ABSTRACT
This study evaluated the prognostic significance of the maximum standardized uptake value of the primary site (pSUVmax) in 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) scans of patients with oropharyngeal or hypopharyngeal cancer who were treated using definitive radiotherapy. The study included 86 patients who were primarily treated with radiotherapy for oropharyngeal or hypopharyngeal cancer. Sixty-nine patients underwent concurrent chemotherapy. The associations between pre-treatment pSUVmax and treatment outcomes were evaluated. The most appropriate pSUVmax cut-off value for predicting disease-free survival (DFS) and local control (LC) was selected using receiver operating characteristic (ROC) curves. The median follow-up time for surviving patients was 60 months, while the median survival time in the entire patient cohort was 55 months. A pSUVmax cut-off value of 9.0 showed the best discriminative performance. Five-year OS and DFS rates were 65.9% and 60.0%, respectively. In univariate analyses, pSUVmax (p = 0.009), T-stage (p = 0.001), N-stage (p = 0.039), and clinical stage (p = 0.017) were identified as significant prognostic predictors for DFS. The multivariate analysis did not identify any statistically significant factors, but the association between pSUVmax and DFS was borderline significant (p = 0.055). Interestingly, pSUVmax was predictive of local controllability in T1–T2 disease (p = 0.024), but there was no significant association for T3–T4 disease (p = 0.735). In this study, pSUVmax was predictive of DFS and LC in patients with oropharyngeal or hypopharyngeal cancer that was treated with definitive radiotherapy. pSUVmax was strongly associated with LC in T1–T2 disease.
Key Words: pharyngeal cancer, prognostic predictor, definitive radiotherapy, 18F-FDG-PET, standardized uptake value (SUV)
INTRODUCTION
Oropharyngeal and hypopharyngeal cancer are regarded as comparatively rare disease.1) The National Comprehensive Cancer Network (NCCN) guidelines note some differences for each disease,2) but treatment selection is broadly similar for these cancers: For T1–T2 N0 disease, definitive radiation therapy alone (or with additional chemotherapy in some cases) or an operation is selected. For T3–T4 or N+ disease, definitive radiotherapy with chemotherapy or an operation is selected. The reported prognostic predictors for oropharyngeal cancer include age, race, stage, history of cigarette smoking, status of lymph node metastases, and human papilloma virus (HPV) status.3) The reported prognostic predictors for hypopharyngeal cancer include age, stage, and performance status (PS).1, 4, 5)
Some reports have mentioned that the maximum standardized uptake value (SUVmax) in 18F-fluorodeoxygulucose positron emission tomography (FDG-PET) may be a prognostic predictor in patients with head and neck cancer.6-9) Xie et al. examined previous reports in a meta-analysis, concluding that pSUVmax is a significant prognostic predictor for overall survival (OS), disease-free survival (DFS), and local control (LC) in patients with head and neck cancer.10) However, most studies have not focused on individual types of head and neck cancer,6-10) and very few reports have focused on pharyngeal cancer.11-14) Moreover, although some reports noted that pSUVmax was a prognostic predictor for LC,6, 9, 15) no report has analyzed the association between pSUVmax and LC as stratified by T-stage. As radiation oncologists, we would like to know whether the recurrence rate of primary tumors differs according to the pSUVmax, even between cases of the same T-stage. If the recurrence rate of tumors with higher pSUVmax is greater than that of tumors with lower pSUVmax, it may be necessary to increase the intensity of treatment, for example by administering a dose escalation or providing radiotherapy or surgery in combination with chemotherapy.
This study had 2 central aims. First, we sought to determine whether pSUVmax is a prognostic predictor in patients with oropharyngeal or hypopharyngeal cancer that was treated with definitive radiotherapy and followed-up for 5 years, as mentioned in previous reports.6-15) Second, we sought to determine whether the association between pSUVmax and LC differed according to T-stage.
MATERIALS AND METHODS
Patients
This retrospective study was based on a review of the medical records of 136 patients who had oropharyngeal or hypopharyngeal cancer and received definitive radiotherapy at our institution between May 2006 and October 2011. All patients underwent a FDG-PET study before initial radiotherapy. The following patients were excluded from our analysis of the efficacy of FDG-PET examination: (1) 41 patients who were treated with a radical operation prior to radiotherapy, (2) 3 patients who had distant metastases, (3) 2 patients who had double cancers of the oropharynx and hypopharynx, and (4) 4 patients who were lost follow-up. Thus, a total of 86 patients were included in our analysis. Of these patients, 17 (20%) received radiotherapy alone and 69 (80%) received a combination of radiotherapy and chemotherapy.
As performed along with a routine physical examination, the pretreatment systematic evaluations included nasopharyngolaryngoscopy, tissue biopsy, gastrointestinal endoscopy, serum chemistry, chest radiography, contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI) of the head and neck, and the FDG-PET scan. Clinical staging and treatment choices were determined at our institution’s Head and Neck Cancer Board conference, using the information that had been derived from these examinations. This conference involved head and neck surgeons, radiation oncologists, medical oncologists, and radiologists.
Routine follow-up evaluations were performed every 1 month for the first year, every 3 months for next 2 years, and every 6 months thereafter. The follow-up included a routine physical examination and nasopharyngolaryngoscopy. Moreover, the assessment included repeated CT or MRI every 3–6 months, and PET or PET-CT every 6–12 months. Patient and tumor characteristics are summarized in Table 1. All patients provided prior written informed consent to undergo FDG-PET imaging and receive treatments. Our institution’s review board approved this retrospective study (No. 1401). The study and all procedures were performed in accordance with the Helsinki Declaration of 1975, as revised in 1983.
Table 1.
Patient characteristics (n = 86)
| Characteristics | n | % |
|---|---|---|
| Total no. of patients | 86 | |
| Age, years | median 65 (range 31–96) | |
| Sex | ||
| Male/Female | 73/13 | 85%/15% |
| Performance status | ||
| 0–1/2–3 | 72/14 | 84%/16% |
| Site of primary tumor | ||
| Oropharyngeal/Hypopharyngeal | 51/35 | 59%/41% |
| TNM classification (UICC 2009) | ||
| T-stage | ||
| T1/T2/T3/T4 | 12/45/15/14 | 14%/53%/17%/16% |
| N-stage | ||
| N0/N1/N2/N3 | 21/14/47/4 | 24%/16%/55%/5% |
| Clinical stage | ||
| StageI/StageII/StageIII/StageIVa–b | 3/14/13/56 | 3%/16%/15%/66% |
| Value of pSUVmax | median 8.0 (range 1.7–25.7) | |
| Content of treatment | ||
| RT only/CCRT | 17/69 | 20%/80% |
| Described dose (Gy) | median 70 (range 60–74.4) | |
| Histology | ||
| Squamous cell carcinoma | 83 | 97% |
| Lymphoepithelial carcinoma | 2 | 2% |
| Carcinoma (NOS) | 1 | 1% |
Abbreviations: UICC, Union for International Cancer Control; pSUVmax, the maximum standardized uptake value of the primary site; RT, Radiotherapy; CCRT, Concurrent radiotherapy; NOS, not otherwise specified.
FDG-PET study
FDG-PET scans were performed with a PET scanner (Philips Allegro, Philips Medical System, Best, the Netherlands) that provided 45 trans-axial images at 4-mm intervals over a distance of 18.0 cm. The field of view and pixel size of the reconstructed images were 57.6 cm and 4.0 mm, respectively. The PET scans were acquired in a 3-dimensional mode with a matrix size of 128 × 128. Patients fasted for at least 6 hours before the PET study. None of the patients had a blood glucose level that exceeded 200 mg/dL before PET. Approximately 1 hour after IV administration of 222 to 333 MBq (6 to 9 mCi) of FDG, a static emission scan was performed with 2.5 to 3 min of acquisition in each bed position, covering the upper thigh to the ear with a total of 9–10 bed positions. Then, a transmission scan using a 137Cs ring was performed over the same area for 23 s per bed position. PET images were reconstructed using an ordered-subset expectation maximization iterative reconstruction algorithm (RAMLA).
After the FDG-PET scan had been completed, patients were moved to the CT room. The CT device was a multi-detector row CT system with an acceleration voltage of 120 kVp and a current of 80 mA. Both reconstructed PET and CT data were transferred to a workstation running viewing-dedicated software (Syntegra; SUN Microsystems, Milpitas, CA, USA) to create fused PET and CT images.
Image analysis
For the semiquantitative analysis of FDG uptake, regions of interest (ROIs) were manually drawn on the transaxial PET images around the focal FDG uptake zone in the primary tumor, as defined on the target lesions of the primary site on the transaxial PET images. The pSUVmax was then calculated using the following formula: SUV = activity concentration (kBq/ml) per injected dose (kBq) per body weight (kg). The measurements of pSUVmax were performed by experienced nuclear medicine physicians. For the survival analysis, the cut-off value of pSUVmax was determined based on receiver operating characteristic (ROC) curves.
Definitions of survival times
OS was defined as the time between initial diagnosis and death from any cause. DFS was defined as the time between diagnosis and the first recurrence of the disease (loco-regional or distant recurrence), or death from any cause. LC was defined as the time between diagnosis and the first recurrence of the primary lesion.
Statistical analysis
The data were analyzed using SPSS software, version 19 (SPSS Inc., Chicago, IL, USA). Pearson’s χ2 test was used to assess measures of association in frequency tables. Spearman’s correlation coefficient was used to analyze the correlations of pSUVmax with T-stage and clinical stage. OS, DFS, and LC were evaluated using Kaplan-Meier estimates and the log-rank test. In a multivariate analysis of DFS, the Cox proportional hazards model was used to assess the effects of patient characteristics and other potential predictive factors of significance. A P-value of 0.05 or less was considered statistically significant and all statistical tests were 2-sided.
RESULTS
Of the 86 included patients, 51 had oropharyngeal cancer and 35 had hypopharyngeal cancer. Seventeen patients were diagnosed with stage I–II disease and 69 patients were diagnosed with stage III disease to stage IVa–b disease. Sixty-nine patients underwent concurrent chemotherapy (CCRT) and the remaining 17 patients were treated with radiotherapy alone because of advanced age, decreased respiratory function, or decreased renal function. In the CCRT group, the median described dose was 70 Gy (range, 60–70 Gy) in 2 Gy per fraction, with 5 fractions per week. The chemotherapy regimen was single-agent cisplatin for 2 or 3 cycles during radiotherapy. In the radiotherapy-alone group, the median described dose was 70 Gy in 35 fractions (range, 60–74.4 Gy). The field of therapy for each patient included the primary tumor site and regional lymph nodes. Therefore, whole-neck irradiation was used (40–50 Gy) and then a shrinking field was adopted as a boost irradiation. The patients’ ages ranged from 31 to 96 years, with a median age of 65 years.
The median duration of follow-up for surviving patients was 60 months (range, 13–94 months). During the period of the follow-up evaluations, 29 patients developed recurrences: 12 had local recurrence only, 4 had local and regional lymph node recurrences, 2 had local and distant metastases, 1 had local and lymph node recurrences with distant metastases, 2 had regional lymph node recurrence only, 6 had distant metastases only, and 2 had regional lymph node recurrence with distant metastases. Twenty-three patients died of the disease and 5 patients died from other causes. Additionally, 5 patients were lost to follow-up. Although we did not determine whether these 5 patients had survived or died, 4 of them had no recurrence at the time of last follow-up, and the remaining patient had local recurrence and distant metastases at the time of last follow-up. The median survival time was 55 months in the entire cohort of included patients, while the 5-year OS and DFS were 65.9% and 60.0%, respectively.
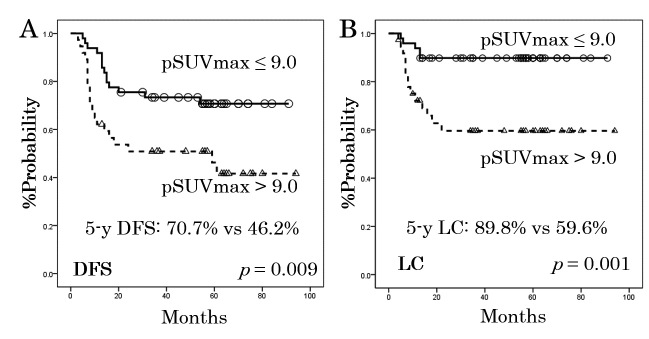
The median pSUVmax was 8.0 (range, 1.7–25.7). Based on the ROC curve analysis, the appropriate pSUVmax cut-off values for DFS and LC were both determined to be 9.0. Figure 1 shows the rates of DFS and LC as stratified by the pSUVmax cut-off value of 9.0. As can be seen from the DFS and LC curves, patients with higher pSUVmax developed significantly more recurrences than those with lower pSUVmax (DFS: 70.7% vs. 46.2% at 5-years, p = 0.009; LC: 89.8% vs. 59.6%, p = 0.001).
Fig. 1.
Disease-free survival (DFS, panel A) and local control (LC, panel B) rates for the 86 patients, as stratified according to the standardized uptake value for the primary lesion (pSUVmax).
Table 2 shows the correlations between various factors and pSUVmax, using the value of 9.0 as a cut-off. T-stage (p < 0.0001), N-stage (p = 0.041), and clinical stage (p = 0.004) were significantly correlated with pSUVmax. Moreover, the correlations of pSUVmax with T-stage and clinical stage were analyzed using Spearman’s correlation coefficient. There was a significant correlation between pSUVmax and T-stage (r = 0.557, p < 0.001). On the other hand, there was a weaker correlation between pSUVmax and clinical stage (r = 0.341, p = 0.001).
Table 2.
Associations between pSUVmax (using 9.0 as a cut-off value) and clinical parameters
| ≤ 9.0 (n = 49) | > 9.0 (n = 37) | p-value* | |
|---|---|---|---|
| Age, years | |||
| < 70/≥ 70 | 35/14 | 23/14 | p = 0.364 |
| Sex | |||
| Male/Female | 41/8 | 32/5 | p = 0.718 |
| Tumor site | |||
| OPC/HPC | 27/22 | 24/13 | p = 0.362 |
| PS | |||
| ≤ 1/≥ 2 | 43/6 | 29/8 | p = 0.244 |
| Histology | |||
| Squamous/Other | 46/3 | 37/0 | p = 0.126 |
| Chemotherapy | |||
| +/– | 37/12 | 32/5 | p = 0.206 |
| T-stage | |||
| T1–2/T3–4 | 43/6 | 14/23 | p < 0.0001 |
| N-stage | |||
| N0/N1–3 | 16/33 | 5/32 | p = 0.041 |
| Clinical stage | |||
| I–II/III–IVa-b | 15/34 | 2/35 | p = 0.004 |
Abbreviations: OPC, oropharyngeal cancer; HPC, hypopharyngeal cancer; PS, Performance status; pSUVmax, the maximum standardized uptake value of the primary site.
* χ2 test
In the univariate analyses, T-stage (p = 0.001), N-stage (p = 0.039), clinical stage (p = 0.015), and pSUVmax (p = 0.009) were significant prognostic predictors for DFS (Table 3). Moreover, a multivariate analysis of DFS was performed, which included N-stage, clinical stage, and pSUVmax. Although the analysis did not reveal any statistically significant associations with DFS, we observed that pSUVmax showed borderline statistical significance as a prognostic factor for DFS (Table 3, hazard ratio = 0.499, 95% confidence interval = 0.245–1.014, p = 0.055).
Table 3.
Univariate and multivariate analyses of disease-free survival
| Factor | n | Number of recurrences/ deaths | p-value* | p-value** | HR (95% CI) |
|---|---|---|---|---|---|
| Age, years | |||||
| < 70/≥ 70 | 58/28 | 23/11 | 0.941 | ||
| Gender | |||||
| Male/Female | 73/13 | 32/2 | 0.091 | ||
| Tumor site | |||||
| OPC/HPC | 51/35 | 18/16 | 0.377 | ||
| PS | |||||
| ≤ 1/≥ 2 | 72/14 | 25/9 | 0.079 | ||
| Histology | |||||
| SCC/Other | 83/3 | 34/0 | 0.204 | ||
| Chemo | |||||
| +/– | 69/17 | 25/9 | 0.129 | ||
| T-stage | |||||
| T1–2/T3–4 | 57/29 | 16/18 | 0.001 | ||
| N-stage | |||||
| N0/N1–3 | 21/65 | 4/30 | 0.039 | 0.731 | 0.774 (0.180–3.333)/1 |
| c stage | |||||
| I–II/III–IVa-b | 17/69 | 2/32 | 0.017 | 0.297 | 0.340 (0.045–2.588)/1 |
| pSUVmax | |||||
| ≤ 9.0/> 9.0 | 49/37 | 14/20 | 0.009 | 0.055 | 0.499 (0.245–1.014)/1 |
* Univariate analyses using the log-rank test
**Multivariate analyses using the Cox regression model
Abbreviations: n, number of patients; HR, hazard rate; CI, confidence interval; OPC, oropharyngeal cancer; HPC, hypopharyngeal cancer; PS, Performance status; SCC, squamous cell carcinoma; chemo, chemotherapy; c stage, clinical stage; pSUVmax, the maximum standardized uptake value of the primary site.
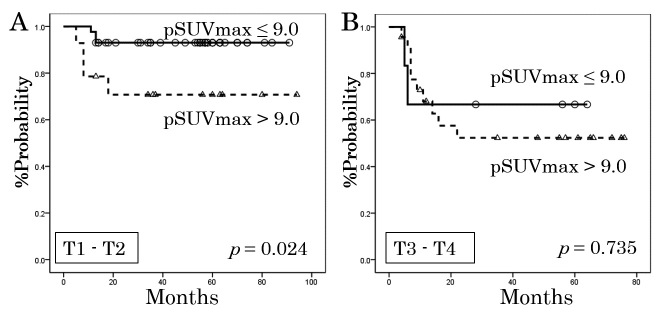
We additionally performed an analysis of LC that was stratified by T-stage (Figure 2). Among patients with T1–T2 disease, those with pSUVmax ≤9.0 and those with pSUVmax >9.0 had significantly different rates of LC (p = 0.024). However, among patients with T3–T4 disease, there was no statistically significant difference between the pSUVmax groups (p = 0.735).
Fig. 2.
Local control rates for the 57 patients with T1–T2 disease (A) and the 29 patients with T3–T4 disease (B), as stratified according to the standardized uptake value for the primary lesion (pSUVmax).
DISCUSSION
There is a large difference between definitive radiotherapy and a radical operation in terms of whether a pathological diagnosis is obtained. If it is possible for us to know the condition of the disease in detail (e.g., whether the primary lesion has invaded the neighborhood, the details of histological type, and the presence of extra-nodal spread), such as is possible during an operation, then we can judge the need for later additional treatment. Thus, the existence of a minimally invasive predictor of radiotherapy’s effectiveness could be useful for determining the intensity of treatment that should be provided to the patient, including whether an operation is necessary. In this regard, pSUVmax may be a useful predictive factor.
In this study, pSUVmax was an important prognostic predictor for DFS in patients who had oropharyngeal or hypopharyngeal cancer and were treated with definitive radiotherapy. Many reports have mentioned that pSUVmax may be a prognostic factor in patients with head and neck cancer.6-10) Nonetheless, the burdens of various primary tumors might differ, thus affecting FDG uptake, treatment response, and survival, all of which could cause biases. Table 4 summarizes the previous reports that have focused on pharyngeal cancer.11-14) In these reports, it was shown that pSUVmax was related to survival rates, and the results of the present study are similar. A report by Kim et al. is especially interesting.13) They analyzed 52 patients who had squamous cell carcinoma (SCC) of the oropharynx and were treated with surgical resection plus radiotherapy (n = 31) or radical radiotherapy plus chemotherapy (n = 21), concluding that patients with high pSUVmax may obtain a survival benefit from primary surgery followed by radiotherapy with or without chemotherapy, instead of from concomitant chemoradiotherapy. Thus, high 18F-FDG uptake may be useful for identifying pharyngeal cancers that require more aggressive treatment. Although we excluded patients with surgical resection from the present study to reduce the potential for confounding, an analysis that includes their data and carefully adjusts for confounding should be performed in the future.
Table 4.
Summary of reports in the literature on the efficacy of pre-treatment pSUVmax for patients with pharyngeal cancer
| Author | n | Subject | Treatment | Median follow-up (month) | Cut-off value of pSUVmax | p-value* |
|---|---|---|---|---|---|---|
| Lee(11) (2008) | 41 | NPC | RT/CCRT | 40 | 8 | p < 0.007 (DFS) |
| Lin(12) (2012) | 62 | OPC+HPC | RT/CCRT | 24 | 11 | p < 0.04 (PRFS) |
| Kim(13) (2007) | 52 | HPC | Ope/RT/CCRT | 36 | 6 | p = 0.036 (DFS) |
| Suzuki(14) (2014) | 49 | OPC+HPC | Ope/RT/CCRT | 33 | 8 | p < 0.04 (OS) |
| Present study | 86 | OPC+HPC | RT/CCRT | 60 | 9 | p = 0.009 (DFS) |
Abbreviations: n, numbers of patients; pSUVmax, the maximum standardized uptake value of the primary site; NPC, nasopharyngeal cancer; OPC, oropharyngeal cancer; HPC, hypopharyngeal cancer; RT, radiotherapy alone; CCRT, concurrent radiotherapy; Ope, operation; DFS, disease-free survival; PRFS, primary tumor relapse-free survival; OS, overall survival.
*Univariate analysis using the log-rank test
Interestingly, pSUVmax was predictive of LC rates in T1–T2 disease; even for the relatively small T1–T2 tumors, patients with higher pSUVmax had a worse LC rate than did patients with lower pSUVmax. Therefore, patients with tumors that have higher FDG uptake might need both more aggressive treatments and stricter follow-up. On the other hand, in our subgroup of patients with T3–T4 tumors, pSUVmax with a cut-off value of 9.0 was not observed to be significantly predictive of LC. The underlying reasons for the discrepancy between T1–T2 and T3–T4 disease remain unclear. One possible explanation is that the number of our patients who had T3–T4 tumors in the group with pSUVmax ≤ 9.0 (n = 6) did not provide enough power for the statistical analysis. Lin et al. analyzed the correlation between pSUVmax and the gross tumor volume (GTV) of the primary site in oropharyngeal and hypopharyngeal cancers, observing 2-year primary relapse-free survival rates of 92% and 47% for patients with a GTV ≤ 15 ml and pSUVmaxs of ≤ 11 and > 11, respectively (p = 0.014).12) This report provides indirect support for the results of the stratified analysis of T-stage in our study. Although no previous report has performed a similar stratified analysis of T-stage, some reports have mentioned significant associations between T-stage and the standardized uptake value of the primary site.6, 14, 16)Haerleet al. reported a significant association between pSUVmax and T-stage (p < 0.001) in head and neck cancer.16) Allal et al. reported that T1–T2 tumors had a lower median standardized uptake value than T3–T4 tumors (p = 0.004) in cases of head and neck cancer.6) In an analysis of pharyngeal cancer, Suzuki et al. reported that patients with pSUVmax ≥ 8 had clinical T3–T4 stage disease more frequently than patients with pSUVmax < 8 (p < 0.03).14) Our results are consistent with these previous reports, demonstrating significant associations between T-stage and pSUVmax (Table 2). In other words, higher T-stages are associated with higher pSUVmax.
Although SUVmax is convenient to measure and widely used, it has an important disadvantage: as a single-pixel measure that evaluates the most intense FDG uptake in the tumor, it may not be reflective of the total uptake for the whole tumor. Recently, metabolic tumor volume (MTV) and total lesion glycolysis (TLG) have been investigated as novel indexes for analyses of standardized uptake value and tumor volume.19-23) (MTV is defined as the volume of tissues with increased FDG uptake, while TLG is calculated by taking the product of the tumor volume and the mean standardized uptake value.) Moon et al. reported that primary tumor TLG was an independent predictor for OS, whereas primary tumor MTV was not.19) Garsa et al. found that primary tumor MTV was a significant predictor for OS and DFS, and that primary tumor TLG was a significant predictor for OS.20) However, Higgins et al. reported that TLG was not prognostic.21) Accordingly, there is currently no established agreement regarding the prognostic potential of MTV or TLG. In the present study of pSUVmax, we were not able to investigate MTV and TLG as prognostic predictors. Hence, this topic should be considered in the future.
The limitations to this study include its single-institution retrospective design and relatively small sample size (although a sufficient median duration of follow-up was employed). Thus, this study may have missed some confounding factors and include some biases.
In conclusion, in this study, pSUVmax was predictive of DFS and LC in patients with oropharyngeal or hypopharyngeal cancer that was treated with definitive radiotherapy. pSUVmax was strongly associated with LC in T1–T2 disease.
Conflicts of interest: none to declare
REFERENCES
- 1). Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer, 2001; 94: 153–156. [DOI] [PubMed]
- 2). Pfister DG, Spencer S, Brizel DM, Burtness B, Busse PM, Caudell JJ, et al Head and neck cancers, version 2.2014. Clinical practice guidelines in oncology. J Natl Compr Canc Netw, 2014; 12: 1454–1487. [DOI] [PubMed]
- 3). Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med, 2010; 363: 24–35. [DOI] [PMC free article] [PubMed]
- 4). Jakobsen J, Hansen O, Jørgensen KE, Bastholt L. Lymph node metastases from laryngeal and pharyngeal carcinomas–calculation of burden of metastasis and its impact on prognosis. Acta Oncol, 1998; 37: 489–493. [DOI] [PubMed]
- 5). Khuri FR, Lippman SM, Spitz MR, Lotan R, Hong WK. Molecular epidemiology and retinoid chemoprevention of head and neck cancer. J Natl Cancer Inst, 1997; 89: 199–211. [DOI] [PubMed]
- 6).Allal AS, Slosman DO, Kebdani T, Allaoua M, Lehmann W, Dulguerov P. Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-[18F] fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys, 2004; 59: 1295–1300. [DOI] [PubMed]
- 7).Schwartz DL, Rajendran J, Yueh B, Coltrera MD, LeBlanc M, Eary J, et al FDG-PET prediction of head and neck squamous cell cancer outcomes. Arch Otolaryngol Head Neck Surg, 2004; 130: 1361–1367. [DOI] [PubMed]
- 8).Machtay M, Natwa M, Andrel J, Hyslop T, Ann PR, Lavarino J, et al Pretreatment FDG-PET standardized uptake value as a prognostic factor for outcome in head and neck cancer. Head Neck, 2009; 31: 195–201. [DOI] [PubMed]
- 9).Torizuka T, Tanizaki Y, Kanno T, Futatsubashi M, Naitou K, Ueda Y, et al Prognostic value of 18F-FDG PET in patients with head and neck squamous cell cancer. AJR Am J Roentgenol, 2009; 192: 156–160. [DOI] [PubMed]
- 10).Xie P, Li M, Zhao H, Sun X, Fu Z, Yu J. 18F-FDG PET or PET-CT to evaluate prognosis for head and neck cancer: a meta-analysis. J Cancer Res Clin Oncol, 2011; 137: 1085–1093. [DOI] [PubMed]
- 11).Lee SW, Nam SY, Im KC, Kim JS, Choi EK, Ahn SD, et al Prediction of prognosis using standardized uptake value of 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography for nasopharyngeal carcinomas. Radiother Oncol, 2008; 87: 211–216. [DOI] [PubMed]
- 12).Lin SC, Liao CY, Kao CH, Yen KY, Yang SN, Wang YC, et al Pretreatment maximal standardized uptake value of the primary tumor predicts outcome to radiotherapy in patients with pharyngeal cancer. J Radiat Res, 2012; 53: 462–468. [DOI] [PubMed]
- 13). im SY, Roh JL, Kim MR, Kim JS, Choi SH, Nam SY, et al Use of 18F-FDG PET for primary treatment strategy in patients with squamous cell carcinoma of the oropharynx. J Nucl Med, 2007; 48: 752–757. [DOI] [PubMed]
- 14).Suzuki H, Kato K, Fujimoto Y, Itoh Y, Hiramatsu M, Naganawa S, et al Prognostic value of 18F-fluorodeoxyglcose uptake before treatment for pharyngeal cancer. Ann Nucl Med, 2014; 28: 356–362. [DOI] [PubMed]
- 15). Liao CT, Chang JT, Wang HM, Ng SH, Hsueh C, Lee LY, et al Pretreatment primary tumor SUVmax measured by FDG-PET and pathologic tumor depth predict for poor outcomes in patients with oral cavity squamous cell carcinoma and pathologically positive lymph nodes. Int J Radiat Oncol Biol Phys, 2009; 73: 764–771. [DOI] [PubMed]
- 16). Haerle SK, Huber GF, Hany TF, Ahmad N, Schmid DT. Is there a correlation between 18F-FDG-PET standardized uptake value, T-classification, histological grading and the anatomic subsites in newly diagnosed squamous cell carcinoma of the head and neck? Eur Arch Otorhinolaryngol, 2010; 267: 1635–1640. [DOI] [PubMed]
- 17).Monn SH, Choi JY, Lee HJ, Son YI, Baek CH, Ahn YC, et al Prognostic value of 18F-FDG PET/CT in patients with squamous cell carcinoma of the tonsil: comparisons of volume-based metabolic parameters. Head Neck, 2013; 35: 15–22. [DOI] [PubMed]
- 18).Garsa AA, Chang AJ, DeWees T, Spencer CR, Adkins DR, Dehdashti F, et al Prognostic value of 18F-FDG PET metabolic parameters in oropharyngeal squamous cell carcinoma. J Radiat Oncol, 2013; 2: 27–34. [DOI] [PMC free article] [PubMed]
- 19).Higgins KA, Hoang JK, Roach MC, Chino J, Yoo DS, Turkington TG, et al Analysis of pretreatment FDG-PET SUV parameters in head-and-neck cancer: tumor SUVmean has superior prognostic value. Int J Radiat Oncol Biol Phys, 2012; 82: 548–553. [DOI] [PubMed]
- 20).Chung MK, Jeong HS, Park SG, Jang JY, Son YI, Choi JY, et al Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res, 2009; 15: 5861–5868. [DOI] [PubMed]
- 21). Kao CH, Lin SC, Hsieh TC, Yen KY, Yang SN, Wang YC, et al Use of pretreatment metabolic tumour volumes to predict the outcome of pharyngeal cancer treated by definitive radiotherapy. Eur J Nucl Med Mol Imaging, 2012; 39: 1297–1305. [DOI] [PubMed]
- 22). Tang C, Murphy JD, Khong B, La TH, Kong C, Fischbein NJ, et al Validation that metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys, 2012; 83: 1514–1520. [DOI] [PMC free article] [PubMed]
- 23). Ryu IS, Kim JS, Roh JL, Cho KJ, Choi SH, Nam SY, etal Prognostic significance of preoperative metabolic tumour volume and total lesion glycolysis measured by 18F-FDG PET/CT in squamous cell carcinoma of the oral cavity. Eur J Nucl Med Mol Imaging, 2014; 41: 452–461. [DOI] [PubMed]