Abstract
Adult Leydig cells develop from undifferentiated mesenchymal-like stem cells (stem Leydig cells, SLCs) present in the interstitial compartment of the early postnatal testis. Putative SLCs also have been identified in peritubular and perivascular locations of the adult testis. The latter cells, which normally are quiescent, are capable of regenerating new Leydig cells upon the loss of the adult cells. Recent studies have identified several protein markers to identify these cells, including nestin, PDGFRα, COUP-TFII, CD51 and CD90. We have shown that the proliferation of the SLCs is stimulated by DHH, FGF2, PDGFBB, activin and PDGFAA. Suppression of proliferation occurred with TGFβ, androgen and PKA signaling. The differentiation of the SLCs into testosterone-producing Leydig cells was found to be regulated positively by DHH (Desert hedgehog), lithium-induced signaling and activin; and negatively by TGFβ, PDGFBB, FGF2, Notch and Wnt signaling. DHH, by itself, was found to induce SLC differentiation into LH-responsive steroidogenic cells, suggesting that DHH plays a critical role in the commitment of SLC into the Leydig lineage. These studies, taken together, address the function and regulation of low turnover stem cells in a complex, adult organ, and also have potential application to the treatment of androgen deficiency.
Keywords: Leydig cell, stem cell, DHH, CD90, COUP-TFII
1. Introduction
Testosterone, produced by the Leydig cells of the mammalian testis, is essential for the development of the male reproductive system and for maintenance of male reproductive function throughout life (Nef and Parada, 2000; Smith and Walker, 2014). Testosterone deficiency in the adult contributes to symptoms that include increased body fat and fatigue, and decreased muscle mass, cognitive function and immune response (Malkin et al. 2004; Snyder, 2008; McHenry, 2012; Bobjer et al., 2013; Huhtaniemi, 2014). It is now recognized that adult Leydig cells ultimately derive from stem cells (referred to herein as stem Leydig cells or SLCs). Progress has been made in the last decade to advance our understanding of the regulation of the proliferation and differentiation of the SLCs in both the neonatal and adult testes. The works summarized in this review are based primary on the rodents. Their relevancies to human have been reviewed elsewhere recently (Teerds and Huhtaniemi, 2015).
2. Stem Leydig Cells in Prepubertal and Adult Testes
2.1. Adult Leydig cell formation
In the rat, the adult Leydig cells (ALCs) have been shown to develop from SLCs that are present in the prepubertal testis. Four distinct stages of ALC development have been described: SLCs, progenitor Leydig cells (PLCs), immature Leydig cells (ILCs), and ALCs. These stages are common to both the postnatal development of ALCs, and to ALC restoration in Leydig cell-depleted adult testes (Chen et al., 2010).
SLCs
SLCs were originally identified as spindle-shaped, platelet-derived growth factor receptor alpha (PDGFRα)-positive cells in the peritubular regions of postnatal day 7 (PND 7) rat testes (Ge et al., 2006). SLCs also are reported to surround the vasculature in the interstitial compartment of the testis (Haider and Sevros, 1998; Davidoff et al., 2004, 2009). Hardy and colleagues isolated putative SLCs by immuno-selecting for luteinizing hormone receptor (LHR)-negative, PDGFRα-positive cells in the PND 7 rat testis. Over 99% of the cells thus selected were found to be 3β-hydroxysteroid dehydrogenase (3β-HSD or HSD3B1)-negative, LHR-negative, and PDGFRα-positive. When cultured in growth factor-containing expansion medium, the cells maintained a stable 3βHSD−/LHR−/PDGFRα+ phenotype for more than 6 months. However, when switched to differentiation medium containing PDGF-BB, LH, thyroid hormone and IGF-1, the cells expressed the Leydig cell steroidogenic enzymes cholesterol side-chain cleavage (P450scc or CYP11A1), 3β-HSD, 17α-hydroxylase (P450c17 or CYP17A1), and also LHR and steroidogenic acute regulatory protein (StAR), and began to produce testosterone (Ge et al., 2006).
The ability of the cells to differentiate into Leydig cells was further confirmed in vivo. The isolated putative SLCs were tagged with a fluorescent tracking dye, carboxyfluorescein diacetate, and then injected into the adult rat testis from which Leydig cells had been eliminated by administering the Leydig cell toxin ethane dimethanesulfonate (EDS). Ten days after the cells were injected into the Leydig cell-depleted testes, fluorescently labeled cells were found in the interstitial compartment that had become 3β-HSD positive, indicating that injected cells had begun to differentiate in vivo. These results, taken together, indicated that the 3βHSD−/LHR−/PDGFRα+ cells that were isolated from PND 7 testes indeed were able to become Leydig cells both in vitro and in vivo (Ge et al., 2006).
PLCs
By PND 21, some of the SLCs were found to have given rise to PLCs. The PLCs retain a spindle shape, but express LHR, 3α-hydroxysteroid dehydrogenase (3α-HSD or AKR1C14), and 3β-HSD (HSD3B1) (Haider et al., 1986; Shan et al., 1995). The proliferation of the PLCs plays an important role in establishing the adult ALC number (Hardy et al., 1989; Siril et al., 2000). Due to high levels of 5α-reductase and 3α-HSD (AKR1C14), and low levels of 17β-HSD (HSD17B3), androsterone is the major steroid product of the PLCs (Ge and Hardy, 1998).
ILCs
PLCs proliferate and their progeny continue to differentiate such that by PND 28, the PLCs transform from spindle-shaped to round, contain numerous lipid inclusions, and form a population of ILCs. The number of such cells increases to ~13–14 million per testis (Hardy et al., 1989). During the transformation from PLCs to ILCs, the smooth ER content of the cells expands greatly, conferring an ultrastructure similar to that of ALCs. Concurrent with the expansion of the smooth ER, the levels of 3β-HSD, P450scc, and P450c17 increase (Haider et al., 1986; Dupont et al., 1993; Shan et al., 1993), and the cells develop an increased capacity for testosterone production (Zirkin and Ewing, 1987). Due to the presence of testosterone metabolizing enzymes 3α-HSD and 5α-reductase, the ILCs primarily produce the testosterone metabolite 5α-androstane-3α, 17β-diol (3α-diol), not testosterone.
ALCs
ILCs, once formed, typically divide only once between days 28 and day 56 to form the adult population of 25 million ALCs per testis (Benton et al., 1995). ALCs do not normally proliferate (Keeney et al., 1988; Teerds et al., 1989), but are capable of being regenerated if the original ALC population is eliminated experimentally (Kerr et al., 1987; Sharpe et al., 1990). The nuclei of ALCs are large and round, with condensed euchromatin and one or two nucleoli. Compared to ILCs, the ALCs have a greater abundance of smooth ER and are largely devoid of lipid inclusions (Zirkin and Ewing, 1987).
The activities of androgen metabolizing enzymes decline as ALCs form (Inano and Tamaoki, 1966; Steinberger and Fischer, 1969; Murono, 1989). The cells express LHR and the steroidogenic enzymes 3β-HSD, 3α-HSD, and 17β-HSD. By postnatal day 30 and thereafter, the cells also express 11β-HSD type 1 (Haider et al., 1986; Hardy et al., 1989; Dupont et al., 1993; Neumann et al., 1993; Ge et al., 1997; Mendis-Handagama and Ariyaratne, 2001). The decrease in androgen metabolism and continued increase in levels of testosterone biosynthetic enzymes culminate in the predominance of testosterone over 5α-reduced products in the ALCs. In Leydig cells from 90 day-old adults, testosterone production is 150 times greater than that by PLCs at 21 days of age, and five times greater than that by ILCs at 35 days of age (Shan et al., 1993).
2.2. Identity and location of stem Leydig cells in the adult testis
The normal formation of ALCs in the prepubertal testis and the regeneration of ALCs in the adult testis appear to be similar (Teerds and Rijntjes, 2007; Chen et al., 2010) with ALCs in both cases apparently deriving from SLCs. Early morphological studies of EDS-treated rats suggested that the Leydig cells restored to the adult testis may arise from fibroblasts, lymphatic endothelial cells, and/or pericytes (Jackson et al., 1986; Kerr et al., 1987). Subsequent studies reported that there are spindle-shaped cells in perivascular and peritubular locations in the interstitial compartment involved in ALC development (Haider et al., 1995; Ariyaratne et al., 2003; Davidoff et al., 2004, 2009). There are studies suggesting that cells located along the outer surface of the seminiferous tubules (peritubular cells) are the precursor cells to Leydig cell regeneration after EDS (O'Shaughnessy et al., 2008; Stanley et al., 2012; Li et al., 2016), while other studies have suggested that the predominant cells involved are those associated with testicular blood vessels, namely vascular smooth muscle cells and pericytes (Davidoff et al., 2004, 2009). The relative contributions of cells from these different locations to the establishment of the ALC population remain uncertain.
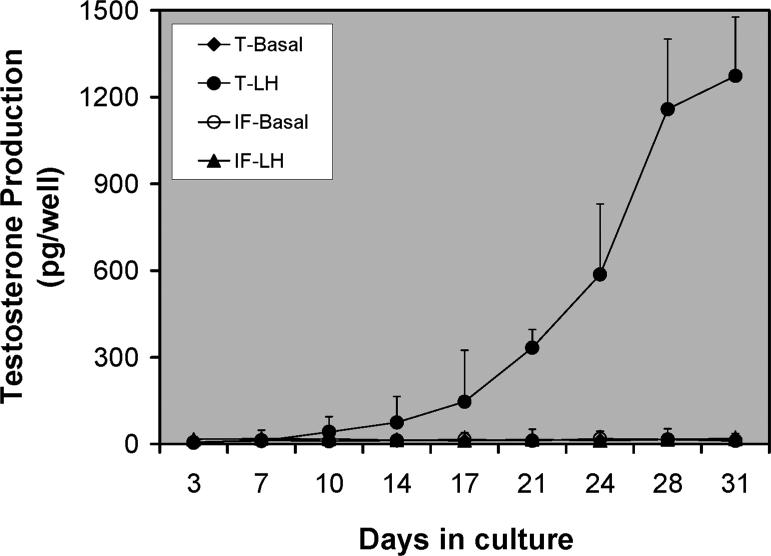
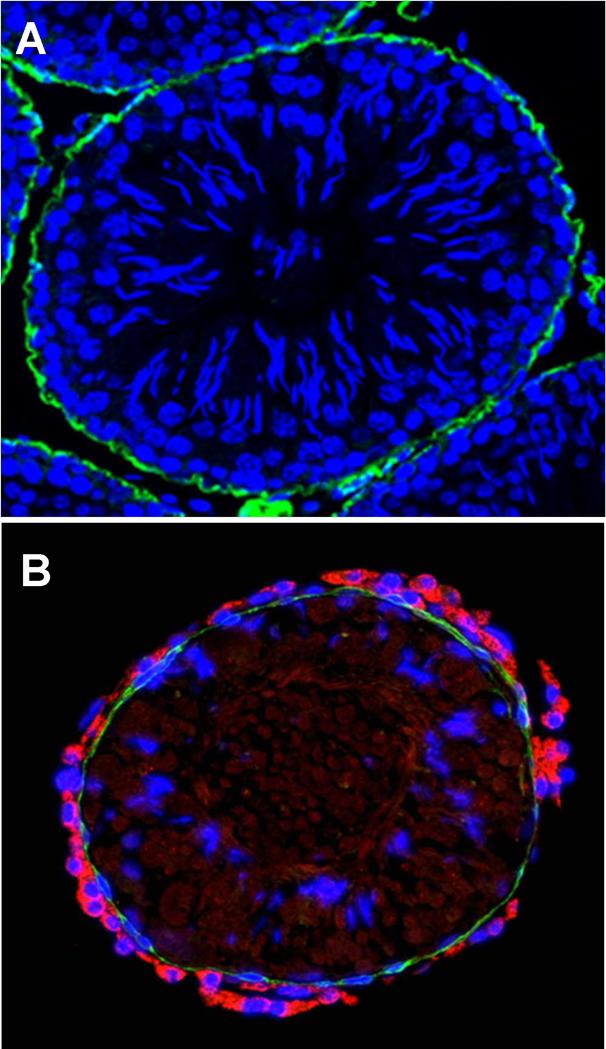
The peritubular origin hypothesis, which is consistent with the studies of normal Leydig cell development during the prepubertal period (Haider et al., 1995; Russell et al., 1995; Ariyaratne and Mendis-Handagama, 2000; Ge et al., 2006), suggests that Leydig cells differentiate during postnatal days 10–15 from fibroblast-like cells of the multi-layered tubule wall, and that the products (PLCs or ILCs) move to areas within the interstitial compartment where they continue to divide and differentiate to achieve the final population of ALCs (Russell et al., 1995). But there are studies indicating that Leydig cells differentiate from perivascular smooth muscle cells, pericytes or even vascular endothelial cells (Haider and Sevros, 1998; Davidoff et al., 2004, 2009). To examine whether cells from one or both of the intratesticular locations give rise to functional Leydig cells, we established an in vitro system in which the seminiferous tubules and interstitial tissue were physically separated and cultured separately (Stanley et al., 2012; Odeh et al., 2014; Li et al., 2016; Zhang et al., 2013). No testosterone was detected in the culture medium of either tubules or interstitial tissue during the first 2 weeks of culture. Testosterone began to be produced by cells associated with the tubules after the 2-week period, and increased through 4 weeks (Fig. 1). In contrast, testosterone was not found in the culture medium containing interstitial tissue even at 4 weeks (Fig. 1; Stanley et al., 2012). Examination of the cultured tubules by microscopy revealed that after 2 weeks, cells with the appearance of Leydig cells had appeared on the surfaces of tubules, outside the myoid cell layer. As seen in Figure 2, co-staining of the cultured tubules with the Leydig cell marker protein CYP11A1 and myoid cell marker α-SMA confirmed that new Leydig cells (red) had been generated outside the single layer of myoid cells (green; compare A and B) (Li et al., 2016). Interestingly, this peritubular location of SLCs was also observed in the human testis, suggesting it may be a common phenomenon across different species (Landreh et al., 2014).
Figure 1.
Differentiation of stem Leydig cells associated with seminiferous tubules (T) and interstitium (IF) in vitro. Testosterone production over time by isolated tubules and interstitium cultured separately with luteinizing hormone (LH). (Adapted from Stanley et al., 2012 with permission)
Figure 2.
Cross section of tubules before (A) and after (B) culture for 4 weeks with LH, co-stained for α-SMA (green, myoid cells) and CYP11A1 (red, Leydig cells). (Adapted from Li et al., 2016 with permission)
Although functional Leydig cells were not derived from the cultured interstitial tissue, this does not rule out the possibility that the interstitial tissue contains stem cells capable of differentiating. For example, if niche factors from the tubules were required for SLC proliferation and/or differentiation, such factors would not be present in preparations of interstitial tissue cultured in the absence of the tubules. To examine this possibility, we carried out a co-culture experiment in which the tubules and interstitial tissues were cultured in separate chambers that allowed a free exchange of paracrine factors between the two tissues (Chen et al., 2016). The co-culture of the interstitium with seminiferous tubules resulted in the production of testosterone-producing Leydig cells in the interstitial tissue. The formation of testosterone-producing Leydig cells by seminiferous tubules in the absence of interstitial tissue, and by the interstitial tissue only when paracrine factors were provided by the tubules in co-culture experiments, strongly suggest that the interstitium has precursor cells but lacks critical factors provided by the seminiferous tubules. This emphasizes the uniqueness of the intratesticular environment in the development of Leydig cells, a conclusion that is consistent with previous observations that stem cells from other sources can be induced to form Leydig cells in vivo if transplanted into the interstitial compartment of the testis (Yazawa et al., 2006; Lue et al., 2007; Yang et al., 2015). The nature of the paracrine factors, and the cells from which they are derived, are not known as yet.
These results are consistent with in vivo observations that cells from both seminiferous tubules and interstitial tissue may contribute to ALC development. Thus, after EDS treatment to eliminate preexisting Leydig cells, Leydig cell regeneration appears to occur both around the tubules and around blood vessels of germ cell-containing tubules (Haider and Servos, 1998; Yan et al., 2000; Davidoff et al., 2004). Interestingly, in the case of atrophic tubules (i.e. tubules containing few or no germ cells), most new Leydig cells appear around the tubules (Kerr and Donachie, 1986; O'Leary et al., 1986; Sharpe et al., 1990; O'Shaughnessy et al., 2008). These observations suggest that the regressed tubules may secrete different factors than tubules with normal complements of germ cells, thereby affecting nearby SLCs differently.
2.3. Stem Leydig cell markers
As discussed below, a number of proteins have been shown to be expressed by putative SLCs in the interstitial compartment of prepubertal or adult testes, including nestin, PDGFRα, COUP-TFII (chicken ovalbumin upstream promoter transcription factor-II), CD51 and CD90. Among these, only COUP-TFII has been defined as specific to SLCs by genetic lineage tracing experiments. Cell surface markers can be used to isolate SLCs. Unfortunately, however, few among the cell surface proteins identified as associated with SLCs are SLC-specific. Rather, most are also expressed either by other testicular cells or by more advanced cells in the Leydig lineage. Other surface markers, such as CD51 and CD90, have been tested only in a single species and/or age. Additionally, it remains possible that there may be more than one SLC type that gives rise to ALCs, and these cells may express different surface marker proteins.
Nestin
Nestin is an intermediate filament protein that is expressed in stem/progenitor cells of the developing and adult central and peripheral nervous system (Messam, 2000; Liu 2002), as well as other tissues such as skeletal muscle, skin, and pancreatic islet (Sejersen and Lendahl 1993; Zulewski et al., 2001; Li et al., 2003). Nestin also is expressed in putative SLCs in both fetal and adult testes (Davidoff et al., 2004; Jiang et al., 2014). Following EDS treatment of adults, the nestin of blood vessel smooth muscle cells and pericytes increased, coincident with the time course of Leydig precursor cell proliferation, and later nestin-positive cells on vessel walls expressed the Leydig cell lineage marker CYP11A1 (Davidoff et al., 2004). At this time, nestin-positive cells also were detected in cells in peritubular locations. The subsequent appearance of Leydig cells was associated with reduced nestin expression. This developmental relationship between Leydig cell maturation and loss of nestin expression also was found in the fetal testis (Ricci et al., 2012) and in the early postnatal testis (Jiang et al., 2014).
The identification of nestin as a stem Leydig cell marker was reinforced by a recent study which showed nestin promtor-driven GFP expression (Nes-GFP+) in cells specific to the interstitial compartment (Jiang et al., 2014). These Nes-GFP+ cells also expressed LIFR (leukemia inhibitory factor receptor) and PDGFRα, but not Leydig cell lineage markers, and were found to be capable of clonogenic self-renewal and extensive proliferation in vitro. Depending upon conditions, the cells were able to differentiate into Leydig cells with the ability to produce testosterone. Moreover, when transplanted into the testes of Leydig cell-disrupted animals, the Nes-GFP+ cells colonized the interstitium and increased testosterone production, thereby accelerating meiotic and post-meiotic germ cell recovery. More than 90% of these Nes-GFP+ cells expressed CD51, suggesting that the latter might be useful for the isolation of SLCs from non-transgenic mice (Jiang et al., 2014).
Smooth muscle actin/desmin
In addition to expressing neural cell markers, SLCs have been reported to express muscle cell markers including smooth muscle actin (α-SMA) and desmin (Davidoff et al., 2004; 2009; Stanley et al., 2011; Landreh et al., 2013). However, the expressions of these proteins were not always observed in putative SLCs (Kilcoyne et al., 2014; Li et al., 2016), suggesting that cells considered to be SLCs may not always be identified correctly. For example, myoid cells and pericytes, which would express these proteins, may be in close physical proximity to SLCs and therefore mistaken for SLCs. Thus, it remains uncertain whether α-SMA+/desmin+ cells localized to perivascular and peritubular locations in the testis can form Leydig cells. Indeed, most recent studies have found that SLCs may not express these smooth muscle proteins (Kilcoyne et al., 2014; Li et al., 2016). There also may be a number of developmental stages of Leydig cell precursors, and the different markers (nestin, α-SMA, desmin) may be characteristic of different sub-populations of these cells, with nestin in the earlier stages (e.g. SLCs) and α-SMA/desmin in the more advanced stages (e.g. PLCs) (Landreh et al., 2013).
SF1
It is well known that SF1 (NR5A1) is crucial for the development of gonads and adrenal glands (Luo et al, 1994; Hatano et al, 1996). In the early developing fetal testis, SF1 expressing cells give rise to both Sertoli and Leydig cells (Barsoum and Yao, 2010; Barsoum et al., 2013). Deletion of WT1 from adult Sertoli cells resulted in a transdifferentiation of the cells into Leydig-like steroidogenic cells (Zhang et al., 2015), supporting their common origin. SF1 is important in the development of ALCs; without it, Leydig cell differentiation does not occur (Koskimies et al., 2002; Park et al., 2007; Val et al., 2003). A number of studies have reported that forced expression of SF1 was sufficient to turn non-steroidogenic cells (mesenchymal, embryonic stem cells) into steroidogenic cells, suggesting that SF1 is necessary, and indeed sufficient, for the development of steroidogenic cells (Yazawa et al., 2006, 2009; Gondo et al., 2008; Yang et al., 2015). However, although Leydig cell precursor cells may express SF1 and be important for the formation of Leydig cells, SF1 does not appear to be an appropriate marker to identify/isolate SLCs from the adult testis since its expression is not limited in Leydig cell lineage (Kato et al., 2012).
PDGFR-α/β
PDGF-AA and PDGF-BB were detected within the testicular cords of the day 20 fetal rat testis, while staining for PDGFRα and PDGFβ was seen in cells between the cords (Gnessi et al., 1995). A similar distribution pattern was observed in the day 5 rat testis, with intense staining for PDGF-AA and PDGF-BB localized within the Sertoli cells and for PDGFRα and PDGFβ in gonocytes and peritubular locations. In the adult, however, Leydig cells were found to express both PDGF and receptor. Similar expression profiles were seen in the human testis (Basciani et al., 2002). These observations suggest that PDGF ligands and receptors may play important roles in the development of Leydig cells. Although the expressions of PDGFRα and PDGFRβ are not specific for the Leydig cell lineage, PDGFRα and PDGFRβ are expressed in Leydig cell precursor cells from the fetal period through adulthood. Due to their expressions on the cell surface, these proteins have the potential to be used to enrich precursor cells for Leydig cell lineage cells, and thus to isolate the cells. Indeed, PDGFRα has served as a selection marker to purify putative SLCs. It was shown that cells from neonatal and adult testes purified in this way were able to proliferate for months without differentiating, but also could be induced to differentiate into Leydig cells under the appropriate culture conditions (Ge et al., 2006; Stanley et al., 2012; Landreh et al., 2013).
Although the PDGFRα+ cells isolated from day 7 rat testes had characteristics expected of stem cells and were able to differentiate into Leydig cells, it is possible that the cells in fact represent an intermediate stage between SLCs and PLCs. For example, though the PDGFRα+ cells isolated from day 7 testes were negative for Leydig cell markers (Ge et al., 2006), soon thereafter they were found to express low levels of steroidogenic proteins (e.g. CYP11A1), and produced some progesterone (Landreh et al., 2013). Additionally, culturing these cells with cAMP agonist rapidly up-regulated the expression of steroidogenic enzymes and increased steroidogenesis in vitro. These results indicated that although the cells did not express LHR, they had developed a cAMP/PKA-steroidogenic enzyme regulatory pathway, and therefore were not entirely uncommitted to a differentiation pathway (Landreh et al., 2013). Moreover, very few of the COUP-TFII+ stem cells in the fetal testis were found to express PDGFRα, and only some COUP-TFII+ cells in the adult testis after EDS treatment were PDGFRα+, suggesting that PDGFRα+ cells may represent SLCs that have begun to commit to the Leydig cell lineage (Kilcoyne et al., 2014). This conclusion is supported by the observation that PDGFRα gene expression was found to increase upon SLC transition to PLCs (Odeh et al., 2014).
COUP-TFII
COUP-TFII (aka NR2F2) is a nuclear receptor that has been shown to play an important role in mesoderm formation, including the development of blood vessels (Davis et al., 2013; Wu et al., 2016). Mesenchymal stem cells are multipotent cells with the capacity to give rise to multiple cell types such as adipocytes, osteoblasts, chondrocytes, and myocytes. Although the molecular events responsible for lineage specification and differentiation remain uncertain, COUP-TFII has been shown to be involved (Xie et al., 2011; 2013). Thus, inactivation of COUP-TFII in mesenchymal progenitor cells has been shown to induce osteoblast and myoblast development while impairing adipogenic and chondrogenic programs. Further studies indicated that COUP-TFII functions through the combined modulation of Wnt signaling and PPARγ and Sox9 expression (Xie et al., 2011). Interestingly, these are also important regulators in Leydig cell development, suggesting that COUP-TFII may play a significant role in defining the Leydig cell lineage from stem cells that also have the capacity to differentiate into other cells (Jiang et al., 2014). Indeed, Qin et al. (2008) showed that the induced knockout of COUP-TFII in prepubertal male mice resulted in failure of adult Leydig cell development, but that its knockout in adult mice, when the adult Leydig cells were fully developed, did not affect the function of the ALCs. These results suggest that COUP-TFII plays an important role in ALC development, but not in the maintenance of fully developed ALCs. Further analysis revealed that Leydig cell differentiation in the prepubertal knockout was arrested at the stem/progenitor cell stage, suggesting that COUP-TFII may affect the differentiation of stem/progenitor cells (Qin et al., 2008).
COUP-TF-II has been proposed to be a potentially useful marker to identify SLCs/PLCs both in early development and in the adult (Kilcoyne et al., 2014), in part because it is expressed by these cells across species, including mice, marmoset and human, and because it is not expressed by such testicular cells as macrophages, pericytes, endothelial cells, peritubular myoid cells, fibroblasts or hematopoietic cells. Lineage tracing studies showed that COUP-TFII+ progenitor cells in the fetal mouse testis developed into ALCs in the adult (Kilcoyne et al., 2014). To date, COUP-TFII is the only SLC/PLC marker to be confirmed as such by lineage tracing. However, whether all Leydig cell progenitors have the capacity to develop into ALCs, and whether there are other COUP-TFII negative progenitor cells that may also contribute to the adult Leydig cell population, remain uncertain.
CD51
As discussed above, nestin was the first identified SLC marker (Davidoff et al., 2004; 2009). In a transgenic mouse model, Nes-GFP+ putative SLCs in the neonatal mouse testis were found to have the ability to differentiate into Leydig cells (Jiang et al., 2014). Further characterization of the SLCs required their purification, and therefore SLC-specific surface proteins needed to be identified. The surface protein CD51 was found to be present in over 90% of Nes-GFP+ cells in the transgenic neonatal mouse testis. Using CD51 as a SLC marker, cells were isolated with properties very similar to those of the Nes-GFP+ cells, including their ability to differentiate into Leydig cells and also into cells of the adipogenic, osteogenic, and chondrogenic lineages. Nes-GFP+ cells were also found to express putative SLC surface proteins in addition to CD51, including P75NTR and PDGFRα. None, however, was as specific for the Nes-GFP+ SLCs as CD51. Whether or not CD51 can be used to isolate SLCs from adult testes or from different species has not yet been reported.
CD90
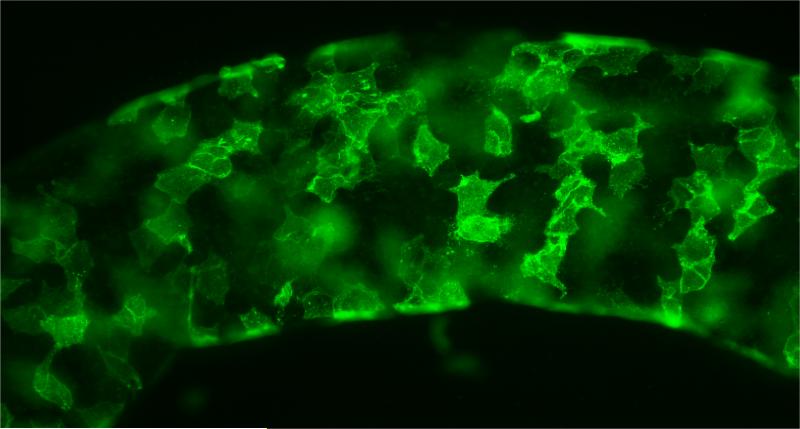
We and others (Chen et al., 1996; Kerr et al., 1986; Teerds and Rijnties, 2007) reported that adult rat testes contain stem cells that give rise to ALCs by 8-10 weeks following the elimination of the preexisting ALCs. The process by which the ALCs are restored is similar or identical to the development of ALCs that occurs after birth and through puberty (Teerds and Rijnties, 2007; Chen et al., 2010). We identified 15 cell surface proteins whose message levels were highly expressed in SLCs and then turned off at the PLC stage (Stanley et al., 2011; Li et al., 2016). Four among these (CD90, p75NTR, CD51, and PDGFRα) were tested for their specificity for SLCs. Among these, CD90 localized to cells on the surfaces of the seminiferous tubules (Fig. 3), which is where the stem cells reside. These cells did not co-stain for the myoid cell protein desmin (Li et al., 2016). After collagenase treatment of the tubules, the cells obtained were stained for CD90 and sorted by flow cytometry. The CD90+ cells represented about 0.4% of the total cells. The ability of CD90+ and CD90− cells to differentiate into steroidogenic cells was assessed by culturing the cells with LH for 3 weeks plus or minus the Desert hedgehog (DHH) agonist SAG (smoothened agonist). CD90+ but not CD90− cells were able to form testosterone-producing cells in the presence of LH plus SAG. These results indicated that the CD90+ cells on the tubule surfaces were SLCs that can be induced to differentiate into Leydig cells. It is not yet known whether CD90 expression is a characteristic of SLCs at all ages and in all species.
Figure 3.
CD90-positive cells (green) on the surface of freshly isolated seminiferous tubule. (Adapted from Li et al., 2016 with permission)
3. Stem Leydig Cell Growth and Regulation
3.1. Stem Leydig cell niche
Adult stem cells are defined by their capacity to proliferate, self-renew and generate differentiated, tissue-specific cells throughout an organism's lifetime. Their functions are regulated by extracellular and intracellular signals (Li and Xie, 2005; David, 2006). The stem cell “niche” refers to a defined anatomic region that regulates stem cell renewal and differentiation, and thus how particular stem cells function in tissue maintenance and repair without becoming depleted (Li and Xie, 2005; David, 2006). In the rat testis, ALCs, once formed, turn over slowly under normal circumstances, and few die. However, loss of the adult Leydig cell population occurs within 3-4 days in response to treating a rat with a single dose of the alkylating agent EDS. This loss is followed by the repopulation of the testis by testosterone-producing Leydig cells (Molenaar et al., 1986; Kerr et al., 1987; Sharpe et al., 1990). Previous studies have shown that the new Leydig cells derive from the proliferation and subsequent differentiation of SLCs (Teerds et al., 1990; Myers and Abney, 1991; Gaytan et al., 1992; Miyano et al., 1997; Yan et al., 2000). As indicated above, some studies have reported that the SLCs reside on the outer surfaces of the seminiferous tubules (O'Shaughnessy et al., 2008; Stanley et al., 2012), while others have suggested that they also are associated with blood vessels (Davidoff et al., 2004, 2009). As yet, little is known about the SLC niche, and in particular about the mechanisms by which SLC quiescence, proliferation and differentiation are regulated.
Sertoli cells are the source of many of the secreted factors that control SLC function (see below), including DHH and PDGF (Gnessi et al., 1995; Szczepny et al., 2006; Huang and Yao, 2010; Clark et al., 2000; Martin, 2016). Germ cells may also contribute to the niche, either by secreting factors or by modifying Sertoli cell function (Kerr and Donachie, 1986; O'Leary et al., 1986; Sharpe et al., 1990). The failure of SLCs associated with blood vessels to produce Leydig cells when cultured in the absence of the seminiferous tubules may be a consequence of the absence of paracrine factors from the tubules that are required to stimulate SLC proliferation and differentiation. In addition to cells associated with seminiferous tubules, cells in the interstitial compartment may also be involved in SLC function. For example, the ALCs themselves may, in some way, suppress SLC proliferation and differentiation. The triggering SLC proliferation and differentiation that results from the experimental loss of ALCs could be a direct response of the SLCs to the loss of suppressive Leydig cell products (e.g. testosterone, estradiol) (Abney and Myers, 1991; Zhai et al., 1996), or an indirect response resulting from changes elicited in other niche-associated cells. For example, the loss of Leydig cells might affect cells in the seminiferous tubules and/or LH secretion by the pituitary, which, in turn, might affect the Leydig cell niche.
3.2. Stem Leydig cell quiescence vs. proliferation vs. differentiation
SLCs isolated from the seminiferous tubules and cultured with LH alone failed to differentiate into Leydig cells, suggesting that the seminiferous tubules are required to provide niche factors necessary to regulate the proliferation and/or differentiation of the SLCs (Li et al., 2016). Microarray analysis of gene expression during the differentiation of neonatal SLCs to PLCs revealed signaling molecules and receptors that were significantly up- or down-regulated (Li et al., 2016). Also up- or down-regulated were molecules that had been identified in cell and/or animal studies as playing roles in Leydig cell development and/or involved in stem cell function in organs outside the testis. Using cultured seminiferous tubules, we tested the possible effects of factors associated with the tubules on SLC proliferation, differentiation, or both (Li et al., 2016). This was made possible because in this in vitro system, proliferation occurs during the first week of culture, and differentiation during weeks 2-3. This separation in time made it possible to determine the effects of added factors on one process and/or the other.
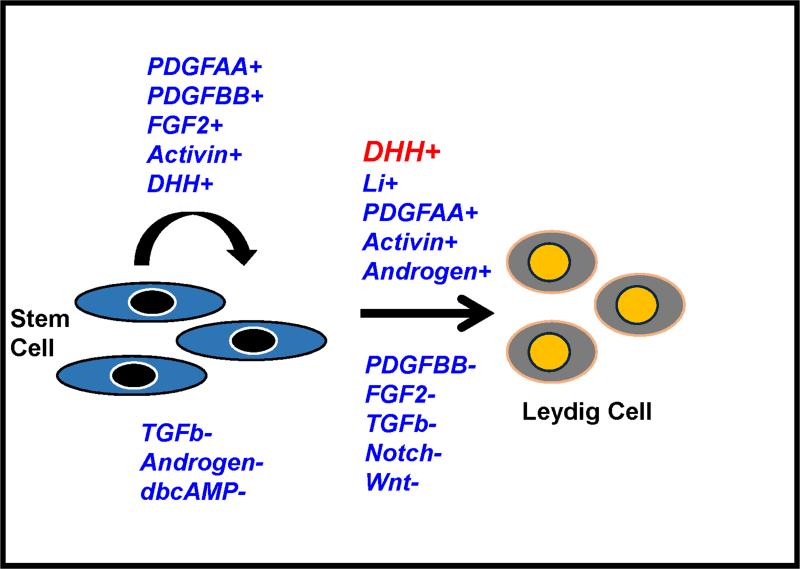
As illustrated in Figure 4, the proliferation of SLCs was stimulated by DHH, FGF2, PDGFBB, activin and PDGFAA. Suppression of proliferation occurred with TGFβ, androgen and PKA signaling. The differentiation of the SLCs into testosterone-producing Leydig cells was regulated positively by DHH (Desert hedgehog), lithium-induced signaling and activin; and negatively by TGFβ, PDGFBB, FGF2, Notch and Wnt signaling. DHH was found to function as a commitment factor, inducing the transition of stem cells to the progenitor stage and thus into the Leydig cell lineage. Interestingly, PDGFBB and FGF2, the two most potent stem Leydig cell proliferation stimulators, inhibited stem Leydig cell differentiation, suggesting that these factors may play roles in controlling the balance between stem and differentiated cells. The factors that affect stem Leydig cell proliferation and differentiation are summarized in Figure 4.
Figure 4.
Proposed regulatory model of stem Leydig cell proliferation and differentiation. The positive and negative factors are followed by ‘+’ and ‘−’, respectively. DHH (red) was the most significant factor in the differentiation of SLCs. (Adapted from Li et al., 2016 with permission)
The critical role of LH in ALC steroidogenic function is well established. However, it is unlikely that LH alone could commit stem Leydig cells to the differentiation pathway because the stem cells do not express the LH receptor. We found SAG, which stimulates DHH, and lithium, to have strong differentiation effects. Consequently, we hypothesized that one or both might play an important role in the early commitment of stem cells, perhaps inducing the expression of the LH receptor. When tubules were cultured without LH for 3 weeks, no Leydig cells were formed. Similarly, lithium was not able to induce stem cell differentiation in the absence of LH. However, when tubules were incubated with DHH agonist in the absence of LH from weeks 2-3, differentiation of the stem cells into testosterone-producing cells occurred. These results suggest that DHH may be the critical commitment factor that triggers the transition of SLCs into PLCs and thus into a differentiation pathway. The expression of LH receptors by the progenitor cells makes these cells responsive to LH signaling, which is essential for the further maturation of the cells and thus testosterone production. As yet, the molecular mechanisms by which these factors act remain uncertain. Based on the results obtain from the in vitro system that we developed, and results published by others, we hypothesize that there are positive (stimulatory) and negative (inhibitory) factors that function to regulate SLCs. Normally, the balance leads to inhibition such that the SLCs remain quiescent in the adult testis. But upon loss of ALCs, the balance shifts to the stimulatory side, with increase in positive factors and/or decrease in negative factors, resulting in SLCs gaining the ability to proliferate.
4. Summary
Leydig cells are the testosterone-producing cells of the testis. In the rat, the ALC population develops from SLCs in the interstitial compartment of the neonatal testis. Four developmental stages have been identified in the development of ALCs, including transitions from SLCs to PLCs, PLCs to ILCs, and ILCs to ALCs. SLCs also are present in the adult testis, in peritubular and perivascular locations. The SLCs, which normally are quiescent, are capable of regenerating new Leydig cells upon the loss of the adult cells. The regeneration process is very similar to the development of ALCs during the pubertal period. Despite the complexity of the adult testis, progress has been made in identifying SLCs, the SLC niche, and factors that initiate SLC proliferation and differentiation. Several proteins have been identified as expressed by SLCs, including nestin, PDGFRα, COUP-TFII, CD51 and CD90. Recent studies have indicated that DHH is of particular importance in regulating the commitment of SLCs into the Leydig lineage, able to induce SLC differentiation into LH receptor-expressing and thus LH-responsive steroidogenic cells.
SLCs have been shown to be capable of giving rise to testosterone-producing Leydig cells in vivo, in locations outside testis (Chen et al., 2016). This could have clinical implications. Hypogonadism, which is common in aging men, may be linked to a number of metabolic and quality-of-life changes, including decreased lean body mass and bone mineral density, increased visceral fat, decreased libido and sexual function, altered mood, and fatigue (Carruthers, 2009; Lang et al., 2012; Morris and Channer, 2012). These changes can be partially overcome by exogenous testosterone administration (Katznelson et al., 1996; Wang et al., 1996; Steidle et al., 2003; Page et al., 2005; Seftel et al., 2015). However, there are reports that administering testosterone may increase the risks of cardiovascular disorders and prostate tumorigenesis (Klotz, 2015; Yeap et al., 2015). Thus the availability of therapies that increase serum testosterone levels more physiologically, without the need to administer testosterone, could be of benefit to the many men with primary hypogonadism. It may be possible to elevate serum testosterone levels in individuals, and thus to treat testosterone deficiency, by implanting stem cells at locations outside testis. However, the basic studies discussed above suggest that generating Leydig cells from stem cells outside the testis apparently requires critical seminiferous tubule-derived proliferation/differentiation factors. The nature and the regulation of such potential factors thus have both basic and clinical implications.
Highlights.
-
1)
Stem Leydig cells (PLC) are present in the peritubular and the perivascular locations of testis.
-
2)
SLC may be able to be identified by their expressions of nestin, PDGFRα, COUP-TFII, CD51 or CD90.
-
3)
SLC proliferation is stimulated by DHH, FGF2, PDGFBB, activin and PDGFAA, and inhibited by TGFβ.
-
4)
SLC differentiation is regulated by desert hedgehog (DHH) and lithium-induced signaling.
Acknowledgments
This work was supported by NIH grant R37 AG21092 (B.R.Z), and by National Natural Science Foundation of China grants NSFC31271252 (H.C.) and NSFC81471411 (H.C.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- Abney TO, Myers RB. 17 beta-estradiol inhibition of Leydig cell regeneration in the ethane dimethylsulfonate-treated mature rat. J Androl. 1991;12:295–304. [PubMed] [Google Scholar]
- Ariyaratne S, Kim I, Mills N, Mason I, Mendis-Handagama C. Effects of ethane dimethane sulfonate on the functional structure of the adult rat testis. Arch Androl. 2003;49:313–326. doi: 10.1080/01485010390204922. [DOI] [PubMed] [Google Scholar]
- Ariyaratne HB, Mendis-Handagama SM. Changes in the testis interstitium of Sprague Dawley rats from birth to sexual maturity. Biol Reprod. 2000;62:680–690. doi: 10.1095/biolreprod62.3.680. [DOI] [PubMed] [Google Scholar]
- Barsoum IB, Kaur J, Ge RS, Cooke PS, Yao HH. Dynamic changes in fetal Leydig cell populations influence adult Leydig cell populations in mice. FASEB J. 2013;27:2657–66. doi: 10.1096/fj.12-225060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum IB, Yao HH. Fetal Leydig cells: progenitor cell maintenance and differentiation. J Androl. 2010;31:11–5. doi: 10.2164/jandrol.109.008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basciani S, Mariani S, Arizzi M, Ulisse S, Rucci N, Jannini EA, Della Rocca C, Manicone A, Carani C, Spera G, Gnessi L. Expression of platelet-derived growth factor-A (PDGF-A), PDGF-B, and PDGF receptor-alpha and -beta during human testicular development and disease. J Clin Endocrinol Metab. 2002;87:2310–9. doi: 10.1210/jcem.87.5.8476. [DOI] [PubMed] [Google Scholar]
- Benton LX, Shan LX, Hardy MP. Differentiation of adult Leydig cells. J Steroid Biochem Mol Biol. 1995;53:61–68. doi: 10.1016/0960-0760(95)00022-r. [DOI] [PubMed] [Google Scholar]
- Bobjer J, Katrinaki M, Tsatsanis C, Lundberg GY, Giwercman A. Negative association between testosterone concentration and inflammatory markers in young men: A nested cross-sectional study. PLoS ONE. 2013;8:e61466. doi: 10.1371/journal.pone.0061466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers M. Time for international action on treating testosterone deficiency syndrome. Aging Male. 2009;12:21–28. doi: 10.1080/13685530802699067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Huhtaniemi I, Zirkin BR. Depletion and repopulation of Leydig cells in the testes of aging brown Norway rats. Endocrinology. 1996;137:3447–3452. doi: 10.1210/endo.137.8.8754773. [DOI] [PubMed] [Google Scholar]
- Chen H, Jin S, Huang S, Folmer J, Liu J, Ge R, Zirkin BR. Transplantation of alginate-encapsulated seminiferous tubules and interstitial tissue into adult rats: Leydig stem cell differentiation in vivo? Mol Cell Endocrinol. 2016;S0303-7207(16):30353–7. doi: 10.1016/j.mce.2016.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Stanley E, Jin S, Zirkin BR. Stem Leydig cells: from fetal to aged animals. Birth Defects Res C Embryo Today. 2010;90:272–283. doi: 10.1002/bdrc.20192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod. 2000;63:1825–1838. doi: 10.1095/biolreprod63.6.1825. [DOI] [PubMed] [Google Scholar]
- David T, Scadden The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Müller D. Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol. 2004;167:935–44. doi: 10.1083/jcb.200409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff MS, Middendorff R, Müller D, Holstein AF. The neuroendocrine Leydig cells and their stem cell progenitors, the pericytes. Adv Anat Embryol Cell Biol. 2009;205:1–107. [PubMed] [Google Scholar]
- Davis RB, Curtis CD, Griffin CT. BRG1 promotes COUP-TFII expression and venous specification during embryonic vascular development. Development. 2013;140:1272–81. doi: 10.1242/dev.087379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont E, Labrie F, Luu-The V, Pelletier G. Ontogeny of 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase (3 beta-HSD) in rat testis as studied by immunocytochemistry. Anat Embryol (Berl) 1993;187:583–589. doi: 10.1007/BF00214437. [DOI] [PubMed] [Google Scholar]
- Gaytan F, Aceitero J, Lucena C, Aguilar E, Pinilla L, Garnelo P, Bellido C. Simultaneous proliferation and differentiation of mast cells and Leydig cells in the rat testis. Are common regulatory factors involved? J Androl. 1992;13:387–397. [PubMed] [Google Scholar]
- Ge RS, Dong Q, Sottas CM, Papadopoulos V, Zirkin BR, Hardy MP. In search of rat stem Leydig cells: identification, isolation, and lineage-specific development. Proc Natl Acad Sci USA. 2006;103:2719–2724. doi: 10.1073/pnas.0507692103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge RS, Hardy MP. Variation in the end products of androgen biosynthesis and metabolism during postnatal differentiation of rat Leydig cells. Endocrinology. 1998;139:3787–3795. doi: 10.1210/endo.139.9.6183. [DOI] [PubMed] [Google Scholar]
- Ge RS, Hardy DO, Catterall JF, Hardy MP. Developmental changes in glucocorticoid receptor and 11beta-hydroxysteroid dehydrogenase oxidative and reductive activities in rat Leydig cells. Endocrinology. 1997;138:5089–95. doi: 10.1210/endo.138.12.5614. [DOI] [PubMed] [Google Scholar]
- Gnessi L, Emidi A, Jannini EA, Carosa E, Maroder M, Arizzi M, Ulisse S, Spera G. Testicular development involves the spatiotemporal control of PDGFs and PDGF receptors gene expression and action. J Cell Biol. 1995;131:1105–21. doi: 10.1083/jcb.131.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondo S, Okabe T, Tanaka T, et al. Adipose tissue-derived and bone marrow-derived mesenchymal cells develop into different lineage of steroidogenic cells by forced expression of steroidogenic factor 1. Endocrinology. 2008;149:4717–4725. doi: 10.1210/en.2007-1808. [DOI] [PubMed] [Google Scholar]
- Haider SG, Laue D, Schwochau G, Hilscher B. Morphological studies on the origin of adult-type Leydig cells in rat testis. Ital J Anat Embryol. 1995;100:535–41. [PubMed] [Google Scholar]
- Haider SG, Passia D, Overmeyer G. Studies on the fetal and postnatal development of rat Leydig cells employing 3 β-hydroxysteroid dehydrogenase activity. Acta Histochem Suppl. 1986;32:197–202. [PubMed] [Google Scholar]
- Haider SG, Servos G. Ultracytochemistry of 3beta-hydroxysteroid dehydrogenase in Leydig cell precursors and vascular endothelial cells of the postnatal rat testis. Anat Embryol (Berl) 1998;198:101–110. doi: 10.1007/s004290050168. [DOI] [PubMed] [Google Scholar]
- Hardy MP, Zirkin BR, Ewing LL. Kinetic studies on the development of the adult population of Leydig cells in testes of the pubertal rat. Endocrinology. 1989;124:762–770. doi: 10.1210/endo-124-2-762. [DOI] [PubMed] [Google Scholar]
- Hatano O, Takakusu A, Nomura M, Morohashi K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells. 1996;1:663–71. doi: 10.1046/j.1365-2443.1996.00254.x. [DOI] [PubMed] [Google Scholar]
- Huang CC, Yao HH. Diverse functions of Hedgehog signaling in formation and physiology of steroidogenic organs. Mol. Reprod. Dev. 2010;77:489–96. doi: 10.1002/mrd.21174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtaniemi I. Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl. 2014;16:192–202. doi: 10.4103/1008-682X.122336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inano H, Tamaoki BI. Bioconversion of steroids in immature rat testes in vitro. Endocrinology. 1966;79:579–590. doi: 10.1210/endo-79-3-579. [DOI] [PubMed] [Google Scholar]
- Jackson AE, O'Leary PC, Ayers MM, de Kretser DM. The effects of ethylene dimethane sulphonate (EDS) on rat Leydig cells: evidence to support a connective tissue origin of Leydig cells. Biol Reprod. 1986;35:425–437. doi: 10.1095/biolreprod35.2.425. [DOI] [PubMed] [Google Scholar]
- Jiang MH, Cai B, Tuo Y, Wang J, Zang ZJ, Tu X, Gao Y, Su Z, Li W, Li G, Zhang M, Jiao J, Wan Z, Deng C, Lahn BT, Xiang AP. Characterization of Nestin-positive stem Leydig cells as a potential source for the treatment of testicular Leydig cell dysfunction. Cell Res. 2014;24:1466–1485. doi: 10.1038/cr.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Esaki M, Matsuzawa A, Ikeda Y. NR5A1 is required for functional maturation of Sertoli cells during postnatal development. Reproduction. 2012;143:663–72. doi: 10.1530/REP-11-0365. [DOI] [PubMed] [Google Scholar]
- Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81:4358–4365. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- Keeney DS, Mendis-Handagama SM, Zirkin BR, Ewing LL. Effect of long term deprivation of luteinizing hormone on Leydig cell volume, Leydig cell number, and steroidogenic capacity of the rat testis. Endocrinology. 1988;123:2906–2915. doi: 10.1210/endo-123-6-2906. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Bartlett JM, Donachie K, Sharpe RM. Origin of regenerating Leydig cells in the testis of the adult rat. An ultrastructural, morphometric and hormonal assay study. Cell Tissue Res. 1987;249:367–377. doi: 10.1007/BF00215521. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Donachie K. Regeneration of Leydig cells in unilaterally cryptorchid rats: evidence for stimulation by local testicular factors. Cell Tissue Res. 1986;245:649–655. doi: 10.1007/BF00218568. [DOI] [PubMed] [Google Scholar]
- Kilcoyne KR, Smith LB, Atanassova N, Macpherson S, McKinnell C, van den Driesche S, Jobling MS, Chambers TJ, De Gendt K, Verhoeven G, O'Hara L, Platts S, Renato de Franca L, Lara NL, Anderson RA, Sharpe RM. Fetal programming of adult Leydig cell function by androgenic effects on stem/progenitor cells. Proc Natl Acad Sci USA. 2014;111:E1924–1932. doi: 10.1073/pnas.1320735111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L. Testosterone therapy and prostate cancer–safety concerns are well founded. Nat Rev Urol. 2015;12:48–54. doi: 10.1038/nrurol.2014.338. [DOI] [PubMed] [Google Scholar]
- Koskimies P, Levallet J, Sipilä P, Huhtaniemi I, Poutanen M. Murine relaxin-like factor promoter: functional characterization and regulation by transcription factors steroidogenic factor 1 and DAX-1. Endocrinology. 2002;143:909–19. doi: 10.1210/endo.143.3.8683. [DOI] [PubMed] [Google Scholar]
- Landreh L, Spinnler K, Schubert K, Häkkinen MR, Auriola S, Poutanen M, Söder O, Svechnikov K, Mayerhofer A. Human testicular peritubular cells host putative stem Leydig cells with steroidogenic capacity. J Clin Endocrinol Metab. 2014;99:E1227–35. doi: 10.1210/jc.2013-4199. [DOI] [PubMed] [Google Scholar]
- Landreh L, Stukenborg JB, Söder O, Svechnikov K. Phenotype and steroidogenic potential of PDGFRα-positive rat neonatal peritubular cells. Mol Cell Endocrinol. 2013;372:96–104. doi: 10.1016/j.mce.2013.03.019. [DOI] [PubMed] [Google Scholar]
- Lang PO, Samaras D, Samaras N. Testosterone replacement therapy in reversing “andropause”: what is the proof-of-principle? Rejuvenation Res. 2012;15:453–465. doi: 10.1089/rej.2012.1316. [DOI] [PubMed] [Google Scholar]
- Lue Y, Erkkila K, Liu PY, Ma K, Wang C, Hikim AS, Swerdloff RS. Fate of bone marrow stem cells transplanted into the testis: potential implication for men with testicular failure. Am J Pathol. 2007;2007;170:899–908. doi: 10.2353/ajpath.2007.060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G, Hoffman RM. Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci U S A. 2003;100:9958–61. doi: 10.1073/pnas.1733025100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang Z, Jiang Z, Guo J, Zhang Y, Li C, Chung J, Folmer J, Liu J, Lian Q, Ge R, Zirkin BR, Chen H. Regulation of seminiferous tubule-associated stem Leydig cells in adult rat testes. Proc Natl Acad Sci U S A. 2016;113:2666–71. doi: 10.1073/pnas.1519395113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xie T. Stem Cell Niche: Structure and Function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Liu K, Wang Z, Wang H, Zhang Y. Nestin expression and proliferation of ependymal cells in adult rat spinal cord after injury. Chin Med J (Engl) 2002;115:339–41. [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–90. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- Martin LJ. Cell Interactions and Genetic Regulation That Contribute to Testicular Leydig Cell Development and Differentiation. Mol Reprod Dev. 2016;83:470–87. doi: 10.1002/mrd.22648. [DOI] [PubMed] [Google Scholar]
- McHenry MC. Testosterone deficiency in older men: a problem worth treating. Consult Pharm. 2012;27:152–163. doi: 10.4140/TCP.n.2012.152. [DOI] [PubMed] [Google Scholar]
- Mendis-Handagama SM, Ariyaratne HBC. Differentiation of the adult Leydig cell population in the postnatal testis. Biol Reprod. 2001;65:660–671. doi: 10.1095/biolreprod65.3.660. [DOI] [PubMed] [Google Scholar]
- Messam CA, Hou J, Major EO. Coexpression of nestin in neural and glial cells in the developing human CNS defined by a human-specific anti-nestin antibody. Exp Neurol. 2000;161:585–96. doi: 10.1006/exnr.1999.7319. [DOI] [PubMed] [Google Scholar]
- Miyano M, Ito Y, Fujihira S, Matsuo T, Ueno H, Mori H. Restoration of Leydig cells after repeated administration of ethane dimethanesulfonate in adult rats. Pathol Int. 1997;47:478–488. doi: 10.1111/j.1440-1827.1997.tb04527.x. [DOI] [PubMed] [Google Scholar]
- Molenaar R, de Rooij DG, Rommerts FF, van der Molen HJ. Repopulation of Leydig cells in mature rats after selective destruction of the existent Leydig cells with ethylene dimethane sulfonate is dependent on luteinizing hormone and not follicle-stimulating hormone. Endocrinology. 1986;118:2546–2554. doi: 10.1210/endo-118-6-2546. [DOI] [PubMed] [Google Scholar]
- Morris PD, Channer KS. Testosterone and cardiovascular disease in men. Asian J Androl. 2012;14:428–435. doi: 10.1038/aja.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murono EP. Maturational changes in steroidogenic enzyme activities metabolizing testosterone and dihydrotestosterone in two populations of testicular interstitial cells. Acta Endocrinol. 1989;121:477–483. doi: 10.1530/acta.0.1210477. [DOI] [PubMed] [Google Scholar]
- Myers RB, Abney TO. Interstitial cell proliferation in the testis of the ethylene dimethane sulfonate-treated rat. Steroids. 1991;56:91–96. doi: 10.1016/0039-128x(91)90130-n. [DOI] [PubMed] [Google Scholar]
- Nef S, Parada LF. Hormones in male sexual development. Genes Dev. 2000;14:3075–3086. doi: 10.1101/gad.843800. [DOI] [PubMed] [Google Scholar]
- Neumann A, Haider SG, Hilscher B. Temporal coincidence of the appearance of elongated spermatids and of histochemical reaction of 11 beta-hydroxysteroid dehydrogenase in rat Leydig cells. Andrologia. 1993;25:263–9. doi: 10.1111/j.1439-0272.1993.tb02723.x. [DOI] [PubMed] [Google Scholar]
- Odeh HM, Kleinguetl C, Ge R, Zirkin BR, Chen H. Regulation of the proliferation and differentiation of Leydig stem cells in the adult testis. Biol Reprod. 2014;90:123. doi: 10.1095/biolreprod.114.117473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary P, Jackson AE, Averill S, de Kretser DM. The effects of ethane dimethane sulphonate (EDS) on bilaterally cryptorchid rat testes. Mol Cell Endocrinol. 1986;45:183–90. doi: 10.1016/0303-7207(86)90146-2. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Morris ID, Baker PJ. Leydig cell re-generation and expression of cell signaling molecules in the germ cell-free testis. Reproduction. 2008;135:851–858. doi: 10.1530/REP-07-0529. [DOI] [PubMed] [Google Scholar]
- Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502–1510. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- Park SY, Tong M, Jameson JL. Distinct roles for steroidogenic factor 1 and desert hedgehog pathways in fetal and adult Leydig cell development. Endocrinology. 2007;148:3704–3710. doi: 10.1210/en.2006-1731. [DOI] [PubMed] [Google Scholar]
- Qin J, Tsai MJ, Tsai SY. Essential roles of COUP-TFII in Leydig cell differentiation and male fertility. PLoS One. 2008;3:e3285. doi: 10.1371/journal.pone.0003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci G, Guglielmo MC, Caruso M, Ferranti F, Canipari R, Galdieri M, Catizone A. Hepatocyte growth factor is a mouse fetal Leydig cell terminal differentiation factor. Biol Reprod. 2012;87:146. doi: 10.1095/biolreprod.112.104638. [DOI] [PubMed] [Google Scholar]
- Russell LD, de França LR, Hess R, Cooke P. Characteristics of mitotic cells in developing and adult testes with observations on cell lineages. Tissue Cell. 1995;27:105–128. doi: 10.1016/s0040-8166(95)80015-8. [DOI] [PubMed] [Google Scholar]
- Seftel AD, Kathrins M, Niederberger C. Critical update of the 2010 endocrine society clinical practice guidelines for male hypogonadism: a systematic analysis. Mayo Clin Proc. 2015;90:1104–1115. doi: 10.1016/j.mayocp.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Sejersen T, Lendahl U. Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci. 1993;106:1291–300. doi: 10.1242/jcs.106.4.1291. [DOI] [PubMed] [Google Scholar]
- Shan L, Hardy DO, Catterall JF, Hardy MP. Effects of luteinizing hormone (LH) and androgen on steady state levels of messenger ribonucleic acid for LH receptors, androgen receptors, and steroidogenic enzymes in rat Leydig cell progenitors in vivo. Endocrinology. 1995;136:1686–1693. doi: 10.1210/endo.136.4.7895679. [DOI] [PubMed] [Google Scholar]
- Shan LX, Phillips DM, Bardin CW, Hardy MP. Differential regulation of steroidogenic enzymes during differentiation optimizes testosterone production by adult rat Leydig cells. Endocrinology. 1993;133:2277–2283. doi: 10.1210/endo.133.5.8404681. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Maddocks S, Kerr JB. Cell-cell interactions in the control of spermatogenesis as studied using Leydig cell destruction and testosterone replacement. Am J Anat. 1990;188:3–20. doi: 10.1002/aja.1001880103. [DOI] [PubMed] [Google Scholar]
- Siril Ariyaratne HB, Chamindrani Mendis-Handagama S, Buchanan Hales D, Ian Mason J. Studies on the onset of Leydig precursor cell differentiation in the prepubertal rat testis. Biol Reprod. 2000;63:165–171. doi: 10.1095/biolreprod63.1.165. [DOI] [PubMed] [Google Scholar]
- Smith LB, Walker WH. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol. 2014;30:2–13. doi: 10.1016/j.semcdb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PJ. Might testosterone actually reduce mortality? J Clin Endocrinol Metab. 2008;93:32–33. doi: 10.1210/jc.2007-2506. [DOI] [PubMed] [Google Scholar]
- Stanley E, Lin CY, Jin S, Liu J, Sottas CM, Ge R, Zirkin BR, Chen H. Identification, proliferation, and differentiation of adult Leydig stem cells. Endocrinology. 2012;153:5002–10. doi: 10.1210/en.2012-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E, Johnston DS, Fan J, Papadopoulos V, Chen H, Ge RS, Zirkin BR, Jelinsky SA. Stem Leydig cell differentiation: gene expression during development of the adult rat population of Leydig cells. Biol Reprod. 2011;85:1161–1166. doi: 10.1095/biolreprod.111.091850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, Bachand R. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88:2673–2681. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]
- Steinberger E, Ficher M. Differentiation of steroid biosynthetic pathways in developing testes. Biol Reprod. 1969;1:119–133. doi: 10.1095/biolreprod1.supplement_1.119. [DOI] [PubMed] [Google Scholar]
- Szczepny A, Hime GR, Loveland KL. Expression of hedgehog signalling components in adult mouse testis. Dev. Dyn. 2006;235:3063–70. doi: 10.1002/dvdy.20931. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, de Rooij DG, Rommerts FF, Wensing CJ. Development of a new Leydig cell population after the destruction of existing Leydig cells by ethane dimethane sulphonate in rats: an autoradiographic study. J Endocrinol. 1990;126:229–236. doi: 10.1677/joe.0.1260229. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, Rijntjes E. Dynamics of Leydig cell regeneration after EDS, a model for postnatal Leydig cell development. In: Payne AH, Hardy MP, editors. The Leydig cell in health and disease. Humana Press Inc.; Totowa, NJ: 2007. pp. 91–115. [Google Scholar]
- Teerds KJ, De Rooij DG, Rommerts FF, van der Tweel I, Wensing CJ. Turnover time of Leydig cells and other interstitial cells in testes of adult rats. Arch Androl. 1989;23:105–111. doi: 10.3109/01485018908986831. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, Huhtaniemi IT. Morphological and functional maturation of Leydig cells: from rodent models to primates. Hum Reprod Update. 2015;21:310–28. doi: 10.1093/humupd/dmv008. [DOI] [PubMed] [Google Scholar]
- Underhill GH, Bhatia SN. High-throughput analysis of signals regulating stem cell fate and function. Curr Opin Chem Biol. 2007;11:357–366. doi: 10.1016/j.cbpa.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Val P, Lefrançois-Martinez AM, Veyssière G, Martinez A. SF-1 a key player in the development and differentiation of steroidogenic tissues. Nucl Recept. 2003;1:8, 1–23. doi: 10.1186/1478-1336-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Eyre DR, Clark R, Kleinberg D, Newman C, Iranmanesh A, Veldhuis J, Dudley RE, Berman N, Davidson T, Barstow TJ, Sinow R, Alexander G, Swerdloff RS. Sublingual testosterone replacement improves muscle mass and strength, decreases bone resorption, and increases bone formation markers in hypogonadal men–a clinical research center study. Clin Endocrinol Metab. 1996;81:3654–3662. doi: 10.1210/jcem.81.10.8855818. [DOI] [PubMed] [Google Scholar]
- Wu SP, Yu CT, Tsai SY, Tsai MJ. Choose your destiny: Make a cell fate decision with COUP-TFII. J Steroid Biochem Mol Biol. 2016;157:7–12. doi: 10.1016/j.jsbmb.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Qin J, Lin SH, Tsai SY, Tsai MJ. Nuclear receptor chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) modulates mesenchymal cell commitment and differentiation. Proc Natl Acad Sci U S A. 2011;108:14843–8. doi: 10.1073/pnas.1110236108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Tang K, Yu CT, Tsai SY, Tsai MJ. Regulatory potential of COUP-TFs in development: stem/progenitor cells. Semin Cell Dev Biol. 2013;24:687–93. doi: 10.1016/j.semcdb.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Kero J, Huhtaniemi I, Toppari J. Stem cell factor functions as a survival factor for mature Leydig cells and a growth factor for precursor Leydig cells after ethylene dimethane sulfonate treatment: implication of a role of the stem cell factor/c-Kit system in Leydig cell development. Dev Biol. 2000;227:169–182. doi: 10.1006/dbio.2000.9885. [DOI] [PubMed] [Google Scholar]
- Yang Y, Su Z, Xu W, Luo J, Liang R, Xiang Q, Zhang Q, Ge RS, Huang Y. Directed mouse embryonic stem cells into leydig-like cells rescue testosterone-deficient male rats in vivo. Stem Cells Dev. 2015;24:459–70. doi: 10.1089/scd.2014.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa T, Mizutani T, Yamada K, et al. Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinology. 2006;147:4104–4111. doi: 10.1210/en.2006-0162. [DOI] [PubMed] [Google Scholar]
- Yazawa T, Inanoka Y, Mizutani T, et al. Liver receptor homolog-1 regulates the transcription of steroidogenic enzymes and induces the differentiation of mesenchymal stem cells into steroidogenic cells. Endocrinology. 2009;150:3885–3893. doi: 10.1210/en.2008-1310. [DOI] [PubMed] [Google Scholar]
- Yeap BB. Testosterone and cardiovascular disease risk. Curr Opin Endocrinol Diabetes Obes. 2015;22:193–202. doi: 10.1097/MED.0000000000000161. [DOI] [PubMed] [Google Scholar]
- Zhai J, Lanclos KD, Abney TO. Estrogen receptor messenger ribonucleic acid changes during Leydig cell development. Biol Reprod. 1996;55:782–788. doi: 10.1095/biolreprod55.4.782. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen M, Wen Q, Li Y, Wang Y, Wang Y, Qin Y, Cui X, Yang L, Huff V, Gao F. Reprogramming of Sertoli cells to fetal-like Leydig cells by Wt1 ablation. Proc Natl Acad Sci U S A. 2015;112:4003–4008. doi: 10.1073/pnas.1422371112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang H, Yang Y, Liu H, Zhang Q, Xiang Q, Ge R, Su Z, Huang Y. NGF induces adult stem Leydig cells to proliferate and differentiate during Leydig cell regeneration. Biochem Biophys Res Commun. 2013;436:300–305. doi: 10.1016/j.bbrc.2013.05.098. [DOI] [PubMed] [Google Scholar]
- Zirkin BR, Ewing LL. Leydig cell differentiation during maturation of the rat testis: a stereological study of cell number and ultrastructure. Anat Rec. 1987;219:157–163. doi: 10.1002/ar.1092190208. [DOI] [PubMed] [Google Scholar]
- Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Müller B, Vallejo M, Thomas MK, Habener JF. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521–33. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]