Abstract
Serine/threonine protein phosphatases control dephosphorylation of numerous cardiac proteins, including a variety of ion channels and calcium-handling proteins, thereby providing precise post-translational regulation of cardiac electrophysiology and function. Accordingly, dysfunction of this regulation can contribute to the initiation, maintenance and progression of cardiac arrhythmias. Atrial fibrillation (AF) is the most common heart rhythm disorder and is characterized by electrical, autonomic, calcium-handling, contractile, and structural remodeling, which include, among other things, changes in the phosphorylation status of a wide range of proteins. Here, we review AF-associated alterations in the phosphorylation of atrial ion channels, calcium-handling and contractile proteins, and their role in AF-pathophysiology. We highlight the mechanisms controlling the phosphorylation of these proteins and focus on the role of altered dephosphorylation via local type-1, type-2A and type-2B phosphatases (PP1, PP2A, and PP2B, also known as calcineurin, respectively). Finally, we discuss the challenges for phosphatase research, potential therapeutic significance of altered phosphatase-mediated protein dephosphorylation in AF, as well as future directions.
Keywords: protein phosphatases, atrial fibrillation, ion channels, calcium handling, myofilaments
1. Introduction
1.1. Atrial fibrillation
Atrial fibrillation (AF) significantly affects morbidity and mortality as a risk factor for worsening heart failure (HF) and stroke [1–3]. AF affects more than 33 million people worldwide, with an incidence that is expected to increase further as the population ages [4]. A large number of cardiovascular and non-cardiovascular diseases, as well as AF itself, increase the risk of AF by promoting proarrhythmic atrial remodeling, giving rise to a progressive atrial cardiomyopathy [1,5,6]. Clinically, this progression of AF is reflected by its classification into paroxysmal AF (pAF), persistent AF or long-standing persistent (chronic) AF (cAF), depending on the duration of the arrhythmia [3].
Conceptually, abnormal ectopic (triggered) activity and reentry are the main arrhythmogenic mechanisms underlying AF [2,7,8] (Figure 1). Atrial ectopic activity is predominantly promoted by (sub)cellular Ca2+-handling abnormalities resulting in delayed afterdepolarizations (DADs) that may trigger atrial action potentials (AP) independent of the normal atrial activation. Sustained high-frequent ectopic activity can maintain AF through fibrillatory conduction [7]. In addition, ectopic activity can initiate reentry in a vulnerable substrate characterized by a short refractory period and slow and/or heterogeneous conduction [7]. Clinical AF is likely maintained through a combination of ectopic and reentrant mechanisms, with significant inter-patient variability in the exact balance between both mechanisms [2,9].
Fig. 1. Schematic overview of the general concepts of AF pathophysiology.
AF is induced and maintained by ectopic activity and reentry. Reentry requires an arrhythmogenic substrate and a suitable trigger to initiate it. The trigger usually involves premature beats or rapid ectopic firing. Numerous risk factors including genetics and cardiovascular and non-cardiovascular comorbidities cause alterations in atrial function and structure (remodeling) increasing the likelihood of ectopic activity and reentry. The remodeled substrate include effective refractory period (ERP) changes, conduction slowing and atrial fibrosis, ectopic activity is believed to result from abnormal automaticity, early (EADs) and delayed (DADs) afterdepolarizations and activation of autonomic nervous system (ANS). Increased activity of serine/threonine protein phosphatases, altered local phosphatase targeting and the subsequent alterations in protein phosphorylation are suggested to mediated the effects of atrial remodeling promoting the induction and maintenance of AF by increasing the likelihood of ectopic activity and reentry.
At the molecular level, atrial remodeling is mediated by various pathways [2,10,11]. Dysfunctional post-translational regulation through reversible protein phosphorylation may contribute both to the acute initiation of AF and to the substrate promoting AF maintenance [12,13] (Figure 1). The majority of all proteins in human beings undergo reversible phosphorylation, predominantly at serine or threonine residues [14,15]. Most proteins in the heart have multiple phosphorylation sites and the phosphorylation state of each site depends on a dynamic local balance between multiple kinases and phosphatases organized as holoenzymes in macromolecular complexes [16]. Combined, the phosphorylation state of all sites determines the function of each holoenzyme and allows precise control of atrial electrophysiology and contractility.
Type-1 phosphatases (PP1) and type-2 phosphatases (PP2), notably PP2A and PP2B (calcineurin; Cn) are the best characterized phosphatases [17] and make up the majority of phosphatase activity in the heart [18]. The catalytic subunits of these phosphatases are organized in multi-protein complexes with different regulatory and anchoring subunits that target the holoenzyme to specific subcellular locations to ensure precise local regulation of individual targets. This review will focus on the regulation of atrial electrophysiology, Ca2+ handling and contractility through PP1, PP2A and calcineurin and their potential roles in AF.
1.2. Cellular electrophysiology and excitation-contraction coupling in the atrium
For a detailed overview of cardiac cellular electrophysiology and excitation-contraction coupling, the interested reader is referred to [19–21]. Similar to ventricular cardiomyocytes, the upstroke of the atrial AP is mediated by an inward Na+-current (INa). Subsequently, the L-type Ca2+-channel is rapidly activated, resulting in an additional inward current (ICa,L). The Ca2+ entering the atrial cardiomyocyte via the L-type Ca2+-channels triggers a much larger Ca2+ release from the sarcoplasmic reticulum (SR) through type-2 ryanodine receptor (RyR2) channels. The total increase in cytosolic Ca2+ gives rise to the systolic Ca2+-transient and initiates cardiomyocyte contraction. Repolarization is mediated by the voltage- and time-dependent activation of atrial K+-currents, including the transient-outward current (Ito), the ultra-rapid, rapid and slow delayed-rectifier K+-currents (IKur, IKr, and IKs, respectively), two-pore K+-current (IK2P) and inward-rectifier K+-current (IK1). The acetylcholine-activated inward-rectifier K+-current (IK,ACh) furthermore contributes to faster repolarization during increased parasympathetic tone. Relaxation of atrial cardiomyocytes occurs when cytosolic Ca2+ levels decline due to reuptake of Ca2+ into the SR by the SR Ca2+-ATPase type-2a (SERCA2a) and extrusion from the cell by the Na+/Ca2+ exchanger type-1 (NCX1), with a small additional contribution from the plasmalemmal Ca2+-ATPase. Finally, homeostasis of Na+ and K+ is achieved through the Na+-K+-ATPase. Virtually all of these channels/transporters or their regulatory subunits can be modulated through reversible phosphorylation by protein kinases, notably protein kinase-A (PKA), protein kinase-C (PKC), and Ca2+/calmodulin-dependent protein kinase-II (CaMKII), and dephosphorylation by PP1, PP2A and, to a lesser extent, Cn.
Despite the overall similarities between atrial and ventricular electrophysiology and Ca2+-handling, there are important electrophysiological differences between both chambers. Several ion currents (IKur, IK2P and IK,ACh), are almost exclusively present in the atria, resulting in a more triangular AP morphology compared to ventricular cardiomyocytes [20]. These channels are currently explored as targets for new antiarrhythmic drugs with atrial-specific effects [8,22]. Similarly, the inhibitory SERCA2a-regulator sarcolipin is predominantly expressed in the atria [23] and atrial cardiomyocytes generally have a less well-developed system of transverse membrane invaginations (T-tubules) that bring L-type Ca2+-channels into close proximity of RyR2 located towards the center of the cardiomyocyte [24], modulating the atrial Ca2+-transient. Protein phosphatases are also differentially expressed between atria and ventricles, with increased PP1 and PP2A mRNA levels and increased PP2A, but unchanged PP1 protein expression in the right ventricle compared to the right atrium [25]. This difference may affect the basal phosphorylation state of atrial versus ventricular ion channels, thereby contributing to atrial/ventricular electrophysiological differences [26].
2. Remodeling of phosphatase activity and expression in AF
2.1 PP1
PP1 is ubiquitously expressed in most cell types, including atrial cardiomyocytes. Table 1 summarizes the alterations in PP1 in different AF paradigms. The global expression of PP1 is unchanged in cAF patients compared to sinus rhythm controls [27,28], but its enzymatic activity is increased [29]. In pAF patients, PP1 catalytic subunit expression is borderline-significantly increased or unchanged [30,31]. The dog models of atrial tachycardia remodeling (ATR) and ventricular tachypacing-induced congestive HF (CHF), well-known experimental models with increased susceptibility to AF [32], show qualitatively similar PP1 alterations, despite the important differences in atrial remodeling between both models [33]. Taken together, these data suggest that increased PP1 activity is a typical finding for conditions associated with increased risk of AF. Three isoforms of catalytic PP1 subunits (PP1α/PPP1CA, PP1γ/PPP1CC, and PP1δ also known as PP1β/PPP1CB) have been identified [34] (see Supplementary Table 1 for an overview of protein phosphatase nomenclature). The mRNA levels of all three isoforms are either unchanged or reduced in cAF patients compared to sinus rhythm patients [28]. The absence of a clear increase in the expression of PP1 catalytic subunits in cAF patients, as well as different animal models, despite the increase in PP1 activity (Table 1), points to post-translational regulation of PP1 activity in AF.
Table 1.
Alterations in PP1 activity and expression in AF patients and animal models.
| PP1 | ||||
|---|---|---|---|---|
| Human pAF | Human cAF | Experimental Modelsn | ||
| Enzymatic activity | +62% [29] +49% [28] (PP1+PP2A) +48% [39] |
Goat: ↔ [118] Dog ATR: +28% [119] Dog CHF: +83% [120] |
||
| Global protein levels | ↔[30] | ↔[27,28] | Goat: ↔ [118] Dog ATR: ↔ [119] Dog CHF: ↔ [120] |
|
| Specific subunits: | ||||
| PPP1CA (PP1α) | mRNA | -37%[28] | ||
| Protein | ↔[31] | +150%[39] | Rabbit RAP: +32% [121] | |
| PPP1CB (PP1β) | mRNA | -48%[28] | ||
| PPP1CC (PP1γ) | mRNA | ↔[28] | ||
| PPP1R1A (I-1) | mRNA | ↔[39] | ||
| Phospho | Rel T35 +800%[39] Rel S67 ↔[39] |
|||
| PPP1R2 (I-2) | mRNA | ↔[30] | ↔[39] | |
| PPP1R3A (RGL) | mRNA | ↔[39] | ||
| PPP1R9B (spinophilin-1) |
mRNA | ↔[39] | ||
| PP2A | ||||
| Human pAF | Human cAF | Experimental Models | ||
| Enzymatic activity | +60%[29] +49%[28] (PP1+PP2A) +38%[39] |
Goat: ↔[118] Dog ATR: ↔[119] Dog CHF: ↔[120] |
||
| Global protein levels | ↔[31] | ↔[27] +57%[28] +30%[39] |
Goat: ↔[118] Dog ATR: ↔[119] Dog CHF: ↔[120] |
|
| Specific subunits: | ||||
| PPP2CA (PP2A- Cα) |
mRNA | -58%[28] | ||
| Protein | Rabbit RAP: ↔[121] | |||
| PPP2CB (PP2A- Cβ) |
mRNA | ↔[28] | ||
| PPP2R3A (PR130) |
mRNA | ↔[39] | ||
| PPP2R1A (PP2A–A/PR65) |
Protein | ↔[28] | ||
| PP2B (Calcineurin) | ||||
| Human pAF | Human cAF | Experimental Models | ||
| Enzymatic activity | +80%[44] | Goat: +272%[122] Dog ACMs (3Hz): +151%[45] Rat: Stretched ACMs: +76%[46] Human: In vitro paced atrial tissue (2Hz): +72%[44] |
||
| Global protein levels | Rat: Stretched ACMs: +43%[46] | |||
| Specific subunits: | ||||
| PPP3CA (CnA- α) |
mRNA | +60%[43] | +68%[43] +55%[123] |
|
| Protein | +140%[43] | +215%[43] +37%[123] (60kD) +32%[123] (45kD) |
Human: In vitro paced atrial tissue (2Hz): +39%[44]; |
|
| PPP3CB (CnA-β) | mRNA | +55%[44] +60%[123] |
Human: In vitro paced atrial tissue (2Hz): +100%[44] |
|
| Protein | +83 [44] | |||
| PPP3R (CnB) | mRNA | +236%[43] | +285%[43] | |
| Protein | +164%[43] | +300%[43] | ||
ACM: atrial cardiomyocytes; ATR: atrial tachycardia remodeling; CHF: congestive heart failure; RAP: rapid atrial pacing
PP1 catalytic subunits form dimers with numerous regulatory subunits that either target the holoenzyme to specific substrates or regulate its enzymatic activity [12,17]. The composition and role of the PP1 interactome in cardiovascular disease is reviewed in detail in [35], but there is only limited information available about AF-associated remodeling of PP1 regulatory subunits (Table 1). One notable exception to this is the regulatory subunit I-1 (PPP1R1A), a selective and potent PP1 inhibitor that facilitates crosstalk between different protein phosphatases and kinases [36,37]. PKA-dependent phosphorylation of Thr35 activates I-1. As such, PKA-dependent I-1 activation and subsequent PP1 inhibition form a positive feedback loop amplifying the phosphorylation of several substrates during β-adrenoceptor (β-AR) stimulation. Dephosphorylation of I-1 Thr35 is mediated by PP2A and Cn, thereby creating additional crosstalk between different phosphatases [38]. I-1 Thr35-phosphorylation levels are strongly increased (~10 fold) in cAF patients [39], likely resulting in local reductions in PP1 activity. Furthermore, PKCα phosphorylates I-1 on Ser67, decreasing I-1 activity and substrate phosphorylation, but Ser67 phosphorylation levels are unchanged in cAF patients [39]. Thus, AF-related changes in I-1-mediated regulation (inhibition) of PP1 are unlikely to explain the increase in global PP1 activity, but may produce local reductions in PP1 activity contributing to AF-associated hyperphosphorylation of specific targets, as discussed below.
2.2 PP2A
Similar to PP1, PP2A is ubiquitously expressed [40] and its activity is increased in cAF vs. sinus rhythm patients, although the magnitude of this increase appears smaller than for PP1 (Table 1). PP2A can exist as a dimer of a catalytic (PP2A–C) and scaffold (PP2A–A) subunits, or as a trimer of scaffolding, catalytic and regulatory subunits. PP2A–A (PPP2R1) expression is unchanged in cAF patients, but the expression of PP2A–C (PPP2C) is increased, potentially explaining the increased enzymatic activity [28]. By contrast, PP2A–C expression is unchanged in pAF patients [31]. Immunostaining of PP2A–A and PP2A–C can be observed throughout the cardiomyocyte, whereas regulatory subunits have specific subcellular localizations, for example B56ε (PP2R5E) and PR72/PR130 (PPP2R3A) near the Z-line, or B56γ (PP2R5C) and PR53 (PP2R4) near the nucleus, indicating that functional regulation by PP2A is spatially-heterogeneous [40]. Regulatory subunits may also play a critical inhibitory role in the regulation of PP2A activity in the heart. For example, B56α (PPP2R5A) overexpression decreases phosphatase activity and increases RyR2 phosphorylation, whereas loss of B56α results in PP2A–mediated RyR2 dephosphorylation [41]. However, there is limited information about remodeling of PP2A regulatory subunit expression or distribution in AF, particularly in pAF patients.
Further regulation of PP2A activity can occur through phosphorylation of PP2A–C on Tyr307, which destabilizes the interaction with the regulatory subunit and reduces phosphatase activity, or methylation of Leu309, which increases activity [40]. Both modifications are promoted by conditions of oxidative stress [40], but despite the evidence for increased oxidative stress in AF [42], PP2A–C methylation levels between cAF and sinus rhythm patients are similar [39].
2.3 PP2B (Calcineurin)
Calcineurin (PP2B, Cn) is a Ca2+-dependent phosphatase consisting of catalytic (CnA) and regulatory (CnB) subunits that link Ca2+- and phosphorylation-dependent signaling pathways [15]. Three different CnA isoforms (CnAα/PPP3CA, CnAβ/PPP3CB, and CnAγ/PPP3CC) have been identified, of which CnAα and CnAβ are ubiquitously expressed. CnB is expressed by two genes (CnBα/PPP3R1 and CnBβ/PPP3R2) [34]. Both expression and activity of CnA are increased in pAF [43] and cAF patients [44] as well as in cardiomyocytes paced at 3 Hz compared to those paced at 1-Hz [44,45] (Table 1), suggesting that upregulation of CnA occurs in response to AF-related tachycardia. Other studies have shown that increased atrial stretch may also upregulate Cn activity [46], highlighting the multi-factorial nature of AF-related Cn regulation. Both mRNA and protein levels of CnAβ are significantly increased in cAF patients compared to sinus rhythm controls [44] and CnB expression is similarly enhanced [43].
3. Altered regulation of cardiac electrophysiology and contraction by protein phosphatases in AF
Figure 2 schematically depicts the protein phosphatase complexes regulating the local phosphorylation of individual ion channels and Ca2+-handling proteins, thereby modulating atrial electrophysiology and contraction in AF.
Fig. 2. Schematic overview of protein phosphatase complexes regulating the local phosphorylation of individual ion channels and Ca2+-handling proteins.
Adapted from Heijman et al. [12].
3.1. Modulation of APD by protein phosphatases
Reentry-promoting APD shortening is a hallmark of AF-associated atrial electrical remodeling and is mediated by transcriptional, translational and post-translational regulation of atrial ion channels [2]. A reduction in depolarizing ICa,L, likely developing as an adaptive response to the Ca2+-overload during initial stages of atrial tachycardia, is an important contributor to APD shortening in cAF patients [28] and ATR animal models [47]. Moreover, in the long term, this reduction likely contributes to the reduced Ca2+-transient amplitude and atrial hypo-contractility associated with AF [48–50]. The molecular mechanisms underlying reduced ICaL are diverse [51], including, among other things, increased phosphatase-mediated channel dephosphorylation [28] (Figure 3). Similarly, indirect evidence using pharmacological inhibitors identified impaired src-kinase regulation of ICa,L together with an increased phosphatase activity, suggesting that a complex alteration in the kinase/phosphatase balance leads to ICa,L dysregulation in cAF patients [29]. The PP2A-catalytic subunit can bind directly to the poreforming L-type Ca2+ -channel α1C-subunit, in close proximity to the PKA phosphorylation site Ser1928, but this interaction can be further modulated by multiple regulatory subunits and other phosphatases [52,53]. Disruption of PP2A binding using inhibitory peptides increase ICa,L, suggesting an important inhibitory role for channel-bound PP2A [53]. Similarly, data from guinea pig ventricular membrane patches suggest that PKA and phosphatases attached on or near the L-type Ca2+-channel regulate basal ICa,L [54]. However, inhibition of PP2A by okadaic acid increases open probability of single ICa,L channels only in sinus rhythm patients, but not in cAF patients, despite the increase in global PP2A activity [27], whereas the same procedure increases whole-cell ICa,L stronger in cAF than in sinus rhythm patients [28], suggesting that the mechanisms of PP2A-mediated ICa,L regulation may be more complex and could differ in specific subpopulations of AF patients. Moreover, phosphorylation of the Ser1928 site does not appear to be required for β-AR-mediated regulation of ICa,L and various other potential phosphorylation sites have been proposed [55]. Thus, the molecular mechanisms acutely regulating ICa,L remain incompletely understood. Phosphatases may also reduce expression of L-type Ca2+ channels. In particular, Ca2+-dependent activation of Cn and subsequent dephosphorylation of nuclear factor of activated T-cells (NFAT) promote translocation of NFAT to the nucleus, inhibiting transcription of L-type Ca2+-channels [45,56]. This may contribute to the AF-related reduction in L-type Ca2+-channel expression in some [27,57,58], but not all patient cohorts [28,29].
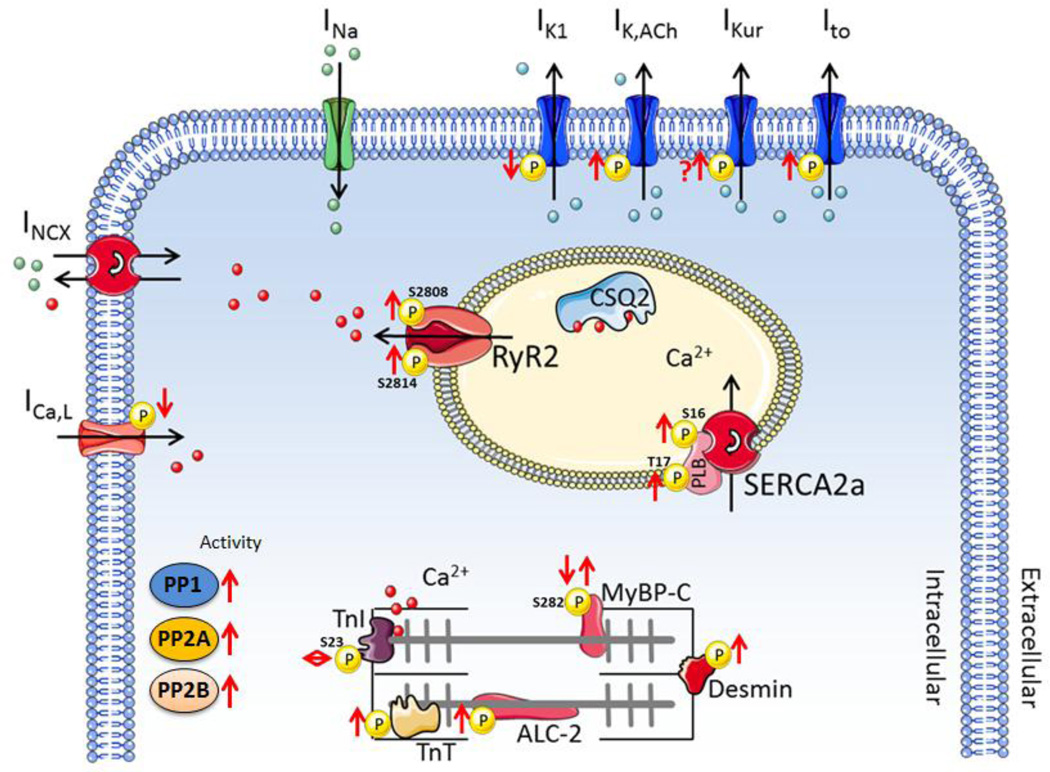
Fig. 3. Schematic overview of altered protein phosphorylation and phosphatase activity in cAF patients.
The total inward-rectifier K+ current is larger in cAF patients due to both increased IK1 and the development of a receptor-independent, constitutive IK,ACh [59,60]. The increase in IK1 appears in large part due to upregulation of Kir2.1/Kir2.3 protein expression, which may be partly mediated by the Ca2+/Cn/NFAT pathway. In particular, nuclear translocation of NFAT inhibits transcription of the inhibitory microRNA miR-26, thereby de-repressing Kir2.1 expression [61]. In addition, okadaic acid produced a small reduction in basal inward-rectifier K+-current in cAF, but not sinus rhythm patients [62], providing indirect evidence that the AF-related increase in phosphatases may also acutely augment the basal inward-rectifier K+-current. In contrast, PP2A (but not PP1 or Cn) is a component of the type-2 muscarinic-receptor complex that counteracts receptor phosphorylation (inactivation) by G-protein-coupled receptor kinases, thereby augmenting IK,ACh [63]. Accordingly, IK,ACh was smaller in the presence of okadaic acid in atrial cardiomyocytes from sinus rhythm patients [62], but open probability of constitutively active IK,ACh channels was increased [64]. In cAF, however, okadaic acid did not affect carbachol-activated IK,ACh suggesting potentially impaired function of G-protein-coupled receptor kinases [62] and highlighting the complexity of phosphorylation-dependent regulation of IK,ACh in cAF.
Augmentation of a number of other repolarizing currents may contribute to APD shortening in cAF. Increased IKs upon PKA-dependent phosphorylation contributes to AP-shortening during sympathetic stimulation and IKs is increased in cAF patients [65], with additional evidence for altered sympathetic regulation of IKs [66]. However, this increase has been attributed to enhanced β1-AR expression [66] and there is no information about altered dephosphorylation of IKs by PP1, which is specifically targeted to the IKs macromolecular complex by the anchoring protein Yotiao [67], in cAF patients. We have recently identified IK2P as an atrial-predominant K+-current involved in APD regulation [68]. Expression of K2P3.1 channels and functional IK2P were increased in cAF patients in our cohort, contributing significantly to AF-associated APD shortening [68]. By contrast, others have found reduced IK2P in a cohort of AF patients with mitral valve disease, which could be rescued by exogenous application of phosphatases [69], suggesting that abnormal regulation of K2P channel phosphorylation may affect IK2P in certain patient populations. However, also for IK2P it is unknown whether this abnormal phosphorylation is due to reduced dephosphorylation and, if so, which phosphatases and anchoring proteins are involved.
In contrast to IK1, IKs and IK2P, Ito is decreased in most cAF patients [66,70–73] and IKur in some [66,72,73], but not all studies [70,71], contributing to a prolongation of early atrial repolarization and resulting in the typical triangular shape of the atrial AP in cAF patients. CaMKII inhibition with KN-93 abolishes the differences in Ito between sinus rhythm and cAF patients [74], suggesting that the pore-forming subunit Kv4.3 of Ito might be CaMKII hyperphosphorylated in cAF patients. Whether reduced local phosphatase activity also contributes is unknown. Application of okadaic acid increased the sustained component of the outward K+-current (consisting for a large part of IKur) in atrial cardiomyocytes from sinus rhythm patients [74]. Like ICa,L, Cn is known to inhibit functional expression of Ito in cardiomyocytes [56], so the increased Cn activity in cAF may furthermore contribute to the reduced Ito.
The Na+-K+-ATPase is electrogenic, producing a repolarizing current that contributes to regulation of atrial APD and reentry dynamics [75]. PKA-dependent phosphorylation of Ser68 and PKC-dependent phosphorylation of Ser63 or Ser68 on phospholemman reduce its inhibition of the Na+-K+-ATPase [76]. PP1, but not PP2A, dephosphorylates phospholemman at Ser68 and this is controlled by the PP1 inhibitor I-1 [77]. Phospholemman phosphorylation at both Ser63 and Ser68 is decreased in rabbits with rapid atrial pacing (RAP)-induced atrial remodeling (Table 2), which would be expected to reduce Na+-K+-ATPase activity and contribute to intracellular Na+ and Ca2+ overload [78], but the role of potential phosphatase remodeling was not addressed and data from human atrial samples are not available.
Table 2.
AF-associated changes in phosphorylation
| Phospho Site |
Human AF |
Animal Model | Kinase | PP | Reference | |
|---|---|---|---|---|---|---|
| Sarcoplasmic reticulum proteins (SR) | ||||||
|
RyR2 |
Ser2808 | cAF: ↑; pAF: ↓ |
RAP: ↑ TG Mice: ↔ AVB: ↔ AF: ↔ CHF: ↔ AoB: ↔ ATR: ↔ |
PKA |
PP1, PP2A |
Greiser et al., JCI;2014 [78] Chiang et al., Cardiovasc Res;2014 [89] Vest et al., Circulation;2005 [93] Chelu et al., JCI;2009 [94] Voigt et al., Circulation;2012 [50] Neef et al., Circ Res;2010 [95] Li et al., Circulation;2014 [124] Guo et al., JCE;2014 [125] Shan et al., Circ Res;2012 [126] Li et al., Circ Res;2012 [127] Greiser et al., JMCC;2009 [118] Yeh et al., Circ Arrhythmia Electrophysiol;2008 [120] Zhang et al. PloS One;2015 [128] Lugenbiel et al., Plos One;2015 [129] Wakili et al., Circ Arrhythmia Electrophysiol;2010 [119] |
| Ser2814 | cAF: ↑; pAF: ↔ |
RAP: ↔, ↑ TG Mice: ↑ AF:↑ AVB: ↑ CHF: ↔ AoB: ↔ ATR: ↔ |
CaMKII | PP1, PP2A |
||
| Ser2030 | RAP: ↔ | PKA | PP1, PP2A |
|||
|
PLB |
Ser16 | pAF: ↑ | AF: ↓ AVB: ↓ RAP: ↔ CHF: ↔ AoB: ↔ ATR: ↔ |
PKA | PP2A | Voigt et al., Circulation; 2014 [31] Chelu et al., JCI;2009 [94] Neef et al., Circ Res;2010 [95] Li et al., Circulation;2014 [124] Greiser et al., JCI;2014 [78] Li et al., Circ Res;2012 [127] Greiser et al., JMCC;2009 [118] Yeh et al., Circ Arrhythmia Electrophysiol;2008 [120] El-Armouche et al. Circulation;2006 [39] Zhang et al. PloS One;2015 [128] Lugenbiel et al., Plos One;2015 [129] Wakili et al., Circ Arrhythmia Electrophysiol;2010 [119] |
| Thr17 | cAF: ↑; pAF: ↔ |
RAP: ↔ TG Mice: ↔, ↑ AF: ↔ AVB: ↔ CHF: ↑ ATR: ↔ |
CaMKII | PP1, PP2A |
||
| Plasma membrane proteins | ||||||
|
PLM |
Ser63 | RAP: ↓ | PKA | PP1 | Greiser et al., JCI;2014 [78] | |
| Ser68 | RAP: ↓ | PKC | PP1 | |||
| Myofilament proteins | ||||||
|
TnI |
Ser23/24 |
cAF: ↔ |
RAP: ↓ CHF: ↔ ATR: ↔ AF: ↔ AVB: ↓ |
PKA, PKC |
PP1, PP2A |
Yeh et al., Circ Arrhythmia Electrophysiol;2008 [120] El-Armouche et al., Circulation;2006 [39] Belus et al., Circ Res; 2010 [105] Greiser et al., JMCC;2009[118] Greiser et al., JCI;2014 [78] Wakili et al., Circ Arrhythmia Electrophysiol;2010 [119] |
|
cMyBPC |
Ser282 | cAF: ↓, ↑ | CHF: ↓ ATR: ↓ AF: ↔ AVB: ↓ RAP: ↔ |
PKA, PKC |
PP1, PP2A |
Yeh et al., Circ Arrhythmia Electrophysiol;2008 [120] El-Armouche et al., Circulation;2006 [39] Belus et al., Circ Res;2010 [105] Greiser et al., JMCC;2009 [118] Greiser et al., JCI;2014 [78] Wakili et al., Circ Arrhythmia Electrophysiol;2010 [119] |
ATR: atrial tachycardia remodeling; AVB: atrio-ventricular block; CaMKII: Ca2+/calmodulin-dependent protein kinase-II; CHF: congestive heart failure; PKA: protein kinase-A; PKC: protein kinase-C; RAP: rapid atrial pacing; TG: transgenic;
3.2. Modulation of cell-to-cell communication by protein phosphatases
Reduced INa, loss of electrical cell-to-cell communication via gap-junctions or interruption of muscle-bundle integrity by fibrosis all impair conduction and promote reentrant activity that can maintain AF [2]. Gap-junction proteins consist of connexins, which contain multiple phosphorylation sites for several kinases [79,80]. Both PP1 and PP2A co-localize with connexins and increased phosphatase activity in HF decreases gap-junction coupling through multiple mechanisms [81]. In a large animal model of AF, connexin gene transfer restores conduction velocity and prevents AF [82]. In cAF patients, the overall immunocytochemical staining of connexin 40 and connexin 43 in atrial samples is reduced, but lateral staining is significantly increased [83]. In agreement, connexins are dephosphorylated and redistributed to the lateral membranes in human atrial samples from patients with atrial dilatation, a common feature in AF patients [84]. However, these findings are independent of the underlying rhythm [84]. In contrast, a different study observed increased connexin 40 phosphorylation, but reduced total connexin 40 levels, in AF patients with atrial dilatation compared to sinus rhythm patients with or without atrial dilatation [85], suggesting that other factors may further modulate connexin phosphorylation.
Atrial hypertrophy and fibrosis are major components of atrial structural remodeling that promote reentrant activity [1,2,10]. The AF-associated increase in Cn activity likely contributes to atrial hypertrophy via activation of the NFAT pathway [44,56]. Furthermore, this pathway may be involved in remodeling of the extracellular matrix in response to atrial stretch through activation of matrix metalloproteases [46]. In agreement, atrial samples from cAF patients are characterized by pronounced changes in collagen synthesis and degradation pathways, including increased expression of matrix metalloproteases [86]. Dephosphorylation of a set of conserved serine residues on class IIa histone deacetylases by a PP2A complex, consisting of Cα, Aα and B55α subunits, controls their nuclear translocation where they repress the activity of prohypertrophic genes in the ventricle [87], but the precise roles of PP1/PP2A in atrial hypertrophy and fibrosis remain largely unknown.
3.3. AF-related modulation of Ca2+-handling by protein phosphatases
SR Ca2+ release occurs via RyR2 channels. In addition to post-translational regulation by oxidation and S-nitrosylation, the RyR2 channel is phosphorylated on Ser2030 and Ser2808 by PKA, and on Ser2814 by CaMKII [88]. Dephosphorylation of these sites is mediated by PP1, which is targeted to the complex by its regulatory subunit spinophilin-1 (PPP1R9B) [89], and by PP2A, targeted through its regulatory subunit PR130 (PPP2R3A) [67,90], through B56α binding to ankyrin-B [91], or through the muscle-specific A-kinase-anchoring protein (mAKAP/AKAP6), which binds both RyR2 and PP2A-B56δ (PPP2R5B) [92]. RyR2 phosphorylation at both Ser2808 and Ser2814 is increased in cAF patients [50,93–95], and Ser2814 phosphorylation (but not Ser2808 phosphorylation) is increased in most animal models with increased AF susceptibility (Table 2). Although the functional relevance of RyR2 hyperphosphorylation remains somewhat controversial, accumulating evidence suggests that RyR2 hyperphosphorylation promotes SR Ca2+ leak, spontaneous Ca2+-release events and DADs [88,96], thereby increasing the likelihood of triggered activity. Although the RyR2 hyperphosphorylation in cAF patients is likely in part due to increased PKA and CaMKII activity [50,95], a reduction in RyR2-associated phosphatases may also contribute. For example, microRNA-1 and microRNA-133-mediated reduction in B56α expression results in reduced RyR2-associated PP2A levels in HF [90]. Furthermore, recent work has shown that mice with reduced PP1 in the RyR2 complex due to genetic knock-out of spinophilin-1 have increased RyR2 phosphorylation at Ser2814 (but unchanged Ser2808 phosphorylation), more spontaneous SR Ca2+-release events and an enhanced susceptibility to pacing-induced AF [89]. Although global spinophilin-1 mRNA levels are unchanged in cAF patients [39], our preliminary data suggest that the amount of PP1 associated to the RyR2 complex is decreased in cAF patients [97]. Finally, the Thr35-hyperphosphorylation of I-1 in cAF patients is expected to reduce PP1 activity in the SR microdomain, contributing to RyR2 hyperphosphorylation [12,37]. Taken together, the decreased local phosphatase activity in the RyR2 microdomain likely facilitates triggered-activity-promoting Ca2+-handling abnormalities in cAF. By contrast, RyR2 phosphorylation at Ser2814 is not different in pAF patients compared to sinus rhythm controls, whereas Ser2808-mediated RyR2 phosphorylation is strongly reduced [31]. RyR2 open probability is nonetheless increased, potentially due to non-phosphorylation-related changes in the macromolecular complex [98]. Interestingly, dephosphorylation of RyR2 through exogenous application of phosphatases also increases SR Ca2+ leak, suggesting that increased phosphorylation and dephosphorylation of RyR2 both may enhance RyR2 [99]. Clearly much more molecular work is needed to dissect the precise role of altered RyR2 phosphorylation for channel function in health and disease.
SR Ca2+ uptake is decreased in cAF patients, potentially contributing to the smaller Ca2+-transient amplitude and atrial hypocontractility [50]. This reduced SR Ca2+ uptake is likely mediated in part by reduced SERCA2a expression and increased phospholamban (PLB) expression, resulting in a significant increase in PLB-mediated SERCA2a inhibition in cAF [39]. On the other hand, despite the increase in global PP1 and PP2A activities, phosphorylation of PLB at both Ser16 (PKA-site) and Thr17 (CaMKII-site) is higher in cAF patients, which should reduce the inhibitory consequences of enhanced total PLB (Table 2). Similarly, the expression of sarcolipin is reduced in cAF patients, which would increase SERCA2a activity [100]. The increased PLB phosphorylation is likely in part due to enhanced Thr35-phosphorylation (activity) of I-1, reducing PP1-mediated PLB dephosphorylation [39]. PP1β is the most significant PP1 isoform involved in regulating SR Ca2+ cycling in rat cardiomyocytes [101], but other studies found that individual genetic deletion of PP1α, PP1β or PP1γ does not alter PLB phosphorylation [102], suggesting partial redundancy of individual isoforms. The RGL (PPP1R3A) subunit targets PP1 to the SR, where it controls dephosphorylation of PLB, but RGL mRNA levels are unchanged in cAF patients [39]. Overexpression of B56α results in reduced PLB phosphorylation during β-AR stimulation, suggesting a potential role for B56α in targeting PP2A to PLB [103]. In contrast to cAF, SR Ca2+ uptake is increased in pAF, possibly due to increased Ser16-phosphorylation of PLB, contributing to increased SR Ca2+ load and the related increase in proarrhythmic spontaneous SR Ca2+-release events [31]. Furthermore, different AF animal models exhibit either decreased, unchanged or increased PLB phosphorylation (Table 2), making the interpretation of the role of altered phosphatase-mediated PLB dephosphorylation for AF paradigms challenging.
3.4. Modulation of contraction by protein phosphatases
In addition to the reduced Ca2+-transient amplitude, remodeling of contractile proteins, including altered expression of titin and myosin heavy chain isoforms, may contribute to AF-associated changes in atrial contractility [104,105] (Figure 3). Atrial contractility is also regulated by phosphorylation of contractile proteins, including the inhibitory troponin subunit (TnI), which influences Ca2+-sensitivity of the myofilaments and cMyBP-C, which determines Ca2+-sensitivity and kinetics of cross-bridge cycling [106]. cAF patients appear to have increased overall phosphorylation of troponin-T [104,105], cMyBP-C and desmin [105]. Increased phosphorylation of atrial light chain-2, the atrial-specific variant of myosin light chain-2, has been reported in some [105], but not all [104] studies.
PP1 and PP2A are the major phosphatases controlling dephosphorylation of myofilament proteins. PP1 preferentially dephosphorylates Ser23/24 on TnI, whereas PP2A induces a more uniform dephosphorylation [107]. Among the PP1 isoforms, PP1β appears to be preferentially targeted to the myofilaments and loss of PP1β increases phosphorylation of myosin light chain 2 and cMyBP-C [102]. The B56α subunit may contribute to PP2A targeting to the myofilaments, with B56α overexpression resulting in reduced TnI and cMyBP-C phosphorylation [103]. TnI phosphorylation at Ser23/24 is unchanged in cAF patients compared to sinus rhythm controls [105] and in multiple animal models of AF (Table 2). cMyBP-C phosphorylation at Ser282 is increased in cAF patients compared to sinus rhythm controls with dilated atria [105], but is decreased in a different cohort when compared to sinus rhythm patients with normal atrial dimensions [39] and in multiple AF animal models (Table 2). These data suggest complex remodeling of myofilament phosphorylation in response to AF and atrial dilatation, which are likely region specific (e.g., in left vs. right atrium) [39,104,105].
4. Challenges and future perspectives
4.1 Challenges in the dissection of the precise role of specific phosphatases in AF
There is emerging evidence that altered phosphorylation of proteins involved in atrial electrophysiology and contraction contributes to atrial arrhythmogenesis in cAF patients (Figure 3). One major obstacle in the field of phosphatase research is the fact that the proper study of phosphatase function requires preserved physiological target protein phosphorylation, which is not always possible for technical reasons. In addition, although pharmacological studies using putative inhibitors of different phosphatases have provided valuable evidence for the potential role of protein phosphatases for cardiac regulation, their limited specificity precludes definite conclusions [34]. For example, okadaic acid inhibits PP3-PP6 phosphatases with a similar efficacy as PP2A, suggesting that the PP2A-ascribed roles for cardiac function might be biased by potential inhibition of additional phosphatases. However, even with improved pharmacological tools, it will remain challenging to identify the precise role of phosphatases in the regulation of individual ion channels or Ca2+-handling proteins, given the redundancy between phosphatases in the regulation of individual sites and the numerous phosphorylation sites that influence function of a single protein. Finally, the large variability between different clinical forms of AF, as well as between AF patients and animal models, makes generic statements about the role of phosphatases in AF challenging. Instead, sub-groups should be analyzed to determine the roles of atrial rate, dilatation and other (clinical) factors in the AF-related regulation of phosphatases.
4.2 Targeting phosphatases as therapeutic opportunities for AF
Inhibition of tyrosine kinase by ibrutinib, employed in patients with chronic lymphocytic leukemia or mantle cell lymphoma has been associated with significantly increased risk of AF [108]. Although increased global PP1 and PP2A levels may also contribute to the formation of an AF-promoting substrate, it is likely that global phosphatase inhibition (e.g., using pharmacological inhibitors of catalytic subunits) will not be effective and may have pronounced adverse side effects, including promotion of AF itself. Direct and specific targeting of holoenzymes (e.g., through the associated regulatory subunits), on the other hand, may allow regulation of the phosphorylation state of a specific substrate, and this is the most frequently pursued strategy for phosphatase drug discovery. Several examples of specific modulators for PP1-regulatory subunits for the treatment of diabetes [109], hypertension [110], Parkinson’s disease or drug addiction [111] have been described. In addition, several studies have shown the therapeutic potential of protein phosphatase modulation via adenoviral-mediated gene therapy in both small animal [37] and large animal HF models [112]. Thus, inhibition of phosphatases may represent a novel opportunity to treat cardiovascular diseases, including AF, in the near future.
Given the central role for Ca2+-handling abnormalities in animal models of AF and atrial samples from AF patients, targeting the phosphorylation of Ca2+-handling proteins could be a promising antiarrhythmic strategy [2,113]. Although the functional relevance of RyR2 hyperphosphorylation remains a topic of debate [88], the proarrhythmic nature of RyR2 mutations and the antiarrhythmic efficacy of some drugs modulating RyR2 activity, suggest it could be an interesting target [99,113]. Furthermore, although our knowledge is far from complete, the RyR2 macromolecular complex is perhaps the best characterized, with multiple potential targets to modulate RyR2 function and some information about how to selectively modulate individual phosphorylation sites via alterations in phosphatase holoenzymes. Interestingly, the RyR2 hyperphosphorylation suggests that increased PP1, PP2A and Cn activities might be anti- instead of proarrhythmic. This indicates that we should increase local phosphatase activity as a therapeutic strategy.
4.3 Future perspectives
The molecular basis of increased phosphatase activity in AF is unknown. Since phosphatase activity is similarly increased in both ATR animal models and AF patients (Table 1), it is very likely that the upregulation is the consequence of AF itself, potentially to prevent unphysiological hyperphosphorylation of cellular proteins. Conversely, CHF dogs have atrial structural remodeling, which also promotes AF development, and increased PP1 activity, suggesting that besides the high atrial rate the underlying structural heart disease can also increase PP1 activity. The hemodynamic changes during HF lead to atrial dilatation and stretch and stretching of cultured neonatal rat atrial cardiomyocytes results in an upregulation of Cn [114]. Abnormal atrial metabolism may also play an important role in AF-promoting atrial remodeling [115] and specific mitochondria-associated phosphatases regulate mitochondrial function and signaling [116,117], suggesting a potential role for mitochondrial phosphatases in AF. Thus, besides potential contribution to AF progression and maintenance, the increase in phosphatases associated with AF-predisposing clinical conditions may also contribute to AF induction. The increase in PP1 activity under AF-promoting conditions in the absence of clear changes in the expression of catalytic subunits (Table 1), suggests functional regulation of phosphatase activity, for instance through phosphorylation or other forms of post-translational regulation. The exact (neurohumoral) factors and signaling pathways involved and their remodeling in AF will require further study. Finally, currently most information about phosphatase mediated regulation of atrial electrophysiology and contractility is based on global associations using pharmacological phosphatase inhibition, with limited information about the direct relationship between phosphatase function and specific phosphorylation sites (perhaps with the exception of RyR2 Ser2808 and Ser2814). Recent work has identified a large number of putative PP1 regulatory subunits [30]. Future studies will likely help to obtain a better overview of the specific holoenzyme compositions responsible for targeting phosphatases to individual substrates. Taken together, further extensive work is required to dissect the precise role of PP1, PP2A and Cn in AF promotion and persistence.
Supplementary Material
Highlights.
Atrial fibrillation (AF) is associated with profound atrial remodeling
Global cardiomyocyte PP1, PP2A and calcineurin activities are increased in AF patients
PP1, PP2A and calcineurin are involved in proarrhythmic atrial remodeling
Methodological challenges exist to elucidate the precise roles of phosphatases in AF
Phosphatases represent a potential therapeutic target for AF
Acknowledgments
The authors’ work is supported by the Netherlands Organization for Scientific Research (ZonMW Veni 91616057 to J.H.), the National Institutes of Health (HL089598, HL091947, HL117641, HL129570 to X.H.T.W. and HL131517 to D.D), the American Heart Association (13EAI-14560061 to X.H.T.W.) and the DZHK (German Center for Cardiovascular Research to D.D).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: AF: atrial fibrillation; AP: action potential; β-AR: β-adrenoceptor; cAF: chronic AF; CaMKII: Ca2+/calmodulin-dependent protein kinase type-II; cMyBP-C: cardiac myosin-binding protein-C; Cn: calcineurin; DAD: delayed afterdepolarization; HF: heart failure; ICa,L: L-type Ca2+-current; IK1: basal inward-rectifier K+-current; IK2P: two-pore domain K+-current; IK,ACh: acetylcholine-dependent inward-rectifier K+-current; IKr: rapid delayed-rectifier K+-current; IKs: slow delayed-rectifier K+-current; IKur: ultra-rapid delayed-rectifier K+-current; INa: Na+-current; Ito: transient-outward K+-current; NCX1: Na+-Ca2+-exchanger; pAF: paroxysmal AF; PKA: protein kinase-A; PKC: protein kinase-C; PLB: phospholamban; PP1: protein phosphatase type-1; PP2A: protein phosphatase type-2A; RyR2: ryanodine receptor type-2; SERCA2a: sarcoplasmic reticulum Ca2+-ATPase; SR: sarcoplasmic reticulum; TnI: troponin-I;
Conflicts of Interest
X.H.T.W. is a founding partner of Elex Biotech, a start-up company that developed drug molecules that target ryanodine receptors for the treatment of cardiac arrhythmia disorders. D.D. is consultant for OMEICOS Therapeutics that develops drug molecules targeting the ω-fatty acid metabolism as an antiarrhythmic therapeutic strategy. J.H. and S.G declared no conflicts of interest.
References
- 1.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 2.Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114:1483–1499. doi: 10.1161/CIRCRESAHA.114.302226. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 4.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on Atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. 2016;18:1455–1490. doi: 10.1093/europace/euw161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. doi: http://dx.doi.org/10.1161/01.CIR.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 7.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 8.Heijman J, Algalarrondo V, Voigt N, Melka J, Wehrens XHT, Dobrev D, et al. The value of basic research insights into atrial fibrillation mechanisms as a guide to therapeutic innovation: a critical analysis. Cardiovasc Res. 2016;109:467–479. doi: 10.1093/cvr/cvv275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schotten U, Dobrev D, Platonov PG, Kottkamp H, Hindricks G. Current controversies in determining the main mechanisms of atrial fibrillation. J Intern Med. 2016;279:428–438. doi: 10.1111/joim.12492. [DOI] [PubMed] [Google Scholar]
- 10.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 11.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91:265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 12.Heijman J, Dewenter M, El-Armouche A, Dobrev D. Function and regulation of serine/threonine phosphatases in the healthy and diseased heart. J Mol Cell Cardiol. 2013;64:90–98. doi: 10.1016/j.yjmcc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Chen P-S, Chen LS, Fishbein MC, Lin S-F, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114:1500–1515. doi: 10.1161/CIRCRESAHA.114.303772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen P. The origins of protein phosphorylation. Nat Cell Biol. 2002;4:E127–E130. doi: 10.1038/ncb0502-e127. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Balycheva M, Faggian G, Glukhov AV, Gorelik J. Microdomain-specific localization of functional ion channels in cardiomyocytes: an emerging concept of local regulation and remodelling. Biophys Rev. 2015;7:43–62. doi: 10.1007/s12551-014-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Virshup DM, Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Mol Cell. 2009;33:537–545. doi: 10.1016/j.molcel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 18.El-Armouche A, Eschenhagen T. Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Fail Rev. 2009;14:225–241. doi: 10.1007/s10741-008-9132-8. [DOI] [PubMed] [Google Scholar]
- 19.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 20.Schmitt N, Grunnet M, Olesen S-P. Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia. Physiol Rev. 2014;94:609–653. doi: 10.1152/physrev.00022.2013. [DOI] [PubMed] [Google Scholar]
- 21.Bartos DC, Grandi E, Ripplinger CM. Ion Channels in the Heart. Compr Physiol. 2015;5:1423–1464. doi: 10.1002/cphy.c140069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voigt N, Dobrev D. Atrial-Selective Potassium Channel Blockers. Card Electrophysiol Clin. 2016;8:411–421. doi: 10.1016/j.ccep.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Vangheluwe P, Schuermans M, Zádor E, Waelkens E, Raeymaekers L, Wuytack F. Sarcolipin and phospholamban mRNA and protein expression in cardiac and skeletal muscle of different species. Biochem J. 2005;389:151–159. doi: 10.1042/BJ20050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobrev D, Teos LY, Lederer WJ. Unique atrial myocyte Ca2+ signaling. J Mol Cell Cardiol. 2009;46:448–451. doi: 10.1016/j.yjmcc.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lüss H, Klein-Wiele O, Bokník P, Herzig S, Knapp J, Linck B, et al. Regional expression of protein phosphatase type 1 and 2A catalytic subunit isoforms in the human heart. J Mol Cell Cardiol. 2000;32:2349–2359. doi: 10.1006/jmcc.2000.1265. [DOI] [PubMed] [Google Scholar]
- 26.Molina CE, Heijman J, Dobrev D. Differences in Left Versus Right Ventricular Electrophysiological Properties in Cardiac Dysfunction and Arrhythmogenesis. Arrhythmia Electrophysiol Rev. 2016;5:14–19. doi: 10.15420/aer.2016.8.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein G, Schröder F, Vogler D, Schaefer A, Haverich A, Schieffer B, et al. Increased open probability of single cardiac L-type calcium channels in patients with chronic atrial fibrillation. role of phosphatase 2A. Cardiovasc Res. 2003;59:37–45. doi: 10.1016/s0008-6363(03)00357-2. [DOI] [PubMed] [Google Scholar]
- 28.Christ T, Boknik P, Wöhrl S, Wettwer E, Graf EM, Bosch RF, et al. L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation. 2004;110:2651–2657. doi: 10.1161/01.CIR.0000145659.80212.6A. [DOI] [PubMed] [Google Scholar]
- 29.Greiser M, Halaszovich CR, Frechen D, Boknik P, Ravens U, Dobrev D, et al. Pharmacological evidence for altered src kinase regulation of ICa,L in patients with chronic atrial fibrillation. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:383–392. doi: 10.1007/s00210-007-0174-6. [DOI] [PubMed] [Google Scholar]
- 30.Chiang DY, Lebesgue N, Beavers DL, Alsina KM, Damen JMA, Voigt N, et al. Alterations in the interactome of serine/threonine protein phosphatase type-1 in atrial fibrillation patients. J Am Coll Cardiol. 2015;65:163–173. doi: 10.1016/j.jacc.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, et al. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129:145–156. doi: 10.1161/CIRCULATIONAHA.113.006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishida K, Michael G, Dobrev D, Nattel S. Animal models for atrial fibrillation: clinical insights and scientific opportunities. Europace. 2010;12:160–172. doi: 10.1093/europace/eup328. [DOI] [PubMed] [Google Scholar]
- 33.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 34.Herzig S, Neumann J. Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol Rev. 2000;80:173–210. doi: 10.1152/physrev.2000.80.1.173. [DOI] [PubMed] [Google Scholar]
- 35.Chiang DY, Heck AJR, Dobrev D, Wehrens XHT. Regulating the regulator: Insights into the cardiac protein phosphatase 1 interactome. J Mol Cell Cardiol. 2016;101:165–172. doi: 10.1016/j.yjmcc.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Armouche A, Rau T, Zolk O, Ditz D, Pamminger T, Zimmermann W-H, et al. Evidence for protein phosphatase inhibitor-1 playing an amplifier role in beta-adrenergic signaling in cardiac myocytes. FASEB. 2003;17:437–439. doi: 10.1096/fj.02-0057fje. [DOI] [PubMed] [Google Scholar]
- 37.Weber S, Meyer-Roxlau S, El-Armouche A. Role of protein phosphatase inhibitor-1 in cardiac beta adrenergic pathway. J Mol Cell Cardiol. 2016;101:116–126. doi: 10.1016/j.yjmcc.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 38.El-Armouche A, Bednorz A, Pamminger T, Ditz D, Didié M, Dobrev D, et al. Role of calcineurin and protein phosphatase-2A in the regulation of phosphatase inhibitor-1 in cardiac myocytes. Biochem Biophys Res Commun. 2006;346:700–706. doi: 10.1016/j.bbrc.2006.05.182. [DOI] [PubMed] [Google Scholar]
- 39.El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, et al. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation. 2006;114:670–680. doi: 10.1161/CIRCULATIONAHA.106.636845. [DOI] [PubMed] [Google Scholar]
- 40.DeGrande ST, Little SC, Nixon DJ, Wright P, Snyder J, Dun W, et al. Molecular mechanisms underlying cardiac protein phosphatase 2A regulation in heart. J Biol Chem. 2013;288:1032–1046. doi: 10.1074/jbc.M112.426957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Little SC, Curran J, Makara MA, Kline CF, Ho H-T, Xu Z, et al. Protein phosphatase 2A regulatory subunit B56α limits phosphatase activity in the heart. Sci Signal. 2015;8:ra72. doi: 10.1126/scisignal.aaa5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Wagoner DR. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J Cardiovasc Pharmacol. 2008;52:306–313. doi: 10.1097/FJC.0b013e31817f9398. [DOI] [PubMed] [Google Scholar]
- 43.Zhao F, Zhang S, Chen L, Wu Y, Qin J, Shao Y, et al. Calcium- and integrin-binding protein-1 and calcineurin are upregulated in the right atrial myocardium of patients with atrial fibrillation. Europace. 2012;14:1726–1733. doi: 10.1093/europace/eus149. [DOI] [PubMed] [Google Scholar]
- 44.Bukowska A, Lendeckel U, Hirte D, Wolke C, Striggow F, Röhnert P, et al. Activation of the calcineurin signaling pathway induces atrial hypertrophy during atrial fibrillation. Cell Mol Life Sci CMLS. 2006;63:333–342. doi: 10.1007/s00018-005-5353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi XY, Yeh Y-H, Xiao L, Burstein B, Maguy A, Chartier D, et al. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circ Res. 2008;103:845–854. doi: 10.1161/CIRCRESAHA.108.175463. [DOI] [PubMed] [Google Scholar]
- 46.Saygili E, Rana OR, Meyer C, Gemein C, Andrzejewski MG, Ludwig A, et al. The angiotensin-calcineurin-NFAT pathway mediates stretch-induced up-regulation of matrix metalloproteinases-2/-9 in atrial myocytes. Basic Res Cardiol. 2009;104:435–448. doi: 10.1007/s00395-008-0772-6. [DOI] [PubMed] [Google Scholar]
- 47.Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res. 1997;81:512–525. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]
- 48.Schotten U, Ausma J, Stellbrink C, Sabatschus I, Vogel M, Frechen D, et al. Cellular mechanisms of depressed atrial contractility in patients with chronic atrial fibrillation. Circulation. 2001;103:691–698. doi: 10.1161/01.cir.103.5.691. [DOI] [PubMed] [Google Scholar]
- 49.Wettwer E, Hála O, Christ T, Heubach JF, Dobrev D, Knaut M, et al. Role of IKur in controlling action potential shape and contractility in the human atrium: influence of chronic atrial fibrillation. Circulation. 2004;110:2299–2306. doi: 10.1161/01.CIR.0000145155.60288.71. [DOI] [PubMed] [Google Scholar]
- 50.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dobrev D, Carlsson L, Nattel S. Novel molecular targets for atrial fibrillation therapy. Nat Rev Drug Discov. 2012;11:275–291. doi: 10.1038/nrd3682. [DOI] [PubMed] [Google Scholar]
- 52.Hall DD, Feekes JA, Arachchige Don AS, Shi M, Hamid J, Chen L, et al. Binding of protein phosphatase 2A to the L-type calcium channel Cav1.2 next to Ser1928, its main PKA site, is critical for Ser1928 dephosphorylation. Biochemistry (Mosc) 2006;45:3448–3459. doi: 10.1021/bi051593z. [DOI] [PubMed] [Google Scholar]
- 53.Xu H, Ginsburg KS, Hall DD, Zimmermann M, Stein IS, Zhang M, et al. Targeting of protein phosphatases PP2A and PP2B to the C-terminus of the L-type calcium channel Ca v1.2. Biochemistry (Mosc) 2010;49:10298–10307. doi: 10.1021/bi101018c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu J, Yu L, Minobe E, Lu L, Lei M, Kameyama M. PKA and phosphatases attached to the CaV1.2 channel regulate channel activity in cell-free patches. Am J Physiol Cell Physiol. 2016;310:C136–C141. doi: 10.1152/ajpcell.00157.2015. [DOI] [PubMed] [Google Scholar]
- 55.Yang L, Katchman A, Samad T, Morrow JP, Weinberg RL, Marx SO. β-adrenergic regulation of the L-type Ca2+ channel does not require phosphorylation of α1C Ser1700. Circ Res. 2013;113:871–880. doi: 10.1161/CIRCRESAHA.113.301926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Tandan S, Hill JA. Calcineurin-dependent ion channel regulation in heart. Trends Cardiovasc Med. 2014;24:14–22. doi: 10.1016/j.tcm.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brundel BJ, van Gelder IC, Henning RH, Tuinenburg AE, Deelman LE, Tieleman RG, et al. Gene expression of proteins influencing the calcium homeostasis in patients with persistent and paroxysmal atrial fibrillation. Cardiovasc Res. 1999;42:443–454. doi: 10.1016/s0008-6363(99)00045-0. [DOI] [PubMed] [Google Scholar]
- 58.Brundel BJ, Van Gelder IC, Henning RH, Tieleman RG, Tuinenburg AE, Wietses M, et al. Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation. Circulation. 2001;103:684–690. doi: 10.1161/01.cir.103.5.684. [DOI] [PubMed] [Google Scholar]
- 59.Dobrev D, Graf E, Wettwer E, Himmel HM, Hála O, Doerfel C, et al. Molecular basis of downregulation of G-protein-coupled inward rectifying K+ current (IK,ACh) in chronic human atrial fibrillation: decrease in GIRK4 mRNA correlates with reduced IK,ACh and muscarinic receptor-mediated shortening of action potentials. Circulation. 2001;104:2551–2557. doi: 10.1161/hc4601.099466. [DOI] [PubMed] [Google Scholar]
- 60.Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, et al. The G protein-gated potassium current IK,ACh is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112:3697–3706. doi: 10.1161/CIRCULATIONAHA.105.575332. [DOI] [PubMed] [Google Scholar]
- 61.Luo X, Pan Z, Shan H, Xiao J, Sun X, Wang N, et al. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J Clin Invest. 2013;123:1939–1951. doi: 10.1172/JCI62185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Voigt N, Friedrich A, Bock M, Wettwer E, Christ T, Knaut M, et al. Differential phosphorylation-dependent regulation of constitutively active and muscarinic receptor-activated IK,ACh channels in patients with chronic atrial fibrillation. Cardiovasc Res. 2007;74:426–437. doi: 10.1016/j.cardiores.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Shui Z, Boyett MR, Zang WJ, Haga T, Kameyama K. Receptor kinase-dependent desensitization of the muscarinic K+ current in rat atrial cells. J Physiol. 1995;487:359–366. doi: 10.1113/jphysiol.1995.sp020885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Makary S, Voigt N, Maguy A, Wakili R, Nishida K, Harada M, et al. Differential protein kinase C isoform regulation and increased constitutive activity of acetylcholine-regulated potassium channels in atrial remodeling. Circ Res. 2011;109:1031–1043. doi: 10.1161/CIRCRESAHA.111.253120. [DOI] [PubMed] [Google Scholar]
- 65.Caballero R, de la Fuente MG, Gómez R, Barana A, Amorós I, Dolz-Gaitón P, et al. In humans, chronic atrial fibrillation decreases the transient outward current and ultrarapid component of the delayed rectifier current differentially on each atria and increases the slow component of the delayed rectifier current in both. J Am Coll Cardiol. 2010;55:2346–2354. doi: 10.1016/j.jacc.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 66.González de la Fuente M, Barana A, Gómez R, Amorós I, Dolz-Gaitón P, Sacristán S, et al. Chronic atrial fibrillation up-regulates β1-Adrenoceptors affecting repolarizing currents and action potential duration. Cardiovasc Res. 2013;97:379–388. doi: 10.1093/cvr/cvs313. [DOI] [PubMed] [Google Scholar]
- 67.Marx SO, Kurokawa J, Reiken S, Motoike H, D’Armiento J, Marks AR, et al. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 68.Schmidt C, Wiedmann F, Voigt N, Zhou X-B, Heijman J, Lang S, et al. Upregulation of K2P3.1 K+ Current Causes Action Potential Shortening in Patients With Chronic Atrial Fibrillation. Circulation. 2015;132:82–92. doi: 10.1161/CIRCULATIONAHA.114.012657. [DOI] [PubMed] [Google Scholar]
- 69.Harleton E, Besana A, Chandra P, Danilo P, Rosen TS, Rosen MR, et al. TASK-1 current is inhibited by phosphorylation during human and canine chronic atrial fibrillation. Am J Physiol Heart Circ Physiol. 2015;308:H126–H134. doi: 10.1152/ajpheart.00614.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kühlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–131. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 71.Workman AJ, Kane KA, Rankin AC. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovasc Res. 2001;52:226–235. doi: 10.1016/s0008-6363(01)00380-7. doi: http://dx.doi.org/10.1016/S0008-6363(01)00380-7. [DOI] [PubMed] [Google Scholar]
- 72.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 73.Brandt MC, Priebe L, Böhle T, Südkamp M, Beuckelmann DJ. The ultrarapid the transient outward K+ current in human atrial fibrillation. Their possible role in postoperative atrial fibrillation. J Mol Cell Cardiol. 2000;32:1885–1896. doi: 10.1006/jmcc.2000.1221. [DOI] [PubMed] [Google Scholar]
- 74.Tessier S, Karczewski P, Krause EG, Pansard Y, Acar C, Lang-Lazdunski M, et al. Regulation of the transient outward K+ current by Ca2+/calmodulin-dependent protein kinases II in human atrial myocytes. Circ Res. 1999;85:810–819. doi: 10.1161/01.res.85.9.810. [DOI] [PubMed] [Google Scholar]
- 75.Sánchez C, Corrias A, Bueno-Orovio A, Davies M, Swinton J, Jacobson I, et al. The Na+/K+ pump is an important modulator of refractoriness and rotor dynamics in human atrial tissue. Am J Physiol Heart Circ Physiol. 2012;302:H1146–H1159. doi: 10.1152/ajpheart.00668.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pavlovic D, Hall AR, Kennington EJ, Aughton K, Boguslavskyi A, Fuller W, et al. Nitric oxide regulates cardiac intracellular Na+ and Ca2+ by modulating Na/K ATPase via PKCε and phospholemman-dependent mechanism. J Mol Cell Cardiol. 2013;61:164–171. doi: 10.1016/j.yjmcc.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El-Armouche A, Wittköpper K, Fuller W, Howie J, Shattock MJ, Pavlovic D. Phospholemman-dependent regulation of the cardiac Na/K-ATPase activity is modulated by inhibitor-1 sensitive type-1 phosphatase. FASEB. 2011;25:4467–4475. doi: 10.1096/fj.11-184903. [DOI] [PubMed] [Google Scholar]
- 78.Greiser M, Kerfant B-G, Williams GSB, Voigt N, Harks E, Dibb KM, et al. Tachycardia-induced silencing of subcellular Ca2+ signaling in atrial myocytes. J Clin Invest. 2014;124:4759–4772. doi: 10.1172/JCI70102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dhein S. Role of connexins in atrial fibrillation. Adv Cardiol. 2006;42:161–174. doi: 10.1159/000092568. [DOI] [PubMed] [Google Scholar]
- 80.Márquez-Rosado L, Solan JL, Dunn CA, Norris RP, Lampe PD. Connexin43 phosphorylation in brain, cardiac, endothelial and epithelial tissues. Biochim Biophys Acta. 2012;1818:1985–1992. doi: 10.1016/j.bbamem.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ai X, Jiang A, Ke Y, Solaro RJ, Pogwizd SM. Enhanced activation of p21 -activated kinase 1 in heart failure contributes to dephosphorylation of connexin 43. Cardiovasc Res. 2011;92:106–114. doi: 10.1093/cvr/cvr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Igarashi T, Finet JE, Takeuchi A, Fujino Y, Strom M, Greener ID, et al. Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation. 2012;125:216–225. doi: 10.1161/CIRCULATIONAHA.111.053272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54:361–379. doi: 10.1016/s0008-6363(02)00273-0. doi: http://dx.doi.org/10.1016/S0008-6363(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 84.Rucker-Martin C, Milliez P, Tan S, Decrouy X, Recouvreur M, Vranckx R, et al. Chronic hemodynamic overload of the atria is an important factor for gap junction remodeling in human and rat hearts. Cardiovasc Res. 2006;72:69–79. doi: 10.1016/j.cardiores.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 85.Nao T, Ohkusa T, Hisamatsu Y, Inoue N, Matsumoto T, Yamada J, et al. Comparison of expression of connexin in right atrial myocardium in patients with chronic atrial fibrillation versus those in sinus rhythm. Am J Cardiol. 2003;91:678–683. doi: 10.1016/s0002-9149(02)03403-3. [DOI] [PubMed] [Google Scholar]
- 86.Polyakova V, Miyagawa S, Szalay Z, Risteli J, Kostin S. Atrial extracellular matrix remodelling in patients with atrial fibrillation. J Cell Mol Med. 2008;12:189–208. doi: 10.1111/j.1582-4934.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ling S, Sun Q, Li Y, Zhang L, Zhang P, Wang X, et al. CKIP-1 inhibits cardiac hypertrophy by regulating class II histone deacetylase phosphorylation through recruiting PP2A. Circulation. 2012;126:3028–3040. doi: 10.1161/CIRCULATIONAHA.112.102780. [DOI] [PubMed] [Google Scholar]
- 88.Dobrev D, Wehrens XHT. Role of RyR2 phosphorylation in heart failure and arrhythmias: Controversies around ryanodine receptor phosphorylation in cardiac disease. Circ Res. 2014;114:1311–1319. doi: 10.1161/CIRCRESAHA.114.300568. discussion 1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chiang DY, Li N, Wang Q, Alsina KM, Quick AP, Reynolds JO, et al. Impaired local regulation of ryanodine receptor type 2 by protein phosphatase 1 promotes atrial fibrillation. Cardiovasc Res. 2014;103:178–187. doi: 10.1093/cvr/cvu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Belevych AE, Sansom SE, Terentyeva R, Ho H-T, Nishijima Y, Martin MM, et al. MicroRNA-1 and −133 increase arrhythmogenesis in heart failure by dissociating phosphatase activity from RyR2 complex. PloS One. 2011;6:e28324. doi: 10.1371/journal.pone.0028324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Terentyev D, Belevych AE, Terentyeva R, Martin MM, Malana GE, Kuhn DE, et al. miR-1 overexpression enhances Ca2+ release and promotes cardiac arrhythmogenesis by targeting PP2A regulatory subunit B56alpha and causing CaMKII-dependent hyperphosphorylation of RyR2. Circ Res. 2009;104:514–521. doi: 10.1161/CIRCRESAHA.108.181651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dodge-Kafka KL, Bauman A, Mayer N, Henson E, Heredia L, Ahn J, et al. cAMP-stimulated protein phosphatase 2A activity associated with muscle A kinase-anchoring protein (mAKAP) signaling complexes inhibits the phosphorylation and activity of the cAMP-specific phosphodiesterase PDE4D3. J Biol Chem. 2010;285:11078–11086. doi: 10.1074/jbc.M109.034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vest JA, Wehrens XHT, Reiken SR, Lehnart SE, Dobrev D, Chandra P, et al. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- 94.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, et al. CaMKII-Dependent Diastolic SR Ca2+ Leak and Elevated Diastolic Ca2+ Levels in Right Atrial Myocardium of Patients With Atrial Fibrillation. Circ Res. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 96.Backx PH. Complexity, confusion and controversy continue complicating the contribution of RyR2 channel phosphorylation to heart function. J Physiol. 2014;592:1911–1912. doi: 10.1113/jphysiol.2014.272575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schirmer I, Heijman J, Voigt N, Dobrev D. Altered Composition of the Ryanodine Receptor Channel Complex in Patients with Chronic Atrial Fibrillation (Abstract) Heart Rhythm. 2015;12:S50. [Google Scholar]
- 98.Beavers DL, Wang W, Ather S, Voigt N, Garbino A, Dixit SS, et al. Mutation E169K in junctophilin-2 causes atrial fibrillation due to impaired RyR2 stabilization. J Am Coll Cardiol. 2013;62:2010–2019. doi: 10.1016/j.jacc.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Terentyev D, Hamilton S. Regulation of sarcoplasmic reticulum Ca2+ release by serine-threonine phosphatases in the heart. J Mol Cell Cardiol. 2016;101:156–164. doi: 10.1016/j.yjmcc.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shanmugam M, Molina CE, Gao S, Severac-Bastide R, Fischmeister R, Babu GJ. Decreased sarcolipin protein expression and enhanced sarco(endo)plasmic reticulum Ca2+ uptake in human atrial fibrillation. Biochem Biophys Res Commun. 2011;410:97–101. doi: 10.1016/j.bbrc.2011.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aoyama H, Ikeda Y, Miyazaki Y, Yoshimura K, Nishino S, Yamamoto T, et al. Isoform-specific roles of protein phosphatase 1 catalytic subunits in sarcoplasmic reticulum-mediated Ca2+ cycling. Cardiovasc Res. 2011;89:79–88. doi: 10.1093/cvr/cvq252. [DOI] [PubMed] [Google Scholar]
- 102.Liu R, Correll RN, Davis J, Vagnozzi RJ, York AJ, Sargent MA, et al. Cardiac-specific deletion of protein phosphatase 1β promotes increased myofilament protein phosphorylation and contractile alterations. J Mol Cell Cardiol. 2015;87:204–213. doi: 10.1016/j.yjmcc.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kirchhefer U, Brekle C, Eskandar J, Isensee G, Kučerová D, Müller FU, et al. Cardiac function is regulated by B56α-mediated targeting of protein phosphatase 2A (PP2A) to contractile relevant substrates. J Biol Chem. 2014;289:33862–33873. doi: 10.1074/jbc.M114.598938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eiras S, Narolska NA, van Loon RB, Boontje NM, Zaremba R, Jimenez CR, et al. Alterations in contractile protein composition and function in human atrial dilatation and atrial fibrillation. J Mol Cell Cardiol. 2006;41:467–477. doi: 10.1016/j.yjmcc.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 105.Belus A, Piroddi N, Ferrantini C, Tesi C, Cazorla O, Toniolo L, et al. Effects of chronic atrial fibrillation on active and passive force generation in human atrial myofibrils. Circ Res. 2010;107:144–152. doi: 10.1161/CIRCRESAHA.110.220699. [DOI] [PubMed] [Google Scholar]
- 106.Kuster DWD, Bawazeer AC, Zaremba R, Goebel M, Boontje NM, van der Velden J. Cardiac myosin binding protein C phosphorylation in cardiac disease. J Muscle Res Cell Motil. 2012;33:43–52. doi: 10.1007/s10974-011-9280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Solaro RJ, Kobayashi T. Protein phosphorylation and signal transduction in cardiac thin filaments. J Biol Chem. 2011;286:9935–9940. doi: 10.1074/jbc.R110.197731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Armstrong CG, Doherty MJ, Cohen PT. Identification of the separate domains in the hepatic glycogen-targeting subunit of protein phosphatase 1 that interact with phosphorylase a, glycogen and protein phosphatase 1. Biochem J. 1998;336:699–704. doi: 10.1042/bj3360699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 111.Yger M, Girault J-A. DARPP-32, Jack of All Trades… Master of Which? Front Behav Neurosci. 2011;5:56. doi: 10.3389/fnbeh.2011.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fish KM, Ladage D, Kawase Y, Karakikes I, Jeong D, Ly H, et al. AAV9.I-1c delivered via direct coronary infusion in a porcine model of heart failure improves contractility and mitigates adverse remodeling. Circ Heart Fail. 2013;6:310–317. doi: 10.1161/CIRCHEARTFAILURE.112.971325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Heijman J, Voigt N, Ghezelbash S, Schirmer I, Dobrev D. Calcium Handling Abnormalities as a Target for Atrial Fibrillation Therapeutics: How Close to Clinical Implementation? J Cardiovasc Pharmacol. 2015;66:515–522. doi: 10.1097/FJC.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 114.De Jong AM, Maass AH, Oberdorf-Maass SU, De Boer RA, Van Gilst WH, Van Gelder IC. Cyclical stretch induces structural changes in atrial myocytes. J Cell Mol Med. 2013;17:743–753. doi: 10.1111/jcmm.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ghezelbash S, Molina CE, Dobrev D. Altered atrial metabolism: an underappreciated contributor to the initiation and progression of atrial fibrillation. J Am Heart Assoc. 2015;4:e001808. doi: 10.1161/JAHA.115.001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu G, Ren S, Korge P, Choi J, Dong Y, Weiss J, et al. A novel mitochondrial matrix serine/threonine protein phosphatase regulates the mitochondria permeability transition pore and is essential for cellular survival and development. Genes Dev. 2007;21:784–796. doi: 10.1101/gad.1499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Van Hoof C, Goris J. Phosphatases in apoptosis: to be or not to be, PP2A is in the heart of the question. Biochim Biophys Acta. 2003;1640:97–104. doi: 10.1016/s0167-4889(03)00029-6. doi: http://dx.doi.org/10.1016/S0167-4889(03)00029-6. [DOI] [PubMed] [Google Scholar]
- 118.Greiser M, Neuberger H-R, Harks E, El-Armouche A, Boknik P, de Haan S, et al. Distinct contractile and molecular differences between two goat models of atrial dysfunction: AV block-induced atrial dilatation and atrial fibrillation. J Mol Cell Cardiol. 2009;46:385–394. doi: 10.1016/j.yjmcc.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 119.Wakili R, Yeh Y-H, Yan Qi X, Greiser M, Chartier D, Nishida K, et al. Multiple potential molecular contributors to atrial hypocontractility caused by atrial tachycardia remodeling in dogs. Circ Arrhythm Electrophysiol. 2010;3:530–541. doi: 10.1161/CIRCEP.109.933036. [DOI] [PubMed] [Google Scholar]
- 120.Yeh Y-H, Wakili R, Qi X-Y, Chartier D, Boknik P, Kääb S, et al. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ Arrhythm Electrophysiol. 2008;1:93–102. doi: 10.1161/CIRCEP.107.754788. [DOI] [PubMed] [Google Scholar]
- 121.Laszlo R, Winkler C, Wöhrl S, Laszlo S, Eick C, Schreieck J, et al. Influence of verapamil on tachycardia-induced alterations of PP1 and PP2A in rabbit atrium. Exp Clin Cardiol. 2007;12:175–178. [PMC free article] [PubMed] [Google Scholar]
- 122.Lin C-C, Lin J-L, Lin C-S, Tsai M-C, Su M-J, Lai L-P, et al. Activation of the calcineurin-nuclear factor of activated T-cell signal transduction pathway in atrial fibrillation. Chest. 2004;126:1926–1932. doi: 10.1378/chest.126.6.1926. [DOI] [PubMed] [Google Scholar]
- 123.Zhao Y, Cui G-M, Zhou N-N, Li C, Zhang Q, Sun H, et al. Calpain-Calcineurin-Nuclear Factor Signaling and the Development of Atrial Fibrillation in Patients with Valvular Heart Disease and Diabetes. J Diabetes Res. 2016:4639654. doi: 10.1155/2016/4639654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li N, Chiang DY, Wang S, Wang Q, Sun L, Voigt N, et al. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation. 2014;129:1276–1285. doi: 10.1161/CIRCULATIONAHA.113.006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guo X, Yuan S, Liu Z, Fang Q. Oxidation- and CaMKII-mediated sarcoplasmic reticulum Ca2+ leak triggers atrial fibrillation in aging. J Cardiovasc Electrophysiol. 2014;25:645–652. doi: 10.1111/jce.12395. [DOI] [PubMed] [Google Scholar]
- 126.Shan J, Xie W, Betzenhauser M, Reiken S, Chen B-X, Wronska A, et al. Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2012;111:708–717. doi: 10.1161/CIRCRESAHA.112.273342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li N, Wang T, Wang W, Cutler MJ, Wang Q, Voigt N, et al. Inhibition of CaMKII phosphorylation of RyR2 prevents induction of atrial fibrillation in FKBP12.6 knockout mice. Circ Res. 2012;110:465–470. doi: 10.1161/CIRCRESAHA.111.253229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang H, Cannell MB, Kim SJ, Watson JJ, Norman R, Calaghan SC, et al. Cellular Hypertrophy and Increased Susceptibility to Spontaneous Calcium-Release of Rat Left Atrial Myocytes Due to Elevated Afterload. PloS One. 2015;10:e0144309. doi: 10.1371/journal.pone.0144309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lugenbiel P, Wenz F, Govorov K, Schweizer PA, Katus HA, Thomas D. Atrial fibrillation complicated by heart failure induces distinct remodeling of calcium cycling proteins. PloS One. 2015;10:e0116395. doi: 10.1371/journal.pone.0116395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.