Abstract
Aerobic fitness has previously been related to cognitive control in preadolescents; however, these investigations have generally relied on global measures of performance. Thus, we have little understanding of how aerobic fitness may relate to trial-by-trial modulations in cognitive control. This study utilized congruency sequence effects (CSEs), which characterize how behavior on the current trial is influenced by the previous trial, to investigate the relation of aerobic fitness on varying levels of cognitive control. One hundred eighty-seven children completed tests of aerobic fitness and a flanker task. Regressions were performed to determine relationships between CSE sequences and aerobic fitness while controlling for other potential confounding factors (e.g., age, sex, IQ). Lower-fit children were less able to modulate cognitive control during sequences requiring relatively less cognitive control. Additionally, lower-fit children were less able to adjust for variable levels of cognitive control during relatively more difficult sequences. Lastly, lower-fit children had longer reaction times (RTs) for all sequences in the condition requiring greater amounts of cognitive control. These findings corroborate the importance of aerobic fitness for cognitive control in school-aged children, and extend the literature by demonstrating a relationship between fitness and trial-by-trial modulations in control demands.
Keywords: physical activity, cognitive control, inhibitory control, trial-by-trial effects
1. Introduction
Reduced aerobic fitness levels in children (Olds, Tomkinson, Leger, & Cazorla, 2006; Salmon & Timperio, 2007) remain a growing concern as opportunities for physical activity are continuously being obviated from the school day (Castelli et al., 2014; Howie & Pate, 2012). Such a trend is particularly worrisome as sedentary behaviors have increased (Vaynman & Gomez-Pinilla, 2006) along with rates of obesity and type-2 diabetes (Eisenmann, 2003). Surprisingly, these changes have occurred despite findings that less aerobically fit children exhibit poorer performance on tests of academic achievement and other cognitive outcomes (Buck, Hillman, & Castelli, 2008; Castelli, Hillman, Buck, & Erwin, 2007; Chaddock, Erickson, Prakash, Kim, et al., 2010; Chaddock, Hillman, Buck, & Cohen, 2011; Chomitz et al., 2009; Dwyer, Sallis, Blizzard, Lazarus, & Dean, 2001; Eveland-Sayers, Farley, Fuller, Morgan, & Caputo, 2009; Hillman, Buck, Themanson, Pontifex, & Castelli, 2009a; Monti, Hillman, & Cohen, 2012; Scudder et al., 2014), leading many to suggest that schools should reconsider sacrificing daily physical activity opportunities for additional classroom time (Durant et al., 2009). Additionally, previous research has indicated that aerobic fitness plays an important role in the brain health of children (Chaddock, Pontifex, Hillman, & Kramer, 2011). This line of research has indicated that lower aerobic fitness is associated with smaller volumes in the dorsal striatum and globus pallidus, which are regions of the basal ganglia that are involved in cognitive control (Chaddock, Erickson, Prakash, VanPatter, et al., 2010), as well as smaller bilateral hippocampal volume, a brain region integral to aspects of memory (Chaddock, Erickson, Prakash, Kim, et al., 2010). Furthermore, measures of brain function have been collected using event related brain potentials (ERPs; Hillman, Buck, et al., 2009; Hillman, Castelli, & Buck, 2005; Pontifex et al., 2011) and functional magnetic resonance imaging (fMRI; Chaddock, Erickson, et al. 2012; Voss et al., 2011) have indicated beneficial associations between aerobic fitness and brain networks underlying cognitive control processes.
Cognitive control is one aspect of cognition that has received much attention due to its relationship with educational outcomes (Diamond, Barnett, Thomas, & Munro, 2007; Howie & Pate, 2012) and health behaviors (Diamond, 2013). It refers to top-down, goal directed behavior, and is comprised of inhibitory control (the ability to gate out distracting information or refrain from executing a prepotent response), working memory (the ability to store, maintain, and manipulate information within a brief period of time), and cognitive flexibility (the ability to shift attention and alter response strategy in response to changing task demands). Cognitive control is of considerable importance in children due to its underlying beneficial associations with academic performance (Diamond et al., 2007; Diamond & Lee, 2011) and protracted developmental trajectory throughout childhood (Luna, 2009). In particular, development and integration of the anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (DLPFC) has been shown to subserve cognitive control in preadolescents (Luna, 2009). Further, health behaviors and outcomes such as physical activity, aerobic fitness, and body composition have been found to relate to cognitive control performance (Hillman, Khan, & Kao, 2015). As such, there is continued interest in gaining a more comprehensive understanding of the beneficial relationship between aerobic fitness and cognitive control in children.
The Eriksen flanker task has been used extensively to study aspects of cognitive control, (Eriksen & Eriksen, 1974) and has helped reveal the importance of demographic factors, such as socioeconomic status (SES), that influence its development (Mezzacappa, 2004). In one version of this paradigm, participants are presented with an array of five arrows and are instructed to respond according to the directionality of the central, target arrow. Stimulus-congruent trials, which place low demand on cognitive control, involve flanking stimuli that are oriented in the same direction as the central target stimulus, whereas stimulus-incongruent flanking stimuli are oriented opposite to the target and require greater cognitive control to overcome perceptual interference. As a result, stimulus-incongruent trials result in greater difficulty as evidenced by longer reaction time (RT) and lower accuracy compared to stimulus-congruent trials (Hillman, Pontifex, et al., 2009; Pontifex et al., 2011; Voss et al., 2011). Task difficulty can be further increased by introducing a response-compatibility manipulation, wherein participants are instructed to respond either in the same direction (response-compatible) or in the opposite direction (response-incompatible) of the central target stimulus (Friedman, Nessler, Cycowicz, & Horton, 2009). Studies investigating fitness effects on cognitive control as indexed using the flanker task have found that lower-fit children demonstrate poorer overall performance (longer RT and decreased accuracy) when compared to their higher-fit peers (Chaddock, Erickson, Prakash, VanPatter, et al., 2010; Chaddock, Hillman, et al., 2012; Hillman, Buck, et al., 2009a; Pontifex et al., 2011; Scudder et al., 2014; Voss et al., 2011). Additionally, lower-fit children are disproportionately affected by tasks that require greater cognitive control demands, resulting in poorer performance compared to higher-fit children (Chaddock, Erickson, Prakash, VanPatter, et al., 2010; Kamijo et al., 2011; Pontifex et al., 2011; Scudder et al., 2014; Voss et al., 2011). A study conducted by Pontifex et al. (2011) found that lower-fit children were less able to flexibly modulate cognitive control as evidenced by a lack of modulation of the event related negativity (ERN) component, which has been localized to the dorsal portion of the ACC and is thought to reflect action-monitoring processes to enact top-down compensatory mechanisms in response to conflict or erroneous behaviors (Gehring, Liu, Orr, & Carp, 2011). Additionally, these children experienced greater response conflict, reduced attentional allocation, and slower processing speed, as indexed by increased N2 amplitude, decreased P3 amplitude, and increased latencies, respectively (Pontifex et al., 2011). Taken together, these findings suggest that not only is lower aerobic fitness associated with decreased overall cognitive control performance and less optimal neuroelectric profiles, but that these associations are greatest as task demands increase. Despite the robustness of these findings, researchers have yet to understand why this pattern of behavior occurs. Although some studies have suggested that higher- and lower-fit participants may elicit different cognitive control strategies to maintain performance (Pontifex et al., 2011; Voss et al., 2011), the observed findings may also be explained, in part, by the ability to overcome specific cognitive control demands encountered from sequential modulation of trial-by-trial congruency, also known as the ‘Gratton Effect’ or ‘congruency sequence effect’.
Congruency sequence effects (CSEs) allow insight into cognitive control ability under varying levels of cognitive demand, making them particularly useful for unveiling further details about the selective differences observed between higher- and lower-fit children. CSEs identify trial sequences that require greater levels of cognitive control, and are more likely to cause a behavioral misstep resulting in an incorrect response or delayed RT. The initial discovery of CSEs was reported by Gratton, Coles, and Donchin (1992), who discovered that stimulus-incongruent trials preceded by a stimulus-incongruent trial showed better performance than those preceded by stimulus-congruent trials. Typically, stimulus-congruent trials (n) preceded by a stimulus-congruent or stimulus-incongruent trial (n − 1) are described as cC and iC, respectively, with the preceding trial represented by a lower case letter. Similarly, stimulus-incongruent trials (n) preceded by a stimulus-congruent or stimulus-incongruent trial (n − 1) are described as cI and iI, respectively. Typical CSEs findings show that cC sequences are associated with the fastest and most accurate responses, while cI sequences are associated with the slowest and least accurate responses. Additionally, iC and iI sequences result in RTs that fall in the middle, with iI sequences having longer RTs and lower accuracy.
Accordingly, the present study sought to investigate whether CSEs are influenced by aerobic fitness given previous findings that lower-fit children demonstrate poorer performance on measures of cognitive control (Chaddock, Erickson, Prakash, Kim, et al., 2010; Chaddock, Erickson, Prakash, VanPatter, et al., 2010; Chaddock, Hillman, et al., 2011; Chaddock, Hillman, et al., 2012; Hillman, Buck, et al., 2009a; Pontifex et al., 2011; Scudder et al., 2014; Voss et al., 2011), particularly during high cognitive demand trials (Chaddock, Erickson, Prakash, VanPatter, et al., 2010; Pontifex et al., 2011; Voss et al., 2011). It was hypothesized that lower-fit children would exhibit poorer performance during sequences with the highest cognitive demands. In particular, lower-fit children would demonstrate longer RT and reduced accuracy during the cI sequence, as they would be less able to upregulate inhibitory control on incongruent trials following low conflict generated by the preceding congruent trial. Further, we predicted that higher-fit children would benefit from the upregulation of cognitive control after a higher demand trial and be better able to inhibit additional conflicting information during iI sequences. Collectively, the results from this study will help determine how greater aerobic fitness is associated with more efficient online adjustments of cognitive control.
2. Methods
2.1. Participants
A total of 187 healthy preadolescent children between the ages of 8–9 from the East-Central Illinois region were used in this analysis. This sample was drawn from a larger sample of 229 participants from the FITKids intervention trial (Hillman et al., 2014). Of the 229 participants, 25 were removed for not meeting aerobic fitness testing (VO2peak) criteria, 4 were removed for incomplete health and demographic information used in the analysis, and 13 were removed for outlier data in overall flanker performance (see Inhibitory Control Task section for criteria). Only cross sectional data derived from baseline measures were investigated for this study. Participants and their legal guardian signed informed assent and consent waivers approved by the Institutional Review Board (IRB) of the University of Illinois at Urbana-Champaign. Guardians were asked to complete a health history and demographics questionnaire as well as other documentation indicating that their child had normal or corrected-to-normal vision, was free of neurological diseases, and had no physical disabilities that could be exacerbated by exercise participation (Physical Activity Readiness Questionnaire [PAR-Q]; Thomas, Reading, & Shephard, 1992).
2.2. Procedure
On the first visit, demographic information including age, gender, race/ethnicity, pubertal stage (Taylor et al., 2001), and socioeconomic status (SES) were collected (Birnbaum et al., 2002; Hillman et al., 2012; see Table 1). SES was determined using a trichotomous index based on the following: (1) participation in free or reduced-price meal program at school, (2) the highest level of education obtained by the mother and father, and (3) number of parents who worked full-time (Birnbaum et al., 2002; Hillman et al., 2012). Participants completed the Kaufman Brief Intelligence Test (K-BIT; Kaufman & Kaufman, 1990) to assess IQ, and the PAR-Q to screen for health issues that may be exacerbated by physical exercise. Participants were then fitted with a Polar heart rate monitor (Model A1, Polar Electro, Finland), had their height and weight measured (Tanita WB-300 Plus digital scale and stadiometer; Tanita Corp, Tokyo, Japan), and completed a maximal exercise test to assess aerobic fitness. On the second visit, participants performed a modified flanker task to assess cognitive control, and received $10/hour for their participation.
Table 1.
Mean (SD) Values for Participant Demographic Data
| Measure | All | Lower fit | Higher fit |
|---|---|---|---|
| N | 187 (92 females) | 94 (60 females) | 93 (32 females) |
| Low SES (%) | 73 (39) | 47 (50) | 26 (28) |
| Age, y | 8.9 (0.6) | 8.9 (0.6) | 8.9 (0.6) |
| IQ | 112.0 (13.8) | 110.3 (13.1) | 113.7 (14.3) |
| Pubertal Timing | 1.4 (0.5) | 1.6 (0.5) | 1.3 (0.5) |
| VO2peak (mL/kg/min) | 38.6 (6.9) | 33.1 (4.4) | 44.1 (3.8) |
Note: A median split of the participants, based on VO2peak is provided for informational purposes.
IQ = K-BIT, Kaufman Brief Intelligence Test.
2.3. Aerobic fitness assessment
Aerobic fitness was assessed using a test of maximal oxygen consumption (VO2peak) measured on a motor-driven treadmill following a modified Balke protocol (American College of Sports Medicine [ACSM], 2010). This test employed a computerized indirect calorimetry system while participants’ walked/ran on a motor-driven treadmill at a constant speed with a 2.5% incremental grade increase every 2 minutes until volitional exhaustion. A Polar heart rate monitor (Model A1; Polar Electro, Finland) was used to measure heart rate throughout the test. Ratings of perceived exertion (RPE) were assessed every 2 min with the children’s OMNI scale (Utter, Robertson, Nieman, & Kang, 2002). Relative peak oxygen consumption was expressed in ml/kg/min and was based upon maximal effort as evidenced by: (1) a plateau in oxygen consumption corresponding to an increase of less than 2 ml/kg/min despite an increase in workload, (2) a peak heart rate ≥ 185 bpm (ACSM, 2010) and a heart rate plateau (Freedson & Goodman, 1993); (3) RER ≥ 1.0 (Bar-Or, 1983); and/or (4) ratings on the children’s OMNI scale of perceived exertion ≥ 8 (Utter et al., 2002).
2.4. Cognitive control task
Cognitive control was assessed using a modified flanker task, which has been previously used to measure children’s ability to inhibit unnecessary or interfering information in the stimulus environment (Mezzacappa, 2004; Pontifex et al., 2011; Voss et al., 2011). All stimuli were presented focally at a distance of approximately 1 m using Neuroscan Stim software version 4.5 (Compumedics, Charlotte, NC) and consisted of a child-friendly goldfish graphic amid bilaterally flanking goldfish. Stimulus-congruency was varied by manipulating the direction of the flanking fish in relation to the central target fish. Flanking stimuli were either stimulus-congruent (i.e., facing the same direction) or stimulus-incongruent (i.e., facing the opposite direction) to the central target stimulus. Stimuli, presented on a blue background, were 3 cm tall and appeared for 200 ms with a fixed inter-trial interval of 1700 ms.
In addition, two conditions were administered that manipulated response-compatibility. In the response-compatible condition participants were instructed to respond as quickly and accurately as possible with a thumb press according to the directionality of the target, while during the response-incompatible condition participants were instructed to respond in the opposite direction of the target. Two blocks of each response-compatibility condition were completed, and each block consisted of 75 trials with equiprobable stimulus-congruent and stimulus-incongruent trials. Response accuracy and reaction time (RT) were collected to assess behavioral performance. Outliers in the dependent variables were removed if they were less than 50% for overall accuracy and greater than 3 standard deviations for RT across response-compatible and response-incompatible conditions.
2.5. Congruency Sequence Effects
Sequential effect trial types were categorized by taking the n − 1 trial type (stimulus-congruent or stimulus-incongruent) and the current trial type (stimulus-congruent or stimulus-incongruent), which created four sequence trial types for each compatibility condition: (1) a congruent trial preceded by a congruent trial (cC), (2) a congruent trial preceded by an incongruent trial (iC), (3) an incongruent trial preceded by a congruent trial (cI), and (4) an incongruent trial preceded by an incongruent trial (iI), with the preceding trial represented by a lower case letter. The number of trials per condition ranged from 29–43. Response accuracy and RT were collected from the current trial (n) for each sequence trial type.
2.6. Statistical Analyses
A 2 (response-compatibility) × 2 (previous stimulus-congruency) × 2 (current stimulus-congruency) repeated measures ANOVA was conducted to determine overall performance for each sequence type between the two response-compatibility conditions. Follow-up paired t-tests were used to investigate sequence differences within each response-compatibility. Bonferroni correction for multiple comparisons was used to adjust for six comparisons (p = .008). Initial Pearson product-moment correlation analyses (Table 2) were conducted for the dependent variables from the flanker task (RT and accuracy for response-compatible and response-incompatible conditions across the cC, iC, cI, and iI sequences), fitness, age, sex (coded as 0 = female, 1 = male), SES, IQ, and pubertal timing to identify covariates for inclusion in the regression analysis. Hierarchical regression analyses were utilized to investigate variance in flanker task performance as it related to aerobic fitness (Step 2), independent of the variance associated with descriptive factors (Step 1). Assumptions of linearity, equality of variance, independence, and normality were plotted, inspected, and verified using Studentized residuals. Multicolinearity was not observed among any of the independent variables.
Table 2.
Intercorrelations Be tween Varia bles for Ac curacy and RT
| Subscale | Fitness | Age | IQ | Gender | SES | Pubertal Timing |
|---|---|---|---|---|---|---|
| Accuracy | ||||||
| Compatible cC | 0.20** | 0.23** | 0.13 | −0.07 | 0.02 | −0.09 |
| Compatible iC | 0.12 | 0.19** | 0.09 | −0.02 | 0.07 | −0.08 |
| Compatible cI | 0.07 | 0.15* | 0.07 | −0.05 | 0.03 | −0.04 |
| Compatible iI | 0.14 | 0.24** | 0.1 | −0.03 | 0.05 | 0.01 |
| Incompatible cC | 0.17* | 0.19** | 0.21** | 0.1 | 0.11 | −0.06 |
| Incompatible iC | 0.26** | 0.12 | 0.25** | 0.11 | 0.13 | −0.12 |
| Incompatible cI | 0.20** | 0.12 | 0.26** | 0.08 | 0.07 | −0.09 |
| Incompatible iI | 0.17* | 0.1 | 0.20** | 0.14 | 0.05 | −0.14 |
| RT | ||||||
| Compatible cC | −0.06 | −0.22** | −0.10 | −0.04 | 0.01 | 0.08 |
| Compatible iC | −0.10 | −0.25** | −0.11 | −0.06 | 0.00 | 0.11 |
| Compatible cI | −0.07 | −0.23** | −0.11 | −0.03 | 0.01 | 0.11 |
| Compatible iI | −0.01 | −0.19** | −0.14 | −0.07 | 0.02 | 0.1 |
| Incompatible cC | −0.25** | −0.16* | −0.21** | −0.15* | −0.14 | 0.05 |
| Incompatible iC | −0.24** | −0.21** | −0.24** | −0.13 | −0.11 | 0.07 |
| Incompatible cI | −0.23** | −0.19* | −0.25** | −0.12 | −0.12 | 0.08 |
| Incompatible iI | −0.24** | −0.20** | −0.21** | −0.13 | −0.14 | 0.05 |
p < .05.
p < .01.
3. Results
3.1. General congruency sequence effects
3.1.1. Accuracy
Figure 1 plots overall performance for each sequence. Main effects of response-compatibility [F(1,186) = 5.61, p = .019; η2 = .029], previous stimulus-congruency [F(1,186) = 24.29, p < .001; η2 = .116], and current stimulus-congruency [F(1,186) = 177.33, p < .001; η2 = .488] were superseded by two-way interactions of response-compatibility × previous stimulus-congruency [F(1,186) = 14.82, p < .001; η2 = .074], response-compatibility × current stimulus-congruency [F(1,186) = 34.31, p < .001; η2 = .156], and previous stimulus-congruency × current stimulus-congruency [F(1,186) = 41.10, p < .001; η2 = .181]. The 3-way interaction of response-compatibility × previous stimulus-congruency × current stimulus-congruency was not significant [F(1,186) = 2.73, p = .100; η2 = .014].
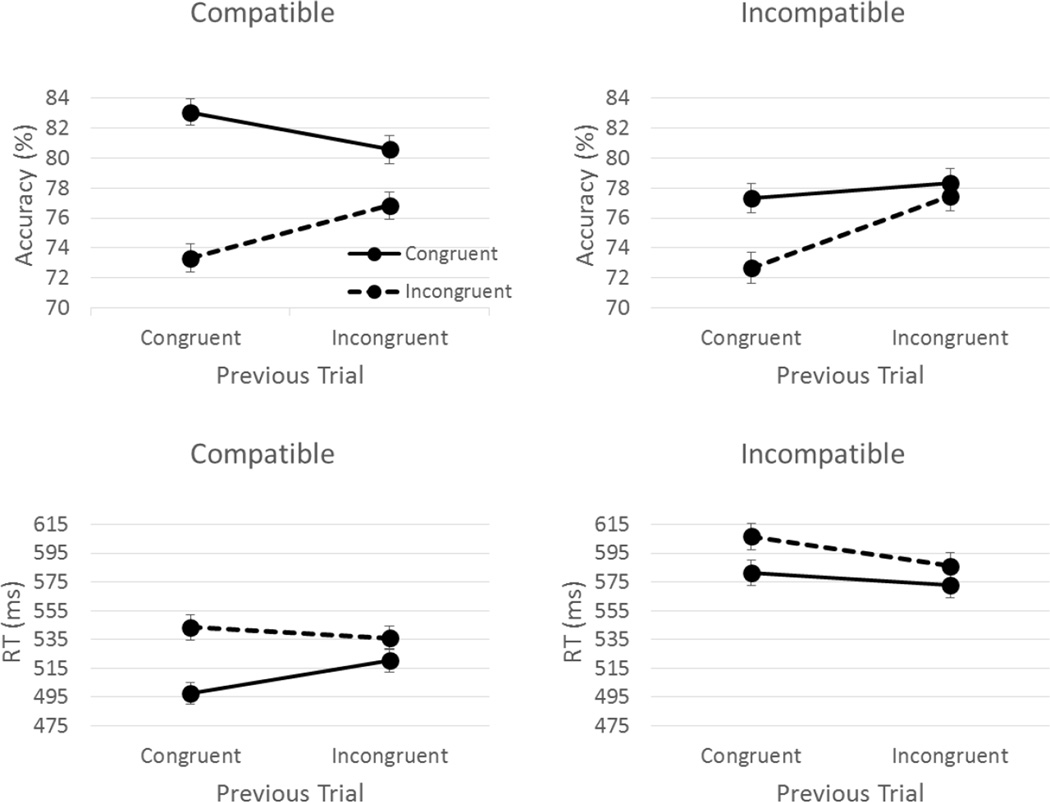
Figure 1.
CSEs within response-compatible and response-incompatible conditions. Mean accuracy and RT with error bars plotted as a function of the previous and current trial stimulus-congruency.
Post hoc tests of the response-compatibility × previous stimulus-congruency interaction indicated that the stimulus-congruent stimuli elicited increased accuracy during the response-compatible condition than the response-incompatible condition [t(186) = 3.81, p < .001; compatible congruent M = 78.2, incompatible congruent M = 75.0]. Additionally, post hoc tests of the response-compatibility × current stimulus-congruency interaction also indicated increased stimulus-congruent accuracy during the response-compatible condition than the response-incompatible condition [t(186) = 4.31, p < .001; compatible congruent M = 81.9, incompatible congruent M = 77.8]. Post hoc tests of the previous stimulus-congruency ×current stimulus-congruency revealed that there was no difference between cC and iC [t(186) = 1.65, p = .100; cC M = 80.2, iC M = 79.4] but cI had significantly lower accuracy than iI [t(186) = 7.33, p < .001; cI M = 73.00, iI M = 77.15].
3.1.2. RT
Main effects of response-compatibility [F(1,186) = 88.91, p < .001; η2 = .323], current stimulus-congruency [F(1,186) = 168.1, p < .001; η2 = .475], and a marginal effect of previous stimulus-congruency [F(1,186) = 3.28, p = .072; η2 = .017] were superseded by two-way interactions of response-compatibility × previous stimulus-congruency [F(1,186) = 48.14, p < .001; η2 = .206], response-compatibility × current stimulus-congruency [F(1,186) = 10.44, p = .001; η2 = .053], and previous stimulus-congruency × current stimulus-congruency [F(1,186) = 41.29, p < .001; η2 = .182]. Further, these two-way interactions were superseded by a 3-way interaction of response-compatibility × previous stimulus-congruency × current stimulus-congruency [F(1,186) = 7.42, p = .007; η2 = .038].
The three-way interaction was decomposed by comparing previous stimulus-congruency × current stimulus-congruency within each response-compatibility (compatible and incompatible). For the response-compatible condition, cC had shorter RTs than iC [t(186) = 7.70, p < .001; cC M = 497.40, iC M = 520.65] and iI had shorter RTs than cI [t(186) = 2.30, p = .022; iI M = 536.05, cI M = 543.38]. However, within the response-incompatible condition cC had longer RTs than iC [t(186) = 2.63, p = .009; cC M = 581.11, iC M = 572.51], though iI had shorter RTs than cI [t(186) = 5.05, p < .001; iI M = 586.13, cI M = 606.46].
3.2. Response accuracy regression
Table 2 summarizes the Pearson product-moment correlation analyses for inclusion in step 1 of the regression analyses. Table 3 summarizes regression results for response accuracy. Further, given that the step 1 results are not central to the study aims, they are provided in Table 3, rather than described herein. Hierarchical regression analysis indicated that individuals with lower-fitness exhibited poorer response accuracy across response-compatible cC (pr = .241), response-compatible iI (pr = .153), response-incompatible iC (pr = .210), and response-incompatible cI (pr = .158), t’s ≥ 2.10, p’s ≤ .04, β’s ≥ .161; independent of the demographic factors entered into step 1. No associations were found between fitness and response-compatible iC and cI or response-incompatible cC and iI (pr ≤ .130, t ≤ 1.77, p ≥ .08, β ≤ .138).
Table 3.
Summary of Hierarchic al Regress ion Analyses for Flanker Accuracy
| cC | iC | cI | iI | |||||
|---|---|---|---|---|---|---|---|---|
| Model and variable | ΔR2 | β | ΔR2 | β | ΔR2 | β | ΔR2 | β |
| Compatible | ||||||||
| Step 1 | .079** | .048* | .033 | .072** | ||||
| Age | .240** | .201** | .159* | .246** | ||||
| IQ | .148* | .105 | .090 | .127 | ||||
| Sex | −.068 | −.013 | −.046 | −.019 | ||||
| Step 2 | .053** | .016 | .007 | .022* | ||||
| Fitness | .252** | .138 | .092 | .161* | ||||
| Incompatible | ||||||||
| Step 1 | .097** | .094** | .094** | .072** | ||||
| Age | .215** | .147* | .148* | 0.125 | ||||
| IQ | .222** | .259** | .268** | .207** | ||||
| Sex | .101 | 0.109 | .081 | 0.134 | ||||
| Step 2 | .015 | .040** | .023* | 0.011 | ||||
| Fitness | .133 | .218** | .164* | 0.115 | ||||
p < .05.
p < .01.
3.3. Reaction time regression
Table 4 summarizes results for RT. Further, given that the step 1 results are not central to the study aims, they are provided in Table 4 rather than described in the text. Hierarchical analysis indicated that individuals with lower-fitness exhibited longer RTs across all response-incompatible sequences cC (pr = −.192), iC (pr = −.191), cI (pr = −.182), and iI (pr = −.190), t’s ≥ 2.50, p’s ≤ .01, β’s ≥ −.186; independent of the demographic factors entered into step 1. No significant findings were observed for response-compatible sequences, (pr’s ≤ −.077, t’s ≤ 1.04, p’s ≥ .30, β’s ≤ −.080).
Table 4.
Summary of Hierarchical Regression Analyses for Flanker RT
| cC | iC | cI | iI | |||||
|---|---|---|---|---|---|---|---|---|
| Model and variable | ΔR2 | β | ΔR2 | β | ΔR2 | β | ΔR2 | β |
| Compatible | ||||||||
| Step 1 | .067** | .084** | .070** | .065** | ||||
| Age | −.236** | −.262** | −.239** | −.205** | ||||
| IQ | −.124 | −.132 | −.128 | −.154* | ||||
| Sex | −.044 | −.070 | −.041 | −.071 | ||||
| Step 2 | .002 | .005 | .002 | .000 | ||||
| Fitness | −.042 | −.080 | −.048 | −.013 | ||||
| Incompatible | ||||||||
| Step 1 | .103** | .127** | .119** | .109** | ||||
| Age | −.190** | −.242** | −.215** | −.225** | ||||
| IQ | −.225** | −.251** | −.261** | −.224** | ||||
| Sex | −.154* | −.128 | −.121 | −.133 | ||||
| Step 2 | .033** | .032** | .029* | −.032* | ||||
| Fitness | −.198** | −.194** | −.186* | −.196* | ||||
p < .05.
p < .01.
4. Discussion
Empirical evidence has indicated that lower aerobic fitness is associated with decreased cognitive control (Buck et al., 2008; Castelli et al., 2007; Chaddock, Erickson, Prakash, Kim, et al., 2010; Chaddock, Hillman, et al., 2011; Chomitz et al., 2009; Dwyer et al., 2001; Eveland-Sayers et al., 2009; Hillman, Buck, et al., 2009a; Monti et al., 2012; Pontifex et al., 2011; Scudder et al., 2014; Voss et al., 2011) that is not only related to changes in brain structures such as decreased brain volume in select subcortical structures such as the basal ganglia and hippocampus (Chaddock, Erickson, Prakash, Kim, et al., 2010; Chaddock, Erickson, Prakash, VanPatter, et al., 2010), but also brain functions reflected by alterations in neuroelectric components such as N2, P3, and ERN amplitude (Hillman, Buck, et al., 2009b; Pontifex et al., 2011), and N2 and P3 latency (Pontifex et al., 2011). To further understand this relationship, we investigated the temporal dynamics of cognitive control by utilizing CSEs. Response accuracy and RT findings from the compatible flanker condition demonstrated a similar pattern of results to that observed in the literature (Egner, 2007). However, within the response-incompatible condition a differential pattern of effects was observed, but the classic CSE pattern still remained.
Previous findings using response-compatibility manipulations (e.g., Simon tasks) have revealed CSEs similar to those expected during flanker tasks (Egner, 2007). In the current study, the response-incompatible task combined multiple levels of cognitive control. That is, the response-compatible flanker condition introduced perceptual conflict when flanking arrows were stimulus-incongruent to the central target. However, the response-incompatible condition introduced stimulus-response conflict by requiring inhibition of the prepotent response (i.e., responding in the opposite direction of the target arrow) in addition to the inhibitory control requirements associated with perceptual interference and the activation of multiple response mappings. This increased demand for inhibitory control may account for the different pattern of effects in the response-incompatible condition.
Relative to group differences in aerobic fitness, lower-fit children exhibited lower accuracy in the response-compatible cC and iI sequences and response-incompatible iC and cI sequences, suggesting that fitness is selectively associated with different aspects of cognitive control based, in part, on the difficulty of task demands. Lower-fit children also exhibited longer RTs during all response-incompatible sequences (cC, iC, cI, iI), suggesting a generalized relationship between fitness and response speed during conditions placing greater demand on the control over stimulus-response mappings. In tasks requiring cognitive control, children are typically more impulsive and maintain RT at the cost of accuracy on more difficult trials (Davidson, Amso, Anderson, & Diamond, 2006). Thus, it is not surprising that accuracy effects were observed between fitness groups across both response-compatible and response-incompatible conditions, while RT differences were only observed during the response-incompatible condition that required the greatest amount of cognitive control.
The CSEs observed herein replicate prior accuracy results (Hillman, Buck, et al., 2009a; Voss et al., 2011), indicating that lower-fit children demonstrate overall lower performance during the response-compatible flanker task. That is, lower-fit children exhibited lower response accuracy in cC and iI sequences, which contributes to worse performance in both stimulus-congruent and stimulus-incongruent trials. The reduced response accuracy of cC and iI sequences for lower-fit children may be the result of poorer ability to modulate cognitive control. In addition to the response-compatible condition, the current findings revealed the relation of aerobic fitness to response accuracy during the response-incompatible condition for sequences requiring the flexible regulation of cognitive control (i.e., cI and iC sequences). These results not only replicated Pontifex et al. (2011), such that higher-fit children exhibited greater response accuracy compared to lower-fit children during the response-incompatible condition, but also demonstrated that this relationship may be attributed to the increased response accuracy during cI and iC sequences for higher-fit relative to lower-fit children.
Further, the current CSEs on RT are consistent with previous fitness studies, such that no differences in RT have been demonstrated between lower- and higher-fit children in response-compatible flanker tasks (Chaddock, Erickson, Prakash, VanPatter, et al., 2010; Hillman, Buck, et al., 2009a; Pontifex et al., 2011). Although Chaddock, Erickson, Prakash, VanPatter, et al. (2010) reported that lower-fit children exhibited increased interference RT (i.e. incongruent RT – congruent RT) compared to higher-fit children, the study did not demonstrate raw RT differences between the groups. The current findings further revealed that lower-fit children exhibited longer RTs than their higher-fit counterparts during the response-incompatible condition, suggesting that they were less able to flexibly adjust to greater task demands and less efficient at inhibiting incorrect prepotent responses.
One interpretation of CSEs is described by the conflict-monitory theory (Botvinick, Braver, Barch, Carter, & Cogen, 2001), which holds that CSEs arise when conflicting information (i.e., conditions requiring greater amounts of inhibitory control) is detected and inhibitory control is modulated to meet these demands. As such, inhibitory control is temporarily upregulated following greater amounts of conflict (i.e., stimulus-incongruent trials) and temporarily downregulated following lower amounts of conflict (i.e., stimulus-congruent trials). This results in the classic CSE improvement in stimulus-incongruent responses when followed by a previously incongruent trial as opposed to a previously congruent trial (Gratton et al., 1992). Within the framework of the conflict-monitoring theory, CSEs provide a window in which to examine online adjustments in inhibitory control (Duthoo, Abrahamse, Braem, Boehler, & Notebaert, 2014). Given that the current findings show a positive association between aerobic fitness and response accuracy for iC and cI sequences in the response-incompatible condition, it is plausible that children with higher aerobic fitness exhibited a greater capability for online adjustment of inhibitory control relative to their lower-fit counterparts. Additionally, the conflict-monitoring theory has clear predictions regarding the neurobiological underpinnings and holds that the ACC is responsible for monitoring under such environmental demands, while the DLPFC is responsible for adjustments in top-down cognitive control (Botvinick et al., 2001). Although speculative, because previous research has indicated association between aerobic fitness and cognitive control through functional changes in ACC (for review see Chaddock-Heyman, Hillman, Cohen, & Kramer, 2014) the current findings may be explained by increased integration of these systems in higher-fit children, allowing for improved conflict monitoring and possibly more efficient adjustments to varying levels of cognitive control demand, particularly during the most difficult sequences.
An alternative explanation is provided by the feature integration hypothesis (Hommel, Proctor, & Vu, 2004; Mayr, Awh, & Laurey, 2003), which holds that during a trial, stimulus and response features become temporarily bound together into a single episodic memory representation. Thus, on subsequent trials, if common features of the stimulus-response representation are detected then the other features will automatically be activated. As a consequence, the necessity of updating working memory may be different between sequences with complete stimulus repetition and sequences with partial stimulus repetition. That is, cC and iI sequences involve complete stimulus repetitions and require less working memory updating, resulting in superior task performance. In contrast, iC and cI sequences involve only partial stimulus repetitions and require more working memory updating, resulting in inferior task performance. However, to the best of our knowledge there are no existing studies investigating the neurobiological underpinnings of the feature integration explanation for CSEs.
In the current study it is impossible to differentiate between the effects described by the conflict-monitoring theory and feature integration hypothesis, and it is likely that both adjustments in inhibitory control (conflict-monitoring theory) and updating of working memory (feature integration) contribute to cognitive control performance during CSEs (Duthoo et al., 2014). Given that performance accuracy in cC and iI sequences may be associated with feature integration (Hommel et al., 2004; Mayr et al., 2003) from the previous n − 1 trial, the increased response accuracy of response-compatible cC and iI sequences for higher-fit children may be the result of the ability to better modulate inhibitory control, while still benefiting from stimulus repetition. In contrast, lower-fit children engaged in inhibitory control at the expense of potentially blocking the feature integration from the n − 1 trial, fail to benefit from the stimulus repetition of the previous trial (i.e., less flexibility of inhibitory control may lead to over-inhibition of the feature integration on the n − 1 trial). Additionally, because the success in response-incompatible cI and iC sequences may be associated with blocking the feature integration from the n − 1 trial and the additional demand of inhibitory control elicited by stimulus-response mapping compatibility, the increased response accuracy of response-incompatible cI and iC sequences in higher-fit children may reflect their ability to more flexibly regulate inhibitory control while inhibiting the prepotent response caused by the feature integration from the n − 1 trial. In contrast, because inhibitory control requirements increased due to the response-incompatible stimulus-response mapping, competition for cognitive resources in lower-fit children may lead to insufficient inhibitory control allocation during feature integration from the n − 1 trial, introducing greater interference during cI and iC sequences and resulting in less accurate responses. However, these interpretations are speculative because little evidence exists to determine the brain regions involved in the feature integration account of CSEs. That being said, the pattern of CSEs in relation to aerobic fitness in the current study still provides preliminary evidence as a basis for future study to investigate the relationship of aerobic fitness with conflict-monitoring and feature integration accounts separately.
5. Study Limitations
To the best of our knowledge, this study is the first attempt to investigate the association of childhood aerobic fitness with modulations in cognitive control using CSEs. However, as indicated above, the task employed does not allow for teasing apart predictions related to the feature integration hypothesis from conflict-monitoring theory, thus future research should explore methods for dissociating between conflict adaptation and feature integration, to facilitate determination of whether aerobic fitness affects these two processes differently. Additionally, neuroelectric measures would help elucidate differences in the allocation of attentional resources during different levels of cognitive control demand between higher- and lower-fit children. Further, the causal relationship between aerobic fitness and trial-by-trial modulation of inhibitory control and working memory cannot be concluded due to the cross-sectional nature of this study, which warrants the need for future longitudinal research.
6. General Conclusions
In conclusion, the current study used CSEs to demonstrate a beneficial relationship between aerobic fitness and varying levels of cognitive control in preadolescent children. Lower-fit children were less able to utilize aspects of feature integration and adjust for variable levels of inhibition during task conditions requiring relatively less cognitive control. Additionally, lower-fit children were less able to modulate cognitive control while also adjusting to changes in features from trial n − 1 to trial n during the relatively more difficult sequences. As schools continue to provide less physical activity time during the school day (Castelli et al., 2014; Howie & Pate, 2012) and children are becoming less aerobically fit due to increased participation in sedentary lifestyles (Olds et al., 2006; Salmon & Timperio, 2007; Vaynman & Gomez-Pinilla, 2006), these findings add support to the growing body of literature indicating the importance of aerobic fitness to cognitive health during childhood.
Research Highlights.
Children have become increasingly less aerobically fit.
Previous research has demonstrated relationships between fitness and cognitive control.
Congruency sequence effects investigate modulations of cognitive control.
Lower fitness was differentially associated with cognitive control demands.
Acknowledgments
This research was supported by a grant from the National Institutes of Health (NIH) grant ROI HD055352 (to Charles Hillman).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors declare no potential conflicts of interest or competing financial interests.
References
- American College of Sports Medicine (ACSM) ACSM's guidelines for exercise testing and prescription. 8th. New York: Loppincott Williams & Wilkins; 2010. [Google Scholar]
- Bar-Or O. Pediatric sports medicine for the practitioner: From physiologic principles to clinical applications. New York: Springer-Verlag; 1983. [Google Scholar]
- Birnbaum AS, Lytle LE, Murray DM, Story M, Perry CL, Boutelle KN. Survey Development for Assessing Correlated of Young Adolescents' Eating. American Journal of Health Behavior. 2002;26(4):284–295. doi: 10.5993/ajhb.26.4.5. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cogen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Buck SM, Hillman CH, Castelli DM. The relation of aerobic fitness to stroop task performance in preadolescent children. Medicine and Science in Sports and Exercise. 2008;40(1):166–172. doi: 10.1249/mss.0b013e318159b035. [DOI] [PubMed] [Google Scholar]
- Castelli DM, E CE, Hwang J, Barcelona JM, Glowacki EC, Calvert HG, Nicksic HM. VII. The history of physical activity and academic performance research: Informing the future. Monographs of the Society for Research in Child Development. 2014;79(4):119–148. doi: 10.1111/mono.12133. [DOI] [PubMed] [Google Scholar]
- Castelli DM, Hillman CH, Buck SM, Erwin HE. Physical fitness and academic achievement in third- and fifth-grade students. Journal of Sport & Exercise Psychology. 2007;29(2):239–252. doi: 10.1123/jsep.29.2.239. [DOI] [PubMed] [Google Scholar]
- Chaddock-Heyman L, Hillman CH, Cohen NJ, Kramer AF. III. The importance of physical activity and aerobic fitness for cognitive control and memory in children. Monographs of the Society for Research in Child Development. 2014;79(4):25–50. doi: 10.1111/mono.12129. [DOI] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, Kramer AF. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172–183. doi: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, VanPatter M, Voss MW, Pontifex MB, Kramer AF. Basal ganglia volume is associated with aerobic fitness in preadolescent children. Developmental Neuroscience. 2010;32(3):249–256. doi: 10.1159/000316648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Voss MW, VanPatter M, Pontifex MB, Kramer AF. A functional MRI investigation of the association between childhood aerobic fitness and neurocognitive control. Biological Psychology. 2012;89(1):260– 268. doi: 10.1016/j.biopsycho.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Chaddock L, Hillman CH, Buck SM, Cohen NJ. Aerobic fitness and executive control of relational memory in preadolescent children. Med Sci Sports Exerc. 2011;43(2):344– 349. doi: 10.1249/MSS.0b013e3181e9af48. [DOI] [PubMed] [Google Scholar]
- Chaddock L, Hillman CH, Pontifex MB, Johnson CR, Raine LB, Kramer AF. Childhood aerobic fitness predicts cognitive performance one year later. Journal of Sports Sciences. 2012;30(5):421–430. doi: 10.1080/02640414.2011.647706. [DOI] [PubMed] [Google Scholar]
- Chaddock L, Pontifex MB, Hillman CH, Kramer AF. A review of the relation of aerobic fitness and physical activity to brain structure and function in children. Journal of the International Neuropsychological Society. 2011;17(6):975–985. doi: 10.1017/S1355617711000567. [DOI] [PubMed] [Google Scholar]
- Chomitz VR, Slining MM, McGowan RJ, Mitchell SE, Dawson GF, Hacker KA. Is there a relationship between physical fitness and academic achievement? Positive results from public school children in the northwestern united states. Journal of School Health. 2009;79(1):30–37. doi: 10.1111/j.1746-1561.2008.00371.x. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44(11):2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annual Review Psychology. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318(5855):1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011;333(6045):959–964. doi: 10.1126/science.1204529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant N, Harris SK, Doyle S, Person S, Saelens BE, Kerr J, Sallis JF. Relation of School Environment and Policy to Adolescent Physical Activity. Journal of School Health. 2009;79(4):153–159. doi: 10.1111/j.1746-1561.2008.00384.x. [DOI] [PubMed] [Google Scholar]
- Duthoo W, Abrahamse EL, Braem S, Boehler CN, Notebaert W. The heterogeneous world of congruency sequence effects: an update. Frontiers in Psychology. 2014;5 doi: 10.3389/fpsyg.2014.01001. 1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer T, Sallis JF, Blizzard L, Lazarus R, Dean K. Relation of academic performance to physical activity and fitness in children. Pediatric Exercise Science. 2001;(13):225–237. [Google Scholar]
- Egner T. Congruency sequence effects and cognitive control. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(4):380–390. doi: 10.3758/cabn.7.4.380. [DOI] [PubMed] [Google Scholar]
- Eisenmann JC. Secular trends in variables associated with the metabolic syndrome of North American children and adolescents: a review and synthesis. American Journal of Human Biology. 2003;15:786–794. doi: 10.1002/ajhb.10214. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16(1):143–149. [Google Scholar]
- Eveland-Sayers BM, Farley RS, Fuller DK, Morgan DW, Caputo JL. Physical fitness and academic achievement in elementary school children. Journal of Physical Activity and Health. 2009;6:99–104. doi: 10.1123/jpah.6.1.99. [DOI] [PubMed] [Google Scholar]
- Freedson PS, Goodman TL. Measurement of oxygen consumption. In: Rowland TW, editor. Pediatric laboratory exercise testing: Clinical guidelines. Champaign, IL: Human Kinetics; 1993. pp. 91–113. [Google Scholar]
- Friedman D, Nessler D, Cycowicz YM, Horton C. Development of and change in cognitive control: a comparison of children, young adults, and older adults. Cognitive, Affective, & Behavioral Neuroscience. 2009;9(1):91–102. doi: 10.3758/CABN.9.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Liu Y, Orr JM, Carp J. The Error-Related Negativity (ERN/Ne) In: Kappenman ES, Luck SJ, editors. The Oxford Handbook of Event-Related Potential Components. Oxford University Press; 2011. [Google Scholar]
- Gratton G, Coles MGH, Donchin E. Optimizing the use of information: Strategic control of activation of responses. Journal of Experimental Psychology: General. 1992;121(4):480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Buck SM, Themanson JR, Pontifex MB, Castelli DM. Aerobic fitness and cognitive development: Event-related brain potential and task performance indices of executive control in preadolescent children. Developmental Psychology. 2009a;45(1):114–129. doi: 10.1037/a0014437. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Buck SM, Themanson JR, Pontifex MB, Castelli DM. Aerobic fitness and cognitive development: Event-related brain potential and task performance indices of executive control in preadolescent children. Dev Psychol. 2009b;45(1):114–129. doi: 10.1037/a0014437. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Castelli DM, Buck SM. Aerobic fitness and neurocognitive function in healthy preadolescent children. Med Sci Sports Exerc. 2005;37(11):1967–1974. doi: 10.1249/01.mss.0000176680.79702.ce. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Khan NA, Kao SC. The Relationship of Health Behaviors to Childhood Cognition and Brain Health. Annals of Nutrition and Metabolism. 2015;66(Suppl 3):1–4. doi: 10.1159/000381237. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Pontifex MB, Castelli DM, Khan NA, Raine LB, Scudder MR, Kamijo K. Effects of the FITKids randomized controlled trial on executive control and brain function. Pediatrics. 2014;134(4):e1063–e1071. doi: 10.1542/peds.2013-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman CH, Pontifex MB, Motl RW, O’Leary KC, Johnson CR, Scudder MR, Castelli DM. From ERPs to academics. Developmental Cognitive Neuroscience. 2012;2:S90–S98. doi: 10.1016/j.dcn.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman CH, Pontifex MB, Raine LB, Castelli DM, Hall EE, Kramer AF. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience. 2009;159(3):1044–1054. doi: 10.1016/j.neuroscience.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B, Proctor RW, Vu KP. A feature-integration account of sequential effects in the Simon task. Psychological Research. 2004;68(1):1–17. doi: 10.1007/s00426-003-0132-y. [DOI] [PubMed] [Google Scholar]
- Howie EK, Pate RR. Physical activity and academic achievement in children: A historical perspective. Journal of Sport and Health Science. 2012;1(3):160–169. [Google Scholar]
- Kamijo K, Pontifex MB, O'Leary KC, Scudder MR, Wu CT, Castelli DM, Hillman CH. The effects of an afterschool physical activity program on working memory in preadolescent children. Dev Sci. 2011;14(5):1046–1058. doi: 10.1111/j.1467-7687.2011.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Manual for the Kaufman Brief Intelligence Test. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Luna B. Developmental changes in cognitive control through adolescence. Advances in Child Development and Behavior. 2009;37:233–278. doi: 10.1016/s0065-2407(09)03706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nature Neuroscience. 2003;6(5):450–452. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- Mezzacappa E. Alerting, Orienting, and Executive Attention: Developmental Properties and Sociodemographic Correlates in an Epidemiological Sample of Young, Urban Children. Child Development. 2004;75(5):1373–1386. doi: 10.1111/j.1467-8624.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- Monti JM, Hillman CH, Cohen NJ. Aerobic fitness enhances relational memory in preadolescent children: the FITKids randomized control trial. Hippocampus. 2012;22(9):1876–1882. doi: 10.1002/hipo.22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds T, Tomkinson G, Leger L, Cazorla G. Worldwide variation in the performance of children and adolescents: an analysis of 109 studies of the 20-m shuttle run test in 37 countries. Journal of Sports Sciences. 2006;24(10):1025–1038. doi: 10.1080/02640410500432193. [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Raine LB, Johnson CR, Chaddock L, Voss MW, Cohen NJ, Hillman CH. Cardiorespiratory fitness and flexible modulation of cognitive control in preadolescent children. Journal of Cognitive Neuroscience. 2011;23(6):1332–1345. doi: 10.1162/jocn.2010.21528. [DOI] [PubMed] [Google Scholar]
- Salmon J, Timperio A. Prevalence, trends and environmental influences on child and youth physical activity. Medicine and Sport Sciences. 2007;50:183–199. doi: 10.1159/000101391. [DOI] [PubMed] [Google Scholar]
- Scudder MR, Lambourne K, Drollette ES, Herrmann SD, Washburn RA, Donnelly JE, Hillman CH. Aerobic capacity and cognitive control in elementary school-age children. Medicine & Science in Sports & Exercise. 2014;46(5):1025–1035. doi: 10.1249/MSS.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SJC, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatric and Perinatal Epidemiology. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q) Canadian Journal of Sports Sciences. 1992;17(4):338–345. [PubMed] [Google Scholar]
- Utter AC, Robertson RJ, Nieman DC, Kang J. Children's OMNI scale of perceived exertion: Walking/running evaluation. Medicine & Science in Sports & Exercise. 2002;34(1):139–144. doi: 10.1097/00005768-200201000-00021. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. Revenge of the "sit": how lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. Journal of Neuroscience Research. 2006;84(4):699–715. doi: 10.1002/jnr.20979. [DOI] [PubMed] [Google Scholar]
- Voss MW, Chaddock L, Kim JS, Vanpatter M, Pontifex MB, Raine LB, Kramer AF. Aerobic fitness is associated with greater efficiency of the network underlying cognitive control in preadolescent children. Neuroscience. 2011;199:166–176. doi: 10.1016/j.neuroscience.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]