Abstract
Coronary artery disease (CAD) remains a significant global public health burden despite advancements in prevention and therapeutic strategies. Common non-invasive imaging modalities, anatomic and functional, are available for the assessment of patients with stable chest pain. Exercise electrocardiography is a long-standing method for evaluation for CAD and remains the initial test for the majority of patients who can exercise adequately with a baseline interpretable electrocardiogram. The addition of cardiac imaging to exercise testing provides incremental benefit for accurate diagnosis for CAD and is particularly useful in patients who are unable to exercise adequately and/or have uninterpretable electrocardiograms. Radionuclide myocardial perfusion imaging and echocardiography with exercise or pharmacological stress provide high sensitivity and specificity in the detection and further risk stratification of patients with CAD. Recently, coronary computed tomography angiography has demonstrated its growing role to rule out significant CAD given its high negative predictive value. Although less available, stress cardiac magnetic resonance provides a comprehensive assessment of cardiac structure and function and provides a high diagnostic accuracy in the detection of CAD. The utilization of non-invasive testing is complex due to various advantages and limitations, particularly in the assessment of low- and intermediate-risk patients with chest pain, where no single study is suitable for all patients. This review will describe currently available non-invasive modalities, along with current evidence-based guidelines and appropriate use criteria in the assessment of low- and intermediate-risk patients with suspected, stable CAD.
Keywords: Coronary artery disease, Risk stratification, Non-invasive imaging, Exercise electrocardiography, Radionuclide myocardial perfusion imaging, Stress echocardiography, Coronary computed tomography angiography, Cardiac magnetic resonance imaging
Introduction
Despite recent trends showing a decline in cardiovascular morbidity and mortality, cardiovascular disease (CVD) remains the leading cause of death in the United States. Every year, approximately 635,000 Americans will have a new coronary event, 300,000 will have a recurrent event, and an estimated 155,000 will suffer a silent first myocardial infarction [1]. Chest pain remains the most common reason for ER visits in men older than 15 years and is the second most frequent cause of ER encounters in adults with more than eight million visits a year [2]. Early recognition and diagnosis remains crucial to the survival of these patients. Unfortunately, 2% (160,000) of patients are discharged inadvertently from the ER and are not recognized as having a coronary event [3]. This group of patients carries a higher risk of death than those who are hospitalized with an acute coronary syndrome (ACS).
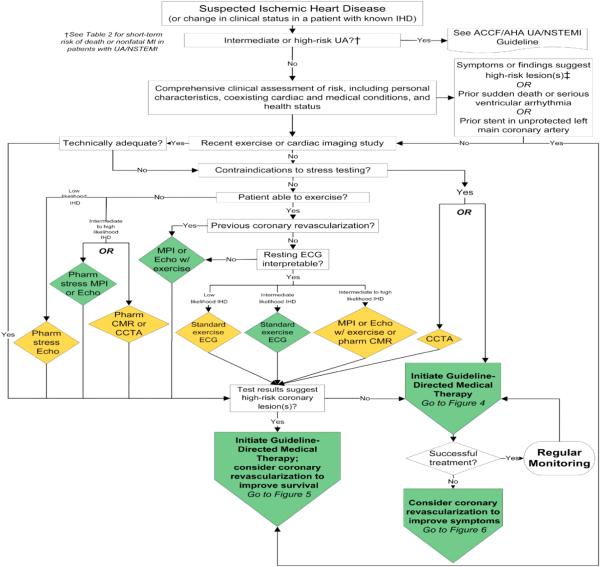
New diagnostic strategies (chest pain units, new highly sensitive biomarkers, and risk questionnaires) have been utilized in the assessment of patients with chest pain; however, evaluation of chest pain in low- and intermediate-risk patients continues to be a diagnostic challenge. Patel et al. [4] showed that only slightly more than one-third of symptomatic patients without known CAD who underwent elective invasive coronary angiography (ICA) had obstructive coronary disease. However, patients with a positive non-invasive test were moderately more likely to have obstructive CAD than those who did not undergo any testing and were referred only based on risk factors and symptoms at the discretion of the referring physician. Thus, although the current gold standard for diagnosis of CAD, ICA is not indicated for the initial step for all patients with chest pain, particularly those at low-to-intermediate risk (Fig.) [5].
Fig.
Diagnosis of patients with suspected IHD. Colors correspond to the class of recommendations in the ACCF/AHA (Table) [5]. The algorithms do not represent a comprehensive list of recommendations (see full guideline text Ref. [5] for all recommendations and additional Tables and Figures). †See Table 2 of Ref. [5] for short-term risk of death or non-fatal MI in patients with UA/NSTEMI. ‡CCTA is reasonable only for patients with intermediate probability of IHD. CCTA, computed coronary tomography angiography; CMR, cardiac magnetic resonance; ECG, electrocardiogram; Echo, echocardiography; IHD, ischemic heart disease; MI, myocardial infarction; MPI, myocardial perfusion imaging; Pharm, pharmacological; UA, unstable angina; UA/NSTEMI, unstable angina/non-ST-elevation myocardial infarction. (Adapted with permission from Fihn et al. [5].) (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
There are several well-established non-invasive testing modalities for the diagnosis of CAD including exercise stress electrocardiography (ExECG) testing, echocardiography and radionuclide myocardial perfusion imaging (MPI) using exercise or pharmacologic stress, coronary computed tomography angiography (CCTA), and cardiac magnetic resonance imaging (CMR). These non-invasive modalities vary with respect to several factors including, but not limited to, diagnostic accuracy, availability, costs, ionizing radiation exposure, and clinical expertise. This review will further explore the role of non-invasive testing, both anatomic and functional, for detection of myocardial ischemia and CAD in patients with low- and intermediate-risk chest pain.
Non-invasive imaging modalities
Exercise stress electrocardiography
ExECG remains the recommended initial diagnostic test modality by the American College of Cardiology and American Heart Association (ACC/AHA) guidelines on exercise testing, diagnosis, and management of stable ischemic heart disease in patients with intermediate pre-test probability who are able to exercise and have an interpretable rest electrocardiogram (ECG) [5]. Two decades ago, stress testing that involved ExECG was the most common form of stress either with or without imaging, accounting for more than two-third of total exercise stress testing [10]. However, despite current recommendations, ExECG is now underutilized and the growth of imaging testing continues to rise every year in the United States [6]. In 2009, more than nine million myocardial perfusion imaging (MPI) studies were performed at a cost of more than $1 billion [7]. Andrus and Welch [8] found that the use of stress test imaging ordered by cardiologists increased significantly among Medicare beneficiaries between the period of 1999 and 2008; nuclear cardiology stress testing increased 3.2-fold and stress echocardiography around 11%. Utilizing cross-sectional data from the National Ambulatory Medical Care and National Hospital Ambulatory Care Surveys from 1993–2010, Ladapo et al. [9] found a similar trend; cardiac stress test with imaging increased from 59% in 1993–1995 to 87% in 2008–2010. Furthermore, 35% were considered inappropriate, with associated annual costs and estimated harms of $501 million and 491 future cases of cancer.
The use of ExECG can identify and help stratify patients with intermediate-risk probability for cardiac events despite its relative low sensitivity and specificity. Detrano et al. [11] reported a sensitivity of 68% and specificity of 77% for the detection of coronary artery disease (CAD) in a meta-analysis that included over 24,000 patients and 22 years of research. Exercise-induced ST-segment depression is considered the strongest predictor of future cardiac events along with the duration of exercise and exercise capacity [12]. The Duke Treadmill Score (DTS) is a widely accepted tool used by many clinicians providing added prognostic information by combining exercise duration and ST changes and angina during exercise [13]. However, DTS is less useful when the test result falls in the intermediate-risk category, which leads to physicians ordering additional imaging to increase the test's accuracy [14].
The information obtained during exercise, even if the patient is not able to achieve target heart rate, enhances the ability to provide an accurate risk assessment. Bourque et al. [15] found that in a prospective cohort of patients referred for SPECT MPI with intermediate-to-high clinical risk for CAD, achieving ≥10 METs with no ischemic ST-segment depression was associated with 0.4% prevalence of significant ischemia (≥10% left ventricular ischemia), very low rates of cardiac death (0.1%/year), and non-fatal MI (0.7%/year). A subsequent study was able to show similar results in those who achieve ≥10 METs during ExECG regardless of peak exercise heart rate achieved [16]. There are other markers obtained during ExECG that could be used for prognostication. Christman et al. [17] found a low rate of composite end point of cardiovascular death, non-fatal MI, or coronary revascularization in patients with positive ExECG with rapid ST-segment recovery. Uthamalingam et al. [18] demonstrated that ≥1-mm ST-segment elevation in lead AVR is a strong predictor of obstructive left main (LM) or ostial left anterior descending artery (LAD). A strategy based on exercise capacity achievement by eliminating MPI in such patients could provide significant reduction of unnecessary testing and substantial cost savings. The preference of exercise over a pharmacological stress-testing approach is based on multiple studies showing the prognostic power that exercise provides. Rozanski et al. [19] showed that exercise capacity was the single most important predictor of cardiac events between the two approaches. Furthermore, Poulin et al. [20] showed that the inability to perform any level of exercise during stress MPI is associated with higher mortality risk.
Stress radionuclide perfusion imaging
Pharmacological stress MPI when used in the right subset of patients is as relevant as exercise stress MPI. Navare et al. [21] found that exercise stress MPI and pharmacological stress MPI have comparable ability to diagnose and risk stratify patients when used accordingly. Stress MPI provides excellent diagnostic accuracy for CAD with sensitivity and specificity of 87–89% and 70–79%, respectively [22]. The average sensitivity and specificity for the detection of CAD is 88% and 69%, and for exercise MPI and for adenosine MPI, 90% and 81%, respectively. The use of adenosine as a vasodilator for stress MPI is widely accepted [23] and more recently regadenoson has shown similar perfusion results in multi-center clinical trials making it faster and with less frequent side effects for patients [24].
While the use of pharmacological stress agents is relatively safe (<1–3% risk of serious complication), the FDA added an update into the safety information profile of regadenoson and adenosine in 2013 after serious complications were reported [25,26]. Clinicians may order pharmacological stress tests over exercise as submaximal exercise could lead to underestimation of size defect and false-negative studies. In patients who are able to exercise, a strategy of symptom-limited exercise with regadenoson as needed MPI is a feasible option that is safe and well tolerated and could provide additional prognostic information for this subset of patients [27–29]. Disadvantages of stress MPI include radiation exposure, long acquisition times, high cost, and poor spatial resolution that limits detection of sub-endocardial ischemia [30,31]. In addition, artifacts secondary to breast tissue, respiratory motion or sub-diaphragmatic attenuation are known to occur. Techniques such as attenuation correction has allowed for improvements in characterization of perfusion defections by reducing artifacts created by surrounding tissue particularly in women and obese patients [22,30,31].
Cardiac positron emission tomography (PET) is an additional established modality for the detection of CAD, although less available than SPECT and stress echocardiography. However, it has established diagnostic and prognostic capabilities in patients with suspected CAD [30,32,33]. Previous studies have demonstrated the range of sensitivity and specificity of PET for the diagnosis of CAD compared to ICA (>50% stenosis) of 83–100% and 73–100%, respectively [32]. In addition, with an overall diagnostic accuracy of 90% (84–98%), patients with normal studies have been demonstrated to have low risk (<1% annual cardiac event rate). Growing evidence supports the role of PET in its ability to accurately risk stratify patients suspected of underlying CAD. Bateman et al. compared PET and SPECT in two matched-patient cohorts and demonstrated improved diagnostic accuracy for PET (87%) over SPECT (71%) for detecting 50% stenosis on ICA [34]. Advantages of PET over SPECT include lower radiation exposure, higher spatial and temporal resolution, and less attenuation artifacts allowing for improved image quality [30,32,33]. However, attenuation-correction artifacts still occur (30–60% cases) from patient cardiac and respiratory motion leading to transmission–emission imaging misalignment [32]. Limited availability, increased cost, and less expertise in this emerging modality are notable disadvantages [30,32,33].
Stress echocardiography
Stress echocardiography is a widely used non-invasive modality in the diagnosis of patients with suspected CAD. Along with detection of ischemia via wall motion abnormalities, assessment of left ventricular (LV) and right ventricular systolic function, LV diastolic dysfunction, valvular heart disease, and hypertrophic obstructive cardiomyopathy is obtainable [30,31]. Similar to MPI, echocardiography may be performed with exercise ECG or pharmacologic stress using administration of β-adrenergic agonists such as dobutamine or vasodilators (adenosine) [30,31,34]. In addition, patients with permanent pacemakers may undergo stress testing by increasing the pacing rate until target heart rate (THR) is achieved [34]. The sensitivity and specificity of exercise stress echocardiography for the diagnosis of CAD is 88% and 79%, respectively. The diagnostic accuracy of dobutamine stress echo is similar with sensitivity and specificity of 81% and 80%, respectively [30]. A meta-analysis comparing stress echocardiography with stress myocardial perfusion imaging demonstrated a higher sensitivity with stress MPI (84% vs 80%) but lower specificity (77% vs 86%) [34]. Stress echocardiography is a well-validated tool for prognosis and risk stratification as studies have demonstrated similar low annual event rate (<1%) after a normal study comparable with stress MPI [34].
There are several advantages of the use of stress echo including wide availability, relative low cost, and lack of ionizing radiation [30,31,34]. The presence of resting regional wall motion abnormalities and inter-observer variability limits its diagnostic accuracy. In addition, suboptimal image quality due to patient body habitus, lung disease, or respiratory motion can present a problem. Given the importance of endocardial border detection, the use of ultrasound contrast agents have been shown to be safe and effective in improving image quality [30,31]. In addition, patients with underlying tachyarrhythmias or uncontrolled hypertension are contraindicated for dobutamine stress echocardiography [30,31,34].
Coronary computed tomography angiography
CCTA continues to increase its potential role in the diagnosis of patients with low-to-intermediate risk for CAD. CCTA has demonstrated rapid development as a cardiac imaging modality for the assessment of coronary artery disease with advancements from early generation 4- and 16-slice to 64- and now 320-slice scanners [22,30,31]. The sensitivity and specificity of CCTA is 82–99% and 89–98% [22], with a high negative predictive value of >90% making it a preferred modality to rule out CAD in low- and intermediate-risk patients [35].
CCTA has shown its utility in triaging patients with chest pain in the ER, improving efficiency of clinical decision-making, however, with increased downstream of diagnostic testing and radiation exposure with no savings in the overall cost of care [36]. In the Prospective Multi-center Imaging Study for Evaluation of Chest Pain (PROMISE) study, a randomized trial evaluating outpatient non-invasive testing, Douglas et al. [37] demonstrated that for symptomatic patients suspected of CAD who required non-invasive testing, a strategy of anatomical assessment (CCTA) is similar to that of a functional test (ExECG, stress echocardiography or MPI), supporting CCTA as a reasonable diagnostic modality for patients with low-to-intermediate risk. Prior to the PROMISE trial, Nielsen et al. [38] published a systemic review and meta-analysis examining the diagnostic accuracy and outcomes after CCTA compared with conventional functional testing (ExECG and SPECT) in patients with suspected stable CAD. The authors demonstrated that CCTA had significantly higher diagnostic performance for the detection of CAD when compared to ExECG and SPECT. Similarly, participants who underwent CCTA in the meta-analysis were more likely to have further downstream testing as in PROMISE. The results from the meta-analysis are supported by previous data evaluating coronary anatomy burden vs ischemic burden. Mancini et al. [39] in the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial, with 621 participants who underwent SPECT and ICA, showed that anatomic burden was consistently found to be an independent predictor of death, MI, or non-ST-segment elevation acute coronary syndromes, whereas ischemic burden was not.
Additional advantages of CCTA include non-invasive assessment of plaque morphology and functional characteristics. Prior studies, with ICA and intravascular ultrasound (IVUS), have demonstrated that increased plaque burden is associated with future cardiovascular events [40]. CCTA may be used to assess individual and global plaque burden along with identifying high-risk features such as large plaque volume, low CT attenuation, positive remodeling, and spotty calcification, all of which increase vulnerability of rupture and subsequent cardiovascular event [40–42]. CCTA may also be used to assess the ratio of flow in diseased artery to expected flow normal artery, known as fractional flow reserve (FFR), an established marker of ischemia severity and need for revascularization when used during ICA. Hlatky et al. [36] showed the potential role of computed tomography fractional flow reserve (CT-FFR) in patients with suspected CAD, demonstrating that this could be utilized in triaging patients for invasive procedures more efficiently and more accurately than conventional care. A recent review and meta-analysis by Gonzalez et al. [43] demonstrated the increased specificity of CT-FFR compared to CCTA for the detection of functionally significant stenosis, 72% vs 42%, respectively, as defined by invasive FFR in patients with known or suspected CAD.
The Assessment by Coronary Computed Tomographic Angiography of Individual Undergoing Invasive Coronary Angiography (ACCURACY) trial demonstrated reduced specificity (86% vs 53%) for detection of ≥50% stenosis in subjects with calcium scores vs >400 Agatston units, respectively [44]. Thus, the diagnostic utility of CCTA is limited in particular patient populations where the degree of stenosis cannot be assessed. These subgroups include older patients due to coronary calcification, or those with chronic kidney disease or prior coronary stents [22,30]. Radiation exposure is an important limitation of CCTA as a cardiac imaging modality; however, studies have shown effective strategies that reduce radiation dose while maintaining adequate assessment of the coronary anatomy [45,46]. Additional disadvantages include limited availability and reduced image quality in the presence of arrhythmias or increased heart rate [22,30].
Stress cardiac magnetic resonance imaging
Although not as widely available as other modalities, stress cardiac magnetic resonance imaging (CMR) provides a robust and comprehensive assessment of myocardial perfusion imaging for the detection of CAD and accurate quantification of left ventricular morphology and function, along with myocardial tissue characterization [31,47]. CMR has the advantage of assessment of sub-endocardial perfusion abnormalities where radionuclide MPI may be falsely negative due to balanced multi-vessel CAD [22,30]. In addition, CMR does not expose the patient to ionizing radiation. Pharmacologic stress perfusion CMR has a sensitivity of 83–91% and a specificity of 81–86% using invasive coronary angiography as the gold standard [22]. Dobutamine stress functional CMR is less sensitive but more specific; however, it is used less often presently because of patient comfort factors and the desired use of gadolinium contrast for infarct detection and viability imaging.
Randomized controlled trials assessing the cost-effectiveness of stress CMR have been performed in intermediate-risk patients presenting to the emergency department with chest pain. Miller et al. [48] demonstrated that the median direct cost of the hospital visit was lower in the observation unit-cardiac MRI (OU-CMR) group compared to the inpatient care group. Follow-up analysis at 1 year demonstrated that cardiac related healthcare cost remained significantly lower for the OU-CMR group compared to the inpatient care group ($3101 vs $4742, p = 0.004) [49]. In addition, similar to PET, patients with a negative stress CMR have a low annual event rate (<1%) for cardiovascular death or non-fatal MI demonstrating its excellent prognostic ability and risk stratification in patients with suspected CAD [50]. Additional limitations include patient factors such as severe claustrophobia and individuals with cardiac devices such as permanent pacemakers, implantable cardioverter defibrillators, and certain intracranial aneurysm clips, along with limited functional analysis in the presence of arrhythmias. For patients with stage four or five chronic kidney disease, the administration of gadolinium is contraindicated due to the association with nephrogenic systemic fibrosis [22,30,31].
Modality selection
Initial clinical evaluation of patients with chest pain should include a detailed history, physical examination, electrocardiogram (ECG), and chest radiography. The focus of the clinician is to exclude the most common and potentially life-threatening conditions such as acute coronary syndrome, aortic dissection, pulmonary embolism, and spontaneous pneumothorax [5,51]. Thorough clinical assessment suggestive of these conditions will require early aggressive medical therapy and invasive testing, serial cardiac biomarkers, D-dimer, and/or computed tomography (CT) [51]. Stable patients excluded from these conditions may require further evaluation of suspected CAD.
After initial comprehensive assessment, patients are able to be categorized based on their pre-test probability of CAD as low (<10% pre-test probability), intermediate (10–90% pre-test probability), or high (>90% pre-test probability) risk. This pre-test probability is an estimate of the likelihood of CAD based upon factors such as characterization of chest pain (typical vs atypical angina), age, gender, and/or conventional risk factors [5,31]. Well-known risk models that have been used for decades such as the Framingham risk score [31] and Diamond–Forrester (DF) [5] score have been validated and recommended by the ACC/AHA guidelines on the management of patients with suspected CAD [5]. Patients at low, intermediate, and high risks have estimated predicted 10-year CAD risk of less than 10%, 10–20%, and greater than 20%, respectively [5,52]. Further decision-making for selection of non-invasive testing modality should incorporate pre-test probability, patient clinical characteristics such as ability to exercise, and presence of resting ECG abnormalities.
According to the ACCF/AHA 2012 guidelines, patients with suspected CAD requiring further evaluation, exercise ECG testing is recommended in intermediate-risk patients, while considered reasonable in low-risk patients who can exercise with interpretable resting ECG [5]. Exercise stress with radionuclide MPI or echocardiography are also recommended in patients with intermediate risk who have an uninterpretable ECG, while exercise stress with radionuclide MPI is not recommended in low-risk patients. As an initial test, exercise stress with radionuclide MPI or echocardiography is reasonable in intermediate-risk patients who can exercise with interpretable resting ECG, whereas pharmacological stress with CMR is considered reasonable in patients who can exercise with uninterpretable ECG. Exercise stress echocardiography may be reasonable in low-risk patients who can exercise with interpretable ECG.
Regarding patients who are unable to exercise, pharmacological stress with radionuclide MPI or echocardiography is recommended for intermediate-risk patients [5]. Pharmacological stress echocardiography is reasonable in patients with low risk, while pharmacological stress CMR is reasonable in patients with intermediate risk. CCTA is considered appropriate in patients with low-to-intermediate risk. In addition, CCTA is reasonable in intermediate-risk patients who have continued symptoms after prior normal test or inconclusive exercise or pharmacological stress or are unable to undergo functional stress imaging [5]. The ACCF/AHA guidelines are comparable to the most recent revised 2013 Multi-modality Appropriate Use Criteria (AUC) for the Detection and Risk Assessment of Stable Ischemic Heart disease published in 2014 [47] (Table). In scenarios where patients are suitable for multiple modalities, clinical decisions are generally made by physician judgment based upon minimizing risks and costs to patient, local availability, and expertise.
Table.
Detection of CAD/risk assessment: Symptomatic. (Adapted with permission from Wolk et al. [52].)
| Indication text | Exercise ECG | Stress RNI | Stress echo | Stress CMR | Calcium scoring | CCTA | Invasive coronary angiography | |
|---|---|---|---|---|---|---|---|---|
| 1. | Low pre-test probability of CAD | A | R | M | R | R | R | R |
| ECG interpretable and able to exercise | ||||||||
| 2. | Low pre-test probability of CAD | A | A | M | R | M | R | |
| ECG uninterpretable or unable to exercise | ||||||||
| 3. | Intermediate pre-test probability of CAD | A | A | A | M | R | M | R |
| ECG interpretable and able to exercise | ||||||||
| 4. | Intermediate pre-test probability of CAD | A | A | A | R | A | M | |
| ECG uninterpretable or unable to exercise | ||||||||
| 5. | High pre-test probability of CAD | M | A | A | A | R | M | A |
| ECG interpretable and able to exercise | ||||||||
| 6. | High pre-test probability of CAD | A | A | A | R | M | A | |
| ECG uninterpretable or unable to exercise | ||||||||
Appropriate use key: A = appropriate; M = may be appropriate; R = rarely appropriate. A = appropriate; CAD = coronary artery disease; CCTA = coronary computed tomography angiography; CMR = cardiac magnetic resonance; ECG = electrocardiogram; Echo = echocardiography; M = may be appropriate; R = rarely appropriate; RNI = radionuclide imaging.
Future directions
Novel approaches to assess cardiac risk in patients who are unable to exercise are being developed in the nuclear and cardiac magnetic resonance (CMR) fields. Murthy et al. [53] described the use of the non-invasive quantitative assessment of coronary vasodilator function with positron emission tomography (PET) as an independent predictor of cardiac death in patients with known or suspected CAD. Lee and Johnson [54] described the role of CMR in the quantification of absolute myocardial blood flow and the ability to assess coronary flow reserve without the need of radiation. Similarly, functional assessment of coronary arteries under pharmacological stress with adenosine known as CT perfusion (CTP) is also an emerging technique. Although initial studies were small and were conducted at single center, recent meta-analysis showed increased specificity when compared to CCTA for detecting functionally significant stenosis as defined by invasive FFR [39]. As there is currently no single study that provides both anatomic and functional assessment with high diagnostic accuracy, hybrid techniques are being evaluated to combine the strengths while addressing the limitations of multi-modality imaging. Such hybrid techniques may include SPECT/CCTA, PET-CCTA, or PET/MR. The use of hybrid imaging techniques may allow for a single study to obtain both anatomical and functional data with improved diagnostic accuracy and decrease the need for further downstream or unnecessary invasive testing by ICA, the current gold standard. Large cohort studies evaluating these hybrid techniques are needed to examine the outcomes, cost-effectiveness, and potential adverse effects in patients presenting with low- and intermediate-risk chest pain.
Conclusion
As CVD remains the leading cause of morbidity and mortality, the accurate diagnosis of CAD in patients presenting with stable chest pain remains an important public health issue. ICA is the current gold standard for the detection of obstructive CAD; however, given increased risks and cost, noninvasive cardiac testing is a more appropriate first-line diagnostic step. Current non-invasive methods provide similar diagnostic and prognostic accuracy, with varying cost, availability, and limitations, making the selection in low- and intermediate-risk patients complex. Current guidelines support the use of exercise ECG as the initial test for low-to-intermediate risk chest pain for further evaluation of stable CAD. The addition of imaging to exercise testing provides incremental benefit for accurate diagnosis of obstructive CAD with an acceptable increase in cost and should be reserved for patients who are unable to exercise and/or have uninterpretable ECGs. Growing evidence supports the role of CCTA as an alternative to stress imaging given its ability to rule out CAD. Overall, physician judgment should determine the most appropriate non-invasive modality for an individual with respect to patient safety, accessibility, and cost-effectiveness in order to minimize risk of future adverse cardiac events in patients with suspected CAD.
Acknowledgments
Dr. Kramer is funded by National Institutes of Health (Grant Nos. U01HL117006-01A1 and 5R01 HL075792). Dr. Balfour is funded by National Institutes of Health (Grant No 5T32EB003841).
Footnotes
The authors have indicated there are no conflicts of interest.
REFERENCES
- [1].Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- [2].Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl Health Stat Report. 2008;7:1–38. [PubMed] [Google Scholar]
- [3].Amsterdam EA, Kirk JD, Bluemke DA, Diercks D, Farkouh ME, Garvey JL, et al. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122:1756–76. doi: 10.1161/CIR.0b013e3181ec61df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–95. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- [6].United States Government Accountability Office [accessed 22.01.16];Report to congressional requesters. Medicare Part B imaging services: rapid spending and growth and shift to physician offices indicate need for CMS to consider additional management practices. 2008 Jun; Available at: http://www.gao.gov/new.items/d08452.pdf.
- [7].De Gonzalez AB, Kim K-P, Smith-Bindman R, McAreavey D. Myocardial perfusion scans: projected population cancer risks from current levels of use in the U.S. Circulation. 2010;122:2403–10. doi: 10.1161/CIRCULATIONAHA.110.941625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Andrus BW, Welch HG. Medicare services provided by cardiologists in the United States: 1999–2008. Circ Cardiovasc Qual Outcomes. 2012;5:31–6. doi: 10.1161/CIRCOUTCOMES.111.961813. [DOI] [PubMed] [Google Scholar]
- [9].Ladapo JA, Blecker S, Douglas PS. Physician decision making and trends in the use of cardiac stress testing in the United States: an analysis of repeated cross-sectional data. Ann Intern Med. 2014;161:482–90. doi: 10.7326/M14-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gharacholou SM, Pellikka PA. Trends in noninvasive testing for coronary artery disease: less exercise, less information. Am J Med. 2015;128:5–7. doi: 10.1016/j.amjmed.2014.08.019. [DOI] [PubMed] [Google Scholar]
- [11].Detrano R, Gianrossi R, Froelicher V. The diagnostic accuracy of the exercise electrocardiogram: a meta-analysis of 22 years of research. Prog Cardiovasc Dis. 1989;32:173–206. doi: 10.1016/0033-0620(89)90025-x. [DOI] [PubMed] [Google Scholar]
- [12].Bourque JM, Beller GA. Value of exercise ECG for risk stratification in suspected or known CAD in the era of advanced imaging technologies. JACC Cardiovasc Imaging. 2015;8:1309–21. doi: 10.1016/j.jcmg.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mark DB, Shaw L, Harrell FE, Jr, Hlatky MA, Lee KL, Bengtson JR, et al. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med. 1991;325:849–53. doi: 10.1056/NEJM199109193251204. [DOI] [PubMed] [Google Scholar]
- [14].Cheezum MK, Subramaniyam PS, Bittencourt MS, Hulten EA, Ghoshhajra BB, Shah NR, et al. Prognostic value of coronary CTA vs. exercise treadmill testing: results from the Partners registry. Eur Heart J Cardiovasc Imaging. 2015;16:1338–46. doi: 10.1093/ehjci/jev087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bourque JM, Holland BH, Watson DD, Beller GA. Achieving an exercise workload of 4 or = 10 metabolic equivalents predicts a very low risk of inducible ischemia: does myocardial perfusion imaging have a role? J Am Coll Cardiol. 2009;54:538–45. doi: 10.1016/j.jacc.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bourque JM, Charlton GT, Holland BH, Belyea CM, Watson DD, Beller GA. Prognosis in patients achieving ≥10 METS on exercise stress testing: was SPECT imaging useful? J Nucl Cardiol. 2011;18:230–7. doi: 10.1007/s12350-010-9323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Christman MP, Bittencourt MS, Hulten E, Saksena E, Hainer J, Skali H, et al. Yield of downstream tests after exercise treadmill testing: a prospective cohort study. J Am Coll Cardiol. 2014;63:1264–74. doi: 10.1016/j.jacc.2013.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Uthamalingam S, Zheng H, Leavitt M, Pomerantsev E, Ahmado I, Gurm GS, et al. Exercise-induced ST-segment elevation in ECG lead aVR is a useful indicator of significant left main or ostial LAD coronary artery stenosis. JACC Cardiovasc Imaging. 2011;4:176–86. doi: 10.1016/j.jcmg.2010.11.014. [DOI] [PubMed] [Google Scholar]
- [19].Rozanski A, Gransar H, Hayes SW, Min J, Friedman JD, Thomson LE, et al. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991 to 2009. J Am Coll Cardiol. 2013;61:1054–65. doi: 10.1016/j.jacc.2012.11.056. [DOI] [PubMed] [Google Scholar]
- [20].Poulin MF, Alexander S, Doukky R. Prognostic implications of stress modality on mortality risk and cause of death in patients undergoing office-based SPECT myocardial perfusion imaging. J Nucl Cardiol. 2016;23:202–11. doi: 10.1007/s12350-014-0064-5. [DOI] [PubMed] [Google Scholar]
- [21].Navare SM, Mather JF, Shaw LJ, Fowler MS, Heller GV. Comparison of risk stratification with pharmacologic and exercise stress myocardial perfusion imaging: a meta-analysis. J Nucl Cardiol. 2004;11:551–61. doi: 10.1016/j.nuclcard.2004.06.128. [DOI] [PubMed] [Google Scholar]
- [22].Alexanderson-Rosas E, Guinto-Nishimura G, Cruz-Mendoza J, Oropeza-Aguilar M, Fuente, et al. Current and future trends in multimodality imaging of coronary artery disease. Expert Revi Cardiovasc Therapy. 2015;13:715–31. doi: 10.1586/14779072.2015.1039991. [DOI] [PubMed] [Google Scholar]
- [23].Iskandrian AE, Bateman TM, Belardinelli L, Blackburn B, Cerqueira MD, Hendel RC, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter internationaltrial. J Nucl Cardiol. 2007;14:645–58. doi: 10.1016/j.nuclcard.2007.06.114. [DOI] [PubMed] [Google Scholar]
- [24].Thompson RC, Patil H, Thompson EC, Thomas GS, Al-Amoodi M, Kennedy KF, et al. Regadenoson pharmacologic stress for myocardial perfusion imaging: a three-way comparison between regadenoson administered at peak exercise, during walk recovery, or no-exercise. J Nucl Cardiol. 2013;20:214–21. doi: 10.1007/s12350-012-9660-4. [quiz 222–226] [DOI] [PubMed] [Google Scholar]
- [25].Rosenblatt J, Mooney D, Dunn T, Cohen M. Asystole following regadenoson infusion in stable outpatients. J Nucl Cardiol. 2014;21:862–8. doi: 10.1007/s12350-014-9898-0. [DOI] [PubMed] [Google Scholar]
- [26].Dilsizian V, Gewirtz H, Paivanas N, Kitsiou AN, Hage FG, Crone NE, et al. Serious and potentially life threatening complications of cardiac stress testing: physiological mechanisms and management strategies. J Nucl Cardiol. 2015;22:1198–213. doi: 10.1007/s12350-015-0141-4. [DOI] [PubMed] [Google Scholar]
- [27].Ross MI, Wu E, Wilkins JT, Gupta D, Shen S, Aulwes D, et al. Safety and feasibility of adjunctive regadenoson injection at peak exercise during exercise myocardial perfusion imaging: the Both Exercise and Regadenoson Stress Test (BERST) trial. J Nucl Cardiol. 2013;20:197–204. doi: 10.1007/s12350-013-9679-1. [DOI] [PubMed] [Google Scholar]
- [28].Partington SL, Lanka V, Hainer J, Blankstein R, Skali H, Forman DE, et al. Safety and feasibility of regadenoson use for suboptimal heart rate response during symptom-limited standard Bruce exercise stress test. J Nucl Cardiol. 2012;19:970–8. doi: 10.1007/s12350-012-9562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Thomas GS, Thompson RC, Miyamoto MI, Ip TK, Rice DL, Milikien D, et al. The RegEx trial: a randomized, double-blind, placebo- and active-controlled pilot study combining regadenoson, a selective A(2A) adenosine agonist, with low-level exercise, in patients undergoing myocardial perfusion imaging. J Nucl Cardiol. 2009;16:63–72. doi: 10.1007/s12350-008-9001-9. [DOI] [PubMed] [Google Scholar]
- [30].Mastouri R, Sawada SG, Mahenthiran J. Current noninvasive imaging techniques for detection of coronary artery disease. Expert Rev Cardiovasc Ther. 2010;8:77–91. doi: 10.1586/erc.09.164. [DOI] [PubMed] [Google Scholar]
- [31].Mieres JH, Makaryus AN, Redberg RF, Shaw LJ. Noninvasive cardiac imaging. Am Fam Physician. 2007;75:1219–28. [PubMed] [Google Scholar]
- [32].Di Carli MF, Murthy VL. Cardiac PET/CT for the evaluation of known or suspected coronary artery disease. Radiographics. 2011;31:1239–54. doi: 10.1148/rg.315115056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dorbala S, Di Carli MF. Cardiac PET perfusion: prognosis, risk stratification, and clinical management. Semin Nucl Med. 2014;44:344–57. doi: 10.1053/j.semnuclmed.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG. American society of echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021–41. doi: 10.1016/j.echo.2007.07.003. [DOI] [PubMed] [Google Scholar]
- [35].Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, et al. Coronary CT angiography versus standard, evaluation in acute chest pain. N Engl J Med. 2012;367:299–308. doi: 10.1056/NEJMoa1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hlatky MA, De Bruyne B, Pontone G, Patel MR, Norgaard BL, Byrne RA, et al. Quality-of-life and economic outcomes of assessing, fractional flow reserve with computed tomography angiography: PLATFORM. J Am Coll Cardiol. 2015;66:2315–23. doi: 10.1016/j.jacc.2015.09.051. [DOI] [PubMed] [Google Scholar]
- [37].Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, et al. Outcomes of anatomical versus functional testing for coronary, artery disease. N Engl J Med. 2015;372:1291–300. doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nielsen LH, Ortner N, Norgaard BL, Achenbach S, Leipsic J, Abdulla J. The diagnostic accuracy and outcomes after coronary computed tomography angiography vs. conventional functional testing in patients with stable angina pectoris: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2014;15:961–71. doi: 10.1093/ehjci/jeu027. [DOI] [PubMed] [Google Scholar]
- [39].Mancini GB, Hartigan PM, Shaw LJ, Berman DS, Hayes SW, Bates ER, et al. Predicting outcome in the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation): coronary anatomy versus ischemia. JACC Cardiovasc Interv. 2014;7:195–201. doi: 10.1016/j.jcin.2013.10.017. [DOI] [PubMed] [Google Scholar]
- [40].Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol. 2014;11:390–402. doi: 10.1038/nrcardio.2014.60. [DOI] [PubMed] [Google Scholar]
- [41].Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–92. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- [42].Narula J, Nakano M, Virmani R, Kolodgie FD, Petersen R, Newcomb R, et al. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol. 2013;61:1041–51. doi: 10.1016/j.jacc.2012.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gonzalez JA, Lipinski MJ, Flors L, Shaw PW, Kramer CM, Salerno M. Meta-analysis of diagnostic performance of coronary computed tomography angiography, computed tomography perfusion, and computed tomography-fractional flow reserve in functional myocardial ischemia assessment versus invasive fractional flow reserve. Am J Cardiol. 2015;116:1469–78. doi: 10.1016/j.amjcard.2015.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (assessment by coronary computed tomographic angiography of individuals undergoing invasive coronary angiography) trial. J Am Coll Cardiol. 2008;52:1724–32. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- [45].Sun Z. Multislice CT angiography in coronary artery disease: technical developments, radiation dose and diagnostic value. World J Cardiol. 2010;2:333–43. doi: 10.4330/wjc.v2.i10.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sun Z, Fau CG, Ng KH. Coronary CT angiography: current status and continuing challenges. Br J Radiol. 2012;85:495–510. doi: 10.1259/bjr/15296170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shaw PW, Kramer CM. The case for CMR. J Nucl Cardiol. 2015;22:968–70. doi: 10.1007/s12350-015-0147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Miller CD, Hwang W, Hoekstra JW, Case D, Lefebvre C, Blumstein H, et al. Stress cardiac magnetic resonance imaging with observation unit care reduces cost for patients with emergent chest pain: a randomized trial. Ann Emerg Med. 2010;56:209–19 e2. doi: 10.1016/j.annemergmed.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Miller CD, Hwang W, Case D, Hoekstra JW, Lefebvre C, Blumstein H, et al. Stress CMR imaging observation unit in the emergency department reduces 1-year medical care costs in patients with acute chest pain: a randomized study for comparison with inpatient care. JACC Cardiovasc Imaging. 2011;4:862–70. doi: 10.1016/j.jcmg.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lipinski MJ, McVey CM, Berger JS, Kramer CM, Salerno M. Prognostic value of stress cardiac magnetic resonance imaging in patients with known or suspected coronary artery disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2013;62:826–38. doi: 10.1016/j.jacc.2013.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hoffmann U, Akers SR, Brown RKJ, Cummings KW, Cury RC, Greenberg SB, et al. ACR appropriateness criteria acute nonspecific chest pain—low probability of coronary artery disease. J Am Coll Radiol. 2015;12:1266–71. doi: 10.1016/j.jacr.2015.09.004. [DOI] [PubMed] [Google Scholar]
- [52].Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63:380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- [53].Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–24. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lee DC, Johnson NP. Quantification of absolute myocardial blood flow by magnetic resonance perfusion imaging. JACC Cardiovasc Imaging. 2009;2:761–70. doi: 10.1016/j.jcmg.2009.04.003. [DOI] [PubMed] [Google Scholar]