Abstract
Histone lysine methylation is an epigenetic mark to index chromosomal subdomains. In Drosophila, H3-K9 di- and trimethylation is mainly controlled by the heterochromatic SU(VAR)3-9 HMTase, a major regulator of position-effect variegation (PEV). In contrast, H3-K27 methylation states are independently mediated by the Pc-group enzyme E(Z). Isolation of 19 point mutants demonstrates that the silencing potential of Su(var)3-9 increases with its associated HMTase activity. A hyperactive Su(var)3-9 mutant, pitkinD, displays extensive H3-K9 di- and trimethylation within but also outside pericentric heterochromatin. Notably, mutations in a novel Su(var) gene, Su(var)3-1, severely restrict Su(var)3-9-mediated gene silencing. Su(var)3-1 was identified as “antimorphic” mutants of the euchromatic H3-S10 kinase JIL-1. JIL-1Su(var)3-1 mutants maintain kinase activity and do not detectably impair repressive histone lysine methylation marks. However, analyses with seven different PEV rearrangements demonstrate a general role of JIL-1Su(var)3-1 in controlling heterochromatin compaction and expansion. Our data provide evidence for a dynamic balance between heterochromatin and euchromatin, and define two distinct mechanisms for Su(var) gene function. Whereas the majority of Su(var)s encode inherent components of heterochromatin that can establish repressive chromatin structures [intrinsic Su(var)s], Su(var)3-1 reflects gain-of-function mutants of a euchromatic component that antagonize the expansion of heterochromatic subdomains [acquired Su(var)s].
Keywords: Su(var) genes, Su(var)3-9, JIL-1, heterochromatin, position-effect variegation, histone lysine methylation
Control of higher-order chromatin structure is important for epigenetic gene regulation and functional differentiation of chromosomal domains during development. In Drosophila and Schizosaccharomyces pombe, genetic dissection of heterochromatic gene silencing identified fundamental mechanisms controlling DNA packaging with histones and other chromosomal proteins into repressive chromatin structures (Reuter and Spierer 1992; Allshire et al. 1994). In Drosophila, >50 Suppressor of position-effect variegation [Su(var)] loci exist, of which ∼15 have been molecularly defined. Su(var) genes encode structural components of heterochromatin, such as the zinc finger protein Su(var)3-7 (Delattre et al. 2000; Jaquet et al. 2002) and the chromo domain protein HP1 (Eissenberg et al. 1990; Eissenberg and Elgin 2000), but also enzymes that can modify histone N termini (tails), such as the histone deacetylase HDAC1 (DeRubertis et al. 1996; Mottus et al. 2000; Czermin et al. 2001) and the histone methyltransferase (HMTase) Su(var)3-9 (Rea et al. 2000; Schotta et al. 2002). SU(VAR)3-9, HP1, and SU(VAR)3-7 are inherent components of heterochromatin. In a genetic hierarchy, Su(var)3-9 is dominant over the other two genes indicating an important role for histone lysine methylation in heterochromatin formation (Schotta et al. 2002).
Histone lysine methylation can be present in mono-, di-, or trimethylation states (Santos-Rosa et al. 2002; Peters et al. 2003), and combinations of active (H3-K4, H3-K36, H3-K79) or repressive (H3-K9, H3-K27, and H4-K20) modifications can index distinct chromosomal subdomains. Hallmarks of constitutive heterochromatin in mammals are H3-K9 trimethylation, H3-K27 monomethylation (Peters et al. 2003; Rice et al. 2003), and H4-K20 trimethylation (Schotta et al. 2004). The major HMTases for H3-K9 trimethylation at pericentric heterochromatin are Suv39h enzymes (Peters et al. 2003). Ezh2 is the major H3-K27 HMTase to index the inactive X-chromosome (Xi) and Pc-dependent gene silencing (Plath et al. 2003; Silva et al. 2003); however, it is not known whether Ezh2 contributes to pericentric H3-K27 monomethylation in mammals.
In Drosophila, H3-K9 dimethylation was found to be enriched at heterochromatin (Schotta et al. 2002). The development of highly specific antibodies that can discriminate mono-, di-, and trimethylation of H3-K9 versus H3-K27 (Peters et al. 2003) now allowed us to characterize all H3-K9 and H3-K27 methylation states in Drosophila. Our data reveal that Su(var)3-9 mainly controls H3-K9 di- and trimethylation at pericentric heterochromatin, whereas all H3-K27 methylation is mediated by Enhancer of zeste [E(z)]. The identification and characterization of novel point mutants in Su(var)3-9 that show differential HMTase activities demonstrate that the silencing potential of Su(var)3-9 is mainly determined by the kinetic properties of the HMTase reaction. Importantly, we identified the PEV modifier Su(var)3-1 as a key antagonist for Su(var)3-9-dependent gene silencing. Su(var)3-1 mutants are novel alleles of the euchromatic H3-S10 kinase JIL-1 (Wang et al. 2001). In these mutants, H3-S10 phosphorylation is not altered, indicating that the kinase function of JIL-1Su(var)3-1 is intact. However, condensation of the male X-chromosome and expansion of heterochromatic subdomains are impaired, suggesting that JIL-1Su(var)3-1 may regulate the balance between euchromatin and heterochromatin. Although JIL-1 is not an intrinsic component of heterochromatin, JIL-1Su(var)3-1 mutants display the strongest PEV modifier effect and are dominant over Su(var)3-9. Our data define Su(var)3-1 as a novel class of Su(var) genes, in which gain-of-function mutants of a euchromatic component antagonize the expansion of heterochromatic subdomains.
Results
H3-K9 and H3-K27 methylation states in Drosophila heterochromatin
To investigate the distribution of distinct H3-K9 and H3-K27 methylation states in Drosophila heterochromatin, we used highly specific antibodies that can discriminate mono-, di-, and trimethylation of H3-K9 versus H3-K27 (Peters et al. 2003) in immunofluorescence analyses. Polytene chromosomes allow the mapping of these marks at a very high resolution because they have a clearly discernible chromocenter where all chromosomes are joined. Attached to this chromocenter is the largely heterochromatic fourth chromosome.
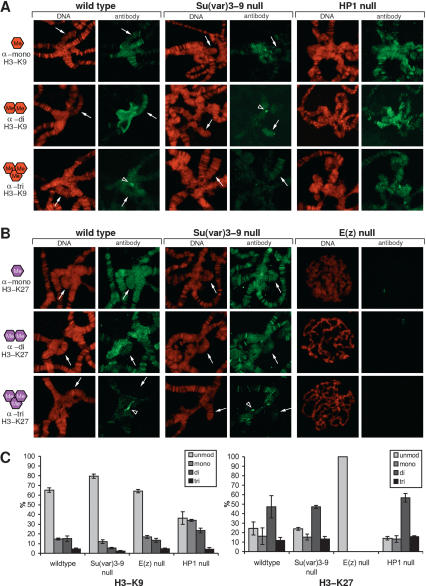
In wild-type chromosomes, the prominent mark of the chromocenter and the fourth chromosome is H3-K9 dimethylation (Fig. 1A). In addition, H3-K9 dimethylation is associated with some euchromatic bands and telomeres (Supplementary Fig. S1). H3-K9 monomethylation is also found in the chromocenter (Fig. 1A) and is associated with many euchromatic bands (Supplementary Fig. S1). H3-K9 trimethylation shows a very distinct heterochromatic distribution with a strong enrichment in the chromocenter core (Fig. 1A, arrowhead). The chromocenter core region is also marked by CID, a centromere-specific histone variant in Drosophila (Henikoff et al. 2000; data not shown), and likely represents centromeric heterochromatin.
Figure 1.
Control of H3-K9 and H3-K27 methylation states in Drosophila heterochromatin. (A) Distribution of H3-K9 mono-, di-, and trimethylation in the chromocenter region of salivary gland polytene chromosomes from wild-type, Su(var)3-9-null, and HP1-null third instar larvae. (B) Distribution of H3-K27 mono-, di-, and trimethylation in the chromocenter region of polytene chromosomes from wild-type and Su(var)3-9-null as well as in whole chromosomes of E(z)-null third instar larvae. DNA was stained with propidium iodide. Arrows point to the fourth chromosome; arrowheads indicate the chromocenter core region. (C) Comparative mass spectometric analyses of H3-K9 and H3-K27 mono-, di-, and trimethylation levels in H3 peptide fragments isolated from nuclear extracts of wild-type, Su(var)3-9-null, E(z)-null, and HP1-null mutant larvae. For each genotype three different extracts were analyzed (error bar indicates standard deviation). The relative level of a particular H3-K9 methylation state is indicated with respect to the sum of all H3-K9 and H3-K27 modifications present in the H3 peptide populations.
In Su(var)3-9-null mutants, H3-K9 monomethylation is not significantly altered. In contrast, H3-K9 di- and trimethylation are strongly reduced in the chromocenter but not in the fourth chromosome (Fig. 1A). Notably, the chromocenter core that is enriched for H3-K9 trimethylation in wild-type chromosomes is prominently marked with H3-K9 dimethylation in Su(var)3-9 mutants (Fig. 1A, arrowhead).
HP1 is an interaction partner of SU(VAR)3-9 and important to retain SU(VAR)3-9 at pericentric heterochromatin (Schotta et al. 2002). To test whether HP1 is involved in the control of Su(var)3-9-dependent H3-K9 di- and trimethylation, we analyzed these methyl-marks in Su(var)2-5 (HP1) mutants. In the chromocenter, H3-K9 mono-, di-, and trimethylation appear unaltered (Fig. 1A); however, there is a dramatic increase in H3-K9 mono- and dimethylation along euchromatic arms (Supplementary Fig. S1).
All three H3-K27 methylation states are present at pericentric heterochromatin. H3-K27 mono- and dimethylation are broadly associated with the chromocenter region, whereas H3-K27 trimethylation displays a strong accumulation at the chromocenter core (Fig. 1B). Additionally, H3-K27 mono- and dimethylation can be detected at almost all bands in euchromatin, whereas only ∼100 bands are marked by H3-K27 trimethylation (Supplementary Fig. S2). H3-K27 methylation states are not significantly altered in Su(var)3-9-null mutants (Fig. 1B); however, no H3-K27 methylation can be detected in E(z)-null mutants (Fig. 1B).
Immunofluorescence analyses and the different abundance of H3-K9 and H3-K27 methylation states were confirmed by mass spectrometric analyses of bulk nuclear extracts from wild-type and mutant larvae (Fig. 1C). Consistent with previous studies (Schotta et al. 2002) demonstrating dispersed localization of SU(VAR)3-9, H3-K9 methylation levels are significantly increased in the HP1-null background.
Together, the data demonstrate that pericentric heterochromatin in Drosophila is largely characterized by H3-K9 di- and H3-K27 mono- and dimethylation. Only the chromocenter core displays a focal enrichment for the trimethylated state (H3-K9 and H3-K27 trimethylation). We provide evidence for the existence of Su(var)3-9-independent H3-K9 monomethylation at the chromocenter and of additional H3-K9 HMTases that target the fourth chromosome. In contrast, E(z) mediates all three H3-K27 methylation states but does not appear to affect H3-K9 methylation. Although E(Z) has been detected at a limited number of bands (Carrington and Jones 1996), the high abundance of H3-K27 methylation indicates that E(Z) complexes might not reside at all sites after accomplishing the methylation.
The silencing potential of Su(var)3-9 mutants correlates with their associated H3-K9 HMTase activity
To identify novel regulatory mechanisms for Su(var)3-9-dependent gene silencing, we performed EMS mutagenesis experiments and isolated 56 novel suppressor mutants together with 19 Su(var)3-9 alleles. Sequence analyses of these novel Su(var)3-9 alleles revealed that most mutants cluster around the SET domain (Fig. 2A; Supplementary Fig. S3). Although the screen was rather saturated, we could not detect mutants in the SU(VAR)3-9 chromo domain.
Figure 2.
SU(VAR)3-9 HMTase activity correlates with its ability to induce gene silencing. (A) Protein structure of SU(VAR)3-9 and positions of the molecularly characterized mutations. Point mutations resulting in amino acid exchanges are in red. (B) Genetic classification of Su(var)3-9 mutants. Su(var)3-9mut alleles were crossed to two extra Su(var)3-9 copies. Null mutants show the characteristic white mottled phenotype, whereas hypomorphic alleles partially enhance white repression. Su(var)3-9ptn is a hypermorphic mutant resulting in complete white and partial silencing of the roughest gene. (C) H3-K9 di- and trimethylation in chromocenter regions of heterozygous Su(var)3-917/Su(var)3-9mut mutants. Null mutants [exemplified by Su(var)3-910] show the same loss of H3-K9 di- and trimethylation as the Su(var)3-917/Su(var)3-917-null mutant. H3-K9 methylation levels are elevated in hypomorphic mutants [exemplified by Su(var)3-922]. Very high levels of H3-K9 di- and trimethylation are found in hypermorphic Su(var)3-9ptn. Arrowheads indicate the chromocenter core. (D) In vitro HMTase activity of recombinant SU(VAR)3-9 mutant proteins. The amount of incorporated label was measured by scintillation counting. (E) Time-course analysis of HMTase reactions with wild-type (wt) and SU(VAR)3-9ptn proteins. Incorporation of the radioactive label was measured after different timepoints by scintillation counting.
In the course of these studies we also identified the exceptionally strong PEV enhancer pitkinD (Kuhfittig et al. 2001) as a novel Su(var)3-9 allele. The pitkinD mutation causes extensive chromatin compaction in early embryos and induces ectopic recruitment of SU(VAR)3-9 with many euchromatic sites (Kuhfittig et al. 2001). P-element-induced male recombination (cf. Materials and Methods) indicated that the pitkin locus maps to region 88E, near Su(var)3-9. Sequence analysis revealed a point mutation (R529S) within the SU(VAR)3-9 SET domain. To verify that this mutation is, indeed, pitkinD, we analyzed transgenic flies expressing SU(VAR)3-9R529S. After remobilization, four independent lines were selected that display a very strong PEV enhancer effect that was equivalent to three additional Su(var)3-9 gene copies (data not shown). Since these data classify the pitkinD mutation as a hypermorphic Su(var)3-9 allele, we propose to rename this mutant Su(var)3-9ptn.
Based on their ability to induce white gene silencing in the PEV rearrangement In(1)wm4, the other Su(var)3-9 mutants were categorized into null and hypomorphic alleles. One wild-type copy of Su(var)3-9 fails to induce white gene repression (the eyes remain red), whereas two wild-type copies partially silence the white gene and display the characteristic white mottled phenotype (Fig. 2B). Next, we crossed these two wild-type copies with our novel Su(var)3-9mut mutants. In this combination, some mutant alleles could moderately enhance white gene silencing and were regarded as hypomorphic mutants (Fig. 2B). In contrast, mutant alleles that did not alter the In(1)wm4 phenotype were regarded as null alleles (Fig. 2B).
Next, we tested whether the reduced silencing potential of hypomorphic Su(var)3-9 mutants possibly correlated with their ability to induce H3-K9 di- and trimethylation at pericentric heterochromatin. In the homozygous Su(var)3-917-null mutant, pericentric H3-K9 dimethylation is nearly absent and H3-K9 trimethylation is entirely lost from the chromocenter (Fig. 2C). All mutants that were classified as null alleles show a similar loss of H3-K9 methylation. In contrast, hypomorphic alleles show slightly elevated H3-K9 di- and trimethylation at the chromocenter (Fig. 2C). Importantly, hypermorphic Su(var)3-9ptn induces strong H3-K9 di- and trimethylation at pericentric heterochromatin (Fig. 2C) and, additionally, very prominent signals at many euchromatic sites (Supplementary Fig. S4A). Mass spectrometric analyses of Su(var)3-9ptn nuclear extracts further demonstrate that the overall levels of H3-K9 di- and trimethylation are significantly increased (Supplementary Fig. S4B).
To examine the mutant proteins in a more direct manner, we performed in vitro HMTase assays with 16 different point mutants. All loss-of-function mutants are catalytically inactive, whereas mutant proteins of hypomorphic alleles are less active than wild-type protein (Fig. 2D). SU(VAR)3-9ptn displayed a higher HMTase activity (Fig. 2D).
Finally, we characterized reaction kinetics of the wild-type HMTase versus the SU(VAR)3-9ptn mutant. In the starting phase of the reaction, SU(VAR)3-9ptn methylates approximately twice as many H3 peptides as the wild-type HMTase and reaches a plateau within ∼15 min (Fig. 2E). These data characterize SU(VAR)3-9ptn as a hyperactive H3-K9 HMTase and strongly suggest that its hypermorphic phenotype (chromatin compaction and enhanced gene silencing) is caused by enhanced enzymatic activity.
Identification of JIL-1 mutants as novel regulators of PEV-based gene silencing
In our mutant screens to isolate novel Su(var)3-9 alleles, we also identified other suppressor mutants that impair Su(var)3-9-dependent silencing. The strongest of these mutants has been identified as an allele of the previously described Su(var)3-1 gene (Wustmann et al. 1989). Su(var)3-1 mutants strongly suppress gene silencing in the In(1)wm4 PEV rearrangement (Fig. 3A, left panel) and even counteract gene repression that is caused by overexpression of prominent regulators of heterochromatin formation, such as Su(var)3-9, Su(var)2-5 (HP1), and Su(var)3-7 (Fig. 3A, right panels). Using male recombination, we mapped the Su(var)3-1 locus to a small region comprising four genes (Mocs1, CG7839, CG6302, and JIL-1) between the two P-element insertions P{EP}EP3688 and P{EP}EP3657. Sequencing of candidate open reading frames (ORFs) in all six Su(var)3-1 alleles revealed nonsense mutations in the C-terminal part of JIL-1 that would generate truncated proteins (Fig. 3B). We therefore propose naming these mutants JIL-1Su(var)3-1.
Figure 3.
Su(var)3-1 mutants are antimorphic alleles of the JIL-1 kinase. (A) Genomic extra copies of Su(var)3-9, Su(var)2-5, and Su(var)3-7 display enhanced silencing of the white gene in In(1)wm4 (upper row), which is impaired in the presence of the strong PEV suppressor mutant Su(var)3-1 (lower row). (B) Protein structure of JIL-1. All mutational lesions in Su(var)3-1 alleles result in a C-terminally truncated JIL-1 protein. (S/T) Serine/threonine kinase domain; (S/T/Y) Serine/threonine/tyrosine kinase domain. (C) Polytene chromosomes of wild-type and Su(var)3-1 mutant larvae were stained with pH3S10-specific antibodies. DNA was stained with propidium iodide. (D) X-chromosome decondensation phenotype of Su(var)3-1 mutants. In male homozygous mutant larvae, the X-chromosome is largely decondensed and partially loses its banding structure. The arrowhead points to the X-chromosome. The affected chromosome shows association with MOF, a specific marker for the male X-chromosome. DNA was stained with propidium iodide. Bar, 10 μm.
JIL-1 is a kinase that controls H3-S10 phosphorylation (pH3S10) in interphase nuclei (Wang et al. 2001). In JIL-1-null mutants, euchromatic pH3S10 is impaired, whereas mitotic pH3S10 is not altered. We analyzed pH3S10 in polytene chromosomes of JIL-1Su(var)3-1 mutants by immunofluorescence using a pH3S10-specific antibody. In wild-type chromosomes, pH3S10 is associated with many interbands along chromosomal arms. In JIL-1Su(var)3-1 mutants, we did not observe a significant change of this pattern (Fig. 3C), indicating that the kinase function of the truncated JIL-1Su(var)3-1 protein is intact.
JIL-1-null mutants are characterized by condensation of polytene chromosomes, an effect that is most apparent for the male X-chromosome (Wang et al. 2001). To test for changes in the polytene chromosome structure of JIL-1Su(var)3-1 mutants, we analyzed chromosomal squashes of wild-type versus homozygous mutant larvae. Strikingly, the X-chromosome is largely decondensed in JIL-1Su(var)3-1 males (Fig. 3D). The identity of this chromosome was confirmed by immunofluorescence stainings for MOF, a protein that is specifically associated with the male X-chromosome (Fig. 3D). These data show that truncated JIL-1Su(var)3-1 acts against the wild-type protein and classify JIL-1Su(var)3-1 mutants as antimorphic JIL-1 alleles.
JIL-1Su(var)3-1 prevents heterochromatin expansion over a large chromosomal domain
In order to test whether the altered chromosomal structure in JIL-1Su(var)3-1 mutants would coincide with changes in the pattern of repressive histone lysine methylation marks, we performed IF analyses of polytene chromosomes from wild-type versus mutant larvae. In homozygous JIL-1Su(var)3-1 mutants, no obvious differences in the patterns of H3-K9 and H3-K27 methylation states or of H4-K20 trimethylation have been detected (Supplementary Fig. S5), which was confirmed by mass spectrometric analyses of nuclear extracts (data not shown).
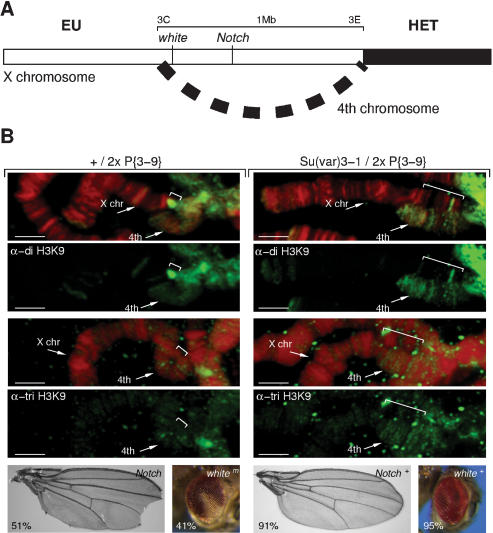
One possibility to explain the strong derepression of the white gene in In(1)wm4 by JIL-1Su(var)3-1 could be a shift in the expansion of the heterochromatic structure. We analyzed the effect of JIL-1Su(var)3-1 mutants on heterochromatin expansion in the PEV rearrangement T(1;4)wm258-21, in which heterochromatization of a large euchromatic region can be monitored. T(1;4)wm258-21 is a complex rearrangement in which a part of the X-chromosome (cytological region 3C-3E) comprising ∼1 Mb of DNA is brought near to pericentric heterochromatin (Fig. 4A). Because of a translocation of the fourth chromosome, this region can specifically be detected in polytene chromosome spreads. It contains several genes, of which inactivation of Notch (clipped wings) and white (white eyes) can be monitored phenotypically. In the presence of two extra Su(var)3-9 copies, the whole region becomes highly compacted and prominently marked by H3-K9 dimethylation (Fig. 4B, bracket, left panel). The repressed state of this region can also be detected by inactivation of Notch and, to a lesser extent, of the more distant white gene (Fig. 4B, left panel). Notably, heterochromatin does not expand along the fourth chromosome, which is modified by different HMTase complexes that might be critical to the spreading effect. Next, we crossed the two extra copies of Su(var)3-9 with JIL-1Su(var)3-1 and examined the polytene structure at T(1;4)wm258-21. Surprisingly, the whole region that was inactivated in the presence of the two extra Su(var)3-9 copies is not compacted and shows a normal banding pattern (Fig. 4B, right panel). Derepression of this region is further supported by reactivation of the Notch and white marker genes (Fig. 4B, right panel). These data illustrate that Su(var)3-9-mediated expansion of heterochromatin can be impaired by truncated JIL-1Su(var)3-1, suggesting an important function for the C terminus of JIL-1 in regulating heterochromatin formation.
Figure 4.
Su(var)3-1 mutants impair expansion of heterochromatin. (A) Translocation T(1;4)wm258-21 contains the X-chromosomal region 3C-3E comprising ∼1 Mb of DNA, which can be detected because of contacts to the fourth chromosome. Heterochromatin expansion inactivates the two marker genes Notch (clipped wings) and white (white eyes). (B) Immunocytological analysis of H3-K9 di- and trimethylation at T(1;4)wm258-21. (Left panel) In the presence of two extra Su(var)3-9 copies, region 3C-3E becomes compacted (bracket) and marked with H3-K9 dimethylation but not H3-K9 trimethylation. Transcriptional repression is indicated by a large number of Notch wings (51%) and white mottled sectored eyes (41%). (Right panel) The presence of Su(var)3-1 mutations impairs expansion of heterochromatin (bracket), resulting in a normal banding pattern, loss of H3-K9 dimethylation, and restored transcriptional activity as indicated by wild-type (Notch+) wings (91%) and red (white+) eyes (95%).
The compaction of a large chromosomal domain in T(1;4)wm258-21 allowed us to examine distinct contributions of H3-K9 di- and trimethylation to heterochromatin expansion. The active and decondensed region that displays a normal banding pattern does not show significant signals for H3-K9 di- or trimethylation (Fig. 4B, bracket, right panel). In contrast, Su(var)3-9-induced con densation of this chromosomal region is accompanied by strong H3-K9 dimethylation; however, we could not detect significant H3-K9 trimethylation (Fig. 4B, bracket, left panel). These data demonstrate that in Drosophila, H3-K9 dimethylation is the major mark to induce chromatin condensation and expansion of heterochromatic structures. H3-K9 trimethylation plays a minor role for heterochromatin formation, and the strong enrichment for this mark in the chromocenter core rather supports the argument for a function of H3-K9 trimethylation in organizing chromatin structure at centromeres.
JIL-1Su(var)3-1 generally antagonizes Su(var)3-9 gene function at PEV epialleles
In order to test whether JIL-1Su(var)3-1 generally impairs Su(var)3-9-mediated heterochromatin expansion, we tested the effect of JIL-1Su(var)3-1 mutants on seven different PEV rearrangements. In the first class of rearrangements, chromosomal aberrations bring an endogenous gene locus (marker gene) near to pericentric heterochromatin (Table 1). In wild type, these marker genes are largely silenced, detectable by white mottled eyes [In(1)wm4], yellow abdomen with few black spots [Dp(1;f)γ238], or long thoracic bristles due to inactivation of the dominant Sbv mutant [T(2;3)Sbv], respectively. JIL-1Su(var)3-1 can release silencing of these marker genes and even antagonizes Su(var)3-9-mediated gene silencing (Table 1; Supplementary Fig. S6).
Table 1.
JIL-1Su(var)3-1 generally antagonizes Su(var)3-9 mediated heterochromatin expansion
| % wild-type expression of marker gene in
|
||||||
|---|---|---|---|---|---|---|
| PEV epialleles | Marker gene(s) | Chr. | Phenotype when marker is fully expressed | P{3-9} +/+ | Su (var) 3-1; +/+ | Su (var) 3-1; P {3-9} +/+ |
| Class I | ||||||
| In(1)Wm4 | white | X | Red eyes | 0% | 100% | 95% |
| Dp(1;f)γ238 | yellow | X | Black abdominal pigmentation | 6% | 70% | 45% |
| T(2;3)SbV | stubble | 3 | Short bristles | 2% | 64% | n.d. |
| T(1;4)Wm258-21 | Notch | X | Clipped wings | 49% | 100% | 91% |
| white | Red eyes | 41% | 100% | 95% | ||
| Class II | ||||||
| In(3L)BL1 | lacZ-transgene | 3 | lacZ expression | 26% | 73% | 60% |
| w+ | Red eyes | 33% | 86% | 58% | ||
| Tp(3;Y)BL2 | lacZ-transgene | Y | lacZ expression | 26% | 68% | 53% |
| w+ | Red eyes | 25% | 98% | 68% | ||
| T(2;3)V21e | tandem repeats of w+ transgene
|
3
|
Red eyes
|
22%
|
85%
|
n.d.
|
| PlacW(92E) | ||||||
Expression of marker genes in different PEV epialleles was monitored in the presence of two Su(var)3-9 extra copies [P{3-9} +/+], JIL-1Su(var)3-1 mutants [Su(var)3-1 +/+], and the combination of two Su(var)3-9 extra copies with JIL-1Su(var)3-1 mutants [Su(var)3-1; P{3-9}+/+]. Expression of the respective marker genes was evaluated according to Materials and Methods. Representative phenotypes are depicted in Supplementary Figure 6.
In the second class of PEV rearrangements, transgene insertions containing lacZ and white marker genes are brought near to heterochromatic regions (Table 1). In these rearrangements we have analyzed expression of marker genes in different developmental stages, lacZ expression in larval salivary gland tissues, and white expression in adult flies. Both lacZ and white are silenced by overexpression of Su(var)3-9, but strongly derepressed in the presence of JIL-1Su(var)3-1 mutants (Table 1; Supplementary Fig. S6). Also in these rearrangements, Su(var)3-9-mediated expansion of heterochromatin is restricted by JIL-1Su(var)3-1, which is indicated by reactivation of both marker genes in flies containing genomic extra copies of Su(var)3-9 together with JIL1Su(var)3-1 mutants.
These data demonstrate that JIL-1Su(var)3-1 antagonizes Su(var)3-9-mediated heterochromatin expansion at different genomic regions and developmental stages. We therefore conclude that JIL-1Su(var)3-1 is a general regulator for the balance between euchromatin and heterochromatin.
Discussion
Su(var) genes as inherent components of heterochromatin
The identification of Su(var) genes in Drosophila revealed many important proteins regulating higher-order chromatin structure. SU(VAR)3-9, HP1, and SU(VAR)3-7 are inherent components of heterochromatin that can establish and maintain a heterochromatic chromatin structure. By genetic means, Su(var)3-9 is the dominant component over Su(var)2-5 (HP1) and Su(var)3-7 (Schotta et al. 2002), indicating a major function of the associated HMTase in the formation of repressive chromatin regions.
In Drosophila, heterochromatic gene silencing is mainly determined by H3-K9 dimethylation. In agreement with its in vitro HMTase activity (Eskeland et al. 2004), Su(var)3-9 regulates H3-K9 di- and trimethylation at pericentric heterochromatin. In addition, other H3-K9-specific HMTases must exist that mediate H3-K9 dimethylation at the fourth chromosome and in the chromocenter core of Su(var)3-9 mutants. HP1 is another important control factor for H3-K9 methylation because in Su(var)2-5 mutants, H3-K9 mono- and dimethylation are dramatically increased along chromosomal arms. Although SU(VAR)3-9 shows extensive association with heterochromatic and euchromatic regions in HP1-null cells, the shift in these methylation patterns could also be mediated by other H3-K9-specific HMTases. At pericentric heterochromatin, H3-K9 methylation states appear unaltered; however, a slight reduction of H3-K9 dimethylation that is not detectable by immunofluorescence might be the cause of the HP1 Su(var) effect. Alternatively, impairment of H4-K20 trimethylation at pericentric heterochromatin (Schotta et al. 2004) could cause the loss of silencing in HP1 mutants.
The analysis of Su(var)3-9 mutants revealed novel hypomorphic and null alleles that show differential effects on H3-K9 methylation states. A direct correlation between their HMTase activities and the amount of gene silencing demonstrates that establishment of heterochromatic structures is to a large extent determined by the kinetic properties of the SU(VAR)3-9 HMTase reaction. Consistently, hyperactive SU(VAR)3-9ptn induces enhanced H3-K9 di- and trimethylation, concomitant with an expansion of heterochromatin. This results in severe phenotypes such as dominant female sterility caused by overcompaction of the whole chromatin in early embryos (Kuhfittig et al. 2001). In this study we show that Su(var)3-9ptn mutants display around 100-200 additional bands with enhanced H3-K9 di- and trimethylation in the arms of polytene chromosomes. These bands likely correspond to endogenous SU(VAR)3-9-binding sites (Greil et al. 2003) and demonstrate that hyperactive SU(VAR)3-9ptn can mediate the expansion of repressive structures also at chromosomal arms.
A loss-of-function Su(var) mutant reduces gene silencing by removing one or several intrinsic components of heterochromatin. Su(var)3-9ptn induces enhanced gene silencing, and it was therefore surprising to encode a mutant of a “classical” Su(var) gene. Based on our functional characterization, we identified this mutant as the first hypermorphic (gain-of-function) allele of an intrinsic Su(var) gene. Many more PEV modifier mutants that cause enhanced gene silencing have been isolated. Molecular characterization of some of these genes revealed components of active chromatin, such as transcription factors (Dorn et al. 1993); however, further characterization of this class of genes might also uncover other hypermorphic mutants of intrinsic Su(var) genes and contribute to the understanding of heterochromatin regulation.
JIL-1Su(var)3-1 represents a novel class of PEV modifier mutants with acquired Su(var) function
Our mutant screen to identify genes that impair Su(var)3-9-mediated gene silencing led to the molecular identification of Su(var)3-1, the strongest Su(var) gene to date. Su(var)3-1 can restrict heterochromatin formation and generally dominate over the strong silencing effect of Su(var)3-9 extra copies. Su(var)3-1 carries C-terminal truncations of the H3-S10 kinase JIL-1 that do not affect its ability to phosphorylate H3-S10 but selectively impair the expansion of heterochromatin.
Intrinsic Su(var) genes impair or weaken the heterochromatic structure. Loss-of-function Su(var)3-9 alleles or mutations in HDAC1 reduce H3-K9 dimethylation (Czermin et al. 2001), whereas HP1 mutants impair H4-K20 trimethylation (Schotta et al. 2004) at pericentric heterochromatin. JIL-1 is not an intrinsic component of heterochromatin (Wang et al. 2001) and does not affect any of the known repressive histone lysine methylation marks at pericentric heterochromatin. Nevertheless, JIL-1Su(var)3-1 alleles display a strong Su(var) effect. We therefore categorize JIL-1Su(var)3-1 as a novel type of mutants with acquired Su(var) function. In contrast to intrinsic Su(var)s that encode components directly involved in heterochromatin formation, acquired Su(var) mutants would antagonize heterochromatin formation by stabilizing the euchromatic state or preventing heterochromatin expansion.
Our data assign a novel function to the C terminus of the JIL-1 kinase that is independent of its H3-S10 kinase activity. Lack of the C terminus could prevent phosphorylation or interaction with an as-yet-unknown target protein. Alternatively, the C terminus could comprise control functions that prevent excessive phosphorylation of this target. There are many possible target proteins that could be involved in higher-order chromatin formation. For example, the striking X-chromosome decondensation phenotype of JIL-1Su(var)3-1 mutants has also been described for iswi and nurf301, subunits of the NURF complex (Deuring et al. 2000; Badenhorst et al. 2002). Particularly, nurf301 mutants display a dominant Su(var) effect, indicating that chromatin remodeling processes are involved in the regulation of heterochromatin. Another possible target of the JIL-1Su(var)3-1 kinase might be the histone variant H2A.Z. In budding yeast, H2A.Z is required to prevent spreading of heterochromatin into euchromatin (Meneghini et al. 2003). This protective function might be modulated by JIL-1Su(var)3-1-mediated phosphorylation of H2A.Z. Finally, some intrinsic Su(var)s such as SU(VAR)3-9 and HP1 are phosphoproteins (Eissenberg et al. 1994; Aagaard et al. 2000) and JIL-1Su(var)3-1-mediated phosphorylation of those proteins might affect their function in heterochromatin expansion.
The balance between euchromatin and heterochromatin
Based on our data we propose a model for a dynamic balance between euchromatin and heterochromatin (Fig. 5). In PEV rearrangements, the boundary between these two states is determined by the antagonistic functions of euchromatic regulators (e.g., JIL-1) and the SU(VAR)3-9 HMTase that mediates H3-K9 dimethylation, the major mark of heterochromatin (Fig. 5A). The boundary between euchromatin and heterochromatin is not static and depends on the activity and abundance of inherent components of heterochromatin, such as, for example, hyperactive SU(VAR)3-9ptn (Fig. 5B) or overexpression of Su(var)3-9 (Fig. 5C). In contrast, hypoactive or null mutants of Su(var)3-9 weaken heterochromatin formation and favor the propagation of the euchromatic state. In JIL-1Su(var)3-1 mutants, heterochromatin expansion is severely repressed, even under conditions of elevated Su(var)3-9 function, and is most likely antagonized by a currently unknown proteins that are potential targets of the gain-of-function JIL-1Su(var)3-1 kinase. Dependent on the phosphorylation state of these targets, either heterochromatin expansion can be blocked or the propagation of the euchromatic state is highly stabilized (Fig. 5D).
Figure 5.
Su(var) genes regulate the balance between euchromatin and heterochromatin. The antagonistic functions of acquired and intrinsic Su(var) genes establish a boundary between euchromatin and heterochromatin. Hyperactive SU(VAR)3-9ptn (B) or overexpression of Su(var)3-9 (C) can expand heterochromatin, whereas JIL-1Su(var)3-1 stabilizes the euchromatic state by acting on an unknown target protein X (D). Please see main text for details.
In this work we have significantly extended mechanistic insights into the formation of heterochromatin and discovered Su(var)3-1 as a novel class of PEV modifiers that controls the balance between euchromatic and heterochromatic subdomains. Because of the dominant role of JIL-1Su(var)3-1 over major components of heterochromatin [intrinsic Su(var)s], the putative JIL-1 mutant targets are predicted to have important functions in gene silencing and the higher-order structuring of chromatin. The ongoing analysis on the full definition of Su(var) gene function is therefore likely to reveal both the genetic and molecular hierarchy that dictates epigenetic gene control.
Materials and methods
Drosophila cultures, stocks, and genetic analysis
Flies were reared on standard medium at 25°C. Chromosomes and mutations not noted here are described in FlyBase (http://flybase.net). Su(var)3-9 and Su(var)3-1 mutations were isolated by their dominant suppressor effect on white variegation of In(1)wm4 in the background of E(var) mutations or transgenic copies of Su(var)3-9 after EMS (2.5 mM) mutagenesis (Reuter et al. 1986; Tschiersch et al. 1994). The effects of Su(var)3-9 mutations on white gene silencing in wm4 were quantified in transheterozygotes with an additional P[(ry+) Su(var)3-9 11.5kb]100E genomic copy (Tschiersch et al. 1994). For molecular analysis, genomic DNA of Su(var)3-906/Su(var)3-9x heterozygotes was used to amplify the coding region of the Su(var)3-9 locus. Due to the DNA fragment inserted into Su(var)3-906 (Schotta et al. 2002), only PCR products of Su(var)3-9x are generated. Sequence information for all mutant alleles is available at GenBank (AJ290956).
PitkinD, originally assigned to region 67C due to a modifier of dominant female sterility was finally mapped by P-transposase-induced male recombination near Su(var)3-9 with the help of EP elements. w/Y; CyO, H{w+mC =P2-3}HoP2.1/+; ru ptnD e/EP(3) males were crossed to +/+; ru e/ru e females. Recombinant + e/ru e and ru +/ru e flies were tested for the presence of ptnD (PEV enhancer effect and dominant female sterility) by crosses to wm4 flies. The full-length Su(var)3-9 cDNA containing the ptnD mutation was introduced into pP{GS[ry+, hsEGFP]} (Schotta and Reuter 2000). Independent pP{GS[ry+, hs cDNA Su(var)3-9ptn EGFP]} transgenes were mobilized in crosses with TM3, ryRK Sb e [P(ry+)2-3](99B) and derivative lines with ptnD-specific mutant effects established.
HP1-null Df(2L)TE128X11/Su(var)2-504 larvae survive up to third instar and were identified as GFP-negative larvae in the progeny of CyO GFP/Df(2L)TE128X11 × CyO GFP/Su(var)2-504. E(z)-null larvae were generated by a cross of y; mwh E(z)5 red e/TM3, y+ Sb Ser × E(z)15 red/TM3, Sb Ser. Transheterozygous mwh E(z)5 red e/E(z)15 red larvae were identified by their red mutant Malpighian tube phenotype.
In vitro histone methyltransferase assays and mass spectrometry analysis
HMTase activities of recombinant GST-tagged proteins were analyzed according to Czermin et al. (2001). For the methyltransferase assays 1 μg of recombinant protein, 1 μg of histone H3 peptide (amino acids 1-20; Upstate Biotech), and 0.5 μL of SAM (1 μCi/mL; Amersham) were incubated for various times at 30°C in 25 μL of HIM buffer (Czermin et al. 2001). Mass spectrometry analysis of reaction products was performed according to Peters et al. (2003).
Nuclear extracts were prepared from wild-type, SU(VAR)3-9-null [Su(var)3-917/Su(var)3-917], HP1-null [Df(2L)TE128X11/Su(var)2-504], E(Z)-null [E(z)5/E(z)15], Su(var)3-9ptn [Su(var)3-9ptn/+ and Su(var)3-9ptn/Su(var)3-917] third instar larvae and separated by 18% SDS-PAGE. Following Coomassie staining, histone H3 bands were excised and processed according to Peters et al. (2003).
Immunostaining of polytene chromosomes
Preparation and staining of polytene chromosomes was performed as described (Schotta et al. 2002). Chromosomes were incubated with rabbit polyclonal α-mono-, α-di-, and α-trimethyl H3-K9 or H3-K27, respectively (1:50-1:100/5% dry milk), α-phospho H3-S10 (Upstate; 1:50/5% dry milk) antibodies at 4°C overnight, followed by incubation with Alexa Fluor 488 conjugated (Molecular Probes) secondary antibody for 2 h at 37°C (1:50/5% dry milk). Preparations were examined with confocal laser scanning microscopy (LSM 510; Zeiss).
Molecular cloning of Su(var)3-1 and analysis of heterochromatic silencing
Su(var)3-1 was mapped to the genomic region of JIL-1 at 86A by P-transposase-induced male recombination using a series of EP(3) P-element insertions. After a cross of homozygous se ss females with +/Y; CyO, H{w+mC = P2-3}HoP2.1/+; se Su(var)3-102 ss e ro/EP(3) males, se+ and +ss recombinant chromosomes were selected and consecutively tested for the presence of Su(var)3-102 by a cross to wm4 flies. The coding sequence of JIL-1 was PCR-amplified from genomic DNA of homozygous Su(var)3-1mut flies and subjected to sequence analyses.
Suppression of heterochromatization by Su(var)3-1 was studied in T(1;4)wm258-21/T(1;4)w70l26.5 larvae (Reuter et al. 1982) with an autosomal constitution of either Su(var)3-102 e ro/P[(ry+) Su(var)3-9 11.5kb]96A + P[(ry+) Su(var)3-9 11.5kb]100E or TM6B, Sb/P[(ry+) Su(var)3-9 11.5kb]96A + P[(ry+) Su(var)3-9 11.5kb]100E generated by a cross of T(1;4)wm258-21/FM1, y31d wa dm B; Su(var)3-102 e ro/TM6B, Sb females with T(1;4)w70l26.5/Y; P[(ry+) Su(var)3-9 11.5kb]96A + P[(ry+) Su(var)3-9 11.5kb]100E males. P[(ry+) Su(var)3-9 11.5kb]96A + P[(ry+) Su(var)3-9 11.5kb]100E contains two additional genomic Su(var)3-9 copies inserted in 96A and 100E. T(1;4)wm258-21/T(1;4)w70l26.5 and T(1;4)w70l26.5/FM1, y31d wa dm B larvae were distinguished by yellow versus white Malpighian tubes.
The epistatic effect of Su(var)3-102 on SU(VAR)3-9-induced gene silencing was studied in Dp(1;f)g238 (Le et al. 1995) variegating for yellow, T(2;3)V21e1A with repeat-dependent white gene silencing (Dorer and Henikoff 1994), T(2;3)BL1, and T(Y;2)BL2, showing P[(w+), HS-lacZ] transgene silencing (Lu et al. 1998). Su(var)3-102-dependent compensation of PEV enhancement was studied in genotypes with two additional genomic Su(var)3-9 copies (see Supplementary Fig. S6). Expression levels of marker genes were determined by classifying the phenotypes into 5 categories (0 = no expression, 5 = full expression).
Acknowledgments
We are grateful to S. Henikoff for CID antibody and A. Shearn for fly strains. Research in the laboratory of G.R. is supported by grants from the Deutsche Forschungsgemeinschaft (DFG). This work was further supported by a Marie Curie Intra-European Fellowship to Gunnar Schotta. Studies conducted in the lab of T.J. were supported by the IMP through Boehringer Ingelheim, and by grants from the Vienna Economy Promotion Fund (WWFF), the European Union (EU-network HPRN-CT 2000-00078), and the Austrian GEN-AU initiative, which is financed by the Austrian Ministry of Education, Science and Culture.
Supplemental material is available at http://www.genesdev.org.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.323004.
References
- Aagaard L., Schmid, M., Warburton, P., and Jenuwein, T. 2000. Mitotic phosphorylation of SUV39H1, a novel component of active centromeres, coincides with transient accumulation at mammalian centromeres. J. Cell Sci. 113: 817-829. [DOI] [PubMed] [Google Scholar]
- Allshire R.C., Javerzat, J.P., Redhead, N.J., and Cranston, G. 1994. Position effect variegation at fission yeast centromeres. Cell 76: 157-169. [DOI] [PubMed] [Google Scholar]
- Badenhorst P., Voas, M., Rebay, I., and Wu, C. 2002. Biological functions of the ISWI chromatin remodeling complex NURF. Genes & Dev. 16: 3186-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington E.A. and Jones, R.S. 1996. The Drosophila Enhancer of zeste gene encodes a chromosomal protein: Examination of wild-type and mutant protein distribution. Development 122: 4073-4083. [DOI] [PubMed] [Google Scholar]
- Czermin B., Schotta, G., Hulsmann, B.B., Brehm, A., Becker, P.B., Reuter, G., and Imhof, A. 2001. Physical and functional association of SU(VAR)3-9 and HDAC1 in Drosophila. EMBO Rep. 2: 915-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delattre M., Spierer, A., Tonka, C.-H., and Spierer, P. 2000. The genomic silencing of position-effect variegation in Drosophila melanogaster: Interaction between the heterochromatin-associated proteins Su(var)3-7 and HP1. J. Cell Sci. 113: 4253-4261. [DOI] [PubMed] [Google Scholar]
- DeRubertis F., Kadosh, D., Henchoz, S., Pauli, D., Reuter, G., Struhl, K., and Spierer, P. 1996. The histone deacetylase RPD3 counteracts genomic silencing in Drosophila and yeast. Nature 384: 589-591. [DOI] [PubMed] [Google Scholar]
- Deuring R., Fanti, L., Armstrong, J.A., Sarte, M., Papoulas, O., Prestel, M., Daubresse, G., Verardo, M., Moseley, S.L., Berloco, M., et al. 2000. The ISWI chromatin-remodeling protein is required for gene expression and the maintenance of higher order chromatin structure in vivo. Mol. Cell 5: 355-365. [DOI] [PubMed] [Google Scholar]
- Dorer D.R. and Henikoff, S. 1994. Expansions of transgene repeats cause heterochromatin formation and gene silencing in Drosophila. Cell 77: 993-1002. [DOI] [PubMed] [Google Scholar]
- Dorn R., Krauss, V., Reuter, G., and Saumweber, H. 1993. The enhancer of position-effect variegation of Drosophila, E(var)3-93D, codes for a chromatin protein containing a conserved domain common to several transcriptional regulators. Proc. Natl. Acad. Sci. 90: 11376-11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J.C. and Elgin, S.C. 2000. The HP1 protein family: Getting a grip on chromatin. Curr. Opin. Genet. Dev. 10: 204-210. [DOI] [PubMed] [Google Scholar]
- Eissenberg J.C, James, T., Foster-Hartnett, D., Hartnett, T., Ngan, V., and Elgin, S.C.R. 1990. Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc. Natl. Acad. Sci. 87: 9923-9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J.C., Ge, Y.W., and Hartnett, T. 1994. Increased phosphorylation of HP1, a heterochromatin-associated protein of Drosophila, is correlated with heterochromatin assembly. J. Biol. Chem. 269: 21315-21321. [PubMed] [Google Scholar]
- Eskeland R., Czermin, B., Boeke, J., Bonaldi, T., Regula, J.T., and Imhof, A. 2004. The N-terminus of Drosophila SU(VAR)3-9 mediates dimerization and regulates its methyltransferase activity. Biochemistry 43: 3740-3749. [DOI] [PubMed] [Google Scholar]
- Greil F., van der Kraan, I., Delrow, J., Smothers, J.F., de Wit, E., Bussemaker, H.J., van driel, R., Henikoff, S., and van Steensel, B. 2003. Distinct HP1 and Su(var)3-9 complexes bind to sets of developmentally coexpressed genes depending on chromosomal location. Genes & Dev. 17: 2825-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Ahmad, K., Platero, J.S., and van Steensel, B. 2000. Heterochroatic deposition of centromeric histone H3-like proteins. Proc. Natl Acad. Sci. 97: 716-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquet Y., Delattre, M., Spierer, A., and Spierer, P. 2002. Functional dissection of the Drosophila modifier of variegation Su(var)3-7. Development 129: 3975-3982. [DOI] [PubMed] [Google Scholar]
- Kuhfittig S., Szabad, J., Schotta, G., Hoffmann, J., Máthé, E., and Reuter, G. 2001. pitkinD a novel gain-of-function enhancer of position-effect variegation affects chromatin regulation during oogenesis and early embryogenesis in Drosophila. Genetics 157: 1227-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le M.H., Duricka, D., and Karpen, G.H. 1995. Islands of complex DNA are widespread in Drosophila centric heterochromatin. Genetics 141: 282-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B.Y., Ma, J., and Eissenberg, J.C. 1998. Developmental regulation of heterochromatin-mediated gene silencing in Drosophila. Development 125: 2223-2234. [DOI] [PubMed] [Google Scholar]
- Meneghini M.D., Wu, M., and Madhani, H.D. 2003. Conserved histone variant H2A.Z protects euchromatin from the ectopic spread of silent heterochromatin. Cell 112: 725-736. [DOI] [PubMed] [Google Scholar]
- Mottus R., Sobel, R.E., and Grigliatti, T.A. 2000. Mutational analysis of a histone deacetylase in Drosophila melanogaster: Missense mutations suppress gene silencing associated with position effect variegation. Genetics 154: 657-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A.H.F.M., Kubicek, S., Mechtler, K., O'Sullivan, J., Derijck, A.A.H.A., Perez-Burgos, L., Kohlmaier, A., Opravil, S., Tachibana, M., Shinkai, Y., et al. 2003. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol. Cell 12: 1577-1589. [DOI] [PubMed] [Google Scholar]
- Plath K., Mlynarczyk-Evans, S., Nusinow, D.A., and Panning, B. 2003. Xist RNA and the mechanism of X chromosome inactivation. Annu. Rev. Genet. 36: 233-278. [DOI] [PubMed] [Google Scholar]
- Rea S., Eisenhaber, F., O'Carroll, D., Stahl, B.D., Sun, Z-W., Schmid, M., Opravil, S., Mechtler, K., Ponting, C.P., Allis, C.D., et al. 2000. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406: 593-599. [DOI] [PubMed] [Google Scholar]
- Reuter G. and Spierer, P. 1992. Position effect variegation and chromatin proteins. Bioessays 14: 605-612. [DOI] [PubMed] [Google Scholar]
- Reuter G., Werner, W., and Hoffmann, H.-J. 1982. Mutants affecting position-effect heterochromatinization in Drosophila melanogaster. Chromosoma 85: 539-551. [DOI] [PubMed] [Google Scholar]
- Reuter G., Dorn, R., Wustmann, G., Friede, B., and Rauh, G. 1986. Third chromosome suppressor of position-effect variegation loci in Drosophila melanogaster. Mol. Gen. Genet. 202: 481-487. [Google Scholar]
- Rice J.C., Briggs, S.D., Ueberheide, B., Barber, C.M., Shabanowitz, J., Hunt, D.F., Shinkai, Y., and Allis, C.D. 2003. Histone methyltransferases direct different degrees of methylation to define distinct chromatin domains. Mol. Cell 12: 1591-1598. [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H., Schneider, R., Bannister, A.J., Sherriff, J., Bernstein, B.E., Emre, N.C., Schreiber, S.L., Mellor, J., and Kouzarides, T. 2002. Active genes are trimethylated at K4 of histone H3. Nature 419: 407-411. [DOI] [PubMed] [Google Scholar]
- Schotta G. and Reuter, G. 2000. Controlled expression of tagged proteins in Drosophila using a new modular P-element vector system. Mol. Gen. Genet. 262: 916-920. [DOI] [PubMed] [Google Scholar]
- Schotta G., Ebert, A., Krauss, V., Fischer, A., Hoffmann, J., Rea, S., Jenuwein, T., and Reuter, G. 2002. Central role of Drosophila SU(VAR)3-9 in histone H3-K9 methylation and heterochromatic gene silencing. EMBO J. 21: 1121-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotta G., Lachner, M., Sarma, K., Ebert, A., Sengupta, R., Reuter, G., Reinberg, D., and Jenuwein, T. 2004. A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes & Dev. 18: 1251-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J., Mak, W., Zvetkova, I., Appanah, R., Neterova, T.B., Webster, Z., Peters, A.H., Jenuwein, T., Otte, A.P., and Brockdorff, N. 2003. Establishment of histone h3 methylation on the inactive X chromosome requires transient recruitment of Eed-Enx1 polycomb group complexes. Dev. Cell 4: 481-495. [DOI] [PubMed] [Google Scholar]
- Tschiersch B., Hofmann, A., Krauss, V., Dorn, R., Korge, G., and Reuter, G. 1994. The protein encoded by the Drosophila position-effect variegation suppressor gene Su(var)3-9 combines domains of antagonistic regulators of homeotic gene complexes. EMBO J. 13: 3822-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang, W., Jin, Y., Johansen, J., and Johansen, K.M. 2001. The JIL-1 tandem kinase mediates histone H3 phosphorylation and is required for maintenance of chromatin structure in Drosophila. Cell 105: 433-443. [DOI] [PubMed] [Google Scholar]
- Wustmann G., Szidonya, J., Taubert, H., and Reuter, G. 1989. The genetics of position-effect variegation modifying loci in Drosophila melanogaster. Mol. Gen. Genet. 217: 520-527. [DOI] [PubMed] [Google Scholar]