Abstract
Mucopolysaccharidoses (MPSs) and mucolipidoses (ML) are groups of lysosomal storage disorders in which lysosomal hydrolases are deficient leading to accumulation of undegraded glycosaminoglycans (GAGs), throughout the body, subsequently resulting in progressive damage to multiple tissues and organs. Assays using tandem mass spectrometry (MS/MS) have been established to measure GAGs in serum or plasma from MPS and ML patients, but few studies were performed to determine whether these assays are sufficiently robust to measure GAG levels in dried blood spots (DBS) of patients with MPS and ML.
Material and methods
In this study, we evaluated GAG levels in DBS samples from 124 MPS and ML patients (MPS I = 16; MPS II = 21; MPS III = 40; MPS IV = 32; MPS VI =10; MPS VII = 1; ML= 4), and compared them with 115 age-matched controls. Disaccharides were produced from polymer GAGs by digestion with chondroitinase B, heparitinase, and keratanase II. Subsequently, dermatan sulfate (DS), heparan sulfate (HS-0S, HS-NS), and keratan sulfate (mono-sulfated KS, di-sulfated KS, and ratio of di-sulfated KS in total KS) were measured by MS/MS.
Results
Untreated patients with MPS I, II, VI, and ML had higher levels of DS compared to control samples. Untreated patients with MPS I, II, III, VI, and ML had higher levels of HS-0S; and untreated patients with MPS II, III and VI and ML had higher levels of HS-NS. Levels of KS were age dependent, so although levels of both mono-sulfated KS and di-sulfated KS were generally higher in patients, particularly for MPS II and MPS IV, age group numbers were not sufficient to determine significance of such changes. However, the ratio of di-sulfated KS in total KS was significantly higher in all MPS patients younger than 5 years old, compared to age-matched controls. MPS I and VI patients treated with HSCT had normal levels of DS, and MPS I, VI, and VII treated with ERT or HSCT had normal levels of HS-0S and HS-NS, indicating that both treatments are effective in decreasing blood GAG levels.
Conclusion
Measurement of GAG levels in DBS is useful for diagnosis and potentially for monitoring the therapeutic efficacy in MPS.
Keywords: mucopolysaccharidoses, mucolipidoses, glycosaminoglycans, tandem mass spectrometry, hematopoietic stem cell transplantation
2. Introduction
Mucopolysaccharidoses (MPSs) are a group of lysosomal storage disorders (LSDs) caused by a deficiency of lysosomal hydrolases responsible for the catabolism of glycosaminoglycans (GAGs)[1, 2]. Mucolipidoses (ML) are related diseases caused by a deficiency of N-acetylglucosaminyl-1-phosphotransferase. This enzyme deficiency produces unphosphorylated lysosomal enzymes, which leads to inhibition of reuptake of enzymes and accumulation of GAGs and lipids. MPSs and ML are classified according to the enzyme deficiency (Table 1).
Table 1.
Classification of MPS and ML
| MPS | Enzyme deficiency | GAG or sphingolipid accumulated |
|---|---|---|
| MPS I | α-L-Iduronidase (IDS) | DS & HS |
| MPS II | Iduronate-2-sulfatase (IDS) | DS & HS |
| MPS IIIA | Heparan-N-sulfatase | HS |
| MPS IIIB | α-N-acetylglucosaminidase | HS |
| MPS IIIC | Acetyl-CoA- α- glucosaminidase acetyltransferase |
HS |
| MPS IIID | N-acetylglucosamine-6- sulfatase |
HS |
| MPS IVA | N-acetylgalactosamine-6- sulfate sulfatase |
C6S & KS |
| MPS IVB | β-galactosidase | KS |
| MPS VI | N-acetylgalactosamine-4- sulfatase |
DS |
| MPS VII | β-glucuronidase | DS, CS, HS |
| MPS IX | Hyaluronidase | HA |
| ML II | N-acetylglucosaminyl-1- phosphotransferase |
GAGs & sphingolipids |
| ML III | N-acetulglucosamine-1- phosphotransferase |
DS: dermatan sulfate; CS: chondroitin sulfate; HS: heparan sulfate; HA: hyaluronan; KS: keratan sulfate
The MPSs and ML are progressive LSDs that share many clinical features such as: coarse faces, neurological impairment (MPS I, II, III, VII and ML II), skeletal dysplasia (all, but maybe mild in MPS III), hepatosplenomegaly, joint rigidity, and heart valvular disease [3]. MPSs are usually asymptomatic at birth, and the initial signs and symptoms appear with progression of the disease during the first one or two years of age. Mucolipidoses II (ML II; I-cell disease) is fatal during childhood or the first decade of life, and can even produce intra-uterine fractures, while ML III has a milder somatic phenotype with slower progression throughout childhood but leads to severe neurodegeneration with a fatal outcome during adulthood [2, 4].
ML II and III are caused by impaired trafficking of several lysosomal enzymes [2, 5]. The prevalence of ML is variable among different populations: 0.3 cases per 100,000 live births in Australia, 0.16 per 100,000 live births in Portugal, and 0.08 per 100,000 live births in the Netherlands [6, 7]. The incidence in Quebec, Canada is very high, 1:6,184, due to a founder effect [8]. The combined incidence of MPSs is 1:25,000 live births, and therefore more common than ML [9].
Enzyme replacement therapy (ERT) is available commercially in practice for MPS I, II, VI, and IVA [10–13]. Hematopoietic stem cell transplantation (HSCT) is recommended for MPS I [14, 15]. Several studies indicate that HSCT will also improve outcomes for MPS II [16–18], MPS IVA [19, 20], MPS VI [21] and MPS VII [22].
Levels of GAGs in patients with MPS have been studied for several decades. Initially, total urinary GAGs were measured using a variety of dye methods [23–34]. Although these methods were useful and cost-effective; they gave high false-positive rates [35], could not be easily applied to measure GAGs in blood and/or tissues due to the presence of proteins and other interferent molecules, and could not distinguish specific GAG(s) [36]. Measurement of total urinary GAG using a dimethylmethylene blue (DMMB) method did not distinguish a substantial number of MPS IVA patients from age-matched controls [37–41]. The development of ELISA methods in early 90’s made it possible to measure HS and KS in blood and urine of MPS and ML patients [40, 42–44]. We used an ELISA method to show that KS levels in blood are elevated not only in MPS IV, but also in other types of MPS and ML [44]. However, ELISA assays are expensive and cannot distinguish subclasses of HS and KS. Since 2001, protocols have been developed for GAG analysis using tandem mass spectrometry (MS/MS). Two main branches of GAG detection methods by MS/MS have been developed: detection of digested disaccharides (direct or labeled with aniline) [45–50] and chemically depolymerized GAGs by methanolysis and/or butanolysis [51–56]. Such MS/MS methods have been used to measure specific GAGs in blood and urine of MPS and ML patients [51, 54, 55, 57–65]. MS/MS provides a sensitive, specific, and reproducible GAG analysis and allows measurement of several GAGs simultaneously, indicating its potential for use in mass screening, prognosis, and monitoring therapeutic effect in patients with MPS and ML. More recently, MS/MS methods have been developed to measure GAGs in dried blood spots (DBS) [57, 61, 66, 67].
In this study, we have simultaneously determined levels of dermatan sulfate (DS), heparan sulfate (HS-0S, HS-NS), and keratan sulfate (mono, di-sulfated, and ratio di-sulfated in total KS) in DBS of control subjects and patients with MPS I, II, III, IV, VI, VII; and ML II and III by liquid chromatography tandem mass spectrometry (LC/MS/MS). We have also evaluated GAG levels in ERT and HSCT treated patients with some types of MPS.
3. Material and Methods
3.1 Enzymes and standards
Chondroitinase B, heparitinase, keratanase II, chondrosine (internal standard-IS), and the unsaturated disaccharides: heparan ΔDi-0S [2-acetamido-2-deoxy-4-O-(4-deoxy-a-L-threo-hex-4-enopyranosyluronic acid)-D-glucose] (HS-0S), heparan ΔDi-NS [2-deoxy-2-sulfamino 4-O-(4-deoxy-α- L-threo-hex-4-enopyranosyluronic acid)-D-glucose] (HS-NS), chondro Δ Di-4S [2-acetamido-2-deoxy 3-O-(β-D-gluco-4-enepyranosyluronic acid)-4-O-D-sulfo-galactose] (Di-4S), mono-sulfated KS [Galβ1-4GlcNAc(6S)], and di-sulfated KS [Gal(6S) Galβ1-4GlcNAc(6S) were all provided by Seikagaku Co (Tokyo, Japan). Stock solutions of HS-0S (100 µg/mL), HS-NS (100 µg/mL), Di-4S (250 µg/mL), mono- and di-sulfated KS (1,000 µg/mL), and IS (5 µg/mL) were prepared in ddH20 (Millipore Milli-Q Reference A+ System).
3.2 Samples
Whole blood was collected with EDTA by venipuncture and 150 µL of blood was spotted onto filter paper to create DBS. DBS from 106 untreated MPS and ML patients (MPS I = 7; MPS II = 21; MPS IIIA = 12, MPS IIIB = 17, MPS IIIC = 6, MPS III (undefined) = 2; MPS IVA = 28, MPS IVB = 2; MPS VI = 7; MLII = 3; ML III=1), 18 treated MPS (MPS I with ERT = 6, MPS I with ERT+HSCT = 2, MPS I with HSCT = 1; MPS IIIA with HSCT = 1, MPS IIIB with HSCT = 2; MPS IVA with HSCT = 2; MPS VI with ERT = 2, MPS VI with HSCT = 1; MPS VII with HSCT = 1), and 115 control subjects. Diagnosis of MPS and ML was made with enzyme assay.
DBS from MPS patients were provided by Shimane University (Japan), Gifu University (Japan), St. Mary`s Hospital (UK), and Kasturba Medical College Manipal University (India). Control samples were obtained from 15 volunteer subjects from Hospital de Clínicas de Porto Alegre (Brazil) and from subjects who had blood draws for clinical testing for non-metabolic disease from Shimane University (Japan). Informed consent was obtained at each Institute for all patient and control samples according to IRB approval at each institute..
All de-identified samples were shipped to Nemours/AIDHC and stored at −20 °C until the GAG assay was conducted. This study was approved by the Nemours IRB (protocol # 281498).
3.3 Sample preparation
Two disks (3.3mm) were cut from each DBS sample with a DBS puncher (PerkinElmer®; Waltham, MA) and placed into wells of a 96 well Omega 10K filter plate containing 100 µL of 0.1% BSA. Samples were pre-treated as previously described by Kubaski et al., 2016 [48, 67–70] using a standardized method for extraction of GAGs from DBS. Briefly, samples were incubated with enzymes (chondroitinase B, heparitinase and keratanase II) to digest polymeric GAGs to their constituent disaccharides at 37°C overnight. The next day, disaccharides were collected by centrifugation of digests through a filter plate for 15 min at 2,500 g into receiver plates prior to LC/MS/MS analysis.
3.4 LC/MS/MS
The apparatus consisted of a 1290 Infinity LC system with a 6460 triple quad mass spectrometer (Agilent Technologies, Palo Alto, CA). Disaccharides were separated on a Hypercarb column (2.0 mm i.d. 50 mm length; 5 µm particles; Thermo Scientific, USA), thermostated at 60 °C. The method was modified from that developed by Oguma et al. [49]. The mobile phase was a gradient elution of 148 mM of ammonia (pH 11.0) (solution A) to acetonitrile 90% acetonitrile (solution B). The flow rate was 0.7 mL/min, and the gradient was as follows: 0 min 100% solution A, 1 min 50% solution A, 2 min 50% solution A, 2.20 min 0% solution A, 2.60 min 0% solution A, 2.61 min 100% solution A, 5 min 100% solution A. The mass spectrometer was operated with electrospray ionization in the negative ion mode (Agilent Jet Stream technology) with drying gas temperature 350 °C, drying gas flow 11 L/min, nebulizer pressure 58 psi, sheath gas temperature 400 °C, sheath gas flow 11 L/min, capillary voltage 4,000 V, nozzle voltage 2,000 V. Specific precursor and product ions, m/z, were used to quantify each disaccharide respectively (IS, 354.3, 193.1; DS, 378.3, 175.1; mono-sulfated KS, 462, 97; di-sulfated KS 542, 462; HS-NS 416, 138; HS-0S 378.3, 175.1) [49, 54, 71, 72]. DS was measured as Di-0S after digestion of Di-4S by a 4S-sulfatase present in the preparation of chondroitinase B. The injection volume was 5 µL with a running time of 5 min per sample. Ratio of di-sulfated KS in total KS was calculated as di-sulfated KS divided by (mono-sulfated KS + di-sulfated KS) × 100%.
3.5 Statistical analysis
For KS (mono and di-sulfated KS, ng/mL; ratio di-sulfated KS in total KS, %) analyses, patients were grouped by age as follows: 0–5 (years), 5–10 (years), 10–15 (years), 15–30 (years), and >30 years. Age-matched data was summarized using mean and standard deviation (SD). Untreated patients were compared to controls and treated patients (ERT, HSCT, and/or ERT+HSCT) using student t-test at the level of significance of 0.05 performed using Graphpad Prism 7.0a.
4. Results
Ages of the patients with MPS were as follows: untreated MPS I (mean: 3 ± 2 years; range: 3 months to 5 years; n = 7), MPS I with ERT (mean: 26 ±18 years; range: 1.1 to 37.9 years; n = 6), MPS I with HSCT (12.8 years; transplant age = 2.5 years, n = 1), MPS I with ERT+HSCT (ages 4 and 15 years; transplant age: 2.3 and 3.8 years; n = 2); untreated MPS II (mean: 9 ± 7 years; range: 1.1 to 29 years; n = 21); untreated MPS III (mean: 13 ± 8 years; range: 1 to 34.2 years; n = 37), MPS IIIA with HSCT (ages 8 and 17 years, transplant age: 7 and 12 years; n = 2), MPS IIIB with HSCT (age: 21, transplant age: 2; n = 2); untreated MPS IVA (mean: 15 ± 14 years; range: 7 months to 56 years; n = 28), MPS IVA with HSCT (ages: 25.7 and 26.3 years; transplant ages: 4 and 15 years old; n = 2); untreated MPS IVB (ages 9 months and 48 years; n = 2); untreated MPS VI (mean: 6 ± 7 years; range: 2 months to 22 years; n = 7), MPS VI with ERT (ages: 3.5 and 9.4 years; n = 2), MPS VI with HSCT (age: 21.9 years, unknown transplant age; n = 1); MPS VII with HSCT (age: 30 years; transplant age: 12 years; n = 1); untreated ML (mean: 6 ± 6 years; age range: 0.2 to 14 years; n = 4); controls (mean: 12 ± 12 years; age range: 1 month to 57 years; n = 115).
4.1. Distribution of GAG levels in untreated MPS and ML patients compared to controls
No age-dependent differences in DS, HS-0S or HS-NS were detected in control subjects, or MPS and ML patients. Consequently, levels of these GAGs in all untreated patients with each MPS or ML were compared with levels in control subjects.
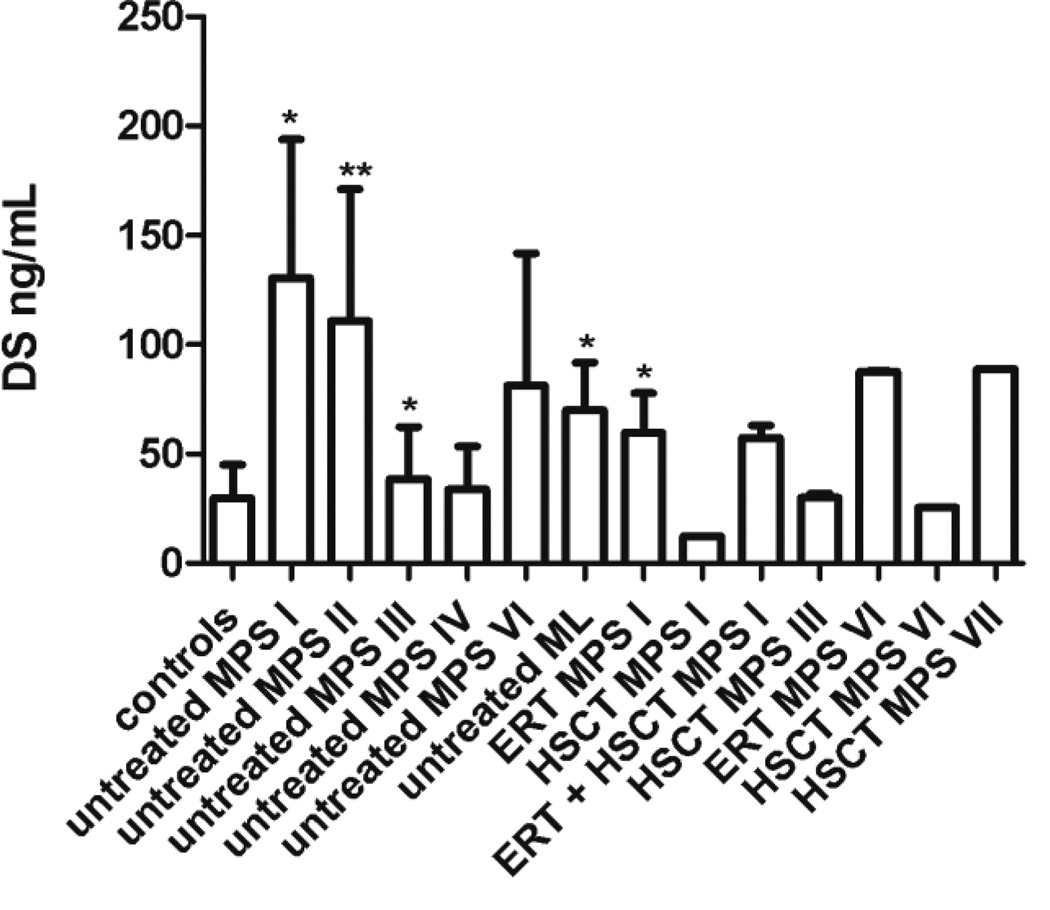
Mean levels of DS were higher than controls in untreated MPS I (p = 0.006), MPS II (p < 0.0001), MPS III (p = 0.04) (Fig. 1). Levels of DS in 6 of 7 MPS I, 20 of 21 MPS II, 2 of 37 MPS III, 3 of 6 MPS VI, and 3 of 4 ML were higher than the mean + 2SD of the control group. DS was not significantly higher in untreated MPS IV patients.
Figure 1. Dermatan sulfate (DS) levels in treated vs. untreated patients.
Untreated patients were compared with controls; treated patients were compared with untreated. Values that were significantly increased in untreated patients or decreased by treatment are marked *p < 0.05; **p < 0.0001
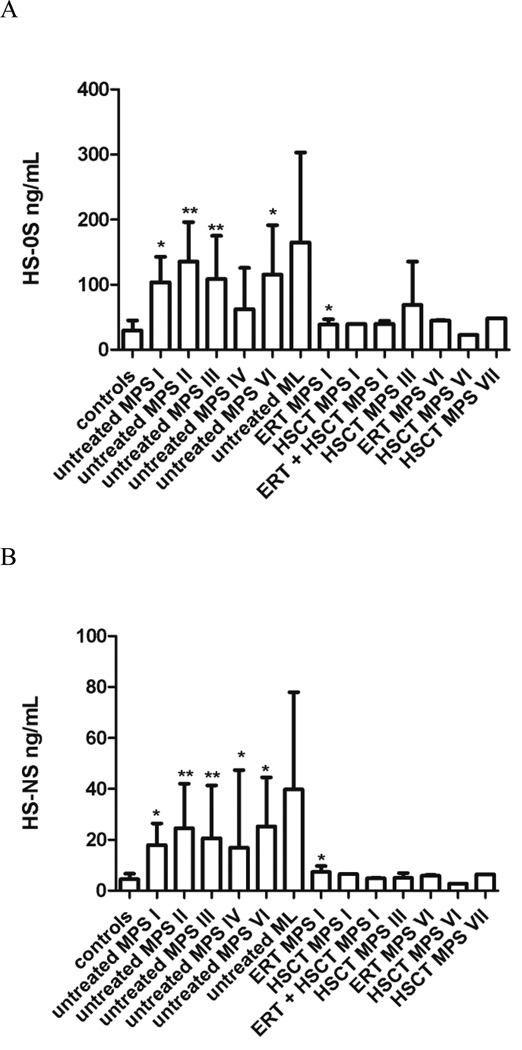
Levels of HS-0S were higher than controls in untreated MPS I (p = 0.004), MPS II (p < 0.0001), MPS III (p < 0.0001), MPS VI (p = 0.03) (Fig. 2A). Levels of HS-0S in 5 of 7 MPS I, 20 of 21 MPS II, 24 of 37 MPS III, 4 of 6 MPS VI, and 3 of 4 ML were higher than the mean + 2SD of the control group.
Figure 2. Heparan sulfate (HS-NS and HS-0S) levels in treated vs. untreated patients.
Untreated patients were compared with controls; treated patients were compared with untreated. A shows levels of HS-NS and B shows levels of HS-0S as mean and standard deviation. Values that were significantly increased in untreated patients or decreased by treatment are marked *p < 0.05; **p < 0.001
Levels of HS-NS were also higher than controls in untreated MPS I (p = 0.006), MPS II (p < 0.0001), MPS III (p < 0.0001), MPS IV (p = 0.04), and MPS VI (p = 0.03) (Fig 2B). Levels of HS-0S in 6 of 7 MPS I, 19 of 21 MPS II, 30 of 37 MPS III, 10 of 30 MPS IV, 4 of 6 MPS VI, and all 4 ML were higher than the mean + 2SD of the control group.
Di-sulfated KS levels vary with age in control samples, being high from newborn until 15 years of age and then decreasing with age (Table 2a). Although the numbers of patients in each age group is low, di-sulfated KS levels are also lower in older patients. Di-KS levels in MPS patients were generally higher than in age-matched controls (Table 2a) although only 12 of 21 MPS II, 10 of 37 MPS III, 12 of 30 MPS IV, and 4 of MPS VI were more than mean + 2SD of the control groups.
Table 2.
Levels of KS in controls and untreated patients
| A. Di-sulfated KS (ng/mL) | ||||||
|---|---|---|---|---|---|---|
| Age (years) |
Controls | MPS I | MPS II | MPS III | MPS IV | ML |
| Newborns | 27 ± 47 | 39 ± 20 | 84 | 29 ± 7 | n/a | n/a |
| 0–5 | 33 ± 21 | 55 ± 30 | 245 ± 474 | 92 ± 88 | 107 ± 139 | 42 |
| 5–10 | 40 ± 20 | n/a | 84 ± 41 | 44 ± 23 | 126 ± 200 | 25 |
| 10–15 | 41 ± 19 | n/a | 295 ± 557 | 106 ± 165 | 497 ± 869* | 46 |
| 15–30 | 21 ± 11 | n/a | 122 | 44 ± 20* | 38 | n/a |
| >30 | 19 ± 15 | n/a | n/a | 56 | 56 ± 37 | n/a |
| B. Mono-sulfated KS (ng/mL) | ||||||
|---|---|---|---|---|---|---|
| Age (years) |
Controls | MPS I | MPS II | MPS III | MPS IV | ML |
| Newborns | 128 ± 161 | 172 ± 82 | 208 | 86 ± 27 | n/a | n/a |
| 0–5 | 188 ± 75 | 216 ± 127 | 398 ± 415 | 257 ± 186 | 332 ± 408 | 100 |
| 5–10 | 152 ± 63 | n/a | 313 ± 163 | 106 ± 49 | 225 ± 190 | 115 |
| 10–15 | 140 ± 69 | n/a | 560 ± 720 | 315 ± 383 | 259 ± 177 | 182 |
| 15–30 | 67 ± 32 | n/a | 317 | 121 ± 60* | 96 | n/a |
| >30 | 49 ± 24 | n/a | n/a | 143 ± 85 | 123 ± 61* | n/a |
| C. Ratio of di-sulfated KS in total KS (%) | ||||||
|---|---|---|---|---|---|---|
| Age (years) |
Controls | MPS I | MPS II | MPS III | MPS IV | ML |
| Newborns | 17 ± 6 | 26 ± 15*** | 27 ± 12*** | 28 ± 11*** | n/a | n/a |
| 0–5 | 15 ± 8 | 23 ± 5* | 26 ± 10* | 25 ± 5*** | 23 ± 4** | 30 ± 2 |
| 5–10 | 21 ± 7 | n/a | 21 ± 1 | 24 ± 4 | 29 ± 8* | 18 |
| 10–15 | 23 ± 5 | n/a | 26 ± 8 | 30 ± 9* | 32 ± 21 | 20 |
| 15–30 | 23 ± 7 | n/a | 28 | 26 ± 4 | 29 | n/a |
| >30 | 28 ± 7 | n/a | n/a | 29 | 24 ± 9 | n/a |
Mono-sulfated KS levels also varied with age in the control group, with lower levels after the age of 15 years (Table 2b). Levels of mono-KS were generally higher in MPS patients than in age-matched controls, although differences were only significant in older MPS III and IV patients (Table 2b). Only 12 of 21 MPS II, 7 of 37 MPS III, 4 of 30 MPS IV, 2 of 6 MPS VI were more than mean + 2SD of the age-matched control groups.
The ratio of di-sulfated KS in total KS gradually increased with age in the control group (Table 2c). As seen previously in newborn samples [67], this ratio was higher in DBS from MPS I, II, and III patients aged 0 – 5 than in age-matched controls. This was also the case for MPS IV children in this age group. Differences were not significant in older patients.
4.2. GAG levels in untreated vs. treated patients with MPS and ML
Levels of HS-0S and HS-NS were generally lower in all treated patients compared to untreated patients although due to the limited sample size, only the ERT treated MPS I patients showed a significant reduction in these GAGs (Fig 2). Levels of DS were less affected by treatment although lower levels were seen in HSCT treated MPS I and VI patients (Fig 1). Two MPS IVA patients were treated by HSCT. Patient 1 was 25 years old (transplanted at 4 years of age) and patient 2 was 26 years old (transplanted at 15 years of age). In patients 1 and 2 respectively, levels of di-sulfated KS were 21 ng/mL, 4 ng/mL; levels of mono-sulfated KS 56 ng/mL, 79 ng/mL; and ratio of di-sulfated KS 27% and 5%. Levels of mono-sulfated, di-sulfated KS, and ratio of di-sulfated KS were more similar to age-matched controls than untreated MPS IV patients (Table 2).
5. Discussion
We have demonstrated the usefulness and significance of assay of disaccharides from DBS by LC/MS/MS as a diagnostic approach and therapeutic monitoring tool for MPS and ML. We measured several GAGs (DS, HS, and KS) simultaneously and found that the majority of untreated MPS and ML patients had higher levels of at least one GAG compared to age-matched controls. We have also shown a reduction of GAGs in patients treated with ERT and/or HSCT in MPS I, VI, and VII when compared to other untreated patients, suggesting the potential use of this method for treatment monitoring. Samples from individual patients pre- and post-therapy were not available for this study.
An important consideration when selecting a method to analyze GAG levels is related to the choice of specimens. Urine has been extensively used in clinical practice due to convenience for collection and accessibility [32, 41, 64, 73]. DBS may offer a useful alternative to urine for measurement of GAGs, including easy access of specimens, simplicity of transport, and potential for use in multiple assays including measurement of enzyme activity. DBS samples are routinely used for newborn screening for several metabolic disorders [74].
Limitations for use of DBS are related to procedures for DBS preparation. Although each 3.3 mm disc from a DBS corresponds to approximately 3.6 µL of blood [75], variations in sample collection methods amongst different centers might affect blood volume per spot. Also, protocols are typically standardized for serum or plasma use, and consequently, extra validation is required for use of DBS. Another important consideration for clinical use is that measurements of GAGs in DBS will reflect the concentration of analytes in both the plasma and the cellular fraction of whole blood, whereas the cells are removed in serum or plasma. Consequently levels of GAGs in DBS may not directly correspond with levels in plasma or serum. Another disadvantage of DBS is that the volume of sample is small and may not be sufficient for early stage research that typically requires more samples for protocol validation [76].
As expected, and described previously in serum (or plasma) and urine samples [40, 43, 57, 59, 61, 77], the analysis of DBS samples revealed that MPS I, II, III, VI, and ML patients had an elevation of DS compared to controls (Fig. 1). These patients also had elevated HS-0S and HS-NS, although elevations were not statistically significant for the ML patients. The data support the use of DBS to measure DS and HS levels to screen for MPS and ML patients. DS was not significantly elevated in DBS from MPS IV patients, but levels of HS-NS were elevated, indicating that these patients may also be detected in a screen for HS in DBS.
Levels of KS are age-dependent, and results of this study confirm that levels of KS are lower in both controls and patients more than 15 years old. MPS II and MPS IV patients generally had higher levels of mono- and di-sulfated KS than controls. However, due to limited numbers of patients in each age group, most differences were not statistically significant. By contrast, the ratio of di-sulfated KS in total KS was significantly higher than controls for all MPS patients (I, II, III, and IV) in the 0 – 5 year age group. These results are consistent with our previous findings that the ratio of di-KS in total KS is elevated in newborn DBS of MPS I, II, and III patients [67]. This ratio was also higher in the ML patients, consistent with our previous observation of a patient with ML II who had elevated KS levels [44].
It is well-established that each type of MPS results in characteristic accumulated of specific GAG(s) based on the deficient enzyme (Table I, [1]). More recent studies show that secondary elevations of other GAGs also occur. For example, elevation of KS level in blood and urine is diagnostic for MPS IV since the deficient enzyme is directly involved in catabolism of KS; but KS is also elevated in several other types of MPS [55, 58, 77–79]. Notably, in the current study the secondary KS elevation in DBS of MPS II patients is as high as the levels seen in patients with MPS IVA. Elevation of the ratio of di-KS in total KS is not a specific biomarker for MPS IV, but is a marker in newborns and young patients for several forms of MPS including I, II, III, and IV.
Although MPS are progressive disorders that often take years to present clinically, there is considerable evidence from both humans [80–83] and animal models [84, 85] that biochemical storage commences in the fetus. Results of this study showing elevation of GAGs in young patients support these observations.
It is well known that total urinary GAG is reduced by ERT [10, 11, 13, 86, 87]. However, studies examining the effects of either ERT or HSCT treatment on specific GAG levels are limited. We previously showed that HS levels are more effectively reduced by HSCT than by ERT in MPS II patients [88]. In the present study, we evaluated the effect of ERT and/or HSCT on specific GAGs in MPS I, III, VI, and VII. For all treated patients, levels of HS-0S and HS-NS were similar to control levels, indicating efficient reduction of GAGs in blood for both types of treatment and each form of MPS. The results are less clear for DS, with values remaining high for the two ERT treated MPS VI patients and the HSCT-treated MPS VII patient. More extensive studies are required with many patients treated with ERT and/or HSCT to determine whether levels of HS, and possibly other GAGs, are consistently reduced and whether this reduction translates into better outcomes for patients.
Because most MPS patients are not diagnosed at birth, DBSs of newborn MPS patients are rare and consequently it is difficult to define cutoff values of GAGs for newborn screening. In this study, we show that as levels of DS and HS do not vary significantly with age, most older patients still have levels of DS and HS that are distinguishable from controls using a cutoff of mean + 2SD of the controls. With a limited number of newborn MPS samples, we were able to define cutoffs for HS and DS that effectively discriminated MPS I, II, and III patients from controls [67]. Previous studies have reported the validity of GAG measurement in urine and CSF in which a good discrimination between MPS patients and controls were seen [51, 52, 54–56, 64]. However, some of the older patients in the present study were indistinguishable from the controls. The reason for low levels of GAGs in our patients is not known, but management of their condition by palliative care or anti-inflammatory treatments could lower GAG levels in the bloodstream. It is also possible that their genotype and phenotype were less severe with lower GAG accumulation.
In conclusion, this study has demonstrated that DBS, when available, can be used as a convenient source of patient samples for rapid and simultaneous measurement of multiple GAGs by MS/MS and that they could be used for diagnosis of severe forms of MPS and ML and, potentially, also for therapeutic monitoring. Furthermore, it is important to note that MPS patients with attenuated phenotypes are likely to have lower GAG levels and that this could be a potential limitation, with potential false-negative results when using DBS for diagnosis and/or treatment monitoring on those patients. Longitudinal studies should be conducted in order to elucidate the feasibility of GAG monitoring with DBS samples.
Highlights.
DBS is feasible for GAG measurement by tandem mass spectrometry;
Untreated patients had higher GAG levels compared to control samples;
Treated patients had lower GAG levels compared to untreated patients;
GAG in DBS seem to be feasible for diagnosis and therapeutic monitoring;
Acknowledgments
This work was supported by grants from Japanese MPS Society, the Austrian MPS Society, The Bennett Foundation, and International Morquio Organization (Carol Ann Foundation). R.W.M. and S.T. were supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of NIH under grant numbers P20GM103464 and P30GM114736. S.T. was supported by National Institutes of Health grant 1R01HD065767. F.K. was supported by INAGEMP and Conselho Nacional de Desenvolvimento Científico e Tecnológico, from Brazil (CNPq). The content of the article has not been influenced by the sponsors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
Francyne Kubaski, Yasuyuki Suzuki, Kenji Orii, Roberto Giugliani, Heather J. Church, Robert W. Mason, Vũ Chí Dũng, Can Thi Bich Ngoc, Seiji Yamaguchi, Hironori Kobayashi, Katta M. Girisha, Toshiyuki Fukao, Tadao Orii, and Shunji Tomatsu declare that they have no conflict of interests.
Contributions to the project:
Francyne Kubaski has contributed to the concept and planning of the project, collection of data, data analysis, the draft of the manuscript, and reporting of the work described.
Yasuyuki Suzuki has contributed to the concept and planning of the project, collection of samples, and reporting of the work described.
Kenji Orii has contributed to the concept and planning of the project, collection of samples, and reporting of the work described.
Roberto Giugliani has contributed to the concept and planning of the project, collection of samples, and reporting of the work described.
Heather J. Church has contributed to the concept and planning of the project, collection of samples, and reporting of the work described.
Robert W. Mason has contributed to the concept and planning of the project, collection of data, analysis of data, and reporting of the work described.
Vũ Chí Dũng has contributed to the concept and planning of the project, collection of samples, and reporting of the work described.
Can Thi Bich Ngoc has contributed to the concept and planning of the project, collection of samples, and reporting of the work described.
Seiji Yamaguchi has contributed to the concept and planning of the project, collection of samples, and reporting of the work described.
Hironori Kobayashi has contributed to the concept and planning of the project, collection of samples, and reporting of the work described.
Girisha KM has contributed to the concept and planning of the project, collection of samples, and reporting of the work described.
Toshiyuki Fukao has contributed to the concept and planning of the project, collection of samples, and reporting of the work described.
Tadao Orii has contributed to the concept and planning of the project, collection of samples, and reporting of the work described.
Shunji Tomatsu is a Principal Investigator for this project and has contributed to the concept and planning of the project, analysis of data, and reporting of the work described.
All authors reviewed and approved the final version of the manuscript.
References
- 1.Neufeld Elizabeht, Muenzer Joseph. The Mucopolysaccharidoses, 8th ed. New York: McGraw-Hill; 2001. [Google Scholar]
- 2.Braulke T, Raas-Rotchschild A, Kornfeld S, Sly WS. I-cell disease, pseudo-Hurler polydystrophy: disorders of lysosomal enzyme phosphorylation, localization. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic, Molecular Bases of Inherited Disease, 8 Ed. New York: McGraw-Hill; 2001. pp. 3469–3482. assoc. eds. I-cell disease and pseudo-Hurler polydystrophy: disorders of lysosomal enzyme phosphorylation and localization 3469-82. [Google Scholar]
- 3.Wraith JE. Mucopolysaccharidoses and mucolipidoses. Handb Clin Neurol. 2013;113:1723–1729. doi: 10.1016/B978-0-444-59565-2.00042-3. [DOI] [PubMed] [Google Scholar]
- 4.Cathey SS, Leroy JG, Wood T, Eaves K, Simensen RJ, Kudo M, Stevenson RE, Friez MJ. Phenotype and genotype in mucolipidoses II and III alpha/beta: a study of 61 probands. J. Med. Genet. 2010;47:38–48. doi: 10.1136/jmg.2009.067736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomatsu S, Gutierrez MA, Ishimaru T, Peña OM, Montaño AM, Maeda H, Velez-Castrillon S, Nishioka T, Fachel AA, Cooper A, Thornley M, Wraith E, Barrera LA, Laybauer LS, Giugliani R, Schwartz IV, Frenking GS, Beck M, Kircher SG, Paschke E, Yamaguchi S, Ullrich K, Isogai K, Suzuki Y, Orii T, Noguchi A. Heparan sulfate levels in mucopolysaccharidoses and mucolipidoses. J. Inherit. Metab. Dis. 2005;28:743–757. doi: 10.1007/s10545-005-0069-y. [DOI] [PubMed] [Google Scholar]
- 6.Pinto R, Caseiro C, Lemos M, Lopes L, Fontes A, Ribeiro H, Pinto E, Silva E, Rocha S, Marcão A, Ribeiro I, Lacerda L, Ribeiro G, Amaral O, Sá Miranda MC. Prevalence of lysosomal storage diseases in Portugal. Eur. J. Hum. Genet. 2004;12:87–92. doi: 10.1038/sj.ejhg.5201044. [DOI] [PubMed] [Google Scholar]
- 7.Coutinho MF, Encarnação M, Gomes R, da Silva Santos L, Martins S, Sirois-Gagnon D, Bargal R, Filocamo M, Raas-Rothschild A, Tappino B, Laprise C, Cury GK, Schwartz IV, Artigalás O, Prata MJ, Alves S. Origin and spread of a common deletion causing mucolipidosis type II: insights from patterns of haplotypic diversity. Clin. Genet. 2011;80:273–280. doi: 10.1111/j.1399-0004.2010.01539.x. [DOI] [PubMed] [Google Scholar]
- 8.De Braekeleer M. Hereditary disorders in Saguenay-Lac-St-Jean (Quebec, Canada) Hum. Hered. 1991;41:141–146. doi: 10.1159/000153992. [DOI] [PubMed] [Google Scholar]
- 9.Tomatsu S, Fujii T, Fukushi M, Oguma T, Shimada T, Maeda M, Kida K, Shibata Y, Futatsumori H, Montaño AM, Mason RW, Yamaguchi S, Suzuki Y, Orii T. Newborn screening and diagnosis of mucopolysaccharidoses. Mol. Genet. Metab. 2013;110:42–53. doi: 10.1016/j.ymgme.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wraith JE, Clarke LA, Beck M, Kolodny EH, Pastores GM, Muenzer J, Rapoport DM, Berger KI, Swiedler SJ, Kakkis ED, Braakman T, Chadbourne E, Walton-Bowen K, Cox GF. Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human alpha-L-iduronidase (laronidase) J. Pediatr. 2004;144:581–588. doi: 10.1016/j.jpeds.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 11.Muenzer J, Wraith JE, Beck M, Giugliani R, Harmatz P, Eng CM, Vellodi A, Martin R, Ramaswami U, Gucsavas-Calikoglu M, Vijayaraghavan S, Wendt S, Wendt S, Puga AC, Puga A, Ulbrich B, Shinawi M, Cleary M, Piper D, Conway AM, Conway AM, Kimura A. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet. Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 12.Harmatz P, Giugliani R, Schwartz I, Guffon N, Teles EL, Miranda MCS, Wraith JE, Beck M, Arash L, Scarpa M, Yu Z, Wittes J, Berger KI, Newman MS, Lowe AM, Kakkis E, Swiedler SJ. Enzyme replacement therapy for mucopolysaccharidosis VI: a phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. J. Pediatr. 2006;148:533–539. doi: 10.1016/j.jpeds.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Hendriksz CJ, Burton B, Fleming TR, Harmatz P, Hughes D, Jones SA, Lin S, Mengel E, Scarpa M, Valayannopoulos V, Giugliani R, Slasor P, Lounsbury D, Dummer W. Efficacy and safety of enzyme replacement therapy with BMN 110 (elosulfase alfa) for Morquio A syndrome (mucopolysaccharidosis IVA): a phase 3 randomised placebo-controlled study. J. Inherit. Metab. Dis. 2014;37:979–990. doi: 10.1007/s10545-014-9715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muenzer J, Wraith JE, Clarke LA. Mucopolysaccharidosis I: management and treatment guidelines. Pediatrics. 2009;123:19–29. doi: 10.1542/peds.2008-0416. [DOI] [PubMed] [Google Scholar]
- 15.Giugliani R, Federhen A, Rojas MVM, Vieira T, Artigalás O, Pinto LL, Azevedo AC, Acosta A, Bonfim C, Lourenço CM, Kim CA, Horovitz D, Bonfim D, Norato D, Marinho D, Palhares D, Santos ES, Ribeiro E, Valadares E, Guarany F, de Lucca GR, Pimentel H, de Souza IN, Correa J, Fraga JC, Goes JE, Cabral JM, Simionato J, Llerena J, Jardim L, Giuliani L, Carlos Santana da Silva Luiz, Santos ML, Moreira MA, Kerstenetzky M, Ribeiro M, Ruas N, Barrios P, Aranda P, Honjo R, Boy R, Costa R, Souza C, Alcantara FF, Avilla SGA, Fagondes S, Martins AM. Mucopolysaccharidosis I, II, and VI: Brief review and guidelines for treatment. Genet. Mol. Biol. 2010;33:589–604. doi: 10.1590/S1415-47572010005000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araya K, Sakai N, Mohri I, Kagitani-Shimono K, Okinaga T, Hashii Y, Ohta H, Nakamichi I, Aozasa K, Taniike M, Ozono K. Localized donor cells in brain of a Hunter disease patient after cord blood stem cell transplantation. Mol. Genet. Metab. 2009;98:255–263. doi: 10.1016/j.ymgme.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka A, Okuyama T, Suzuki Y, Sakai N, Takakura H, Sawada T, Tanaka T, Otomo T, Ohashi T, Ishige-Wada M, Yabe H, Ohura T, Suzuki N, Kato K, Adachi S, Kobayashi R, Mugishima H, Kato S. Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan. Mol. Genet. Metab. 2012;107:513–520. doi: 10.1016/j.ymgme.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Luan Z, Jiang H, Fang J, Qin M, Lee V, Chen J. Allogeneic Hematopoietic Stem Cell Transplantation in Thirty-Four Pediatric Cases of Mucopolysaccharidosis-A Ten-Year Report from the China Children Transplant Group. Biol. Blood Marrow Transplant. 2016;22:2104–2108. doi: 10.1016/j.bbmt.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Chinen Y, Higa T, Tomatsu S, Suzuki Y, Orii T, Hyakuna N. Long-term therapeutic efficacy of allogenic bone marrow transplantation in a patient with mucopolysaccharidosis IVA. Mol Genet Metab Rep. 2014;1:31–41. doi: 10.1016/j.ymgmr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yabe H, Tanaka A, Chinen Y, Kato S, Sawamoto K, Yasuda E, Shintaku H, Suzuki Y, Orii T, Tomatsu S. Hematopoietic stem cell transplantation for Morquio A syndrome. Mol. Genet. Metab. 2016;117:84–94. doi: 10.1016/j.ymgme.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herskhovitz E, Young E, Rainer J, Hall CM, Lidchi V, Chong K, Vellodi A. Bone marrow transplantation for Maroteaux-Lamy syndrome (MPS VI): long-term follow-up. J. Inherit. Metab. Dis. 1999;22:50–62. doi: 10.1023/a:1005447232027. [DOI] [PubMed] [Google Scholar]
- 22.Yamada Y, Kato K, Sukegawa K, Tomatsu S, Fukuda S, Emura S, Kojima S, Matsuyama T, Sly WS, Kondo N, Orii T. Treatment of MPS VII (Sly disease) by allogeneic BMT in a female with homozygous A619V mutation. Bone Marrow Transplant. 1998;21:629–634. doi: 10.1038/sj.bmt.1701141. [DOI] [PubMed] [Google Scholar]
- 23.Humbel R, Etringer S. A colorimetric method for the determination of sulfated glycosaminoglycans. R R B. 1974;11:21–24. R. Humbel, S. Etringer, A colorimetric method for the determination of sulfated glycosaminoglycans, Rev. Roum. Biochim. 11 (1974) 21-4. [Google Scholar]
- 24.Gold EW. A simple spectrophotometric method for estimating glycosaminoglycan concentrations. Anal. Biochem. 1979;99:183–188. doi: 10.1016/0003-2697(79)90061-7. [DOI] [PubMed] [Google Scholar]
- 25.Gold EW. The quantitative spectrophotometric estimation of total sulfated glycosaminoglycan levels. Formation of soluble alcian blue complexes. Biochim. Biophys. Acta. 1981;673:408–415. doi: 10.1016/0304-4165(81)90472-4. [DOI] [PubMed] [Google Scholar]
- 26.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 27.Panin G, Naia S, Dall'Amico R, Chiandetti L, Zachello F, Catassi C, Felici L, Coppa GV. Simple spectrophotometric quantification of urinary excretion of glycosaminoglycan sulfates. Clin. Chem. 1986;32:2073–2076. [PubMed] [Google Scholar]
- 28.Berry HK. Screening for mucopolysaccharide disorders with the Berry spot test. Clin. Biochem. 1987;20:365–371. doi: 10.1016/s0009-9120(87)80088-7. [DOI] [PubMed] [Google Scholar]
- 29.de Jong JG, Wevers RA, Laarakkers C, Poorthuis BJ. Dimethylmethylene blue-based spectrophotometry of glycosaminoglycans in untreated urine: a rapid screening procedure for mucopolysaccharidoses. Clin. Chem. 1989;35:1472–1477. [PubMed] [Google Scholar]
- 30.de Jong JG, Hasselman JJ, van Landeghem AA, Vader HL, Wevers RA. The spot test is not a reliable screening procedure for mucopolysaccharidoses. Clin. Chem. 1991;37:572–575. [PubMed] [Google Scholar]
- 31.Björnsson S. Quantitation of proteoglycans as glycosaminoglycans in biological fluids using an alcian blue dot blot analysis. Anal. Biochem. 1998;256:229–237. doi: 10.1006/abio.1997.2494. [DOI] [PubMed] [Google Scholar]
- 32.Whitley CB, Spielmann RC, Herro G, Teragawa SS. Urinary glycosaminoglycan excretion quantified by an automated semimicro method in specimens conveniently transported from around the globe. Mol. Genet. Metab. 2002;75:56–64. doi: 10.1006/mgme.2001.3271. [DOI] [PubMed] [Google Scholar]
- 33.Mabe P, Valiente A, Soto V, Cornejo V, Raimann E. Evaluation of reliability for urine mucopolysaccharidosis screening by dimethylmethylene blue and Berry spot tests. Clin. Chim. Acta. 2004;345:135–140. doi: 10.1016/j.cccn.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Whitley CB, Utz JRJ. Maroteaux-Lamy syndrome (mucopolysaccharidosis type VI): a single dose of galsulfase further reduces urine glycosaminoglycans after hematopoietic stem cell transplantation. Mol. Genet. Metab. 2010;101:346–348. doi: 10.1016/j.ymgme.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Muenzer J. Mucopolysaccharidoses. Adv Pediatr. 1986;33:269–302. [PubMed] [Google Scholar]
- 36.Tomatsu S, Fujii T, Fukushi M, Oguma T, Shimada T, Maeda M, Kida K, Shibata Y, Futatsumori H, Montaño AM, Mason RW, Yamaguchi S, Suzuki Y, Orii T. Newborn screening and diagnosis of mucopolysaccharidoses. Mol. Genet. Metab. 2013;110:42–53. doi: 10.1016/j.ymgme.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitley CB, Ridnour MD, Draper KA, Dutton CM, Neglia JP. Diagnostic test for mucopolysaccharidosis. I. Direct method for quantifying excessive urinary glycosaminoglycan excretion. Clin. Chem. 1989;35:374–379. [PubMed] [Google Scholar]
- 38.Whitley CB, Draper KA, Dutton CM, Brown PA, Severson SL, France LA. Diagnostic test for mucopolysaccharidosis. II. Rapid quantification of glycosaminoglycan in urine samples collected on a paper matrix. Clin. Chem. 1989;35:2074–2081. [PubMed] [Google Scholar]
- 39.Chuang C, Lin H, Wang T, Tsai C, Liu H, Lin S. A modified liquid chromatography/tandem mass spectrometry method for predominant disaccharide units of urinary glycosaminoglycans in patients with mucopolysaccharidoses. Orphanet J Rare Dis. 2014;9:135. doi: 10.1186/s13023-014-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomatsu S, Okamura K, Taketani T, Orii KO, Nishioka T, Gutierrez MA, Velez-Castrillon S, Fachel AA, Grubb JH, Cooper A, Thornley M, Wraith E, Barrera LA, Giugliani R, Schwartz IV, Frenking GS, Beck M, Kircher SG, Paschke E, Yamaguchi S, Ullrich K, Isogai K, Suzuki Y, Orii T, Kondo N, Creer M, Noguchi A. Development and testing of new screening method for keratan sulfate in mucopolysaccharidosis IVA. Pediatr. Res. 2004;55:592–597. doi: 10.1203/01.PDR.0000113767.60140.E9. [DOI] [PubMed] [Google Scholar]
- 41.Gray G, Claridge P, Jenkinson L, Green A. Quantitation of urinary glycosaminoglycans using dimethylene blue as a screening technique for the diagnosis of mucopolysaccharidoses: an evaluation. Ann. Clin. Biochem. 2007;44:360–363. doi: 10.1258/000456307780945688. [DOI] [PubMed] [Google Scholar]
- 42.Tomatsu S, Dieter T, Schwartz IV, Sarmient P, Giugliani R, Barrera LA, Guelbert N, Kremer R, Repetto GM, Gutierrez MA, Nishioka T, Serrato OP, Montaño AM, Yamaguchi S, Noguchi A. Identification of a common mutation in mucopolysaccharidosis IVA: correlation among genotype, phenotype, and keratan sulfate. J. Hum. Genet. 2004;49:490–494. doi: 10.1007/s10038-004-0178-8. [DOI] [PubMed] [Google Scholar]
- 43.Tomatsu S, Gutierrez MA, Ishimaru T, Peña OM, Montaño AM, Maeda H, Velez-Castrillon S, Nishioka T, Fachel AA, Cooper A, Thornley M, Wraith E, Barrera LA, Laybauer LS, Giugliani R, Schwartz IV, Frenking GS, Beck M, Kircher SG, Paschke E, Yamaguchi S, Ullrich K, Isogai K, Suzuki Y, Orii T, Noguchi A. Heparan sulfate levels in mucopolysaccharidoses and mucolipidoses. J. Inherit. Metab. Dis. 2005;28:743–757. doi: 10.1007/s10545-005-0069-y. [DOI] [PubMed] [Google Scholar]
- 44.Tomatsu S, Okamura K, Maeda H, Taketani T, Castrillon SV, Gutierrez MA, Nishioka T, Fachel AA, Orii KO, Grubb JH, Cooper A, Thornley M, Wraith E, Barrera LA, Laybauer LS, Giugliani R, Schwartz IV, Frenking GS, Beck M, Kircher SG, Paschke E, Yamaguchi S, Ullrich K, Haskins M, Isogai K, Suzuki Y, Orii T, Kondo N, Creer M, Okuyama T, Tanaka A, Noguchi A. Keratan sulphate levels in mucopolysaccharidoses and mucolipidoses. J. Inherit. Metab. Dis. 2005;28:187–202. doi: 10.1007/s10545-005-5673-3. [DOI] [PubMed] [Google Scholar]
- 45.Oguma T, Toyoda H, Toida T, Imanari T. Analytical method for keratan sulfates by high-performance liquid chromatography/turbo-ionspray tandem mass spectrometry. Anal. Biochem. 2001;290:68–73. doi: 10.1006/abio.2000.4940. [DOI] [PubMed] [Google Scholar]
- 46.Oguma T, Toyoda H, Toida T, Imanari T. Analytical method of chondroitin/dermatan sulfates using high performance liquid chromatography/turbo ionspray ionization mass spectrometry: application to analyses of the tumor tissue sections on glass slides. Biomed. Chromatogr. 2001;15:356–362. doi: 10.1002/bmc.74. [DOI] [PubMed] [Google Scholar]
- 47.Oguma T, Toyoda H, Toida T, Imanari T. Analytical method of heparan sulfates using high-performance liquid chromatography turbo-ionspray ionization tandem mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 2001;754:153–159. doi: 10.1016/s0378-4347(00)00601-0. [DOI] [PubMed] [Google Scholar]
- 48.Oguma T, Tomatsu S, Okazaki O. Analytical method for determination of disaccharides derived from keratan sulfates in human serum and plasma by high-performance liquid chromatography/turbo-ionspray ionization tandem mass spectrometry. Biomed. Chromatogr. 2007;21:356–362. doi: 10.1002/bmc.760. [DOI] [PubMed] [Google Scholar]
- 49.Oguma T, Tomatsu S, Montano AM, Okazaki O. Analytical method for the determination of disaccharides derived from keratan, heparan, and dermatan sulfates in human serum and plasma by high-performance liquid chromatography/turbo ionspray ionization tandem mass spectrometry. Anal. Biochem. 2007;368:79–86. doi: 10.1016/j.ab.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 50.Lawrence R, Brown JR, Al-Mafraji K, Lamanna WC, Beitel JR, Boons G, Esko JD, Crawford BE. Disease-specific non-reducing end carbohydrate biomarkers for mucopolysaccharidoses. Nat. Chem. Biol. 2012;8:197–204. doi: 10.1038/nchembio.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Auray-Blais C, Bhérer P, Gagnon R, Young SP, Zhang HH, An Y, Clarke JTR, Millington DS. Efficient analysis of urinary glycosaminoglycans by LC-MS/MS in mucopolysaccharidoses type I, II and VI. Mol. Genet. Metab. 2011;102:49–56. doi: 10.1016/j.ymgme.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, Young SP, Auray-Blais C, Orchard PJ, Tolar J, Millington DS. Analysis of glycosaminoglycans in cerebrospinal fluid from patients with mucopolysaccharidoses by isotope-dilution ultra-performance liquid chromatography-tandem mass spectrometry. Clin. Chem. 2011;57:1005–1012. doi: 10.1373/clinchem.2010.161141. [DOI] [PubMed] [Google Scholar]
- 53.Trim PJ, Lau AA, Hopwood JJ, Snel MF. A simple method for early age phenotype confirmation using toe tissue from a mouse model of MPS IIIA. Rapid Commun. Mass Spectrom. 2014;28:933–938. doi: 10.1002/rcm.6861. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, Wood T, Young SP, Millington DS. A straightforward, quantitative ultra-performance liquid chromatography-tandem mass spectrometric method for heparan sulfate, dermatan sulfate and chondroitin sulfate in urine: an improved clinical screening test for the mucopolysaccharidoses. Mol. Genet. Metab. 2015;114:123–128. doi: 10.1016/j.ymgme.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 55.Auray-Blais C, Lavoie P, Maranda B, Boutin M. Evaluation of urinary keratan sulfate disaccharides in MPS IVA patients using UPLC-MS/MS. Bioanalysis. 2016;8:179–191. doi: 10.4155/bio.15.239. [DOI] [PubMed] [Google Scholar]
- 56.Auray-Blais C, Lavoie P, Tomatsu S, Valayannopoulos V, Mitchell JJ, Raiman J, Beaudoin M, Maranda B, Clarke JTR. UPLC-MS/MS detection of disaccharides derived from glycosaminoglycans as biomarkers of mucopolysaccharidoses. Anal. Chim. Acta. 2016;936:139–148. doi: 10.1016/j.aca.2016.06.054. [DOI] [PubMed] [Google Scholar]
- 57.Tomatsu S, Montaño AM, Oguma T, Dung VC, Oikawa H, de Carvalho TG, Gutiérrez ML, Yamaguchi S, Suzuki Y, Fukushi M, Sakura N, Barrera L, Kida K, Kubota M, Orii T. Dermatan sulfate and heparan sulfate as a biomarker for mucopolysaccharidosis I. J. Inherit. Metab. Dis. 2010;33:141–150. doi: 10.1007/s10545-009-9036-3. [DOI] [PubMed] [Google Scholar]
- 58.Tomatsu S, Montaño AM, Oguma T, Dung VC, Oikawa H, de Carvalho TG, Gutiérrez ML, Yamaguchi S, Suzuki Y, Fukushi M, Kida K, Kubota M, Barrera L, Orii T. Validation of keratan sulfate level in mucopolysaccharidosis type IVA by liquid chromatography-tandem mass spectrometry. J. Inherit. Metab. Dis. 2010;33(Suppl 3):35. doi: 10.1007/s10545-009-9013-x. [DOI] [PubMed] [Google Scholar]
- 59.Tomatsu S, Montaño AM, Oguma T, Dung VC, Oikawa H, Gutiérrez ML, Yamaguchi S, Suzuki Y, Fukushi M, Barrera LA, Kida K, Kubota M, Orii T. Validation of disaccharide compositions derived from dermatan sulfate and heparan sulfate in mucopolysaccharidoses and mucolipidoses II and III by tandem mass spectrometry. Mol. Genet. Metab. 2010;99:124–131. doi: 10.1016/j.ymgme.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 60.Shimada T, Tomatsu S, Yasuda E, Mason RW, Mackenzie WG, Shibata Y, Kubaski F, Giugliani R, Yamaguchi S, Suzuki Y, Orii K, Orii T. Chondroitin 6-Sulfate as a Novel Biomarker for Mucopolysaccharidosis IVA and VII. JIMD Rep. 2014;16:15–24. doi: 10.1007/8904_2014_311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimada T, Kelly J, LaMarr WA, van Vlies N, Yasuda E, Mason RW, Mackenzie W, Kubaski F, Giugliani R, Chinen Y, Yamaguchi S, Suzuki Y, Orii KE, Fukao T, Orii T, Tomatsu S. Novel heparan sulfate assay by using automated high-throughput mass spectrometry: Application to monitoring and screening for mucopolysaccharidoses. Mol. Genet. Metab. 2014;113:92–99. doi: 10.1016/j.ymgme.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shimada T, Tomatsu S, Mason RW, Yasuda E, Mackenzie WG, Hossain J, Shibata Y, Montaño AM, Kubaski F, Giugliani R, Yamaguchi S, Suzuki Y, Orii KE, Fukao T, Orii T. Di-sulfated Keratan Sulfate as a Novel Biomarker for Mucopolysaccharidosis II, IVA, and IVB. JIMD Rep. 2015;21:1–13. doi: 10.1007/8904_2014_330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawrence R, Brown JR, Al-Mafraji K, Lamanna WC, Beitel JR, Boons G, Esko JD, Crawford BE. Disease-specific non-reducing end carbohydrate biomarkers for mucopolysaccharidoses. Nat. Chem. Biol. 2012;8:197–204. doi: 10.1038/nchembio.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Auray-Blais C, Lavoie P, Zhang H, Gagnon R, Clarke JTR, Maranda B, Young SP, An Y, Millington DS. An improved method for glycosaminoglycan analysis by LC-MS/MS of urine samples collected on filter paper. Clin. Chim. Acta. 2012;413:771–778. doi: 10.1016/j.cca.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Langereis EJ, Wagemans T, Kulik W, Lefeber DJ, van Lenthe H, Oussoren E, van der Ploeg Ans T, Ruijter GJ, Wevers RA, Wijburg FA, van Vlies N. A Multiplex Assay for the Diagnosis of Mucopolysaccharidoses and Mucolipidoses. PLoS ONE. 2015;10:e0138622. doi: 10.1371/journal.pone.0138622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Ruijter J, de Ru MH, Wagemans T, Ijlst L, Lund AM, Orchard PJ, Schaefer GB, Wijburg FA, van Vlies N. Heparan sulfate and dermatan sulfate derived disaccharides are sensitive markers for newborn screening for mucopolysaccharidoses types I, II and III. Mol. Genet. Metab. 2012;107:705–710. doi: 10.1016/j.ymgme.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 67.Kubaski F, Mason RW, Nakatomi A, Shintaku H, Xie L, van Vlies NN, Church H, Giugliani R, Kobayashi H, Yamaguchi S, Suzuki Y, Orii T, Fukao T, Montaño AM, Tomatsu S. Newborn screening for mucopolysaccharidoses: a pilot study of measurement of glycosaminoglycans by tandem mass spectrometry. J. Inherit. Metab. Dis. 2016 doi: 10.1007/s10545-016-9981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oguma T, Tomatsu S, Okazaki O. Analytical method for determination of disaccharides derived from keratan sulfates in human serum and plasma by high-performance liquid chromatography/turbo-ionspray ionization tandem mass spectrometry. Biomed. Chromatogr. 2007;21:356–362. doi: 10.1002/bmc.760. [DOI] [PubMed] [Google Scholar]
- 69.Shimada T, Tomatsu S, Yasuda E, Mason RW, Mackenzie WG, Shibata Y, Kubaski F, Giugliani R, Yamaguchi S, Suzuki Y, Orii K, Orii T. Chondroitin 6-Sulfate as a Novel Biomarker for Mucopolysaccharidosis IVA and VII. JIMD Rep. 2014;16:15–24. doi: 10.1007/8904_2014_311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shimada T, Tomatsu S, Mason RW, Yasuda E, Mackenzie WG, Hossain J, Shibata Y, Montaño AM, Kubaski F, Giugliani R, Yamaguchi S, Suzuki Y, Orii KE, Fukao T, Orii T. Di-sulfated Keratan Sulfate as a Novel Biomarker for Mucopolysaccharidosis II, IVA, and IVB. JIMD Rep. 2015;21:1–13. doi: 10.1007/8904_2014_330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saad OM, Ebel H, Uchimura K, Rosen SD, Bertozzi CR, Leary JA. Compositional profiling of heparin/heparan sulfate using mass spectrometry: assay for specificity of a novel extracellular human endosulfatase. Glycobiology. 2005;15:818–826. doi: 10.1093/glycob/cwi064. [DOI] [PubMed] [Google Scholar]
- 72.Wei W, Niñonuevo MR, Sharma A, Danan-Leon LM, Leary JA. A comprehensive compositional analysis of heparin/heparan sulfate-derived disaccharides from human serum. Anal. Chem. 2011;83:3703–3708. doi: 10.1021/ac2001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alonso-Fernández JR, Fidalgo J, Colón C. Neonatal screening for mucopolysaccharidoses by determination of glycosaminoglycans in the eluate of urine-impregnated paper: preliminary results of an improved DMB-based procedure. J. Clin. Lab. Anal. 2010;24:149–153. doi: 10.1002/jcla.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Almannai M, Marom R, Sutton VR. Newborn screening: a review of history, recent advancements, and future perspectives in the era of next generation sequencing. Curr. Opin. Pediatr. 2016;28:694–699. doi: 10.1097/MOP.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 75.Blanchard S, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis I. Clin. Chem. 2008;54:2067–2070. doi: 10.1373/clinchem.2008.115410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44:899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- 77.Tomatsu S, Shimada T, Mason RW, Montaño AM, Kelly J, LaMarr WA, Kubaski F, Giugliani R, Guha A, Yasuda E, Mackenzie W, Yamaguchi S, Suzuki Y, Orii T. Establishment of glycosaminoglycan assays for mucopolysaccharidoses. Metabolites. 2014;4:655–679. doi: 10.3390/metabo4030655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tomatsu S, Okamura K, Maeda H, Taketani T, Castrillon SV, Gutierrez MA, Nishioka T, Fachel AA, Orii KO, Grubb JH, Cooper A, Thornley M, Wraith E, Barrera LA, Laybauer LS, Giugliani R, Schwartz IV, Frenking GS, Beck M, Kircher SG, Paschke E, Yamaguchi S, Ullrich K, Haskins M, Isogai K, Suzuki Y, Orii T, Kondo N, Creer M, Okuyama T, Tanaka A, Noguchi A. Keratan sulphate levels in mucopolysaccharidoses and mucolipidoses. J. Inherit. Metab. Dis. 2005;28:187–202. doi: 10.1007/s10545-005-5673-3. [DOI] [PubMed] [Google Scholar]
- 79.Shimada T, Tomatsu S, Mason RW, Yasuda E, Mackenzie WG, Hossain J, Shibata Y, Montaño AM, Kubaski F, Giugliani R, Yamaguchi S, Suzuki Y, Orii KE, Fukao T, Orii T. Di-sulfated Keratan Sulfate as a Novel Biomarker for Mucopolysaccharidosis II, IVA, and IVB. JIMD Rep. 2015;21:1–13. doi: 10.1007/8904_2014_330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Crow J, Gibbs DA, Cozens W, Spellacy E, Watts RW. Biochemical and histopathological studies on patients with mucopolysaccharidoses, two of whom had been treated by fibroblast transplantation. J. Clin. Pathol. 1983;36:415–430. doi: 10.1136/jcp.36.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wiesmann UN, Spycher MA, Meier C, Liebaers I, Herschkowitz N. Prenatal mucopolysaccharidosis II (Hunter): a pathogenetic study. Pediatr. Res. 1980;14:749–756. doi: 10.1203/00006450-198005000-00008. [DOI] [PubMed] [Google Scholar]
- 82.Beck M, Braun S, Coerdt W, Merz E, Young E, Sewell AC. Fetal presentation of Morquio disease type A. Prenat. Diagn. 1992;12:1019–1029. doi: 10.1002/pd.1970121207. [DOI] [PubMed] [Google Scholar]
- 83.Baldo G, Matte U, Artigalas O, Schwartz IV, Burin MG, Ribeiro E, Horovitz D, Magalhaes TP, Elleder M, Giugliani R. Placenta analysis of prenatally diagnosed patients reveals early GAG storage in mucopolysaccharidoses II and VI. Mol. Genet. Metab. 2011;103:197–198. doi: 10.1016/j.ymgme.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 84.Rowan DJ, Tomatsu S, Grubb JH, Haupt B, Montaño AM, Oikawa H, Sosa AC, Chen A, Sly WS. Long circulating enzyme replacement therapy rescues bone pathology in mucopolysaccharidosis VII murine model. Mol. Genet. Metab. 2012;107:161–172. doi: 10.1016/j.ymgme.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tomatsu S, Alméciga-Díaz CJ, Montaño AM, Yabe H, Tanaka A, Dung VC, Giugliani R, Kubaski F, Mason RW, Yasuda E, Sawamoto K, Mackenzie W, Suzuki Y, Orii KE, Barrera LA, Sly WS, Orii T. Therapies for the bone in mucopolysaccharidoses. Mol. Genet. Metab. 2015;114:94–109. doi: 10.1016/j.ymgme.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clarke LA, Wraith JE, Beck M, Kolodny EH, Pastores GM, Muenzer J, Rapoport DM, Berger KI, Sidman M, Kakkis ED, Cox GF. Long-term efficacy and safety of laronidase in the treatment of mucopolysaccharidosis I. Pediatrics. 2009;123:229–240. doi: 10.1542/peds.2007-3847. [DOI] [PubMed] [Google Scholar]
- 87.Harmatz P, Giugliani R, Schwartz I, Guffon N, Teles EL, Miranda MCS, Wraith JE, Beck M, Arash L, Scarpa M, Yu Z, Wittes J, Berger KI, Newman MS, Lowe AM, Kakkis E, Swiedler SJ. Enzyme replacement therapy for mucopolysaccharidosis VI: a phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. J. Pediatr. 2006;148:533–539. doi: 10.1016/j.jpeds.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 88.Shimada T, Kelly J, LaMarr WA, van Vlies N, Yasuda E, Mason RW, Mackenzie W, Kubaski F, Giugliani R, Chinen Y, Yamaguchi S, Suzuki Y, Orii KE, Fukao T, Orii T, Tomatsu S. Novel heparan sulfate assay by using automated high-throughput mass spectrometry: Application to monitoring and screening for mucopolysaccharidoses. Mol. Genet. Metab. 2014;113:92–99. doi: 10.1016/j.ymgme.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]