProThera Biologics, Providence RI, USA) at a dilution of 1:5,000. The immunoblot membranes were incubated with primary antibody at 4°C overnight. Peroxidase-labeled secondary goat anti-rabbit antibody (Alpha Diagnostic, San Antonio, TX, USA) was incubated for 1 hour at room temperature in a dilution of 1:10,000. Binding of the secondary antibody was detected with enhanced chemiluminescence (ECL plus, Western Blotting Detection reagents, Amersham Pharmacia Biotech, Inc., Piscataway, NJ, USA) before exposure to autoradiography film (Daigger, Vernon Hills, IL, USA).
The samples were normalized to a reference protein standard that was obtained from a homogenate protein pool from the brain of one adult sheep. For the purpose of the current report, we refer to these samples as the internal control protein samples. As we previously described (Kim et al., 2006; Malaeb et al., 2007; Sadowska et al., 2009; Spasova et al., 2014), these internal control samples served as controls for quality of loading, transfer of the samples, normalization of the densitometric values, and permitted accurate comparisons among the different immunoblots (Kim et al., 2006; Malaeb et al., 2007; Spasova et al., 2014). The use of the internal control protein samples permit comparisons among large groups of animals and over a large number of different immunoblots. The experimental autoradiographic integrated optical density (IOD) values were expressed as a ratio to the internal control and enabled normalized comparisons among the groups. This methodology correlates well with values that were normalized as ratios to β-actin and vinculin (Kim et al., 2006; Sadowska et al., 2015). Each immunoblot included samples from the four groups and three internal control samples. The internal control samples were included in three lanes, as the first, middle, and last samples on each immunoblot. We calculated a coefficient of variation for the internal control samples on each immunoblot. The experimental values were accepted as valid only when the percent coefficient of variation for the internal control samples was less than 20% on the immunoblot. Human plasma-derived IAIPs served as a positive control for all immunoblots to ascertain that the antibody correctly identified the ovine proteins. Molecular weight standards (Bio-Rad Laboratories, Hercules, CA USA) were also included in each immunoblot. The R-20 pAb detected IAIPs bands at 125 and 250 kDa (PaI and IaI) in all brain regions. Uniformity in inter lane loading was also established by Coomassie blue (Sigma, St. Louis, MO, USA) staining of the polyacrylamide gels and uniformity of transfer to the polyvinylidene diflouride membranes was confirmed by Ponceau S staining (Sigma, St. Louis, MO, USA, Data not shown). Anti-glyceraldehyde-3-phosphate dehydrogenase-antibody (GAPDH, mouse monoclonal, Imgenex, San Diego, CA, USA) and anti-β-actin primary antibody (Bio-Rad Laboratories, Hercules, CA) were used as well to ensure that equal amounts of protein were applied to each lane. A loading control was not used for the CSF because GAPDH and β-actin were not detected in CSF.
Samples from the brain regions of each group were placed on the immunoblots to permit identical standard conditions among of the brain samples from the different reperfusion groups. However, for the purpose of the graphic illustration, we selected the specific immunoblots that the most closely represented the mean values for each bar graph of each group in Figures 1 and 2. The white vertical lines were used to delineate the representative bands cut from the different immunoblots, for the entire immunoblots please see the supplementary Figure 1. On the other hand, Figures 3-5 show the entire original immunoblots above the bar graphs.
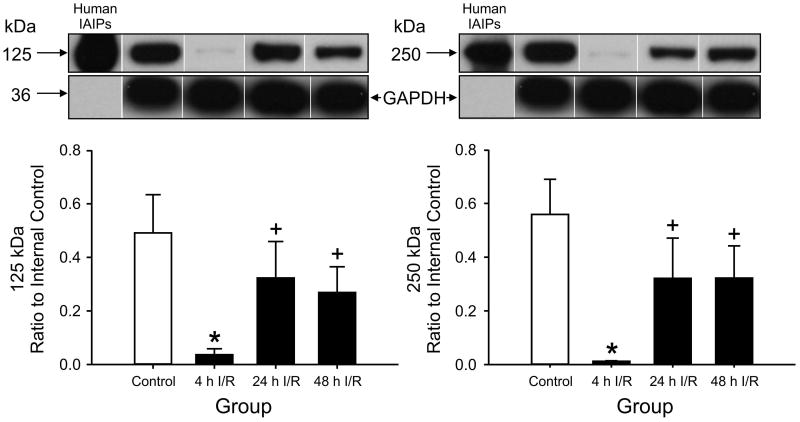
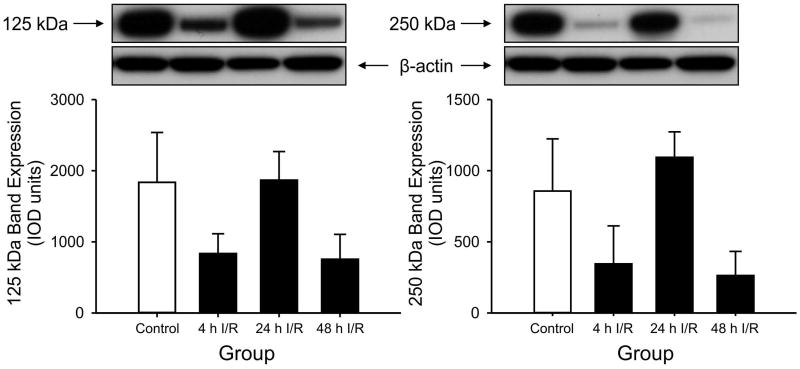
Figure 1.
125 kDa PaI and 250 kDa IaI expression in ovine cerebral cortex plotted as the ratio to the internal control standard in the sham control, 4h, 24h, and 48h ischemia-reperfusion (I/R) groups. 125 kDa PaI and 250 kDa IaI: Expression was lower in the 4 h I/R group, Purified human IAIP shown as a positive control. *P<0.05 versus the control group, +P<0.05 versus 24 h and 48 h I/R. Values are mean ± SEM.
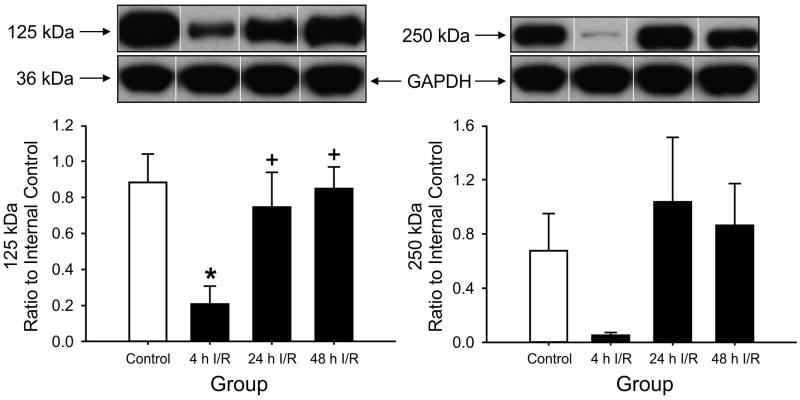
Figure 2.
125 kDa PαI and 250 kDa IαI expression in ovine cerebellum plotted as the ratio to the internal control standard. 125 kDa PaI Expression was lower in 4 h I/R group, *P<0.05 versus control group, +P<0.05 versus 24 h and 48 h I/R. Values are mean ± SEM.
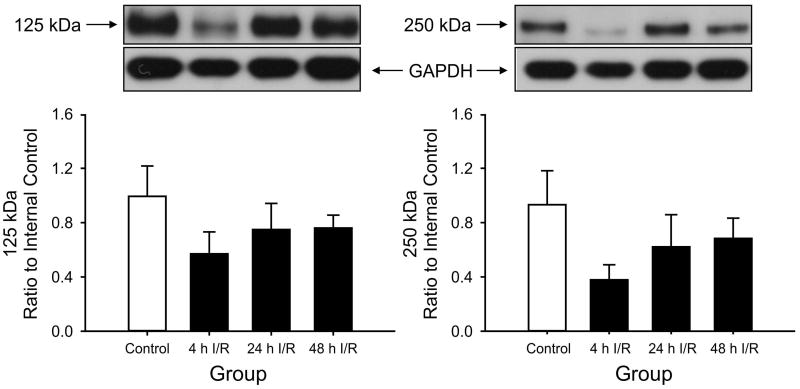
Figure 3.
125 kDa PαI and 250 kDa IαI expression in cervical spinal cord plotted as the ratio to the internal control standard. 125 kDa PaI and 250 kDa IaI: Changes in expressions did not reach significance. Values are mean ± SEM.
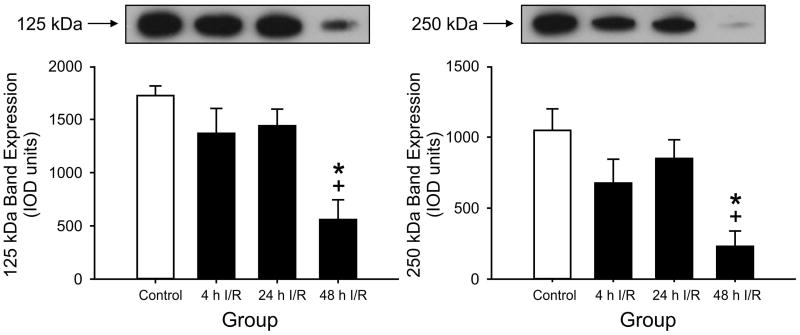
Figure 5.
125 kDa PaI and 250 kDa IaI expression plotted as arbitrary OD units in CSF for the control, 4 h, 24 h and 48 h I/R groups. 125 kDa PaI and 250 kDa PaI: Expression is lower at 48 h of I/R than in the 4 h and 24 h I/R groups, *P<0.05 the control group, +P<0.05 versus the 4 h and 24 h I/R groups. Values are mean ± SEM.
Densitometric Analysis
Band intensities were analyzed with a Gel-Pro Analyzer (Media Cybernetics, Silver Spring, MD, USA). All experimental samples were normalized to the respective average autoradiographic integrated optical density (IOD) values of the three internal control samples. However, the band intensities were expressed as arbitrary autoradiographic integrated optical density (IOD) units for choroid plexus and CSF because we did not have choroid plexus or CSF from adult sheep. The final values represented averages of the densitometry values obtained from the different immunoblots (n=5) and were presented as a ratio to the internal control sample except for choroid plexus and CSF.
Statistical Analysis
All results were expressed as means ± standard error of the mean (SEM). Two-way analysis of variance (ANOVA) was used to compare the differences among the groups. The factors were ischemia-reperfusion (I/R) group (control, 4, 24, and 48 hours I/R) and protein expression (125 kDa PaI and 250 kDa IaI). Two-way analysis of variance (ANOVA) was also used to determine the plasma concentrations over time among the study groups. If a significant effect of group was found by ANOVA, the Fisher least significant difference test was utilized to identify specific differences among the groups. A value of P<0.05 was considered statistically significant.
Results
IAIPs Detection by ELISA in Ovine Plasma
IAIPs were detected by our competitive ELISA in the fetal sheep plasma from the control and 4, 24 and 48 I/R groups. Serial samples were obtained just before and after the ischemic insults until the end of the studies at 4, 24, or 48 hours after ischemia (Chen et al., 2012). The baseline plasma levels did not differ among the groups (mean value for all groups: 156 ± 5.8 μg/ml) and were not affected by the brain ischemia-reperfusion related insults after any of the different reperfusion exposure times.
IAIPs Detection by Western Immunoblot in Cerebral Cortex, Cerebellum, Choroid Plexus, Cervical Spinal Cord, and CSF
IAIPs were detected in cerebral cortex, cerebellum, cervical spinal cord, choroid plexus, and CSF as the 125 kDa PaI and 250 kDa IaI protein bands by Western immunoblot using the specific antibody against IAIPs (Fig.1-5). Human IAIPs were used as a positive control in each immunoblot as shown in Figure 1 and its location was identical in all immunoblot for each brain region. The expression of the PaI and IaI were both significantly lower in the cerebral cortex at 4 hours after ischemia compared with the values in the sham control group. After 24 and 48 hours of reperfusion, the values of PaI and IaI expression appeared to increase and return toward values that did not differ compared with the sham control group (Fig. 1).
Likewise, the expression of the PaI in the cerebellum was reduced significantly in the group exposed to 4 hours of reperfusion compared with the control group (Fig. 2). However, although the IaI expression showed a similar pattern of change among the reperfusion groups, the SEM for this protein band was wide, and the changes did not show statistical significance. Likewise, the expressions of the PaI and IaI proteins did not exhibit statistically significant changes in the cervical spinal cord (Fig. 3) or in the choroid plexus (Fig. 4).
Figure 4.
125 kDa PaI and 250 kDa IaI expression plotted as arbitrary OD units in the choroid plexus for the different groups. 125 kDa PaI and 250 kDa PaI: Changes in expressions did not reach significance. Values are mean ± SEM.
Inspection of Fig. 1, 2, and 3 compared with Fig. 5 suggests that the pattern of expression of IAIPs in the cerebral cortex, cerebellum, and spinal cord differed from those of the CSF. In CSF, the values of PaI and IaI expression after 4 and 24 hours of reperfusion did not differ from the values in the control group. However, at 48 hours after ischemia, the expression of the PaI protein was significantly lower compared with the control, 4-hour, and 24-hour reperfusion groups and the expression of the IaI protein was lower compared with the control and 24-hour reperfusion groups (Fig. 5).
Discussion
The main purpose of our study was to examine the levels of IAIPs in the CNS after ischemic-reperfusion injury in fetal sheep as an initial approach to understand endogenous changes in these critical molecules after ischemic insults in fetal brain. Recently, we reported that IAIPs were identified for the first time in plasma, cerebral cortex, choroid plexus, CSF and other non-neural organs from early in fetal development through the neonatal period, and up to maturity in adult sheep as both the 125 kDa PaI and 250 kDa IaI IAIP protein moieties, and that IAIP genes and proteins are also present in fetal and neonatal rodent brain (Spasova et al., 2014; Chen et al., 2016). Accordingly, we reasoned that the endogenous presence of both IAIP moieties in sheep and rodent brain over a wide span of development suggested that these proteins most likely have important immunomodulatory functions in normal brain (Fries and Blom, 2000; Lim et al., 2003). Moreover, although the exact functions of the endogenous IAIPs in the CNS are not known, their presence in high amounts and in a wide variety of CNS cell types suggested a potentially important role in brain development and potentially injury repair (Spasova et al., 2014; Chen et al., 2016).
The findings of our study are novel because previous work has not reported changes in the expression of IAIPs after ischemia in the CNS of any species. The major findings of this study were as follows. 1. Ischemia with reperfusion results in acute decreases in cerebral cortical and cerebellar IAIP levels 4 hours after ischemia. 2. IAIP expressions return toward non-ischemic control levels 24 and 48 hours after ischemia. 3. The expression of IAIPs in CSF remained similar to control levels at 4 and 24 hours after the ischemic insult but exhibited a significant decrease 48 hours after ischemia. 4. Serum IAIPs expression levels were not affected by brain ischemia.
Inflammation is increasingly recognized as an important factor in normal brain development and during the evolution of injury in the developing brain (Ferriero, 2004; Hagberg et al., 2012). Although inflammation contributes to injury, it also participates in repair processes (Foster-Barber and Ferriero, 2002; Kendall and Peebles, 2005; Stridh et al., 2011; Hagberg et al., 2012; Hagberg et al., 2015). Pro-inflammatory cytokines are important mediators in pathways associated with perinatal brain injury caused by a variety of insults including hypoxic-ischemic and inflammatory related brain damage (Dammann and O'Shea, 2008; Malaeb and Dammann, 2009; Elovitz et al., 2011; McAdams and Juul, 2012). Moreover, we have previously shown that ischemia for 30 minutes with reperfusion for 48 and 72 hours results in increases in IL-1β protein expression in the cerebral cortex of fetal sheep (Sadowska et al., 2012). In addition, we have shown that the high mobility group box 1 (HMGB1), which has recently been identified as a pro-inflammatory cytokine after extracellular release from necrotic tissue (Wang et al., 1999; Yang et al., 2005), is translocated from the nucleus to the cytosolic compartment after ischemic brain injury in fetal sheep. Others have shown that hypoxia-ischemia also results in increases in caspase expression, microglia and gliosis in fetal sheep brain (Hutton et al., 2007; Riddle et al., 2011; Jellema et al., 2013; Jellema et al., 2013). Therefore, there is evidence to suggest that inflammatory markers are increased in the brain of fetal sheep after hypoxia-ischemia.
In human neonates, sepsis, necrotizing enterocolitis (NEC), and mechanical ventilation are associated with increases in pro-inflammatory cytokines, cerebral palsy, and in lower mental and psychomotor development (Stoll et al., 2004; Shah et al., 2008; Bose et al., 2013; O'Shea et al., 2013). IAIPs are decreased during systemic inflammation such as sepsis and NEC (Chaaban et al., 2009; Chaaban et al., 2010) and both disorders are associated with increased incidences of brain injury (Stoll et al., 2004; Shah et al., 2008). These findings raise the interesting possibility that decreases in IAIPs could potentially contribute to the development of the associated brain injury (Stoll et al., 2004; Shah et al., 2008).
IAIPs are anti-inflammatory proteins that inhibit destructive serine proteases, block pro-inflammatory cytokines, augment anti-inflammatory cytokine production, block complement activation during inflammation and neutralize the cytotoxicity of extracellular histones (Fries and Blom, 2000; Chaaban et al., 2015). Furthermore, they are broad spectrum immunomodulators that increase survival after systemic infection, exhibit cytoprotective properties in somatic cells and CNS, and improve recovery after brain injury (Yano et al., 2003; Opal et al., 2005; Chaaban et al., 2009; Chaaban et al., 2010; Singh et al., 2010; Wang et al., 2013). In addition, we have recently shown that exogenous IAIPs reduce cortical neuronal death, improve plasticity and behavioral outcomes in neonatal rats exposed to HI brain injury (Threlkeld et al., 2014; Gaudet et al., 2016). Although there is a paucity of information regarding the characteristics and significance of endogenous IAIPs in the brain, recent evidence demonstrates that the Inter-alpha-Trypsin Inhibitor Heavy Chain 4 is decreased in the serum of human patients after acute ischemic stroke (Kashyap et al., 2009). In contrast to the findings in adult patients after stroke (Kashyap et al., 2009), ischemic-reperfusion brain injury was not associated with changes in the plasma IAIPs levels in our fetal sheep.
In the current study, we have shown that ischemia results in acute reductions in the endogeneous expression of IAIPs in fetal brain with levels returning toward control values 24 and 48 hours after ischemia. Given that IAIPs decrease during systemic inflammation, and that inflammation is an important component of ischemia-reperfusion related brain injury, we speculate that the rapid decrease in IAIPs after ischemia suggests that these molecules are utilized, consumed and/or degraded as a result of the ischemia-related inflammation in brain.
The increase in IAIPs after 24 and 48 hours of reperfusion most likely represents local endogenous IAIPs synthesis within the brain parenchyma because IAIPs are relatively large molecules that most likely do not readily cross the fetal blood-brain barrier (Stonestreet et al., 1996). Moreover, IAIPs are most likely readily produced within the brain parenchyma, because we have shown that they are widely expressed both in sheep and rodents during normal brain development and have shown the presence of IAIP genes in cultured mouse neurons (Spasova et al., 2014; Chen et al., 2016).
Treatment with ulinastatin (bikunin) has been shown to have protective effects against ischemic-reperfusion injury in the liver, intestine, kidney, heart, lung and brain (Li et al., 1993; Li et al., 1994; Nakahama et al., 1996; Yano et al., 2003). Ulinastatin (bikunin) also has unique immunomodulatory effects by reducing TNF-α during reperfusion after ischemic injury and systemic inflammation (Li et al., 1993; Li et al., 1994; Nakahama et al., 1996). Additionally, blood-derived IAIPs augment survival and exhibit anti-inflammatory properties in a model of neonatal sepsis (Singh et al., 2010). Therefore, we postulate that endogenous IAIPs could also represent anti-inflammatory molecules within the brain parenchyma that are consumed, utilized or deactivated by ishemia and resynthesized by brain shortly after ischemia as a component of endogenous reparative processes. Our current findings in conjunction with our recent publications would appear to suggest that IAIPs may function as endogenous protective factors, and also are efficacious when administered exogenously as a therapeutic treatment strategy (Threlkeld et al., 2014; Chen et al., 2016; Gaudet et al., 2016). Therefore, it appears that both endogenous and exogenous IAIPs have important characteristics in brain injury. Hence, IAIPs could be analogous to other previously reported neuroprotective agents such as FGF-2, which has been shown to have endogenous protective functions and also to exert neuroprotective properties when given as an exogenous treatment strategy (Nozaki et al., 1993; Petersson et al., 2002; Noda et al., 2014).
The function of the choroid plexus and its product cerebral spinal fluid (CSF) has been thought to provide physical protection to the brain and facilitate removal of brain metabolites through the drainage of CSF. However, more recent studies suggest that the choroid plexus and CSF play a much more active role in the development, homeostasis, and repair of the CNS (Bagnard et al., 2001; Johanson et al., 2008). CSF is produced from choroid plexus as a plasma ultra-filtrate and from interstitial fluid drainage from CNS tissue. CSF also provides the CNS with a variety of biologically active growth, neurotrophic, and angiogenic factors essential for brain development (Bagnard et al., 2001; Johanson et al., 2008) and is also involved in responses to brain injury and subsequent repair processes (Bondy et al., 1992; Diaz-Ruiz et al., 1993; Gonzalez et al., 1995; Johanson et al., 1999; Raballo et al., 2000; Justicia et al., 2001; Reid and Ferretti, 2003; Sun et al., 2003; Chesnutt et al., 2004). Previous studies have reported that many species including humans have very high CSF protein concentrations during the perinatal period that are most likely important for normal brain growth and development (Saunders, 1977; Dziegielewska et al., 1980; Dziegielewska et al., 1981; Dziegielewska et al., 1991; Dziegielewska et al., 2000; Iwama et al., 2000; Gato et al., 2001). In our previous study, we demonstrated high levels of IAIPs in the CSF of fetal sheep (Spasova et al., 2014). The high protein concentrations in fetal CSF are most likely a result of local production by choroid plexus rather than immaturity of the blood-brain or blood-CSF barriers because both barriers form very early during development in the fetus (Saunders, 1977; Dziegielewska et al., 1980; Stonestreet et al., 1996; Johanson et al., 2011). The IAIPs contained within the CSF most likely have important biological functions similar to growth factors and cytokines that could influence the development of neuroepithelial cells (Gato et al., 2004; Gato and Desmond, 2009; Johanson et al., 2011). The delayed decreases in CSF IAIP levels 48 hours after ischemia is of great interest and could represent ischemia-related interference with choroid plexus transport of IAIPs, even though the choroid plexus samples did not show significant reductions in IAIPs expression. Alternatively, the delayed reductions in CSF IAIP levels could also reflect corresponding reductions in interstitial fluid IAIP levels secondary to reduced IAIPs expression in the brain after ischemia.
The ubiquitous nature of IAIPs in multiple regions of sheep brain in including cerebral cortex, cerebellum, choroid plexus, and CSF during the development suggests that these molecules have important characteristics as endogenous anti-inflammatory molecules with region specific differential production or modulation during development (Spasova et al., 2014; Chen et al., 2016). In addition, treatment with exogenous IAIPs during brain injury also has important neuroprotective properties (Threlkeld et al., 2014; Gaudet et al., 2016). Future studies are required to examine the precise role of endogenous IAIPs in the brain and to determine the mechanisms by which exogenous IAIPs are neuroprotective (Threlkeld et al., 2014). Furthermore, it would also be of great interest in future studies to examine the effect of ischemia on IAIPs at additional time intervals after ischemia, and to examine inflammatory changes as a function of changes in endogenous levels of IAIPs in the fetal sheep brain.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number 1R01-HD-057100, R21 NS095130-01, R21NS096525, by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 RR018728 and P20GM103537, P30GM114750 and Marshall-Klaus Research Award to M.S. 2011-2012. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Support: National Institute of General Medical Sciences of the National Institutes of Health: 1R01-HD-057100, R21 NS095130, R21NS096525, by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health: P20 RR018728 and P20GM103537, P30GM114750, 1R21NS095130 and Marshall-Klaus Research Award to M.S. 2011-2012.
Footnotes
Disclosures: Yow-Pin Lim is employed by ProThera Biologics and has equity interest in the company. No conflicts of interest, financial or otherwise are declared by Mariya S. Spasova, Xiaodi Chen, Grazyna B. Sadowska, Edward R. Horton or Barbara S. Stonestreet.
References
- Back SA, Riddle A, Hohimer AR. Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white-matter injury. J Child Neurol. 2006;21:582–589. doi: 10.1177/08830738060210070101. [DOI] [PubMed] [Google Scholar]
- Bagnard D, Vaillant C, Khuth ST, Dufay N, Lohrum M, Puschel AW, Belin MF, Bolz J, Thomasset N. Semaphorin 3A-vascular endothelial growth factor-165 balance mediates migration and apoptosis of neural progenitor cells by the recruitment of shared receptor. J Neurosci. 2001;21:3332–3341. doi: 10.1523/JNEUROSCI.21-10-03332.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow RM. The foetal sheep: morphogenesis of the nervous system and histochemical aspects of myelination. J Comp Neurol. 1969;135:249–262. doi: 10.1002/cne.901350302. [DOI] [PubMed] [Google Scholar]
- Bondy C, Werner H, Roberts CT, Jr, LeRoith D. Cellular pattern of type-I insulin-like growth factor receptor gene expression during maturation of the rat brain: comparison with insulin-like growth factors I and II. Neuroscience. 1992;46:909–923. doi: 10.1016/0306-4522(92)90193-6. [DOI] [PubMed] [Google Scholar]
- Bose CL, Laughon MM, Allred EN, O'Shea TM, Van Marter LJ, Ehrenkranz RA, Fichorova RN, Leviton A. Systemic inflammation associated with mechanical ventilation among extremely preterm infants. Cytokine. 2013;61:315–322. doi: 10.1016/j.cyto.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaaban H, Keshari RS, Silasi-Mansat R, Popescu NI, Mehta-D'Souza P, Lim YP, Lupu F. Inter-alpha inhibitor protein and its associated glycosaminoglycans protect against histone-induced injury. Blood. 2015;125:2286–2296. doi: 10.1182/blood-2014-06-582759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaaban H, Shin M, Sirya E, Lim YP, Caplan M, Padbury JF. Inter-alpha inhibitor protein level in neonates predicts necrotizing enterocolitis. J Pediatr. 2010;157:757–761. doi: 10.1016/j.jpeds.2010.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaaban H, Singh K, Huang J, Siryaporn E, Lim YP, Padbury JF. The role of inter-alpha inhibitor proteins in the diagnosis of neonatal sepsis. J Pediatr. 2009;154:620–622. doi: 10.1016/j.jpeds.2008.10.008. e621. [DOI] [PubMed] [Google Scholar]
- Chen X, Rivard L, Naqvi S, Nakada S, Padbury JF, Sanchez-Esteban J, Stopa EG, Lim YP, Stonestreet BS. Expression and localization of Inter-alpha Inhibitors in rodent brain. Neuroscience. 2016;324:69–81. doi: 10.1016/j.neuroscience.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Threlkeld SW, Cummings EE, Juan I, Makeyev O, Besio WG, Gaitanis J, Banks WA, Sadowska GB, Stonestreet BS. Ischemia-reperfusion impairs blood-brain barrier function and alters tight junction protein expression in the ovine fetus. Neuroscience. 2012;226:89–100. doi: 10.1016/j.neuroscience.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnutt C, Burrus LW, Brown AM, Niswander L. Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev Biol. 2004;274:334–347. doi: 10.1016/j.ydbio.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res. 2014;75:376–380. doi: 10.1038/pr.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammann O, O'Shea TM. Cytokines and perinatal brain damage. Clin Perinatol. 2008;35:643–663. v. doi: 10.1016/j.clp.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Ruiz C, Perez-Tomas R, Domingo J, Ferrer I. Immunohistochemical localization of transforming growth factor-alpha in choroid plexus of the rat and chicken. Neurosci Lett. 1993;164:44–46. doi: 10.1016/0304-3940(93)90853-d. [DOI] [PubMed] [Google Scholar]
- Dziegielewska KM, Evans CA, Lorscheider FL, Malinowska DH, Mollgard K, Reynolds ML, Saunders NR. Plasma proteins in fetal sheep brain: blood-brain barrier and intracerebral distribution. J Physiol. 1981;318:239–250. doi: 10.1113/jphysiol.1981.sp013861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska KM, Evans CA, Malinowska DH, Mollgard K, Reynolds ML, Saunders NR. Blood-cerebrospinal fluid transfer of plasma proteins during fetal development in the sheep. J Physiol. 1980;300:457–465. doi: 10.1113/jphysiol.1980.sp013172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska KM, Habgood MD, Mollgard K, Stagaard M, Saunders NR. Species-specific transfer of plasma albumin from blood into different cerebrospinal fluid compartments in the fetal sheep. J Physiol. 1991;439:215–237. doi: 10.1113/jphysiol.1991.sp018664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska KM, Knott GW, Saunders NR. The nature and composition of the internal environment of the developing brain. Cell Mol Neurobiol. 2000;20:41–56. doi: 10.1023/a:1006943926765. [DOI] [PubMed] [Google Scholar]
- Elovitz MA, Brown AG, Breen K, Anton L, Maubert M, Burd I. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci. 2011;29:663–671. doi: 10.1016/j.ijdevneu.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Foster-Barber A, Ferriero DM. Neonatal encephalopathy in the term infant: neuroimaging and inflammatory cytokines. Ment Retard Dev Disabil Res Rev. 2002;8:20–24. doi: 10.1002/mrdd.10009. [DOI] [PubMed] [Google Scholar]
- Fries E, Blom AM. Bikunin--not just a plasma proteinase inhibitor. Int J Biochem Cell Biol. 2000;32:125–137. doi: 10.1016/s1357-2725(99)00125-9. [DOI] [PubMed] [Google Scholar]
- Fries E, Kaczmarczyk A. Inter-alpha-inhibitor, hyaluronan and inflammation. Acta Biochim Pol. 2003;50:735–742. [PubMed] [Google Scholar]
- Gato A, Desmond ME. Why the embryo still matters: CSF and the neuroepithelium as interdependent regulators of embryonic brain growth, morphogenesis and histiogenesis. Dev Biol. 2009;327:263–272. doi: 10.1016/j.ydbio.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Gato A, Martin C, Alonso MI, Martinez-Alvarez C, Moro JA. Chondroitin sulphate proteoglycan is involved in lens vesicle morphogenesis in chick embryos. Exp Eye Res. 2001;73:469–478. doi: 10.1006/exer.2001.1060. [DOI] [PubMed] [Google Scholar]
- Gato A, Martin P, Alonso MI, Martin C, Pulgar MA, Moro JA. Analysis of cerebro-spinal fluid protein composition in early developmental stages in chick embryos. J Exp Zool A Comp Exp Biol. 2004;301:280–289. doi: 10.1002/jez.a.20035. [DOI] [PubMed] [Google Scholar]
- Gaudet CM, Lim YP, Stonestreet BS, Threlkeld SW. Effects of Age, Experience and Inter-alpha Inhibitor Proteins on Working Memory and Neuronal Plasticity after Neonatal Hypoxia-Ischemia. Behav Brain Res. 2016 doi: 10.1016/j.bbr.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AM, Berry M, Maher PA, Logan A, Baird A. A comprehensive analysis of the distribution of FGF-2 and FGFR1 in the rat brain. Brain Res. 1995;701:201–226. doi: 10.1016/0006-8993(95)01002-x. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol. 2012;71:444–457. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Mallard C, Ferriero DM, Vannucci SJ, Levison SW, Vexler ZS, Gressens P. The role of inflammation in perinatal brain injury. Nat Rev Neurol. 2015;11:192–208. doi: 10.1038/nrneurol.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton LC, Castillo-Melendez M, Walker DW. Uteroplacental inflammation results in blood brain barrier breakdown, increased activated caspase 3 and lipid peroxidation in the late gestation ovine fetal cerebellum. Dev Neurosci. 2007;29:341–354. doi: 10.1159/000105475. [DOI] [PubMed] [Google Scholar]
- Iwama H, Ohmori S, Tsutsumi T. Detectable concentrations of urinary trypsin inhibitor in cerebrospinal fluid. J Neurosurg Anesthesiol. 2000;12:29–32. doi: 10.1097/00008506-200001000-00006. [DOI] [PubMed] [Google Scholar]
- Jellema RK, Lima Passos V, Ophelders DR, Wolfs TG, Zwanenburg A, De Munter S, Nikiforou M, Collins JJ, Kuypers E, Bos GM, Steinbusch HW, Vanderlocht J, Andriessen P, Germeraad WT, Kramer BW. Systemic G-CSF attenuates cerebral inflammation and hypomyelination but does not reduce seizure burden in preterm sheep exposed to global hypoxia-ischemia. Exp Neurol. 2013;250:293–303. doi: 10.1016/j.expneurol.2013.09.026. [DOI] [PubMed] [Google Scholar]
- Jellema RK, Lima Passos V, Zwanenburg A, Ophelders DR, De Munter S, Vanderlocht J, Germeraad WT, Kuypers E, Collins JJ, Cleutjens JP, Jennekens W, Gavilanes AW, Seehase M, Vles HJ, Steinbusch H, Andriessen P, Wolfs TG, Kramer BW. Cerebral inflammation and mobilization of the peripheral immune system following global hypoxia-ischemia in preterm sheep. J Neuroinflammation. 2013;10:13. doi: 10.1186/1742-2094-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Duncan JA, 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Stopa EG, McMillan PN. The blood-cerebrospinal fluid barrier: structure and functional significance. Methods Mol Biol. 2011;686:101–131. doi: 10.1007/978-1-60761-938-3_4. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Szmydynger-Chodobska J, Chodobski A, Baird A, McMillan P, Stopa EG. Altered formation and bulk absorption of cerebrospinal fluid in FGF-2-induced hydrocephalus. Am J Physiol. 1999;277:R263–271. doi: 10.1152/ajpregu.1999.277.1.R263. [DOI] [PubMed] [Google Scholar]
- Justicia C, Perez-Asensio FJ, Burguete MC, Salom JB, Planas AM. Administration of transforming growth factor-alpha reduces infarct volume after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 2001;21:1097–1104. doi: 10.1097/00004647-200109000-00007. [DOI] [PubMed] [Google Scholar]
- Kashyap RS, Nayak AR, Deshpande PS, Kabra D, Purohit HJ, Taori GM, Daginawala HF. Inter-alpha-trypsin inhibitor heavy chain 4 is a novel marker of acute ischemic stroke. Clin Chim Acta. 2009;402:160–163. doi: 10.1016/j.cca.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Kendall G, Peebles D. Acute fetal hypoxia: the modulating effect of infection. Early Hum Dev. 2005;81:27–34. doi: 10.1016/j.earlhumdev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Kim CR, Sadowska GB, Petersson KH, Merino M, Sysyn GD, Padbury JF, Stonestreet BS. Effects of postnatal steroids on Na+/K+-ATPase activity and alpha1- and beta1-subunit protein expression in the cerebral cortex and renal cortex of newborn lambs. Reprod Fertil Dev. 2006;18:413–423. doi: 10.1071/rd05114. [DOI] [PubMed] [Google Scholar]
- Li XK, Matin AF, Suzuki H, Uno T, Yamaguchi T, Harada Y. Effect of protease inhibitor on ischemia/reperfusion injury of the rat liver. Transplantation. 1993;56:1331–1336. doi: 10.1097/00007890-199312000-00008. [DOI] [PubMed] [Google Scholar]
- Li XK, Suzuki H, Kimura T, Kawabe A, Uno T, Harada Y. Ulinastatin, a protease inhibitor, attenuates intestinal ischemia/reperfusion injury. Transplant Proc. 1994;26:2423–2425. [PubMed] [Google Scholar]
- Lim YP, Bendelja K, Opal SM, Siryaporn E, Hixson DC, Palardy JE. Correlation between mortality and the levels of inter-alpha inhibitors in the plasma of patients with severe sepsis. J Infect Dis. 2003;188:919–926. doi: 10.1086/377642. [DOI] [PubMed] [Google Scholar]
- Lim YP, Josic D, Callanan H, Brown J, Hixson DC. Affinity purification and enzymatic cleavage of inter-alpha inhibitor proteins using antibody and elastase immobilized on CIM monolithic disks. J Chromatogr A. 2005;1065:39–43. doi: 10.1016/j.chroma.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Malaeb S, Dammann O. Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol. 2009;24:1119–1126. doi: 10.1177/0883073809338066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaeb SN, Sadowska GB, Stonestreet BS. Effects of maternal treatment with corticosteroids on tight junction protein expression in the cerebral cortex of the ovine fetus with and without exposure to in utero brain ischemia. Brain Res. 2007;1160:11–19. doi: 10.1016/j.brainres.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams RM, Juul SE. The role of cytokines and inflammatory cells in perinatal brain injury. Neurol Res Int. 2012:561494. doi: 10.1155/2012/561494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahama H, Obata K, Sugita M. Ulinastatin ameliorates acute ischemic renal injury in rats. Ren Fail. 1996;18:893–898. doi: 10.3109/08860229609047715. [DOI] [PubMed] [Google Scholar]
- Noda M, Takii K, Parajuli B, Kawanokuchi J, Sonobe Y, Takeuchi H, Mizuno T, Suzumura A. FGF-2 released from degenerating neurons exerts microglial-induced neuroprotection via FGFR3-ERK signaling pathway. J Neuroinflammation. 2014;11:76. doi: 10.1186/1742-2094-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki K, Finklestein SP, Beal MF. Delayed administration of basic fibroblast growth factor protects against N-methyl-D-aspartate neurotoxicity in neonatal rats. Eur J Pharmacol. 1993;232:295–297. doi: 10.1016/0014-2999(93)90788-j. [DOI] [PubMed] [Google Scholar]
- O'Shea TM, Shah B, Allred EN, Fichorova RN, Kuban KC, Dammann O, Leviton A, Investigators ES. Inflammation-initiating illnesses, inflammation-related proteins, and cognitive impairment in extremely preterm infants. Brain Behav Immun. 2013;29:104–112. doi: 10.1016/j.bbi.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal SM, Artenstein AW, Cristofaro PA, Jhung JW, Palardy JE, Parejo NA, Lim YP. Inter-alpha-inhibitor proteins are endogenous furin inhibitors and provide protection against experimental anthrax intoxication. Infect Immun. 2005;73:5101–5105. doi: 10.1128/IAI.73.8.5101-5105.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal SM, Lim YP, Siryaporn E, Moldawer LL, Pribble JP, Palardy JE, Souza S. Longitudinal studies of inter-alpha inhibitor proteins in severely septic patients: a potential clinical marker and mediator of severe sepsis. Crit Care Med. 2007;35:387–392. doi: 10.1097/01.CCM.0000253810.08230.83. [DOI] [PubMed] [Google Scholar]
- Petersson KH, Pinar H, Stopa EG, Faris RA, Sadowska GB, Hanumara RC, Stonestreet BS. White matter injury after cerebral ischemia in ovine fetuses. Pediatr Res. 2002:768–776. doi: 10.1203/00006450-200206000-00019. [DOI] [PubMed] [Google Scholar]
- Raballo R, Rhee J, Lyn-Cook R, Leckman JF, Schwartz ML, Vaccarino FM. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J Neurosci. 2000;20:5012–5023. doi: 10.1523/JNEUROSCI.20-13-05012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid S, Ferretti P. Differential expression of fibroblast growth factor receptors in the developing murine choroid plexus. Brain Res Dev Brain Res. 2003;141:15–24. doi: 10.1016/s0165-3806(02)00635-1. [DOI] [PubMed] [Google Scholar]
- Riddle A, Dean J, Buser JR, Gong X, Maire J, Chen K, Ahmad T, Cai V, Nguyen T, Kroenke CD, Hohimer AR, Back SA. Histopathological correlates of magnetic resonance imaging-defined chronic perinatal white matter injury. Ann Neurol. 2011;70:493–507. doi: 10.1002/ana.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowska GB, Ahmedli N, Chen X, Stonestreet BS. Ontogeny of tight junction protein expression in the ovine cerebral cortex during development. Neuroscience. 2015;310:422–429. doi: 10.1016/j.neuroscience.2015.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowska GB, Stopa EG, Stonestreet BS. Ontogeny of connexin 32 and 43 expression in the cerebral cortices of ovine fetuses, newborns, and adults. Brain Res. 2009;1255:51–56. doi: 10.1016/j.brainres.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowska GB, Threlkeld SW, Flangini A, Sharma S, Stonestreet BS. Ontogeny and the effects of in utero brain ischemia on interleukin-1beta and interleukin-6 protein expression in ovine cerebral cortex and white matter. Int J Dev Neurosci. 2012;30:457–463. doi: 10.1016/j.ijdevneu.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders NR. Ontogeny of the blood-brain barrier. Exp Eye Res. 1977;25(Suppl):523–550. doi: 10.1016/s0014-4835(77)80046-8. [DOI] [PubMed] [Google Scholar]
- Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, Inder TE. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153:170–175. doi: 10.1016/j.jpeds.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Shu Y, Li R, Yang Y, Dai Y, Qiu W, Chen Y, Zhao Z, Lu Z, Hu X. Urinary trypsin inhibitor levels are reduced in cerebrospinal fluid of multiple sclerosis and neuromyelitis optica patients during relapse. Neurochem Int. 2015;81:28–31. doi: 10.1016/j.neuint.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Shu Y, Yang Y, Qiu W, Lu Z, Li Y, Bao J, Feng M, Hu X. Neuroprotection by ulinastatin in experimental autoimmune encephalomyelitis. Neurochem Res. 2011;36:1969–1977. doi: 10.1007/s11064-011-0520-4. [DOI] [PubMed] [Google Scholar]
- Singh K, Zhang LX, Bendelja K, Heath R, Murphy S, Sharma S, Padbury JF, Lim YP. Inter-alpha inhibitor protein administration improves survival from neonatal sepsis in mice. Pediatr Res. 2010;68:242–247. doi: 10.1203/PDR.0b013e3181e9fdf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasova MS, Sadowska GB, Threlkeld SW, Lim YP, Stonestreet BS. Ontogeny of inter-alpha inhibitor proteins in ovine brain and somatic tissues. Exp Biol Med (Maywood) 2014;239:724–736. doi: 10.1177/1535370213519195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD, National Institute of Child H, Human Development Neonatal Research N Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- Stonestreet BS, Patlak CS, Pettigrew KD, Reilly CB, Cserr HF. Ontogeny of blood-brain barrier function in ovine fetuses, lambs, and adults. Am J Physiol. 1996;271:R1594–1601. doi: 10.1152/ajpregu.1996.271.6.R1594. [DOI] [PubMed] [Google Scholar]
- Stridh L, Smith PL, Naylor AS, Wang X, Mallard C. Regulation of toll-like receptor 1 and -2 in neonatal mice brains after hypoxia-ischemia. J Neuroinflammation. 2011;8:45. doi: 10.1186/1742-2094-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlkeld SW, Gaudet CM, La Rue ME, Dugas E, Hill CA, Lim YP, Stonestreet BS. Effects of inter-alpha inhibitor proteins on neonatal brain injury: Age, task and treatment dependent neurobehavioral outcomes. Exp Neurol. 2014;261:424–433. doi: 10.1016/j.expneurol.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci SJ, Hagberg H. Hypoxia–ischemia in the immature brain. Journal of Experimental Biology. 2004;207:3149–3154. doi: 10.1242/jeb.01064. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Wang X, Xue Q, Yan F, Li L, Liu J, Li S, Hu S. Ulinastatin as a neuroprotective and anti-inflammatory agent in infant piglets model undergoing surgery on hypothermic low-flow cardiopulmonary bypass. Paediatr Anaesth. 2013;23:209–216. doi: 10.1111/pan.12073. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc Biol. 2005;78:1–8. doi: 10.1189/jlb.1104648. [DOI] [PubMed] [Google Scholar]
- Yano T, Anraku S, Nakayama R, Ushijima K. Neuroprotective effect of urinary trypsin inhibitor against focal cerebral ischemia-reperfusion injury in rats. Anesthesiology. 2003;98:465–473. doi: 10.1097/00000542-200302000-00028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.