Abstract
Diverse extrinsic and intrinsic cues must be integrated within a developing organism to ensure appropriate growth at the cellular and organismal level. In Drosophila, the insulin receptor/TOR/S6K signaling network plays a fundamental role in the control of metabolism and cell growth. Here we show that scylla and charybdis, two homologous genes identified as growth suppressors in an EP (enhancer/promoter) overexpression screen, act as negative regulators of growth. The simultaneous loss of both genes generates flies that are more susceptible to reduced oxygen concentrations (hypoxia) and that show mild overgrowth phenotypes. Conversely, scylla or charybdis overactivation reduces growth. Growth inhibition is associated with a reduction in S6K but not PKB/Akt activity. Together, genetic and biochemical analysis places Scylla/Charybdis downstream of PKB and upstream of TSC. Furthermore, we show that scylla and charybdis are induced under hypoxic conditions and that scylla is a target of Drosophila HIF-1 (hypoxia-inducible factor-1) like its mammalian counterpart RTP801/REDD1, thus establishing a potential cross-talk between growth and oxygen sensing.
Keywords: Insulin signaling, S6 kinase, TSC, hypoxia, RTP801, REDD1
The control of growth in response to intrinsic and extrinsic factors is important for the development of every organism. Deregulated growth can have devastating consequences such as tumor formation. The evolutionarily conserved Insulin/IGF receptor (Inr)/Target of Rapamycin (TOR) signaling network plays an important role in modulating growth, metabolism, reproduction, and life span in response to intracellular and extracellular signals in species ranging from invertebrates to humans (Garofalo 2002; Jacinto and Hall 2003). In Drosophila, viable mutant combinations of positive components of the Drosophila Inr cascade such as Inr, chico (the homolog of vertebrate IRS1-4), PKB (Protein kinase B, also known as Akt) and PDK1 (3-phosphoinositide-dependent protein kinase-1) lead to developmentally delayed and proportionally reduced small flies, displaying a reduction in cell size and number (Bohni et al. 1999; Brogiolo et al. 2001; Rintelen et al. 2001). On the other hand, loss of PTEN (phosphatase and tensin homolog on chromosome ten), which antagonizes PI3K activity by dephosphorylating the 3′-position of phosphoinositides, leads to hypertrophy and hyperplasia (Goberdhan et al. 1999; Huang et al. 1999; Gao et al. 2000). In humans, loss of the tumor suppressor PTEN is observed frequently in glioblastomas, prostate cancers, and endometrial cancers, and PTEN germline mutations are linked to dominant hamartoma syndromes like Cowden syndrome, Lhermitte-Duclose disease, Proteus syndrome, and Bannayan-Zonana syndrome (Sulis and Parsons 2003). Genetic studies in Drosophila indicate that PKB has a crucial role in signaling downstream of PTEN since flies completely lacking PTEN function can be rescued to viability by lowering PKB activity (Stocker et al. 2002).
The TOR/S6K (S6 kinase) branch of the growth modulatory network is negatively regulated by the tumor suppressor Tsc1 (hamartin)/Tsc2 (tuberin) complex. Tuberous sclerosis complex (TSC) is an autosomal-dominant disorder characterized by the formation of hamartomas, benign tumors that arise in various tissues (Pan et al. 2004). In Drosophila, cells devoid of Tsc1/Tsc2 function are increased in size (Gao and Pan 2001; Potter et al. 2001; Tapon et al. 2001). Tsc2 and, more weakly, Tsc1 were found to physically associate with dTOR, thereby inhibiting dTOR kinase activity (Gao et al. 2002). Other studies reported an inhibitory role of PKB on Tsc1-Tsc2 by PKB-mediated phosphorylation of Tsc2 (Inoki et al. 2002; Manning et al. 2002). Recently, the small GTPase Rheb (Ras homolog enriched in brain) has been identified as a new positive growth effector acting downstream of Tsc1-Tsc2 and upstream of TOR. Mechanistically, Tsc2 acts as GTPase-activating protein (GAP) toward Rheb (Garami et al. 2003; Inoki et al. 2003a; Saucedo et al. 2003; Stocker et al. 2003; Tee et al. 2003; Zhang et al. 2003). The molecular mechanism how Rheb relays the signal to TOR is currently unknown. dTOR mutants show a growth deficit that is more pronounced in endoreplicative tissues than in mitotic tissues (Oldham et al. 2000; Zhang et al. 2000). An effector of mTOR is S6K, which upon activation by mTOR phosphorylates ribosomal protein S6. S6K-mediated S6 phosphorylation has been thought to lead to a preferential translation of mRNAs encoding ribosomal proteins and proteins of the translational apparatus although the significance of this S6K function has been questioned recently (Tang et al. 2001; Stolovich et al. 2002; Pende et al. 2004). Inr/TOR signaling activity culminates in the regulation of translation rate by controlling S6K and the translational repressor 4E-BP1 (Gingras et al. 2004). S6K mutant flies are small but in contrast to mutants of the Inr pathway, only cell size is reduced without a change in cell number (Montagne et al. 1999). Therefore, loss of S6K function reduces growth and body size to a lesser extent than loss of other positive components acting further upstream in the cascade.
Growth is modulated by extrinsic factors such as nutrients, temperature, and hypoxia (Palos and Blasko 1979; Frazier et al. 2001; Azevedo et al. 2002). However, their link to the Inr/TOR signaling network are not well defined. Although it is known that starvation results in a reduction in the levels of insulin-like peptides and a reduction in S6K activity in Drosophila (Oldham et al. 2000; Ikeya et al. 2002), little is known about whether temperature or hypoxia regulates the activity of this pathway. It is conceivable that mutations in genes coding for factors mediating the modulation of growth in response to external stimuli have escaped detection because they do not exhibit a very strong phenotype under standard culture conditions. For example, overexpression of the Forkhead transcription factor FOXO3a, the human homolog of Drosophila dFOXO, produces a very subtle small eye phenotype under normal nutrient conditions. Under starvation, however, this phenotype is massively exacerbated, inducing a further eye size reduction and cell death. Furthermore, dFOXO mutant flies are viable and do not show an (over)growth phenotype in an otherwise wild-type background under normal conditions (Junger et al. 2003).
Genes like dFOXO were missed in genetic loss-of-function screens aimed at identifying growth-regulatory genes (1) because they have only mild or missing mutant phenotypes under normal conditions and/or (2) because their function is masked by redundancy.
Genes acting in a redundant manner can be identified in a complementary gain-of-function approach using EP (enhancer/promoter) elements (Rorth 1996). Screening >4000 novel EP lines, we found two insertions in the scylla locus as suppressors of a PKB/PDK1-dependent eye overgrowth phenotype. We identified a second gene in the Drosophila genome with homology to scylla named charybdis. Homologs of these genes also exist in mammals, and they have been implicated in the response of a cell to hypoxia or more generally as stress-induced genes having either pro- or anti-apoptotic functions. We present evidence that scylla and charybdis, like some of their mammalian homologs, are induced by hypoxia and that Scylla and Charybdis act as partially redundant negative regulators of growth by controlling S6K but not PKB activity.
Results
Identification of scylla and charybdis
In order to identify novel genes involved in growth regulation by the Inr/TOR pathway, we performed an EP overexpression screen using a double-headed EP element. We used a genetically sensitized system involving coexpression of PKB and PDK1 (achieved by using EP837 that drives endogenous PDK1) in the eye, which leads to a big eye phenotype (Fig. 1B). Pilot experiments demonstrated that overexpression of PTEN or a dominant-negative version of the catalytic subunit of Drosophila PI3K, Dp110, were not able to suppress the PKB/PDK1-dependent phenotype. Thus, our screening system is likely to identify components acting downstream of or in parallel to PKB/PDK1. For example, coexpression of Tsc1/Tsc2 strongly suppresses the phenotype of our tester flies (data not shown).
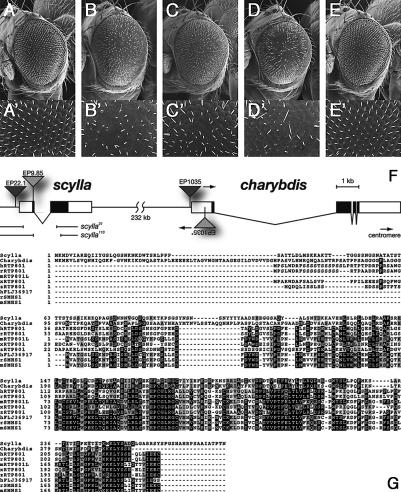
Figure 1.
Overexpression of scylla and its paralog charybdis suppresses the hyperactivity of the Inr pathway. (A-E) scylla (C) and/or charybdis (D) overexpression (achieved by EPscy and EPchar, respectively) suppress the PKB/PDK1-dependent big eye phenotype (B). Genotypes: y w; GMR-Gal4/+ (A,A′); y w; GMR-Gal4 UAS-PKB/+; EP837 (≈UAS-PDK1)/+ (B,B′); y w; GMR-Gal4 UAS-PKB/+; EP837/EPscy (C,C′); y w; GMR-Gal4 UAS-PKB/+; EP837/EPchar (D,D′); y w; GMR-Gal4, UAS-PKB/+; EP837/EPscy EPchar (E,E′). (F) Genomic organization of scylla and charybdis and corresponding mutant alleles. (G) Several homologs of Scylla/Charybdis are present in humans (h), rat (r), mouse (m), Xenopus (x), and zebrafish (z). Charybdis bears a coiled-coil domain (amino acids 168-188) not predicted in Scylla. However, the evolutionarily most highly conserved, C-terminal part of the gene family lacks similarities to known protein domains.
We identified two EP insertions (EP22.1, hereafter named EPscy, and EP9.85) in the scylla locus as suppressors of the PKB/PDK1 bulging eye phenotype (Fig. 1C). BLASTP search with the Scylla amino acid sequence revealed another homologous protein encoded in the Drosophila genome termed Charybdis. scylla (scy, CG7590) and charybdis (char, CG7533) are separated by ∼232 kb of genomic DNA. Their gene products share a high degree of homology (38% identity, 49% similarity), suggestive of a gene duplication event (Fig. 1F,G). We checked whether charybdis overexpression would behave similarly to scylla in the PKB/PDK1 overexpression assay using EP1035 (hereafter named EPchar). Indeed, the big eye phenotype of the tester system is also suppressed by EPchar (Fig. 1D). UAS transgenes with either the scylla or charybdis cDNA recapitulate the suppression phenotype of the corresponding EP element (data not shown). Coexpression of scylla and charybdis further ameliorates the suppression phenotype to a nearly wild-type situation (Fig. 1E). Notably, scylla or charybdis overexpression on their own using a panel of different eye/wing Gal4 drivers reduced adult organ size. Coexpression of the caspase inhibitors p35 or DIAP1 did not rescue the small eye phenotype induced by expression of either scylla or charybdis in the eye. Moreover, no elevated cell death in eye imaginal disks overexpressing scylla/ charybdis under control of the GMR-Gal4 driver was observed by acridine orange staining (data not shown). This suggests that apoptosis is not the cause for the eye size reduction. Thus, scylla and charybdis overexpression antagonizes the growth-promoting effects of PKB/PDK1 and is sufficient to negatively regulate growth.
scylla and charybdis are both expressed during embryogenesis in dynamic, partially overlapping patterns. In contrast to the broadly expressed scylla mRNA, charybdis transcripts are predominantly restricted to neurons of the CNS and PNS as assessed by mRNA in situ hybridization. During late larval stages scylla mRNA is uniformly expressed without apparent tissue-specific distribution, whereas charybdis mRNA expression could not be detected in third instar imaginal disks (data not shown).
Scylla and Charybdis belong to an evolutionarily conserved protein family
scylla codes for a 280-amino acid polypeptide with a predicted molecular weight (MW) of ∼31 kDa, and the Charybdis protein contains 299 amino acid residues (MW ∼32 kDa). Since EP9.85 is located in the coding region of scylla, thus generating a Scylla protein with an N-terminal truncation of 12 amino acids, we tested whether a UAS-scy transgene encoding the truncated form of Scylla behaves as the full-length protein. Overexpression of UAS-scyΔ1-12 and UAS-scywt showed the same effects as EPscy (data not shown), indicating that amino acids 1-12 are dispensable for the growth-suppressing function of Scylla.
Homologs of Scylla/Charybdis exist throughout the animal kingdom including humans, rat, mouse, Xenopus, and zebrafish. Reminiscent of the situation in Drosophila, mammals possess several paralogous proteins with homology to Scylla/Charybdis. At least two homologs (RTP801/REDD1 and FLJ3691, a hypothetical protein presumably corresponding to REDD2 [Ellisen et al. 2002]) exist in humans, two (RTP801 and SMHS1) in rat, and three (RTP801L, Dig2, and SMHS1) in mice. The overall homology is highest toward the C terminus (Fig. 1G). Charybdis contains a predicted coiled-coil structure (amino acids 168-188).
In mammals, RTP801/REDD1 and other members of the family are induced upon various forms of cellular stress including hypoxia, DNA damage, and dexamethasone treatment (Ellisen et al. 2002; Shoshani et al. 2002; Wang et al. 2003). In fact, RTP801/REDD1 is a direct target of HIF-1, a heterodimeric transcription factor that plays a pivotal role for survival in response to hypoxia (Shoshani et al. 2002). Furthermore, RTP801/REDD1 is controlled by p53 and p63 (Ellisen et al. 2002). Depending on the experimental setup and cell context, RTP801 overexpression either inhibits or increases apoptosis, suggesting a complex regulation and/or dependence on the developmental program of the cell (Shoshani et al. 2002).
Scylla and Charybdis inhibit growth and are important under hypoxic conditions
To investigate the function of Scylla and Charybdis in more detail, we generated loss-of-function mutations in both genes and complemented the analysis with overexpression studies.
Partial scylla deletions were obtained by imprecise excisions of EP9.85, which is integrated in the scylla open reading frame (ORF) and therefore already represents a scylla allele (hereafter named scyEP9.85) (Fig. 1F). For charybdis, we used a local hop strategy of EPchar to obtain char180, constituting a new EP insertion (EP1035*) in the charybdis 5′-untranslated region (UTR) plus the original EPchar (see Materials and Methods). Quantitative real time-PCR showed that in char180 homozygotes, charybdis mRNA expression is decreased to ∼25% of wild-type levels (data not shown). We assume that this charybdis allele is a strong hypomorph, as mRNA levels were only slightly more reduced (to 23% or 15%, respectively) when RNA was extracted from flies heterozygous for char180 over either one of two independent deficiencies uncovering charybdis and scylla (data not shown).
All scylla mutant combinations and the char180 homozygotes are viable and fertile without apparent mutant phenotype. scylla and char180 mutant animals have the same weight as control flies. Measurement of wing size and hair density in the adult wing of scylla mutants revealed no differences in cell size and cell number as compared to control animals (data not shown). We created scylla loss-of-function clones in imaginal disks using FLP/FRT-mediated mitotic recombination to test the effect on growth properties of the mutant tissue. One would expect a growth advantage of cells in clones lacking a bona fide negative growth regulator, as is the case for PTEN. However, larval scylla mutant clones were the same size as their wild-type sister clones. Likewise, clones obtained in adult eyes revealed no increase in cell size of scylla or char180 mutant ommatidia (data not shown). Thus, loss of Scylla or Charybdis function is dispensable for growth under normal conditions. It is conceivable that Scylla and Charybdis act in a redundant manner. Therefore, it was important to create scylla charybdis double mutants.
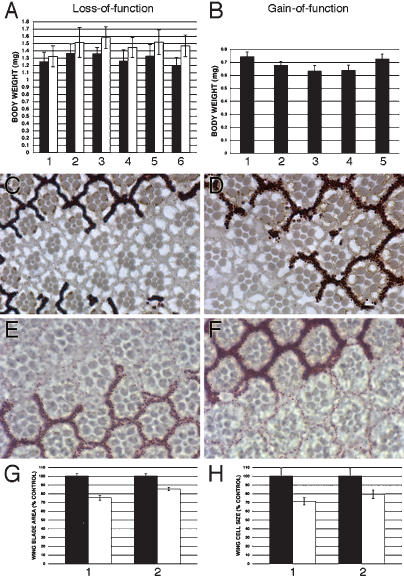
We combined char180 with three of our scylla alleles by meiotic recombination. All double-mutant scylla charybdis combinations produced viable adult flies. Weight analysis of heteroallelic scylla charybdis flies demonstrated that simultaneous loss of Scylla and Charybdis significantly increases body weight. Consistent results were obtained by combining one copy of scyEP9.85/31/113 char180 and the deficiency Df(3L)vin4 uncovering scylla and charybdis. Mutant females were on average 6%-23% and males 9%-17% heavier than control flies (Fig. 2A; data not shown). Conversely, ubiquitous scylla/charybdis overexpression using the Act5CGal4 driver generated flies that are decreased in size and weight (15.1% using EPscy and 14.3% using UAS-char#53) (Fig. 2B, data only shown for males). The majority of scylla/charybdis-overexpressing flies eclosed with a minor delay (0.5-1 d) (data not shown).
Figure 2.
Scylla and Charybdis inhibit growth. (A) Simultaneous loss of Scylla and Charybdis leads to an increase in body weight. Control flies (black bars) are represented by either of the two possible double mutants over the TM3 balancer chromosome (first three black columns) or the deficiency chromosome (Df(3L)vin4) over TM3 (three last black columns) and were derived from the same vial as the heteroallelic double-mutant flies, p < 0.009 using Student's t-test (two-tailed). Between 32 and 65 flies were weighed per genotype. Genotypes (only white bars indicated) are as follows: y w; scy113 char180/scyEP9.85 char180 (1); y w; scy31 char180/scyEP9.85 char180 (2); y w; scy31 char180/scy113 char180 (3); y w/+; scyEP9.85 char180/Df(3L)vin4 (4); y w/+; scy113 char180/Df(3L)vin4 (5); y w/+; scy31 char180/Df(3L)vin4 (6). (B) Ubiquitous scylla and charybdis overexpression generates smaller flies that have reduced body weights. In all our overexpression studies, we obtained the strongest effects using EPscy or UAS-char#53, driving scylla and charybdis, respectively. A representative experiment of three independent weighing analyses is shown (n = 35 for each genotype; p < 0.0001 for UAS-scy#2, EPscy, UAS-char#53, and p < 0.02 for EPchar). Data are only shown for males, but significant weight reduction is also observed in females. Genotypes are as follows: y w; Act5CGal4/+ (control) (1); y w; UAS-scy#2/+; Act5CGal4/+ (2); y w; Act5CGal4/EPscy (3); y w; Act5CGal4/UAS-char#53 (4); y wAct5CGal4/EPchar (5). (C-F) Tangential sections through the adult eye showing photoreceptor and associated ommatidial cells. Clones of cells either overexpressing scylla/charybdis or lacking the function of both are recognizable by the absence of red pigment. scylla-overexpressing cells (C) or charybdis-overexpressing cells (D) in the eye are smaller than neighboring wild-type cells, and no patterning defects are observed. Conversely, scylla charybdis double-mutant cells show a mild increase in cell size (E,F). Genotypes are as follows: y w hsflp; GMR > w+ > Gal4/EPscy (C); y w hsflp; UAS-char#56/+; GMR > w+ > Gal4/+ (D); y w hsflp; FRT80 scy113 char180/FRT80 w+ (E); y w hsflp; FRT80 scyEP9.85 char180/FRT80 w+ (F). (G,H) Quantification of wing cell size and cell number of flies overexpressing scylla. Hair density in a defined square area next to the junction of the posterior cross-vein and vein five was determined, and from this number cell size was calculated. The cell number corresponds to the ratio of total wing area and cell size. scylla overexpression reduces only cell size but not cell number. Black bars always represent the control (y w; Act5CGal4/+), and white bars represent scylla overexpression (y w; Act5CGal4/EPscy). Numbers indicate the sex: 1 for males, and 2 for females. We analyzed 10 wings of each genotype.
To assess whether charybdis/scylla overexpression affects cell size, we generated scylla and charybdis gain-of-function flip-out clones in the eye, marked by the absence of the red pigment. A moderate reduction in cell size was observed in cells overexpressing scylla or charybdis (Fig. 2C,D). Ommatidia overexpressing scylla or charybdis exhibit no patterning defects. On the other hand, simultaneous removal of Scylla and Charybdis in clones of photoreceptor cells resulted in slightly enlarged cells (Fig. 2E,F). Consistently, when most of the head capsule and the eyes were made homozygous by means of the eyflp/FRT system in an otherwise heterozygous mutant background, a mild big head phenotype was generated in the double mutant but not in either single mutant (Supplementary Fig. S1).
The gain-of-function clonal analysis in the eye showed that cell size is reduced upon forced scylla/charybdis expression, but it did not address the question whether cell number is affected. To clarify this issue, the wing size and cell number of flies that ubiquitously overexpress scylla were measured. The insect wing is a double-layered epithelial structure, and each cell in the wing secretes a single hair (trichome). Therefore, by counting the number of trichomes per defined area, hair density can be taken as a measure for cell number. Overall wing size was reduced by ∼25% in males or ∼15% in females overexpressing scylla (Fig. 2G). By extrapolating the number of cells per measured area, we found that cell size is decreased by ∼29% in males and ∼21% in females (Fig. 2H). We conclude that the size reduction brought about by overexpressing scylla is caused by a reduction in cell size. Cell density is even slightly increased (5.8% in males, 7.6% in females) (data not shown). Our data show that Scylla and Charybdis have a growth-inhibitory role and that they share some functional redundancy.
RTP801/REDD1 was shown to be induced by hypoxia (Shoshani et al. 2002). This prompted us to investigate the effects of hypoxia on the scylla charybdis double mutants. scylla charybdis mutant larvae were raised on normal food at room temperature in a hypoxia chamber containing 9% oxygen during their entire development. In general, control flies as well as scylla charybdis homozygotes were 3-4 d delayed in development under these hypoxic conditions. However, whereas adult homozygous scylla charybdis double mutants could readily be recovered under standard culture conditions in normoxia, homozygous mutants of three independent scylla charybdis allelic combinations (char180 in combination with scy31, scy113, or scyEP9.85) were strongly underrepresented compared to normoxic conditions. We also observed the appearance of an increased number of dead pupae in the vials (data not shown). The scylla113 char180 double-mutant combination had the strongest effect, and only two escapers (out of 326 scored flies) hatched, whereas under normoxia flies with this genotype were recovered with nearly the expected Mendelian ratio. The eclosed homozygotes raised under hypoxia did not show obvious morphological defects.
Thus, whereas simultaneous loss of Scylla and Charybdis under normoxic conditions results in a slight increase in growth, their absence under reduced oxygen concentrations severely compromises larval development. This indicates that Scylla and Charybdis have a critical function for survival under hypoxic conditions.
scylla and charybdis are up-regulated under hypoxic conditions and scylla is a target of Drosophila HIF-1
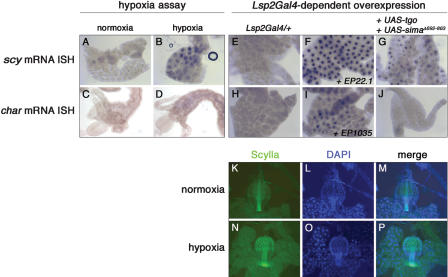
The transcription factor HIF-1 is the key regulator of changes in gene expression in response to hypoxia (Bruick 2003). It consists of two bHLH-PAS domain protein subunits (HIF-1α and HIF-1β). Under conditions of low oxygen, the HIF-1 protein complex is stabilized and binds to Hypoxia Response Elements (HRE), short regulatory DNA sequences (core recognition sequence 5′-TACGTG-3′) located in the genomic region of target genes. Both the scylla and charybdis loci possess several HREs. Since RTP801/REDD1, a mammalian homolog of scylla and charybdis, is a direct target gene of HIF-1 and is induced under hypoxic conditions, we wondered whether this function is evolutionarily conserved. We subjected wild-type larvae to hypoxia (between 2% and 5% O2) and checked for the induction of scylla and charybdis expression. It is mainly the endoreplicative tissue such as fat body, gut, salivary glands, and tracheae that respond to changes in oxygen concentrations (Lavista-Llanos et al. 2002). scylla mRNA expression was up-regulated in the larval fat body and in the gut after hypoxia (Fig. 3B; data not shown). charybdis, on the other hand, was mildly induced in the midgut but not in the fat body (Fig. 3D; data not shown). We also tested whether hypoxia had an effect on Scylla protein levels and distribution. To detect the endogenous Scylla protein, we took advantage of a transgenic Scylla-reporter line (a so-called protein trap line). This protein trap line bears a promoter-less green fluorescent protein (GFP)-reporter transgene in the scylla locus generating a Scylla-GFP fusion protein (Morin et al. 2001). Scylla-GFP is expressed in most larval tissues. Under normoxic conditions, nuclear accumulation of Scylla protein is observed in some cells of the endoreplicative tissue (data not shown). Consistent with our mRNA data, upon exposure of third instar larvae to various hypoxia conditions, we observed an up-regulation and nuclear localization of Scylla protein in the fat body (data not shown) and in the gut (Fig. 3K-P).
Figure 3.
scylla and charybdis are induced by hypoxia, and scylla is a target of Drosophila HIF-1. (A-D) Larvae were incubated in a hypoxia chamber for either 17 h under 2% O2 or for 7 h under 2.8% O2 conditions leading to the induction of scylla (B) and charybdis (D) expression as assessed by mRNA in situ hybridization (ISH). Since scylla mRNA is detected in the gut under normoxia, it has been difficult to assess whether scylla transcript levels are further induced under hypoxia (data not shown). (E-J) Directed coexpression of Tgo and Sima in the fat body up-regulates scylla (G) but not charybdis (J) transcription. (F,I) Positive controls for scylla and charybdis, respectively. (K-P) Scylla protein localizes to the nucleus and is up-regulated in the proventriculus (N) and the fat body (not shown) in hypoxia. Genotypes are as follows: y w (A-D); y w; Lsp2Gal4/+ (E,H); y w; Lsp2Gal4/EPscy (F); y w; UAS-tgo/+; Lsp2Gal4/UAS-simaΔ692-863 (G,J); y w; Lsp2Gal4/EPchar (I). (K-P) Scylla fly trap larvae grown in normoxia (K-M) or incubated in hypoxia (2.8% O2 for 8 h; N-P).
In Drosophila, the bHLH-PAS family comprises Period (Per), Trachealess (Trh), Single-minded (Sim), Spineless (Ss), Dysfusion (Dys), Tango (Tgo), and Similar (Sima). Tgo is the ubiquitously expressed HIF-1β ortholog, which dimerizes with any of the α-subunits. Sima has been shown to fulfill analogous functions to its mammalian homolog HIF-1α (Lavista-Llanos et al. 2002).
To test whether scylla/charybdis transcription is regulated by bHLH-PAS proteins that recognize the same DNA stretch, we overexpressed sim, trh, or sima together with tgo using the Lsp2Gal4-driver that is active specifically in the fat body during the third larval stage. For Sima, we used a form lacking the oxygen-dependent degradation domain (ODD), rendering it refractory to proteolytic destruction under normoxic conditions. Only the coexpression of tgo and sima induced scylla but not charybdis expression as assessed by mRNA in situ hybridization (Fig. 3G,J; data not shown). This does not preclude, however, the possibility that charybdis is a target of Tgo-Sima as we detected its endogenous induction upon hypoxia only in the gut but not in the fat body. Since neither expression of trh with tgo nor sim induced scylla or charybdis expression, the regulation of scylla by the Tgo-Sima heterodimer is specific. Thus, scylla and charybdis, like their mammalian homolog RTP801/REDD1, are induced by hypoxia, and at least scylla appears to be a direct target gene of the HIF-1 homolog Tgo-Sima.
Scylla acts downstream of PKB but upstream of TSC
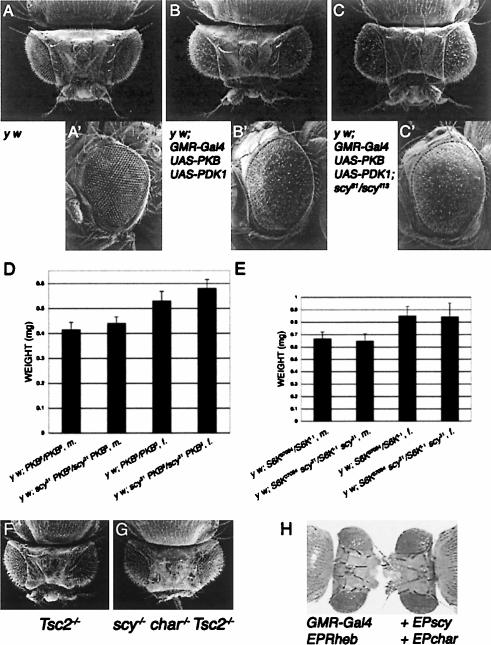
Although loss of Scylla function does not produce a mutant phenotype on its own, we tested whether it would alter the PKB/PDK1 overexpression eye phenotype. Indeed, loss of Scylla function enhanced the PKB/PDK1 overgrowth phenotype (Fig. 4B,C). Thus, Scylla is essential for attenuating the increased growth in response to hyperactivation of the Inr pathway. Furthermore, loss of Scylla partially suppressed the growth reduction associated with reduced PKB function as assessed by comparing weights of PKB3 single mutants to scy31 PKB3 double mutants (Fig. 4D). In contrast, complete loss of Scylla in a heteroallelic S6K combination did not rescue the S6K single mutant phenotype indicating that S6K is epistatic over scylla (Fig. 4E).
Figure 4.
Scylla attenuates growth in response to elevated Inr pathway activity downstream of PKB but upstream of TSC. (A-C) A PKB/PDK1-overexpression eye phenotype is enhanced in a scylla mutant background. Genotypes are as follows: y w (A,A′); y w; GMR-Gal4 UAS-PKB UAS-PDK1/+ (B,B′); y w; GMR-Gal4 UAS-PKB UAS-PDK1/+; scylla31/scylla113 (C,C′). (D) Loss of scylla partially suppresses the weight loss of homozygous PKB3 mutants. Weight increase in the double mutants versus PKB3 single mutants is ∼6% (males) and 10% (females) for this experiment. One representative of four independent weighing experiments is shown; p < 0.0001, n = 60 (males) and 90 (females). (E) No difference in body weight between a hypomorphic S6K mutant combination and S6K scylla double mutants was observed (n = 30). (F,G) scylla charybdis Tsc2 triple-mutant heads resemble Tsc2 single mutants and do not show additive growth effects, indicating that Scylla and Charybdis act upstream of Tsc2. Genotypes are as follows: y w eyflp; FRT80 gig192/FRT80 cl3L w+ (F); y w eyflp; FRT80 scyEP9.85 char180 gig192/FRT80 cl3L w+ (G). (H) A Rheb-dependent bulging eye phenotype cannot be suppressed by scylla/charybdis coexpression. Genotypes are as follows: y w; GMR-Gal4/+; EPRheb/+ (left); y w; GMR-Gal4/+; EPRheb/EPscy EPchar (right).
Moreover, overexpression of scylla and charybdis not only suppressed the growth phenotype caused by over-activation of the Inr pathway in the eye but to a certain extent also rescued the lethality associated with the ubiquitous increase in Inr pathway activity due to either overexpression of PKB or loss of PTEN. scylla rescued the male-specific lethality caused by ubiquitous expression of PKB and organismal lethality associated with the partial but not complete loss of PTEN function. Similarly, PKB-associated male lethality was also rescued by charybdis overexpression (Supplementary Tables 1, 2). This indicates that scylla and charybdis have the capacity to act as potent negative regulators of insulin signaling downstream of PKB and PDK1.
Several lines of evidence suggest that Scylla and Charybdis act upstream of TSC and Rheb. Tsc1/2 mutant flies can be rescued to adulthood by reducing S6K signaling (Radimerski et al. 2002a), and a mere reduction of one TOR copy in a Tsc1 mutant context results in a rescue to the pupal stage (Gao et al. 2002). We examined whether ubiquitous scylla overexpression could rescue the larval lethality of heteroallelic Tsc1/2 mutant combinations (Tsc12G3/Tsc1Q87X and Tsc256/Tsc2192) using the daGal4 or Act5CGal4 drivers in combination with a UAS-scy transgene or EPscy at 18°C, 25°C, and 29°C. Ubiquitous overexpression of scylla/charybdis in a Tsc1/2 mutant background did in no case extend larval development beyond first/second instar, and these larvae died at the same time as Tsc1/2 mutants. Moreover, the big head phenotype of Tsc2192 (and Tsc256) induced by the eyflp/FRT system was not further enhanced in scyEP9.85 char180 Tsc2 triple-mutant heads (Fig. 4F,G). We have shown before (Supplementary Fig. S1) that heads composed almost entirely of scylla charybdis double-mutant cells are enlarged. Conversely, GMRGal4-driven co-overexpression of Tsc1, Tsc2, and scylla or charybdis in the eye did not further reduce the small eye phenotype induced by coexpression of Tsc1 and Tsc2 on their own (data not shown). The absence of an additive growth effect upon loss of Tsc2, scylla, and charybdis or overexpression of Tsc1/2 and scylla or charybdis suggests that they function in the same pathway. These results are consistent with the idea that Scylla and Charybdis act upstream of the TSC complex (see also next section). Our conclusion is further supported by the fact that neither a Rheb-dependent bulging eye phenotype nor organismal lethality could be suppressed by scylla/charybdis coexpression (Fig. 4H; data not shown).
Scylla decreases S6K but not PKB activity
To test whether the placement of Scylla between PKB and TSC can be corroborated biochemically, we investigated the effect of scylla overexpression on PKB and S6K activity.
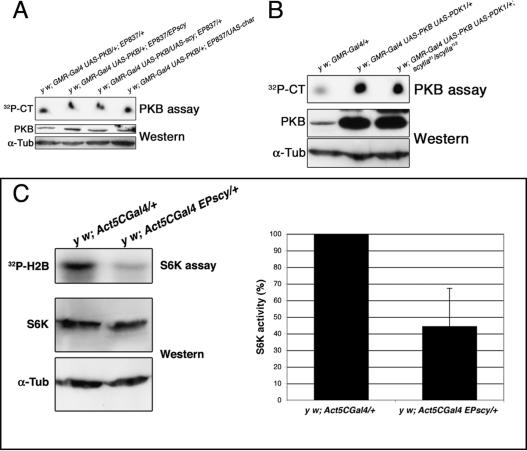
We tested PKB activity of adult female heads overexpressing scylla or charybdis in conjunction with PKB and PDK1 under control of the GMR-Gal4 enhancer. The same experimental setup has previously been used to demonstrate that PDK1 increases PKB activity (Cho et al. 2001). Total fly head protein was extracted and PKB activity was assayed by incorporation of 32P-labeled phosphate into a synthetic PKB substrate (Crosstide, CT). Although scylla/charybdis overexpression substantially suppressed the PKB/PDK1-induced bulging eye phenotype (Fig. 1C), PKB activity was not reduced in these eyes (Fig. 5A). Moreover, PKB activity was also unaffected in a scylla-/- background (Fig. 5B).
Figure 5.
Scylla reduces S6K activity, but neither Scylla nor Charybdis down-regulates PKB activity. (A) Head extracts of flies overexpressing PKB and PDK1 in conjunction with either scylla or charybdis in the eye were assayed for PKB activity. No reduction could be detected (experiment has been performed twice). Genotypes are indicated above the autoradiograph. (CT) Crosstide, a synthetic PKB substrate. (B) Eye-specific PKB/PDK1 coexpression in a scylla mutant background does not lead to elevated PKB activity (three experiments have been done). (C) Ubiquitous scylla overexpression decreases S6K activity by 56% on average, p < 0.006. S6K activity of second instar larvae was determined by the incorporation of radioactively labeled phosphate into the H2B substrate. Control (Act5CGal4/+) activity was set to 100%. Five independent experiments were performed.
These results are consistent with the placement of Scylla downstream of PKB. To test the effect of Scylla on S6K activity, second instar larvae expressing scylla under the control of Act5CGal4 were collected, and larval extracts were assayed for S6K activity. On average, S6K activity was down-regulated by >50% (Fig. 5C). Although there may also be a slight reduction in total S6K protein levels, this effect cannot account for the much stronger reduction in S6K activity. Taken together with the genetic evidence presented above, these results strongly support the argument that Scylla acts between PKB and TSC to regulate S6K activity. Furthermore, in an accompanying paper, Brugarolas et al. (2004) provide direct biochemical evidence that a functional TSC complex is required for RTP801/REDD1 to affect S6 phosphorylation. Altogether, these data indicate that Scylla functions upstream of TSC.
Scylla and Charybdis prolong life span under starvation conditions
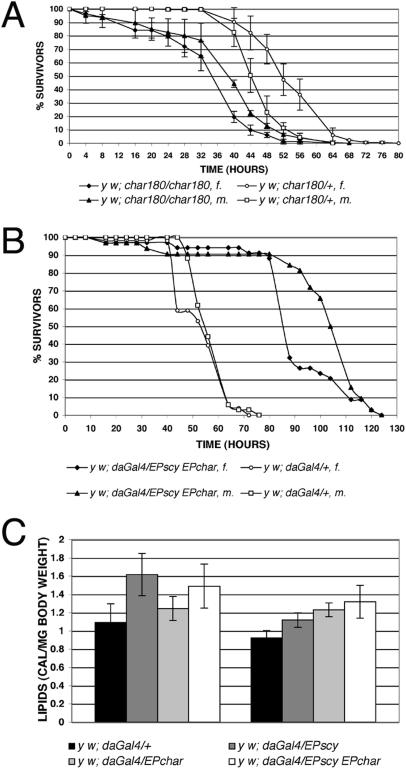
Zinke et al. (2002) have performed a whole-genome DNA microarray analysis of 2-d-old larvae (48 h AEL) grown on normal food that were subsequently subjected to a starvation regime for different time periods. Under these conditions, upon 12 h of starvation, scylla and charybdis expression were found to be on average 6.3 times and 4.6 times up-regulated, respectively (downloadable Supplemental Material at http://www.fzk.de/embo). Therefore, we tested the effects of starvation on the viability of scylla and charybdis mutants, as well as on flies overexpressing both genes by exposing adult flies to a water-only diet. Various scylla heteroallelic combinations did not show elevated susceptibility to starvation (data not shown). However, char180 mutants lived significantly shorter lives than control flies, suggesting that Charybdis has a protective effect for the animal under nutrient-deprived conditions (Fig. 6A). Strikingly, forced expression of scylla/charybdis extended mean life span by up to twofold (Fig. 6B). We analyzed lipid and glycogen content of these flies to see whether energy stores were altered. Indeed, flies overexpressing scylla and/or charybdis showed significantly elevated lipid levels (Fig. 6C). We also measured glycogen levels but could not detect any statistically significant changes, although there was a tendency toward increased glycogen content (data not shown).
Figure 6.
Scylla and Charybdis prolong life span under starvation conditions. (A) Homozygous char180 mutants are more susceptible to starvation than control animals. The average and standard deviations of three independent experiments are shown (n = 114 for char180/char180, m.; n = 145 for char180/+, m.; n = 82 for char180/char180, f.; and n = 127 for char180/+, f.). (B) Two- to three-day-old adult flies coexpressing scylla and charybdis were starved, and survivors were counted every 4/8 h. Simultaneous overexpression of both proteins strongly extended life span compared to control flies. A representative example of three independent experiments is shown. (C) Ubiquitous expression of scylla, charybdis, and/or both together using the daGal4 driver is accompanied by elevated lipid levels in males (left bar cluster) and females (right bar cluster; p < 0.065 for daGal4/EP1035, m.; for all other genotypes p < 0.0009; n = 10). (m.) Males; (f.) females.
Discussion
Here we provide evidence that two related proteins, Scylla and Charybdis, are negative modulators of Inr/TOR signaling in response to different external stress situations including hypoxia and starvation. scylla and charybdis single mutants do not show obvious growth phenotypes. scylla charybdis double-mutant flies are also viable and fertile and exhibit a slight increase in body size. Viability of the double mutants is strongly reduced, however, when they are reared under hypoxic conditions. Thus, although Scylla and Charybdis are largely dispensable for normal development, they have a critical role for the endurance of prolonged hypoxia. We show that scylla is transcriptionally induced as a target of Drosophila HIF-1 (the Tango-Similar dimer) and that scylla and charybdis are up-regulated under hypoxic conditions like their mammalian homolog RTP801/REDD1. Furthermore, we show that Scylla negatively regulates Inr/TOR signaling by reducing S6K but not PKB activity.
scylla/charybdis and their orthologs are stress-induced genes
RTP801 was described as a hypoxia/HIF-1-inducible factor with a role in apoptosis (Shoshani et al. 2002). We find that scylla/charybdis, like their mammalian homolog, are induced by hypoxia, and that scylla is a direct target of Drosophila HIF-1. However, we have no indication that scylla/charybdis overexpression promotes cell death. Coexpression of the caspase inhibitors p35 or DIAP1 did not rescue the small eye phenotype induced by expression of either scylla or charybdis in the eye. Moreover, acridine orange staining revealed no increased cell death upon scylla/charybdis coexpression (data not shown). Accordingly, we never observed signs of necrotic eye tissue upon scylla/charybdis overexpression.
Dig2, a murine Scylla/Charybdis homolog, is also a stress-responsive protein induced by a variety of treatments including dexamethasone, thapsigargin, tunicamycin, and heat shock (Wang et al. 2003). Together with the finding by Zinke et al. (2002) that scylla/charybdis expression is increased during starvation conditions and our analysis, showing that Scylla and Charybdis act as growth inhibitors, these data support a model wherein Scylla and Charybdis, induced by stresses like hypoxia and starvation, act to dampen growth under certain stress conditions.
Do Scylla and Charybdis act together?
Overexpressing either scylla or charybdis on their own is sufficient to reduce growth. Coexpression of both proteins seemed to have a slight cooperative effect (Fig. 1G) on the PKB/PDK1-dependent eye phenotype with respect to ommatidial structure. Thus, an obvious question is whether Scylla and Charybdis bind to each other and exert their effect only in the presence of the other. Notably, eye-specific charybdis overexpression in a scylla-/- background resulted in the same phenotype as in a wild-type situation (data not shown). This indicates that Charybdis can act independently of Scylla. This is further supported by their mostly nonoverlapping mRNA expression patterns. Indeed, charybdis but not scylla is expressed in neuromuscular junctions (M. Knirr and C. Schuster, pers. comm.). Moreover, under hypoxia only scylla was induced in the fat body but not charybdis, indicating that they may be differentially regulated.
Scylla/Charybdis act downstream of PKB but upstream of TSC to regulate S6K activity
Several lines of evidence indicate that Scylla and Charybdis feed into the Inr pathway downstream of PKB: (1) Scylla antagonizes PKB/PDK1-induced overgrowth in the eye, but in vitro kinase assays demonstrate that Scylla and Charybdis do not reduce PKB kinase activity, nor does the loss of Scylla enhance PKB kinase activity. (2) scylla or charybdis coexpression can rescue PKB-induced developmental lethality, and ubiquitous scylla expression suppresses the lethality associated with hypomorphic PTEN mutants. (3) The weight reduction of hypomorphic PKB3 flies is partially rescued by the simultaneous absence of Scylla function. (4) Eye-specific PKB/PDK1 expression in a scylla-/- background leads to a mild enhancement of the eye phenotype. (5) Overexpression of PTEN or Dp110DN does not suppress the big eye phenotype of the tester system, suggesting that our screening system identifies components that act downstream of PKB.
The S6K assay demonstrated that Scylla is capable of reducing S6K activity. Thus, Scylla acts upstream of S6K. This result is consistent with our in vivo results. (1) scylla overexpression in the wing reduces wing size by decreasing cell size but not cell number (in fact, cell number is slightly increased), and (2) a S6K scylla mutant combination has the same weight as S6K single mutants. S6K mutants are smaller because of a reduction in cell size but not cell number, making it distinct from other Inr signaling pathway mutants (Montagne et al. 1999). Scylla and Charybdis do not control S6K activity directly but require its upstream regulator TSC. Tsc1/2 mutants cannot be rescued by overexpression of scylla, and the big head phenotype caused by loss of TSC function is not enhanced by the absence of Scylla and Charybdis. Coexpression of scylla or charybdis and Tsc1/2 does not further decrease eye size compared to Tsc1 and Tsc2 co-overexpression on their own. Moreover, a Rheb-dependent big eye phenotype or lethality induced by ubiquitous Rheb expression cannot be suppressed by scylla expression. These results indicate that Scylla regulates S6K activity by acting upstream of Tsc1/2 and Rheb. This function appears to be conserved between mammals and flies because RTP801/REDD1 can reduce S6 phosphorylation only in the presence of TSC (Brugarolas et al. 2004).
Our findings indicate that scylla/charybdis overexpression mainly affects the TSC/TOR/S6K branch of the pathway downstream of PKB. The PKB-FOXO axis appears not to be influenced by Scylla and Charybdis. Eye-specific overexpression of scylla/charybdis in conjunction with FOXO was unable to induce 4EBP (Supplementary Fig. S2). In contrast, simultaneous expression of FOXO and PTEN or a dominant-negative form of PI3K led to a strong induction of the reporter gene (Junger et al. 2003).
Consistent with an interplay between the Inr and TOR/S6K pathways, Inr lethality is suppressed by heterozygosity of Tsc1 (Gao and Pan 2001). Furthermore, overexpressed PKB phosphorylates and inactivates Tsc2 and thereby activates S6K (Inoki et al. 2002; Manning et al. 2002). Our finding that scylla overexpression is sufficient to rescue the lethality associated with PKB overexpression indicates that the lethality caused by PKB overexpression is due to the hyperactivation of the TOR/S6K pathway. Thus, oncogenic activation of PI3K/PKB signaling seems to be mainly mediated by TOR/S6K signaling. This may explain the beneficial effect of Rapamycin treatment (or its derivatives CCl-779 and RAD001) on PTEN-deficient tumors or cells overexpressing PKB (Neshat et al. 2001; Podsypanina et al. 2001; Shi et al. 2002; Majumder et al. 2004).
The TSC/TOR pathway as an integrator of growth and metabolism
TSC and TOR receive multiple inputs reflecting the metabolic state of the cell (Hay and Sonenberg 2004). AMP-activated kinase (AMPK) is a heterotrimeric kinase that is activated by high AMP/ATP ratios in the cell (Carling 2004). ATP depletion induces Tsc2-phosphorylation, and it was found that AMPK could interact with and phosphorylate Tsc2 (Inoki et al. 2003b). Interestingly, loss of Tsc2 in MEFs and U2OS osteosarcoma cells under low serum and prolonged hypoxia conditions results in HIF-1α accumulation and concomitantly increased expression of HIF-1 targets in a Rapamycin-dependent manner (Brugarolas et al. 2003). Arsham et al. (2003) showed that mTOR is regulated by decreased oxygen concentration resulting in a dephosphorylation of mTOR at Ser 2481, an mTOR autophosphorylation site. This effect was accompanied by reduced S6K phosphorylation but did not correlate with changes in adenine nucleotide levels and AMPK phosphorylation. Hence, these findings suggest a role for AMPK/Tsc2/mTOR in the integration of oxygen sensing/energy metabolism and growth.
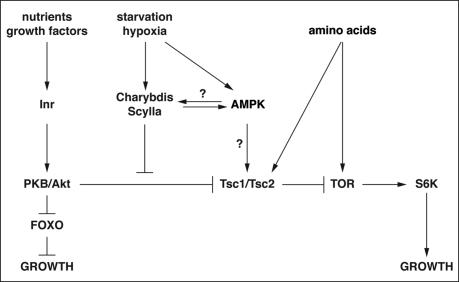
Model of Scylla/Charybdis function
Frei and Edgar (2004) found mutations in the gene encoding Drosophila HIF-1 prolyl hydroxylase (Hph), the enzyme rendering HIF-1α a substrate for proteasomal destruction under normoxic conditions, as dominant suppressors of a Cyclin D/Cdk4-induced bulging eye phenotype. Cells defective for hph show a growth deficit, and its overexpression stimulated growth. The authors suggested that the growth-promoting function of Hph is independent of HIF-1α/Sima. Our results raise the possibility that the Sima target scylla is important under hypoxia for growth inhibition.
We have shown that directed expression of Tgo-Sima in the fat body induces scylla expression. That this regulation is physiologically relevant can be inferred from three findings. First, scylla is also induced under hypoxic conditions. Second, directed expression of other bHLH-PAS proteins like Tgo-Trh or Sim alone did not induce scylla expression. Third, survival of flies lacking scylla and charybdis function is severely compromised under hypoxic conditions. Figure 7 summarizes our current model of Scylla and Charybdis function. scylla and charybdis are induced in response to external stress stimuli (e.g., hypoxia and starvation) to inhibit growth downstream of PKB but upstream of Tsc1/2. Scylla suppresses growth by reducing S6K activity. This could be achieved by relieving the inhibitory effect of PKB on Tsc2. Alternatively, Scylla/Charybdis could be negatively regulated targets of PKB. This is unlikely, however, since Scylla and Charybdis lack PKB consensus phosphorylation sites. AMPK, activated by drops in energy levels, may also contribute to the induction process of scylla and charybdis for growth inhibition, presumably under prolonged stress exposure. However, it is also possible that AMPK is controlled by Scylla and/or Charybdis. AMPK decreases protein synthesis by inhibition of S6K in a Rapamycin-sensitive manner, suggesting that mTOR is involved in mediating AMPK signaling (Kimura et al. 2003). AMPK also phosphorylates Tsc2, an event important for the cellular energy response pathway (Inoki et al. 2003b).
Figure 7.
Model of scylla/charybdis regulation and their effects. scylla and charybdis are stress-induced, negative growth modulators that inhibit growth under adverse environmental conditions. They are likely to feed into Inr/TOR/S6K signaling downstream of PKB but upstream of TSC to relieve the inhibitory effect of PKB on the TSC complex, thereby inhibiting growth. Our results do not exclude the possibility that S6K activity also responds to stress independent of Scylla/Charybdis function. Furthermore, Scylla/Charybdis may also regulate or be regulated by AMPK. See text for details.
RTP801/REDD1 may act as a hypoxia-dependent tumor suppressor
In tumors, hypoxic microenvironments are often encountered. Tumor hypoxia is associated with poor prognosis and resistance to radiation-induced cell death. Mutations in the tumor suppressor von Hippel-Lindau (VHL), the subunit of an E3 ubiquitin ligase complex that recognizes proline-hydroyxlated residues in HIF-1α, lead to the formation of a variety of tumors including clear cell carcinomas of the kidney, pheochromocytomas, and hemangioblastomas (Safran and Kaelin 2003). VHL-defective tumors exhibit increased HIF-1α expression. The induction of RTP801/REDD1 in cells exposed to hypoxia in tumors raises the possibility that these genes may play a role in tumor development. RTP801/REDD1 may act as a tumor suppressor. Cells having lost RTP801/REDD1 function may not stop growing under hypoxic conditions and hence risk accumulating further mutations that promote their tumorigenic state. The analysis of RTP801/REDD1 expression or mutations in a variety of tumor cell lines should help to test this hypothesis.
Materials and methods
EP element mapping and generation of transgenic flies
The insertion sites of EP9.85 and EPscy were determined by plasmid rescue. To obtain UAS-scy, we PCR-amplified a fragment containing the coding region of scylla out of a reverse-transcribed embryonic RNA pool (kindly provided by K. Nairz, University of Zurich, Switzerland); all primer sequences available on request). The PCR fragment was subcloned into the pCRII-TOPO vector using the TOPO TA cloning kit (Invitrogen). The scylla cDNA was cut out of pCRII-Topo as an Asp718/XbaI fragment and inserted into the pUAST vector (Brand and Perrimon 1993) to yield pJR3. At position 414 of the coding region, our sequence differs from that submitted by S. Chauvet, C. Maurel-Zaffran, R. Miassod, N. Julliens, J. Pradel, and D. Aragnol (submitted sequence to the EMBL GenBank DDBJ databases) and results in an A → G exchange. However, the codon still codes for Glu and represents either a silent mutation or a polymorphism.
The shortened scylla construct UAS-scyΔ1-12 was PCR-generated by using pJR3 as the DNA source with an ATG translational start site introduced by PCR mutagenesis. EP9.85, inserted 36 bp after the predicted translational start, shows the same effects as EPscy, which lies further upstream of the scylla transcription start site. We consider it likely that the last 3 nt of EP9.85 (i.e., ATG) serve as starter methionine to which amino acid 13 of the original Scylla protein is connected in frame.
To generate the UAS-char transgene, we ordered EST LP03309 (Research Genetics) corresponding to charybdis and checked the sequence for mutations. charybdis cDNA was excised from pOT2 as an EcoRI/XhoI fragment and inserted into the pUAST vector.
Transgenic flies were generated by P element-mediated germline transformation. Constructs were injected into yw embryos. At least two independent lines could be established for each construct.
Generation of mutants
For the generation of scylla mutants, we mobilized EP9.85 (marked with y+) inserted in the scylla ORF by supplying Δ2-3 transposase. Jump starter males were mated with y w; D/TM3 females, and single F1 y- males were crossed to y w; D/TM3 virgins. Approximately 400 stocks were established, and each stock was molecularly investigated for deletions by single-fly PCR using a panel of different primer pairs. Besides the mentioned scy31 and scy113 deletions, we found three additional smaller deficiencies.
char180 was identified in a local hop screen, mobilizing the original w+-marked EPchar insertion (causing a faint orange eye color). After the mobilization, we scored animals having a darker w+ eye color than EPchar and allowed recombination to occur by crossing single females to y w males. Only lines in which all the offspring consistently displayed the darker eye color (i.e., where presumably no recombination has occurred) were further analyzed by PCR using primers that would amplify the charybdis genomic locus (but not when a new EP had inserted). Out of 331 single females showing a darker eye color, 112 lines were established and analyzed by single-fly PCR. Only char180 was identified (contains the original EPchar element plus a new inverted EP insertion [indicated as EP1035* in Fig. 1F] ∼450 bp upstream of the char ORF). Quantitative RT-PCR of homozygous char180 flies revealed a 75% reduction in mRNA levels (Q RT-PCR data available on request).
Fly stocks
The following fly stocks and transgenes have been used in the course of this study: GMR-Gal4 (gift of M. Freeman, MRC Laboratory of Molecular Biology, Cambridge, UK); UAS-PKB, UAS-PDK1, EP837, and GMR > w+ > Gal4 (Rintelen et al. 2001); UAS-PTEN (Huang et al. 1999); UAS-Dp110DN (Leevers et al. 1996); Act5CGal4, Lsp2Gal4, ZCL0611 (Scylla fly trap) and Df(3L)vin4 (all from the Bloomington Drosophila Stock Center); EP1035 (from the Szeged Drosophila Stock Center); eyflp; FRT80 w+ cl3L/TM6B (Newsome et al. 2000); akt3 (Stocker et al. 2002); S6Kl-1/TM6B and S6K07084/TM6B (Montagne et al. 1999); PTEN2L100/CyO, y+, and PTEN2L117/CyO, y+ (Oldham et al. 2000, 2002); PTENdj189/CyO, y+ (Gao et al. 2000); Tsc1Q87X/TM6B, y+ (Tapon et al. 2001); Tsc12G3 (H. Stocker, unpubl.); Tsc256/TM6B, y+, and Tsc2192/TM6B, y+ (Ito and Rubin 1999); UAS-tgo, UAS-trh, and UAS-sim (gifts of B. Shilo, Department of Molecular Genetics, Weizmann Institute of Science, Rehovot, Israel); UAS-simaΔ692-863 (Lavista-Llanos et al. 2002); daGal4/CyO (A. Wodarz, unpubl.); Thor1 (Bernal and Kimbrell 2000).
All crosses were performed at 25°C if not stated otherwise.
Phenotypic analyses
Freshly eclosed adult flies were collected, separated according to sex, and placed for 24 h on normal fly food before weighing. Weighing was performed using a Mettler Toledo MX5 micro-balance.
scylla-overexpressing wings were mounted in Euparal Mounting solution (TAAB Laboratories). Wing area was determined using Photoshop and NIH Image 1.61 to count pixels. Relative cell number and cell size was determined by extrapolating the counted hairs in a defined area (0.1 mm)2 on the dorsal side of the wing proximal to the posterior cross-vein and posterior to vein five.
Adult flies used for scanning electron microscopy were stored in 70% acetone before they were critical-point dried and coated with gold to be examined under the scanning microscope.
Adult fly heads were cut in half using a razor blade and shortly stored in Ringers on ice. Eyes were then fixed as described in Basler et al. (1991).
Heat-shock-induced Gal4 overexpression clones (y w hsflp; GMR > w+ > Gal4, where > corresponds to FRT sites) were induced 24-48 h after egg laying (AEL) by a 1-h heat shock at 37°C. The heat shock induces FLP/FRT-mediated mitotic recombination leading to the excision of the w+ marker. scylla charybdis double-mutant clones were induced 24-48 h AEL by a 30-min heat shock at 38°C.
Starvation experiments and lipid analysis
Freshly eclosed flies were separated according to sex, left on normal food for 2 d, and subsequently transferred to empty plastic vials sealed with a water-soaked foam stopper. Dead flies were counted every 4/8 h. Determination of lipid and glycogen levels was done essentially as previously described (Bohni et al. 1999).
Hypoxia treatment
Larvae were exposed to the indicated O2 concentration at room temperature or at 25°C in a closed chamber connected to a gas-mixing pump (Digamix gas mixing pump, type M302 a-F, H Wösthoff e.H.G) that mixes air and N2, or to a ventilation pump (VENT2 pump, EMKA Technologies) that pushes a nitrogen/air mixture into the chamber. Dissection and fixation of larval tissues for mRNA in situ hybridizations and immunostainings were carried out immediately after hypoxia exposure. Scylla protein trap-larvae were dissected in Ringers, and larval tissue was fixed in 4% paraformaldehyde/PBS for 1 h by gentle agitation at room temperature. After a subsequent washing step, nuclei were stained by incubation for 10 min in DAPI (0.5 μg/mL)/PBS followed by three washing steps with PBS. Fluorescence images were recorded using a Zeiss Axiophot microscope.
mRNA in situ hybridizations were done essentially as described (Lehmann and Tautz 1994; O'Neill and Bier 1994). In vitro transcription was done using the DIG RNA labeling kit (Roche). DNA fragments corresponding to parts of the scylla and charybdis full-length mRNAs were PCR-amplified and sub-cloned into pCRII-TOPO vector. For the in vitro transcription, SP6 and T7 polymerases were used to produce sense and anti-sense RNAs.
PKB-/S6K in vitro activity assays and Western blotting
PKB assay: For each sample, 50 heads of 2-3-d-old female flies were removed with a forceps and collected on a cooled aluminum block placed on dry ice before the heads were transferred to Eppendorf tubes and flash-frozen in liquid nitrogen. Heads were squashed in 400 μL of extraction buffer (Stocker et al. 2003) using a plastic pestle and subsequently treated as described previously (Radimerski et al. 2002b). One-hundred micrograms of total protein was used for the PKB assay.
For the S6K assay, second instar larvae were collected by floatation in 30% glycerol. Following a brief wash in water, batches of 20-30 larvae were transferred to 1.5-mL Eppendorf tubes and flash-frozen in liquid nitrogen. The S6K assay was done essentially as described (Oldham et al. 2000). Either 25 or 40 μg of total protein was used for the S6K assay with H2B (Histone type VII-S, Sigma: H4255) as a substrate. Protein concentrations were determined using the RC DC Protein Assay (Bio-Rad).
32P incorporation into Crosstide or H2B was quantified and background-corrected using a PhosphorImager and image-quant software (Molecular Dynamics).
Western blotting: The following antibodies and dilutions were used: S6K D-20 antibody (Montagne et al. 1999) at 1:200 dilution, PKB antibody (Andjelkovic et al. 1995) at 1:1000 and mouse anti-α-tubulin (Sigma) at 1:2000. HRP-conjugated secondary antibodies (Dako A/S, Glostrup) were diluted 1:2000. Since the same PKB antibody used in immunoprecipitation was also used in Western blotting, HRP-conjugated protein-A (ICN Biomedicals) at 1:5000 was used instead of secondary antibody. Signals were detected using ECL Western blotting detection reagents (Amersham Biosciences).
Acknowledgments
We are greatly indebted to Thomas Radimerski for his support and the introduction into the PKB- and S6K activity assays and critical reading of the manuscript, George Thomas for the dS6K antibodies and diverse reagents, and Brian Hemmings for the dPKB antibody and the PKB substrate Crosstide. We appreciate the support of Jorge Soliz and Max Gassmann for letting us use the hypoxia chamber. We thank Kathrin Doepfner for the help in generating the char180 mutant; Christof Hugentobler for excellent technical support; Pablo Wappner, Benny Shilo, and Stephen T. Crews for fly stocks; Matthias Knirr and Christoph M. Schuster for fly stocks and sharing results prior to publication; Christian Frei and Hugo Stocker for critical reading of the manuscript and insightful comments; and the entire Hafen lab for discussions.
Supplemental material is available at http://www.genesdev.org.
Article published online ahead of print. Article and publication date are at http://www.genesdev.org/cgi/doi/10.1101/gad.322704.
References
- Andjelkovic M., Jones, P.F., Grossniklaus, U., Cron, P., Schier, A.F., Dick, M., Bilbe, G., and Hemmings, B.A. 1995. Developmental regulation of expression and activity of multiple forms of the Drosophila RAC protein kinase. J. Biol. Chem. 270: 4066-4075. [DOI] [PubMed] [Google Scholar]
- Arsham A.M., Howell, J.J., and Simon, M.C. 2003. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J. Biol. Chem. 278: 29655-29660. [DOI] [PubMed] [Google Scholar]
- Azevedo R.B., French, V., and Partridge, L. 2002. Temperature modulates epidermal cell size in Drosophila melanogaster. J. Insect Physiol. 48: 231-237. [DOI] [PubMed] [Google Scholar]
- Basler K., Christen, B., and Hafen, E. 1991. Ligand-independent activation of the sevenless receptor tyrosine kinase changes the fate of cells in the developing Drosophila eye. Cell 64: 1069-1081. [DOI] [PubMed] [Google Scholar]
- Bernal A. and Kimbrell, D.A. 2000. Drosophila Thor participates in host immune defense and connects a translational regulator with innate immunity. Proc. Natl. Acad. Sci. 97: 6019-6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohni R., Riesgo-Escovar, J., Oldham, S., Brogiolo, W., Stocker, H., Andruss, B.F., Beckingham, K., and Hafen, E. 1999. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell 97: 865-875. [DOI] [PubMed] [Google Scholar]
- Brand A.H. and Perrimon, N. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401-415. [DOI] [PubMed] [Google Scholar]
- Brogiolo W., Stocker, H., Ikeya, T., Rintelen, F., Fernandez, R., and Hafen, E. 2001. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 11: 213-221. [DOI] [PubMed] [Google Scholar]
- Brugarolas J.B., Vazquez, F., Reddy, A., Sellers, W.R., and Kaelin Jr., W.G. 2003. TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell 4: 147-158. [DOI] [PubMed] [Google Scholar]
- Brugarolas J., Lei, K., Hurley, R.L., Manning, B.D., Reiling, J.H., Hafen, E., Witters, L.A., Ellisen, L.W., and Kaelin Jr., W.G. 2004. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes & Dev. (this issue). [DOI] [PMC free article] [PubMed]
- Bruick R.K. 2003. Oxygen sensing in the hypoxic response pathway: Regulation of the hypoxia-inducible transcription factor. Genes & Dev. 17: 2614-2623. [DOI] [PubMed] [Google Scholar]
- Carling D. 2004. The AMP-activated protein kinase cascade—A unifying system for energy control. Trends Biochem. Sci. 29: 18-24. [DOI] [PubMed] [Google Scholar]
- Cho K.S., Lee, J.H., Kim, S., Kim, D., Koh, H., Lee, J., Kim, C., Kim, J., and Chung, J. 2001. Drosophila phosphoinositide-dependent kinase-1 regulates apoptosis and growth via the phosphoinositide 3-kinase-dependent signaling pathway. Proc. Natl. Acad. Sci. 98: 6144-6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisen L.W., Ramsayer, K.D., Johannessen, C.M., Yang, A., Beppu, H., Minda, K., Oliner, J.D., McKeon, F., and Haber, D.A. 2002. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Mol. Cell 10: 995-1005. [DOI] [PubMed] [Google Scholar]
- Frazier M.R., Woods, H.A., and Harrison, J.F. 2001. Interactive effects of rearing temperature and oxygen on the development of Drosophila melanogaster. Physiol. Biochem. Zool. 74: 641-650. [DOI] [PubMed] [Google Scholar]
- Frei C. and Edgar, B.A. 2004. Drosophila cyclin D/Cdk4 requires Hif-1 prolyl hydroxylase to drive cell growth. Dev. Cell 6: 241-251. [DOI] [PubMed] [Google Scholar]
- Gao X. and Pan, D. 2001. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes & Dev. 15: 1383-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Neufeld, T.P., and Pan, D. 2000. Drosophila PTEN regulates cell growth and proliferation through PI3K-dependent and -independent pathways. Dev. Biol. 221: 404-418. [DOI] [PubMed] [Google Scholar]
- Gao X., Zhang, Y., Arrazola, P., Hino, O., Kobayashi, T., Yeung, R.S., Ru, B., and Pan, D. 2002. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat. Cell Biol. 4: 699-704. [DOI] [PubMed] [Google Scholar]
- Garami A., Zwartkruis, F.J., Nobukuni, T., Joaquin, M., Roccio, M., Stocker, H., Kozma, S.C., Hafen, E., Bos, J.L., and Thomas, G. 2003. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol. Cell 11: 1457-1466. [DOI] [PubMed] [Google Scholar]
- Garofalo R.S. 2002. Genetic analysis of insulin signaling in Drosophila. Trends Endocrinol. Metab. 13: 156-162. [DOI] [PubMed] [Google Scholar]
- Gingras A.C., Raught, B., and Sonenberg, N. 2004. mTOR signaling to translation. Curr. Top. Microbiol. Immunol. 279: 169-197. [DOI] [PubMed] [Google Scholar]
- Goberdhan D.C., Paricio, N., Goodman, E.C., Mlodzik, M., and Wilson, C. 1999. Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes & Dev. 13: 3244-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N. and Sonenberg, N. 2004. Upstream and downstream of mTOR. Genes & Dev. 18: 1926-1945. [DOI] [PubMed] [Google Scholar]
- Huang H., Potter, C.J., Tao, W., Li, D.M., Brogiolo, W., Hafen, E., Sun, H., and Xu, T. 1999. PTEN affects cell size, cell proliferation and apoptosis during Drosophila eye development. Development 126: 5365-5372. [DOI] [PubMed] [Google Scholar]
- Ikeya T., Galic, M., Belawat, P., Nairz, K., and Hafen, E. 2002. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr. Biol. 12: 1293-1300. [DOI] [PubMed] [Google Scholar]
- Inoki K., Li, Y., Zhu, T., Wu, J., and Guan, K.L. 2002. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4: 648-657. [DOI] [PubMed] [Google Scholar]
- Inoki K., Li, Y., Xu, T., and Guan, K.L. 2003a. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes & Dev. 17: 1829-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K., Zhu, T., and Guan, K.L. 2003b. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577-590. [DOI] [PubMed] [Google Scholar]
- Ito N. and Rubin, G.M. 1999. gigas, a Drosophila homolog of tuberous sclerosis gene product-2, regulates the cell cycle. Cell 96: 529-539. [DOI] [PubMed] [Google Scholar]
- Jacinto E. and Hall, M.N. 2003. Tor signalling in bugs, brain and brawn. Nat. Rev. Mol. Cell. Biol. 4: 117-126. [DOI] [PubMed] [Google Scholar]
- Junger M.A., Rintelen, F., Stocker, H., Wasserman, J.D., Vegh, M., Radimerski, T., Greenberg, M.E., and Hafen, E. 2003. The Drosophila Forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J. Biol. 2: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N., Tokunaga, C., Dalal, S., Richardson, C., Yoshino, K., Hara, K., Kemp, B.E., Witters, L.A., Mimura, O., and Yonezawa, K. 2003. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells 8: 65-79. [DOI] [PubMed] [Google Scholar]
- Lavista-Llanos S., Centanin, L., Irisarri, M., Russo, D.M., Gleadle, J.M., Bocca, S.N., Muzzopappa, M., Ratcliffe, P.J., and Wappner, P. 2002. Control of the hypoxic response in Drosophila melanogaster by the basic helix-loop-helix PAS protein similar. Mol. Cell. Biol. 22: 6842-6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevers S.J., Weinkove, D., MacDougall, L.K., Hafen, E., and Waterfield, M.D. 1996. The Drosophila phosphoinositide 3-kinase Dp110 promotes cell growth. EMBO J. 15: 6584-6594. [PMC free article] [PubMed] [Google Scholar]
- Lehmann R. and Tautz, D. 1994. In situ hybridization to RNA. Methods Cell. Biol. 44: 575-598. [DOI] [PubMed] [Google Scholar]
- Majumder P.K., Febbo, P.G., Bikoff, R., Berger, R., Xue, Q., McMahon, L.M., Manola, J., Brugarolas, J., McDonnell, T.J., Golub, T.R., et al. 2004. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat. Med. 10: 594-601. [DOI] [PubMed] [Google Scholar]
- Manning B.D., Tee, A.R., Logsdon, M.N., Blenis, J., and Cantley, L.C. 2002. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell 10: 151-162. [DOI] [PubMed] [Google Scholar]
- Montagne J., Stewart, M.J., Stocker, H., Hafen, E., Kozma, S.C., and Thomas, G. 1999. Drosophila S6 kinase: A regulator of cell size. Science 285: 2126-2129. [DOI] [PubMed] [Google Scholar]
- Morin X., Daneman, R., Zavortink, M., and Chia, W. 2001. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl. Acad. Sci. 98: 15050-15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neshat M.S., Mellinghoff, I.K., Tran, C., Stiles, B., Thomas, G., Petersen, R., Frost, P., Gibbons, J.J., Wu, H., and Sawyers, C.L. 2001. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc. Natl. Acad. Sci. 98: 10314-10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome T.P., Asling, B., and Dickson, B.J. 2000. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development 127: 851-860. [DOI] [PubMed] [Google Scholar]
- Oldham S., Montagne, J., Radimerski, T., Thomas, G., and Hafen, E. 2000. Genetic and biochemical characterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes & Dev. 14: 2689-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S., Stocker, H., Laffargue, M., Wittwer, F., Wymann, M., and Hafen, E. 2002. The Drosophila insulin/IGF receptor controls growth and size by modulating PtdInsP(3) levels. Development 129: 4103-4109. [DOI] [PubMed] [Google Scholar]
- O'Neill J.W. and Bier, E. 1994. Double-label in situ hybridization using biotin and digoxigenin-tagged RNA probes. Biotechniques 17: 870, 874-875. [PubMed] [Google Scholar]
- Palos L.A. and Blasko, G. 1979. Effect of hypoxia on the development of Drosophila melanogaster (Meigen). Aviat. Space Environ. Med. 50: 411-412. [PubMed] [Google Scholar]
- Pan D., Dong, J., Zhang, Y., and Gao, X. 2004. Tuberous sclerosis complex: From Drosophila to human disease. Trends Cell Biol. 14: 78-85. [DOI] [PubMed] [Google Scholar]
- Pende M., Um, S.H., Mieulet, V., Sticker, M., Goss, V.L., Mestan, J., Mueller, M., Fumagalli, S., Kozma, S.C., and Thomas, G. 2004. S6K1-/-/S6K2-/- mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol. Cell. Biol. 24: 3112-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsypanina K., Lee, R.T., Politis, C., Hennessy, I., Crane, A., Puc, J., Neshat, M., Wang, H., Yang, L., Gibbons, J., et al. 2001. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/- mice. Proc. Natl. Acad. Sci. 98: 10320-10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C.J., Huang, H., and Xu, T. 2001. Drosophila Tsc1 functions with Tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell 105: 357-368. [DOI] [PubMed] [Google Scholar]
- Radimerski T., Montagne, J., Hemmings-Mieszczak, M., and Thomas, G. 2002a. Lethality of Drosophila lacking TSC tumor suppressor function rescued by reducing dS6K signaling. Genes & Dev. 16: 2627-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radimerski T., Montagne, J., Rintelen, F., Stocker, H., van der Kaay, J., Downes, C.P., Hafen, E., and Thomas, G. 2002b. dS6K-regulated cell growth is dPKB/dPI(3)K-independent, but requires dPDK1. Nat. Cell. Biol. 4: 251-255. [DOI] [PubMed] [Google Scholar]
- Rintelen F., Stocker, H., Thomas, G., and Hafen, E. 2001. PDK1 regulates growth through Akt and S6K in Drosophila. Proc. Natl. Acad. Sci. 98: 15020-15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorth P. 1996. A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proc. Natl. Acad. Sci. 93: 12418-12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safran M. and Kaelin Jr., W.G. 2003. HIF hydroxylation and the mammalian oxygen-sensing pathway. J. Clin. Invest. 111: 779-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucedo L.J., Gao, X., Chiarelli, D.A., Li, L., Pan, D., and Edgar, B.A. 2003. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat. Cell Biol. 5: 566-571. [DOI] [PubMed] [Google Scholar]
- Shi Y., Gera, J., Hu, L., Hsu, J.H., Bookstein, R., Li, W., and Lichtenstein, A. 2002. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 62: 5027-5034. [PubMed] [Google Scholar]
- Shoshani T., Faerman, A., Mett, I., Zelin, E., Tenne, T., Gorodin, S., Moshel, Y., Elbaz, S., Budanov, A., Chajut, A., et al. 2002. Identification of a novel hypoxia-inducible factor 1-responsive gene, RTP801, involved in apoptosis. Mol. Cell. Biol. 22: 2283-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker H., Andjelkovic, M., Oldham, S., Laffargue, M., Wymann, M.P., Hemmings, B.A., and Hafen, E. 2002. Living with lethal PIP3 levels: Viability of flies lacking PTEN restored by a PH domain mutation in Akt/PKB. Science 295: 2088-2091. [DOI] [PubMed] [Google Scholar]
- Stocker H., Radimerski, T., Schindelholz, B., Wittwer, F., Belawat, P., Daram, P., Breuer, S., Thomas, G., and Hafen, E. 2003. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat. Cell Biol. 5: 559-565. [DOI] [PubMed] [Google Scholar]
- Stolovich M., Tang, H., Hornstein, E., Levy, G., Cohen, R., Bae, S.S., Birnbaum, M.J., and Meyuhas, O. 2002. Transduction of growth or mitogenic signals into translational activation of TOP mRNAs is fully reliant on the phosphatidylinositol 3-kinase-mediated pathway but requires neither S6K1 nor rpS6 phosphorylation. Mol. Cell. Biol. 22: 8101-8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulis M.L. and Parsons, R. 2003. PTEN: From pathology to biology. Trends Cell Biol. 13: 478-483. [DOI] [PubMed] [Google Scholar]
- Tang H., Hornstein, E., Stolovich, M., Levy, G., Livingstone, M., Templeton, D., Avruch, J., and Meyuhas, O. 2001. Amino acid-induced translation of TOP mRNAs is fully dependent on phosphatidylinositol 3-kinase-mediated signaling, is partially inhibited by rapamycin, and is independent of S6K1 and rpS6 phosphorylation. Mol. Cell. Biol. 21: 8671-8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N., Ito, N., Dickson, B.J., Treisman, J.E., and Hariharan, I.K. 2001. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell 105: 345-355. [DOI] [PubMed] [Google Scholar]
- Tee A.R., Manning, B.D., Roux, P.P., Cantley, L.C., and Blenis, J. 2003. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr. Biol. 13: 1259-1268. [DOI] [PubMed] [Google Scholar]
- Wang Z., Malone, M.H., Thomenius, M.J., Zhong, F., Xu, F., and Distelhorst, C.W. 2003. Dexamethasone-induced gene 2 (dig2) is a novel pro-survival stress gene induced rapidly by diverse apoptotic signals. J. Biol. Chem. 278: 27053-27058. [DOI] [PubMed] [Google Scholar]
- Zhang H., Stallock, J.P., Ng, J.C., Reinhard, C., and Neufeld, T.P. 2000. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes & Dev. 14: 2712-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Gao, X., Saucedo, L.J., Ru, B., Edgar, B.A., and Pan, D. 2003. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat. Cell Biol. 5: 578-581. [DOI] [PubMed] [Google Scholar]
- Zinke I., Schutz, C.S., Katzenberger, J.D., Bauer, M., and Pankratz, M.J. 2002. Nutrient control of gene expression in Drosophila: Microarray analysis of starvation and sugar-dependent response. EMBO J. 21: 6162-6173. [DOI] [PMC free article] [PubMed] [Google Scholar]