Abstract
Neuronal communication and brain function mainly depend on the fundamental biological events of neurotransmission, including the exocytosis of presynaptic vesicles (SVs) for neurotransmitter release and the subsequent endocytosis for SV retrieval. Neurotransmitters are released through the Ca2+- and SNARE-dependent fusion of SVs with the presynaptic plasma membrane. Following exocytosis, endocytosis occurs immediately to retrieve SV membrane and fusion machinery for local recycling and thus maintain the homeostasis of synaptic structure and sustained neurotransmission. Apart from the general endocytic machinery, recent studies have also revealed the involvement of SNARE proteins (synaptobrevin, SNAP25 and syntaxin), synaptophysin, Ca2+/calmodulin, and members of the synaptotagmin protein family (Syt1, Syt4, Syt7 and Syt11) in the balance and tight coupling of exo-endocytosis in neurons. Here, we provide an overview of recent progress in understanding how these neuron-specific adaptors coordinate to ensure precise and efficient endocytosis during neurotransmission.
Keywords: exocytosis, endocytosis, vesicle recycling, calmodulin, synaptotagmin, SNARE
Neurotransmission based on the exocytosis of synaptic vesicles (SVs) and the subsequent SV membrane retrieval through endocytosis are crucial for efficient neuronal communication, the integrity of neuronal circuits, and normal brain function (Chapman, 2002; Sudhof, 2004; Wu L. G. et al., 2014). With the arrival of an action potential, extra-synaptic Ca2+ flows into the nerve terminals and triggers soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein-dependent vesicle exocytosis (Südhof and Rothman, 2009; Jahn and Fasshauer, 2012; Rizo and Xu, 2015). The released neurotransmitters diffuse across the synaptic cleft and activate or inhibit the postsynaptic compartment. After exocytosis, fused SV components are locally retrieved from the neuronal surface through endocytosis, which is tightly coupled to exocytosis. Precise and efficient endocytosis is critical for the preservation of presynaptic morphology and structural integrity, the replenishment of presynaptic vesicle pools, and sustained neurotransmission during continuous neuronal activity (Saheki and De Camilli, 2012; Wu L. G. et al., 2014; Leitz and Kavalali, 2016).
Several modes of endocytosis operate to ensure a sufficient and precise vesicle-recycling rate during neurotransmission. Clathrin-mediated endocytosis (CME), the best-characterized endocytic pathway, is known to be the predominant route of vesicle retrieval with slow kinetics (time constant: 10–30 s) following exocytosis (Granseth et al., 2006; Jung and Haucke, 2007; McMahon and Boucrot, 2011). The elevated neuronal activity also elicits bulk endocytosis, which internalizes a large area of plasma membrane, forms an endosome-like endocytic structure, and is finally converted into releasable SVs by a mechanism that remains elusive (Clayton et al., 2008; Smith et al., 2008; Saheki and De Camilli, 2012; Wu L. G. et al., 2014). The kiss-and-run mode of exo-endocytosis probably represents the fast component of SV endocytosis, during which SVs release their contents through a transient nanometer-sized fusion pore and are retrieved rapidly without full collapse into the plasma membrane (He and Wu, 2007; Rizzoli and Jahn, 2007; Alabi and Tsien, 2013). In addition, ultrafast endocytosis has been revealed by electron microscopic analysis (Watanabe et al., 2013) and membrane capacitance (Cm) recordings (Wu et al., 2009; Mahapatra et al., 2016), which are not discussed in detail here because of uncertainty about the nature of these endocytic pathways.
Exo-Endocytosis Coupling
Although endocytosis is predominantly a constitutive process in most non-neuronal cells, SV endocytosis is primarily an activity-dependent form of membrane retrieval that is spatiotemporally coupled to exocytosis. Upon depolarization, docked vesicles diminish while clathrin-coated pits and structures associated with bulk endocytosis increase near the release sites (Gad et al., 1998; Gundelfinger et al., 2003; Hosoi et al., 2009; Wang et al., 2016), representing exocytosis and the tightly-coupled endocytosis. Consistently, Cm recordings have revealed endocytosis as a stimulation-dependent form of membrane retrieval, in which exocytosis is represented as a Cm jump upon depolarization and the subsequent Cm decay indicates the process of compensatory endocytosis (Zhang et al., 2004; Wu and Wu, 2007; Yamashita et al., 2010). Importantly, the Cm traces reliably decay back to baseline within seconds to minutes after exocytosis, indicating that endocytosis retrieves an amount similar to that of exocytosed SV membrane (Lou et al., 2008; Yamashita et al., 2010; Wang et al., 2016). Furthermore, blockade of exocytosis by cleaving SNARE proteins with botulinum neurotoxins also abolishes endocytosis (Wu et al., 2005; Yamashita et al., 2005), implying a critical role of exocytosis in the initiation of endocytosis. Given the limitation of Cm recordings in small conventional synapses, the optical imaging of fluorescent dyes such as FM1–43, or dextran uptake, has permitted studies of vesicle recycling in neuronal terminals (Virmani et al., 2003; Deák et al., 2004; Clayton et al., 2010; Wang et al., 2016). Tagging vesicular proteins with pHluorin, a pH-sensitive green fluorescent protein that allows the direct visualization of exocytosis and the subsequent endocytosis in living nerve terminals, has also confirmed the tight coupling of synaptic endocytosis to exocytosis in terms of both timing and amount (Poskanzer et al., 2003; Ferguson et al., 2007; Hua et al., 2011; Yao et al., 2011).
Ca2+/Calmodulin in Exo-Endocytosis Coupling
Although there is extensive evidence that Ca2+ influx plays critical role in compensatory endocytosis, whether and how cytosolic Ca2+ regulates exo-endocytosis coupling is rather controversial. Accumulating evidence has shown that a transient elevation in cytosolic Ca2+ triggers and accelerates both clathrin-dependent and clathrin-independent endocytosis in neurons and neuroendocrine cells (Balaji et al., 2008; Hosoi et al., 2009; Sun et al., 2010; Leitz and Kavalali, 2016). However, the Ca2+-dependence of exo-endocytosis is diverse among different preparations (Wu and Wu, 2014; Wu L. G. et al., 2014). Endocytosis can also occur independent of cytosolic Ca2+ (Ryan et al., 1993; Granseth et al., 2006), and increasing the intracellular Ca2+ concentration slows exo-endocytosis in many cases (von Gersdorff and Matthews, 1994; Leitz and Kavalali, 2011; Armbruster et al., 2013). Nonetheless, the critical roles of cytosolic Ca2+ in SV exocytosis make it inconclusive whether Ca2+ influx directly mediates exo-endocytosis coupling and thus controls the timing and amount of compensatory endocytosis independent of exocytosis, although great efforts have been made to dissect this by manipulating exocytosis (Sun et al., 2002; Wu et al., 2009; Yao et al., 2011). Thus, the exact role of Ca2+ in the coupling of SV exo-endocytosis remains a pending question and needs more thorough investigations.
Several endocytic Ca2+ sensors and effectors have been shown to initiate and mediate Ca2+-dependent endocytosis, in which calmodulin is involved in most forms of endocytosis and synaptotagmin is a dual Ca2+ sensor for both exocytosis and endocytosis. Calcineurin functions as a key mediator of Ca2+/calmodulin in exo-endocytosis by dephosphorylating endocytic proteins known as dephosphins (Cousin and Robinson, 2001; Saheki and De Camilli, 2012). Typically, many proteins involved in different stages of CME (e.g., dynamin, synaptojanin, amphiphysin, epsin and Eps15) are constitutively phosphorylated as an inactive conformation in resting nerve terminals (Liu et al., 1994; Chen et al., 1999; Lee et al., 2004, 2005). During synaptic activity, these dephosphins undergo rapid dephosphorylation by the Ca2+/calmodulin-activated calcineurin to drive endocytosis via their enhanced binding to other endocytic factors or by dephosphorylation-dependent activation (Liu et al., 1994; Slepnev et al., 1998; Anggono et al., 2006; Saheki and De Camilli, 2012). The regulation of CME by calcineurin has been confirmed by the inhibition of slow endocytosis with calcineurin blockers, or the knockdown/knockout of calcineurin (Engisch and Nowycky, 1998; Sun et al., 2010; Armbruster et al., 2013; Wu X. S. et al., 2014). In addition, calcineurin also mediates bulk endocytosis by dephosphorylating dynamin 1 during elevated neuronal activity (Clayton et al., 2009, 2010). It has been proposed that the GTPase activity of dynamin is essential for vesicle fission during CME, bulk endocytosis and kiss-and-run, while its phosphorylation-dephosphorylation cycle is also critical for activity-dependent bulk endocytosis (Marks et al., 2001; Yamashita et al., 2005; Anggono et al., 2006; Clayton and Cousin, 2009; Anantharam et al., 2011). However, the dynamin-dependency of bulk endocytosis remains controversial because it still occurs robustly in the absence of dynamin 1, which might be due to the compensatory effect of other dynamin isoforms (Hayashi et al., 2008; Raimondi et al., 2011; Lou et al., 2012; Fan et al., 2016). Finally, dynamin and the calcineurin-dependent dynamin-syndapin interaction have also been demonstrated to regulate the kiss-and-run mode of exo-endocytosis and the quantal size of neurotransmitter release by limiting the fusion pore dilation under elevated stimulation (Graham et al., 2002; Samasilp et al., 2012).
In addition to calcineurin, myosin light-chain kinase is another co-effector functioning to accelerate both the slow and fast forms of exo-endocytosis through the activity-dependent phosphorylation of myosin at the downstream of Ca2+/calmodulin (Yue and Xu, 2014; Li et al., 2016). A recent study has also defined critical roles of calmodulin in regulating the intrinsic membrane-remodeling activity via a Ca2+-dependent interaction with Rvs167 in yeast and several endocytic N-BAR domain proteins such as endophilins and amphiphysins in mammalian cells (Myers et al., 2016).
Synaptotagmin Proteins in Exo-Endocytosis Coupling
Synaptotagmins (Syts), a family of type I membrane proteins with evolutionarily conserved cytosolic tandem C2 domains (C2A and C2B), are well-characterized Ca2+ sensors that initiate SNARE-dependent vesicle fusion during synaptic transmission and hormone secretion (Chapman, 2002; Gustavsson and Han, 2009; Südhof and Rothman, 2009; Pang and Südhof, 2010). At least 17 mammalian Syt isoforms have been identified, the detailed characterizations of which are summarized in recent reviews (Gustavsson and Han, 2009; Pang and Südhof, 2010). All Syt members bind the clathrin-adaptor protein AP-2 with high affinity (Kd = 0.1–1.0 nM) and some Syts have been shown to function in different endocytic pathways (Zhang et al., 1994; Li et al., 1995; Chapman et al., 1998; Yao et al., 2011). Syt1, the prototypical Syt protein functioning as the primary Ca2+ sensor for exocytosis, has also been proposed to be a major Ca2+-sensing protein that promotes CME upon exocytosis (Haucke et al., 2000; Jarousse and Kelly, 2001; Poskanzer et al., 2003). Cm recordings, electron microscopy, FM uptake and pHluorin assays have reliably revealed dramatic endocytic defects in Syt1-deficient cells from a variety of organisms (Poskanzer et al., 2003; Nicholson-Tomishima and Ryan, 2004; Yao et al., 2011, 2012). Meanwhile, Syt1 has also been demonstrated to bind the μ2 subunit of the endocytic adaptor protein AP-2 and the μ-homology domain of stonin-2 through its C2B domain (Zhang et al., 1994; Haucke et al., 2000; Jarousse and Kelly, 2001; Walther et al., 2001; Kaempf et al., 2015). However, the direct regulation of Syt1 in CME has been challenged due to that the endocytic defects may be secondary to the impaired exocytosis caused by Syt1 deficiency (Poskanzer et al., 2006; Yao et al., 2011). A recent study has provided direct evidence that Syt1 indeed functions as a Ca2+ sensor for SV endocytosis by uncoupling the function of Syt1 in exo- and endocytosis in hippocampal neurons (Yao et al., 2011). Then, with cell-attached Cm recordings, another group validated that Syt1 functions to modulate the Ca2+-dependence of CME probably by AP-2-dependently prolonging the duration of fission pore closure (Yao et al., 2012).
Syt7 is ubiquitously expressed at early stage of development but is later restricted to dividing cells, neuroendocrine cells, and presynaptic neuronal structures (Virmani et al., 2003). Syt7 binds Ca2+ with a high apparent affinity and slow kinetics, and thus mainly functions as a slow Ca2+ sensor to mediate the slow phase of exocytosis known as asynchronous release, as well as fusion-pore expansion and synaptic facilitation (Maximov et al., 2008; Schonn et al., 2008; Liu et al., 2014; Neuland et al., 2014; Wu et al., 2015). Interestingly, Syt7 is extensively spliced and exhibits a broad variety of alternative splice variants, among which the short Syt7 variant lacking both of the C2 domains inhibits CME but accelerates exo-endocytosis in response to intense stimulation, while the regular full-length Syt7 directs synaptic endocytosis into a slow-recycling CME (von Poser et al., 2000; Virmani et al., 2003). A recent study also defined Syt7 as a Ca2+ sensor for SV replenishment (Liu et al., 2014), confirming the regulatory role of Syt7 in SV recycling. Furthermore, Syt7 also plays a critical role in the occurrence of kiss-and-run probably by mediating the push-and-pull regulation of fusion pore dilation (Segovia et al., 2010; Neuland et al., 2014). It has been proposed that Ca2+ binding to the C2A domain of Syt7 is sufficient to trigger fusion-pore opening but the resulting pores are unstable, thus leading to a dramatic increase in kiss-and-run fusion events. In contrast, Ca2+ binding to the C2B domain facilitates the continuous expansion of fusion pores, making Syt7 a critical regulator of the Ca2+-dependent occurrence of kiss-and-run and full-fusion events (Segovia et al., 2010; Neuland et al., 2014).
Syt4 and Syt11 are classified as non-Ca2+-binding Syts because of an aspartate-to-serine substitution in a Ca2+-coordination site of the C2A domain, and they do not bind Ca2+ biochemically (von Poser et al., 1997; Dai et al., 2004; Dean et al., 2009). Syt4 has been reported to regulate fusion-pore and fusion modes in both endocrinal cells and neurons, but the effects fail to reach a consensus in these preparations. Syt4 overexpression favors the occurrence of kiss-and-run and increases the duration of fusion pore dilation in PC12 cells (Wang et al., 2001, 2003; Zhang et al., 2010). Cell-attached Cm recording also revealed prolonged lifetime and smaller downward Cm steps of fission pores during endocytosis (Zhang et al., 2010). In contrast, Syt4 deficiency accelerates the rapid component of endocytosis probably through the enhanced kiss-and-run in the peptidergic nerve terminals of posterior pituitary neurons (Zhang et al., 2009). Similarly, Syt4 inhibits BDNF release in both axons and dendrites but with distinct mechanisms, in which presynaptic Syt4 decreases frequency of spontaneous quantal release while postsynaptic Syt4 limits quantal size by favoring kiss-and-run modes of exo-endocytosis (Dean et al., 2009).
Syt11 is a newly-defined endocytic regulator that inhibits CME and bulk endocytosis in neurons probably through distinct mechanisms (Wang et al., 2016). Disruption of this inhibitory role by Syt11-knockdown induces excessive membrane retrieval, accelerates vesicle pool replenishment, and facilitates sustained neurotransmission, indicating a critical role of Syt11 as a clamp protein to ensure the precise coupling and balance of endocytosis to exocytosis during neurotransmission (Wang et al., 2016). Since Syt11 does not bind Ca2+ biochemically, there may also be a Ca2+-sensitive inhibitor to ensure the precise Ca2+-dependency of exo-endocytosis, especially during sustained neuronal activities.
SNARE Proteins and Synaptophysin in Exo-Endocytosis Coupling
In addition to Ca2+ influx upon depolarization, exocytosis itself is required for the initiation of compensatory SV endocytosis, which is abolished by the cleavage of SNARE proteins essential for exocytosis with botulinum neurotoxins (Hosoi et al., 2009; Xu et al., 2013). A debated issue is that exocytosis-mediated plasma membrane expansion and surface tension reduction may serve to initiate the local membrane curvature (membrane buds) for internalization (Dai et al., 1997; Anantharam et al., 2010; Diz-Muñoz et al., 2013; Hassinger et al., 2017). Meanwhile, the delivery of PI(4,5)P2-lacking SV membranes to the plasma membrane makes these budding sites competent for the recruitment of endocytic scaffolding proteins and the formation of coated pits (Wenk and De Camilli, 2004; McMahon and Gallop, 2005; Haucke et al., 2011; Saheki and De Camilli, 2012; Puchkov and Haucke, 2013). Furthermore, some classical exocytic proteins, especially Syts, SNARE proteins and synaptophysin, also function to couple exo-endocytosis.
SNARE proteins are critical for membrane fusion, while recent studies have also implied a significant contribution of synaptobrevins (also termed VAMPs, vesicle-associated membrane proteins), syntaxin, and SNAP-25 in the coupling of SV exo-endocytosis. Synaptobrevin-2 (VAMP2) deficiency impairs the fast component of compensatory endocytosis and the rapid re-use of SVs in hippocampal neurons (Deák et al., 2004), while the cleavage of VAMP2 and VAMP3 with tetanus toxin blocks both the slow and fast modes of endocytosis in nerve terminals of the calyx of Held (Hosoi et al., 2009; Xu et al., 2013). A recent study has also established an essential role of synaptobrevin in slow endocytosis in hippocampal neurons (Zhang et al., 2013). VAMP4 also plays critical roles in activity-dependent bulk endocytosis in hippocampal neurons (Nicholson-Fish et al., 2015). In addition, an early study also revealed the involvement of t-SNARE proteins (syntaxin and SNAP-25 in targeting membrane) in exo-endocytosis coupling in yeast (Gurunathan et al., 2002). Consistently, SNAP25 knockdown inhibits slow SV endocytosis in hippocampal synapses (Zhang et al., 2013), and the cleavage of SNAP-25 with botulinum neurotoxin E impairs both the fast and slow modes of endocytosis in calyx terminals (Xu et al., 2013). Syntaxin 1 clearance with botulinum neurotoxin C also greatly inhibits SV endocytosis at the calyx (Xu et al., 2013), while syntaxin 1A SUMOylation shows a similar inhibitory effect on SV endocytosis in cortical and hippocampal neurons (Craig et al., 2015).
Synaptophysin is the most abundant SV protein; it is exclusively localized to SVs with uncertain roles in SV exocytosis, endocytosis, synapse formation, and other synaptic functions (Janz et al., 1999; Tarsa and Goda, 2002; Takamori et al., 2006). Synaptophysin interacts with dynamin via its C-terminal cytoplasmic tail region in a Ca2+-dependent manner (Daly et al., 2000; Daly and Ziff, 2002), disruption of which decreases vesicle retrieval and thus neurotransmitter release during intense stimulation, probably due to the impairment of clathrin-independent rapid endocytosis (Daly et al., 2000). A recent study provided direct evidence for the involvement of synaptophysin in exo-endocytosis coupling by using optical imaging of Syt1-pHluorin and SV2-pHluorin. Synaptophysin knockout impairs SV endocytosis during and after sustained neuronal activity, while the C-terminal tail-truncated synaptophysin can only rescue the slow post-stimulus endocytosis (Kwon and Chapman, 2011), indicating the distinct requirement of synaptophysin structural elements in the two phases of exo-endocytosis. These findings validate the critical dual roles of synaptophysin and SNARE proteins in both exocytosis and the exo-endocytosis coupling process; however, which specific endocytic pathways are regulated by these fusion machineries and how these proteins are involved in the compensatory SV endocytosis remain largely elusive.
Conclusion
Recent advances paint an extremely complex picture of the tight exo-endocytosis coupling in neurons. At least three different endocytic pathways, CME, activity-dependent bulk endocytosis, and the kiss-and-run mode of fast endocytosis, cooperate to couple SV endocytosis to exocytosis with different neuronal activities. The Ca2+-calmodulin-calcineurin pathway, synaptophysin and SNARE proteins, Ca2+-binding Syt members, and other positive regulators work together with endocytic inhibitors such as non-Ca2+-binding Syts to provide a fine-tuning mechanism for the efficient and precise coupling of SV endocytosis to exocytosis (Figure 1). Membrane lipid structures and proteins involved in phosphoinositide metabolism also play critical roles in the exo-endocytosis coupling. In addition, scaffolding and effector proteins essential for non-neuronal endocytosis are also necessary for exo-endocytosis coupling in neurons. However, uncertainty about the functions of these endocytic regulators and the co-existence of several other endocytic pathways with distinct kinetics and molecular mediators require a more thorough investigation (Wu et al., 2009; Watanabe et al., 2013; Kononenko and Haucke, 2015). Further studies have been challenged due to the limitation of electrophysiological recordings and live fluorescence imaging assays of single-SV recycling in small nerve terminals. Advances in super-resolution microscopy and correlative light and electron microscopy offer new opportunities in this field. In addition, optogenetic stimulation, two-photon imaging, and acute molecular manipulation in vivo allow a deep functional analysis of the endocytic regulators that associate SV recycling with brain disorders such as Alzheimer disease, Parkinson disease and emotional disorders.
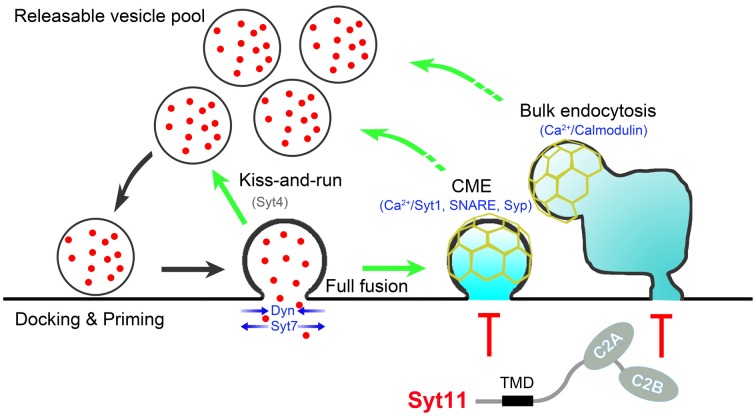
Figure 1.
Schematic presentation showing proteins involved in exo-endocytosis coupling. During neurotransmission, endocytosis occurs immediately after synaptic vesicle (SV) exocytosis via clathrin-mediated endocytosis (CME), kiss-and-run and bulk endocytosis. The Ca2+-calmodulin pathway, synaptophysin (Syp) and SNARE proteins, Ca2+-binding (Syt1, Syt7) and non-Ca2+-binding (Syt4, Syt11) Syts, and other proteins such as dynamin (Dyn) coordinate to control the efficient and precise coupling of SV endocytosis to exocytosis. Syt11 is shown in red due to the nature of negative regulator, and Syt4 is shown in gray since that Syt4 shows different effects on different types fusion-fission events. Scaffolding proteins (e.g., N-BAR/F-BAR/BAR domain-containing proteins (such as FCHo, endophilin, amphiphysin, syndapin), intersectin, Rab3, CDC42, N-WASP, SNX9 and Eps15), downstream effectors (e.g., calcineurin, myosin, CDC2 and CDK5), phosphoinositide metabolism (e.g., PI(4,5)P2, cholesterol, synaptojanin, PI 3-kinase and PIPK1γ), and other general endocytic machineries (clathrin, AP-2, AP180, Epsin and stonin) are excluded to simplify the cartoon.
Author Contributions
ZX drafted the manuscript with help from JLo, JLi, ZC, XK and CW. All authors coordinated, revised and approved the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31400708, 81571235 and 31670843), the Natural Science Foundation of Heilongjiang Province of China (C201453), the Natural Science Foundation of Shandong Province of China (ZR2016CM16) and the Scientific Research Fund of Heilongjiang Provincial Education Department (12531750 and 12531746). XK was supported in part by the start-up funding of Liaocheng University (318051525).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Iain C. Bruce (Peking University) for reading the manuscript.
References
- Alabi A. A., Tsien R. W. (2013). Perspectives on kiss-and-run: role in exocytosis, endocytosis and neurotransmission. Annu. Rev. Physiol. 75, 393–422. 10.1146/annurev-physiol-020911-153305 [DOI] [PubMed] [Google Scholar]
- Anantharam A., Bittner M. A., Aikman R. L., Stuenkel E. L., Schmid S. L., Axelrod D., et al. (2011). A new role for the dynamin GTPase in the regulation of fusion pore expansion. Mol. Biol. Cell 22, 1907–1918. 10.1091/mbc.E11-02-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharam A., Onoa B., Edwards R. H., Holz R. W., Axelrod D. (2010). Localized topological changes of the plasma membrane upon exocytosis visualized by polarized TIRFM. J. Cell Biol. 188, 415–428. 10.1083/jcb.200908010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anggono V., Smillie K. J., Graham M. E., Valova V. A., Cousin M. A., Robinson P. J. (2006). Syndapin I is the phosphorylation-regulated dynamin I partner in synaptic vesicle endocytosis. Nat. Neurosci. 9, 752–760. 10.1038/nn1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster M., Messa M., Ferguson S. M., De Camilli P., Ryan T. A. (2013). Dynamin phosphorylation controls optimization of endocytosis for brief action potential bursts. Elife 2:e00845. 10.7554/eLife.00845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji J., Armbruster M., Ryan T. A. (2008). Calcium control of endocytic capacity at a CNS synapse. J. Neurosci. 28, 6742–6749. 10.1523/JNEUROSCI.1082-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E. R. (2002). Synaptotagmin: a Ca2+ sensor that triggers exocytosis? Nat. Rev. Mol. Cell Biol. 3, 498–508. 10.1038/nrm855 [DOI] [PubMed] [Google Scholar]
- Chapman E. R., Desai R. C., Davis A. F., Tornehl C. K. (1998). Delineation of the oligomerization, AP-2 binding and synprint binding region of the C2B domain of synaptotagmin. J. Biol. Chem. 273, 32966–32972. 10.1074/jbc.273.49.32966 [DOI] [PubMed] [Google Scholar]
- Chen H., Slepnev V. I., Di Fiore P. P., De Camilli P. (1999). The interaction of epsin and Eps15 with the clathrin adaptor AP-2 is inhibited by mitotic phosphorylation and enhanced by stimulation-dependent dephosphorylation in nerve terminals. J. Biol. Chem. 274, 3257–3260. 10.1074/jbc.274.6.3257 [DOI] [PubMed] [Google Scholar]
- Clayton E. L., Anggono V., Smillie K. J., Chau N., Robinson P. J., Cousin M. A. (2009). The phospho-dependent dynamin-syndapin interaction triggers activity-dependent bulk endocytosis of synaptic vesicles. J. Neurosci. 29, 7706–7717. 10.1523/JNEUROSCI.1976-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E. L., Cousin M. A. (2009). The molecular physiology of activity-dependent bulk endocytosis of synaptic vesicles. J. Neurochem. 111, 901–914. 10.1111/j.1471-4159.2009.06384.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E. L., Evans G. J., Cousin M. A. (2008). Bulk synaptic vesicle endocytosis is rapidly triggered during strong stimulation. J. Neurosci. 28, 6627–6632. 10.1523/JNEUROSCI.1445-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E. L., Sue N., Smillie K. J., O’Leary T., Bache N., Cheung G., et al. (2010). Dynamin I phosphorylation by GSK3 controls activity-dependent bulk endocytosis of synaptic vesicles. Nat. Neurosci. 13, 845–851. 10.1038/nn.2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin M. A., Robinson P. J. (2001). The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 24, 659–665. 10.1016/s0166-2236(00)01930-5 [DOI] [PubMed] [Google Scholar]
- Craig T. J., Anderson D., Evans A. J., Girach F., Henley J. M. (2015). SUMOylation of Syntaxin1A regulates presynaptic endocytosis. Sci. Rep. 5:17669. 10.1038/srep17669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H., Shin O. H., Machius M., Tomchick D. R., Südhof T. C., Rizo J. (2004). Structural basis for the evolutionary inactivation of Ca2+ binding to synaptotagmin 4. Nat. Struct. Mol. Biol. 11, 844–849. 10.1038/nsmb817 [DOI] [PubMed] [Google Scholar]
- Dai J., Ting-Beall H. P., Sheetz M. P. (1997). The secretion-coupled endocytosis correlates with membrane tension changes in RBL 2H3 cells. J. Gen. Physiol. 110, 1–10. 10.1085/jgp.110.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C., Ziff E. B. (2002). Ca2+-dependent formation of a dynamin-synaptophysin complex: potential role in synaptic vesicle endocytosis. J. Biol. Chem. 277, 9010–9015. 10.1074/jbc.M110815200 [DOI] [PubMed] [Google Scholar]
- Daly C., Sugimori M., Moreira J. E., Ziff E. B., Llinás R. (2000). Synaptophysin regulates clathrin-independent endocytosis of synaptic vesicles. Proc. Natl. Acad. Sci. U S A 97, 6120–6125. 10.1073/pnas.97.11.6120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deák F., Schoch S., Liu X., Südhof T. C., Kavalali E. T. (2004). Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat. Cell Biol. 6, 1102–1108. 10.1038/ncb1185 [DOI] [PubMed] [Google Scholar]
- Dean C., Liu H., Dunning F. M., Chang P. Y., Jackson M. B., Chapman E. R. (2009). Synaptotagmin-IV modulates synaptic function and long-term potentiation by regulating BDNF release. Nat. Neurosci. 12, 767–776. 10.1038/nn.2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Muñoz A., Fletcher D. A., Weiner O. D. (2013). Use the force: membrane tension as an organizer of cell shape and motility. Trends Cell Biol. 23, 47–53. 10.1016/j.tcb.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engisch K. L., Nowycky M. C. (1998). Compensatory and excess retrieval: two types of endocytosis following single step depolarizations in bovine adrenal chromaffin cells. J. Physiol. 506, 591–608. 10.1111/j.1469-7793.1998.591bv.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F., Funk L., Lou X. (2016). Dynamin 1- and 3-mediated endocytosis is essential for the development of a large central synapse in vivo. J. Neurosci. 36, 6097–6115. 10.1523/JNEUROSCI.3804-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S. M., Brasnjo G., Hayashi M., Wölfel M., Collesi C., Giovedi S., et al. (2007). A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science 316, 570–574. 10.1126/science.1140621 [DOI] [PubMed] [Google Scholar]
- Gad H., Löw P., Zotova E., Brodin L., Shupliakov O. (1998). Dissociation between Ca2+-triggered synaptic vesicle exocytosis and clathrin-mediated endocytosis at a central synapse. Neuron 21, 607–616. 10.1016/s0896-6273(00)80570-x [DOI] [PubMed] [Google Scholar]
- Graham M. E., O’Callaghan D. W., McMahon H. T., Burgoyne R. D. (2002). Dynamin-dependent and dynamin-independent processes contribute to the regulation of single vesicle release kinetics and quantal size. Proc. Natl. Acad. Sci. U S A 99, 7124–7129. 10.1073/pnas.102645099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granseth B., Odermatt B., Royle S. J., Lagnado L. (2006). Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron 51, 773–786. 10.1016/j.neuron.2006.08.029 [DOI] [PubMed] [Google Scholar]
- Gundelfinger E. D., Kessels M. M., Qualmann B. (2003). Temporal and spatial coordination of exocytosis and endocytosis. Nat. Rev. Mol. Cell Biol. 4, 127–139. 10.1038/nrm1016 [DOI] [PubMed] [Google Scholar]
- Gurunathan S., Marash M., Weinberger A., Gerst J. E. (2002). t-SNARE phosphorylation regulates endocytosis in yeast. Mol. Biol. Cell 13, 1594–1607. 10.1091/mbc.01-11-0541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson N., Han W. (2009). Calcium-sensing beyond neurotransmitters: functions of synaptotagmins in neuroendocrine and endocrine secretion. Biosci. Rep. 29, 245–259. 10.1042/BSR20090031 [DOI] [PubMed] [Google Scholar]
- Hassinger J. E., Oster G., Drubin D. G., Rangamani P. (2017). Design principles for robust vesiculation in clathrin-mediated endocytosis. Proc. Natl. Acad. Sci. U S A [Epub ahead of print]. 10.1073/pnas.1617705114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke V., Neher E., Sigrist S. J. (2011). Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat. Rev. Neurosci. 12, 127–138. 10.1038/nrn2948 [DOI] [PubMed] [Google Scholar]
- Haucke V., Wenk M. R., Chapman E. R., Farsad K., De Camilli P. (2000). Dual interaction of synaptotagmin with mu2- and α-adaptin facilitates clathrin-coated pit nucleation. EMBO J. 19, 6011–6019. 10.1093/emboj/19.22.6011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Raimondi A., O’Toole E., Paradise S., Collesi C., Cremona O., et al. (2008). Cell- and stimulus-dependent heterogeneity of synaptic vesicle endocytic recycling mechanisms revealed by studies of dynamin 1-null neurons. Proc. Natl. Acad. Sci. U S A 105, 2175–2180. 10.1073/pnas.0712171105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Wu L. G. (2007). The debate on the kiss-and-run fusion at synapses. Trends Neurosci. 30, 447–455. 10.1016/j.tins.2007.06.012 [DOI] [PubMed] [Google Scholar]
- Hosoi N., Holt M., Sakaba T. (2009). Calcium dependence of exo- and endocytotic coupling at a glutamatergic synapse. Neuron 63, 216–229. 10.1016/j.neuron.2009.06.010 [DOI] [PubMed] [Google Scholar]
- Hua Y., Sinha R., Thiel C. S., Schmidt R., Hüve J., Martens H., et al. (2011). A readily retrievable pool of synaptic vesicles. Nat. Neurosci. 14, 833–839. 10.1038/nn.2838 [DOI] [PubMed] [Google Scholar]
- Jahn R., Fasshauer D. (2012). Molecular machines governing exocytosis of synaptic vesicles. Nature 490, 201–207. 10.1038/nature11320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz R., Südhof T. C., Hammer R. E., Unni V., Siegelbaum S. A., Bolshakov V. Y. (1999). Essential roles in synaptic plasticity for synaptogyrin I and synaptophysin I. Neuron 24, 687–700. 10.1016/s0896-6273(00)81122-8 [DOI] [PubMed] [Google Scholar]
- Jarousse N., Kelly R. B. (2001). The AP2 binding site of synaptotagmin 1 is not an internalization signal but a regulator of endocytosis. J. Cell Biol. 154, 857–866. 10.1083/jcb.200103040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung N., Haucke V. (2007). Clathrin-mediated endocytosis at synapses. Traffic 8, 1129–1136. 10.1111/j.1600-0854.2007.00595.x [DOI] [PubMed] [Google Scholar]
- Kaempf N., Kochlamazashvili G., Puchkov D., Maritzen T., Bajjalieh S. M., Kononenko N. L., et al. (2015). Overlapping functions of stonin 2 and SV2 in sorting of the calcium sensor synaptotagmin 1 to synaptic vesicles. Proc. Natl. Acad. Sci. U S A 112, 7297–7302. 10.1073/pnas.1501627112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko N. L., Haucke V. (2015). Molecular mechanisms of presynaptic membrane retrieval and synaptic vesicle reformation. Neuron 85, 484–496. 10.1016/j.neuron.2014.12.016 [DOI] [PubMed] [Google Scholar]
- Kwon S. E., Chapman E. R. (2011). Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron 70, 847–854. 10.1016/j.neuron.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Voronov S., Letinic K., Nairn A. C., Di Paolo G., De Camilli P. (2005). Regulation of the interaction between PIPKI γ and talin by proline-directed protein kinases. J. Cell Biol. 168, 789–799. 10.1083/jcb.200409028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Wenk M. R., Kim Y., Nairn A. C., De Camilli P. (2004). Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc. Natl. Acad. Sci. U S A 101, 546–551. 10.1073/pnas.0307813100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitz J., Kavalali E. T. (2011). Ca2+ influx slows single synaptic vesicle endocytosis. J. Neurosci. 31, 16318–16326. 10.1523/JNEUROSCI.3358-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitz J., Kavalali E. T. (2016). Ca2+ dependence of synaptic vesicle endocytosis. Neuroscientist 22, 464–476. 10.1177/1073858415588265 [DOI] [PubMed] [Google Scholar]
- Li C., Ullrich B., Zhang J. Z., Anderson R. G., Brose N., Südhof T. C. (1995). Ca2+-dependent and -independent activities of neural and non-neural synaptotagmins. Nature 375, 594–599. 10.1038/375594a0 [DOI] [PubMed] [Google Scholar]
- Li L., Wu X., Yue H. Y., Zhu Y. C., Xu J. (2016). Myosin light chain kinase facilitates endocytosis of synaptic vesicles at hippocampal boutons. J. Neurochem. 138, 60–73. 10.1111/jnc.13635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Bai H., Hui E., Yang L., Evans C. S., Wang Z., et al. (2014). Synaptotagmin 7 functions as a Ca2+-sensor for synaptic vesicle replenishment. Elife 3:e01524. 10.7554/eLife.01524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. P., Sim A. T., Robinson P. J. (1994). Calcineurin inhibition of dynamin I GTPase activity coupled to nerve terminal depolarization. Science 265, 970–973. 10.1126/science.8052858 [DOI] [PubMed] [Google Scholar]
- Lou X., Fan F., Messa M., Raimondi A., Wu Y., Looger L. L., et al. (2012). Reduced release probability prevents vesicle depletion and transmission failure at dynamin mutant synapses. Proc. Natl. Acad. Sci. U S A 109, E515–E523. 10.1073/pnas.1121626109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou X., Paradise S., Ferguson S. M., De Camilli P. (2008). Selective saturation of slow endocytosis at a giant glutamatergic central synapse lacking dynamin 1. Proc. Natl. Acad. Sci. U S A 105, 17555–17560. 10.1073/pnas.0809621105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra S., Fan F., Lou X. (2016). Tissue-specific dynamin-1 deletion at the calyx of Held decreases short-term depression through a mechanism distinct from vesicle resupply. Proc. Natl. Acad. Sci. U S A 113, E3150–E3158. 10.1073/pnas.1520937113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks B., Stowell M. H., Vallis Y., Mills I. G., Gibson A., Hopkins C. R., et al. (2001). GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature 410, 231–235. 10.1038/35065645 [DOI] [PubMed] [Google Scholar]
- Maximov A., Lao Y., Li H., Chen X., Rizo J., Sørensen J. B., et al. (2008). Genetic analysis of synaptotagmin-7 function in synaptic vesicle exocytosis. Proc. Natl. Acad. Sci. U S A 105, 3986–3991. 10.1073/pnas.0712372105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon H. T., Boucrot E. (2011). Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12, 517–533. 10.1038/nrm3151 [DOI] [PubMed] [Google Scholar]
- McMahon H. T., Gallop J. L. (2005). Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 438, 590–596. 10.1038/nature04396 [DOI] [PubMed] [Google Scholar]
- Myers M. D., Ryazantsev S., Hicke L., Payne G. S. (2016). Calmodulin promotes N-BAR domain-mediated membrane constriction and endocytosis. Dev. Cell 37, 162–173. 10.1016/j.devcel.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuland K., Sharma N., Frick M. (2014). Synaptotagmin-7 links fusion-activated Ca2+ entry and fusion pore dilation. J. Cell Sci. 127, 5218–5227. 10.1242/jcs.153742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Fish J. C., Kokotos A. C., Gillingwater T. H., Smillie K. J., Cousin M. A. (2015). VAMP4 is an essential cargo molecule for activity-dependent bulk endocytosis. Neuron 88, 973–984. 10.1016/j.neuron.2015.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Tomishima K., Ryan T. A. (2004). Kinetic efficiency of endocytosis at mammalian CNS synapses requires synaptotagmin I. Proc. Natl. Acad. Sci. U S A 101, 16648–16652. 10.1073/pnas.0406968101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z. P., Südhof T. C. (2010). Cell biology of Ca2+-triggered exocytosis. Curr. Opin. Cell Biol. 22, 496–505. 10.1016/j.ceb.2010.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer K. E., Fetter R. D., Davis G. W. (2006). Discrete residues in the C2B domain of synaptotagmin I independently specify endocytic rate and synaptic vesicle size. Neuron 50, 49–62. 10.1016/j.neuron.2006.02.021 [DOI] [PubMed] [Google Scholar]
- Poskanzer K. E., Marek K. W., Sweeney S. T., Davis G. W. (2003). Synaptotagmin I is necessary for compensatory synaptic vesicle endocytosis in vivo. Nature 426, 559–563. 10.1038/nature02184 [DOI] [PubMed] [Google Scholar]
- Puchkov D., Haucke V. (2013). Greasing the synaptic vesicle cycle by membrane lipids. Trends Cell Biol. 23, 493–503. 10.1016/j.tcb.2013.05.002 [DOI] [PubMed] [Google Scholar]
- Raimondi A., Ferguson S. M., Lou X., Armbruster M., Paradise S., Giovedi S., et al. (2011). Overlapping role of dynamin isoforms in synaptic vesicle endocytosis. Neuron 70, 1100–1114. 10.1016/j.neuron.2011.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J., Xu J. (2015). The synaptic vesicle release machinery. Annu. Rev. Biophys 44, 339–367. 10.1146/annurev-biophys-060414-034057 [DOI] [PubMed] [Google Scholar]
- Rizzoli S. O., Jahn R. (2007). Kiss-and-run, collapse and ‘readily retrievable’ vesicles. Traffic 8, 1137–1144. 10.1111/j.1600-0854.2007.00614.x [DOI] [PubMed] [Google Scholar]
- Ryan T. A., Reuter H., Wendland B., Schweizer F. E., Tsien R. W., Smith S. J. (1993). The kinetics of synaptic vesicle recycling measured at single presynaptic boutons. Neuron 11, 713–724. 10.1016/0896-6273(93)90081-2 [DOI] [PubMed] [Google Scholar]
- Saheki Y., De Camilli P. (2012). Synaptic vesicle endocytosis. Cold Spring Harb. Perspect. Biol. 4:a005645. 10.1101/cshperspect.a005645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samasilp P., Chan S. A., Smith C. (2012). Activity-dependent fusion pore expansion regulated by a calcineurin-dependent dynamin-syndapin pathway in mouse adrenal chromaffin cells. J. Neurosci. 32, 10438–10447. 10.1523/JNEUROSCI.1299-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonn J. S., Maximov A., Lao Y., Südhof T. C., Sørensen J. B. (2008). Synaptotagmin-1 and -7 are functionally overlapping Ca2+ sensors for exocytosis in adrenal chromaffin cells. Proc. Natl. Acad. Sci. U S A 105, 3998–4003. 10.1073/pnas.0712373105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia M., Alés E., Montes M. A., Bonifas I., Jemal I., Lindau M., et al. (2010). Push-and-pull regulation of the fusion pore by synaptotagmin-7. Proc. Natl. Acad. Sci. U S A 107, 19032–19037. 10.1073/pnas.1014070107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepnev V. I., Ochoa G. C., Butler M. H., Grabs D., De Camilli P. (1998). Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science 281, 821–824. 10.1126/science.281.5378.821 [DOI] [PubMed] [Google Scholar]
- Smith S. M., Renden R., von Gersdorff H. (2008). Synaptic vesicle endocytosis: fast and slow modes of membrane retrieval. Trends Neurosci. 31, 559–568. 10.1016/j.tins.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof T. C. (2004). The synaptic vesicle cycle. Annu. Rev. Neurosci. 27, 509–547. 10.1146/annurev.neuro.26.041002.131412 [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Rothman J. E. (2009). Membrane fusion: grappling with SNARE and SM proteins. Science 323, 474–477. 10.1126/science.1161748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. Y., Wu X. S., Wu L. G. (2002). Single and multiple vesicle fusion induce different rates of endocytosis at a central synapse. Nature 417, 555–559. 10.1038/417555a [DOI] [PubMed] [Google Scholar]
- Sun T., Wu X. S., Xu J., McNeil B. D., Pang Z. P., Yang W., et al. (2010). The role of calcium/calmodulin-activated calcineurin in rapid and slow endocytosis at central synapses. J. Neurosci. 30, 11838–11847. 10.1523/JNEUROSCI.1481-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S., Holt M., Stenius K., Lemke E. A., Grønborg M., Riedel D., et al. (2006). Molecular anatomy of a trafficking organelle. Cell 127, 831–846. 10.1016/j.cell.2006.10.030 [DOI] [PubMed] [Google Scholar]
- Tarsa L., Goda Y. (2002). Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc. Natl. Acad. Sci. U S A 99, 1012–1016. 10.1073/pnas.022575999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virmani T., Han W., Liu X., Südhof T. C., Kavalali E. T. (2003). Synaptotagmin 7 splice variants differentially regulate synaptic vesicle recycling. EMBO J. 22, 5347–5357. 10.1093/emboj/cdg514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H., Matthews G. (1994). Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature 370, 652–655. 10.1038/370652a0 [DOI] [PubMed] [Google Scholar]
- von Poser C., Ichtchenko K., Shao X., Rizo J., Südhof T. C. (1997). The evolutionary pressure to inactivate. A subclass of synaptotagmins with an amino acid substitution that abolishes Ca2+ binding. J. Biol. Chem. 272, 14314–14319. 10.1074/jbc.272.22.14314 [DOI] [PubMed] [Google Scholar]
- von Poser C., Zhang J. Z., Mineo C., Ding W., Ying Y., Sudhof T. C., et al. (2000). Synaptotagmin regulation of coated pit assembly. J. Biol. Chem. 275, 30916–30924. 10.1074/jbc.M005559200 [DOI] [PubMed] [Google Scholar]
- Walther K., Krauss M., Diril M. K., Lemke S., Ricotta D., Honing S., et al. (2001). Human stoned B interacts with AP-2 and synaptotagmin and facilitates clathrin-coated vesicle uncoating. EMBO Rep. 2, 634–640. 10.1093/embo-reports/kve134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. T., Grishanin R., Earles C. A., Chang P. Y., Martin T. F., Chapman E. R., et al. (2001). Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science 294, 1111–1115. 10.1126/science.1064002 [DOI] [PubMed] [Google Scholar]
- Wang C. T., Lu J. C., Bai J., Chang P. Y., Martin T. F., Chapman E. R., et al. (2003). Different domains of synaptotagmin control the choice between kiss-and-run and full fusion. Nature 424, 943–947. 10.1038/nature01857 [DOI] [PubMed] [Google Scholar]
- Wang C., Wang Y., Hu M., Chai Z., Wu Q., Huang R., et al. (2016). Synaptotagmin-11 inhibits clathrin-mediated and bulk endocytosis. EMBO Rep. 17, 47–63. 10.15252/embr.201540689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Rost B. R., Camacho-Perez M., Davis M. W., Sohl-Kielczynski B., Rosenmund C., et al. (2013). Ultrafast endocytosis at mouse hippocampal synapses. Nature 504, 242–247. 10.1038/nature12809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk M. R., De Camilli P. (2004). Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc. Natl. Acad. Sci. U S A 101, 8262–8269. 10.1073/pnas.0401874101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. G., Hamid E., Shin W., Chiang H. C. (2014). Exocytosis and endocytosis: modes, functions and coupling mechanisms. Annu. Rev. Physiol. 76, 301–331. 10.1146/annurev-physiol-021113-170305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. S., McNeil B. D., Xu J., Fan J., Xue L., Melicoff E., et al. (2009). Ca2+ and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal. Nat. Neurosci. 12, 1003–1010. 10.1038/nn.2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Wei S., Petersen N., Ali Y., Wang X., Bacaj T., et al. (2015). Synaptotagmin-7 phosphorylation mediates GLP-1-dependent potentiation of insulin secretion from β-cells. Proc. Natl. Acad. Sci. U S A 112, 9996–10001. 10.1073/pnas.1513004112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Wu L. G. (2007). Rapid bulk endocytosis and its kinetics of fission pore closure at a central synapse. Proc. Natl. Acad. Sci. U S A 104, 10234–10239. 10.1073/pnas.0611512104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. S., Wu L. G. (2014). The yin and yang of calcium effects on synaptic vesicle endocytosis. J. Neurosci. 34, 2652–2659. 10.1523/JNEUROSCI.3582-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Xu J., Wu X. S., Wu L. G. (2005). Activity-dependent acceleration of endocytosis at a central synapse. J. Neurosci. 25, 11676–11683. 10.1523/JNEUROSCI.2972-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. S., Zhang Z., Zhao W. D., Wang D., Luo F., Wu L. G. (2014). Calcineurin is universally involved in vesicle endocytosis at neuronal and nonneuronal secretory cells. Cell Rep. 7, 982–988. 10.1016/j.celrep.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Luo F., Zhang Z., Xue L., Wu X. S., Chiang H. C., et al. (2013). SNARE proteins synaptobrevin, SNAP-25 and syntaxin are involved in rapid and slow endocytosis at synapses. Cell Rep. 3, 1414–1421. 10.1016/j.celrep.2013.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Eguchi K., Saitoh N., von Gersdorff H., Takahashi T. (2010). Developmental shift to a mechanism of synaptic vesicle endocytosis requiring nanodomain Ca2+. Nat. Neurosci. 13, 838–844. 10.1038/nn.2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Hige T., Takahashi T. (2005). Vesicle endocytosis requires dynamin-dependent GTP hydrolysis at a fast CNS synapse. Science 307, 124–127. 10.1126/science.1103631 [DOI] [PubMed] [Google Scholar]
- Yao J., Kwon S. E., Gaffaney J. D., Dunning F. M., Chapman E. R. (2011). Uncoupling the roles of synaptotagmin I during endo- and exocytosis of synaptic vesicles. Nat. Neurosci. 15, 243–249. 10.1038/nn.3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L. H., Rao Y., Varga K., Wang C. Y., Xiao P., Lindau M., et al. (2012). Synaptotagmin 1 is necessary for the Ca2+ dependence of clathrin-mediated endocytosis. J. Neurosci. 32, 3778–3785. 10.1523/JNEUROSCI.3540-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue H. Y., Xu J. (2014). Myosin light chain kinase accelerates vesicle endocytosis at the calyx of Held synapse. J. Neurosci. 34, 295–304. 10.1523/JNEUROSCI.3744-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Bhalla A., Dean C., Chapman E. R., Jackson M. B. (2009). Synaptotagmin IV: a multifunctional regulator of peptidergic nerve terminals. Nat. Neurosci. 12, 163–171. 10.1038/nn.2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Z., Davletov B. A., Südhof T. C., Anderson R. G. (1994). Synaptotagmin I is a high affinity receptor for clathrin AP-2: implications for membrane recycling. Cell 78, 751–760. 10.1016/s0092-8674(94)90442-1 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wang D., Sun T., Xu J., Chiang H. C., Shin W., et al. (2013). The SNARE proteins SNAP25 and synaptobrevin are involved in endocytosis at hippocampal synapses. J. Neurosci. 33, 9169–9175. 10.1523/JNEUROSCI.0301-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Xiong W., Zheng H., Wang L., Lu B., Zhou Z. (2004). Calcium- and dynamin-independent endocytosis in dorsal root ganglion neurons. Neuron 42, 225–236. 10.1016/s0896-6273(04)00189-8 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Zhang Z., Jackson M. B. (2010). Synaptotagmin IV modulation of vesicle size and fusion pores in PC12 cells. Biophys. J. 98, 968–978. 10.1016/j.bpj.2009.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]