Abstract
The Eocene La Meseta Formation on Seymour Island, Antarctic Peninsula, is known for its remarkable wealth of fossil remains of chondrichthyans and teleosts. Chondrichthyans seemingly were dominant elements in the Antarctic Paleogene fish fauna, but decreased in abundance from middle to late Eocene, during which time remains of bony fishes increase. This decline of chondrichthyans at the end of the Eocene generally is related to sudden cooling of seawater, reduction in shelf area, and increasing shelf depth due to the onset of the Antarctic thermal isolation. The last chondrichthyan records known so far include a chimeroid tooth plate from TELM 6 (Lutetian) and a single pristiophorid rostral spine from TELM 7 (Priabonian). Here, we present new chondrichthyan records of Squalus, Squatina, Pristiophorus, Striatolamia, Palaeohypotodus, Carcharocles, and Ischyodus from the upper parts of TELM 7 (Priabonian), including the first record of Carcharocles sokolovi from Antarctica. This assemblage suggests that chondrichthyans persisted much longer in Antarctic waters despite rather cool sea surface temperatures of approximately 5°C. The final disappearance of chondrichthyans at the Eocene–Oligocene boundary concurs with abrupt ice sheet formation in Antarctica. Diversity patterns of chondrichthyans throughout the La Meseta Formation appear to be related to climatic conditions rather than plate tectonics.
Introduction
The modern Southern Ocean is delimited by the circum-Antarctic current (= Antarctic Convergence). The Antarctic continent is located within it, and these are amongst the most remote and coldest places in the world. They are key elements in any model of Earth processes and climatic change, as well as sites with unique scientific characteristics (Kriwet, 2005). The extant fish fauna within the Antarctic Convergence is striking in its low taxonomic diversity and high number of endemic taxa. Fishes that have evolved special morphological and physiological traits to survive in the extreme, low sea water temperatures are the dominant elements of this fauna (e.g., Eastman and Grande, 1989; Eastman, 1993; Albertson et al., 2010; Ingram and Mahler, 2011; Marshall, 2012; Near et al., 2012).
Chondrichthyans seemingly were major faunal components of pre-Oligocene Antarctic fish faunas, whereas chondrichthyan records from Neogene strata are still lacking. Similarly, the extant chondrichthyan fauna of the Southern Ocean surrounding Antarctica is extremely impoverished. Up to the present, only a few specimens belonging to three shark species have been reported, mostly off the Kerguelen Plateau (e.g., Gon and Heemstra, 1990), and it still is not established whether these sharks enter the Southern Ocean sporadically or represent permanent residents. Batoid diversity is slightly higher with eight described resident species (Gon and Heemstra, 1990; Long, 1994). Living holocephalans have not yet been recorded from Antarctic waters.
Post-Cretaceous and pre-Oligocene Antarctic fishes are mainly known from Seymour Island, which is located at the northern tip of the Antarctic Peninsula. Here, late early to late Eocene marine sediments of the La Meseta Formation yield the most diverse Paleogene ichthyofauna from the Southern Hemisphere to date, comprising cartilaginous and bony fishes (e.g., Welton and Zinsmeister, 1980; Jerzmanska, 1988; Eastman and Grande, 1989, 1991; Balushkin, 1994; Cione and Reguero, 1995, 1998; Doktor et al., 1996; Long, 1992a, 1992b, 1992c). The lack of post-Eocene fish records results in a gap of approximately 38 million years of unknown evolutionary history of Antarctic fishes between the Eocene–Oligocene boundary and the modern Antarctic fish fauna. Eocene Antarctic fishes consequently are very important for understanding evolutionary and adaptive processes in marine vertebrates related to abiotic causes such as plate-tectonically mediated dispersal and climatic perturbations, including the ‘Early Eocene Climatic Optimum’ and the onset of Antarctic ice-shield formation.
Chondrichthyan remains have previously only been reported from Ypresian to Lutetian (early–middle Eocene) strata, with most Eocene fish specimens occurring within Ypresian horizons, especially the Cucullea allomembers (late Ypresian) of the La Meseta Formation on Seymour Island (Reguero et al., 2012). Long and Stilwell (2000) reported additional selachian teeth from Eocene deposits of Mount Discovery in East Antarctica. All available data from published records imply a consistent increase in species richness of elasmobranchs and holocephalans from the earliest to the latest Ypresian (Tertiary Eocene La Meseta [TELMs] 2–5; Sadler, 1988) followed by rapid declines in both taxonomic diversity and abundance of specimens (e.g., Kriwet, 2005). Remains of chondrichthyans seemingly are very rare in the lower parts of the middle Eocene (Lutetian; TELM 6) and completely absent from upper parts of the middle Eocene (Bartonian; TELM 7) and the late Eocene (Priabonian; TELM 7) of the La Meseta Formation according to our current knowledge. Bony fish remains are quite abundant throughout the La Meseta Formation, including middle and late Eocene strata (e.g., Jerzmanska, 1988, 1991; Eastman and Grande, 1989, 1991; Jerzmanska and Swidnicki, 1992; Long, 1992a; Kriwet and Hecht, 2008). The disappearance of elasmobranchs and holocephalans towards the end of the Eocene in Antarctica generally is thought to be related to progressive cooling, reduction in shelf area, and increasing shelf depth due to the onset of Antarctic isolation through the establishment of the circum-Antarctic current (e.g., Grande and Eastman, 1986). The objective of this study is to describe the stratigraphically youngest and ultimate elasmobranch and chimeroid remains from the uppermost parts of the La Meseta Formation, which is ‘Layer 38’ according to the geological map of Montes et al. (2013). This new assemblage provides new information about Antarctic chondrichthyan diversity patterns during the Eocene and their final disappearance in relation to thermal changes at the Eocene–Oligocene boundary.
Locality and Stratigraphic Setting
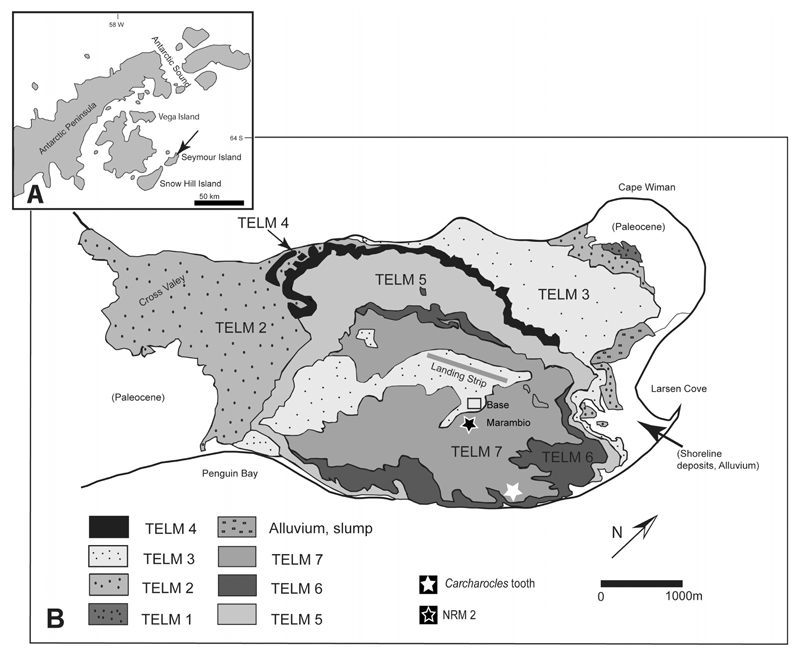
Seymour Island is situated northeast of the Antarctic Peninsula in the Weddell Sea (Fig. 1A–B). It is a rather small island, about 20 km long and 9 km wide, which is not covered by glaciers and is considered one of the most productive Paleogene vertebrate sites in the Southern Hemisphere. Here, Paleogene fossils occur in the La Meseta Formation, which represents the top of the sedimentary infill of the Late Jurassic–Cenozoic James Ross Basin (e.g., Del Valle et al., 1992). Deposition of the La Meseta Formation took place from the Ypresian to the Priabonian according to Sr isotope dating (Dingle and Lavelle, 1998). The mostly unconsolidated sediments of this formation are subdivided into three transgressive-regressive cycles (Units I to III; Elliot and Trautman, 1982), or seven major lithofacies (TELMs 1 to 7), or six allomembers based on erosionally based internal units (Marenssi et al., 1998). These units represent different depositional environments ranging from deltaic and estuarine to shallow marine settings (Marenssi et al., 1998). The chondrichthyan remains described herein are from the uppermost parts of the depositional sequence of the La Meseta Formation and are referred to the upper part (‘Layer 38’ according to the Geological Map of Montes et al., 2013) of the Submeseta Allomember or TELM 7 (Fig. 1B). These sediments are of Priabonian age (late Eocene; TELM 7) and were deposited in a shallow marine, coastal environment (Stilwell and Zinsmeister, 1992, Reguero et al., 2013).
Figure 1. Geological maps of Seymour Island, Antarctica.
A, index map of Seymour Island; B, geological map of Seymour Island, showing units of the Eocene La Meseta Formation. Asterisks indicate localities from where the material described here was obtained.
Materials and Methods
Argentinian-Swedish field parties from a joint project of the Instituto Antártico Argentino (DNA-IAA) and the Swedish Polar Research Secretariat (SPFS) collected the material that forms the focus of this study during three Antarctic summer campaigns between 2011 and 2013. The material consists exclusively of isolated shark teeth and two chimeroid tooth plate remains. The specimens were obtained by surface collecting at three sites exposed in the northern part of Seymour Island, south, southeast, and north of Marambio Base. All specimens described here are deposited in the palaeozoological collections of the Swedish Museum of Natural History (Department of Palaeobiology), with registration numbers prefixed by ‘NRM-PZ.’ The single recovered fin spine was scanned using a Skyscan 1173 (Bruker) desktop micro-CT device at the Department of Paleontology, University of Vienna, at 100 kV, 80 μA for 750 ms with Al filter and an image pixel size of 19.93 μm. Manual segmentation with unconstrained smoothing of digital μCT tiff images, 3D rendering, and analyses were performed using Amira version 5.4.1 (Visualization Sciences Group).
The terminologies and systematic scheme used here for the sharks essentially follows Cappetta (2012). We use the term ‘selachian’ when referring to sharks and ‘batomorph’ when referring to rays. Terms used in the description of the fin spine are according to Jerve et al. (2014). We refer to the saw shark specimen as ‘rostral spine’ rather than ‘rostral tooth’ to clearly distinguish this element from dental structures within the mouth cavity. The terminology employed for the chimeroid specimen follows Stahl (1999). References for fossil occurrences are not exhaustive.
Systematic Paleontology
Class CHONDRICHTHYES Huxley, 1880
Subclass ELASMOBRANCHII Bonaparte, 1838
Order SQUALIFORMES Goodrich, 1909
Family SQUALIDAE Bonaparte, 1834
Genus SQUALUS Linnaeus, 1758
Type Species—Squalus acanthias Linnaeus, 1758; extant.
Stratigraphic Range—Campanian (Late Cretaceous)–Recent.
Fossil Occurrences (Selected)—Europe (Lawley, 1876; Brozobathy and Kalabis, 1970; LeDeux, 1972; Hermann, 1977; Cappetta, 2012); Russia (Glikman, 1964); North and South America (Fitch, 1968; Welton, 1972); Asia (Itoigawa and Nishimoto, 1974; Itoigawa et al., 1985).
Squalus Sp. (Figs. 2–3)
Figure 2. Isolated dorsal fin spine of Squalus sp., NRM-PZ P16000.
A, left lateral view; B, right lateral view; C, posterior view; E, enlargement showing growth bands with original pigmentation; D, anterior view; F, basal view. Scale bars equal 5 mm.
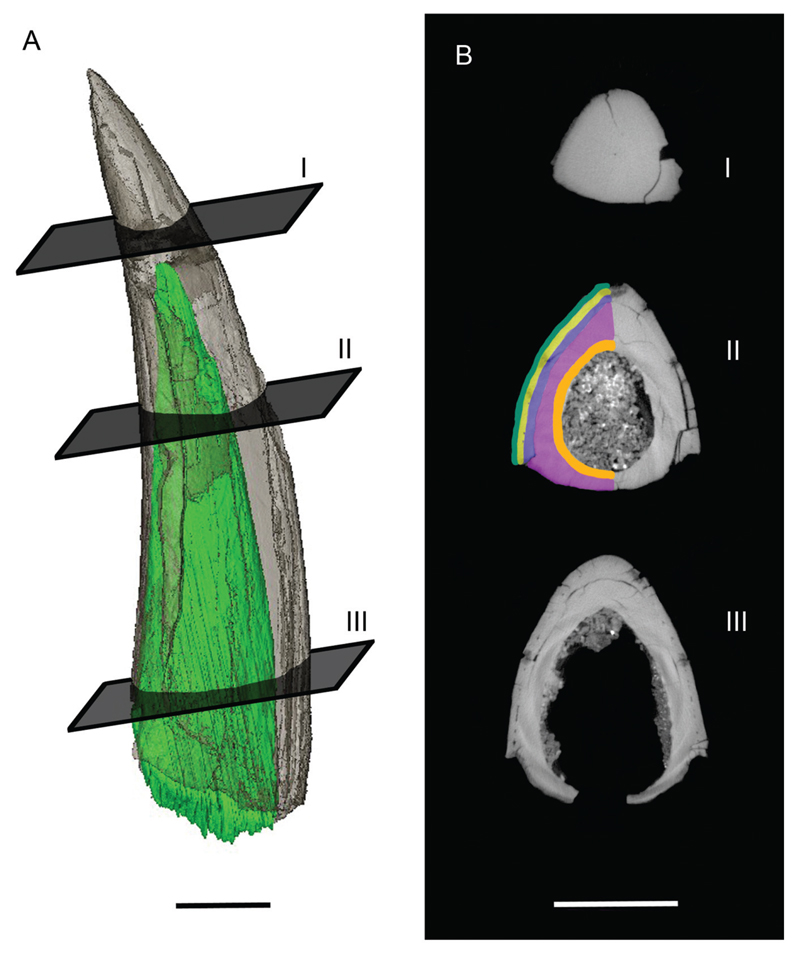
Figure 3. Micro-CT scan of the Squalus sp. fin spine depicted in Figure 2. Hard tissue organization is colored according to Jerve et al. (2014).
A, semitransparent fin spine with 3D reconstruction of the internal cartilage rod (green); B–D, representative horizontal micro-CT slides at different levels of the fin spine (from top to base). Scale bars equal 10 mm.
Material—Single dorsal fin spine, NRM-PZ P16000.
Horizon and Locality—Level 38 within the Submeseta Allomember, close to locality DPV 13/84, Plateau SE Marambio Base, S64°14.650′, W056°36.620′.
Age—Upper TELM 7, Priabonian, late Eocene.
Description—The single specimen of this taxon recovered from the uppermost parts of the La Meseta Formation measures 50.98 mm in height, 8.98 mm in width, and 11.10 mm in depth (Fig. 2A–F). It is slender, elongated, and slightly curved posteriorly (Fig. 2A–B). The apex and the base are damaged and incompletely preserved. This damage allows for the distinction of the cap, trunk, and associated individual layers (Fig. 2F). Most of the preserved fin spine is covered with a continuous and completely smooth enamel-like (= enameloid) superficial layer anterolaterally, but which is lacking posteriorly. The enameled portion and associated tissues form the so-called ‘cap’ (e.g., Maisey, 1979, 1982). The external enameloid layer is preserved in the lower 50% of the total length of spine (TLS) but is secondarily lost in the upper 50% TLS due to abrasion.
The basal edge of the enameled cover is oblique, and the underlying grayish dentine is visible. Numerous grayish bands of variable width are arranged parallel to the basal edge of the cap. These bands result from melanocyte staining, which represents the original cap pigmentation (Fig. 2D). Mantle cavities are present directly beneath the enameloid, implying the occurrence of a mantle dentine (= mantle primordium of Maisey, 1979) (see supplementary movie online). There is some evidence of a transitional layer that separates the enameloid cover and the mantle dentine from the inner trunk dentine layers (Fig. 2F).
Two layers of trunk dentine can be distinguished in the micro-CT scan (Fig. 3). It is not possible to detect their thickness with confidence, because both layers are characterized by the same gray value and the separating layer is difficult to identify. Based on the micro-CT scan and the respective three-dimensional (3D) reconstruction, the diameter of the cartilage rod decreases in size towards the spine tip and disappears in the last 25% TLS.
Posteriorly, the trunk is depressed along its entire height, forming a sulcus between the posterior margins of the lateral enameloid surfaces (Fig. 2F). The trunk itself forms a hollow cone that opens basally. The trunk cavity is displaced posteriorly and extends almost three quarters up along the posterior trunk wall (Fig. 3A).
Remarks—Fin spines are present in many extinct and some extant chondrichthyans, such as holocephalans and several basal squalomorphs (e.g., Centrophorus, Centroscymnus, Deania, Etmopterus, Euprotomicrus, Oxynotus, Squalus) and galeomorph (Heterodontus) selachians (e.g., Maisey, 1974, 1979, 1982; Cione and Pandolfi, 1984; Jerve et al., 2014). The fossil record of chondrichthyans mainly consists of isolated teeth, whereas fin spines are comparatively rare. Up to the present, chondrichthyan fin spines have not been recovered from sediments of the La Meseta Formation on Seymour Island. Consequently, this specimen is an important new contribution because the presence of fin spines suggests that the animal died more or less at the place of fossilization (depending on transportation processes) because fin spines are deeply inserted within the body wall and are not shed, whereas teeth are continuously replaced throughout life, wherever the animal might have lived.
The fin spine is characterized by a smooth and continuous anterolateral enameloid cover, parallel pigmented bands, a posterior non-enameled sulcus, and a posteriorly displaced trunk cavity that extends far up the posteriorly curved trunk. This character combination is very distinctive for fin spines of Squalus and readily distinguishes the Antarctic fin spine from those of Heterodontus, in which the enameled surface covers a smaller area of the fin spine and the trunk cavity is considerably shorter (Maisey, 1979). The presence of an enameloid cover differentiates the specimen from those of Deania and Euprotomicrus, which do not have apical enameloid (Cione and Pandolfi, 1984). In Centrophorus, Deania, and Etmopterus, the enameloid cover is not continuous but only consists of ribs. Fin spines of hybodonts and holocephalans differ from those of selachians in the presence of apicobasally arranged hook-like structures along the posterior margin, and they often have strongly ornamented enameloid surfaces (Maisey, 1982).
The fin spine described herein is apically strongly abraded but still preserves the original pigmentation in the lower half of the enameloid cap. The size of the fin spine and the basal margin of the enameloid cap, which is continuous without any irregularities or gnarled patterns, indicate a healthy adult rather than a senile specimen (Maisey, 1979).
The internal structure as revealed in the micro-CT scan displays the characteristic composition, although distinction of the trunk dentine layers is very difficult. The fin spine from the late Eocene of Antarctica seemingly displays a reverse pattern to what is seen in fin spines of extant Squalus spp. in that the inner trunk layer is narrower than the outer trunk layer (cf. Maisey, 1979:fig. 4B). The importance of this reversal in structure remains unclear.
Figure 4. Isolated lateral tooth of Squatina sp. (NRM-PZ P16005).
A, labial view; B, mesial view; C, occlusal view. Scale bar equals 1 mm.
So far, only teeth assigned to two different species of Squalus, S. woodburnei Long, 1992a, and S. weltoni Long, 1992a, have been described from the La Meseta Formation on Seymour Island (Long, 1992a). Assignment of the fin spine to either of these two species remains impossible at present.
Order SQUATINIFORMES Compagno, 1973
Family SQUATINIDAE Bonaparte, 1838
Genus SQUATINA Duméril, 1806
Type Species—Squalus squatina Linnaeus, 1758.
Stratigraphical Range—Valanginian (Early Cretaceous)–Recent.
Fossil Occurrences (Selected)—Africa (Noubhani and Cappetta, 1997); Antarctica (Kriwet, 2005; Kriwet et al., 2006); Asia (Itoigawa and Nishimoto, 1974); Europe (Cappetta, 2012); North America (Meyer, 1974; Cappetta and Case, 1975); Russia (Glikman, 1964); South America (Suárez et al., 2006).
Squatina Sp. (Fig. 4)
Material—A single tooth, NRM-PZ P16005.
Horizon and Locality—Level 38 within the Submeseta Allomember, locality NRM 2, Slope S Marambio Base, S64°14.778′, W056°37.169′.
Age—Upper TELM 7, Priabonian, late Eocene.
Description—The single specimen displays the characteristic features of teeth attributable to Squatina (Fig. 4A–C). It measures ca. 6.52 mm in mesiodistal width and ca. 3.95 mm in height. The tooth crown is laterally expanded and displays well-developed lateral heels without lateral cusplets or indentations (Fig. 4A). The main cusp is rather bulky basally but slender apically, upright, and slightly bent lingually (Fig. 4B). The cutting edges of the main cusp and lateral heels are strong and continuous. The lateral heels are low in labial and lingual views, with rounded lateral edges (Fig. 4A) that are not curved lingually in occlusal view (Fig. 4C).
The labial face of the crown is convex from side to side and overhangs the root basally with a well-developed prominent and knob-like protuberance (Fig. 4A–B). The protuberance is narrow in labial view and rather short in lateral views, not reaching the root base. The lateral edges of the labial protuberance are smooth and converge medially, forming a rounded basal edge. The tooth crown is completely smooth labially and lingually, and the basal margin of the labial face is continuous and forms a clear boundary between crown and root.
The lingual face of the crown is strongly convex and continues into a very short but broad tongue-shaped uvula that covers the lingual protuberance of the root almost completely (Fig. 4C). The occlusal surface of the uvula is rather flat and slightly damaged. The boundary between crown and tooth is well demarcated lingually but slightly furrowed and marked by a neck collar.
The root is rather massive and mesiodistally elongated. It is hemiaulacorhize, with a central foramen that is connected to a second and smaller foramen by a shallow canal on the lingual surface of the root, forming the mediolingual foramen that opens onto the lingual face of the protuberance just below the neck collar. There are four small marginolingual foramina on each side of the uvula (Fig. 4C).
The root lobes are flared and marginally protrude below the crown in labial view (Fig. 4A). Lingually, they meet to form a broad protuberance. In basal view, the root has a heart-shaped outline. The root lobes are well separated and broadly divergent, forming a shallow labial trough. The basal surfaces of the root lobes are flat and pierced by many very small and incipient foramina.
Remarks—Living angel (or sand devil) sharks represent a peculiar group of bottom-dwelling sharks of moderate body size (total length about 1–2 m) that superficially resemble rays. Twenty-two extant species are known, and the genus is probably globally distributed in tropical and temperate seas (e.g., Compagno et al., 2005; Last and White, 2008; Kriwet et al., 2010; Stelbrink et al., 2010; Klug and Kriwet, 2013). Most living species are highly endemic, with only a few species displaying wider distribution patterns.
The fossil record of squatinids mainly consists of isolated teeth and only rare skeletal remains (Klug and Kriwet, 2013). The oldest remains are isolated teeth from the Valanginian (Early Cretaceous), and 34 fossil species have been described up to now. The sister group of squatinids are the extinct pseudorhinids, which are known by numerous well-preserved complete (holomorphic) specimens from the Jurassic and Early Cretaceous (see Klug and Kriwet, 2013).
Paleogene Gondwana records of Squatina consist of isolated teeth reported from India (late Paleocene–early Eocene; Rana et al., 2005), Antarctica (early–middle Eocene; Welton and Zinsmeister, 1980; Case, 1992; Long, 1992a), Chile (middle–late Eocene; Otero et al., 2012, 2013), North Africa (late Eocene; Underwood et al., 2011), and the Oligocene of Australia (Pfeil, 1984). All previous Antarctic Squatina records were recovered from TELMs 4 and 5 on Seymour Island, and the single Squatina tooth from TELM 7 extends the known stratigraphic range of this taxon into the Priabonian. It displays the general squatinid morphology (e.g., mesiodistally enlarged teeth with lateral heels lacking lateral cusplets, smooth tooth crowns, a labial, knob-like protuberance of the crown extending basally almost to the basal level of the root, hemiaulacorhize root vascularization). The straight cutting edges of the lateral heels in occlusal view, which are not curved lingually, and the distally inclined cusp identify the tooth as coming from a lateral jaw position. It is, however, not possible to assign this tooth to either the upper or lower jaw.
Teeth of squatinids, unfortunately, are morphologically very generalized and display only a few distinct characters for species discrimination (see Klug and Kriwet, 2013, for a detailed discussion of dental characters). Therefore, assignment of the single specimen from TELM 7, as well as all other records from TELMs 4 and 5 to any particular species, would be premature without more complete material. We assume that the teeth from Seymour Island might represent a distinct species considering today’s distribution patterns of squatinids. This, however, requires detailed morphological analyses of all squatinid remains throughout the La Meseta Formation.
Order PRISTIOPHORIFORMES Berg, 1958
Family PRISTIOPHORIDAE Bleeker, 1859
Genus PRISTIOPHORUS Müller and Henle, 1837
Type Species—Pristis cirratus Latham, 1794; extant.
Stratigraphic Range—Albian (Early Cretaceous)–Recent.
Fossil Occurrences (Selected)—Africa (Herman, 1973); Antarctica (Grande and Eastman, 1986; Long, 1992a); Australia and New Zealand (Keyes, 1982); Europe (Jaekel, 1890; Herman et al., 1974; Cigala-Fulgosi, 1986); Near East (Woodward, 1932); North America (Welton, 1972; Barnes et al., 1981; Long, 1992a); Pacific region (Nishimoto and Morozumi, 1979); South America (Cione and Exposito, 1980; De Muizon and De Vries, 1985).
Pristiophorus Lanceolatus (Davis, 1888) (Fig. 5A–C)
Figure 5. Isolated shark teeth from TELM 7.
A–C, single rostral spine of Pristiophorus lanceolatus, NRM-PZ P15924-1; A, ventral view, B, dorsal view; C, anterior view. D–U, seven teeth of Striatolamia sp. cf. macrota. D–F, NRM-PZ P15925; D, labial view; E, lingual view; F, profile view. G–I, NRM-PZ P15924-2; G, labial view; H, lingual view; I, mesial view. J–L, NRM-PZ P15895; J, labial view; K, lingual view; L, mesial view. M–N, NRM-PZ P16002; M, labial view; N, lingual view. O–P, NRM-PZ P16003; O, labial view; P, lingual view. Q–R, NRM-PZ P16004; Q, labial view; R, lingual view. S–U, NRM-PZ P16001; S, labial view; T, lingual view; U, mesial view. V–Y, single lower lateral tooth of Palaeohypotodus sp. cf. rutoti, NRM-PZ P15896; V, labial view; W, lingual view; X, lateral view; Y, profile view. Scale bars equal 5 mm.
Material—A single rostral spine, NRM-PZ P16006.
Horizon and Locality—Level 38 within the Submeseta Allomember, locality NRM 2, Slope S Marambio Base, S64°14.778′, W056°37.169′.
Age—Upper TELM 7, Priabonian, late Eocene.
Description—The specimen recovered from the uppermost parts of the La Meseta Formation is a slightly abraded rostral spine with the basal margin of the basal peduncle being damaged (Fig. 5A–C). The crown, conversely, is completely preserved and shows only minor weathering damage near the tip (Fig. 5A). It measures 15.73 mm in height, 2.62 mm dorsoventrally, and 3.58 mm anteroposteriorly. The spine is elongate, lanceolate in dorsal and ventral views, and weakly curved posteriorly with an almost straight anterior cutting edge that is slightly convex apically, and has a slightly concave posterior cutting edge (Fig. 5A– B). In anterior view, the crown is laterally compressed with apically tapering margins (Fig. 5C). The dorsal and ventral faces are slightly convex transversely, with this convexity increasing towards the base. The surfaces are completely smooth and lack any ornamentation. The crown considerably overhangs the basal peduncle, with a well-developed bulge with almost straight margins in anterior and posterior views but with convexly curved and posteriorly oblique basal margins in dorsal and ventral views. The cutting edges are quite sharp and extend from the apex almost to the base. They fade out a short distance above the bulge.
The basal peduncle is considerably shorter than the crown and only takes up ca. 28% of total spine length. In dorsal and ventral views, the lateral margins of the peduncle are flaring and thus give the basal portion of the peduncle an anteroposteriorly elongated appearance. In anterior view, the lateral margins are concave. Basally, remnants are preserved of the anteroposteriorly directed nutritive canal in which the pulp cavity opens broadly.
Remarks—Living saw sharks of the order Pristiophoriformes are arranged into two genera, Pliotrema Regan, 1906, and Pristiophorus Müller and Henle, 1837, with eight valid species according to Militante (2012).
Saw sharks occur in temperate regions on continental and insular shelves, and upper slopes of the northwest and southeast Atlantic, west Indian Ocean, and west Pacific Ocean, but also in deeper water in the tropics (Compagno et al., 2005). Fossil saw sharks, conversely, seem to have been geographically more widespread but relatively uncommon (Gottfried and Rabarison, 1997). The fossil record extends back to the Albian, but most species have been described from Cenozoic deposits (e.g., Keyes, 1982). Pristiophorus lanceolatus occurs in TELMs 3, 4, 5, and 7. Tambussi et al. (2006) previously indicated the presence of P. lanceolatus in the Anthropornis nordenskjoeldi biozone from the uppermost part of TELM 7.
The smooth and completely unornamented crown readily distinguishes the rostral spine described here from those of Pristiophorus tumidens Woodward, 1932, from the Santonian of Lebanon, and from the Miocene species Pristiophorus suevicus Jaekel, 1890. In Oligocene Pristiophorus lineatus Applegate and Uyeno, 1968, the cutting edges are parallel and the apex is blunt in comparison with P. lanceolatus. Rostral spines of extant Pliotrema and extinct Ikamauius spp. differ in having serrated cutting edges. However, rostral spines of other described and still undescribed species resemble those assigned to P. lanceolatus, and we consider this species an artificial taxon that includes multiple species with similar morphologies. Dental morphologies might provide a better estimate of the real taxonomic assignment and diversity of fossil Pristiophorus spp.
Order LAMNIFORMES Berg, 1958
Family ODONTASPIDIDAE Müller and Henle, 1839
Genus STRIATOLAMIA Glikman, 1964
Type Species—OTODUS MACROTUS Agassiz, 1843.
Stratigraphic Range—Danian (Early Paleocene) to Priabonian (later Eocene).
Fossil Occurrences (Selected)—Africa (Priem, 1907; Arambourg, 1952); Antarctica (Long, 1992a); Europe (Agassiz, 1843; Siverson, 1995; Adnet, 2006; Dutheil et al., 2006; Van den Eeckhaut and De Schutter, 2009; Diedrich, 2012); North America (Leriche, 1942; Ward and Wiest, 1990; Padilla et al., 2014); Russia (Glikman, 1964; Zhelezko and Kozlov, 1999).
Striatolamia Sp. Cf. S. Macrota (Agassiz, 1843) (Fig. 5D–U)
Material—Seven teeth, NRM-PZ P15895, NRM-PZ P15924–15925, NRM-PZ P16001–16004.
Horizon and Localities—Level 38 within the Submeseta Allomember, locality NRM 2, Slope S Marambio Base, S64°14.778′, W056°37.169′; underneath locality NRM 5, Ridge N Marambio Base, S64°13.985′, W056°36.851′.
Age—Upper TELM 7, Priabonian, late Eocene.
Description—All seven specimens are more or less abraded and lack varying portions of the root or cusp apex (Fig. 5D–U). Mesial and distal lateral cusplets are preserved on only three teeth (Fig. 5G–L, S–U). The teeth are of typical odontaspidid appearance, with anterior teeth being long and slender with a sigmoidal profile, whereas the single assignable lateral tooth is less sigmoidal and has more divergent root lobes. The cutting edges are not serrated.
Specimen P15925 (Fig. 5D–F) displays the characteristic crown morphology of a first upper anterior tooth. It is high with a slender central cusp having almost parallel margins in labial view. The apex is acute and slightly bent distally. In profile, the main cusp is slightly sigmoidal and does not jut out labially over the root. In labial view, the cusp is slightly spatulate. The labial crown face is only faintly convex mesiodistally and is devoid of any ornamentation. The labial crown base exhibits a small central depression.
The lingual crown face of the central cusp is weakly convex mesiodistally with a median flattening and displays weak and fine striations, which start at the crown-root junction but do not reach the apex. Apically, the striations become more flexuous. The lateral cutting edges are not well developed (probably due to abrasion) and continuous, extending from the crown tip to almost the central cusp base. The basal crown neck separating the crown from the root is very narrow.
The root is very incompletely preserved, and only a part of the mesial root lobe is preserved. The lingual root protuberance is not well developed, not extending far lingually below the crown, and slightly abraded.
Specimen P15924-2 (Fig. 5G–I) is a second upper anterior tooth. It measures 26.86 mm in total height, 11.26 mm in mesiodistal width, and 7.97 mm labiolingually. The central cusp is inclined distally and sigmoidal in labial and lingual views, with the apex being twisted mesially. In labial view, the central cusp is slightly shorter in height but basally broader than specimen P15925. The enameloid of the labial face is almost completely smooth, lacking vertical striations, except a very short one extending from the base of the labial face a short way apically to the level where the lateral cutting edges fade out. The lateral cutting edges are rather sharp but incomplete and not continuous with the only preserved lateral cusplet, extending from the central cusp apex to the cusp base but fading out distinctly above the cusp-root junction. In profile, the main cusp is sigmoidal, with the apex being twisted labially.
The lingual face of the central cusp is convex mesiodistally with a medial flattening as in specimen P15925. Fine and flexuous striations are present, which start at the crown-root junction. They are rather short on the lingual medial flattening of the crown; but extend very far up the crown laterally, almost reaching the apex. The crown neck is very narrow.
Only one very small but acute lateral cusplet is preserved distally (Fig. 5H). It is separated from the central cusp and has a circular cross-section basally. Cutting edges are not identifiable. The apex is curved towards the central cusp and slightly inclined lingually. The lateral cusplet is positioned lingually with respect to the level of the cutting edge of the central cusp in profile view.
The root is damaged, with only parts of the mesial root lobe remaining. The preserved portion is rather short and is slightly depressed mesiodistally. The lingual protuberance is rather well developed and almost shelf-like. Remnants of the labiolingual nutritive groove are preserved. A small lingual foramen opens on the lingual surface of the protuberance basally.
Specimen P15895 (Fig. 5J–L) comes from the second lower anterior tooth row. It measures 28.20 mm in height, 11.87 mm in mesiodistal width, and 7.91 mm labiolingually.
The central cusp is quite high, slender, and very weakly sigmoidal in labial and lingual views, with the mesial margin being almost straight and the distal one being slightly concave. The apex is lacking but appears to have been twisted mesially, as seen in specimen P15924. In profile view, the crown is slightly sigmoidal and the apex does not jut out over the lingual protuberance.
The labial crown face is very flat and only slightly convex mesiodistally at its base. The medial, vertical ridge originating on the labial face of the central cusp is not well marked but reaches further up than in specimen P15924. The cutting edges are well developed but also do not reach the base of the crown, ending clearly above the crown-root junction. Lingually, the central cusp face is only weakly convex mesiodistally with a pronounced medial flattening. Weak and fine, flexuous striations are present, which reach far up the cusp medially, but do not reach the apex.
A single, distal cusplet is preserved. It is very small and only weakly separated from the central cusp. It is devoid of well-defined cutting edges, and no ornamentation is detectable. The cross-section is circular basally, and the apex curves towards the central cusp.
The root is massive, bilobate, and only slightly damaged. The preserved part of the root constitutes approximately 42% of the preserved total tooth height. The root lobes are divergent and form an asymmetric acute angle. The mesial root lobe appears shorter and broader than the distal one. The distal root lobe is long, rather slender, and basally pointed. Both lobes are mesio-distally compressed. The protuberance is well developed and shelf-like mesiodistally. The nutritive groove is very shallow, and a small foramen opens on the protuberance basally.
Three isolated central cusps (NRM-PZ P16002–16004; Fig. 5M–R) that display the same features are also assigned to this species. They are morphologically very similar and bear a high and slender, almost triangular main cusp, which is more or less sigmoidal in profile view. One crown, NRM-PZ P16002 (Fig. 5M), also displays complete cutting edges that reach the base of the cusp.
Specimen NRM-PZ P16001 (Fig. 5S–U), which probably represents a lower first lateral tooth, is only tentatively assigned to this taxon. It differs from the specimens described above by a more slender, weakly distally curved central cusp, and a vertical nutritive groove separating the rather shallow lingual protuberance into two parts. Additionally, the lateral cutting edges are complete and reach the crown-root junction basally. The basal edge of the labial face is medially concave and irregular, forming small crenulations. The labial face is very flat and completely devoid of any ornamentation, whereas the lingual face is convex mesiodistally with very strong, densely arranged striations extending from the crown neck far up the cusp but not reaching the acute apex. In profile, the cusp is sigmoidal and the apex is not jutting out above the level of the lingual protuberance. The mesial cusplet is small, well separated from the central cusp, devoid of any ornamentation and cutting edges, and inclined towards the central cusp. It is lingually displaced in relation to the cutting edge in profile view. The distal cusplet is lacking. There is no indication of an additional pair of lateral cusplets. The root is imperfect, with only the mesial root lobe being preserved. It is long and slightly mesiodistally flattened, with a pointed basal extremity. The root lobes seemingly are very divergent, forming a broad and shallow, ‘U’-shaped concavity.
Remarks—The fossil genus Striatolamia is known from the early Paleocene up to the late Eocene (Cappetta, 2012). The taxonomic identity and systematic position of Striatolamia is still debated because of dental similarities to living sand tiger sharks of the genus Carcharias (cf. Cunningham, 2000). Striatolamia has traditionally been assigned to Odontaspididae (e.g., Compagno, 1984; Long, 1992a; Adnet, 2006).
Striatolamia was dismissed by Long (1992a) and Purdy (1998), who consider it conspecific with Carcharias. Alternatively, Siver son (1995), Cappetta and Nolf (2005), and Cappetta (2012) assign Striatolamia to Mitsukurinidae, because of similarities to teeth of Anomotodon hermani. We follow the more traditional view and consider Striatolamia a valid taxon (for reasoning, see next paragraph) within Odontaspididae because of the very close similarities to members of this family, as well as the differences in anterior teeth from Anomotodon. Odontaspididae includes Carcharias Rafinesque, 1810, Odontaspis Agassiz, 1838, Palaeohypotodus Glikman, 1964, Brachycarcharias Cappetta and Nolf, 2005, Gluekmanotodus Zhelezko in Zhelezko and Kozlov, 1999, Hypotodus Jaekel, 1895, Jaekelotodus Menner, 1928, Mennerotodus Zhelezko, 1989, Orpodon Cappetta and Nolf, 2005, Sylvestrilamia Cappetta and Nolf, 2005, and Turania Kozlov, 2001, according to Cappetta (2012).
Teeth of Striatolamia differ from those of Carcharias most notably in having very small, rounded, and medially inclined lateral cusplets, less pronounced sigmoidal profile view, cutting edges that are confined to the lateral margins of the central cusp rather than being displaced labiobasally, and more massive root lobes that form an acute divergent angle (see Ferrusquia-Villa-franca et al., 1999; Cunningham, 2000).
Teeth of Odontaspis and Palaeohypotodus are easily differentiated from teeth of Striatolamia by the smooth lingual face of the central cusp and the presence of two pairs of lateral cusplets, of which the first lateral ones closest to the central cusp are distinctly large. Additionally, teeth of Palaeohypotodus depart from teeth of Striatolamia in the presence of coarse, short, and vertical striations at the base of the labial crown face along the crown-root junction. The tooth NRM-PZ P16001 resembles teeth of Palaeohypotodus in the presence of labial crenulae along the labial crown base, but the very small lateral cusplet supports its assignment to Striatolamia. This tooth most likely represents a juvenile or subadult individual.
Teeth of Brachycarcharias have a more robust central cusp and generally larger lateral cusplets, generally accompanied by a second pair of incipient cusplets, whereas teeth of the odontaspidids Gluekmanotodus, Hypotodus, and Jaekelotodus easily can be distinguished by the lack of any labial or lingual crown ornamentation. Teeth of Mennerotodus and Orpodon lack any lingual ornamentation. Additionally, teeth of Orpodon are characterized by a pair of large and robust lateral cusplets and a second incipient pair. In Sylvestrilamia, the cutting edge is continuous between the central cusp and the lateral cusplets.
Teeth of Turania are very similar to those of Carcharias and Striatolamia, but Cappetta (2012) doubts the validity of Turania. Anterior teeth of Turania differ from those of Striatolamia in being comparatively short and less sigmoidal in profile view.
Teeth of Mitsukurina largely agree morphologically with those of Striatolamia. They differ, however, in lacking lateral cusplets in anterior teeth and in the striation pattern. Lateral cusplets are absent in all tooth position in Alopias, Anomotodon, and Woellsteinia. Incipient lateral cusplets occur in Macrorhizodus. Teeth of other lamniforms such as Cretalamna, Isurolamna, and Usakia are devoid of any ornamentation on the labial and lingual central cusp faces.
According to Cappetta (2012), five species of Striatolamia have been described, including S. cederstroemi Siverson, 1995, S. macrota (Agassiz, 1838), S. siberica Zhelezko in Zhelezko and Kozlov, 1999, S. striata (Winkler, 1876), and ‘S.’ whittei (Arambourg, 1952).
Striatolamia cederstroemi from the upper Danian of Sweden has poorly defined lateral cusplets, whereas they are well separated from the main crown in S. striata and S. macrota. Teeth of S. striata, which might represent the ancestor of S. macrota according to Cappetta (2012), differ from teeth of the latter in better-developed lingual striations in all tooth positions. Teeth of ‘S.’ whittei display a rather peculiar morphology of the lateral cusplets and the form of the root lobes, and it thus probably warrants placing this species in its own genus (Zhelezko, 1989; Cappetta, 2012). The teeth from TELM 7 of Seymour Island display the basic characters of S. macrota (Agassiz, 1835) but have a very distinct medial flattening of the lingual crown face and very weak striations, which cover the complete lingual face up to the cutting edges. In this, the teeth differ from typical teeth of S. macrota. However, more material is necessary to establish the significance of these differences, because the labial ornamentation is highly variable in Striatolamia and Carcharias. Therefore, we assign the teeth described here tentatively to this species.
Compared with Eocene Arctic representatives of S. macrota (cf. Padilla et al., 2014), the Antarctic specimens seem to be slightly larger with more pronounced striations. The appearance, nevertheless, is more or less similar in both locations. Teeth assigned to S. macrota from the Paleogene of Morocco resemble teeth from the Arctic and Antarctica but are more delicate in overall appearance and in the striations.
Striatolamia macrota is the dominant species within all TELMs (e.g., Kriwet, 2005; Reguero et al., 2012; Engelbrecht et al., 2014; Kriwet et al., 2014). Dental resemblances to Carcharias and Mitsukurina imply that this species probably occupied a similar trophic niche. A detailed analysis of palaeogeographic occurrences and dental patterns might provide a better understanding of its ecological role.
Genus †PALAEOHYPOTODUS Glikman, 1964
Type Species—Odontaspis rutoti Winkler, 1876; from the middle Paleocene, Selandian, of Belgium.
Stratigraphic Range—Early Paleocene–late Eocene.
Fossil Occurrences (Selected)—Antarctica (Long, 1992a); Europe (Leriche, 1951; Casier, 1967; Gurr, 1962; Herman, 1972; Ward, 1980; Cappetta, 1987; Rayner et al., 2009; Iserbyt and De Schutter, 2012); Greenland (Bendix-Almgreen, 1969); North America (Ward and Wiest, 1990); Africa (Herman, 1973; Cappetta, 2012); central Asia (Glikman, 1964).
†Palaeohypotodus Sp. Cf. P. Rutoti Winkler, 1876 (Fig. 5V–Y)
Material—Single lower lateral tooth, NRM-PZ P15896.
Horizon and Locality—Level 38 within the Submeseta Allomember, locality NRM 2, Slope S Marambio Base, S64°14.778′, W056°37.169′.
Age—Upper TELM 7, Priabonian, late Eocene.
Description—The single specimen is quite small and compact (Fig. 5V–Y). In labial view, the main cusp of P15896 is markedly triangular, with a broad base that uniformly tapers apically (Fig. 5V). The apex of the cusp is very acute. The labial crown face is flat and almost completely devoid of ornamentation except for very short, but rather faint vertical wrinkles along the basal margin of the enamel. The basal enameloid boundary is more or less straight with a slight median depression and does not extend onto the root lobes as in many odontaspidids, but instead overhangs the root medially.
Lingually, the crown face is very convex mesiodistally and completely devoid of ornamentation (Fig. 5W). The lateral cutting edges are noticeably blunt and reduced, accentuating the rounded cross-section of the main cusp. The edges do not reach the base of the crown as far as can be ascertained. Lateral cusplets are not preserved, but their broken bases indicate that they were very broad with rounded bases. The cusplets were closely placed next to the main cusp and seemingly not clearly separated.
In profile view, the crown is strongly inclined lingually but not sigmoidal, with the apex not extending over the lingual root protuberance (Fig. 5X). The crown broadens considerably basally, but in labial view it is slightly constricted above the wrinkles. The transverse section of the main cusp is ‘D’-shaped, from tip to base.
The root is damaged, and only one root lobe is preserved. The root was bilobate and is of holaulacorhize vascularization pattern. The preserved root lobe is slender and long with a vertical lateral margin, giving the root, as far as can be ascertained, a rectangular appearance in labial view. It is depressed mesiodistally, and the basal edge is slightly pointed. Apically, the root lobes are not coalescing but form a very wide and shallow divergence that results in a broad, trough-like appearance in labial view. The lingual protuberance is well developed and rather massive with a shallow and narrow nutritive groove. A small foramen opens into this shallow nutritive groove lingually.
Remarks—Palaeohypotodus was erected by Glikman (1964) for teeth from the Selandian of Belgium and was placed in his new family Jaekolodontidae. Herman (1975) included two other species, P. bronni (Agassiz, 1843) and P. striatula (Dalinkevicius, 1935), from Cretaceous deposits in this genus. Cappetta (1987) alternatively included Palaeohypotodus in Odontaspididae and transferred P. striatula to Synodontaspis (= Carcharias). Cappetta and Nolf (2005) and Cappetta (2012) consider Palaeohypotodus monospecific with only P. rutoti being valid.
Teeth of P. rutoti are characterized by short, vertical wrinkles along the basal edge of the labial crown face, a character that is also present in Cenocarcharias Cappetta and Case, 1999, and Hispidaspis Sokolov, 1978. The latter two, however, are only known from the Cretaceous. Short vertical wrinkles may be present on some teeth of Odontaspis and adult anterior but also juvenile teeth of Striatolamia. These are never as coarse as in Palaeohypotodus. The basolabial wrinkles depicted in the specimen described here resemble the pattern seen in NRM-PZ P16001. Nevertheless, the general morphology of a very convex lingual face, flat labial face with basal bulge overhanging the root, rectangular appearance of root in labial view, and vertical nutritive groove bisecting the lingual protuberance distinguish this specimen from teeth of Striatolamia and other odontaspidids.
It is always difficult to identify a taxon from just a single tooth, but P15896 seems to be very close to Palaeohypotodus. The number (two to three pairs) and size of the lateral cusplets cannot be established, and thus we only tentatively assign the tooth to P. rutoti. The triangular central cusp and the rectangular root indicate that this tooth is from a lower, lateral position. Various teeth of P. rutoti were previously described by Long (1992a). This species was recently described from the Bartonian–Priabonian of southern Chile (Otero and Soto-Acuna, 2015). In the northern hemisphere, this species is restricted to Paleocene strata and the Southern Hemisphere occurrences might represent relict occurrences if one assumes that this species was cool-water adapted (Kriwet et al., 2014).
Family †OTODONTIDAE Glikman, 1964
Genus †CARCHAROCLES Jordan and Hannibal, 1923
Type Species—Squalus auriculatus Blainville, 1818, from the middle Eocene, Lutetian, of Belgium (no precise locality known).
Stratigraphic Range—Ypresian (early Eocene)–Burdigalian (early Miocene).
Fossil Occurrences (Selected)—Africa (Case and Cappetta, 1990; Underwood et al., 2011); Europe (Leriche, 1905, 1910); central Asia (Zhelezko and Kozlov, 1999); North America (Freile et al., 2001; Cicimurri and Knight, 2009); Pacific region (Ueyno et al., 1984; Yabumoto, 1989); Russia (Jaekel, 1895).
†Carcharocles Sokolovi (Jaekel, 1895) (Fig. 6A–B)
Figure 6. Upper lateral tooth of Carcharocles sokolovi, NRM-PZ P16007.
A, labial view; B, lingual view. Scale bar equals 5 mm.
Material—A single incomplete tooth, NRM-PZ P16007.
Horizon and Locality—Level 38 within the Submeseta Allomember, locality DPV 13/84, Plateau SE Marambio Base, S64°14′47″, W56°36′12″.
Age—Upper TELM 7, Priabonian, late Eocene.
Description—The single specimen represents an upper lateral tooth of 54.34 mm height, 60.83 mm mesiodistal length, and 15.41 mm labiolingual width. The crown is labiolingually flattened, quite slender, and slightly inclined distally (Fig. 6A–B). The labial face of the central cusp is very flat, the lingual face, conversely, is very convex mesiodistally, resulting in an almost ‘D’-shaped cross-section of the crown at its base. Unfortunately, the enameloid cover is heavily abraded due to weathering processes. Consequently, the serrated cutting edges, which are important for species identification, are not preserved. The enameloid is best preserved lingually where it is completely smooth apart from some cracks.
The distal lateral cusplet is almost completely lacking, whereas the mesial one is well preserved showing the serration. The cusplet is rather low compared with the central cusp, devoid of any ornamentation, and asymmetrically triangular in labial view, with a very broad base. Mesially, the cusplet is slightly incised, almost dividing it into a larger and a very incipient, smaller part. The serration is very fine and regular along the distal edge of the cusplet and somewhat more irregular and coarser along the mesial edge towards the incision.
The root is heavily damaged but still provides some information about its morphology. It is very low compared with the tooth crown and is best developed at the levels of the root lobes. The root lobes are rather mesiodistally elongated and broadly separated, forming a shallow and broad arc in labial view. The mesial root lobe is shorter and broader with a rounded extremity compared with the distal one, which is narrower and longer. The lingual protuberance is rather damaged but seemingly salient. Nutritive foramina are not discernable due to the state of preservation. The basal face of the root, as far as it can be ascertained, is flattened at the levels of the root lobes. The tooth neck separating the crown from the root lingually is broad and typically chevron-like.
Remarks—The systematic position of extinct macrophagous lamniform sharks represented by large teeth with serrated lateral cutting edges is still debated. The most prominent member of this group, Megaselachus megalodon, was originally assigned to Carcharodon by Agassiz (1835), a view that is still supported by various authors (e.g., Purdy et al., 2001). It was variously placed into Otodus (see Cappetta, 2012, for usage of this name for the lineage; Andrianavalona et al., 2015), Procarcharodon (Casier, 1960), Carcharocles, or Megaselachus (e.g., Jordan and Hanibal, 1923; Glikman, 1964). We follow the view of Cappetta (2012), who placed all extinct macrophagous sharks with serrated cutting edges in a single genus, Otodus, representing the family Otodontidae. Separation of species into different subgenera of Otodus, Otodus s.l., Carcharocles, and Megaselachus, by Cappetta (2012), however, is not reasonable for exclusively fossil groups. Therefore, we consider these subgenera to represent genera.
Members of Otodontidae are known from isolated vertebral centra and teeth, and associated tooth sets, with a fossil record ranging from the Paleocene to the Pliocene. The tooth from the uppermost Eocene of Antarctica described herein is similar to teeth of Otodus, but is distinguished by the presence of the single preserved, serrated lateral cusplet, which identifies it as belonging to a member of Carcharocles. Members of Megaselachus (late Oligocene–Pliocene) are characterized by very regular and fine serrations along the cutting edges and very low lateral cusplets, if any, compared with species of Carcharocles. Members of Carcharocles are geographically widely distributed during the Cenozoic, with several species that were described from Kazakhstan spanning the whole Eocene.
Identification of species within Carcharocles is generally based on serration patterns of the central cusp and the form and serration of lateral cusplets. Species assignment of the tooth presented here is difficult because the serrated cutting edges are not preserved. Two possible candidates known from Gondwana continents during the Eocene are C. auriculatus, which occurs in the Ypresian of Seymour Island (Long, 1992a), in the middle to late Eocene of Chile (Otero et al., 2013), and in the Oligo-Miocene of Australia (Pledge, 1967), and C. sokolovi, which has only been reported from the Priabonian of Egypt (Case and Cappetta, 1990; Underwood et al., 2011). The stratigraphic occurrence of the specimen presented here coincides with the general stratigraphic distribution of C. sokolovi (see above). The rather narrow central cusp, the distinctly mesiodistally elongated and rather low root with basally flattened root lobes, and the serration pattern of the mesially preserved cusplet also support its assignment to C. sokolovi rather than to C. auriculatus. Lateral teeth of C. auriculatus have sturdier roots, which are rather high with almost vertical, slightly concave lateral margins in labial and lingual views, not basally flattened root lobes, a more robust central cusp, and coarser serrated lateral cusplets. Thus, the specimen described here represents the southernmost record of C. sokolovi. The tooth from Antarctica corresponds in size and general morphological appearance to an upper lateral figured by Applegate and Espinosa-Arrubarrena (1996:fig. 9b), who consider the maximum body length of C. sokolovi to reach at least 6 m. This would make this shark one of the ultimate predators in the latest Eocene waters surrounding Antarctica.
Subclass SUBTERBRANCHIALIA Zangerl, 1981
Superorder HOLOCEPHALI Bonaparte, 1832
Order CHIMAERIFORMES Patterson, 1965
Suborder CHIMAEROIDEI Patterson, 1965
Family CALLORHYNCHIDAE Garman, 1901
Subfamily CALLORHYNCHINAE Stahl, 1999
Genus †ISCHYODUS Egerton, 1843
Type Species—Chimaera townsendi Buckland, 1835, from the Bathonian, Great Oolite (Middle Jurassic), Stonesfield, England.
Stratigraphic Range—Middle Jurassic–Miocene.
Fossil Occurrences (Selected)—Antarctic Peninsula (Ward and Grande, 1991); Europe (Leriche, 1902, 1909; Gurr, 1962; Ward, 1980); North America (Cvancara and Hoganson, 1993; Hoganson and Erickson, 2005); Russia (Popov and Ivanov, 1996).
†Ischyodus Dolloi Leriche, 1902 (Fig. 7A–E)
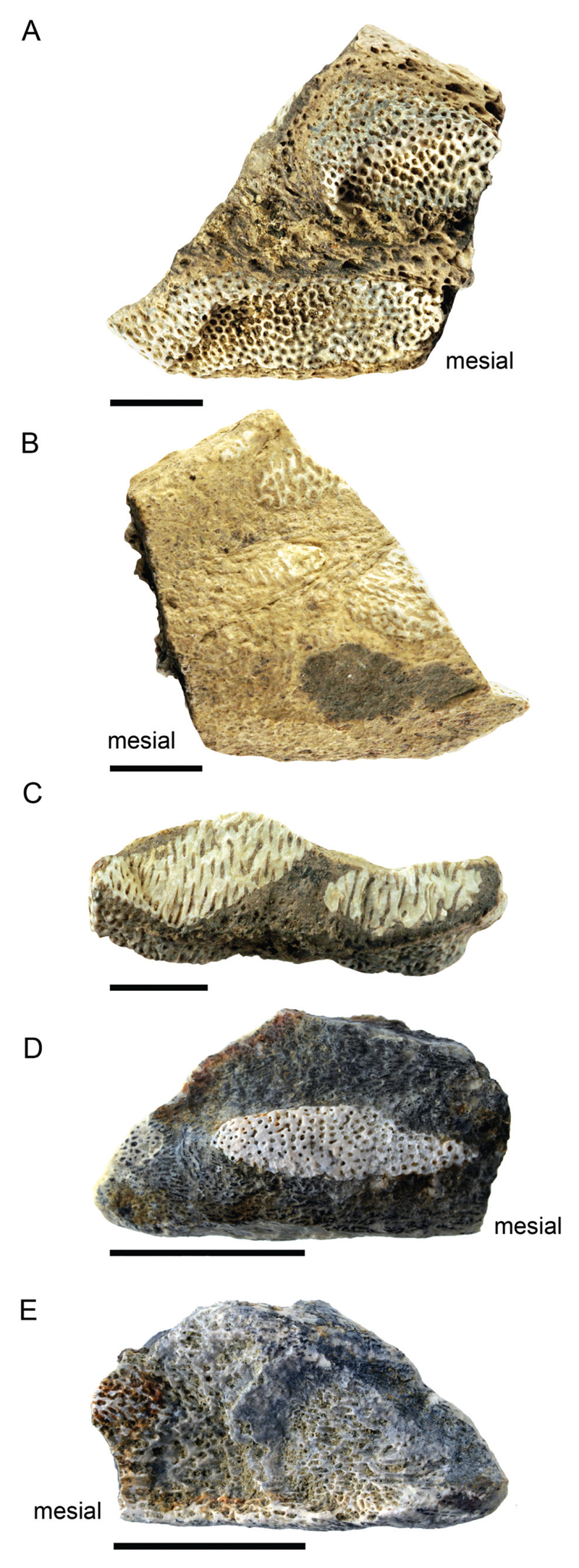
Figure 7. Fragmentary tooth plates of Ischyodus dolloi.
A–C, mandibular tooth plate NRM-PZ P15867; A, occlusal view; B. basal view; C, lingual view. D–E, palatine tooth plate, NRM-PZ P16008; D, oral view; E, aboral view. Scale bars equal 5 mm.
Material—A fragmentary mandibular tooth plate, NRM-PZ P15867, and a fragmentary palatine tooth plate, NRM-PZ P16008.
Horizon and Locality—Level 38 within the Submeseta Allo-member, locality NRM 2, Slope S Marambio Base, S64°14.778′, W056°37.169′; underneath locality NRM 5, Ridge N Marambio Base, S64°13.985′, W056°36.851′.
Age—Upper TELM 7, Priabonian, late Eocene.
Description—Both specimens from TELM 7 are very fragmentary (Fig. 7E). The mesial, distal, and lingual (= basal margin) portions of the mandibular tooth plate are broken off and missing (Fig. 7A–C). The preserved labial edge (= postocclusal margin of Ward and Grande, 1991) is straight and continuous. The symphyseal margin also is lacking.
Only portions of two heavily abraded tritorial pads are preserved, displaying the mesh-like appearance of the pleromic hard tissue that forms the hypermineralized tissue of the pads (Fig. 7A–C). The remnant of the posterior outer tritor is mesio-distally longer than high; the median tritor is rounded lingually and seems to taper mesially. The occlusal surface is undulating in lingual view, because of a depression between the posterior and median tritors.
The second specimen represents a very fragmentary, probably right palatine tooth plate (Fig. 7D–E). The fragment measures ca. 2.5 cm in anteroposterior length and ca. 1.4 cm in width, and is almost subrectangular in occlusal view. It seemingly is more depressed laterally than the mandibular tooth plate. A single tritor, the anterior inner tritor, is completely preserved; all other tritors are lacking. This tritor is elongated, with an anterior, narrow extremity, whereas the posterior part is more robust with a rounded posterior extremity. Basally, the pleromic hard tissue is exposed.
Remarks—Five chimeroids have thus far been recorded from Late Cretaceous and Paleogene strata of Antarctica: Chimaera zangerli Stahl and Chatterjee, 1999, and Callorhinchus torresi Otero et al., 2012, from Maastrichtian strata, and Ischyodus dolloi, Chimaera seymourensis Ward and Grande, 1991, and Callorhinchus stahli Kriwet and Gaździcki, 2003, from Eocene strata. Additionally, Martin and Crame (2006) indicated the presence of an additional, unidentified species of Callorhinchus in the Maastrichtian. The mandibular tooth plate reported here resembles those of Ischyodus dolloi in general appearance and arrangement of the tritorial pads, which are identified as posterior outer and median tritors in combination with the occlusal undulation caused by a depression between these two pads (see also Ward and Grande, 1991; Cicimurri and Ebersole, 2015).
Ischyodus dolloi was originally described from the late Paleocene of Belgium (Leriche, 1902). It occurs also in the Paleocene of France (Leriche, 1909), Paleocene of North America (Cicimurri and Ebersole, 2015), Paleocene–Eocene of Russia (Popov and Ivanov, 1996), and Eocene of England (Gurr, 1962; Ward, 1973). In Antarctica, this species occurs throughout the La Meseta Formation and so far is the only recorded chondrichthyan from TELM 6 (Ward and Grande, 1991; this study). Stahl and Chatterjee (2002) reported I. dolloi from the early Maastrichtian of Seymour Island based on a single right mandibular tooth plate. Hoganson and Erickson (2005) consider that specimen to be too abraded for unambiguous identification, a conclusion with which we agree. Thus, I. dolloi is restricted to the Eocene in Antarctica.
La Meseta Chondrichthyan Diversity Patterns
The chondrichthyan fauna from the La Meseta Formation on Seymour Island includes 29 species (two of which are probably new records: Striatolamia sp. cf. S. macrota and Palaeohypotodus sp. cf. P. rutoti; see above) and is the most diverse Paleogene ichthyofauna from the Southern Hemisphere during the Eocene greenhouse climate known to date. Consequently, this assemblage has the potential to provide important information about the influence of abiotic factors on diversity fluctuations of marine vertebrates in general. During the Eocene, major plate-tectonic and climatic changes occurred, including the Paleocene–Eocene Thermal Maximum, Early Eocene Climatic Optimum (EECO), and separation of Australia from Antarctica.
The overall paleotemperature trend throughout the La Meseta Formation is a decrease from the early to late Eocene (Ivany et al., 2008), and it was suggested that chondrichthyan diversity was influenced by this climatic shift, resulting in their final disappearance before the onset of ice sheet formation (ca. 40–30 Ma) (see Introduction). A detailed review of chondrichthyan occurrences throughout the La Meseta Formation based on published records and results presented here (early Ypresian–late Priabonian), however, shows that cartilaginous fishes did not disappear significantly before the onset of ice sheet formation as previously assumed, but that they occurred at least until the Priabonian, which coincides with the end of the sedimentary sequence of the La Meseta Formation and onset of Antarctic glaciation.
Detailed distribution data about chondrichthyans for TELM 1 (uppermost Thanetian) and TELM 2 (lowermost Ypresian) as reported by Long (1992a) are inconclusive, and we assume that most, if not all, of these remains come from the lowermost Ypresian, but further stratigraphic studies on Seymour Island are required. TELMs 1–3 are characterized by sediments deposited in deltaic settings under comparably high sea surface paleotemperatures, which reached their maximum of ca. 15°C during the EECO at the base of TELM 3 (ca. 51–53 Ma) according to Ivany et al. (2008). Chondrichthyan diversity, however, is lower than expected when compared with modern tropical environments with similar surface temperatures, with only two squaliform and two lamniform sharks and three chimeroids. This, however, also could represent a collecting bias. The occurrences of Deania sp. and Carcharocles auriculatus in the lowermost TELMs represent the oldest records of these species, implying that both probably evolved in the Southern Hemisphere.
Six remains of chondrichthyans were found in TELM 3, including the oldest batomorph record from Antarctica, but it lacks all records of squaliform sharks (Long, 1992a; Table 1). Living squaliform sharks generally live in the deeper waters but may enter shallow marine environments. Nevertheless, they are rather sensitive to seawater temperatures and avoid temperatures above 15°C. Long (1992b) suggested that squaliforms, which he considers to be deep-water/transitional forms, may have lived in adjoining deep-sea areas and conducted seasonal or cyclically diurnal migrations. Migrations into shallow waters in search for food also might be a possible explanation. Absence of squaliforms in TELM 3 thus might correlate with higher surface seawater temperatures, although collecting biases cannot be excluded here.
Table 1.
Stratigraphic occurrences, facies distribution, and climatic conditions of Eocene La Meseta chondrichthyan associations of Seymour Island (Antarctica) based on published records (see the text for references).
| TELM | Facies | Sea surface temperatures | Association |
|---|---|---|---|
| 7 | Shallow marine | ca. 7–8° C | Squalus sp., Squatina sp., Pristiophorus lanceolatus, Carcharocles sokolovi, Palaeohypotodus sp. cf. rutoti, Striatolamia sp. cf. macrota, Ischyodus dolloi |
| Inner estuary channels | ca. 5° C | ||
| 6 | Estuary | ca. 7° C | Ischyodus dolloi |
| ca. 15° C | |||
| 5 | Estuary | ca. 10–11°C | Heptranchias howelli, Hexanchus sp., Centrophorus sp., Dalatias licha, Squalus weltoni, Squalus woodburnei, Pristiophorus lanceolatus, Squatina sp., Pseudoginglymostoma cf. brevicaudatum, Stegostoma cf. fasciatum, Anomotodon multidenticulata, Cetorhinus sp., Isurus praecursor, Lamna cf. nasus, Odontaspis winkleri, Palaeohypotodus rutoti, Striatolamia macrota, Scoliodon sp., Myliobatis sp., Raja/Bathyraja sp., Ischyodus dolloi |
| 4 | Estuary | ca. 10–11°C | Paraorthacodus sp., Heptranchias howelli, Hexanchus sp., Centrophorus sp., Dalatias licha, Deania sp., Squalus weltoni, Squalus woodburnei, Pristiophorus lanceolatus, Squatina sp., Pseudoginglymostoma cf. brevicaudatum, Stegostoma cf. fasciatum, Anomotodon multidenticulata, Carcharocles auriculatus, Cetorhinus sp., Isurus praecursor, Lamna cf. nasus, Odontaspis winkleri, Palaeohypotodus rutoti, Striatolamia macrota, Carcharhinus sp., Scoliodon sp., Myliobatis sp., Pristis sp., Raja/Bathyraja sp., Chimaera seymourensis, Ischyodus dolloi |
| 3 | Delta plain–estuary | ca. 10–11°C | Pristiophorus lanceolatus, Carcharocles auriculatus, Lamna cf. nasus, Striatolamia macrota, Myliobatis sp., Ischyodus dolloi |
| ca. 15° C | |||
| 2 | Delta front | Callorhinchus stahli, Chimaera seymourensis, Ischyodus dolloi | |
| 1–2 | Prodelta?/inner estuarine? | Centrophorus sp., Deania sp., Carcharocles auriculatus, Striatolamia macrota |
Facies interpretation according to Marenssi et al. (2002); sea surface temperatures according to Ivany et al. (2014). For occurrence references, see the text.
Towards the end of TELM 3, temperatures decline to 10–11°C, after which they remain stable throughout TELMs 4–5 (Ivany et al., 2008). The cooler waters probably allowed temperate and cool-temperate sharks, such as Lamna cf. nasus and Palaeohypotodus rutoti, to invade the near-coastal, shallow marine environments of the Antarctic continent and also enabled squaliform sharks to reenter the estuary depositional areas. This resulted in a significant increase in taxonomic diversity of TELM 4 and 5 chondrichthyan assemblages (28 and 21 species, respectively).
The last occurrence of Odontaspis winkleri, but not of Palaeohypotodus rutoti, as previously suggested by Long (1992b) (Table 1), is seen in TELM 5. Interestingly, this Eocene temperate chondrichthyan association is far more diverse than modern associations in comparable environments (see also Case, 1992). Recently published paleoclimatic data for the La Meseta Formation indicate significantly higher mean sea surface temperatures of ca. 16.8°C at 45 Ma, corresponding more or less to the base of the Lutetian, ca. 12.6°C at 42 Ma corresponding to TELM 6, and ca. 14.1° C and 11.3°C for TELM 7 (Douglas et al., 2014). These temperature records correlate much better to a highly diverse chondrichthyan association similar to those found in the tropics today. Nonetheless, the La Meseta Formation associations are dominated by cool- to warm-temperate chondrichthyans or those living in deeper, cooler waters during warmer seasons, and thus it is in good accordance with sea water temperatures as proposed by Ivany et al. (2008). Notably, this Eocene temperate chondrichthyan fauna is more diverse than modern temperate ones.
Douglas et al. (2014) also present an age model for the La Meseta Formation indicating that the Ypresian (TELMs 1, 2, 3, 4, and parts of TELM 5) is missing. This model, however, is not generally accepted and needs further evaluation, because it would have tremendous impacts on all previous biotic considerations.
In the lower parts of TELM 6, a short warming event occurred and temperatures were similar to those at the base of TELM 3 (approximately 15°C), but subsequent cooling throughout the end of TELM 6 and TELM 7 ended with minimum seawater temperature of approximately 5°C at ca. 37 Ma according to Ivany et al. (2008). Temperatures returned to ca. 7–8°C shortly before the end of the Eocene and the onset of Antarctic ice sheet formation. A single chondrichthyan, Ischyodus dolloi, was noted from the upper part of TELM 6 (Ward and Grande, 1991), corresponding with cool-temperate climatic conditions, whereas no chondrichthyans have previously been recorded from the upper parts of TELM 7 (Priabonian). This indicates that chondrichthyans might have disappeared from Antarctic near-coastal waters due to decreasing sea surface temperatures and changing environments before establishment of the circum-Antarctic current (~40–30 Ma) (see also Kriwet, 2005).
The new shark and chimeroid finds from the Priabonian (upper TELM 7) reported herein are thus of great importance, because they verify that several chondrichthyan lineages persisted much longer in the waters surrounding the Antarctic continent. Taxonomic diversity was higher despite rather cool sea surface temperatures of approximately 5°C. All sharks reported here occupied upper trophic levels and represent either littoral-epipelagic or shelf overlapping taxa (Table 2). The chimeroid, Ischyodus, occupied the lowest trophic level and generally is considered to have lived in deeper waters of the oceans and only migrated inshore for spawning (Kriwet and Klug, 2011). Carcharocles sokolovi was a large coastal shark that probably occupied the top-most position within the latest Eocene Antarctic food web. The tooth tentatively assigned to Palaeohypotodus sp. cf. P. rutoti here possibly represents the last record of this cool-temperate shark. The angel shark, Squatina sp., represents a benthic taxon that today inhabits the upper continental and insular shelves down to more than 100 m. The occurrence of this taxon in TELM 7 implies that favorable benthic habitats around the Antarctic continent must have been present.
Table 2.
Environmental distribution and trophic level of late Bartonian Antarctic chondrichthyans.
| Taxon | Living analogue | Zonation | Trophic level |
|---|---|---|---|
| Squalus sp. | Squalus acanthias | Shelf overlap species | 4.1 / 4.4* |
| Squatina sp. | Squatina squatina | Continental shelves | 4.1 / 4* |
| Pristiophorus lanceolatus | Pristiophorus cirratus | Shelf overlap species | 4.2 / 4.2* |
| Striatolamia sp. cf. macrota | Carcharias taurus | Littoral-epipelagic | 4.4 / 4.5* |
| Palaeohypotodus sp. cf. rutoti | Odontaspis ferox | Littoral-epipelagic | 4.4 / 4.2* |
| Carcharocles sokolovi | Carcharodon carcharias | Littoral-epipelagic | 4.3 / 4.5* |
| Ischyodus dolloi | Chimaera spp. | Deep slope/open marine | 3.5–4.1* |
Inferences are based on comparisons with extant analogues displaying similar structures. Zonations according to Compagno (1984) and Compagno et al. (1991); trophic-level assignment according to Ebert and Bizzaro (2007) and www.fishbase.org (asterisk).
The final disappearance of chondrichthyans seemingly concurs with abrupt ice sheet formation on Antarctica, which occurred 34 Ma (Goldner et al., 2014). Decreasing sea temperatures and glaciation resulted in reduced shelf areas, which not only influenced the decrease of chondrichthyans before the end of the Eocene in Antarctica, but also most likely was the driving force behind their ultimate disappearance in the latest Eocene (Priabonian). Diversity patterns of chondrichthyans throughout the La Meseta Formation most likely followed climatic conditions rather than paleogeographic constellations, pending further analyses.
Supplementary Material
SUPPLEMENTAL DATA—Supplemental materials are available for this article for free at www.tandfonline.com/ujvp
Acknowledgments
We would like to thank J. Hagström (NRM) and J. Moly (MLP) for assistance in the field. The Argentinian Antarctic Institute (IAA-DNA), Argentinian Air Force, and Swedish Polar Research Secretariat (SPFS) are acknowledged for logistic support for field work on Seymour Island. We are grateful to Z. Johanson and E. Bernard (Natural History Museum London) for the possibility to study comparative material under their care. Comments by T. D. Cook (University of Alberta, Canada) and C. Duffin (Surrey, U.K.) greatly improved the manuscript. Financial support for this project is provided by the Austrian Science Fund (FWF, grant P26465-B25 to J.K.), the Swedish Research Council (VR grant 2009-4447 to T.M.), the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET grant PIP 0462 to M.R.), and the Argentinian National Agency for Promotion of Science and Technology (ANPCyT grant PICTO-2010-152 0093 to M.R.).
Literature Cited
- Adnet S. Nouvelles faunes de sélaciens (Elasmobranchii, Neoselachii) de l’Éocène des Landes (Sud-Ouest, France). Implication dans les connaissances des communautés d’eaux profondes. Palaeo Ichthyologica. 2006;10:1–128. [Google Scholar]
- Agassiz L. Révue critique des poissons fossiles figurés dans l’Ittiolitologia veronese. Neues Jahrbuch für Mineralogie, Geognosie, Geologie und Petrefakten-Kunde. 1835;1835:290–316. [Google Scholar]
- Agassiz L. Recherches sur les poissons fossiles. Vol. 5. Petitpierre: Neuchâtel et Soleure; 1833–44. p. 1420. (with supplements) [Google Scholar]
- Albertson RC, Yan YL, Titus TA, Pisano E, Vacchi M, Yelick PC, Detrich HW, Postlethwait JH. Molecular pedomorphism underlies craniofacial skeletal evolution in Antarctic notothenioid fishes. BMC Evolutionary Biology. 2010;10:1–12. doi: 10.1186/1471-2148-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrianavalona TH, Ramihangihajason TN, Rasoamiaramanana A, Ward DJ, Ali JR, Samonds KE. Miocene shark and batoid fauna from Nosy Makamby (Mahajanga Basin, Northwestern Madagascar) PLoS ONE. 2015;10:e0129444. doi: 10.1371/journal.pone.0129444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate SP, Espinosa-Arrubarrena L. The fossil history of Carcharodon and its possible ancestor, Cretolamna: a study in tooth identification. In: Klimley AP, Ainley D, editors. Great White Sharks: The Biology of Carcharodon carcharias. Academic Press; San Diego, California: 1996. pp. 19–36. [Google Scholar]
- Applegate SP, Uyeno T. The first discovery of a fossil tooth belonging to the shark genus Heptranchias, with a new Pristiophorus spine, both from the Oligocene of Japan. Bulletin of the National Science Museum, Series C (Geology & Paleontology) 1968;11:195–200. [Google Scholar]
- Arambourg C. Les vertébrés fossiles des gisements de phosphates (Maroc-Algérie-Tunisie) Notes et Mémoires du Service Géologique du Maroc. 1952;92:1–372. [Google Scholar]
- Balushkin AV. Proeleginops grandeastmanorum gen. et sp. nov. (Perciformes, Notothenioidei, Eleginopsidae) from the Late Eocene of Seymour Island (Antarctica) is a fossil notothenioid, not a gadiform. Journal of Ichthyology. 1994;34:10–23. [Google Scholar]
- Barnes LG, Howard H, Hutchinson HJ, Welton BJ. The vertebrate fossils of the marine Cenozoic San Mateo Formation at Oceanside, California. In: Abbott PL, O’Dunn S, editors. Geologic Investigations of the San Diego Coastal Plain. San Diego Association of Geologists; San Diego, California: 1981. pp. 53–70. [Google Scholar]
- Bendix-Almgreen SE. Notes on the Upper Cretaceous and Lower Tertiary fish faunas of northern West Greenland. Meddelelser fra Dansk Geologisk Forening. 1969;19:204–217. [Google Scholar]
- Berg LS. System Rezenten und fossilen Fischartigen und Fisch. Hochschulbücher für Biologie; Berlin: 1958. p. 310. [Google Scholar]
- de Blainville HMD. Sur les ichthyolites ou les poissons fossiles. Nouveau Dictionnaire d’Histoire Naturelle. 1818;27:310–391. [Google Scholar]
- Bleeker P. Enumeratio specierum piscium hucusque in Archipelago indico obervatarum. Acta Societatis Regiae Scientiarum Indo-Neerlandae. 1859;6:1–276. [Google Scholar]
- Bonaparte CL. Iconografia della fauna italica per le quattro classi degli animali vertebrati. Tomo III Pesci Fasc. 1832;1:29–58. [Google Scholar]
- Bonaparte CL. Selachorum tabula analytica. Nuovi Annali delle Scienze Naturali. 1834;1:6–11. [Google Scholar]
- Bonaparte CL. Selachorum tabula analytica. Nuovi Annali delle Scienze Naturali. 1838;1:22–23. [Google Scholar]
- Brozobathy R, Kalabis V. Die Fischzähne aus Pouzdrany (Pouzdrany-Schichten, Oligozän) Acta Musei Moraviae, Scientiae Naturales. 1970;55:41–50. [Google Scholar]
- Buckland W. A notice on the fossil beaks of four extinct species of fishes, referrible to the genus Chimaera, which occur in the Oolitic and Cretaceous Formations of England. Proceedings of the Geological Society of London. 1835;2:205–208. [Google Scholar]
- Cappetta H. Chondrichthyes II: Mesozoic and Cenozoic Elasmobranchii. In: Schultze H-P, editor. Handbook of Paleoichthyology. 3B. Gustav Fischer Verlag; Stuttgart: 1987. p. 193. [Google Scholar]
- Cappetta H. Chondrichthyes: Mesozoic and Cenozoic Elasmobranchii: Teeth. In: Schultze H-P, editor. Handbook of Paleoichthyology. 3E. Verlag Dr. Friedrich Pfeil; Munich: 2012. p. 512. [Google Scholar]
- Cappetta H, Case GR. Contribution à l’étude des Sélaciens du groupe Monmouth (Campanien-Maestrichtien) du New Jersey. Palaeontographica, Abteilung A. 1975;151:1–46. [Google Scholar]
- Cappetta H, Case GR. Additions aux faunes de sélaciens du Crétacé du Texas (Albien supérieur-Campanien) Palaeo Ichthyologica. 1999;9:5–111. [Google Scholar]
- Cappetta H, Nolf D. Révision de quelques Odontaspididae (Neoselachii: Lamniformes) du Paléocène et de l’Éocéne du Bassin de la Mer du Nord. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, Sciences de la Terre. 2005;75:e266. [Google Scholar]
- Case GR, Cappetta H. The Eocene selachian fauna from the Fayum Depression in Egypt. Palaeontographica Abteilung A. 1990;212:1–30. [Google Scholar]
- Case JA. Evidence from fossil vertebrates for a rich Eocene Antarctic marine environment. Antarctic Research Series. 1992;56:119–130. [Google Scholar]
- Casier E. Note sur la collection des poissons paléocènes et éocènes de l’Enclave de Cabinda (Congo) Annales du Musée du Congo Belge, Série A (Minéralogie Géologie, Paléontologie) 1960;1:1–48. [Google Scholar]
- Casier E. Poissons de l’Éocène inferieur de Katharinenhof-Fehmarn (Schleswig-Holstein) Bulletin de l’Institut Royal des Sciences Naturelles de Belgique. 1967;43:1–23. [Google Scholar]
- Cicimurri DJ, Ebersole JA. Paleocene chimaeroid fishes (Chondrichthyes: Holocephali) from the eastern United States, including two new species of Callorhinchus. PaleoBios. 2015;32:1–29. [Google Scholar]
- Cicimurri DJ, Knight JL. Late Oligocene sharks and rays from the Chandler Bridge Formation, Dorchester County, South Carolina, USA. Acta Palaeontologica Polonica. 2009;54:627–647. [Google Scholar]
- Cigala-Fulgosi F. A deep water elasmobranch fauna from a lower Pliocene outcropping (Northern Italy). In: Uyeno T, Arai R, Taniuchi T, Matsuura K, editors. Proceedings of the Second International Conference on Indo-Pacific Fishes; Tokyo. 29 July–3 August 1985; Tokyo: Ichthyological Society of Japan; 1986. pp. 133–139. [Google Scholar]
- Cione AL, Exposito E. Chondrichthyes (Pisces) del ‘Patagoniano’ S.L. de Astra, Golfo de San Jorge, Prov. de Chubut, Argentina. Su significacion paleoclimática y paleobiogeográfica; Congreso Argentino de Paleontología y Bioestratigrafía No. 2 y Congreso Latinoamericano de Paleontologia No. 1, Actas 2; Buenos Aires. 2–6 April 1978; Buenos Aires: Asociación Paleontológica Argentina; 1980. pp. 275–290. [Google Scholar]
- Cione AL, Pandolfi A. A fin spine of Heterodontus from the Patagoniano of Trelew, Chubut, Argentina. Tertiary Research. 1984;6:59–63. [Google Scholar]
- Cione AL, Reguero MA. Extension of the range of hexanchid and isurid sharks in the Eocene of Antarctica and comments on the occurrence of hexanchids in recent waters of Argentina. Ameghiniana. 1995;32:151–157. [Google Scholar]
- Cione AL, Reguero MA. A middle Eocene basking shark (Lamniformes, Cetorhinidae) from Antarctica. Antarctic Science. 1998;10:83–88. [Google Scholar]
- Compagno LJV. Interrelationship of the living elasmobranchs. Zoological Journal of the Linnean Society. 1973;53(1, Supplement):15–61. [Google Scholar]
- Compagno JVL. FAO Species Catalogue. Sharks of the World, Part 1—Hexanchiformes to Lamniformes. 1984;4:1–250. FAO Fisheries Synopsis No.125. [Google Scholar]
- Compagno JVL, Ebert DA, Cowley PD. Distribution of offshore demersal cartilaginous fishes (Class Chondrichthyes) of the west coast of southern Africa, with notes on their systematics. South African Journal of Marine Science. 1991;11:43–139. [Google Scholar]
- Compagno LJV, Dando M, Fowler S. A Field Guide to the Sharks of the World. Harper Collins; London; 2005. p. 368. [Google Scholar]
- Cunningham SB. A comparison of isolated teeth of early Eocene Striatolamia macrota (Chondrichthyes, Lamniformes), with those of a recent sand shark, Carcharias taurus. Tertiary Research. 2000;20:17–32. [Google Scholar]
- Cvancara AM, Hoganson JW. Vertebrates of the Cannon-ball Formation (Paleocene) in North and South Dakota. Journal of Vertebrate Paleontology. 1993;13:1–23. [Google Scholar]
- Dalinkevicius JA. On the fossil fishes of the Lithuanian Chalk. I. Selachii. Mémoires de la Faculté des Sciences de l’Université de Vytautas le Grand. 1935;9:243–305. [Google Scholar]
- Davis JW. On fossil fish-remains from the Tertiary and Cretaceo-Tertiary formations of New-Zealand. Scientific Transactions of the Royal Dublin Society. 1888;4:1–48. [Google Scholar]
- Del Valle RA, Elliot DH, Macdonald DIM. Sedimentary basins on the east flank of the Antarctic Peninsula: proposed nomenclature. Antarctic Science. 1992;4:477–478. [Google Scholar]
- Diedrich CG. Eocene (Lutetian) shark-rich coastal paleoenvironments of the southern North Sea Basin in Europe: biodiversity of the marine Fürstenau Formation including early White and Megatooth Sharks. International Journal of Oceanography. 2012;2012:22. doi: 10.1155/2012/565326. ID 565326. [DOI] [Google Scholar]
- Dingle RV, Lavelle M. Late Cretaceous–Cenozoic climatic variations of the northern Antarctic Peninsula: new geochemical evidence and review. Palaeogeography, Palaeoclimatology, Palaeoecology. 1998;141:215–232. [Google Scholar]
- Doktor M, Gazdzicki A, Jerzmanska A, Porebski SJ, Zastawniak E. A plant and fish assemblage from the Eocene La Meseta Formation of Seymour Island (Antarctic Peninsula) and its environmental implications. Palaeontologia Polonica. 1996;55:127–146. [Google Scholar]
- Douglas PM, Affek HP, Ivany LC, Houben AJ, Sijp WP, Sluijs A, Schouten S, Pagani M. Pronounced zonal heterogeneity in Eocene southern high-latitude sea surface temperatures. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:6582–6587. doi: 10.1073/pnas.1321441111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duméril AHC. Zoologie analytique, ou méthode naturelle de classification des animaux. Allais; Paris: 1806. p. 344. [Google Scholar]
- Dutheil DB, Moreau F, De Plöeg G. Les ichthyofaunes du gisement à ambre de Le Quesnoy (Paléocène et Éocéne du bassin de Paris, France) Cossmanniana. 2006;11:1–13. [Google Scholar]
- Eastman JT. Antarctic Fish Biology: Evolution in a Unique Environment. Academic Press; San Diego, California: 1993. p. 322. [Google Scholar]
- Eastman JT, Grande L. Evolution of the Antarctic fish fauna with emphasis on the recent notothenioids. In: Crame JA, editor. Origin and Evolution of the Antarctic Biota. Vol. 47. Geological Society of London Special Publications; London: 1989. pp. 241–252. [Google Scholar]
- Eastman JT, Grande L. Late Eocene gadiform (Teleostei) skull from Seymour Island, Antarctic Peninsula. Antarctic Science. 1991;3:87–95. [Google Scholar]
- Ebert DA, Bizzarro JJ. Standardized diet compositions and trophic levels of skates (Chondrichthyes: Rajiformes: Rajoidei) Environmental Biology of Fishes. 2007;80:221–237. [Google Scholar]
- Egerton P. On some new species of fossil chimaeroid fishes, with remarks on their general affinities. Proceedings of the Geological Society of London. 1843;4(94):153–157. [Google Scholar]
- Elliot DH, Trautman TA. Lower Tertiary strata on Seymour Island, Antarctic Peninsula. In: Craddock C, editor. Antarctic Geoscience. University of Wisconsin Press, Madison, Wisconsin; 1982. pp. 287–297. [Google Scholar]
- Engelbrecht A, Kriwet J, Mörs T, Reguero M. Diversity of Eocene Antarctic sand tiger sharks (Chondrichthyes, Odontaspididae): climatic controls or implications for nursery areas? Journal of Vertebrate Paleontology, Program and Abstracts. 2014;2014:125. [Google Scholar]
- Fitch JE. Otoliths and other fish remains from the Timmes Point Silt (Early Pleistocene) at San Pedro, California. Contributions in Science, Los Angeles County Museum of Natural History. 1968;146:1–29. [Google Scholar]
- Freile D, DeVore ML, Pramely D. The first record of Carcharocles auriculatus from the Oligocene of Georgia in the context of previous Gulf Coast records. Georgia Journal of Science. 2001;59:128–136. [Google Scholar]
- Ferrusquia-Villafranca I, Applegate SP, Espinosa-Arruharrena L. First Paleogene selachian fauna of the Middle American–Caribbean-Antillean region, La Mesa de Copoya, west-central Chiapas, Mexico. Systematics and paleontological significance. Revista Mexicana de Ciencias Geológicas. 1999;16:155–174. [Google Scholar]
- Garman S. Genera and families of the chimaeroids. Proceedings of the New England Zoölogical Club. 1901;2:75–77. [Google Scholar]
- Glikman LS. Sharks of the Palaeogene and Their Stratigraphic Significance. Nauka Press; Moscow, Russia: 1964. p. 228. [Russian] [Google Scholar]
- Goldner A, Herold N, Huber M. Antarctic glaciation caused ocean circulation changes at the Eocene-Oligocene transition. Nature. 2014;511:574–577. doi: 10.1038/nature13597. [DOI] [PubMed] [Google Scholar]
- Gon O, Heemstra PC, editors. Fishes of the Southern Ocean. Vol. 1. JLB Smith Institute of Ichthyology; Grahamstown, South Africa: 1990. p. 462. [Google Scholar]
- Goodrich ES. The Vertebrata Craniata (first fascicle: cyclostomes and fishes); Part IX. In: Lankester ER, editor. A Treatise on Zoology. Adam and Charles Black; London: 1909. p. 518. [Google Scholar]
- Gottfried MD, Rabarison JA. First Mesozoic Gondwanan record of a sawshark (Chondrichthyes, Pristiophoriformes), from the Late Cretaceous of Madagascar. Journal of Vertebrate Paleontology. 1997;17:750–751. [Google Scholar]
- Grande L, Eastman JT. A review of Antarctic ichthyofaunas in the light of new fossil discoveries. Palaeontology. 1986;29:113–137. [Google Scholar]
- Gurr PR. A new fish fauna form the Wollwich Bottom Bed (Sparnacaian) of Herne Bay, Kent. Proceedings of the Geologist’s Association, London. 1962;73:419–449. [Google Scholar]
- Herman J. Les vertébrés du Landénien Inférieur (Lla ou Heersien) de Maret (Hameau d’Orp-le-Grand) Bulletin de la Société Belge de Géologié, de Paléontologie et d’Hydrologie. 1972;81:191–207. [Google Scholar]
- Herman J. Contribution à la connaissance da la faune ichthyologique des phosphates du Maroc. Annales de la Société Géologique de Belgique. 1973;95:271–284. [Google Scholar]
- Herman J. Les Sélaciens des terrains Néocrétacés et Paléocénes de Belgique et des contrés limitrophes: eléments d’une biostratgraphie intercontinentale. Service Geologique de Belgique Memoire. 1975;15:1–450. [Google Scholar]
- Herman J. Additions to the Eocene fish fauna of Belgium. 3. Revision of the Orectolobiformes. Tertiary Research. 1977;1:127–138. [Google Scholar]
- Herman J, Crochard M, Girardot M. Quelques restes de sélaciens récoltés dans les sables du Kattendijk éa Kallo. I. Selachii-Euselachii. Bulletin de la Société Belge de Géologié. 1974;83:15–31. [Google Scholar]
- Hoganson JW, Erickson JM. A new species of Ischyodus (Chondrichthyes: Holocephali: Callorhynchidae) from Upper Maastrichtian shallow marine facies of the Fox Hills and Hell Creek formations, Williston Basin, North Dakota, USA. Palaeontology. 2005;48:709–721. [Google Scholar]
- Huxley TH. On the application of the laws of evolution to the arrangement of the Vertebrata, and more particularly of the Mammalia. Proceedings of the Zoological Society of London. 1880;1880:649–662. [Google Scholar]
- Ingram T, Mahler DL. Niche diversification follows key innovation in Antarctic fish radiation. Molecular Ecology. 2011;20:4590–4591. doi: 10.1111/j.1365-294x.2011.05321.x. [DOI] [PubMed] [Google Scholar]
- Iserbyt A, De Schutter PJ. Quantitative analysis of Elasmobranch assemblages from two successive Ypresian (Early Eocene) facies at Marke, western Belgium. Geologica Belgica. 2012;15:146–153. [Google Scholar]
- Itoigawa J, Nishimoto H. Fossil elasmobranchs (shark teeth) from the Miocene series of Mizunami. Bulletin of the Mizunami Fossil Museum. 1974;1:243–262. [Google Scholar]
- Itoigawa J, Nishimoto H, Karasawa H, Okumura Y. Miocene fossils of the Mizunami Group, central Japan. 3. Elasmo branchs. Monography of the Mizunami fossil Museum. 1985;5:1–89. [Google Scholar]
- Ivany LC, Lohmann KC, Hasiuk F, Blake DB, Glass A, Aronson RB, Moody RM. Eocene climate record of a high southern latitude continental shelf: Seymour Island, Antarctica. Geological Society of America Bulletin. 2008;120:659–678. [Google Scholar]
- Jaekel O. Ueber die systematische Stellung und über fossile Reste der Gattung Pristiophorus. Zeitschrift der Deutschen Geologischen Gesellschaft. 1890;42:86–120. [Google Scholar]
- Jaekel O. Unter-tertiäre Selachier aus Südrussland. Mémoires du Comité geologique de St Petersburg. 1895;9(4):19–35. [Google Scholar]
- Jerve A, Johanson Z, Ahlberg P, Boisvert C. Embryonic development of the fin spines in Callorhinchus milii (Holocephali); implications for chondrichthyan fin spine evolution. Evolution & Development. 2014;16:339–353. doi: 10.1111/ede.12104. [DOI] [PubMed] [Google Scholar]
- Jerzmanska A. Isolated vertebrae of teleostean fishes from the Paleogene of Antarctica. Polish Polar Research. 1988;9:421–435. [Google Scholar]
- Jerzmanska A. First articulated teleost fish from the Paleogene of West Antarctica. Antarctic Science. 1991;3:309–316. [Google Scholar]
- Jerzmanska A, Swidnicki J. Gadiform remains from the La Meseta Formation (Eocene) of Seymour Island, West Antarctica. Polish Polar Research. 1992;13:241–253. [Google Scholar]
- Jordan DS, Hannibal H. Fossil sharks and rays of the Pacific slope of North America. Bulletin of the Southern California Academy of Sciences. 1923;22:27–63. [Google Scholar]
- Keyes IW. The Cenozoic sawshark Pristiophorus lanceolatus (Davis) (Order Selachii) of New Zealand and Australia, with a review of the phylogeny and distribution of world fossil and extant Pristiophoridae. New Zealand Journal of Geology and Geophysics. 1982;25:459–474. [Google Scholar]
- Klug S, Kriwet J. Node age estimations and the origin of angel sharks, Squatiniformes (Neoselachii, Squalomorphii) Journal of Systematic Palaeontology. 2013;11:91–110. [Google Scholar]
- Kozlov VA. [Additions to the Paleogene elasmobranch fauna of western Kazakhstan. Turania, a new shark genus (Odontaspididae) and a new ray species (genus Archaeomanta, Mobulidae)] Materialy po Stratigrafii i Paleontologii Urala. 2001:83–86. [Russian] [Google Scholar]
- Kriwet J. Additions to the Eocene selachian fauna of Antarctica with comments on Antarctic selachian diversity. Journal of Vertebrate Paleontology. 2005;25:1–7. [Google Scholar]
- Kriwet J, Gaździcki A. New Eocene Antarctic chimeroid fish (Holocephali, Chimaeriformes) Polish Polar Research. 2003;24:29–51. [Google Scholar]
- Kriwet J, Hecht T. A review of early gadiform evolution and diversification: first record of a rattail fish skull (Gadiformes, Macrouridae) from the Eocene of Antarctica, with otoliths preserved in situ. Naturwissenschaften. 2008;95:899–907. doi: 10.1007/s00114-008-0409-5. [DOI] [PubMed] [Google Scholar]
- Kriwet J, Klug S. An embryonic mandibular tooth plate and associated remains of a Late Jurassic chimaeroid (Holocephali, Chimaeriformes) from the Iberian Peninsula. Journal of Vertebrate Paleontology. 2011;31:954–961. [Google Scholar]
- Kriwet J, Endo H, Stelbrink B. On the occurrence of the Taiwan angel shark, Squatina formosa Shen & Ting, 1972 (Chondrichthyes, Squatinidae) from Japan. Zoosystematics and Evolution. 2010;86:117–124. [Google Scholar]
- Kriwet J, Engelbrecht A, Reguero M, Mörs T. Eocene Antarctic fish diversity patterns revisited. XII Annual Meeting of the European Association of Vertebrate Paleontologists; Torino, Italy. 24–28 June 2014; 2014. p. 192. [Google Scholar]
- Kriwet J, Lirio M, Nunez H, Puceat E, Lécuyer C. Late Cretaceous Antarctic fish diversity. In: Pirrie D, Francis JE, Crame JA, editors. Cretaceous-Tertiary High-Latitude Palaeoenvironments, James Ross Basin, Antarctica. Geological Society of London Special Publication 258; London: 2006. pp. 83–100. [Google Scholar]
- Last PR, White WT. Three new angel sharks (Chondrichthyes: Squatinidae) from the Indo-Australian region. Zootaxa. 2008;1734:1–26. [Google Scholar]
- Latham J. An essay on the various species of Sawfish. Transactions of the Linnaean Society of London. 1794;2:273–282. [Google Scholar]
- Lawley R. Nuovi studi sopra ai pesci i altri vertebrati fossili delle Colline Toscane. Tipografia dell Arte della Stampa, Florence. 1876;122 [Google Scholar]
- LeDeux JC. Les Squalidae (Euselachii) miocènes des environs d’Avignon (Vaucluse) Documents des Laboratoires de Géologie de la Faculté des Sciences de Lyon. 1972;52:133–175. [Google Scholar]
- Leriche M. Les poissons tertiaires de la Belgique I. Les poissons paléocènes. Mémoires du Musée Royale du Historie Naturelle du Belgique. 1902;2:1–48. [Google Scholar]
- Leriche M. Les poissons éocènes de la Belgique. Mémoires du Musée Royal d’Histoire Naturelle de Belgique. 1905;3:49–22. [Google Scholar]
- Leriche M. Note préliminaire sur des poissons nouveaux de l’Oligocène Belge. Bulletin de la Société Belge de Géologie, de Palèontologie et d’Hydrologie, Procés-Verbaux. 1909;22:378–384. [Google Scholar]
- Leriche M. Les poissons tertiaires de la Belgique. III. Les poissons oligocènes. Mémoires du Musée Royal d’Histoire Naturelle de Belgique. 1910;5:229–363. [Google Scholar]
- Leriche M. Contribution à l’étude des faunes ichthyologiques marines des terrains tertiaires de la Plaine Côtière Atlantique et du centre des Etats-Unis. Les synchronismes des formations tertiaires des deux côtés de l’Atlantique. Mémoires de la Société géologique de France. 1942;45:1–110. [Google Scholar]
- Leriche M. Les poissons Tertiaires de la Belgique (supplement) Institut Royal des Sciences Naturelles de Belgique, Mémoires. 1951;118:475–600. [Google Scholar]
- Linnaeus C, editor. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Volume 1: Regnum Animale. Editio decima, reformata. Laurentii Salvii; Stockholm: 1758. p. 824. [Google Scholar]
- Long DJ. Sharks from the La Meseta Formation (Eocene), Seymour Island, Antarctic Peninsula. Journal of Vertebrate Paleontology. 1992a;12:11–32. [Google Scholar]
- Long DJ. An Eocene wrasse (Perciformes: Labridae) from Seymour Island. Antarctic Science. 1992b;4:35–237. [Google Scholar]
- Long DJ. Paleoecology of Eocene Antarctic sharks. Antarctic Research Series. 1992c;56:131–139. [Google Scholar]
- Long DJ. Quaternary colonization or Paleogene persistence?: Historical biogeography of skates (Chondrichtyes: Rajidae) in the Antarctic Ichthyofauna. Paleobiology. 1994;20:215–228. [Google Scholar]
- Long DJ, Stilwell JD. Fish remains from the Eocene of Mount Discovery, East Antarctica. In: Stilwell JD, Feldmann RM, editors. Paleobiology and Paleoenvironments of Eocene Rocks, McMurdo Sound, East Antarctica. Antarctic Research Series. Vol. 76. American Geophysical Union; Washington, D.C: 2000. pp. 349–353. [Google Scholar]
- Maisey JG. Chondrichthyan dorsal spines and the relationships of spinate chondrichthyans. Ph.D. dissertation, University of London; London: 1974. p. 592. [Google Scholar]
- Maisey JG. Finspine morphogenesis in squalid and heterodontid sharks. Zoological Journal of the Linnean Society. 1979;66:161–188. [Google Scholar]
- Maisey JG. Studies on the Paleozoic selachian genus Ctenacanthus Agassiz. No. 2, Bythiacanthus St. John and Worthen, Amelacanthus, new genus, Eunemacanthus St. John and Worthen, Sphenacanthus Agassiz, and Wodnika Münster. American Museum Novitates. 1982;2722:1–24. [Google Scholar]
- Marenssi SA, Santillana SN, Rinaldi CA. Stratigraphy of the La Meseta Formation (Eocene), Marambio (Seymour) Island, Antarctica. In: Casadio S, editor. Paleógeno de América del Sur y de la Península Antártica: Asociación Paleontológica Argentina. Vol. 5. Publicación Especial; Buenos Aires, Argentina: 1998. pp. 137–146. [Google Scholar]
- Marshall C. Aspects of protein cold adaptation in Antarctic fish. Adaptation and Evolution in Marine Environments. 2012;1:143–155. [Google Scholar]
- Martin JE, Crame JA. Paleobiological significance of highlatitude Late Cretaceous vertebrate fossils from the James Ross Basin, Antarctica. In: Francis JE, Pirrie D, Crame JA, editors. Cretaceous-Tertiary High-Latitude Paleoenvironments, James Ross Basin, Antarctica. Geological Society London, Special Publications 258; 2006. pp. 109–124. [Google Scholar]
- Menner VV. The Palaeogene sharks of Mangyschlak, Emba and from the east of Oural. Bulletin de la Société des Naturalistes de Moscou, Section Géologique. 1928;6:291–338. [Google Scholar]
- Meyer RE. Late Cretaceous elasmobranchs from the Mississippi and East Texas embayments of the Gulf Coastal Plain. Ph.D. dissertation; University of Texas at Arlington, Texas: 1974. p. 419. [Google Scholar]
- Militante CS. [Accessed July 31, 2015];Pristiophoridae. 2012 Available at http://www.fishbase.org/summary/FamilySummary.php?ID=14.
- Montes M, Nozal F, Santillana S, Marenssi S, Olivero E. Mapa Geológico de la Isla Marambio (Seymour); escala 1:20.000. Serie Cartográfica Geocientífica Antártica. Con texto complementario. Instituto Geológico y Minero de España; Madrid; Instituto Antártico Argentino Buenos Aires: 2013. [Google Scholar]
- de Muizon C, De Vries TJ. Geology and paleontology of late Cenozoic marine deposits in the Sacaco area (Peru) Geologische Rundschau. 1985;74:547–563. [Google Scholar]
- Müller J, Henle J. Gattungen der Haifische und Rochen nach einer von ihm mit Hrn. Henle unternommenen gemeinschaftlichen Arbeit über die Naturgeschichte der Knorpelfische. Berichte der königlichen preussischen Akademie der Wissenschaften zu Berlin. 1837;1837:111–118. [Google Scholar]
- Müller J, Henle J. Systematische Beschreibung der Plagiostomen. Veit and Co; Berlin: 1838–1841. p. 200. [Google Scholar]
- Near TJ, Dornburg A, Kuhn KL, Eastman JT, Pennington JN, Patarnello T, Zane L, Fernández DA, Jones CD. Ancient climate change, antifreeze, and the evolutionary diversification of Antarctic fishes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:434–3439. doi: 10.1073/pnas.1115169109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto H, Morozumi Y. Late Cretaceous elasmobranchs from the Izumi Mountain Range. Bulletin of the Mizunami Fossil Museum. 1979;6:133–140. [Google Scholar]
- Noubhani A, Cappetta H. Systématique, biostratigraphie, évolution et dynamique des faunes. Vol. 8. Palaeo Ichthyologica; 1997. Les Orectolobiformes, Carcharhiniformes et Myliobatiformes (Elasmobranchii, Neoselachii) des Bassins à phosphate du Maroc (Maastrichtien-Lutétien basal) pp. 1–327. [Google Scholar]
- Otero AR, Soto-Acuna S. New chondrichthyans from Bartonian-Priabonian levels of Río de Las Minas and Sierra Dorotea, Mangallanes Basin, Chilean Patagonia. Andean Geology. 2015;42:268–283. [Google Scholar]
- Otero RA, Torres T, Le Roux JP, Herve JP, Fenning CM, Yury Yánez RE, Rubilar-Rogers D. A Late Eocene age proposal for the Loreto Formation (Brunswick Peninsula, southern-most Chile), based on fossil cartilaginous fishes, paleobotany and radiometric evidence. Andean Geology. 2012;39:180–200. [Google Scholar]
- Otero RA, Oyarzún JL, Soto-Acuna S, Yury-Yánez RE, Gutierrez NM, Le Roux JP, Torres T, Hervé F. Neoselachians and Chimaeriformes (Chondrichthyes) from the latest Cretaceous-Paleogene of Sierra Baguales, southernmost Chile. Chronostratigraphic, paleobiogeographic and paleoenvironmental implications. Journal of South American Earth Sciences. 2013;48:13–30. [Google Scholar]
- Padilla A, Eberle JJ, Gottfried MD, Sweet AR, Hutchi-son JH. A sand tiger shark-dominated fauna from the Eocene Arctic greenhouse. Journal of Vertebrate Paleontology. 2014;34:1307–1316. [Google Scholar]
- Patterson C. The phylogeny of the chimaeroids. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 1965;249:101–21. [Google Scholar]
- Pfeil FH. Neoselachian teeth collected from phosphorite-bearing Greensand on Chatham Rise east of New Zealand. Geologisches Jahrbuch, Reihe D. 1984;65:107–115. [Google Scholar]
- Pledge NS. Fossil elasmobranch teeth of South Australia and their stratigraphic distribution. Transactions of the Royal Society of South Australia. 1967;91:135–60. [Google Scholar]
- Popov EV, Ivanov AV. ‘Oscillating tendencies’ in morphogenesis in the Cretaceous-Paleogene Ischyodus (Chimaeroidei, Edaphodontidae). Collection of Papers of the Conference ‘Geological Sciences-96’; Saratove, Russia: Saratov State University; 1996. pp. 53–57. [Russian] [Google Scholar]
- Priem F. Sur des vertébrés de fossils de l’Éocène moyen d’Egypte. Bulletin de la Societé géologique de France. 1907;7:412–419. [Google Scholar]
- Purdy RW. Chondrichthyan fishes from the Paleocene of South Carolina. In: Sanders AE, editor. Paleobiology of the Williamsburg Formation (Black Mingo Group; Paleocene) South of Carolina, U.S.A. Vol. 88. Transactions of the American Philosophical Society; 1998. pp. 122–146. [Google Scholar]
- Purdy RW, Schneider VP, Applegate SP, McLellan JH, Meyer RL, Slaughter R. The Neogene sharks, rays, and bony fishes from Lee Creek Mine, Aurora, North Carolina. In: Ray CE, Bohaska DJ, editors. Geology and Paleontology of the Lee Creek Mine, North Carolina, III. 2001. pp. 71–202. Smithsonian Contributions to Paleobiology No. 90. [Google Scholar]
- Rafinesque CS. Caratteri di alcuni nuovi generi e nuove specie di animali e pinate della Sicilia, con varie osservazioni sopra i medisimi. San Filippo; Palermo: 1810. p. 105. [Google Scholar]
- Rana RS, Kumar K, Singh H, Rose KD. Lower vertebrates from the Late Palaeocene-Earliest Eocene Akli Formation, Giral lignite mine, Barmer District, western India. Current Science. 2005;89:1606–1613. [Google Scholar]
- Rayner TS, Pusey BJ, Pearson RG. Spatio-temporal dynamics of fish feeding in the lower Mulgrave River, north-eastern Queensland: the influence of seasonal flooding, instream productivity and invertebrate abundance. Marine and Freshwater Research. 2009;60:97–111. [Google Scholar]
- Regan CT. Descriptions of new or little known fishes from the coast of Natal. Annals of the Natal Government Museum. 1906;1:1–6. [Google Scholar]
- Reguero MA, Marenssi SA, Santillana SN. Weddellian marine/coastal vertebrate diversity from a basal horizon (Ypresian, Eocene) of the Cucullaea I Allomember, La Meseta formation, Seymour (Marambio) Island, Antarctica. Revista Peruana de Biología. 2012;19:275–284. [Google Scholar]
- Reguero M, Goin F, Acosta Hospitaleche C, Dutra T, Marenssi S. The terrestrial biotic dimension of West Antarctica (WANT) In: Reguero M, Goin F, Acosta Hospitaleche C, Dutra T, Marenssi S, editors. Late Cretaceous/Paleogene West Antarctica Terrestrial Biota and its Intercontinental Affinities. Springer Netherlands; Dordrecht, The Netherlands: 2013. pp. 55–110. [Google Scholar]
- Sadler PM. Geometry and stratification of uppermost Cretaceous and Paleogene units on Seymour Island, northern Antarctic Peninsula. In: Feldmann RM, Woodburne MO, editors. Geology and Paleontology of Seymour Island, Antarctic Peninsula. Geological Society of America Memoirs; 1988. pp. 303–320. 169. [Google Scholar]
- Siverson M. Revision of the Danian cow sharks, sand tiger sharks, and goblin sharks (Hexanchidae, Odontaspididae, and Mitsukurinidae) from southern Sweden. Journal of Vertebrate Paleontology. 1995;15:1–12. [Google Scholar]
- Sokolov MN. Shark teeth as index fossils for the zonal subdivision of the Cretaceous deposits of the Turan Plate. Izdatel’stvo “Nedra”. 1978:1–61. Russian. [Google Scholar]
- Stahl BJ. Chondrichthyes III: Holocephali. In: Schultze H-P, editor. Handbook of Paleoichthyology Volume. Vol. 4. Verlag Dr. Friedrich Pfeil; Munich: 1999. p. 164. [Google Scholar]
- Stahl BJ, Chatterjee S. A Late Cretaceous chimaeroid (Chondrichthyes, Holocephali) from Seymour Island, Antarctica. Palaeontology. 1999;42:979–989. [Google Scholar]
- Stelbrink B, von Rintelen T, Cliff G, Kriwet J. Molecular systematics and global phylogeography of angel sharks (Genus Squatina) Molecular Phylogenetics and Evolution. 2010;54:395–404. doi: 10.1016/j.ympev.2009.07.029. [DOI] [PubMed] [Google Scholar]
- Stilwell JD, Zinsmeister WJ. Molluscan systematics and biostratigraphy. Lower Tertiary La Meseta Formation, Seymour Island, Antarctic Peninsula. American Geophysical Union, Antarctic Research Series. 1992;55:1–192. [Google Scholar]
- Suárez ME, Encinas A, Ward DJ. An Early Miocene elasmobranch fauna from the Navidad Formation, Central Chile, South America. Cainozoic Research. 2006;4:3–18. [Google Scholar]
- Tambussi CP, Acosta Hospitaleche CI, Reguero MA, Marenssi SA. Late Eocene penguins from West Antarctica: systematics and biostratigraphy. In: Francis JE, Pirrie D, Crame JA, editors. Cretaceous–Tertiary High-Latitude Paleoenvironments, James Ross Basin, Antarctica. Geological Society London; London: 2006. pp. 145–161. [Google Scholar]
- Underwood CJ, Ward DJ, King C, Antar SM, Zalmout IS, Gingerich PD. Shark and ray faunas in the Middle and Late Eocene of the Fayum Area, Egypt. Proceedings of the Geologists’ Association. 2011;122:47–66. [Google Scholar]
- Uyeno T, Yabumoto Y, Kuga N. Fossil fishes of Ashiya Group. I. Late Oligocene elasmobranches from the islands of Ainoshima and Kaijima, Kitakyushu. Bulletin of the Kitakyushu Museum of Natural History. 1984;5:135–142. [Google Scholar]
- Van den Eeckhaut G, de Schutter P. The elasmobranch fauna of the Lede Sand Formation at Oosterzele (Lutetian, Middle Eocene of Belgium) Palaeofocus. 2009;1:1–57. [Google Scholar]
- Ward DJ. The English Palaeogene Chimaeroid fishes. Proceedings of the Geologists’ Association. 1973;84:15–330. [Google Scholar]
- Ward DJ. The distribution of sharks, rays and chimaeroids in the English Paleogene. Tertiary Research. 1980;3:13–19. [Google Scholar]
- Ward DJ, Grande L. Chimaeroid fish remains from Seymour Island, Antarctic Peninsula. Antarctic Science. 1991;3:323–330. [Google Scholar]
- Ward DJ, Wiest RLJ. A checklist of Palaeocene and Eocene sharks and rays (Chondrichthyes) from the Pamunkey Group, Maryland and Virginia, U. S.A. Tertiary Research. 1990;12:81–88. [Google Scholar]
- Welton BJ. Fossil sharks in Oregon. The Ore Bin. 1972;34(10):161–170. [Google Scholar]
- Welton BJ, Zinsmeister WJ. Eocene neoselachians from the La Meseta Formation, Seymour Island, Antarctic Peninsula. Contributions in Science of the Natural History Museum of Los Angeles County. 1980;329:1–10. [Google Scholar]
- Winkler TC. Deuxiéme mémoire sur des dents de poissons fossiles du terrain bruxellien. Archives du Musée Teyler. 1876;4:16–48. [Google Scholar]
- Woodward AS. A Cretaceous pristiophorid shark. Journal of Natural History. 1932;10:476–478. [Google Scholar]
- Yabumoto Y. A new Eocene lamnoid shark, Carcharodon nodai, from Omuta in northern Kyushu, Japan. Bulletin Kitakyushu Museum of Natural History. 1989;9:111–116. [Google Scholar]
- Zangerl R. Chondrichthyes I: Paleozoic Elasmobranchii, Chondrichthyes I. In: Schultze H-P, editor. Handbook of Paleoichthyology. 3A. Gustav Fischer Verlag; Stuttgart: 1981. p. 113. [Google Scholar]
- Zhelezko VI. [Phylogenesis of lamnoid sharks of the Palaeogene and their significance for zonal stratigraphy in phylogenetic aspects of palaeontology] Academy of Science USSR, All-Union Palaeontological Society; Leningrad: 1989. pp. 16–17. [Russian] [Google Scholar]
- Zhelezko VI, Kozlov VA. [Elasmobranchii and Palaeogene biostratigraphy of Transural and Central Asia] In: Amon EO, editor. Materialy po stratigrafii i paleontologii Urala 3. UrO RAN; Ekaterinburg, Russia: 1999. pp. 1–324. [Russian with English Abstract] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.