Abstract
Objective
To examine variation in pharmaceutical spending and patient characteristics across prescription drug user groups.
Data Sources
British Columbia's population‐based linked administrative health and sociodemographic databases (N = 3,460,763).
Study Design
We classified individuals into empirically derived prescription drug user groups based on pharmaceutical spending patterns outside hospitals from 2007 to 2011. We examined variation in patient characteristics, mortality, and health services usage and applied hierarchical clustering to determine patterns of concurrent drug use identifying high‐cost patients.
Principal Findings
Approximately 1 in 20 British Columbians had persistently high prescription costs for 5 consecutive years, accounting for 42 percent of 2011 province‐wide pharmaceutical spending. Less than 1 percent of the population experienced discrete episodes of high prescription costs; an additional 2.8 percent transitioned to or from high‐cost episodes of unknown duration. Persistent high‐cost users were more likely to concurrently use multiple chronic medications; episodic and transitory users spent more on specialized medicines, including outpatient cancer drugs. Cluster analyses revealed heterogeneity in concurrent medicine use within high‐cost groups.
Conclusions
Whether low, moderate, or high, costs of prescription drugs for most individuals are persistent over time. Policies controlling high‐cost use should focus on reducing polypharmacy and encouraging price competition in drug classes used by ordinary and high‐cost users alike.
Keywords: Prescription drug costs, high‐cost users, population‐based analysis
Owing to the increased availability, use, and cost of pharmaceuticals, prescription drugs comprise a large and growing share of total health care expenditures in many countries (OECD 2014). As with health services more generally (Roos, Burchill, and Carriere 2003; Rais et al. 2013; Rosella et al. 2014), a small proportion of individuals, termed high‐cost users (HCUs), accounts for the majority of prescription drug costs in health care systems (Hanley and Morgan 2009; Saastamoinen and Verho 2012; CIHI 2014). Research shows that HCUs of health services often incur disproportionately high health care costs for many consecutive years (Monheit 2003; Riley 2007; Joynt et al. 2013). There is comparatively little research on the characteristics of HCUs of prescription drugs over time (Kozyrskyj et al. 2005; Hanley and Morgan 2009).
Given the therapeutic and financial importance of prescription drugs in modern health care, analyses of the distribution and persistence in prescription drug spending can be valuable for health system planning, cost containment, and equitable financing. Health system managers can use information about HCUs of prescription drugs, particularly those with persistent high costs, to more accurately forecast expenditures and detect future cost pressures. A better understanding of drug use by persistent versus transitory or episodic‐HCUs may inform clinical decisions regarding the quality of pharmaceutical therapy as well as facilitate cost mitigation strategies. Additionally, furthering knowledge of persistent‐HCU characteristics will be useful for policy makers targeting inequities in prescription drug spending in countries where prescription drugs are not universally provided, such as Canada and the United States.
Using comprehensive linked health and sociodemographic datasets, we study the level and temporal patterns of individual prescription drug costs for British Columbia's entire population. We classify individuals in the population into prescription drug user groups according to their pharmaceutical costs from 2007 to 2011 and report their sociodemographics, health status, and utilization of other health services. We pay particular attention to patients with persistent high prescription drug costs, those who transition to and from high costs, and those with single episodes of high costs, analyzing the medicines commonly used by these extraordinary groups and comparing their profiles to more ordinary prescription drug users. Finally, we apply hierarchical clustering to determine any patterns of concomitant drug use that might identify high‐cost patients by age and sex.
Methods
Data Sources
We based our analysis on deidentified linked health datasets provided by Population Data BC with approval of relevant data stewards and the University of British Columbia's Behavioural Research Ethics Board (2012–2014). All inferences, opinions, and conclusions drawn in this report are those of the authors, and they do not reflect the opinions or policies of the Data Stewards. Datasets included health care, sociodemographic, and mortality information for all British Columbians except registered First Nations and Inuit, members of the military, and inmates of federal penitentiaries (exclusions combined equal 4 percent of the population). To ensure complete data capture for study subjects, we focused our analysis on individuals living in British Columbia for at least 275 days in each year from 2007 until 2011. We recorded deaths in the fourth quarter of 2011, following fulfillment of the 275‐day requirement, to allow for end‐of‐period mortality.
Our data on prescription drug purchases came from BC PharmaNet, an information system into which pharmacists must enter records of every prescription dispensed outside of acute care hospitals, including those dispensed in long‐term care facilities (Government of British Columbia 2014). Prescription records include information about drug type, dose, quantity dispensed, formulation, and total cost in Canadian dollars.
Acute care hospitals account for approximately 10 percent of total annual prescription drug sales in Canada (Morgan et al. 2013). Failure to capture pharmaceutical use within acute care hospitals may cause patients experiencing long acute hospital stays to be misclassified in terms of their community‐based prescription drug use and cost. To limit this possibility, we excluded individuals who spent the majority of any year (more than 183 days) in an acute care hospital (approximately 0.01 percent of the study population). Beyond this exclusion, we found that annualizing outpatient prescription expenditures to account for time spent in acute care hospitals had no material effect on study findings.
Our medical services data included provider type, service type, cost of service, and one primary diagnosis code (ICD‐9/10) for every fee‐for‐service medical visit by all patients in our dataset. We did not have access to medical services data for care provided on alternative payment mechanisms (e.g., block‐funded or capitation‐funded health clinics). We therefore excluded a small number of geographic areas (e.g., northern and inner‐city communities) that receive 25 percent or more of their medical care from non‐fee‐for‐service providers. This exclusion affected an additional 4 percent of the study population.
Our hospital services data came from the Discharge Abstract Database, which tracks separations from all hospitals in British Columbia. Hospital records contain information about reason for admission, length of stay, level of care, procedures received, and up to 25 diagnoses (ICD‐10). We determined the cost of hospital visits by multiplying the resource intensity weight of a specific hospital stay by the British Columbia government's estimated average costs per weighted case (Morgan et al. 2009).
Derived Variables
We grouped prescription drugs according to the World Health Organization's (WHO) Anatomical Therapeutic Chemical (ATC) drug classification system (WHO Collaborating Centre for Drug Statistics Methodology 2014). To identify polypharmacy, we counted the number of different ATC drug groups at the third level, therapeutic/pharmacological subgroups, that patients filled prescriptions from during each year. We defined broader therapeutic classes by grouping ATC subgroups based on primary indications for use (Appendix S1) (Morgan et al. 2013). Additionally, we identified drugs used to treat acute and chronic illness using WHO classifications (WHO 2011).
We gauged overall patient morbidity using aggregated diagnostic groups (ADGs) of the Johns Hopkins Adjusted Clinical Group (ACG version 10.0) case mix adjustment system (Weiner et al. 1991). ADGs map ICD‐9 and ICD‐10 codes into 32 mutually exclusive groups, eight of which have very high expected resource use and are labeled as major ADGs. We measured overall health status using a count of major ADGs and a count of minor ADGs represented by the ICD codes found in each individual's medical and hospital records, which have been shown to be predictive of mortality and health services utilization (Weiner and Abrams 2009; Hanley, Morgan, and Reid 2010).
We estimated household income based on a combination of household‐specific and area‐based income data (Hanley and Morgan 2008). For 52 percent of the population, we had validated, household‐specific income information from registration files for British Columbia's universal, income‐based public drug subsidy system (Fair PharmaCare, British Columbia, Canada). For the remaining 48 percent of the population, we estimated household income based on the median household income for the Census Dissemination Area in which people lived. Dissemination Areas are contiguous geographic areas with populations ranging from approximately 400 to 700 persons.
As there are no population‐based sources of information on ethnicity that could be linked to British Columbia's health research datasets, we estimated ethnicity using an algorithm developed to identify surnames of South Asian and Chinese origin and validated for use with data from secondary sources (Shah et al. 2010).
Prescription Drug User Groups
We analyzed prescription drug expenditure distributions in each year of the study and developed probability transition matrices depicting individual changes in spending from 2007 to 2011 (Appendix S2). These preliminary analyses revealed reasonable persistence and modest variation in the level of prescription drug spending among individuals whose expenditures were below the 50th percentile of the expenditure distribution, and among individuals whose spending fell between the 50th and 80th percentiles. Spending was reasonably persistent though more varied among patients whose costs fell between the 80th and 90th percentiles, and among patients whose costs were above the 90th percentile. We therefore used the 50th, 80th, and 90th percentiles as thresholds—between what we consider low, average, moderate, and high prescription drug costs—to define nine mutually exclusive prescription drug user groups based on spending levels observed in each year from 2007 to 2011.
We classified individuals as persistent‐HCUs if their prescription drug costs were above the 90th percentile in each of the 5 years from 2007 to 2011. We defined episodic‐HCUs as individuals who had low or average costs (below the 80th percentile) in 2007, then had at least one high‐cost year (above the 90th percentile) between 2008 and 2010, and then had low or average costs again in 2011. We also identified two categories of “transitory‐HCUs” that included people who transitioned to or from episodes of high costs (above the 90th percentile) spanning just the end or just the beginning of our study period. Those transitioning “from HCU” experienced high prescription drug costs in 2007 and transitioned to low or average costs by 2011. Those transitioning “to HCU” had average or low costs in 2007 and high costs by 2011. We could not determine the length of the high‐cost episodes of these transitory‐HCUs because their episodes spanned the bounds of our study period.
People with annual prescription drug costs always above the 80th percentile from 2007 to 2011, but who did not satisfy the criteria for being either a persistent, episodic, or transitory‐HCU were classified as persistent moderate‐cost users. Individuals whose annual prescription drug costs were always below the 80th percentile and were between the 50th and 80th percentiles in one or more years were classified as persistent average‐cost users. Those with annual costs always below the 50th percentile and who filled prescriptions in 1 or more years were classified as persistent low‐cost users. Those who did not fill any prescriptions from 2007 to 2011 were classified as persistent nonusers. Finally, patients whose drug costs varied significantly and included patterns of low‐cost and moderate‐ or high‐cost use that did not satisfy the criteria for any of the previously defined user groups were considered sporadic low‐to‐high‐cost users.
Analyses
For each prescription drug user group, we measured prescription drug, physician, and hospital service usage and costs as well as sociodemographic characteristics, health status, and mortality. We tested for statistically significant differences in these measures across prescription drug user groups using multiple t‐tests, chi‐square tests, and multinomial logistic regression techniques.
We implemented hierarchical cluster analysis to search for patterns of concomitant prescription drug use across various age‐sex strata of key user groups: persistent moderate‐cost users, transitory‐HCUs, episodic‐HCUs, and persistent‐HCUs. Given that we had no prior knowledge of the number of drug clusters, we implemented an agglomerative hierarchical clustering algorithm and weighted average linkage to determine commonly occurring groups of drug classes (Hastie et al. 2005; Cornell et al. 2009; Vu, Finch, and Day 2011; StataCorp 2013). We used a Jaccard‐based dissimilarity matrix and limited our analysis to drug classes with prevalence rates greater than 3 percent to minimize chaining (Vu, Finch, and Day 2011). We visually represent our cluster analysis results using dendrograms for each group of patients assessed.
Results
Approximately 3.5 million individuals fulfilled our inclusion criteria of continuous residency from 2007 to 2011. Owing to birth, death, immigration, or episodes of out‐of‐province residency during the study period, roughly 23 percent of residents of British Columbia in 2011 did not satisfy our inclusion criteria; these excluded individuals accounted for 11 percent of total population‐wide prescription drug spending outside hospitals that year.
Figure 1 illustrates the distribution of prescription drug spending across our study population during 2011. Though use of medicines was quite common, the distribution of prescription drug costs was highly skewed. Approximately 30 percent of the population filled no prescriptions during 2011. The 50th percentile of total prescription drug costs for 2011 was $86; the 80th percentile was $726; the 90th percentile was $1,521; and the 99th percentile was $6,454.
Figure 1.
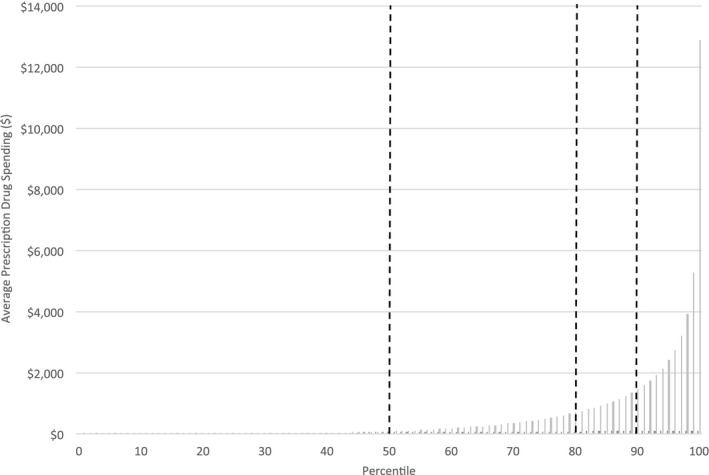
Average Prescription Drug Spending for Population of British Columbia Satisfying Study Inclusion Criteria, by Cost Percentiles in 2011
Prescription Costs by User Group
The cohort size and prescription drug costs of each of our prescription drug user groups are described in Table 1. About 1 in 12 people in our study cohort (8.4 percent) filled no prescriptions in any year from 2007 to 2011 and were classified as persistent nonusers. Approximately one in six people had persistent low prescription drug costs from 2007 to 2011, and almost half of our study cohort (44.2 percent) had persistent average costs. The mean total cost of prescriptions filled in 2011 by persistent low‐cost and persistent average‐cost users was $12 and $131, respectively. Combined, these ordinary prescription drug users, representing 69 percent of the study cohort, accounted for 10 percent of total spending on prescription drugs for our cohort in 2011.
Table 1.
Percentage of Cohort, Total Prescription Drug Costs Accounted for, Demographic Characteristics, and Health Status for Prescription Drug User Groups in 2011
| Persistent Nonusers | Persistent Low‐Cost Users | Persistent Average‐Cost User | Persistent Moderate‐Cost User | Sporadic Low‐to‐High‐Cost Users | Transition to HCU | Transition from HCU | Episodic‐HCU | Persistent‐HCU | |
|---|---|---|---|---|---|---|---|---|---|
| n | 291,940 | 582,763 | 1,528,344 | 262,407 | 484,024 | 67,989 | 27,670 | 12,841 | 202,785 |
| Percentage of study cohort (percent) | 8.4 | 16.8 | 44.2 | 7.6 | 14.0 | 2.0 | 0.8 | 0.4 | 5.9 |
| Percentage of total 2011 prescription drug expenditures (%) | 0.0 | 0.3 | 9.9 | 20.5 | 15.8 | 11.0 | 0.4 | 0.2 | 41.9 |
| Average prescription drug costs | $0 | $12 | $131 | $1,587 | $663 | $3,266 | $267 | $255 | $4,192 |
| Sex (%) | |||||||||
| Female | 40 | 42 | 54 | 55 | 56 | 48 | 55 | 55 | 54 |
| Age (%) | |||||||||
| 0–17 | 22 | 31 | 14 | 1 | 3 | 4 | 2 | 3 | 1 |
| 18–44 | 36 | 40 | 43 | 9 | 25 | 24 | 22 | 38 | 8 |
| 45–64 | 30 | 25 | 32 | 37 | 42 | 41 | 34 | 39 | 34 |
| 65–84 | 7 | 4 | 9 | 45 | 26 | 25 | 29 | 16 | 46 |
| 85+ | 5 | 0 | 1 | 9 | 4 | 5 | 13 | 3 | 10 |
| Ethnicity (%) | |||||||||
| Chinese surname | 21 | 12 | 10 | 6 | 7 | 6 | 6 | 8 | 4 |
| South Asian surname | 2 | 3 | 6 | 3 | 4 | 5 | 3 | 5 | 3 |
| Income (%) | |||||||||
| Quintile 1 | 15 | 15 | 17 | 30 | 23 | 29 | 26 | 23 | 34 |
| Quintile 2 | 23 | 19 | 18 | 19 | 17 | 17 | 20 | 18 | 20 |
| Quintile 3 | 23 | 22 | 20 | 15 | 17 | 16 | 17 | 18 | 15 |
| Quintile 4 | 21 | 23 | 21 | 16 | 18 | 16 | 17 | 18 | 14 |
| Quintile 5 | 16 | 21 | 23 | 20 | 23 | 20 | 18 | 22 | 16 |
| Major ADGs (%) | |||||||||
| 0 | 94 | 88 | 75 | 37 | 54 | 27 | 50 | 49 | 21 |
| 1–2 | 6 | 12 | 24 | 52 | 41 | 56 | 40 | 45 | 58 |
| 3+ | 1 | 0 | 1 | 11 | 6 | 16 | 10 | 7 | 20 |
| Minor ADGs (%) | |||||||||
| 0–1 | 85 | 59 | 29 | 8 | 13 | 8 | 26 | 20 | 6 |
| 2–3 | 12 | 30 | 36 | 27 | 31 | 25 | 29 | 31 | 21 |
| 4–5 | 3 | 9 | 23 | 30 | 29 | 29 | 23 | 27 | 28 |
| 6+ | 1 | 2 | 12 | 35 | 27 | 38 | 22 | 22 | 44 |
| Death in 2011 Q4 (crude), % | 0.27 | 0.06 | 0.17 | 1.84 | 0.99 | 1.98 | 8.54 | 2.94 | 2.12 |
| Death in 2011 Q4 (age‐adjusted), % | 0.28 | 0.27 | 0.34 | 0.80 | 0.70 | 1.46 | 3.48 | 2.72 | 1.03 |
All differences in demographics and spending across prescription drug user groups are statistically significant at p = .001.
At the other extreme, approximately 6 percent of our study cohort had prescription drug costs above the 90th percentile in each year from 2007 to 2011. The mean total cost of prescriptions filled by these persistent‐HCUs in 2011 was approximately $4,200; collectively, this group accounted for 42 percent of total spending on prescription drugs that year.
Less than 1 percent of the study population experienced a discrete episode of high‐cost use during our study period. The mean total cost of prescriptions filled in 2011 by these episodic‐HCUs was just $255, representing 0.2 percent of total prescription drug spending that year. However, in their most recent year of high costs, episodic‐HCUs filled prescriptions worth an average of nearly $3,500.
Approximately 1 percent of the study cohort transitioned from high prescription drug costs in 2007 to low or average costs by 2011. These individuals spent an average of $267 on prescription drugs in 2011 and accounted for 0.4 percent of total drug spending. Yet, in their most recent year of high costs, they had average prescription drug costs totaling $2,663.
Two percent of our study population transitioned from low or average prescription drug costs in 2007 to high costs by 2011. These patients had mean total prescriptions drug costs equal to nearly $3,300 in 2011, and they represented approximately 11 percent of total prescription drug spending.
Approximately 8 percent of the cohort incurred prescription drug costs above the 80th percentile every year between 2007 and 2011 but were not persistent‐HCUs, episodic‐HCUs, or transitory‐HCUs. These persistent moderate‐cost users had average total prescription costs of nearly $1,600 and were responsible for roughly 21 percent of total drug spending in 2011.
Approximately 14 percent of the study cohort did not fall into the above categories. The mean total cost of prescriptions filled by sporadic low‐to‐high‐cost users in 2011 was $663, accounting for 16 percent of total drug costs for our cohort that year. About 70 percent of these sporadic low‐to‐high‐cost users had costs above the 50th percentile in each year of the study and would have been classified as persistent moderate‐cost users had we lowered our threshold for identifying moderate prescription drug costs.
User Group Demographics
Table 1 also describes characteristics of each of our prescription drug user groups. Owing to the very large number of observations used in this population‐based analysis, all differences in characteristics across prescription drug user groups were statistically significant (p < .001). This includes many statistically significant but very small differences (e.g., 3.4 percent of persistent moderate‐cost users had surnames of South Asian origin versus 3.3 percent of persistent‐HCUs who had surnames of South Asian origin). However, some differences warrant more attention than others. For example, both persistent nonusers and low‐cost users were much more likely to be male than other user groups. Additionally, persistent nonusers, persistent low‐cost, and persistent average‐cost users were most likely to be under age 65, to have surnames of Chinese origin, to have midrange incomes, and to have fewer major and minor ADGs.
Persistent moderate‐cost users and persistent‐HCUs were most likely to be older than 65. Persistent moderate‐cost users, individuals who transitioned to HCUs by 2011, and persistent‐HCUs were more likely to have low incomes, many major and minor comorbidities, and to have higher mortality rates than ordinary prescription drug users.
Episodic‐HCUs were significantly younger than persistent‐HCUs. While on average episodic‐HCUs had few comorbidities in 2011, they had approximately the same levels of major and minor comorbidities as persistent‐HCUs during their most recent high‐cost year. Although their morbidity level decreased in 2011, episodic‐HCUs had the second highest mortality rate of any user group. Individuals who transitioned from high to average or low costs by 2011 had the highest mortality rate of any user group. They had comparable population characteristics to episodic‐HCUs, albeit they were more likely to be significantly older. Sporadic low‐to‐high‐cost users had comparable population characteristics to episodic‐HCUs, but they had a much lower mortality rate.
Use and Cost of Prescription Drugs, Medical Services, and Hospital Services
Table 2 summarizes 2011 prescription drug, physician, and hospital service costs and usage for each prescription drug user group. The large majority of pairwise differences in average health service costs and usage per capita across user groups were statistically significant (p < .001). The few differences that were not statistically significant are noted with superscript references in Table 2. Patterns of prescription drug use and costs follow the logic of the user group definitions. Persistent low‐cost users filled an average of about one prescription per year containing an average total of 8 days of therapy, while persistent average‐cost users filled an average of about four prescriptions containing an average total of approximately 130 days of therapy. In comparison, individuals who transitioned to HCUs by 2011 filled an average of 55 prescriptions containing an average total of over 1,250 days of therapy, while persistent‐HCUs filled an average of 92 prescriptions containing an average total of more than 2,200 days of therapy. Persistent‐HCUs were also more likely to experience polypharmacy, filling prescriptions from an average of 10 different drug groups in 2011.
Table 2.
Use and Cost of Prescription Drugs, Physician Services, and Hospital Services per Person in Prescription Drug User Groups, 2011 or Most Recent High‐Cost Year
| Persistent Nonusers | Persistent Low‐Cost Users | Persistent Average‐Cost User | Persistent Moderate‐Cost User | Sporadic Low‐to‐High‐Cost Users | Transition to HCU | Transition from HCU (2011) | Transition from HCU (High‐cost year) | Episodic‐HCU (2011) | Episodic‐HCU (High‐cost year) | Persistent‐HCU | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prescription drugs | |||||||||||
| Average total cost | $0 | $12 | $131 | $1,587 | $663 | $3,266 | $267 | $2,663 | $255 | $3,485 | $4,192 |
| Median total cost | $0 | $0 | $78 | $1,231 | $612 | $2,020 | $264 | $1,857 | $235 | $1,958 | $2,960 |
| Interquartile range of total costs | ($0, $0) | ($0, $22) | ($12, $205) | ($942, $1,693) | ($337, $857) | ($1,622, $2,925) | ($27, $471) | ($1,579, $2,533) | ($57, $438) | ($1,624, $3,090) | ($2,112, $4,533) |
| Average prescriptions filled | 0.0 | 0.6 | 3.7 | 33.7 | 14.3 | 55.0 | 13.0 | 46.7 | 8.5 | 32.5 | 92.4 |
| Percentage for chronic conditions | – | 38.7 | 69.0 | 92.3 | 86.2 | 93.6 | 90.2 | 92.1 | 83.6 | 87.3 | 95.2 |
| Average days of drug supplied | 0.0 | 8.1 | 133.1 | 1,317.1 | 589.2 | 1,251.8 | 376.3 | 1,203.5 | 290.1 | 750.0 | 2,276.2 |
| Percentage for chronic conditions | – | 56.9 | 88.2 | 96.2 | 94.0 | 94.9 | 94.6 | 94.4 | 92.7 | 90.9 | 96.4 |
| Average number of drug groups | 0.0 | 0.5 | 2.0 | 6.8 | 4.4 | 7.2 | 3.2 | 6.9 | 3.1 | 6.5 | 9.8 |
| Physician services | |||||||||||
| Average total cost | $87 | $172 | $452 | $1,386 | $956a | $1,900 | $909b | $2,094 | $930a,b | $2,094 | $1,999 |
| Median total cost | $0 | $59 | $230 | $911 | $578 | $1,233 | $472 | $998 | $536 | $1,397 | $1,389 |
| Interquartile range of total costs | ($0, $33) | ($0, $189) | ($73, $532) | ($478, $1,693) | ($274, $1,149) | ($644, $2,333) | ($150, $1,059) | ($493, $1,999) | ($212, $1,108) | ($694, $2,677) | ($764, $2,459) |
| Average number of providers | 0.9 | 2.1 | 4.4 | 8.9 | 7.1 | 10.9 | 6.1 | 10.0 | 6.8 | 12.2 | 11.4 |
| Average number of visits | 1.5 | 3.1 | 7.7 | 21.7 | 15.2 | 28.5 | 14.6c | 25.5 | 14.1c | 29.6 | 31.2 |
| Percentage of visits to specialists | 39 | 38 | 41 | 48 | 46 | 51 | 43 | 47 | 49 | 58 | 50 |
| Hospital services | |||||||||||
| Average total cost | $112d | $97d | $333 | $1,915 | $1,130 | $3,525 | $2,489 | $3,230e | $1,404 | $4,151 | $3,264e |
| Median total cost | $0 | $0 | $0 | $0 | $0 | $0 | $0 | $0 | $0 | $0 | $0 |
| Interquartile range of total costs | ($0, $0) | ($0, $0) | ($0, $0) | ($0, $328) | ($0, $0) | ($0, $1,274) | ($0, $0) | ($0, $636) | ($0, $0) | ($0, $3,374) | ($0, $959) |
| Rate of hospitalization | 2 | 4 | 10 | 25 | 19 | 33 | 19 | 29 | 20 | 39 | 33 |
| Hospitalizations per user | 1.3f | 1.1 | 1.2 | 1.5 | 1.4g | 1.7j | 1.4h | 1.6I,j | 1.3f,g,h | 1.8 | 1.7i |
| Percentage of urgent visits | 45 | 23 | 21 | 35 | 29 | 47 | 51 | 48 | 33 | 43 | 45 |
| Length of stay per visit | 5.9 | 2.0 | 2.4 | 4.9 | 4.0 | 6.0 | 10.5 | 7.5 | 5.2 | 5.3 | 5.9 |
| Percentage of day visits | 48 | 68 | 64 | 57 | 60 | 45 | 42 | 59 | 53 | 59 | 49 |
All pairwise differences in measures of average use and cost are statistically significant at p = .001, except as noted with superscript references; for example, a = difference between episodic‐HCUs (in high‐cost year) and persistent moderate‐cost users not significant at p = .001. Costs for episodic‐HCUs and transitions from HCU during their most recent high‐cost year are adjusted for inflation. Drug groups measured using the WHO ATC system, third level, therapeutic/pharmacological subgroup.
By definition, episodic‐HCUs and patients who transitioned from HCUs to low‐ or average‐cost users had relatively low prescription drug usage and costs in 2011. However, during their most recent year of high‐cost use, they had much higher levels of use and, especially, costs. In contrast to persistent‐HCUs, episodic‐HCUs filled approximately a third as many prescriptions during their high‐cost year, totaling approximately a third as many days of therapy, but at nearly the same level of total cost. As such, the average cost per day of treatment was much higher for episodic‐HCUs than for persistent‐HCUs. Conversely, in their most recent high‐cost year, average costs per day of treatment for individuals who transitioned from HCUs were much more similar to those for persistent‐HCUs.
Differences in average use and cost of physician services across prescription drug user groups were similar to—though lower in overall magnitude than—the differences in prescription drug use and cost. Notably, many persistent nonusers of prescription drugs received care from physicians. Additionally, during their most recent year of high prescription costs, episodic‐HCUs had higher average physician service costs, saw more providers, and received more care from specialists than persistent‐HCUs.
Patterns of hospital service use and cost across prescription drug user groups also mirrored patterns of prescriptions drug use and cost. High‐ and moderate‐cost users of prescription drugs received more care in hospitals, at higher total cost, than persistent nonusers, low‐cost, and average‐cost users of prescription drugs. Notably, episodic‐HCUs of prescription drugs had the highest rates of hospitalization and associated costs during their most recent high‐cost year. Care for episodic‐HCUs was more likely to be on an inpatient basis and at a higher resource intensity than care received by other user groups, including persistent‐HCUs. While episodic‐HCUs experienced much higher hospital costs during their most recent high‐cost episode than in 2011, individuals who transitioned from HCUs experienced similar average hospital costs in both 2011 and their most recent high‐cost episode. Finally, nonusers of prescription drugs were less likely to be hospitalized but had longer average hospital stays compared to persistent low‐cost and persistent average‐cost users. A relatively large proportion of their hospitalizations, 45 percent, were for urgent unplanned events.
Top Therapeutic Categories for Prescription Spending
Table 3 shows the top 10 therapeutic classes in terms of total prescription drug costs for persistent moderate‐cost users, transitory‐HCUs, episodic‐HCUs, and persistent‐HCUs. The 10 leading drug classes accounted for 63 percent of total cost of prescription drugs for moderate‐cost users, 53 percent of total costs for individuals who transitioned from low‐to‐high‐cost use, 49 percent of costs in their most recent high‐cost year for individuals who transitioned from high‐ to low‐cost use by 2011, 46 percent of costs in their most recent year for episodic‐HCUs, and 60 percent of costs for persistent‐HCUs. A small proportion of individuals from each of these five user groups filled prescriptions for very expensive biologic drugs to treat inflammatory conditions—such as rheumatoid arthritis, psoriasis, and Crohn's disease.
Table 3.
Top Classes of Prescription Drug by Share of Total Drug Costs for Prescription Drug User Group, 2011 or Most Recent High‐Cost Year
| Prescription Drug Class | Percentage of Individuals with One or More Prescriptions | Percentage of Total Prescription Drug Costs |
|---|---|---|
| Persistent‐HCUs (%) | ||
| Antihypertensives | 72 | 11 |
| Drugs for inflammatory conditions | 3 | 8 |
| Cholesterol drugs | 57 | 7 |
| Antidepressants | 41 | 6 |
| Antipsychotics | 16 | 6 |
| Acid‐reducing drugs | 45 | 5 |
| Drugs for respiratory conditions | 26 | 5 |
| Opioids | 36 | 5 |
| Insulins | 14 | 4 |
| Noninsulins | 29 | 3 |
| Episodic‐HCUs (high‐cost year), % | ||
| Cancer drugs | 11 | 18 |
| Drugs for female infertility | 8 | 7 |
| Antidepressants | 27 | 4 |
| Drugs for inflammatory conditions | 2 | 4 |
| Antihypertensives | 25 | 3 |
| Cholesterol drugs | 16 | 3 |
| Antipsychotics | 15 | 2 |
| Anticoagulants | 6 | 2 |
| Pregabalin and gabapentin | 12 | 2 |
| Antibiotics | 52 | 2 |
| Episodic‐HCUs (in 2011), % | ||
| Antihypertensives | 18 | 12 |
| Antidepressants | 15 | 9 |
| Cholesterol drugs | 9 | 8 |
| Antibiotics | 36 | 7 |
| Acid‐reducing drugs | 10 | 4 |
| Opioids | 19 | 4 |
| Drugs for respiratory conditions | 8 | 3 |
| Contraception | 5 | 3 |
| Benzodiazepines | 14 | 3 |
| Nonsteroidal anti‐inflammatory drugs | 13 | 2 |
| Transition from HCU (high‐cost year), % | ||
| Antihypertensives | 45 | 9 |
| Cholesterol drugs | 31 | 7 |
| Antidepressants | 36 | 7 |
| Acid‐reducing drugs | 29 | 5 |
| Antipsychotics | 3 | 4 |
| Cancer drugs | 15 | 4 |
| Drugs for inflammatory conditions | 1 | 4 |
| Drugs for respiratory conditions | 16 | 3 |
| Pregabalin and gabapentin | 11 | 3 |
| Opioids | 28 | 3 |
| Transition from HCU (in 2011), % | ||
| Antihypertensives | 27 | 17 |
| Cholesterol drops | 14 | 10 |
| Antidepressants | 17 | 9 |
| Acid‐reducing drugs | 13 | 6 |
| Antibiotics | 31 | 5 |
| Drugs for respiratory conditions | 8 | 4 |
| Opioids | 17 | 3 |
| Benzodiazepines | 15 | 3 |
| Antipsychotics | 6 | 3 |
| Noninsulins | 6 | 2 |
| Transition to HCU (%) | ||
| Drugs for inflammatory conditions | 3 | 14 |
| Antihypertensives | 45 | 6 |
| Antidepressants | 34 | 6 |
| Cancer drugs | 5 | 5 |
| Cholesterol drugs | 31 | 5 |
| Antipsychotics | 16 | 4 |
| Opioids | 32 | 4 |
| Drugs for respiratory conditions | 19 | 4 |
| Pregabalin and gabapentin | 13 | 3 |
| Acid‐reducing drugs | 26 | 3 |
| Persistent moderate‐cost user (%) | ||
| Antihypertensives | 66 | 18 |
| Cholesterol drugs | 45 | 12 |
| Antidepressants | 29 | 8 |
| Acid‐reducing drugs | 30 | 6 |
| Drugs for respiratory conditions | 17 | 5 |
| Drugs for inflammatory conditions | 1 | 4 |
| Noninsulins | 17 | 3 |
| Opioids | 25 | 3 |
| Antipsychotics | 7 | 2 |
| Benzodiazepines | 26 | 2 |
Costs for episodic‐HCUs and transitions from HCU during their most recent high‐cost year are adjusted for inflation.
Notwithstanding the use of these high‐cost biologic anti‐inflammatory drugs by a small number of patients in the moderate and HCUs groups, much of the spending for persistent moderate‐cost users, individuals who transitioned from high costs, and persistent‐HCUs was driven by the use of several commonly prescribed drug classes. Five of these common drug classes—antihypertensives, cholesterol drugs, antidepressants, acid‐reducing drugs, and drugs for respiratory conditions—accounted for 48 percent of total 2011 spending among the persistent moderate‐cost users, 32 percent of spending among individuals who transitioned from high costs during their most recent high‐cost year, and 34 percent of 2011 spending among persistent‐HCUs. In contrast, these five commonly prescribed drugs only accounted for 23 percent of 2011 spending among individuals who transitioned to high costs and 13 percent of total spending among episodic‐HCUs during their most recent year of high‐cost use.
Drivers of drug costs during the most recent year of high‐cost use for episodic‐HCUs were specialty medicines not as commonly used by persistent moderate and persistent‐HCUs, including outpatient cancer drugs, drugs for female fertility, and biologic drugs for inflammatory conditions. Several of these drugs also drove costs among transitory‐HCUs. Additionally, episodic‐HCUs and individuals who transitioned from high‐costs filled more prescriptions for cardiovascular conditions—including antihypertensives, cholesterol drugs, and anticoagulants—during their most recent high‐cost year than during 2011.
Results of hierarchical clustering across age‐sex groupings are provided in Appendix S3. All results demonstrated a high degree of heterogeneity in the “bundles” of drugs identified by the clustering algorithm. Persistent moderate‐cost users, transitory, episodic, and persistent‐HCUs are highly likely to be on multiple drugs and notwithstanding a small number of logical clusters of certain drug types (e.g., diabetes with cardiovascular drugs; anxiety medicines with antidepressants), cluster analysis suggests weak similarity of drug use clusters. That is, for members of each prescription drug user group, the probability of use of one class of medicines is virtually independent of the use of other classes of medicines.
Discussion
Similar to other health services researchers (Kozyrskyj et al. 2005; Hanley and Morgan 2009; Saastamoinen and Verho 2012), we find that a very small proportion of the population is responsible for the majority of prescription drug spending outside hospitals in British Columbia and that prescription drug expenditure patterns persist over time. Perhaps most notably in this regard, we find that persistent‐HCUs—whose annual prescription drug costs were above the 90th percentile for five consecutive years—accounted for just 6 percent of our study cohort but over 40 percent of total prescription drug spending in 2011.
We find some unique sociodemographic characteristics of ordinary prescription drug users: persistent nonusers and persistent low‐cost users were much more likely to be male. This finding coincides with health services literature, which often shows women use more health care services than men (Bertakis et al. 2000). Additionally, we observe that persistent nonusers, persistent low‐cost, and persistent average‐cost users were more likely to have surnames of Chinese origin. This is consistent with past research showing Chinese men and women in BC are less likely to fill some types of prescriptions than the general population (Morgan et al. 2011; Puyat et al. 2011).
We also identify five distinct types of moderate to HCUs with important similarities and differences. Persistent‐HCUs, individuals who transitioned to HCUs, and persistent moderate‐cost users suffered higher levels of comorbidity relative to the general population, supporting literature indicating that high‐cost patients warrant higher than average levels of service utilization (Reid et al. 2003; Roos et al. 2004; Hanley and Morgan 2009). We also find that most spending on medicines used by persistent moderate‐cost users and persistent‐HCUs was driven by use of multiple, commonly prescribed chronic drug therapies. We observe moderate concomitant drug use (an average of nearly seven distinct drug groups) among persistent moderate‐cost users, transitory‐HCUs, and episodic‐HCUs during their most recent high‐cost year, as well as very high concomitant drug use (an average of nearly 10) among persistent‐HCUs. Cluster analyses revealed substantial heterogeneity in the drug bundles used by all moderate and HCUs.
Our findings concerning major cost drivers for persistent moderate and HCUs may have implications for health care policy and practice. At a policy level, some of the high medicine use among these patients may be mitigated by identifying low‐cost treatment options for their ongoing drug needs. At a practice level, the critical evaluation of concomitant drug use of many different drug groups and an examination of the quality of pharmaceutical therapy may identify opportunities for deprescribing to improve patient outcomes, reduce risks, and save costs, given that individuals using multiple drugs are at increased risk of medication errors and drug‐related problems (Woodward 2003; Øymoen, Pottegård, and Almarsdóttir 2015; Saastamoinen and Verho 2015).
We also find that a small but important cohort of individuals experience well‐defined episodes of high‐cost prescription drug use. People who fit our criteria for being episodic‐HCUs of prescription drugs differed in important ways from other high‐ and moderate‐cost users. On average, episodic‐HCUs were younger, used more specialized physician services, and used more hospital services. Though episodic‐HCUs filled fewer overall prescriptions than other high‐ and moderate‐cost users, they were more likely to fill prescriptions for specialized drugs.
Whether episodic, transitory, or persistent, the high concentration of prescription drug costs documented in this study has policy implications in a country like Canada, where patients often face significant out‐of‐pocket costs for drug treatments. Such financial burdens associated with drug needs may engender inequalities that are inconsistent with ethical principles underlying universal health care systems, especially if they come at a time when patients and families are going through ongoing or acute episodes of serious health care needs.
Study Limitations
Our study is not without limitations. For instance, though our datasets include prescription drug use in long‐term care facilities, they exclude pharmaceuticals used within acute care hospitals. Our results therefore understate episodic high‐cost use of medicines; however, our primary findings with respect to community‐based prescription drug use and cost were not sensitive to different hospital stay exclusions. We had to use proxy information for patient ethnicity and neighborhood‐based information for some patients’ income levels. Results from the use of these data were as expected; however, access to better quality sociodemographic information might better explain determinants of high‐cost medicine use. Finally, our results are based on Canada's drug financing system and may not be as generalizable to jurisdictions with different systems of drug financing.
Conclusion
Prescription drug use and costs are skewed and remarkably persistent for most people in the population, including the small fraction of the population that accounts for the majority of total prescription drug spending. It is far more likely for individuals to transition to HCUs than it is for HCUs to return to ordinary medicine usage patterns. A very small number of HCUs will experience brief episodes of high prescription drug costs. Those that do are likely to use medicines to treat acute health care needs, including cancer, infertility, and serious cardiovascular events. Most HCUs of prescription drugs—and an even larger group of moderate‐cost users of prescription drugs—experience chronic needs that last many years.
Health system managers should anticipate that there will always be HCUs of prescription drugs. Though long‐term, upstream policies preventing the onset of acute and chronic needs for prescription drugs would alleviate some financial pressures in the pharmaceutical component on health care systems, immediate benefits may be attained by focusing on the mix and price of medicines used by these high‐cost patients. Policy makers and health professionals should pay particular attention to the concomitant use of commonly prescribed drugs among people with persistent moderate or high prescription drug costs. Ensuring these drugs are appropriately prescribed and competitively priced could improve health outcomes while reducing financial burdens on the health care system and individual patients.
Supporting information
Appendix SA1: Author Matrix.
Appendix S1. List of ATC Sub‐Groups Identifying Therapeutic Drug Classes.
Appendix S2. Probability Transition Matrices Showing Persistence in Expenditures from 2007 to 2011.
Appendix S3. Prescription Drug Clusters by Age‐Sex Strata.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: The work was supported by the Canadian Institutes of Health Research (grant number: CIHR DCO150GP). The funding agency had no role in product design, methods, data collection, analysis, preparation of the manuscript, or the decision to publish. All opinions and conclusions are those of the authors. Kate Smolina is also funded by the CIHR Banting Postdoctoral Fellowship.
Disclosures: None.
Disclaimers: None.
References
- BC Ministry of Health; BC Vital Statistics Agency [creator] . 2012. –2014. Consolidation File (MSP Registration & Premium Billing); Discharge Abstracts Database (Hospital Separations) and Medical Services Plan (MSP) Payment Information File; PharmaNet; Vital Statistics Deaths. Population Data BC; BC Ministry of Health [publisher]. MOH; Data Stewardship Committee; BC Vital Statistics Agency (2012–2014) [accessed on March 6, 2016]. Available at http://www.popdata.bc.ca/data
- Bertakis, K. D. , Azari R., Helms L. J., Callahan E. J., and Robbins J. A.. 2000. “Gender Differences in the Utilization of Health Care Services.” Journal of Family Practice 49 (2): 147–52. [PubMed] [Google Scholar]
- CIHI . 2014. Prescribed Drug Spending in Canada, 2012: A Focus on Public Drug Programs. Ottawa, ON: CIHI. [Google Scholar]
- Cornell, J. E. , Pugh J. A., Williams J. W. Jr., Kazis L., Lee A. F., Parchman M. L., Zeber J., Pederson T., Montgomery K. A., and Noël P. H.. 2009. “Multimorbidity Clusters: Clustering Binary Data from Multimorbidity Clusters: Clustering Binary Data from a Large Administrative Medical Database.” Applied Multivariate Research 12 (3): 163–82. [Google Scholar]
- Government of British Columbia . 2014. “What Is Pharmanet?” [accessed on September 23, 2015]. Available at http://www2.gov.bc.ca/gov/content/health/health-drug-coverage/pharmacare-for-bc-residents/pharmanet
- Hanley, G. , and Morgan S.. 2008. “On the Validity of Area‐Based Income Measures to Proxy Household Income.” BMC Health Services Research 8 (1): 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley, G. E. , and Morgan S.. 2009. “Chronic Catastrophes: Exploring the Concentration and Sustained Nature of Ambulatory Prescription Drug Expenditures in the Population of British Columbia, Canada.” Social Science & Medicine 68 (5): 919–24. [DOI] [PubMed] [Google Scholar]
- Hanley, G. E. , Morgan S., and Reid R. J.. 2010. “Explaining Prescription Drug Use and Expenditures Using the Adjusted Clinical Groups Case‐Mix System in the Population of British Columbia, Canada.” Medical Care 48 (5): 402–8. [DOI] [PubMed] [Google Scholar]
- Hastie, T. , Tibshirani R., Friedman J., and Franklin J.. 2005. “The Elements of Statistical Learning: Data Mining, Inference and Prediction.” The Mathematical Intelligencer 27 (2): 83–5. [Google Scholar]
- Joynt, K. E. , Gawande A. A., Orav E. J., and Jha A. K.. 2013. “Contribution of Preventable Acute Care Spending to Total Spending for High‐Cost Medicare Patients.” Journal of the American Medical Association 309 (24): 2572–8. [DOI] [PubMed] [Google Scholar]
- Kozyrskyj, A. , Lix L., Dahl M., and Soodeen R.‐A.. 2005. High‐Cost Users of Pharmaceuticals: Who Are They?. Winnipeg, MB: Manitoba Centre for Health Policy, University of Manitoba. [Google Scholar]
- Monheit, A. C. 2003. “Persistence in Health Expenditures in the Short Run: Prevalence and Consequences.” Medical Care 41 (7): III‐53–64. [DOI] [PubMed] [Google Scholar]
- Morgan, S. , Cunningham C., Hanley G., and Mooney D.. 2009. Medical and Hospital Atlas. Vancouver, BC: UBC Centre for Health Services and Policy Research, University of British Columbia. [Google Scholar]
- Morgan, S. , Hanley G., Cunningham C., and Quan H.. 2011. “Ethnic Differences in the Use of Prescription Drugs: A Cross‐Sectional Analysis of Linked Survey and Administrative Data.” Open Medicine 5 (2): e87. [PMC free article] [PubMed] [Google Scholar]
- Morgan, S. , Smolina K., Mooney D., Raymond C., Bowen M. L., Gorczynski C., and Basham K. A. R.. 2013. The Canadian Rx Atlas. Vancouver, BC: UBC Centre for Health Services and Policy Research, University of British Columbia. [Google Scholar]
- OECD . 2014. “OECD Health Statistics 2014—Frequently Requested Data” [accessed on February 15, 2015]. Available at http://www.oecd.org/els/health-systems/oecd-health-statistics-2014-frequently-requested-data.htm
- Øymoen, A. , Pottegård A., and Almarsdóttir A. B.. 2015. “Characteristics and Drug Utilization Patterns for Heavy Users of Prescription Drugs among the Elderly: A Danish Register‐Based Drug Utilization Study.” European Journal of Clinical Pharmacology 71 (6): 751–8. [DOI] [PubMed] [Google Scholar]
- Puyat, J. H. , Hanley G. E., Cunningham C. M., Law M. R., Wong S. T., Sutherland J. M., and Morgan S. G.. 2011. “Ethnic Disparities in Antipsychotic Drug Use in British Columbia: A Cross‐Sectional Retrospective Study.” Psychiatric Services 62 (9): 1026–31. [DOI] [PubMed] [Google Scholar]
- Rais, S. , Nazerian A., Ardal S., Chechulin Y., Bains N., and Malikov K.. 2013. “High‐Cost Users of Ontario's Healthcare Services.” Healthcare Policy 9 (1): 44–51. [PMC free article] [PubMed] [Google Scholar]
- Reid, R. , Evans R., Barer M., Sheps S., Kerluke K., McGrail K., Hertzman C., and Pagliccia N.. 2003. “Conspicuous Consumption: Characterizing High Users of Physician Services in One Canadian Province.” Journal of Health Services Research & Policy 8 (4): 215–24. [DOI] [PubMed] [Google Scholar]
- Riley, G. F. 2007. “Long‐Term Trends in the Concentration of Medicare Spending.” Health Affairs 26 (3): 808–16. [DOI] [PubMed] [Google Scholar]
- Roos, N. , Burchill C., and Carriere K.. 2003. “Who Are the High Hospital Users? A Canadian Case Study.” Journal of Health Services Research & Policy 8 (1): 5–10. [DOI] [PubMed] [Google Scholar]
- Roos, N. P. , Forget E., Walld R., and MacWilliam L.. 2004. “Does Universal Comprehensive Insurance Encourage Unnecessary Use? Evidence from Manitoba Says ‘No’.” Canadian Medical Association Journal 170 (2): 209–14. [PMC free article] [PubMed] [Google Scholar]
- Rosella, L. C. , Fitzpatrick T., Wodchis W. P., Calzavara A., Manson H., and Goel V.. 2014. “High‐Cost Health Care Users in Ontario, Canada: Demographic, Socio‐Economic, and Health Status Characteristics.” BMC Health Services Research 14 (1): 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saastamoinen, L. K. , and Verho J.. 2012. “Drug Expenditure of High‐Cost Patients and Their Characteristics in Finland.” The European Journal of Health Economics 14 (3): 495–502. [DOI] [PubMed] [Google Scholar]
- Saastamoinen, L. K. , and Verho J.. 2015. “Register‐Based Indicators for Potentially Inappropriate Medication in High‐Cost Patients with Excessive Polypharmacy.” Pharmacoepidemiology and Drug Safety 24 (6): 610–8. [DOI] [PubMed] [Google Scholar]
- Shah, B. R. , Chiu M., Amin S., Ramani M., Sadry S., and Tu J. V.. 2010. “Surname Lists to Identify South Asian and Chinese Ethnicity from Secondary Data in Ontario, Canada: A Validation Study.” BMC Medical Research Methodology 10 (1): 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp . 2013. Stata 13 Base Reference Manual. College Station, TX: Stata Press. [Google Scholar]
- Vu, T. , Finch C. F., and Day L.. 2011. “Patterns of Comorbidity in Community‐Dwelling Older People Hospitalised for Fall‐Related Injury: A Cluster Analysis.” BMC Geriatrics 11 (1): 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner, J. , and Abrams C.. 2009. “The Johns Hopkins ACG System Technical Reference Guide.” The Perl Foundation 5: 762–5. [Google Scholar]
- Weiner, J. P. , Starfield B. H., Steinwachs D. M., and Mumford L. M.. 1991. “Development and Application of a Population‐Oriented Measure of Ambulatory Care Case‐Mix.” Medical Care 29 (5): 452–72. [DOI] [PubMed] [Google Scholar]
- WHO . 2011. “WMS 2011—Pharmaceutical Consumption, Annex 2—Classification of Acute and Chronic Disease Medicine” [accessed on February 25, 2015]. Available at http://apps.who.int/medicinedocs/en/d/Js20039en/
- WHO Collaborating Centre for Drug Statistics Methodology . 2014. “Anatomical Therapeutic Chemical Code Classification Index with Defined Daily Doses” [accessed on February 4, 2014]. Available at http://www.whocc.no/atcddd/
- Woodward, M. C. 2003. “Deprescribing: Achieving Better Health Outcomes for Older People through Reducing Medications.” Journal of Pharmacy Practice and Research 33 (4): 323–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Appendix S1. List of ATC Sub‐Groups Identifying Therapeutic Drug Classes.
Appendix S2. Probability Transition Matrices Showing Persistence in Expenditures from 2007 to 2011.
Appendix S3. Prescription Drug Clusters by Age‐Sex Strata.


