Abstract
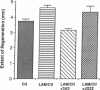
A regeneration chamber was created in vivo by suturing a synthetic tube sealed at its distal end onto the proximal stump of a severed rat sciatic nerve. Nerves regenerated into tubes coated with laminin at a rate of 0.33 mm/day after a lag of about 2 days. At 25 days, regenerating nerves had extended 23% farther into laminin-coated tubes as compared with uncoated ones. Monoclonal antibody 3A3, which functionally interferes with a dual laminin/collagen receptor, inhibited nerve regeneration into laminin-coated tubes by 32%. In contrast, monoclonal antibody JG22, which inhibits chicken matrix receptors, had no significant effect on regeneration. Immunohistochemical studies of teased adult rat sciatic nerves indicate that 3A3 bound to Schwann cells and possibly to axons. In other studies, the heterodimeric, laminin/collagen receptor recognized by 3A3 has been shown to be a member of the integrin superfamily of adhesive receptors. These data provide evidence that an integrin receptor functions in nerve regeneration in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebischer P., Guénard V., Winn S. R., Valentini R. F., Galletti P. M. Blind-ended semipermeable guidance channels support peripheral nerve regeneration in the absence of a distal nerve stump. Brain Res. 1988 Jun 28;454(1-2):179–187. doi: 10.1016/0006-8993(88)90817-7. [DOI] [PubMed] [Google Scholar]
- Akers R. M., Mosher D. F., Lilien J. E. Promotion of retinal neurite outgrowth by substratum-bound fibronectin. Dev Biol. 1981 Aug;86(1):179–188. doi: 10.1016/0012-1606(81)90328-6. [DOI] [PubMed] [Google Scholar]
- Akiyama S. K., Yamada S. S., Yamada K. M. Characterization of a 140-kD avian cell surface antigen as a fibronectin-binding molecule. J Cell Biol. 1986 Feb;102(2):442–448. doi: 10.1083/jcb.102.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman P. G., Mirsky R., Jessen K. R., Timpl R., Duance V. C. Light microscopic immunolocalization of laminin, type IV collagen, nidogen, heparan sulphate proteoglycan and fibronectin in the enteric nervous system of rat and guinea pig. J Neurocytol. 1986 Dec;15(6):733–743. doi: 10.1007/BF01625191. [DOI] [PubMed] [Google Scholar]
- Baron-Van Evercooren A., Kleinman H. K., Ohno S., Marangos P., Schwartz J. P., Dubois-Dalcq M. E. Nerve growth factor, laminin, and fibronectin promote neurite growth in human fetal sensory ganglia cultures. J Neurosci Res. 1982;8(2-3):179–193. doi: 10.1002/jnr.490080208. [DOI] [PubMed] [Google Scholar]
- Berry M., Rees L., Hall S., Yiu P., Sievers J. Optic axons regenerate into sciatic nerve isografts only in the presence of Schwann cells. Brain Res Bull. 1988 Feb;20(2):223–231. doi: 10.1016/0361-9230(88)90182-7. [DOI] [PubMed] [Google Scholar]
- Bozyczko D., Horwitz A. F. The participation of a putative cell surface receptor for laminin and fibronectin in peripheral neurite extension. J Neurosci. 1986 May;6(5):1241–1251. doi: 10.1523/JNEUROSCI.06-05-01241.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Carbonetto S., Gruver M. M., Turner D. C. Nerve fiber growth in culture on fibronectin, collagen, and glycosaminoglycan substrates. J Neurosci. 1983 Nov;3(11):2324–2335. doi: 10.1523/JNEUROSCI.03-11-02324.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonetto S., Stach R. W. Localization of nerve growth factor bound to neurons growing nerve fibers in culture. Brain Res. 1982 Mar;255(3):463–473. doi: 10.1016/0165-3806(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Douville P. J., Harvey W. J., Carbonetto S. Isolation and partial characterization of high affinity laminin receptors in neural cells. J Biol Chem. 1988 Oct 15;263(29):14964–14969. [PubMed] [Google Scholar]
- Edgar D., Timpl R., Thoenen H. The heparin-binding domain of laminin is responsible for its effects on neurite outgrowth and neuronal survival. EMBO J. 1984 Jul;3(7):1463–1468. doi: 10.1002/j.1460-2075.1984.tb01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlsen K. R., Dillner L., Engvall E., Ruoslahti E. The human laminin receptor is a member of the integrin family of cell adhesion receptors. Science. 1988 Sep 2;241(4870):1228–1229. doi: 10.1126/science.2970671. [DOI] [PubMed] [Google Scholar]
- Graf J., Iwamoto Y., Sasaki M., Martin G. R., Kleinman H. K., Robey F. A., Yamada Y. Identification of an amino acid sequence in laminin mediating cell attachment, chemotaxis, and receptor binding. Cell. 1987 Mar 27;48(6):989–996. doi: 10.1016/0092-8674(87)90707-0. [DOI] [PubMed] [Google Scholar]
- Greve J. M., Gottlieb D. I. Monoclonal antibodies which alter the morphology of cultured chick myogenic cells. J Cell Biochem. 1982;18(2):221–229. doi: 10.1002/jcb.1982.240180209. [DOI] [PubMed] [Google Scholar]
- Hall S. M. Regeneration in cellular and acellular autografts in the peripheral nervous system. Neuropathol Appl Neurobiol. 1986 Jan-Feb;12(1):27–46. doi: 10.1111/j.1365-2990.1986.tb00679.x. [DOI] [PubMed] [Google Scholar]
- Hall S. M. The effect of inhibiting Schwann cell mitosis on the re-innervation of acellular autografts in the peripheral nervous system of the mouse. Neuropathol Appl Neurobiol. 1986 Jul-Aug;12(4):401–414. doi: 10.1111/j.1365-2990.1986.tb00151.x. [DOI] [PubMed] [Google Scholar]
- Hammarback J. A., Palm S. L., Furcht L. T., Letourneau P. C. Guidance of neurite outgrowth by pathways of substratum-adsorbed laminin. J Neurosci Res. 1985;13(1-2):213–220. doi: 10.1002/jnr.490130115. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Ide C., Tohyama K., Yokota R., Nitatori T., Onodera S. Schwann cell basal lamina and nerve regeneration. Brain Res. 1983 Dec 12;288(1-2):61–75. doi: 10.1016/0006-8993(83)90081-1. [DOI] [PubMed] [Google Scholar]
- Ignatius M. J., Reichardt L. F. Identification of a neuronal laminin receptor: an Mr 200K/120K integrin heterodimer that binds laminin in a divalent cation-dependent manner. Neuron. 1988 Oct;1(8):713–725. doi: 10.1016/0896-6273(88)90170-5. [DOI] [PubMed] [Google Scholar]
- Junqueira L. C., Montes G. S., Krisztán R. M. The collagen of the vertebrate peripheral nervous system. Cell Tissue Res. 1979 Nov;202(3):453–460. doi: 10.1007/BF00220437. [DOI] [PubMed] [Google Scholar]
- Keynes R. J., Hopkins W. G., Huang L. H. Regeneration of mouse peripheral nerves in degenerating skeletal muscle: guidance by residual muscle fibre basement membrane. Brain Res. 1984 Mar 19;295(2):275–281. doi: 10.1016/0006-8993(84)90976-4. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Ogle R. C., Cannon F. B., Little C. D., Sweeney T. M., Luckenbill-Edds L. Laminin receptors for neurite formation. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1282–1286. doi: 10.1073/pnas.85.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitman N., Wood P., Johnson M. I., Bunge R. P. Schwann cell surfaces but not extracellular matrix organized by Schwann cells support neurite outgrowth from embryonic rat retina. J Neurosci. 1988 Feb;8(2):653–663. doi: 10.1523/JNEUROSCI.08-02-00653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander A. D., Fujii D. K., Gospodarowicz D., Reichardt L. F. Characterization of a factor that promotes neurite outgrowth: evidence linking activity to a heparan sulfate proteoglycan. J Cell Biol. 1982 Sep;94(3):574–585. doi: 10.1083/jcb.94.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundborg G., Dahlin L. B., Danielsen N., Gelberman R. H., Longo F. M., Powell H. C., Varon S. Nerve regeneration in silicone chambers: influence of gap length and of distal stump components. Exp Neurol. 1982 May;76(2):361–375. doi: 10.1016/0014-4886(82)90215-1. [DOI] [PubMed] [Google Scholar]
- Madison R. D., da Silva C., Dikkes P., Sidman R. L., Chiu T. H. Peripheral nerve regeneration with entubulation repair: comparison of biodegradeable nerve guides versus polyethylene tubes and the effects of a laminin-containing gel. Exp Neurol. 1987 Feb;95(2):378–390. doi: 10.1016/0014-4886(87)90146-4. [DOI] [PubMed] [Google Scholar]
- Manthorpe M., Engvall E., Ruoslahti E., Longo F. M., Davis G. E., Varon S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J Cell Biol. 1983 Dec;97(6):1882–1890. doi: 10.1083/jcb.97.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm S. L., Furcht L. T. Production of laminin and fibronectin by Schwannoma cells: cell-protein interactions in vitro and protein localization in peripheral nerve in vivo. J Cell Biol. 1983 May;96(5):1218–1226. doi: 10.1083/jcb.96.5.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischel K. D., Bluestein H. G., Woods V. L., Jr Very late activation antigens (VLA) are human leukocyte-neuronal crossreactive cell surface antigens. J Exp Med. 1986 Aug 1;164(2):393–406. doi: 10.1084/jem.164.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986 Feb 28;44(4):517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Sandrock A. W., Jr, Matthew W. D. An in vitro neurite-promoting antigen functions in axonal regeneration in vivo. Science. 1987 Sep 25;237(4822):1605–1608. doi: 10.1126/science.3306923. [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Thoenen H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J Neurosci. 1985 Sep;5(9):2415–2423. doi: 10.1523/JNEUROSCI.05-09-02415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellswell G. B., Restall D. J., Duance V. C., Bailey A. J. Identification and differential distribution of collagen types in the central and peripheral nervous systems. FEBS Lett. 1979 Oct 15;106(2):305–308. doi: 10.1016/0014-5793(79)80520-7. [DOI] [PubMed] [Google Scholar]
- Smalheiser N. R., Schwartz N. B. Cranin: a laminin-binding protein of cell membranes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6457–6461. doi: 10.1073/pnas.84.18.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., Modderman P. W., Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988 Dec 1;336(6198):487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Risteli L., Ott U., Robey P. G., Martin G. R. Laminin. Methods Enzymol. 1982;82(Pt A):831–838. doi: 10.1016/0076-6879(82)82104-6. [DOI] [PubMed] [Google Scholar]
- Tohyama K., Ide C. The localization of laminin and fibronectin on the Schwann cell basal lamina. Arch Histol Jpn. 1984 Nov;47(5):519–532. doi: 10.1679/aohc.47.519. [DOI] [PubMed] [Google Scholar]
- Tomaselli K. J., Neugebauer K. M., Bixby J. L., Lilien J., Reichardt L. F. N-cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces. Neuron. 1988 Mar;1(1):33–43. doi: 10.1016/0896-6273(88)90207-3. [DOI] [PubMed] [Google Scholar]
- Tomaselli K. J., Reichardt L. F., Bixby J. L. Distinct molecular interactions mediate neuronal process outgrowth on non-neuronal cell surfaces and extracellular matrices. J Cell Biol. 1986 Dec;103(6 Pt 2):2659–2672. doi: 10.1083/jcb.103.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. C., Flier L. A., Carbonetto S. Identification of a cell-surface protein involved in PC12 cell-substratum adhesion and neurite outgrowth on laminin and collagen. J Neurosci. 1989 Sep;9(9):3287–3296. doi: 10.1523/JNEUROSCI.09-09-03287.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. C., Flier L. A. Receptor-mediated active adhesion to the substratum is required for neurite outgrowth. Dev Neurosci. 1989;11(4-5):300–312. doi: 10.1159/000111908. [DOI] [PubMed] [Google Scholar]
- Williams L. R., Longo F. M., Powell H. C., Lundborg G., Varon S. Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: parameters for a bioassay. J Comp Neurol. 1983 Aug 20;218(4):460–470. doi: 10.1002/cne.902180409. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Anderton B. H. Monoclonal antibodies to mammalian neurofilaments. Biosci Rep. 1981 Mar;1(3):263–268. doi: 10.1007/BF01114913. [DOI] [PubMed] [Google Scholar]