Abstract
Mutations in SUCLA2 result in succinyl-CoA ligase (ATP-forming) or succinyl-CoA synthetase (ADP-forming) (A-SCS) deficiency, a mitochondrial tricarboxylic acid cycle disorder. The phenotype associated with this gene defect is largely encephalomyopathy. We describe two siblings compound heterozygous for SUCLA2 mutations, c.985A>G (p.M329V) and c.920C>T (p.A307V), with parents confirmed as carriers of each mutation. We developed a new LC-MS/MS based enzyme assay to demonstrate the decreased SCS activity in the siblings with this unique genotype. Both siblings shared bilateral progressive hearing loss, encephalopathy, global developmental delay, generalized myopathy, and dystonia with choreoathetosis. Prior to diagnosis and because of lactic acidosis and low activity of muscle pyruvate dehydrogenase complex (PDC), sibling 1 (S1) was placed on dichloroacetate, while sibling 2 (S2) was on a ketogenic diet. S1 developed severe cyclic vomiting refractory to therapy, while S2 developed Leigh syndrome, severe GI dysmotility, intermittent anemia, hypogammaglobulinemia and eventually succumbed to his disorder. The mitochondrial DNA contents in skeletal muscle (SM) were normal in both siblings. Pyruvate dehydrogenase complex, ketoglutarate dehydrogenase complex, and several mitochondrial electron transport chain (ETC) complexes activities were low or at the low end of the reference range in frozen SM from S1 and/or S2. In contrast, activities of PDC, other mitochondrial enzymes of pyruvate metabolism, ETC and, integrated oxidative phosphorylation, in skin fibroblasts were not significantly impaired. Although we show that propionyl-CoA inhibits PDC, it does not appear to account for decreased PDC activity in SM. A better understanding of the mechanisms of phenotypic variability and the etiology for tissue-specific secondary deficiencies of mitochondrial enzymes of oxidative metabolism, and independently mitochondrial DNA depletion (common in other cases of A-SCS deficiency), is needed given the implications for control of lactic acidosis and possible clinical management.
Keywords: succinyl-CoA synthetase deficiency, pyruvate dehydrogenase complex deficiency, electron transport chain complexes, propionyl-CoA, liquid chromatography tandem mass spectrometry, SUCLA2
1. Introduction
Mutations in the SUCLA2 gene result in succinyl-CoA ligase (ATP-forming) (EC 6.2.1.5) deficiency, a mitochondrial tricarboxylic acid enzyme converting succinyl-CoA to succinate, ATP and free CoA in the forward direction. This enzyme is also known as succinyl-CoA synthetase (ADP-forming) and A-SCS in the reverse direction, physiologically or when assayed. Succinyl-CoA ligase (SCS or SUCL) is a heterodimer composed of an invariant α-subunit, SUCLG1, and a substrate-specific β-subunit, in which the SUCLA2 and SUCLG2 gene products represent the ADP- and GDP-specific β-subunits of SUCLA2 and SUCLG2, respectively. Only the ADP-specific isoform, SUCLA2, catalyzes the conversion of ADP and succinyl-CoA to succinate and ATP in the tricarboxylic acid cycle. Defects in A-SCS lead to the accumulation of succinyl-CoA, methylmalonyl-CoA and propionyl-CoA and their metabolites such as methylmalonic acid, succinylcarnitine, methylmalonylcarnitine and propionylcarnitine. Most mutations in SUCLA2 are associated with mitochondrial DNA (mtDNA) depletion by an uncertain mechanism. The binding of the nucleoside diphosphate kinase (NDPK) to the SUCLA2 complex may be important in the pathogenesis of mtDNA depletion [1–4].
The SUCLA2 gene is expressed primarily in the brain and skeletal muscle (SM) and the phenotype associated with this gene defect is primarily encephalopathy and myopathy. Leigh disease with bilateral striatal lesions and lactic acidosis has been described in patients with SUCLA2 mutations as well. Other findings in this disorder include hypotonia, myopathy, dystonia, sensorineural hearing loss, peripheral neuropathy and renal Fanconi syndrome. To date, a total of 71 cases due to SUCLA2 mutations have been described in the literature [2, 4–12].
We report two siblings who are compound heterozygous for SUCLA2 mutations c.985A>G (p.M329V) and c.920C>T (p.A307V). To establish the degree of enzyme deficiency in each sibling, we established a novel SCS enzyme assay method, utilizing stable isotope-labeled succinate and liquid chromatography tandem mass spectrometry (LC-MS/MS) as the detection method. Unusually, mtDNA depletion was not detected in SM. We demonstrate for the first time deficiencies of pyruvate dehydrogenase complex (PDC) and other mitochondrial enzymes of oxidative metabolism in SM.
2. Case reports
2.1 Sibling 1(S1)
S1 was born 5 lbs 11 oz. He failed his newborn hearing screen and was subsequently found to have bilateral progressive hearing loss. By three months of age, he exhibited developmental delay and irritability. A brain MRI was normal. Blood lactate (10.6 mM, ref range 0.5–2.2) and pyruvate (0.19 mM, ref range 0.03–0.08) were elevated, and the L/P ratio was 56. Urine organic acids showed a small increase in methylmalonic acid. By six months of age, he had feeding problems and poor growth, and was diagnosed with gastroesophageal reflux disease (GERD). On physical examination the weight, length and head circumference were <3rd %ile, 10th %ile, and 10th %ile, respectively. There was increased muscle tone with elements of dystonia. A repeat plasma lactate was 2.9 mM. The pyruvate level was 0.18 mM. The L/P ratio was 17. The CSF lactate was 3.1 mM (ref range 0.5–2.8). A muscle biopsy was performed at six months of age for investigation of a possible mitochondrial disorder. The pathology showed variation in fiber size and coarse mitochondrial stippling with Gomori trichrome staining, and some myofibers that have subsarcolemmal accumulation of mitochondria and some nearly devoid of cytochrome oxidase staining, suggestive of a mitochondrial myopathy. The predominant feature of electron microscopy was the small size of fibers. Dichloroacetate (DCA) was initiated at 25 mg/kg/day. He was also started on thiamine 100 mg/day, riboflavin 100 mg/day, vitamin E 400 IU/day, vitamin C 500 mg/day, coenzyme Q10 10–20 mg/kg/day, L-carnitine 77 mg/kg/day. Subsequently, plasma lactate decreased to 1.5 mM. At 7 months of age, a brain MRI showed thinning of the corpus callosum.
By one year of age, he received coenzyme Q10 at 240 mg/day and lipoate 400 mg/day. The plasma lactate remained essentially normal. A cochlear implant was performed at 14 months of age. He exhibited severe developmental delay, severe truncal hypotonia, moderate hypotonia of the extremities, bilateral ptosis and facial weakness, with limited upper gaze. There was concern for peripheral neuropathy. Because of poor feeding, a G-tube was placed.
By four years of age, he displayed a pattern of cyclic vomiting. He continued to have profound muscle weakness and hypotonia, and developed scoliosis and kyphosis. The deep tendon reflexes were accentuated in the legs. He had several episodes of aspiration. Pubic and axillary hair development was noted at 6–7 years of age eventually leading to a diagnosis of precocious puberty. There was no language. Thymidine phosphorylase enzyme activity in WBC was normal. Serum methylmalonic acid level was elevated at 1,733 nM (ref range: 73–271), while 2-methylcitrate was normal.
By eight years of age, he developed diabetes mellitus. He could not sit without support. He had bilateral ptosis, oculomotor apraxia, bilateral facial weakness, generalized hypotonia with intermittent increased tone in extremities, bilateral extensor plantar responses and generalized weakness. His medications consisted of Lantus, Prevacid, Zyrtec, L-carnitine (1.2 g/day), DCA and propranolol.
By nine years of age, dystonia was more evident. Baclofen, Reglan and Leucovorin were started. Despite his many medications, the cyclic vomiting syndrome persisted. He had evidence of bone demineralization and at least one fracture. He developed migraine headaches. He manifested an intermittent metabolic acidosis largely associated with elevated lactate levels and that led to sodium bicarbonate administration. At ten years of age, the blood lactate was 4.4 mM and plasma alanine was 504 µM (ref range 161–602).
By 12 years of age, he began to manifest marked choreoathetosis, in addition to the dystonia. Presently, the patient is 14 years old with encephalopathy, generalized myopathy, intermittent hypotonia, intermittent involuntary movement disorder, recurrent emesis, diabetes mellitus, abnormal eye movements, bilateral ptosis and long-tract findings.
2.2 Sibling2 (S2)
S2 was born 5 lbs. 7 oz. He failed the newborn hearing screen and went on to manifest bilateral neurosensory hearing loss. While relatively asymptomatic in the newborn period, the initial blood lactate level at 2 months of age was 8.6 mM and the pyruvate level was 0.14 (L/P ratio 61). The plasma alanine was 332 µM (ref range 120–499). A CSF lactate was 2.0 mM. The CSF pyruvate was 0.09 mM (ref range 0.06–0.19). Developmental delay was evident in the extended neonatal period. On physical exam, he had an exaggerated Moro response and slightly elevated muscle tone in the upper extremities. There was a sacral dimple and a low lying conus medullaris at the upper aspect of the L3 vertebral body was noted. He was started on thiamine 50 mg/day.
At 5 months of age, his height was <3rd %ile. He exhibited motor weakness and could not keep his head erect or roll over. Hypertonia was also noted. At 10 months of age, he had feeding problems, recurrent emesis, GERD, a subacute dystonic reaction to Reglan and metabolic acidosis usually associated with plasma lactate elevations requiring sodium bicarbonate administration. He received a G-tube for feeding. There was a history of constipation. He exhibited bilateral ptosis, mild facial weakness, truncal hypotonia with some mild increase in the muscle tone in the extremities, hyper-reflexia and weight <3rd %ile. A brain MRI showed T2-weighted signal abnormalities in the basal ganglia while MRS revealed a low N-acetylaspartate peak, but no overt lactic acid elevation. A CT of temporal bones showed thinning in bones of left inner ear. Therapy also included coenzyme Q10 150 mg/day, thiamine 100 mg/day, riboflavin 50 mg/day, vitamin E 400 IU/day, vitamin C 250 mg/day, L-carnitine 240 mg/day, Zantac and Prevacid.
By 13 months of age, a port had to be placed to secure fluid intake and nourishment via parenteral nutrition. At 16 months of age a muscle biopsy was performed. It showed fiber type disproportion, mildly abnormal succinate dehydrogenase and electron microscopy which showed increased glycogen. A few days after the muscle biopsy, he was started on a ketogenic diet (3:1 ratio). The ketogenic diet resulted in ketosis (plasma 3-hydroxybutyrate 5.5 mM, ref range 0–0.29 for the non-fasting or fed state) and normal plasma lactate (1.8 mM). Additional medications included potassium supplement, Clarinex, Cephrazole, Bicitra, Protonics plus Pulmicort and Albuterol as needed. By 21 months of age, the ketogenic diet had to be stopped because of severe lethargy. He had had surgery for a cochlear implant, and developed a blood clot in the vicinity of a central line, which necessitated removal. Because of the development of a left subclavian thrombosis, Lovenox was administered. His sleep/wake cycle was severely perturbed. New medications included Clonidine and Klonopin. Bilateral fractures in his shoulders were detected.
At two years of age, a serum methylmalonic acid level was 1,909 nM (ref range: 73–271) and serum 2-methylcitrate level was elevated at 454 nM (ref range: 60–228). CSF lactate was normal. A blood transfusion was administered because of anemia. A bout of pneumonia led to a worsening of the anemia. A systemic yeast infection developed and subsequently, hypogammaglobulinemia was diagnosed and IVIG therapy initiated. A peripheral neuropathy was identified before age 2. Palliative care was instituted. By 3 years of age, the patient was receiving total parenteral nutrition via a central line. His speech was poor and suffered from chronic gagging and choking. Chronic reactive airways disease was diagnosed. He developed gallstones. There was a history of osteopenia, scoliosis and anemia. There was poor visual tracking with obvious ophthalmoplegia. He was not ambulatory and scoliosis, generalized muscle weakness and an involuntary movement disorder were appreciated. By 6 years of age, he spent most of his time hospital because of intermittent chronic pulmonary disease. He was started on a BIPAP device. On physical examination, he had generalized dystonia and tightening of his hamstrings. No deep tendon reflex was elicited. His mitochondrial cocktail was discontinued. His other medications consisted of Baclofen, Methadone, Valium, ferrous sulfate, Senna, Celexa and IVIG. He was restarted on ubiquinol 150 mg/day and leucovorin 10 mg/day. For the next three years, his condition continued to deteriorate. He developed pulmonary insufficiency requiring BIPAP and intermittent O2, blindness, progressive scoliosis, gastroparesis and overall gastrointestinal dysmotility, myopathy, peripheral neuropathy, hypertonia (mixed spasticity and dystonia) with transient use of Baclofen pump, immunodeficiency (humoral), thrombocytopenia and splenomegaly. By seven years of age, physical examination revealed bilateral contractures of the elbows, fingers, knees and ankles, hands in a fisted configuration, pes cavus and eversion with volitional movements of arms that appear jerky and dyskinetic. Further complications included bowel obstruction due to cecal volvulus with subsequent ileocolic anastomosis. Palliative care with transition to home was eventually instituted and he succumbed to his disorder at 9 years of age.
3. Materials and Methods
The proband (S1), both parents, and the affected sibling (S2) were enrolled in the IRB-approved Manton Center for Orphan Disease Research Gene Discovery Core at Boston Children’s Hospital. Informed consent was obtained from the parents/guardians for additional investigative studies by inclusion in the University Hospitals Cleveland Medical IRB-approved Disorders of Pyruvate Metabolism study, for additional functional and/or molecular analyses, before they were performed at CIDEM on pre-existing clinical biopsy specimens.
3.1 Liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis of fibroblast ATP- and GTP-succinyl-CoA synthetase (SCS) activities
3.1.1 Fibroblast cell lines
The fibroblast cell lines were grown in MEM medium (11090-081, GIBCO, USA) with 10% fetal bovine serum (100–106, BenchMark, USA) supplemented with 100 U/ml penicillin and 100 µg/ml streptomycin (15140-122, GIBCO) at a 37°C incubator with humidified atmosphere of 5% CO2. These cells were used for enzymatic analyses and Western blot after being expanded in 15cm dish. Cell pellets were harvested by centrifugation and washed twice with PBS then stored at −80°C freezer for future analysis.
3.1.2 SCS Assay procedure
This novel assay of SCS activity uses D4-succinate, ATP (or GTP) and CoA as substrates, and quantitates directly the reaction product, D4-succinyl-CoA by LC-MS/MS.
The cell pellets were dispensed in 150 µl PBS (10010-023, GIBCO, USA) and lysed at 4°C by a total of 3 rounds of 15s sonication at level 1 (Sonic Dismembrator model 100, Fisher Scientific, USA). The lysate was centrifuged at 4°C and 14,000g for 10min (5402R, Eppendorf). The supernatant was measured for protein concentration (DC protein assay kit, Bio-Rad). Homogenate (final concentration in reaction 1 mg/ml) was incubated at 37°C water bath for 6 min in 50 mM Tris buffer pH 8.0 (154563, Aldrich), 5 mM MgCl2 (M2670, Sigma), 10 mM D4-succinate (DLM-584, Cambridge Isotope Laboratories), 1 mM ATP (A7699, Sigma) or 1 mM GTP (G8877, Sigma), 1 mM CoA (C3144, Sigma) and oligomycin 2 µg/ml (75351, Sigma). Please note it is important to mix stock solutions of Tris buffer, MgCl2 and D4-succinate first then adjust pH to 8.0. Upon completion of the incubation one volume, 15 µl, of reaction mixture was taken out to a tube which consisted of 9 volume mixture of 25 µl of 0.2 M formic acid (695076, Sigma), 10 µl of 50 µM malonyl-CoA (M4263, Sigma) as internal standard and 100 µl of acetonitrile (A955-4, Fisher Scientific) to stop the reaction. The tubes were thoroughly mixed and centrifuged at 14,000g for 10 min. Then 40 µl of supernatant was transferred to an auto-sampler vial containing 100 µl of acetonitrile/water (8:2 vol:vol) for LC-MS/MS analysis.
A series of standards were prepared by mixing 25 µl of 0.2 M formic acid, 10 µl of 50 µM malonyl-CoA, 100 µl of acetonitrile with 15 µl of succinyl-CoA concentrations of 166.7, 55.6, 18.53, 6.17, 3.09 and 1.54 µM. The further steps were same as above.
An AB Sciex QTrap 5500 mass spectrometer equipped with a Shimadzu HPLC of 2 LC-20AD XR pumps and SIL-20AC XR auto-sampler was operated in negative-ion electrospray ionization mode with multiple reaction monitoring scanning. The LC BEH amide column (186004801, Waters) was run at room temperature and the auto-sampler tray was thermostated to 4°C. The parameters for LC-MS/MS are listed in Table 1. LC-MS/MS peak areas were integrated with Analyst 4.0 software. We perform the further data analysis in Excel (Microsoft) and GraphPad Prism. Km and Vmax values were determined using non-linear regression function of GraphPad Prism.
Table 1.
LC-MS/MS settings and conditions
| LC-MS/MS settings and conditions | |||
|---|---|---|---|
|
Mass spectrometer settings | |||
| Curtain Gas | 15 | ||
| Ion Spray Voltage | −4500v | ||
| Dissolvation Temperature |
600°C | ||
| GS1 and GS2 | 20 and 40 respectively | ||
| Declustering potential | −110v | ||
| Collision energy (CE) for different MRM transitions | |||
| Succinyl-CoA | 866.2> 408 (CE −55ev) | ||
| D4-Succinyl-CoA | 870.2 > 408 (CE-55ev) | ||
| Malonyl-CoA | 852.2> 808 (CE −40ev) | ||
| Chromatography Gradient conditions | |||
| Time (min) |
Flow rate (ml/min) |
Solvent A%# |
Solvent B%# |
| 0 | 0.15 | 70 | 30 |
| 2 | 0.15 | 30 | 70 |
| 6.5 | 0.15 | 30 | 70 |
| 7.5 | 0.15 | 70 | 30 |
| 10 | 0.15 | 70 | 30 |
Solvent A, 10mM ammonium formate in acetonitrile/water (90/10, vol/vol); Solvent B, 10mM ammonium formate in acetonitrile/water (20/80, vol/vol)
3.2 Whole Exome Sequencing (WES) Analysis
Sanger sequencing of the genes commonly associated with PDC deficiency was already performed because of functional PDC deficiency noted in SM and was negative. Furthermore, next-generation sequencing of 23 genes associated with pyruvate metabolism was also negative for the following genes: BOLA3, PDHA1, PDHB, DLAT, DLD, PCB, PDHX, PDK1, PDK2, PDK3, PDK4, PDP1 (PPM2C), PDP2 (PPM2C2), LIAS, LIPT1, LIPT2, NFU1, PCK1, PCK2, SLC19A2, SLC19A3, SLC25A19, and TPK1. This led to performance of WES analysis in the CIDEM facility, before knowing about the SUCLA2 mutations.
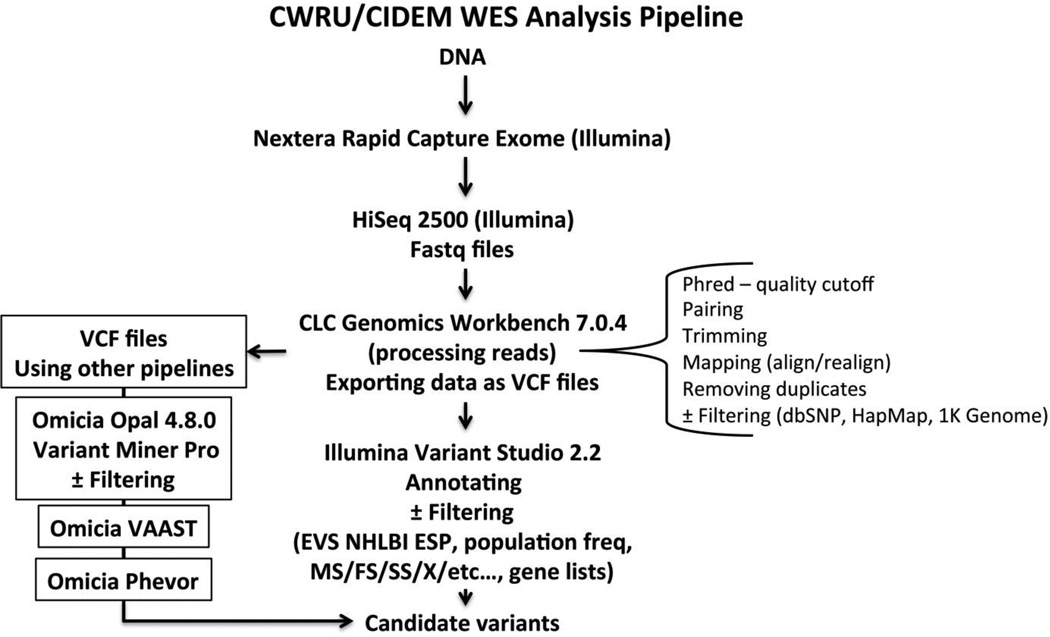
The WES pipeline used here is summarized in Fig. 1. DNA was isolated from fibroblasts using standard methods. Quality control of genomic DNA samples was executed using Qubit dsDNA BR Assay Kit (Invitrogen, USA) and running on a 2% agarose gel. Only DNA of high quality was selected. DNA was diluted to 5 ng/µl and a total of 50 ng DNA was then subjected to Nextera Rapid Capture Exome kit (Illumina, USA). Each DNA sample was enzymatically sheared and then tagged with a unique index-adapter necessary for sequencing. Final libraries were purified and pooled in sets of 12, using 500 ng of each tagged library. Final library preparation included validation using the Agilent High Sensitivity DNA Kit (Agilent, USA), quantification via qPCR (KAPA Biosystems, USA), dilution and denaturation. High-throughput sequencing was carried out using an Illumina HiSeq2500 instrument and a rapid run paired-end flowcell, with an average depth of coverage of 138 ± 22 (mean ± SD; range 106–174, n = 10). Fastq files were uploaded to CLC Genomics Workbench 7.0.4 (CLC Bio, Qiagen Company, USA) for variant calling, analyzing, processing and visualizing reads. Data was exported as VCF files which were uploaded either to Illumina Variant Studio 2.2 for annotation and filtering of variants or, in a parallel way, to Omicia Opal 4.8.0 for annotation and filtering through Omicia Miner Pro followed by ranking and sorting of variants sequentially using Omicia Variant Annotation, Analysis and Search Tool (VAAST) and Omicia Phevor software tools (Omicia Biotechnology Company, USA). Sanger sequencing of the SUCLA2 gene was carried out at the Baylor Miraca Genetics Laboratories (Houston, TX).
Fig. 1.
Summary of the WES pipeline used in this work. MS, missense; FS, frameshift; SS, splice site; and X, stop.
3.3 Acylcarnitine analysis
Ages at muscle biopsy of S1 and S2 were 6.2 and 16.2 months, respectively. SM samples were frozen in liquid nitrogen, shipped on dry ice to CIDEM, and stored at −80°C until analysis. Samples from both siblings were processed and acylcarnitines analyzed using UHPLC-MS/MS as previously described [13, 14].
3.4 Western blot
Fibroblasts were cultured and harvested in the same condition as for LC-MS/MS analysis and lysed in RIPA buffer (R0278 Sigma) with 1% proteinase inhibitor (P8340 Sigma). After constant agitation at cold room for half hour the lysate was sonicated and centrifuged same as in section 3.1.2 and about 30 µg sample were analyzed by SDS-PAGE and immunoblotting. The PVDF membrane was blocked by 5% milk overnight at 4°C then incubated with primary antibody against SUCLA2 (SC-68912 Santa Cruz) 1:200 for 4 hours at 4°C. After washing off the primary antibody the membrane was incubated with 1:2500 dilution of secondary antibody, chicken anti rabbit IgG HRP (SC-2963 Santa Cruz), at room temperature for 1 hour. Signals were detected with ImageQuant LAS4000 (GE Healthcare) after applying the chemiluminescent substrate (34095 ThermoFisher Scientific) to the blot and the result was quantified with Image J and Excel.
3.5 Mitochondrial DNA depletion
Flash frozen SM (~150 mg) was embedded in OCT compound and then used for quantification of mtDNA copy number. Real-time quantitative PCR analysis of mtDNA content was performed at Baylor Genetics Laboratories (Houston, TX) as published [15].
3.6 Pyruvate dehydrogenase complex (PDC), α-ketoglutarate dehydrogenase complex (KDC), pyruvate carboxylase and phosphoenolpyruvate carboxykinase (PEPCK) assays
Assay of PDC, both activated-dephosphorylated and inactivated phosphorylated, KDC, pyruvate carboxylase, PEPCK and dihydrolipoamide dehydrogenase (E3) activities in disrupted blood lymphocytes (for PDC and E3), disrupted cultured skin fibroblasts (for PDC, KDC, E3 and pyruvate carboxylase), or SM (for PDC, E3 and KDC) homogenates, were determined as previously described [16, 17]. PEPCK total enzyme activity (including cytoplasmic isomer of PEPCK, PEPCK-C, and mitochondrial isomer of PEPCK, PEPCK-M) was assayed in disrupted cultured skin fibroblasts by PEP (phosphoenolpyruvate)-dependent 14CO2 fixation as previously described [18]. Purified porcine heart PDC used was from Sigma (P-7032). Control fibroblasts and frozen muscle samples were obtained from unused de-identified extra material retained in CIDEM.
3.7 Assay of propionyl-CoA inhibition of PDC
Coenzyme A, methylmalonyl-CoA and propionyl-CoA were from Sigma-Aldrich (St. Louis, MO; Cat. Nos. C3019, M1762, and P5397, respectively). Succinyl-CoA was from Santa Cruz Biotechnology, Inc. (Paso Robles, CA; Cat. No. sc-215917). For kinetic analyses of propionyl-CoA inhibition of PDC, PDC was assayed with increasing concentration of propionyl-CoA with three different pyruvate (substrate) concentrations (31, 62 and 125 mM as a mixture of 1-14C-labeled and unlabeled pyruvate) by pyruvate dependent 14CO2 release, as previously described [18]. The final concentration of thiamine pyrophosphate (TPP), dithiothreitol (DTT) and CoASH in the reaction mixture were 0.1, 0.8, and 0.64 mM, respectively. The y- and x-axis intercepts (1/Vmax and −1/Km, respectively) and slope (Km/Vmax) from linear regression of data on double reciprocal (Lineweaver-Burk) plots were used to calculate the apparent Vmax and apparent Km values, and subsequently determine Ki, the inhibitor concentration at which the reaction rate (Vmax) is half maximum.
3.8 Electron transport chain (ETC) and oxidative phosphorylation assays
Quantitate oxidative phosphorylation in harvested cultured skin fibroblasts permeabilized with digitonin was measured as described previously [19]. Specific spectrophotometric ETC complex I-IV assays in cultured skin fibroblasts and frozen SM were measured as specific donor-acceptor oxidoreductase activities as described previously [20–22]. The North American Mitochondrial Disease Consortium (NAMDC) criteria for evaluating the significance of ETC activities were followed (https://www.sac-cu.org/NAMDC/Site/DocCenter.aspx and [23]).
4. Results
The two siblings presented here had separate but parallel diagnostic findings in Boston and Cleveland. The Boston path of investigation found initial metabolic and phenotypic findings that led to Sanger sequencing and the finding of compound heterozygosity of two mutations in SUCLA2, and consequently the diagnosis of A-SCS deficiency. It was discovered that mtDNA was not depleted, although this is a common finding in other cases of A-SCS deficiency.
The finding of lactic acidemia led to investigation of a disorder of mitochondrial function in Cleveland, with the finding of PDC and partial ETC deficiencies in SM, but not in lymphocytes or fibroblasts. In Cleveland, failure to identify any mutations within the known PDC genes and normal activity of most other mitochondrial enzymes lead to WES and follow-up analysis of muscle acylcarnitines, which lead to the same diagnostic conclusion that these siblings had heterozygosity for A-SCS deficiency.
4.1 Succinyl-CoA synthetase (SCS) activity
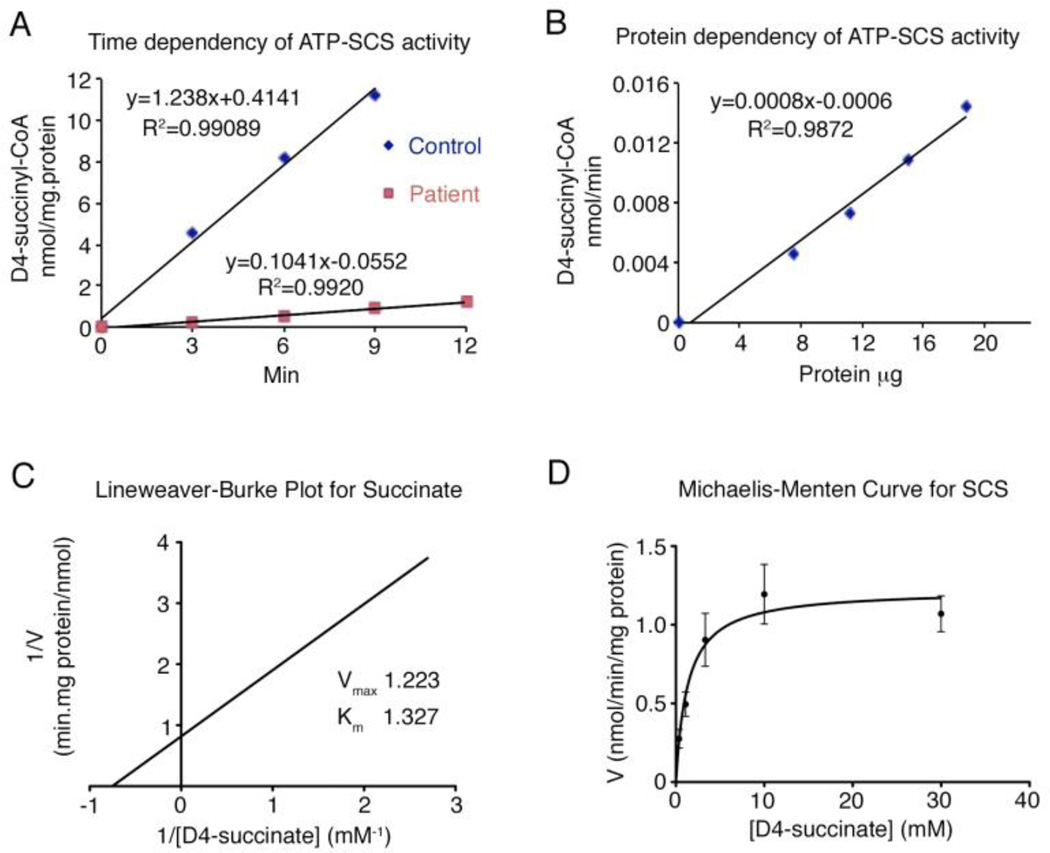
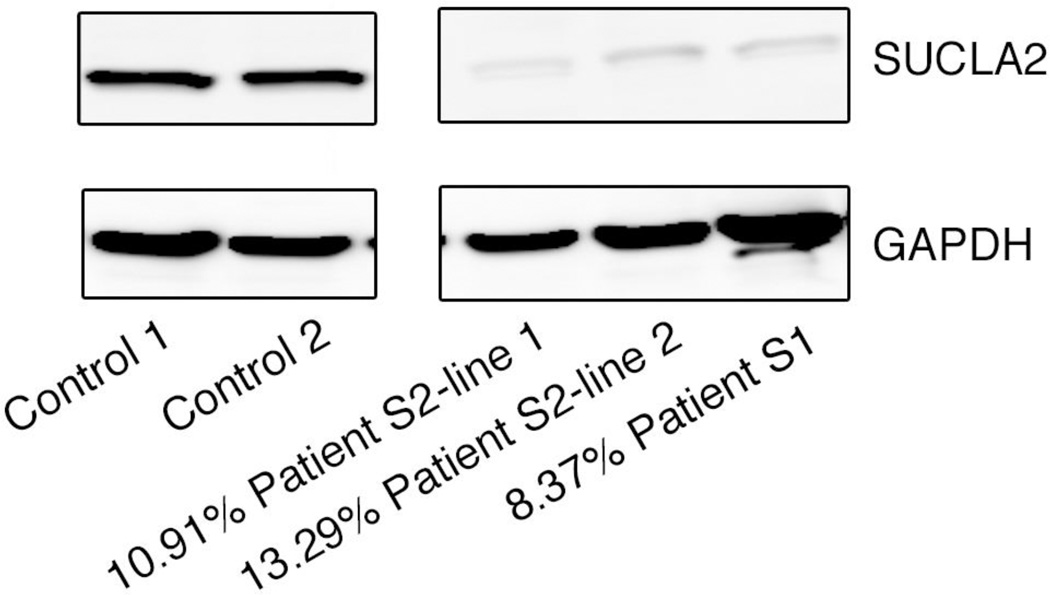
Because the compound heterozygosity of these SUCLA2 variants had not previously been described in an individual and the phenotypes differed in the two siblings, we proceeded with developing the SCS activity assay and performing blot analysis. The LC-MS/MS assay confirmed the defect in the reaction of the ADP forming isoform of SCS activity. The method had linearity from 0 to 9 min and with protein final concentration ranging from 0 to 1.25 mg/ml in the reaction. The apparent Km and Vmax of succinate were determined to be at 1.33 mM and 1.22 nmol/min/mg protein (Fig. 2). We measured both ATP and GTP dependent-SCS activities in 3 control fibroblast lines, 1 line from S1 and 2 lines from patient S2 (Table 2). Consistent with the pathogenicity of mutations of SUCLA2, decreased ATP-SCS activity and normal GTP-SCS activity in the two patients’ fibroblast samples, we found by Western blot analysis that the SUCLA2 protein was also markedly reduced in fibroblasts with 8.4%, 10.9% and 13.3% of control samples in S1, S2 line 1 and S2 line 2, respectively (Fig. 3).
Fig. 2.
A-SCS activity measurement by LC-MS/MS. (A) Time dependency was measured in control line and patient line at 37°C for 0, 3, 6, 9 and 12 min with 50 mM Tris buffer pH 8.0, 5 mM MgCl2, 10 mM D4-succinate, 1 mM ATP, 1 mM CoA, oligomycin 2 µg/ml and homogenate protein concentration 1mg/ml. Activity linearity is shown within 9min. (B) Protein dependency was measured in control line for 6 min at different protein concentrations, 0, 0.5, 0.75, 1 and 1.25 mg/ml. Other conditions were same as (A). Linear for protein concentrations <1.25 mg/ml. (C) and (D) Apparent Km and Vmax of succinate were measured from two control lines with different D4-succinate concentrations, 0.37, 1.11, 3.33, 10 and 30 mM, and 1mg/ml homogenate incubated at 37°C for 6min. Other conditions were same as (A).
Table 2.
ATP-SCS and GTP-SCS activity in control and patient fibroblast cell lines
|
ATP-SCS nmol/min/mg protein |
Control line 1 | Control line 2 | Control line 3 | Patient S2 line 1 | Patient S2 line 2 | Patient S1 |
| Mean ± SD | 1.02±0.45 | 0.98±0.32 | 1.04±0.50 | 0.089±0.052 | 0.177±0.057 | 0.096±0.0013 |
| Replicates | 15 | 13 | 10 | 12 | 7 | 2 |
|
Mean ± SD of 3 control lines |
1.01±0.029 | |||||
|
Percentage of control lines |
8.78% | 17.52% | 9.49% | |||
|
GTP-SCS nmol/min/mg protein |
Control line 1 | Control line 2 | Control line 3 | Patient S2 line 1 | Patient S2 line 2 | Patient S1 |
| Mean ± SD | 8.58±1.90 | 7.60±2.45 | 10.91±3.85 | 13.91±3.42 | 10.51±2.41 | 12.07±6.92 |
| Replicates | 13 | 9 | 7 | 9 | 9 | 3 |
|
Mean ± SD of 3 control lines |
9.03±1.70 | |||||
|
Percentage of control lines |
154.04% | 116.34% | 133.70% | |||
Fig. 3.
Western blot analyses result of decreased SUCLA2 in patient lines. SUCLA2 protein detected by Western Blot and normalized to total GAPDH levels as loading controls. Relative SUCLA2 amounts were calculated using mean value of controls, indicated as percentages.
4.2 Identification of SUCLA2 variants by WES
Target specificity and depth of coverage for the WES analysis that identified the SUCLA2 gene variants were as follows: 72.9% and 60.0% on-target reads and bases, respectively; 99.8% target bases covered at ≥10x; and 108.8 average depth of coverage. There were 2,764,862 reads in total. Omicia Opal Miner Pro identified 13,961 variants, VAAST Solo ranked 426 variants, and Phevor ranked 214 genes with SUCLA2 as #1, after the input of the following phenotype characteristics: chronic lactic acidosis, increased serum lactate, global developmental delay, dystonia, muscular hypotonia, increased CSF lactate, and intellectual disability. Phevor and phenotype/gene association scores were 5.79 and 0.998, respectively. The candidate SUCLA2 variants identified by VAAST were c.985A>G (p.Met329Val) and c.920C>T (p.Ala307Val) with Omicia scores of 0.94 and 0.79, respectively. The Omicia score represents a composite score using PolyPhen, MutationTaster, PhyloP and SIFT in silico prediction algorithms [24]. A score of ≥0.85 = 1% false-positive prediction rate [24]. Furthermore, a variant with an Omicia score of >0.85 is considered likely pathogenic, while one with a score between 0.5 and 0.85 is considered potentially pathogenic [24]. The reads for the candidate SUCLA2 variants were non-overlapping. The c.985A>G (p.Met329Val) and c.920C>T (p.Ala307Val) variants were 178 nt apart (chr13:48,528,397 and chr13:48,528,575, respectively; hg19) and phased in trans, with 100 and 113 depth of coverage, respectively. Other key prediction parameters and allele frequencies of the identified SUCLA2 variants are shown in Supplementary Table 1.
Independently, and unknown before the undertaking of WES analysis, Sanger sequencing of SUCLA2 had identified the same two candidate variants noted above, following a suspicion of succinyl-CoA synthetase deficiency because of elevation of serum methylmalonic acid in both siblings. Parents were subsequently confirmed to be carriers with c.985A>G (p.M329V) and c.920C>T (p.A307V) variants inherited from father and mother, respectively.
4.3 Skeletal muscle (SM) acylcarnitine analyses
The SM quantitative acylcarnitine analyses showed increased succinylcarnitine and methylmalonylcarnitine in both siblings. Succinylcarnitine was 14.2 and 149 nmol/g wet weight in S1 and S2, respectively, with 3.3 nmol/g wet weight as control mean and ref range <9.4. Methylmalonylcarnitine was 6.8 and 11.7 nmol/g wet weight in S1 and S2, respectively, with 0.4 nmol/g wet weight as control mean and ref range <2.7. Propionylcarnitine was increased at 72 nmol/g wet weight in S2 only, with 4.7 nmol/g weight as control mean and ref range <14.3, while propionylcarnitine was not detected in SM of S1. The acetylcarnitine concentrations in SM of S1 and S2 were 145 nmol/g weight and 922 nmol/g weight, respectively, with 484 nmol/g weight as control mean and ref range 158–1069. The increases in succinyl-, methylmalonyl- and propionyl-carnitines were consistent with a biochemical diagnosis of SUCL deficiency.
4.4 Mitochondrial DNA depletion
The mitochondrial DNA contents in SM were 62% and 149% of that of tissue and age-matched controls for patient S1 and S2, respectively.
4.5 Pyruvate dehydrogenase complex (PDC) and other mitochondrial function deficiencies in SM
Prior to the diagnosis of A-SCS deficiency, as part of the differential diagnostic workup, PDC activity was assayed in SM, blood lymphocytes and cultured fibroblasts from both siblings. Functional PDC deficiency was noted only in SM but not in lymphocytes or fibroblasts (Table 3). PDC activities in frozen SM from S1 and S2 were similar, showing 27 ± 17% (range 9–43%; n = 3) and 23% of the control mean for S1 and S2, respectively. Fibroblast pyruvate carboxylase and PEPCK (where the mitochondrial isoform, PEPCK-M, predominates) activities in both siblings were normal. KDC activity in SM of S2 was decreased at 22% of the control mean (there was not enough remaining muscle from S1 to assay KDC). Fibroblast KDC activities in both siblings were normal.
Table 3.
Summary of Other Mitochondrial Enzyme Assays on the Two Siblings
| Patient |
Controls | ||||||
|---|---|---|---|---|---|---|---|
| S1 | S2 | ||||||
| Cell/Tissue | Activity (% mean) |
Average (% mean) |
Activity (% mean) |
Mean ± SD | Range | n | |
| PDC | Lymph | 1.95 (120) | 3.24 (199) | 1.63 ± 0.48 | 0.98–2.72 | 596 | |
| FB | 1.57 (65) | 1.31 (54) | 2.42 ± 0.88 | 1.26–4.42 | 329 | ||
| SM | 0.28 (9) | 27% | 0.73 (23) | 3.17 ± 1.49 | 1.20–6.52 | 340 | |
| 1.37 (43) | |||||||
| 0.94 (30) | |||||||
| KDC | FB | 2.07 (96) | 1.52 (72) | 2.10 ± 1.03 | 0.73–4.58 | 42 | |
| SM | NA | 0.68 (22) | 3.14 ± 1.44 | 0.82–6.70 | 71 | ||
| PC | FB | 1.72 (121) | 103% | 2.74 (193) | 1.42 ± 0.79 | 0.56–3.22 | 338 |
| 1.21 (85) | |||||||
| PEPCK | FB | 0.64 (14) | 3.28 (71) | 4.60 ± 2.56 | 1.09–10.04 | 309 | |
Activity, nmol/min/mg protein; PDC, pyruvate dehydrogenase complex; KDC, α-ketoglutarate dehydrogenase complex; PC, pyruvate carboxylase; PEPCK, phosphoenolpyruvate carboxykinase; Lymph, lymphocytes; FB, fibroblasts; SM, skeletal muscle; NA, not available; and SD, standard deviation.
ETC activities in fibroblasts from S1 and S2 (Table 4), and integrated oxidative phosphorylation in permeabilized fibroblasts in S1 and S2 (data not shown) were essentially normal. SM ETC I-III activity in S1 and S2 were at the low end of the reference range, 25 and 33% of the ref range, respectively (Table 4). SM ETC II-III activity in S2 was also at the low end of the ref range, 19% of the ref range, and SM complex III activity in S2 was 38%, below the ref range, while III in S1 was normal (Table 4).
Table 4.
Electron Transport Chain (ETC) Activity in Fibroblasts and Skeletal Muscle*
| Complexes; enzymatic activity, units below (% of mean) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Tissue | Patient | I-III | “I” | II-III | “II” | III | IV | CS |
| FB | S1 | 10.5 (50%) |
25.9 (101%) |
5.8 (181%) |
72.0 (90%) |
1.3 (65%) |
43.0 (82%) |
|
| FB control |
Mean ± SD (n=144) |
20.9 ± 10.9 | 25.6 ± 4.5 | 3.2 ± 0.8 | 79.8 ± 17.7 | 2.0 ± 0.3 | 52.2 ± 8.8 | |
| Range | 6.1–50.8 | 17.1–33.6 | 1.9–4.3 | 49.7–116.7 | 1.5–2.6 | 36.3–69.9 | ||
| FB | S2 | ND | ND | 16.7 (246%) |
ND | 80.1 (46%) |
1.0 (91%) |
52.5 (122%) |
| FB control |
Mean ± SD (29) |
6.8 ± 3.7 | 173.2 ± 76.7 | 1.1 ± 0.4 | 42.9 ± 10.4 | |||
| Range | 3.6–20.3 | 37.5–311.0 | 0.6–2.5 | 22.9–63.3 | ||||
| SM | S1 |
0.3 (25%) |
48.5(162%) | 1.1 (52%) |
1.2 150%) |
16.9 (111%) |
64.5 (43%) |
12.5 (67%) |
| S2 |
0.4 (33%) |
25.3 (85%) |
0.4 (19%) |
0.5 (63%) |
5.8 (38%) | 61.6 (41%) |
24.5 (132%) |
|
| SM control |
Mean ± SD (n=49) |
1.2 ± 1.1 | 29.9 ± 12.9 | 2.1 ± 1.2 | 0.8 ± 0.4 | 15.2 ± 6.8 | 148.9 ± 67.2 | 18.6 ± 4.7 |
| Range | 0.2–4.7 | 11.5–60.1 | 0.4–4.9 | 0.1–2.0 | 6.8–35.2 | 57.3–373.0 | 9.4–30.0 | |
Oxidative phosphorylation (OxPhos, integrated function) assayed in intact and permeabilized fibroblasts in both sibs were normal (data not shown); Enzyme activity: FB, nmol/min/mg protein and SM, µmol/min/g wet weight. I-III, NADH-cytochrome c reductase (rotenone sensitive); “I”, NADH-ferricyanide reductase; II-III, succinate-cytochrome c reductase (antimycin sensitive); “II”, succinate dehydrogenase; III, decylubiquinol-cytochrome c reductase; IV, cytochrome c oxidase; CS, citrate synthase; ND, not done; and SD: standard deviation. Low CIII in SM of S2 is shown in bold and red. Other SM ETC activities at the low end of the reference range in S1 and S2 are shown in bold and blue. FB ETC assays of S1 and S2 were as previously described [19] and 20], respectively.
4.6 Inhibition of PDC by propionyl-CoA
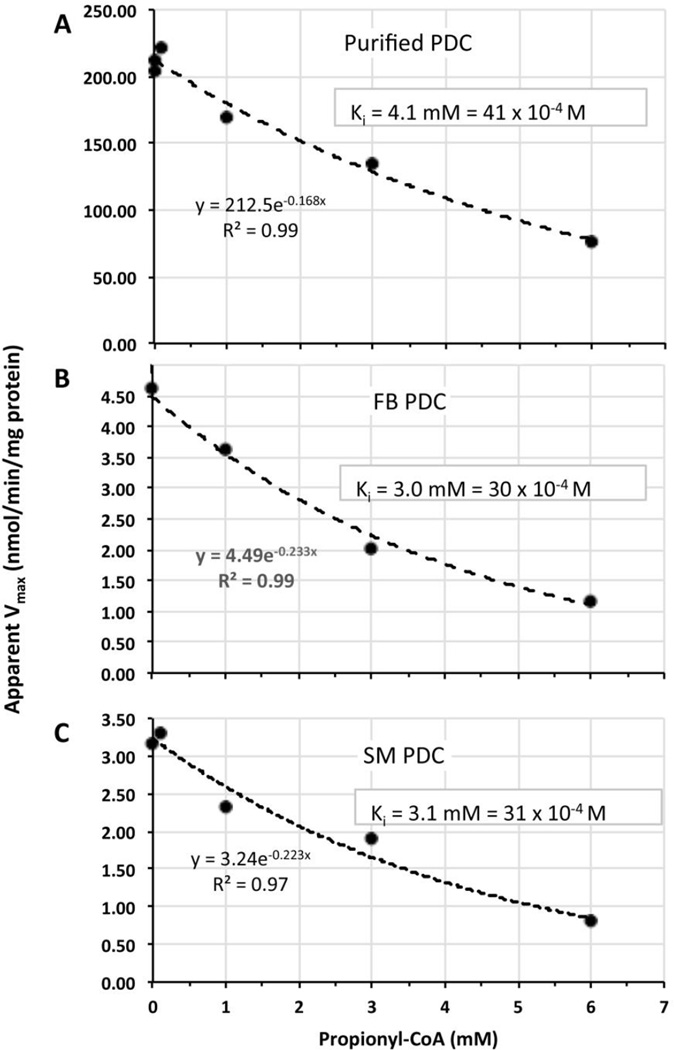
Understanding the etiology for the observed apparent tissue-specific low activities of PDC and other mitochondrial enzymes involved in oxidative metabolism has implications for control of lactic acidemia and other mitochondrial functions. We hypothesized that elevation of a specific acyl-CoA metabolite(s) in SM secondary to A-SCS deficiency, may inhibit PDC function. Thus, we tested whether succinyl-, methylmalonyl- and propionyl-CoA can inhibit PDC in vitro. Succinyl- and methylmalonyl-CoA concentrations up to 1 mM did not inhibit PDC (data not shown). However, propionyl-CoA did inhibit purified PDC as well as PDC in disrupted cultured skin fibroblasts and in SM homogenate (Fig. 4). The PDC Km (for pyruvate) and Vmax measured without propionyl-CoA were 23 mM and 213 nmol/min/mg protein (purified PDC), and the apparent Km 12.4 mM and apparent Vmax 4.6 nmol/min/mg total protein (disrupted fibroblasts) and 18 mM and 3.2 nmol/min/mg total protein (muscle homogenate), respectively (Supplementary Table 2 and Supplementary Fig. 1). The propionyl-CoA Ki or apparent Ki were very similar for these three sources of PDC, 4.1 mM (purified PDC), 3.0 mM (disrupted fibroblasts), and 3.1 mM (SM homogenate), respectively (Fig. 4).
Fig. 4.
Inhibition of PDC by propionyl-CoA. Vmax (nmol/min/mg protein) of PDC (A, purified porcine heart complex); or apparent Vmax (B, complex in disrupted cultured fibroblasts; and C, complex in SM homogenate) vs propionyl-CoA concentration (mM) and their respective determined Ki or apparent Ki
5. Discussion
The c.985A>G and c.920C>T variants of SUCLA2 have been reported before [11, 12] but not together in the compound heterozygous state as described in the two siblings here. While both siblings had a progressive encephalomyopathy, they differed in the severity of other manifestations of their illness. Because of their difference in clinical phenotype, we focused on ascertaining: 1) the degree of deficiency of the enzyme, A-SCS; 2) the magnitude of mtDNA depletion; and 3) the secondary mitochondrial functional abnormalities such as PDC, KDC, and ETC deficiencies.
5.1 Degree of succinyl-CoA synthetase deficiency
We sought to quantify the SCS activity in cultured skin fibroblasts derived from each of the two siblings. To that end, we established a novel LC-MS/MS based enzyme assay using deuterated succinate as substrate. The apparent Km for succinate and Vmax in A-SCS in control human fibroblast samples has not been reported before and we determined them to be 1.3 mM and 1.2 nmol/min/mg protein, respectively. The ligase activity of the same enzyme probably has a different apparent Km for succinyl-CoA and Vmax, and the actual kinetics, to our knowledge, has not been determined in the human fibroblasts. In patient S1, the SUCLA2 protein content on Western blot analysis was 8.4 % of control and A-SCS activity was 9.5% of control while succinyl-CoA synthetase (GDP-forming) (G-SCS) was 134%. In patient S2, the mean SUCLA2 protein content from two S2 fibroblast lines on Western blot analyses was 12% of control while mean A-SCS and G-SCS activities were 13% and 135% of control, respectively. Thus, our work further supports the severe pathogenicity of these two mutations in compound heterozygosity in SUCLA2.
5.2 Magnitude of mitochondrial DNA (mtDNA) depletion
A very common feature of A-SCS deficiency is mtDNA depletion. As the two main targets in this disease are SM and the brain, one would expect significant mitochondrial depletion in these two organs. However, we did not detect significant mtDNA depletion in SM samples from either sibling. This is somewhat unexpected as both siblings had profound myopathy. The pathophysiology of mtDNA depletion in A-SCS deficiency has not been delineated. However, the observed binding of the SUCLA2 complex to the NDPK protein for deoxy-nucleoside triphosphate synthesis may be key to our understanding of the mechanism of mtDNA depletion [25]‥ Restoration of the deoxy-nucleoside triphosphate pools needed for DNA synthesis by deficiency of SUCLA2 in certain target cells which have little activity of SUCLG2, such as SM, may be important in DNA depletion, but that did not occur in these siblings or in previously reported SUCLA2 deficient cultured fibroblasts, despite low activity of cytochrome oxidase [25].
5.3 Secondary PDC deficiency and other mitochondrial function testing
In an effort to further delineate the impact of a primary A-SCS deficiency on mitochondrial oxidative metabolism, additional studies were performed on cultured skin fibroblasts, lymphocytes and SM. Additionally to the primary defect in A-SCS, we find in SM deficiencies of PDC activity from both siblings, low activity of KDC in S2, and low activities of various ETC assays in S1 and/or S2. This was not associated with deficient or low activities of any of these mitochondrial enzymes in cultured fibroblasts, lymphocytes or a generalized decrease of mitochondrial enzyme activity in SM, or depletion of mtDNA in SM. Pyruvate carboxylase and PEPCK enzyme activities were normal in fibroblasts from both siblings. The normal activities of the soluble mitochondrial matrix enzymes citrate synthase and E3, and mitochondrial inner membrane succinate dehydrogenase (complex II) in SM of both siblings, is sufficient evidence against the possibility of deterioration of the biochemical integrity of the specimens affecting PDC and other mitochondrial enzyme activities (https://www.sac-cu.org/NAMDC/Site/DocCenter.aspx and [23]).
Others have also noted low and/or low-normal ETC activities (particularly in complexes I, III and IV) in SM of patients with SUCLA2 deficiency, but these cases also had depletion of mtDNA [5, 12]. Therefore, we conclude that the decrease in PDC, KDC and several ETC activities is specific to SM, secondarily associated with A-SCS deficiency, and not due to muscle sample biochemical integrity or mtDNA depletion.
We found markedly increased propionylcarnitine in SM in one sibling and showed that propionyl-CoA inhibits PDC in disrupted cultured skin fibroblasts, SM homogenate, and in purified porcine heart PDC complex, but the muscle concentration of propionyl-CoA is likely not sufficient to account for associated PDC deficiency. Because we had no remaining muscle (S1) or insufficient amount (S2) for accurate measurement of muscle acyl-CoA content, we resorted to extrapolating the muscle acyl-CoA content from the tissue acylcarnitine levels. In human SM, the content of total carnitine is normally approximately 100-fold higher than total CoA; carnitine vs CoA, 3120 ± 720 vs 31.1 ± 6.3 nmol/gm wet weight (in a normoxia pre-exercise state) [26]. Based on this data, we estimate that the propionyl-CoA content in the SM of patient S2 may be about 0.7 nmol/gm wet weight or 1 µM (see Supplementary Materials for details). This is about 3000-fold lower than the measured Ki of propionyl-CoA on PDC under our conditions (Fig. 4). Furthermore, the reported Ki of acetyl-CoA, 5–10 µM, for end-product inhibition of PDC is 1000–3500 fold lower than the Ki of propionyl CoA measured here [27]. This implies that propionyl-CoA has significantly lower affinity for PDC than acetyl-CoA. Therefore, the elevated concentration of propionyl-CoA in SM from S2, appears not to be sufficient to account for secondary functional PDC deficiency, and SM from S1 did not have an increase of propionylcarnitine but has low activity of PDC. Inhibition of PDC by propionyl-CoA may be relevant to other disorders where propionyl-CoA may increase to much higher levels such as propionic acidemia.
The 13-fold higher concentration of acetylcarnitine compared to propionylcarnitine (in S2) implies that the elevation of acetyl-CoA may be more significantly inhibiting PDC activity. We extrapolate that the acetyl-CoA content in SM of S1 and S2 are 2 µM and 13 µM, respectively. The extrapolated acetyl-CoA content in SM of S2 (but not of S1) would be sufficient for end-product inhibition of PDC. Thus, A-SCS deficiency which causes a block in the tricarboxylic acid cycle, may cause a secondary upstream increase of acetyl-CoA that could inhibit PDC, but would not explain low PDC activity in S1.
The reasons for the observed SM-specific PDC and other deficiencies in the two siblings with primary A-SCS deficiency presented here remains unexplained. Alternative possible mechanisms are: 1) acyl-CoA accumulation within the mitochondrial matrix resulting in a local ≥1000-fold higher concentration of propionyl-CoA or acetyl-CoA; 2) end-product inhibition of PDC by acetyl-CoA accumulation within mitochondria; 3) presence of another inhibitor or factor(s), more generally affecting SM mitochondrial metabolism, including PDC, possibly KDC and ETC, and others not determined with the assays here; and/or 4) presence of other shared deleterious genetic variation(s) in the siblings that might affect PDC.
Recently, a significant decrease in the oxygen consumption rate was observed using pyruvate and malate as substrates in isolated mitochondria from heart tissue of 6-month-old heterozygous Sucla2+/− mice compared to wild type, suggesting a defect in heart muscle PDC or ETC, but direct assay of these functions in heart and SM tissue homogenates were not performed [28]. Assaying PDC, KDC and ETC activities in SM from individuals with A-SCS deficiency and/or conditional homozygous deficient mouse models [28, 29], or other genetic disorders in which acyl-CoAs accumulate could be useful in this respect.
5.4 Study limitations
We were unable to quantify mtDNA depletion in a variety of different muscle samples or other tissues such as liver, or explain the difference between these siblings and other cases of A-SCS deficiency. We limited our analyses of secondary protein/complexes that might be affected by the pathologic process, but there are many proteins and enzyme complexes in mitochondria that were not examined. We did not have an opportunity to assay intact, isolated mitochondrial from fresh SM for overall integrated oxidative phosphorylation. It is possible that the difference in clinical phenotype observed between the two siblings may be due to the fact that each of the siblings received different therapy; patient S1 was treated with DCA, while patient S2 briefly received a ketogenic diet, but this is uncertain. Alternatively, additional mutations (e.g. a polygenic situation with a sporadic de novo deleterious allele) may underlie the phenotypic variability observed between the siblings and a more detailed trio WES or whole genome analysis of siblings and parents may be informative.
6. Summary and Conclusions
The SUCLA2 c.985A>G (p.M329V) and c.920C>T (p.A307V) mutations in a compound heterozygous state are pathogenic, leading to low A-SCS activity and low immunoreactivity of SUCLA2, and are associated with devastating variable phenotypes. The reasons for the SM-specific low activity of PDC, KDC, and several low or low-normal ETC activities, the atypically normal SM mtDNA content, and the differences in some phenotypic manifestations in these two siblings remains unexplained. Although we show that propionyl-CoA inhibits PDC, extrapolated mitochondrial propionyl-CoA is not high enough concentration in SM in either sibling to account for low SM PDC activity. The A-SCS deficiency may cause a secondary upstream increase of acyl-CoAs that might inhibit or otherwise disrupt other mitochondrial oxidative metabolism enzymes. These findings are consistent with the hypothesis that they are not secondary to mtDNA depletion, but may be associated. There is a need for better understanding of the pathogenic mechanisms, phenotypic variability, and the possible impact of therapeutic interventions in this devastating disorder.
Supplementary Material
Highlights.
We describe two siblings compound heterozygous for SUCLA2 mutations, c.985A>G (p.M329V) and c.920C>T (p.A307V).
We developed a new LC-MS/MS-based assay to quantify succinyl-CoA synthetase enzyme activity.
We describe for the first time the presence of a secondary pyruvate dehydrogenase complex deficiency in two siblings with SUCLA2 gene defects.
Acknowledgments
This research was supported in part by the Manton Center for Orphan Disease Research Gene Discovery Core, Boston Children’s Hospital and the Genomics Core Facility of the Case Western Reserve University (CWRU) School of Medicine’s Genetics and Genome Sciences Department, and by funds from the Clinical and Translational Science Collaborative (CTSC) CWRU Core Utilization Pilot Grant 2014 (05496) (to JKB) and NIH RDCRN 5U54NS078059-05 project NAMDC 7413 grant (to JKB and SDD).
Abbreviations
- SUCL
succinyl-CoA ligase
- A-SCS
succinyl-CoA synthetase (ADP-forming)
- SM
skeletal muscle
- PDC
pyruvate dehydrogenase complex
- KDC
α-ketoglutarate dehydrogenase complex
- PEPCK
phosphoenolpyruvate carboxykinase
- ETC
electron transport chain complexes
- WES
whole exome sequencing
- mtDNA
mitochondrial DNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Parts of this work were presented as a talk (by JKB) at the 2016 Society for Inherited Metabolic Disorders (SIMD) meeting.
References
- 1.Kowluru A, Tannous M, Chen HQ. Localization and characterization of the mitochondrial isoform of the nucleoside diphosphate kinase in the pancreatic beta cell: evidence for its complexation with mitochondrial succinyl-CoA synthetase. Arch Biochem Biophys. 2002;398:160–169. doi: 10.1006/abbi.2001.2710. [DOI] [PubMed] [Google Scholar]
- 2.Ostergaard E, Hansen FJ, Sorensen N, Duno M, Vissing J, Larsen PL, Faeroe O, Thorgrimsson S, Wibrand F, Christensen E, Schwartz M. Mitochondrial encephalomyopathy with elevated methylmalonic acid is caused by SUCLA2 mutations. Brain. 2007;130:853–861. doi: 10.1093/brain/awl383. [DOI] [PubMed] [Google Scholar]
- 3.Van Hove JL, Saenz MS, Thomas JA, Gallagher RC, Lovell MA, Fenton LZ, Shanske S, Myers SM, Wanders RJ, Ruiter J, Turkenburg M, Waterham HR. Succinyl-CoA ligase deficiency: a mitochondrial hepatoencephalomyopathy. Pediatr Res. 2010;68:159–164. doi: 10.1203/PDR.0b013e3181e5c3a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elpeleg O, Miller C, Hershkovitz E, Bitner-Glindzicz M, Bondi-Rubinstein G, Rahman S, Pagnamenta A, Eshhar S, Saada A. Deficiency of the ADP-forming succinyl-CoA synthase activity is associated with encephalomyopathy and mitochondrial DNA depletion. Am J Hum Genet. 2005;76:1081–1086. doi: 10.1086/430843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrozzo R, Dionisi-Vici C, Steuerwald U, Lucioli S, Deodato F, Di Giandomenico S, Bertini E, Franke B, Kluijtmans LA, Meschini MC, Rizzo C, Piemonte F, Rodenburg R, Santer R, Santorelli FM, van Rooij A, Vermunt-de Koning D, Morava E, Wevers RA. SUCLA2 mutations are associated with mild methylmalonic aciduria, Leigh-like encephalomyopathy, dystonia and deafness. Brain. 2007;130:862–874. doi: 10.1093/brain/awl389. [DOI] [PubMed] [Google Scholar]
- 6.Jab eri E, Chitsazian F, Ali Shahidi G, Rohani M, Sina F, Safari I, Malakouti Nejad M, Houshmand M, Klotzle B, Elahi E. The novel mutation p.Asp251Asn in the beta-subunit of succinate-CoA ligase causes encephalomyopathy and elevated succinylcarnitine. J Hum Genet. 2013;58:526–530. doi: 10.1038/jhg.2013.45. [DOI] [PubMed] [Google Scholar]
- 7.Morava E, Steuerwald U, Carrozzo R, Kluijtmans LA, Joensen F, Santer R, Dionisi-Vici C, Wevers RA. Dystonia and deafness due to SUCLA2 defect; Clinical course and biochemical markers in 16 children. Mitochondrion. 2009;9:438–442. doi: 10.1016/j.mito.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Lamperti C, Fang M, Invernizzi F, Liu X, Wang H, Zhang Q, Carrara F, Moroni I, Zeviani M, Zhang J, Ghezzi D. A novel homozygous mutation in SUCLA2 gene identified by exome sequencing. Mol Genet Metab. 2012;107:403–408. doi: 10.1016/j.ymgme.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matilainen S, Isohanni P, Euro L, Lonnqvist T, Pihko H, Kivela T, Knuutila S, Suomalainen A. Mitochondrial encephalomyopathy and retinoblastoma explained by compound heterozygosity of SUCLA2 point mutation and 13q14 deletion. Eur J Hum Genet. 2015;23:325–330. doi: 10.1038/ejhg.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navarro-Sastre A, Tort F, Garcia-Villoria J, Pons MR, Nascimento A, Colomer J, Campistol J, Yoldi ME, Lopez-Gallardo E, Montoya J, Unceta M, Martinez MJ, Briones P, Ribes A. Mitochondrial DNA depletion syndrome: new descriptions and the use of citrate synthase as a helpful tool to better characterise the patients. Mol Genet Metab. 2012;107:409–415. doi: 10.1016/j.ymgme.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Nogueira C, Meschini MC, Nesti C, Garcia P, Diogo L, Valongo C, Costa R, Videira A, Vilarinho L, Santorelli FM. A novel SUCLA2 mutation in a Portuguese child associated with “mild” methylmalonic aciduria. J Child Neurol. 2015;30:228–232. doi: 10.1177/0883073814527158. [DOI] [PubMed] [Google Scholar]
- 12.Carrozzo R, Verrigni D, Rasmussen M, de Coo R, Amartino H, Bianchi M, Buhas D, Mesli S, Naess K, Born AP, Woldseth B, Prontera P, Batbayli M, Ravn K, Joensen F, Cordelli DM, Santorelli FM, Tulinius M, Darin N, Duno M, Jouvencel P, Burlina A, Stangoni G, Bertini E, Redonnet-Vernhet I, Wibrand F, Dionisi-Vici C, Uusimaa J, Vieira P, Osorio AN, McFarland R, Taylor RW, Holme E, Ostergaard E. Succinate-CoA ligase deficiency due to mutations in SUCLA2 and SUCLG1: phenotype and genotype correlations in 71 patients. J Inherit Metab Dis. 2016;39:243–252. doi: 10.1007/s10545-015-9894-9. [DOI] [PubMed] [Google Scholar]
- 13.Minkler PE, Stoll MS, Ingalls ST, Kerner J, Hoppel CL. Validated method for the quantification of free and total carnitine, butyrobetaine, and acylcarnitines in biological samples. Anal Chem. 2015;87:8994–9001. doi: 10.1021/acs.analchem.5b02198. [DOI] [PubMed] [Google Scholar]
- 14.Minkler PE, Stoll MS, Ingalls ST, Kerner J, Hoppel CL. Quantitative acylcarnitine determination by UHPLC-MS/MS--Going beyond tandem MS acylcarnitine “profiles”. Mol Genet Metab. 2015;116:231–241. doi: 10.1016/j.ymgme.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venegas V, Wang J, Dimmock D, Wong LJ. Real-time quantitative PCR analysis of mitochondrial DNA content. Curr Protoc Hum Genet. Chapter 19. 2011 doi: 10.1002/0471142905.hg1907s68. Unit 19 17. [DOI] [PubMed] [Google Scholar]
- 16.Chuang DT, Hu CW, Patel MS. Induction of the branched-chain 2-oxo acid dehydrogenase complex in 3T3-L1 adipocytes during differentiation. Biochem J. 1983;214:177–181. doi: 10.1042/bj2140177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerr D, Grahame G, Nakouzi G. Assays of pyruvate dehydrogenase complex and pyruvate carboxylase activity. Methods Mol Biol. 2012;837:93–119. doi: 10.1007/978-1-61779-504-6_7. [DOI] [PubMed] [Google Scholar]
- 18.Atkin BM, Utter MF, Weinberg MB. Pyruvate carboxylase and phosphoenolpyruvate carboxykinase activity in leukocytes and fibroblasts from a patient with pyruvate carboxylase deficiency. Pediatr Res. 1979;13:38–43. doi: 10.1203/00006450-197901000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Ye CL, Hoppel F. Measuring oxidative phosphorylation in human skin fibroblasts. Anal Biochem. 2013;437:52–58. doi: 10.1016/j.ab.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Hoppel CL, Kerr DS, Dahms B, Roessmann U. Deficiency of the reduced nicotinamide adenine dinucleotide dehydrogenase component of complex I of mitochondrial electron transport. Fatal infantile lactic acidosis and hypermetabolism with skeletal-cardiac myopathy and encephalopathy. J Clin Invest. 1987;80:71–77. doi: 10.1172/JCI113066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krahenbuhl S, Chang M, Brass EP, Hoppel CL. Decreased activities of ubiquinol:ferricytochrome c oxidoreductase (complex III) and ferrocytochrome c:oxygen oxidoreductase (complex IV) in liver mitochondria from rats with hydroxycobalamin[c-lactam]-induced methylmalonic aciduria. J Biol Chem. 1991;266:20998–21003. [PubMed] [Google Scholar]
- 22.Krahenbuhl S, Talos C, Wiesmann U, Hoppel CL. Development and evaluation of a spectrophotometric assay for complex III in isolated mitochondria, tissues and fibroblasts from rats and humans. Clin Chim Acta. 1994;230:177–187. doi: 10.1016/0009-8981(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 23.Stacpoole PW, deGrauw TJ, Feigenbaum AS, Hoppel C, Kerr DS, McCandless SE, Miles MV, Robinson BH, Tang PH. Design and implementation of the first randomized controlled trial of coenzyme CoQ(10) in children with primary mitochondrial diseases. Mitochondrion. 2012;12:623–629. doi: 10.1016/j.mito.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coonrod EM, Margraf RL, Russell A, Voelkerding KV, Reese MG. Clinical analysis of genome next-generation sequencing data using the Omicia platform. Expert Rev Mol Diagn. 2013;13:529–540. doi: 10.1586/14737159.2013.811907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller C, Wang L, Ostergaard E, Dan P, Saada A. The interplay between SUCLA2, SUCLG2, and mitochondrial DNA depletion. Biochim Biophys Acta. 2011;1812:625–629. doi: 10.1016/j.bbadis.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Friolet R, Hoppeler H, Krahenbuhl S. Relationship between the coenzyme A and the carnitine pools in human skeletal muscle at rest and after exhaustive exercise under normoxic and acutely hypoxic conditions. J Clin Invest. 1994;94:1490–1495. doi: 10.1172/JCI117488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behal RH, Buxton DB, Robertson JG, Olson MS. Regulation of the pyruvate dehydrogenase multienzyme complex. Annu Rev Nutr. 1993;13:497–520. doi: 10.1146/annurev.nu.13.070193.002433. [DOI] [PubMed] [Google Scholar]
- 28.Kacso G, Ravasz D, Doczi J, Nemeth B, Madgar O, Saada A, Ilin P, Miller C, Ostergaard E, Iordanov I, Adams D, Vargedo Z, Araki M, Araki K, Nakahara M, Ito H, Gal A, Molnar MJ, Nagy Z, Patocs A, Adam-Vizi V, Chinopoulos C. Two transgenic mouse models for beta subunit components of succinate-CoA ligase yielding pleiotropic metabolic alterations. Biochem J. 2016;473:3463–3485. doi: 10.1042/BCJ20160594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donti TR, Stromberger C, Ge M, Eldin KW, Craigen WJ, Graham BH. Screen for abnormal mitochondrial phenotypes in mouse embryonic stem cells identifies a model for succinyl-CoA ligase deficiency and mtDNA depletion. Dis Model Mech. 2014;7:271–280. doi: 10.1242/dmm.013466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.