Abstract
Background
Phosphorylation of the cardiac ryanodine receptor (RyR2) phospho-site S2808 has been touted by the Marks group as a hallmark of heart failure (HF) and a critical mediator of the physiological fight-or-flight response of the heart. In support of this hypothesis, mice unable to undergo phosphorylation at RyR2-S2808 (S2808A) were significantly protected against HF and displayed a blunted response to adrenergic stimulation. However, the issue remains highly controversial because several groups have been unable to reproduce these findings. An important variable not considered before is the genetic background of the mice used to obtain the divergent results.
Methods and results
We backcrossed a RyR2-S2808A mouse into a congenic C57Bl/6J strain, the same strain used by the Marks group to conduct their experiments. We then performed several key experiments to confirm or discard the genetic background of the mouse as a relevant variable, including induction of HF by myocardial infarction and tests of integrity of adrenergic response. Congenic C57Bl/6J harboring the S2808A mutation showed similar echocardiographic parameters that indicated identical progression towards HF compared to wild type controls, and had a normal response to adrenergic stimulation in whole animal and cellular experiments.
Conclusions
The genetic background of the different mouse models is unlikely to be the source of the divergent results obtained by the Marks group in comparison to several other groups. Cardiac adrenergic response and progression towards HF proceed unaltered in mice harboring the RyR2-S2808A mutation. Preventing RyR2-S2808 phosphorylation does not preclude a normal sympathetic response nor mitigates the pathophysiological consequences of MI.
Keywords: ryanodine receptor, phosphorylation, heart failure, myocardial infarction, adrenergic stimulation
1. Introduction
Heart failure (HF) is a complex, multi-factorial syndrome characterized by incapacity of the heart to meet metabolic demands, and is clinically manifested by dyspnea, fatigue, limited exercise capacity and fluid retention.1 Although the underlying mechanisms of HF are multi-varied and complex, dysregulation of intracellular calcium (Ca2+) handling is a defining feature that is thought to be involved in the most prominent pathophysiological observations: structural remodeling of the heart, impaired cardiac contractility, and increased propensity to arrhythmia.2–4
Cardiac contraction requires Ca2+ cycling for excitation-contraction (E-C) coupling, the process transducing electrical signals into mechanical force. A normal contraction occurs when ryanodine receptors (RyR2) release Ca2+ from the sarcoplasmic reticulum (SR) upon activation by a small inward Ca2+ current (ICaL) mediated by L-type Ca2+ channels. Relaxation then ensues from the removal of Ca2+ from the cytosol by the SR Ca pump (SERCA2a) and the Na+/Ca2+ exchanger (NCX). In HF, the abundance, phosphorylation levels and activity of several E-C coupling proteins and their regulatory partners are altered.5,6 In 2000, Marks et al communicated that in HF, RyR2 is hyperphosphorylated at its S2808 phospho-site, which triggers dissociation of the regulatory protein FKBP12.6 and renders the channel hyperactive by inducing long-lasting sub-conductance states.7 In subsequent papers, they provided compelling evidence that S2808 is the only PKA phosphorylation site in RyR27 and that ablation of this site in mice (RyR2-S2808A) blunts the normal cardiac adrenergic response8 and improves the outcome of ischemic HF.9 Thus, Marks et al assigned the Ca2+ mishandling characteristic of HF on RyR2-S2808 hyperphosphorylation and subsequent dissociation of FKBP12.6. However, several laboratories have failed to reproduce key elements of this hypothesis.10,11 Our laboratory independently generated a RyR2-S2808A mouse model and observed a normal progression to HF following transverse aortic constriction.12 Later, Houser’s group reported, using the same mice, normal adrenergic response13 and unaltered progression to HF following myocardial infarction (MI).14
Although the discrepancies between these two mouse models are striking, the underlying causes have not been explored yet. One of the most patent differences between both RyR2-S2808A models is the genetic background: the studies by the Marks laboratory were conducted in C57Bl6 background, while the Valdivia and Houser laboratories used a mixed Sv129/C57Bl6 background.9,12 This variable is relevant, since important phenotypic differences have emerged from identical genetic alterations in various mouse strains. Background genes from the parental strains may interact with the mutated gene, in a manner which could severely compromise the interpretation of the mutant phenotype. In this study, we explored the effect of the genetic background as source of these divergent results. We backcrossed Valdivia’s mouse into a congenic C57Bl/6J strain and followed an experimental approach similar to previous reports using these models.12–14 We found that RyR2-S2808A mice in the C57Bl/6J genetic background (1) have a normal response to adrenergic stimulation at the whole animal and cellular levels, and (2) show identical progression towards HF compared to wild type controls up to four weeks after MI. Therefore, it is unlikely that the genetic background of the mice may be the source of the diametrically opposite results obtained by the Marks and Valdivia/Houser groups. These data support the notion that phosphorylation of S2808 is unlikely to be essential for the cardiac adrenergic response and is perhaps irrelevant in HF progression.
2. Methods
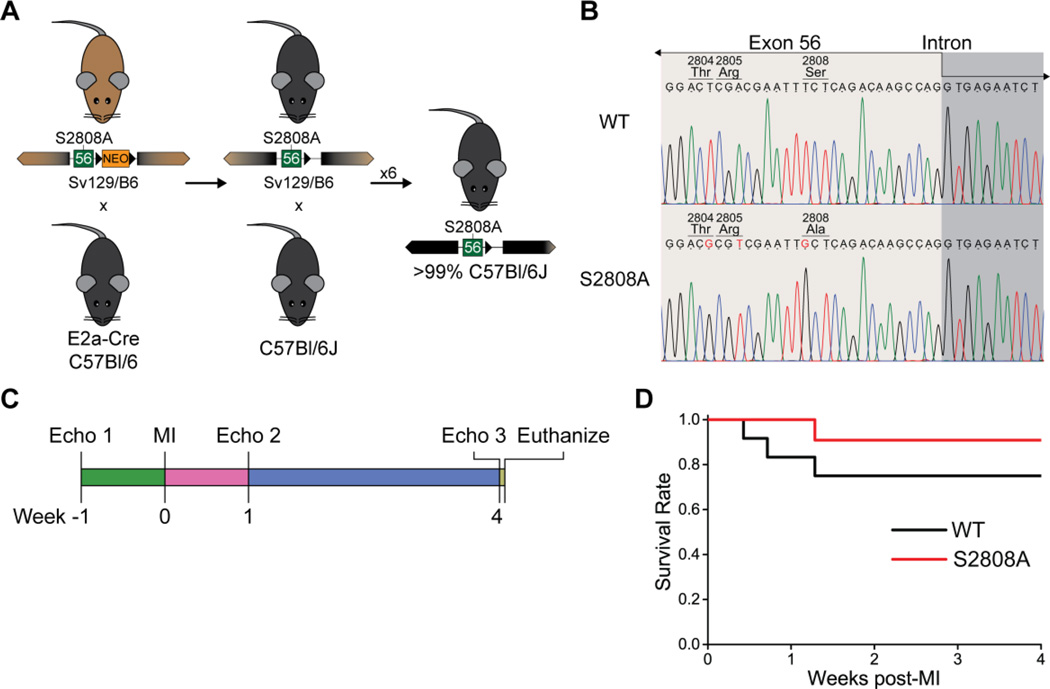
All animal experiments were approved by the University of Michigan Institutional Animal Care and Use Committee. All experiments performed at institutional cores were single blind. The methods used to create the RyR2-S2808A mouse model have been previously published.12 A congenic RyR2-S2808A strain in the C57Bl/6 background was developed as follows (figure 1A): homozygous RyR2-S2808A mice in a mixed Sv129/C57Bl6 background were mated with EIIa-Cre transgenic mice in the C57Bl/6 background, to excise the NEO-resistance cassette. The offspring was backcrossed for 7 additional generations with C57Bl/6J mice to obtain a mouse model with over 99% C57Bl/6 genetic background.15 The new congenic RyR2-S2808A colony was then maintained by breeding homozygotes. Age- and gender-matched C57Bl/6J mice were used as wild type controls.
Figure 1. Experimental model and mouse survival.
A. Backcrossing scheme used to produce a congenic mouse strain derived from the RyR2-S2808A mouse developed in our laboratory. B. Direct sequencing of a portion of exon 55 confirms the presence of the missense mutation in codon 2808 leading to the serine to alanine substitution. Two silent mutations are located in codons 2804 and 2805. C. General scheme used to assess cardiac function in mice undergoing myocardial infarction. In this model, each mouse acts as its own control at basal conditions. D. Kaplan-Meier plot of survival after MI induction. Survival was slightly higher in S2808A than in WT controls, but this difference is not significant (n = 11–12 per genotype, p = 0.298).
A detailed Methods section is available in the Online Supplementary Material.
3. Results
3.1. Direct sequencing of RyR2
For this work, we derived a congenic mouse line in the C57Bl/6J genetic background from the RyR2-S2808A mice generated in our laboratory and first described by Benkusky et al.12 This model has been thoroughly characterized by our laboratory12 and several collaborators.13,14,16,17 All these reports have shown, using Western blots, the absence of S2808 phosphorylation in homozygous mice. However, changes in neighboring amino acids that alter the antibody epitope can produce a similar result. Therefore, we sequenced the genomic region containing the underlying mutation to validate the model from the genetic perspective.
This mouse was created using homologous recombination with a targeting vector containing exons 54–56, and a floxed Neomycin resistance cassette within an intronic region. We amplified and sequenced the region of exon 56 containing the codon of interest, together with a fragment of the downstream intron. Three mutations were identified in exon 56 of S2808A mice (figure 1B): two of them were silent mutations in codons 2804 and 2805; the third was the missense mutation that changed codon 2808 from encoding a serine to alanine substitution. Thus, we confirm that the genetic mutation is at the expected location within Ryr2, and the surrounding exon encodes for the same amino acid sequence as the WT gene (figure S1). The intronic region downstream of exon 56 contains a short deletion within a non-complexity region (dominated by C and A) and an insertion from the targeting vector containing the remaining loxP site from the Cre-mediated deletion of the NEO resistance cassette (figure S1).
3.2. Analysis of cardiac function and structure after myocardial infarction
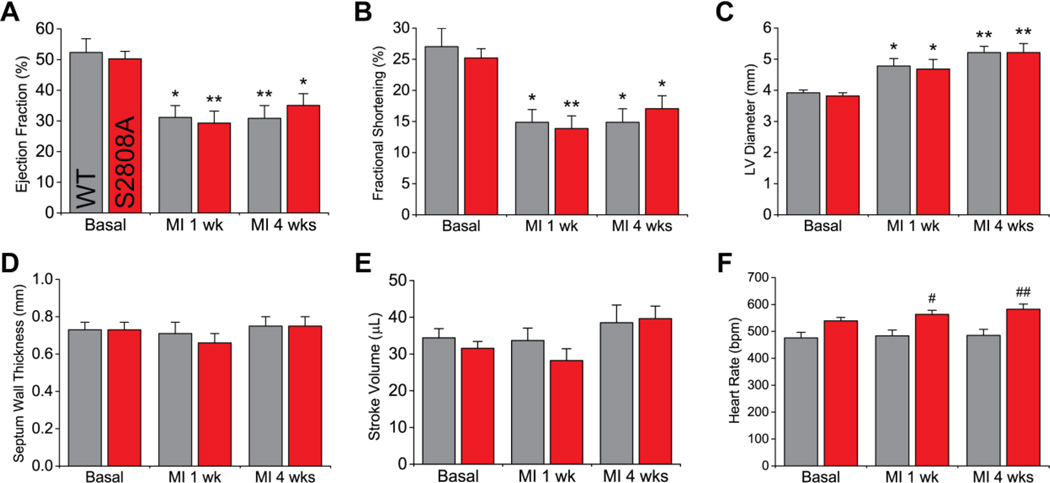
We utilized a similar approach to that used by Wehrens et al.9 and Zhang et al.14 to assess cardiac function after MI in RyR2-S2808A mice. Animals were followed by echocardiography up to 4 weeks after the induction of myocardial infarction by LAD ligation (figure 1C). During this period, survival was not statistically different (p = 0.298) between WT and S2808A mice (9 out of 12, and 10 out of 11, respectively, figure 1D). Figure 2 and table S1 summarize the echocardiographic parameters measured in WT and S2808A before, and one and four weeks after MI. Consistent with previous reports,12–14 we did not observe structural or functional differences between WT and S2808A mice at basal level. Following MI, both groups showed equivalent deteriorating cardiac function that involved a significant decrease in ejection fraction (figure 2A) and fractional shortening (figure 2B), and an increase in LV diameter (figure 2C). The interventricular septum wall thickness (figure 2D) and stroke volume (figure 2E) remained unchanged in both groups. Once established, all deteriorating parameters remained unchanged up until 4 weeks post-MI, with no significant difference between WT and S2808A. This indicates that both groups have a similar progression to HF. Heart rate, on the other hand, was statistically higher in S2808A mice 4 weeks post-MI: WT 485±22 vs. S2808A 582±19 bpm, p = 0.009) (figure 2F). Interestingly, heart rate in S2808A mice appeared to be part of a continuum of processes that was made patent by MI, because it tended to be higher at baseline (WT 476±21 vs. S2808A 539±13 bpm, p = 0.175) and reached statistical significance 1 week post-MI (WT 484±21 vs. S2808A 563±15 bpm, p = 0.047, see Discussion). Finally, cardiac output (table S1) was not statistically different between WT and S2808A mice 4 weeks post-MI (WT 18.2±1.9 vs. S2808A 23.0±2.1 mL/min, p = 0.337), likely reflecting variability in stroke volume (figure 2E).
Figure 2. Cardiac function after MI.
MI due to permanent LAD ligation produced a significant decrease in ejection fraction (A) and fractional shortening (B), and an increase in LV diameter in diastole (C). Septum wall thickness in diastole (E) and stroke volume (E) remained unaltered. Most parameters, measured at one and four weeks after MI, were comparable between S2808A and WT mice, except for heart rate (F) (n = 9 per genotype; * p < 0.05, ** p < 0.01 vs. same genotype basal; # p < 0.05, ## p < 0.01 vs. WT at same time-point).
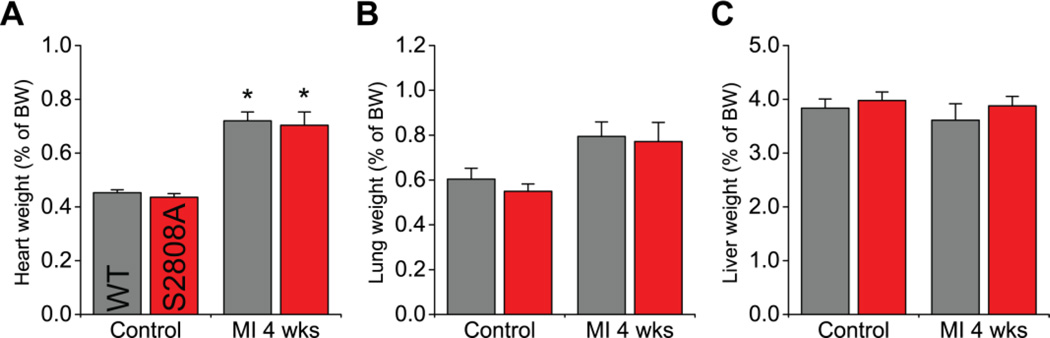
The structural observations described above were accompanied by a significant and comparable increase of heart weight in both groups (figure 3A). Also, both WT and S2808A mice had a similar, non-significant tendency for increased lung weight after MI (figure 3B). Finally, liver weight was not significantly different at any time point (figure 3C). Overall, then, preventing S2808 phosphorylation does not impact the most salient cardiac parameters under basal conditions, nor does it affect their progression towards heart failure after MI.
Figure 3. Heart remodeling after MI.
Heart weight (A), lung weight (B) and liver weight (C) as a percentage of body weight in control mice and 4 weeks after LAD ligation. Only the heart weight was increased in both groups after MI (n = 7–9 per genotype per group; * p < 0.05 vs same genotype control).
3.3. Expression and phosphorylation of E-C coupling proteins
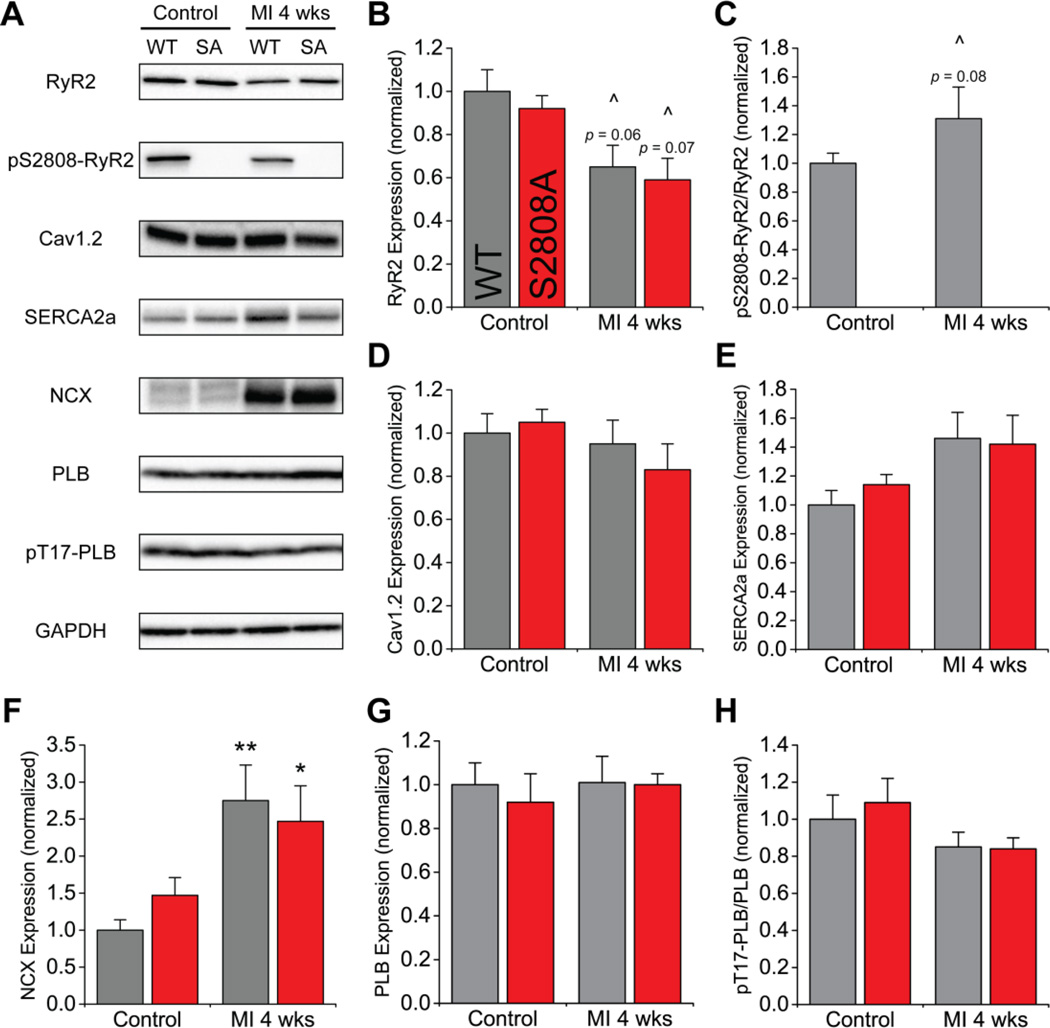
The E-C coupling apparatus relies on a well-organized network of proteins that becomes disrupted during HF. Thus, we measured the abundance and phosphorylation of the major E-C coupling proteins using Western blots. The representative blots and summary plots are shown in figure 4. We observed a similar, non-significant tendency for decreased RyR2 expression in both groups after MI (figure 4B). As expected, we did not observe phosphorylation of S2808 in S2808A mice. WT hearts, on the other hand, had significant basal S2808 phosphorylation and showed a tendency for increased phosphorylation after MI (figure 4C). Among the other E-C coupling proteins studied, we only detected a significant increase in NCX expression after MI in both groups (figure 4F), consistent with other models of HF. However, there were no differences between WT and S2808A in the abundance and phosphorylation of the proteins analyzed, suggesting both mice undergo similar remodeling of the E-C coupling apparatus after MI, and that S2808A does not prevent such changes.
Figure 4. Excitation-contraction coupling protein expression and phosphorylation.
A. Representative blots. B. Expression of RyR2 has a tendency to be decreased 4 weeks after MI. C. Phosphorylation level of RyR2-S2808 determined as a ratio between phosphorylated and total protein. Phosphorylation was absent in S2808A mice, and has a tendency to be increased in wild-type animals. D–G. Expression level of excitation-contraction coupling proteins Cav1.2, SERCA2a, NCX and PLB. Expression of NCX was nearly 2.5-fold increased after MI. H. Phosphorylation level of PLB determined as described above (n = 6–8 per genotype per group; * p < 0.05, ** p < 0.01 vs. same genotype control; ^ tendency with p < 0.1 vs. same group control).
3.4. Cardiac response to adrenergic stimulation
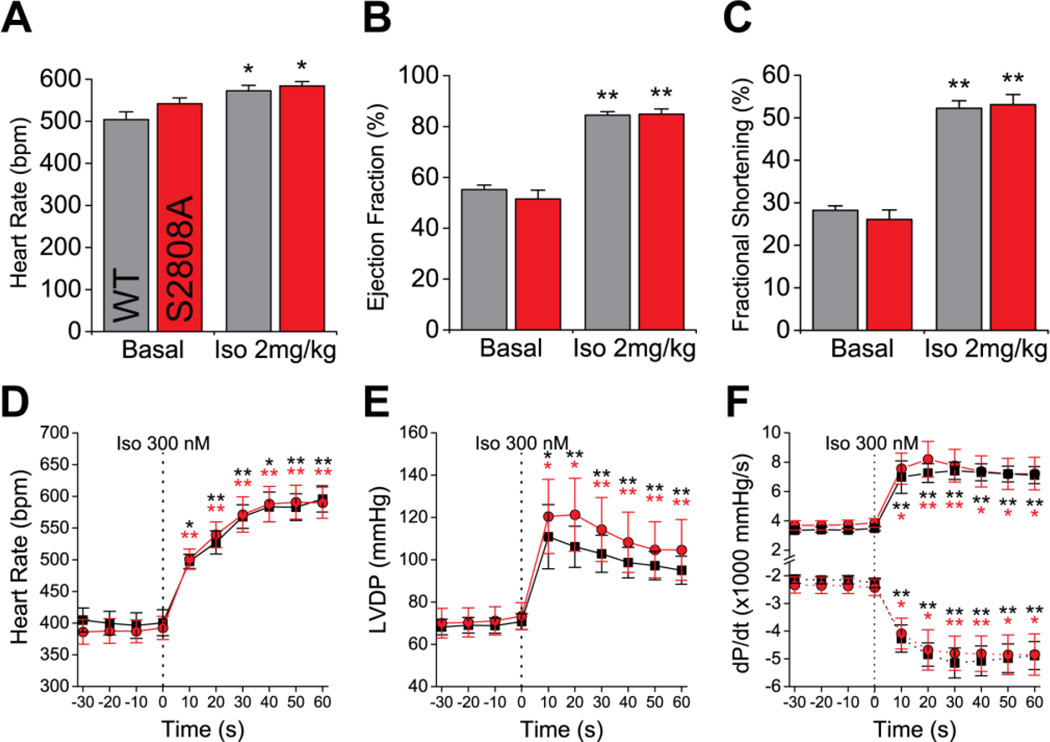
To assess the response of RyR2-S2808A mice to acute adrenergic stimulation, we used echocardiography and analyzed the cardiac function before and after i.p. injection of 2 mg/kg of Isoproterenol (Iso). Consistent with the data shown in figure 2, there were no differences in the basal cardiac function between WT and S2808A mice. Following Iso injection, we observed a robust chronotropic and inotropic response in both groups (figures 5 and S2, table S2), with a significant increase in HR (figure 5A), ejection fraction (EF, figure 5B) and fractional shortening (figure 5C). Interestingly, the stroke volume remained unchanged after Iso (table S2). This was most likely due to insufficient ventricle refilling time, since we also observed a significant decrease of LV diameter and volume in diastole (figure S2A–B). We did not observe differences between WT and S2808A mice injected with Iso. To complement these in vivo results, we used Langendorff perfusions of isolated hearts to assess the adrenergic response in the absence of neurohormonal and hemodynamic regulation (figures 5, table S3). Iso produced a significant increase in HR (figure 5D), LV-developed pressure (LVDP, figure 5E) and dP/dt (figure 5F). Yet again, none of the parameters were different between WT and S2808A mice. Taken together, these data suggest that this phosphorylation site is not essential for the normal adrenergic response.
Figure 5. Cardiac adrenergic response.
A–C. IP injection of isoproterenol (2 mg/kg) in anesthetized mice produces a significant increase in cardiac function, including heart rate (A), ejection fraction (B) and fractional shortening (C) (n = 6 per genotype; * p < 0.05, ** p < 0.01 vs. same genotype basal). D–E. Langendorff-perfused hearts stimulated with 300 nM Iso show a significant increase in heart rate (C), LV-developed pressure (LVPD, D) and dP/dt (E) (n = 5 per genotype; * p < 0.05, ** p < 0.01 vs. same genotype, as indicated by the color, at t = 0).
3.5. Cellular Ca2+ handling kinetics
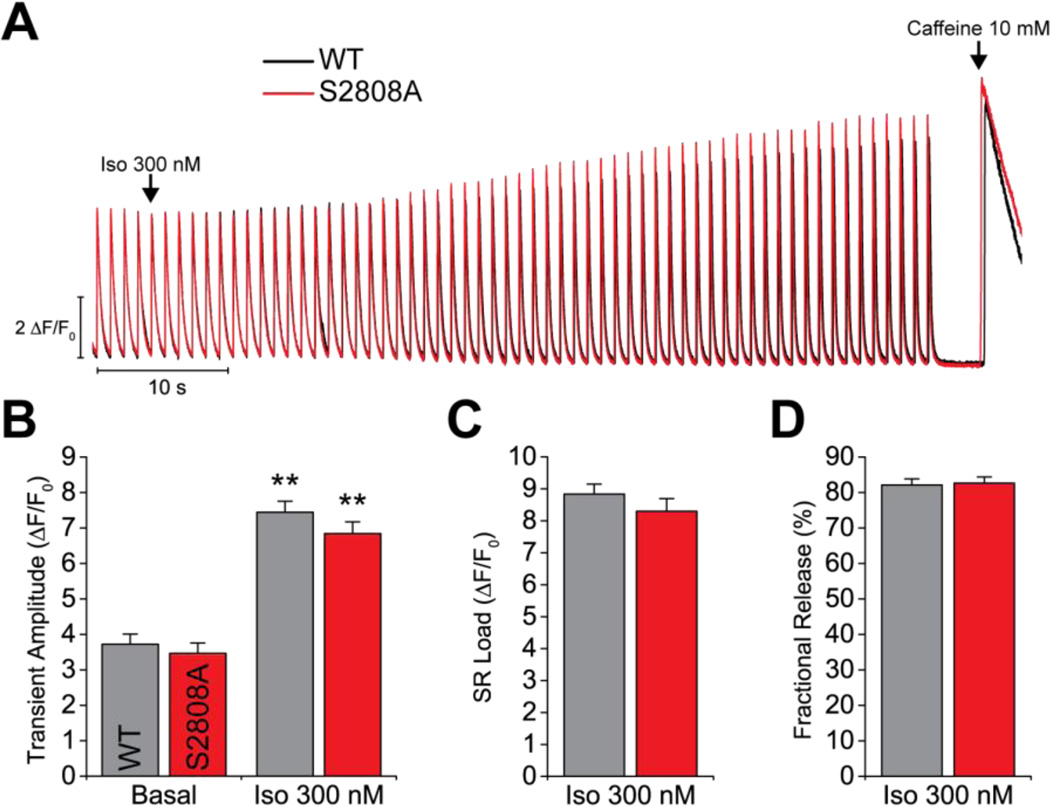
Lastly, we measured the adrenergic response of isolated cardiac myocytes. We loaded the cells with fluo-4 and paced them at 1 Hz. When the Ca2+ transient amplitude was stable, Iso 300 nM was perfused for 1 minute, followed by a caffeine pulse to measure the SR Ca2+ load (figure 6). As expected, Iso produced a significant increase in Ca2+ transient amplitude (figure 6B). This response, however, was identical in WT and S2808A cardiomyocytes. Additionally, we did not observe differences in SR Ca2+ load (figure 6C) or in fractional release (percentage of SR Ca load released during pacing, figure 6D) between groups. These data are consistent with the Isoproterenol stimulation observed in whole animals and further reinforce the notion that the S2808 phospho-site is not critical for adrenergic signaling.
Figure 6. Ca2+ transients and SR Ca2+ load.
A. Representative traces of field stimulation-triggered Ca2+ transients. After obtaining a stable basal response, Iso 300 nM was perfused for 1 min, followed by a pulse of 10 mM caffeine to measure the SR Ca2+ load. B. Quantification of Ca2+ transient amplitude at basal conditions and with Iso stimulation. Both groups showed a significant response to Iso. C–D. Quantification of the SR Ca2+ load and fractional release (Ca2+ transient amplitude/SR Ca2+ load) under Iso stimulation. (N = 2 WT, 3 S2808A; n = 16 cells per genotype; ** p < 0.01 vs. same genotype basal).
4. Discussion
The notion that RyR2-S2808 phosphorylation is a marker of HF in humans and animal models,7 that is a critical mediator of the normal adrenergic response of the heart,8,9 and that it greatly influences the progression and may actually revert major signs of ischemic HF,18 was extensively documented by Marks et al. Since then, extensive research has been devoted at obtaining a better understanding of the underlying mechanisms. While the Marks group has produced elegant and comprehensive studies supporting their hypothesis8,9,18,19 and extended their central tenets to explain pathogenesis in some forms of Ca2+-dependent arrhythmias,22 muscular dystrophy,20 seizures,31 stress-induced cognitive dysfunction,32 and diabetes,33 several laboratories have failed to reproduce fundamental aspects of their model (reviewed in11,21,22) making this a highly controversial topic. One of the most remarkable aspects of this controversy is that RyR2-S2808A mice developed by the Marks laboratory show a blunted response to adrenergic stimulation9 and better outcomes after MI,8 while a different mouse model with the same mutation, generated independently by our laboratory, has a normal adrenergic response13 and normal progression to HF following TAC12 and MI.14 Beyond certain variations in the experimental approach followed by both groups, which have been addressed in previous studies, there are evident differences between both mouse models:9,12 (1) the targeting strategy utilized: Marks used a self-excising ACN vector (containing a neomycin resistance cassette, Cre recombinase and a testes-specific promoter, all flanked by loxP sites), while our laboratory employed the classic “floxed” Neomycin cassette approach, which requires breeding targeted mice with mice expressing Cre-recombinase to excise the cassette; (2) the embryonic stem cells targeted: Marks used MM13 cells from 129S/SvEv mice, we utilized R1 cells derived from (129X1/SvJx129S1/SvImJ)F1 mice; and (3) the genetic background of the final mouse model: Marks backcrossed to the C57Bl/6 background for 10 generations, while we used a Sv129/C57Bl6 mixed background.
Although the impact of the targeting strategies or embryonic stem cells on the final phenotypic outcome is difficult to assess, several instances support the idea that identical mutations expressed in mice of different strains may lead to diverse phenotypes.23,24 Rubinstein et al.,25 for example, observed that C57Bl/6 mice with heterozygous Nav1.1 deletion closely recapitulate the severe epileptic phenotype of human patients with Dravet Syndrome, while the same mutation expressed in Sv129 mice produces a milder phenotype. Moreover, mice with mixed genetic background have an intermediate phenotype. In a more dramatic example, mice with a mutant superoxide dismutase transgene on the mixed B6SJL background show abnormalities resembling human amyotrophic lateral sclerosis lesions that are not evident on the C57Bl/6 background.26 Thus, the mouse strain can greatly alter the phenotype of a mutant mouse, likely because the targeted gene interacts substantially with background genes. Unfortunately, this type of in-depth studies has not been performed in the context of cardiac proteins.
Some authors have suggested the variable beta-adrenergic response of the mouse strains used, as a potential reason for divergent experimental results.27 Thus, in this study we aimed to determine whether the genetic background confers to RyR2-S2808A mice a blunted adrenergic response and protection against HF. The experimental approach was straightforward: backcross the RyR2-S2808A mice with mixed background (Sv129/C57Bl6) RyR2-S2808A mice into a C57Bl/6 strain (same strain used by the Marks Laboratory) and replicate several of the key experiments that have fueled this controversy. This approach allowed us to dissect a single variable (genetic background), potentially responsible for the conflicting results discussed above.
Our initial mouse model has been characterized thoroughly and shared with several collaborators. Nevertheless, we performed gene sequencing of Ryr2 in the congenic mouse strain to validate the genetic integrity of the model. We identified a single missense mutation in exon 56, which introduces a serine to alanine substitution in codon 2807 (S2807A). This codon corresponds to the human S2808. Classically, this site has been referred to as S2808 in the mouse nomenclature, but the latter changed when a revision was introduced to the mouse Ryr2 reference sequence in February 2015. Here we have continued using the same numbering to maintain consistency with previous literature and to avoid confusion. Also, both Benkusky et al.,12 and Wehrens et al.9 reported targeting exon 55 of Ryr2 to generate their respective models. However, based on the most recent reference sequences available (accession numbers NM_023868.2 and NT_039578.8), this region corresponds to exon 56. Our sequenced amplicons align with this exon (figure S1) and show the missense mutation is at the expected codon, as discussed above.
Following this genetic validation, we selected relevant experiments from previous reports to assess the response of our mice to acute and chronic stress (Iso injections and MI, respectively). If the genetic background were the cause of the conflicting results obtained with the two S2808A models, we expected (1) improved cardiac function after MI and (2) a blunted acute response to Iso, compared to WT. Each set of experiments produced the expected results in terms of the response to MI (LV dilation and deteriorating EF) and to adrenergic stimulation (positive chronotropism and inotropism), but S2808A mice behaved indistinguishably from WT controls. The only difference we detected was that S2808A mice showed a significant increase in HR compared to WT, one and four weeks post-MI. This observation was not reported by previous studies using our mouse,14 or by the Marks laboratory.7–9 Although we can only speculate on the potential mechanisms underlying this phenomenon (for example, chronic adrenergic stimulation of RyR2-WT may reduce SR Ca2+ load in pacemaker cells and decrease HR due to decreased entrainment of Ca2+ signals and membrane potential28), the absence of significant differences in cardiac output between genotypes after MI suggest that this increase in HR is not improving cardiac function and, therefore, is not protective against MI. Furthermore, a higher HR in the onset of HF could nudge these mice closer to arrhythmias, making them potentially more vulnerable, not less, to MI. Still, the increase in HR after MI in RyR2-S2808A mice is not logically derived from current models of RyR2 regulation by S2808 phosphorylation and remains to be understood, but it appears from the multitude of other variables that are similar between the two groups (table S1) that it does not affect the progression to HF.
We also measured by Western blots the expression of key proteins of E-C coupling before and after MI in both, WT and S2808A hearts. There were no differences between WT and S2808A before or after MI in any of the proteins analyzed. Expression of RyR2 showed a tendency to be decreased 4 weeks after MI, but the difference did not reach statistical significance. The density of Cav1.2, SERCA2a, and PLB was unchanged after MI, whereas that of NCX increased by ~2.5-fold in both groups of mice. Hyperphosphorylation of RyR2-S2808, a central process in the hypothetical model of Marks,7,9 showed a modest tendency to be increased in WT, but again, the difference was not statistically significant. Lastly, phosphorylation of PLB-T17, a marker of CaMKII activation, tended to decrease but was not modified by MI. All of these observations are in agreement with some, but not all studies that report protein density after MI. There is considerable variability even in closely related models of HF. Hasenfuss6 and Houser et al.5 noted this variability in several reports from human HF. Nevertheless, our observations here are in agreement with those of Zhang,14 who used our S2808A mice (in the Sv129/C57Bl6 background), suggesting that the transfer of the mutation to a different genetic background did not modify expression of other key proteins. More importantly, a side-by-side comparison of WT and S2808A mice shows that preventing phosphorylation of this site does not alter expression of key proteins of E-C coupling before or after MI, as expected if the phosphomutation had the profound changes in E-C coupling postulated by Marks.
On the physiologic response to acute stress, we also failed to detect differences between the two groups in mice, in isolated hearts and in isolated myocytes exposed to Iso. The lack of significant increase in stroke volume (SV) after adrenergic stimulation in both WT and RyR2-S2808A mice was expected when considering that left ventricular volume at diastole (LVVd) and at systole (LVVs) were modified by Isoproterenol, since SV = LVVd − LVVs. Fractional shortening (FS) and ejection fraction (EF), however, which directly reflect the inotropic response of the heart, were substantially increased by Isoproterenol in both groups (table S2), thus indicating that the adrenergic response was fully installed in both groups of mice. Furthermore, the nearly-identical increase in LVDP in isolated hearts perfused with Iso (table S3) supports the idea that both WT and S2808A mice show comparable response to acute stress. Again, these data are in agreement with all reports using our mouse model.13,14 Marks et al. previously proposed a dose-dependent effect of Iso, with only low doses (2 µg/kg) and concentrations (100 nM) producing blunted responses in S2808A mice. However, the idea of a dose-dependent effect on S2808 is not supported by previous reports that used a broad range of adrenergic agonist, as low as 10 nM Iso in isolated hearts13 and myocytes,14 and failed to observe differences in S2808A mice. Here, in our whole-animal experiments we used 2 mg/kg Iso, and for the isolated heart and cellular experiments we used 300 nM Iso, a dose only 3-fold higher than that used by Shan et al.8 and yet, there was no statistical difference between groups. All these data, taken together, argues against S2808 phosphorylation mediating the cardiac fight-or-flight response.
In summary, our data are in general agreement with all previous reports using our RyR2-S2808A mice with mixed Sv129/C57Bl6 background. Therefore, the most logical conclusion is that the genetic background is not the cause of the conflicting results. This leaves the possibility open for other potential reasons, such as animal housing conditions and circadian rhythms, among others proposed by Dobrev & Wehrens.27 However, the studies using the mouse model generated in our laboratory include data obtained by four different laboratories (ourselves,12 Houser,13,14 Niggli17 and Gyorke16) in five different academic institutions, undermining the likelihood of animal housing, circadian rhythms and animal handling as critical variables. Also, they have involved experiments at all integrating levels of physiology (in vivo, ex vivo, cellular and molecular) and encompassing a wide range of experimental approaches. The real causes of the discrepancy thus remain unsolved, but the complexity and variability of data that have become evident through this journey invite us to revise our current model of RyR2 regulation by phosphorylation. A deterministic model in which one site (S2808) controls the fight-or-flight response to stress and prevents ischemic cardiomyopathy, while another site (S2814) defines CaMKII-regulation of Ca2+ release and prevents non-ischemic cardiomyopathy, appears now unsustainable and overly simplistic. Instead, it may be relevant to consider a more comprehensive model of RyR2 regulation that includes all phospho-sites known to date (S2808, S2814 and S2030),22,29 the interplay between these sites at baseline and under stress,30,31 the great likelihood that other critical phospho-sites remain undiscovered,32 and the effect of other post-translational modifications.22 The proximity and location of S2808 and S2814 within the same flexible loop of the putative “phosphorylation hot spot” of RyR2,33 for example, strongly suggest it is unlikely that these sites work independently.
Finally, we consider that validation of experimental models by independent laboratories is paramount to resolve controversies. Our RyR2-S2808A mice, in either the mixed 129Sv/C57Bl6 or C57Bl/6J strains, are available for distribution to all interested parties.
Supplementary Material
Acknowledgments
The services provided by the Physiology Phenotyping Core of the Frankel Cardiovascular Center at the University of Michigan were instrumental in developing this work. We thank Dr. Daniel Michele (Director), Steven Whitesall (microsurgery) and Kimber Converso-Baran (ultrasonography) for their kind support. We also thank Dr. Randall Loaiza for the productive discussions that led to this work.
Funding. Funding for this study was provided by National Institutes of Health grants R01HL055438 and R01HL120108 (to HHV) and American Heart Association Pre-Doctoral Fellowship 14PRE19500012 (to FJA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures. None.
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 2.Piacentino V, 3rd, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 3.Pogwizd SM, Bers DM. Calcium cycling in heart failure: the arrhythmia connection. J Cardiovasc Electrophysiol. 2002;13:88–91. doi: 10.1046/j.1540-8167.2002.00088.x. [DOI] [PubMed] [Google Scholar]
- 4.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 5.Houser SR, Piacentino V, 3rd, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol. 2000;32:1595–1607. doi: 10.1006/jmcc.2000.1206. [DOI] [PubMed] [Google Scholar]
- 6.Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res. 1998;37:279–289. doi: 10.1016/s0008-6363(97)00277-0. [DOI] [PubMed] [Google Scholar]
- 7.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 8.Shan J, Kushnir A, Betzenhauser MJ, Reiken S, Li J, Lehnart SE, Lindegger N, Mongillo M, Mohler PJ, Marks AR. Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest. 2010;120:4388–4398. doi: 10.1172/JCI32726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camors E, Valdivia HH. CaMKII regulation of cardiac ryanodine receptors and inositol triphosphate receptors. Front Pharmacol. 2014;5:101. doi: 10.3389/fphar.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bers DM. Ryanodine receptor S2808 phosphorylation in heart failure: smoking gun or red herring. Circ Res. 2012;110:796–799. doi: 10.1161/CIRCRESAHA.112.265579. [DOI] [PubMed] [Google Scholar]
- 12.Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, Powers PA, Valdivia HH. Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circ Res. 2007;101:819–829. doi: 10.1161/CIRCRESAHA.107.153007. [DOI] [PubMed] [Google Scholar]
- 13.MacDonnell SM, Garcia-Rivas G, Scherman JA, Kubo H, Chen X, Valdivia H, Houser SR. Adrenergic regulation of cardiac contractility does not involve phosphorylation of the cardiac ryanodine receptor at serine 2808. Circ Res. 2008;102:e65–e72. doi: 10.1161/CIRCRESAHA.108.174722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Makarewich CA, Kubo H, Wang W, Duran JM, Li Y, Berretta RM, Koch WJ, Chen X, Gao E, Valdivia HH, Houser SR. Hyperphosphorylation of the cardiac ryanodine receptor at serine 2808 is not involved in cardiac dysfunction after myocardial infarction. Circ Res. 2012;110:831–840. doi: 10.1161/CIRCRESAHA.111.255158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong GT. Speed congenics: applications for transgenic and knock-out mouse strains. Neuropeptides. 2002;36:230–236. doi: 10.1054/npep.2002.0905. [DOI] [PubMed] [Google Scholar]
- 16.Liu B, Ho HT, Velez-Cortes F, Lou Q, Valdivia CR, Knollmann BC, Valdivia HH, Gyorke S. Genetic ablation of ryanodine receptor 2 phosphorylation at Ser-2808 aggravates Ca(2+)-dependent cardiomyopathy by exacerbating diastolic Ca2+ release. J Physiol. 2014;592:1957–1973. doi: 10.1113/jphysiol.2013.264689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ullrich ND, Valdivia HH, Niggli E. PKA phosphorylation of cardiac ryanodine receptor modulates SR luminal Ca2+ sensitivity. J Mol Cell Cardiol. 2012;53:33–42. doi: 10.1016/j.yjmcc.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shan J, Betzenhauser MJ, Kushnir A, Reiken S, Meli AC, Wronska A, Dura M, Chen BX, Marks AR. Role of chronic ryanodine receptor phosphorylation in heart failure and beta-adrenergic receptor blockade in mice. J Clin Invest. 2010;120:4375–4387. doi: 10.1172/JCI37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehnart SE, Terrenoire C, Reiken S, Wehrens XH, Song LS, Tillman EJ, Mancarella S, Coromilas J, Lederer WJ, Kass RS, Marks AR. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc Natl Acad Sci U S A. 2006;103:7906–7910. doi: 10.1073/pnas.0602133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, Matecki S, Lacampagne A, Marks AR. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15:325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houser SR. Role of RyR2 Phosphorylation in Heart Failure and Arrhythmias: Protein Kinase A-Mediated Hyperphosphorylation of the Ryanodine Receptor at Serine 2808 Does Not Alter Cardiac Contractility or Cause Heart Failure and Arrhythmias. Circ Res. 2014;114:1320–1327. doi: 10.1161/CIRCRESAHA.114.300569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valdivia HH. Ryanodine receptor phosphorylation and heart failure: phasing out S2808 and "criminalizing" S2814. Circ Res. 2012;110:1398–1402. doi: 10.1161/CIRCRESAHA.112.270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montagutelli X. Effect of the genetic background on the phenotype of mouse mutations. J Am Soc Nephrol. 2000;11(Suppl 16):S101–S105. [PubMed] [Google Scholar]
- 24.Doetschman T. Influence of genetic background on genetically engineered mouse phenotypes. Methods Mol Biol. 2009;530:423–433. doi: 10.1007/978-1-59745-471-1_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubinstein M, Westenbroek RE, Yu FH, Jones CJ, Scheuer T, Catterall WA. Genetic background modulates impaired excitability of inhibitory neurons in a mouse model of Dravet syndrome. Neurobiol Dis. 2015;73:106–117. doi: 10.1016/j.nbd.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu PH, Raju P, Robinson KA, Gurney ME, Trojanowski JQ, Lee VM. Transgenic mice carrying a human mutant superoxide dismutase transgene develop neuronal cytoskeletal pathology resembling human amyotrophic lateral sclerosis lesions. Proc Natl Acad Sci U S A. 1996;93:3155–3160. doi: 10.1073/pnas.93.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobrev D, Wehrens XH. Role of RyR2 Phosphorylation in Heart Failure and Arrhythmias: Controversies Around Ryanodine Receptor Phosphorylation in Cardiac Disease. Circ Res. 2014;114:1311–1319. doi: 10.1161/CIRCRESAHA.114.300568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neco P, Torrente AG, Mesirca P, Zorio E, Liu N, Priori SG, Napolitano C, Richard S, Benitah JP, Mangoni ME, Gomez AM. Paradoxical effect of increased diastolic Ca(2+) release and decreased sinoatrial node activity in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circulation. 2012;126:392–401. doi: 10.1161/CIRCULATIONAHA.111.075382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao B, Jiang MT, Zhao M, Yang D, Sutherland C, Lai FA, Walsh MP, Warltier DC, Cheng H, Chen SR. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ Res. 2005;96:847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- 30.Loaiza R, Benkusky NA, Powers PP, Hacker T, Noujaim S, Ackerman MJ, Jalife J, Valdivia HH. Heterogeneity of ryanodine receptor dysfunction in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2013;112:298–308. doi: 10.1161/CIRCRESAHA.112.274803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huke S, Bers DM. Ryanodine receptor phosphorylation at Serine 2030, 2808 and 2814 in rat cardiomyocytes. Biochem Biophys Res Commun. 2008;376:80–85. doi: 10.1016/j.bbrc.2008.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.George CH. Sarcoplasmic reticulum Ca2+ leak in heart failure: mere observation or functional relevance? Cardiovasc Res. 2008;77:302–314. doi: 10.1093/cvr/cvm006. [DOI] [PubMed] [Google Scholar]
- 33.Yuchi Z, Lau K, Van Petegem F. Disease mutations in the ryanodine receptor central region: crystal structures of a phosphorylation hot spot domain. Structure. 2012;20:1201–1211. doi: 10.1016/j.str.2012.04.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.