Abstract
Marital status has been reported as an independent prognostic factor for survival in various cancers, but it has been rarely studied in hepatocellular carcinoma (HCC) treated by surgical resection. We retrospectively investigated Surveillance, Epidemiology, and End Results (SEER) population-based data and identified 13,408 cases of HCC with surgical treatment between 1998 and 2013. The patients were categorized according to marital status, as “married,” “never married,” “widowed,” or “divorced/separated.” The 5-year HCC cause-specific survival (HCSS) data were obtained, and Kaplan–Meier methods and multivariate Cox regression models were used to ascertain whether marital status is also an independent prognostic factor for survival in HCC. Patients in the widowed group had the higher proportion of women, a greater proportion of older (>60 years) patients, more frequency in latest year of diagnosis (2008-2013), a greater number of tumors at TNM stage I/II, and more prevalence at localized SEER Stage, all of which were statistically significant within-group comparisons (P < 0.001). Marital status was demonstrated to be an independent prognostic factor by multivariate survival analysis (P < 0.001). Married patients had better 5-year HCSS than did unmarried patients (46.7% vs 37.8%) (P < 0.001); conversely, widowed patients had lowest HCSS compared with all other patients, overall, at each SEER stage, and for different tumor sizes. Marital status is an important prognostic factor for survival in patients with HCC treated with surgical resection. Widowed patients have the highest risk of death compared with other groups.
Keywords: hepatocellular carcinoma, marital status, SEER, survival analysis, surgery
INTRODUCTION
Worldwide, primary liver cancer (LC), which consists of primary hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and combined HCC and ICC, is the fifth most common cancer and the third leading cause of cancer-associated mortality [1]. In Western countries, the rise of HCC has been linked with increasing hepatitis C infection and alcohol consumption [2]; however, the incidence of LC has also been rapidly increasing in Asian countries, such that the incidence among Asians is now twice that among Africans [3].
Extensive research has demonstrated that marital status is an independent prognostic factor of survival in several cancers [4–7]. Li et al reported that unmarried patients with colorectal cancer were at greater risk for cancer-specific mortality and that widowed patients were at highest risk for death compared with other groups [6]. Wang et al showed that marital status was an important prognostic factor in pancreatic cancer and that widowed patients were at the greatest risk for death [7]. A study reported, in primary liver cancer patients, married patients enjoyed survival benefits while widowed persons suffered survival disadvantages in both overall survival and cancer-specific survival [8]. LC is a heterogenous cancer. As we know, HCC and ICC may have different pathogenesis and different biological behavior, or even different long-term survivals. So analysis on survivals of HCC and ICC separately might be more meaningful and reasonable. Yet, the effect of marital status on HCC survival with surgical resection has not been rigorously studied. Therefore, the aim of this study was to explore the relationship between marital status and HCC outcomes, as well as the potential underlying mechanisms. We extracted data from the Surveillance, Epidemiology, and End Results (SEER) cancer registry to investigate the effect of marital status on HCC cause-specific survival (HCSS) in patients with HCC treated by surgical resection.
RESULTS
Baseline patient characteristics
A total of 13,408 eligible patients were identified during the 15-year study period (between 1998 and 2013), including 10,071 male and 3,337 female patients. Of these, 966 (7.2%) were widowed, 8494 (63.4%) were married, 2265 (16.9%) had never married. The 200 (1.5%) individuals who were separated and 1,483 (11.1%) who were divorced were grouped together in the divorced/separated group in our study. Patients in the widowed group had the higher proportion (62.4%) of women within-group comparisons, a greater proportion (84.6%) of older (>60 years) patients, more frequency (49.1%) in latest years of diagnosis (2008-2013), a greater number (29.8%) of TNM stage I/II tumors, and more prevalence (78.1%) at localized SEER Stage, all of which were statistically significant (P < 0.001). The baseline patient demographics and tumor characteristics are described in Table 1.
Table 1. Baseline demographic and tumor characteristics of patients in SEER database.
| Characteristic | Total | Widowed | Married | Never married | Divorced/Separated | P |
|---|---|---|---|---|---|---|
| (n = 13408) N (%) |
(n = 966) N (%) |
(n = 8494) N (%) |
(n = 2265) N (%) |
(n = 1683) N (%) |
||
| Sex | < 0.001 | |||||
| Male | 10071 (75.1) | 363(37.6) | 6743 (79.4) | 1749 (77.2) | 1216 (72.3) | |
| Female | 3337 (24.9) | 603 (62.4) | 1751 (20.6) | 516 (22.8) | 467 (27.7) | |
| Age | < 0.001 | |||||
| ≤60 | 7043 (52.5) | 149 (15.4) | 4307 (50.7) | 1556 (68.7) | 1031 (61.3) | |
| >60 | 6365 (47.5) | 817 (84.6) | 4187 (49.3) | 709 (31.3) | 652 (38.7) | |
| Race | < 0.001 | |||||
| White | 8750 (65.3) | 628 (65.0) | 5387 (63.4) | 1494 (66.0) | 1241 (73.7) | |
| Black | 1412 (10.5) | 95 (9.8) | 647 (7.6) | 451 (19.9) | 219 (13.0) | |
| Other* | 3246 (24.2) | 243 (25.2) | 2460 (29.0) | 320 (14.1) | 223 (13.3) | |
| Year of diagnosis | < 0.001 | |||||
| 1998-2002 | 2029 (15.1) | 177 (18.3) | 1380 (16.2) | 262 (11.6) | 210 (12.5) | |
| 2003-2007 | 4806 (35.8) | 315 (32.6) | 3117 (36.7) | 768 (33.9) | 606 (36.0) | |
| 2008-2013 | 6573 (49.0) | 474 (49.1) | 3997 (47.1) | 1235 (54.5) | 867 (51.5) | |
| Pathological grading | < 0.001 | |||||
| Well/Moderate | 6708 (50.0) | 478 (49.5) | 4376 (51.5) | 1076 (47.5) | 778 (46.2) | |
| Poor/Anaplastic | 1601 (11.9) | 115 (11.9) | 1054 (12.4) | 247 (10.9) | 185 (11.0) | |
| Unknown | 5099 (38.0) | 373 (38.6) | 3064 (36.1) | 942 (41.6) | 720 (42.8) | |
| TNM Stage | < 0.001 | |||||
| I/II | 3708 (27.7) | 288 (29.8) | 2230 (26.3) | 704 (31.1) | 486 (28.9) | |
| III/IV | 593 (4.4) | 42 (4.3) | 365 (4.3) | 121 (5.3) | 65 (3.9) | |
| Unknown | 9107 (67.9) | 636 (65.8) | 5899 (69.4) | 1440 (63.6) | 1132 (67.3) | |
| Tumor Size | ||||||
| <3 cm | 4033 (30.1) | 254 (26.3) | 2444 (28.8) | 752 (33.2) | 583 (34.6) | < 0.001 |
| 3–5 cm | 3289 (24.5) | 218 (22.6) | 2056 (24.2) | 555 (24.5) | 460 (27.3) | |
| >5 cm | 2715 (20.2) | 206 (21.3) | 1775 (20.9) | 465 (20.5) | 269 (16.0) | |
| Not stated | 3371 (25.1) | 288 (29.8) | 2219 (26.1) | 493 (21.8) | 371 (22.0) | |
| SEER Stage | 0.268 | |||||
| Localized | 9941 (74.1) | 754 (78.1) | 6280 (73.9) | 1666 (73.6) | 1241 (73.7) | |
| Regional | 2617 (19.5) | 153 (15.8) | 1678 (19.8) | 452 (20.0) | 334 (19.8) | |
| Distant | 514 (3.8) | 32 (3.3) | 327(3.8) | 92 (4.1) | 63 (3.7) | |
| Unstaged | 336 (2.5) | 27 (2.8) | 209 (2.5) | 55 (2.4) | 45 (2.7) |
Abbreviations: SEER, Surveillance, Epidemiology, and End Results.
Other includes American Indian/Alaska native, Asian/Pacific Islander, and unknown.
Effect of marital status on HCSS
Married patients had better 5-year HCSS than did unmarried patients (46.7% vs 37.8%) (P < 0.001) (Figure 1). The 5-year HCSS was 29.4% in the widowed group, 46.7% in the married group, 39.4% in the never married group, and 40.4% in the divorced/separated group, all significantly different according to the univariate log rank test (P < 0.001) (Figure 2A). Older age (P < 0.001), black race (P < 0.001), poor or undifferentiated pathology grade (P < 0.001), the latest year of diagnosis (P < 0.001), TNM stage III/IV disease (P < 0.001), tumor size >5 cm (P < 0.001), and SEER distant stage (P < 0.001) were regarded as significant risk factors for poor survival on univariate analysis (Table 2). When multivariate analysis with Cox regression was performed, all the aforementioned variables were validated as independent prognostic factors for poor survival (Table 2), as follows: age (>60 years, hazard ratio [HR] 1.399, 95% confidence interval [CI] 1.332–1.469), race (black, HR 1.257, 95% CI 1.167–1.353; other races, HR 0.818, 95% CI 0.773–0.867), year of diagnosis (2003-2007, HR 0.997, 95% CI 0.915–1.086; 2008-2013, HR 0.827, 95% CI 0.748–0.916), pathological grading (poor or undifferentiated tumor, HR 1.458, 95% CI 1.356–1.568, unknown pathology grade, HR 1.386, 95% CI 1.317–1.459), TNM stage (stage III/IV, HR 1.327, 95% CI 1.210–1.455; unknown stage, HR 1.129, 95% CI 0.997–1.278), tumor size (3–5 cm tumor, HR 1.411, 95% CI 1.312–1.516; >5 cm tumor, HR 1.632, 95% CI 1.504–1.771; unstated tumor size, HR 1.456, 95% CI 1.281–1.655), SEER Stage (regional stage, HR 1.427, 95% CI 1.340–1.518; distant stage, HR 2.448, 95% CI 2.188–2.739; unstaged, HR 1.052, 95% CI 0.911–1.216), marital status (married, HR 0.745, 95% CI 0.683–0.812; never married, HR 0.893, 95% CI 0.807–0.988; divorced/separated, HR 0.862, 95% CI 0.776–0.957).
Figure 1. Survival curves in hepatocellular carcinoma patients treated with surgical resection between the unmarried patients and the married patients.
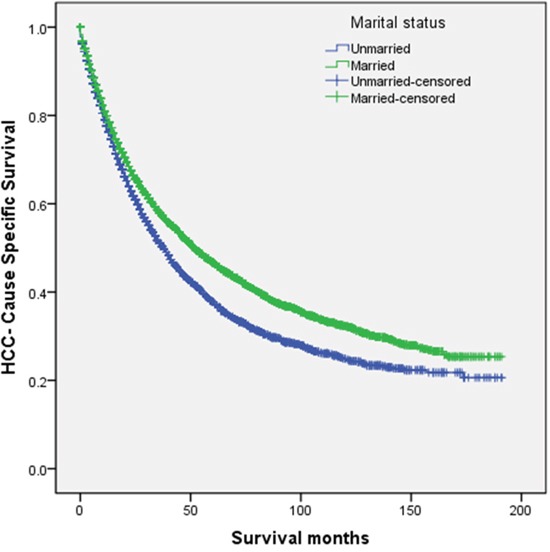
χ2 = 68.610, P < 0.001.
Figure 2. Survival curves in hepatocellular carcinoma patients according to marital status.
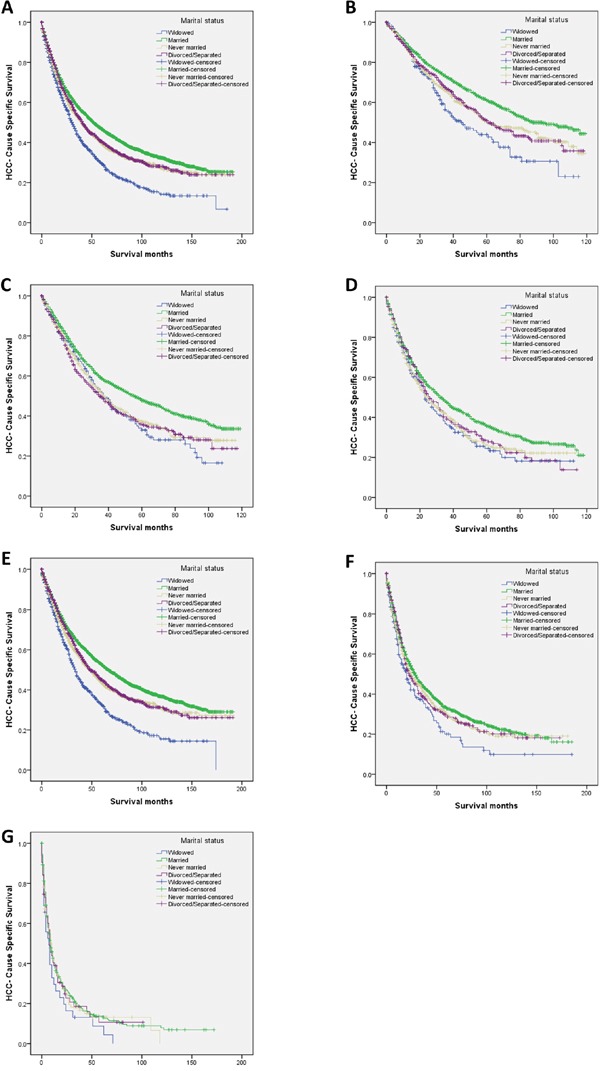
A. Overall: χ2 = 109.915 (P < 0.001); B. tumor diameter <3 cm: χ2 = 35.696 (P < 0.001); C. tumor size 3–5 cm: χ2 = 31.884 (P < 0.001); D. tumor diameter >5 cm: χ2 = 16.972 (P = 0.004); E. SEER localized stage: χ2 = 119.747 (P < 0.001); F. SEER regional stage: χ2 = 11.960 (P = 0.008); and G. SEER distant stage: χ2 = 2.743 (P = 0.433).
SEER: Surveillance, Epidemiology, and End Results.
Table 2. Univariate and multivariate survival analysis for evaluating the influence of marital status on HCC cause-specific survival in SEER database.
| Variable | Total | 5-year CCS | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| 13408 | Log rank χ2 test | P | HR (95%CI) | P | ||
| Sex | 0.010 | 0.921 | NI | |||
| Male | 10071 | 43.5% | ||||
| Female | 3337 | 43.5% | ||||
| Age | 204.008 | < 0.001 | < 0.001 | |||
| ≤60 | 7043 | 49.3% | Reference | |||
| >60 | 6365 | 36.5% | 1.399 (1.332–1.469) | |||
| Race | 83.046 | < 0.001 | < 0.001 | |||
| White | 8750 | 43.8% | Reference | |||
| Black | 1412 | 33.0% | 1.257 (1.167–1.353) | < 0.001 | ||
| Other* | 3246 | 47.1% | 0.818 (0.773–0.867) | < 0.001 | ||
| Year of diagnosis | 125.996 | < 0.001 | < 0.001 | |||
| 1998-2002 | 2029 | 37.6% | Reference | |||
| 2003-2007 | 4806 | 41.9% | 0.997 (0.915–1.086) | 0.937 | ||
| 2008-2013 | 6573 | 46.4% | 0.827 (0.748–0.916) | < 0.001 | ||
| Pathological grading | 266.354 | < 0.001 | < 0.001 | |||
| Well/Moderate | 6708 | 50.4% | Reference | |||
| Poor/Anaplastic | 1601 | 33.9% | 1.458 (1.356–1.568) | < 0.001 | ||
| Unknown | 5099 | 37.3% | 1.386 (1.317–1.459) | < 0.001 | ||
| TNM Stage | 262.301 | < 0.001 | < 0.001 | |||
| I/II | 3708 | 64.8%# | Reference | |||
| III/IV | 593 | 37.2%# | 1.327 (1.210–1.455) | < 0.001 | ||
| Unknown | 9107 | 53.3%# | 1.129 (0.997–1.278) | 0.056 | ||
| Tumor Size | 495.989 | < 0.001 | < 0.001 | |||
| <3 cm | 4033 | 56.7% | Reference | |||
| 3–5 cm | 3289 | 43.4% | 1.411 (1.312–1.516) | < 0.001 | ||
| >5 cm | 2715 | 32.6% | 1.632 (1.504–1.771) | < 0.001 | ||
| Not stated | 3371 | 37.7% | 1.456 (1.281–1.655) | < 0.001 | ||
| SEER Stage | 919.645 | < 0.001 | < 0.001 | |||
| Localized | 9941 | 48.7% | Reference | |||
| Regional | 2617 | 31.3% | 1.427 (1.340–1.518) | < 0.001 | ||
| Distant | 514 | 12.9% | 2.448 (2.188–2.739) | < 0.001 | ||
| Unstaged | 336 | 36.1% | 1.052 (0.911–1.216) | 0.489 | ||
| Marital Status | 109.915 | < 0.001 | < 0.001 | |||
| Widowed | 966 | 29.4% | Reference | |||
| Married | 8494 | 46.7% | 0.745 (0.683–0.812) | < 0.001 | ||
| Never married | 2265 | 39.4% | 0.893 (0.807–0.988) | 0.028 | ||
| Divorced/Separated | 1683 | 40.4% | 0.862 (0.776–0.957) | 0.005 | ||
Abbreviations: HCC, hepatocellular carcinoma; SEER, Surveillance, Epidemiology, and End Results; CCS, cause-specific survival.
Other includes American Indian/Alaska native, Asian/Pacific Islander, and unknown.
3-year CCS. Because TNM stage record according to the AJCC Cancer Staging Manual (7th edition) in the SEER database began from 2009, and ended at 2013, its 5-year CCS did not exist.
NI: not included in the multivariate survival analysis.
Subgroup analysis of the effect of marital status, according to tumor size
We further analyzed the effects of marital status on survival among tumors of different sizes, and we observed three interesting findings: First, tumor size was an independent factor for poor survival, both in univariate and multivariate analysis (P < 0.001). Second, widowed patients had the lowest survival rate in comparisons at all tumor sizes: Widowed patients had a 17.1% reduction in 5-year LCSS compared with married patients for tumors with diameter <3 cm (44.0% vs 61.1%) (P < 0.001), a 14.9% reduction for tumors with diameter 3–5 cm (32.9% vs 47.8%) (P < 0.001), and a 11.2% reduction for tumors with diameter >5 cm (24.5% vs 35.7%) (P < 0.001). Third, there were only very small difference in survival between the never married and divorced/separated patients for any tumor size: At tumor size <3 cm and >5 cm respectively, patients in the never married group had a 0.6% and 2.4% decrease in 5-year HCSS compared with patients in the divorced/separated group; at tumor size 3–5 cm, the never married group showed a 1.4% increase in 5-year HCSS (Table 3, Figure 2B–2D).
Table 3. Univariate and multivariate analysis of marital status on HCC cause-specific survival based on different tumor size.
| Variable | Total | 5-year CCS | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| Log rank χ2 test | P | HR(95% CI) | P | |||
| Tumor Size | ||||||
| <3 cm | 4033 | |||||
| Marital status | 35.696 | < 0.001 | < 0.001 | |||
| Widowed | 254 | 44.0% | Reference | Reference | ||
| Married | 2444 | 61.1% | 22.809 | < 0.001 | 0.612 (0.499–0.750) | < 0.001 |
| Never married | 752 | 50.4% | 4.322 | 0.038 | 0.792 (0.633–0.991) | 0.041 |
| Divorced/Separated | 583 | 51.0% | 4.680 | 0.031 | 0.786 (0.624–0.991) | 0.041 |
| 3–5 cm | 3289 | |||||
| Marital status | 31.884 | < 0.001 | < 0.001 | |||
| Widowed | 218 | 32.9% | Reference | Reference | ||
| Married | 2056 | 47.8% | 11.353 | < 0.001 | 0.725 (0.600–0.877) | < 0.001 |
| Never married | 555 | 37.3% | 0.425 | 0.514 | 0.933 (0.754–1.155) | 0.527 |
| Divorced/Separated | 460 | 35.9% | 0.026 | 0.871 | 0.981 (0.790–1.219) | 0.863 |
| >5 cm | 2715 | |||||
| Marital status | 16.972 | < 0.001 | < 0.001 | |||
| Widowed | 206 | 24.5% | Reference | |||
| Married | 1775 | 35.7% | 8.015 | 0.005 | 0.770 (0.642–0.924) | 0.005 |
| Never married | 465 | 26.2% | 0.259 | 0.611 | 0.947 (0.770–1.164) | 0.603 |
| Divorced/Separated | 269 | 28.6% | 0.709 | 0.400 | 0.913 (0.727–1.146) | 0.432 |
Abbreviations: HCC, hepatocellular carcinoma; CCS, cause-specific survival.
Subgroup analysis of the effect of marital status, according to SEER stage
We also analyzed the effects of marital status on survival at each SEER stage. There were no significant differences in the distribution of SEER stages among the different marital status groups (Table 1). Again, we had three interesting findings: First, marital status was an independent risk factor for poor survival in patients with SEER localized and regional stage disease, both in univariate and multivariate analysis (P < 0.01); marital status was not significant at distant stage disease, in either univariate or multivariate analysis, possibly because of the smaller sample size (n=514)—in particular, there were only 32 widowed patients and 63 divorced/separated patients with distant stage disease. Second, widowed patients again had the lowest survival rate in comparisons at all tumor stages: Widowed patients had a 10.0% reduction in 2-year HCSS compared with married patients for localized stage tumors (62.0% vs 72.0%) (P < 0.001), a 12.0% reduction for regional stage tumors (42.1% vs 54.1%) (P = 0.001), and an 10.5% reduction for distant stage tumors (16.4% vs 26.9%) (P = 0.095). Third, there were almost no differences between the never married and divorced/separated patients for any SEER stage: For localized stage tumors, never married patients had a 3.0% decrease in 2-year HCSS compared with divorced/separated patients; for regional and distant stage tumors, respectively, never married patients had a 1.1% and 1.9% increase in survival compared with divorced/separated patients (Table 4, Figure 2E–2G).
Table 4. Univariate and multivariate analysis of marital status on HCC cause-specific survival based on different SEER Stage.
| Variable | Total | 2-year CCS | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| Log rank χ2 test | P | HR (95% CI) | P | |||
| SEER Stage | ||||||
| Localized | 9941 | |||||
| Marital status | 119.747 | < 0.001 | < 0.001 | |||
| Widowed | 754 | 62.0% | Reference | Reference | ||
| Married | 6280 | 72.0% | 107.518 | < 0.001 | 0.597 (0.541–0.659) | < 0.001 |
| Never married | 1666 | 67.6% | 29.617 | < 0.001 | 0.727 (0.648–0.816) | < 0.001 |
| Divorced/Separated | 1241 | 70.6% | 36.034 | < 0.001 | 0.700 (0.620–0.790) | < 0.001 |
| Regional | 2617 | |||||
| Marital status | 11.960 | 0.008 | 0.009 | |||
| Widowed | 153 | 42.1% | Reference | Reference | ||
| Married | 1678 | 54.1% | 10.627 | 0.001 | 0.723 (0.593–0.881) | 0.001 |
| Never married | 452 | 50.0% | 4.197 | 0.040 | 0.795 (0.637–0.992) | 0.042 |
| Divorced/Separated | 334 | 48.9% | 4.166 | 0.041 | 0.789 (0.626–0.995) | 0.045 |
| Distant | 514 | |||||
| Marital status | 2.743 | 0.433 | 0.464 | |||
| Widowed | 32 | 16.4% | Reference | Reference | ||
| Married | 327 | 26.9% | 2.783 | 0.095 | 0.736 (0.505– 1.073) | 0.111 |
| Never married | 92 | 24.7% | 1.560 | 0.212 | 0.774 (0.508– 1.181) | 0.235 |
| Divorced/Separated | 63 | 22.8% | 1.230 | 0.267 | 0.768 (0.490– 1.204) | 0.250 |
Abbreviations: HCC, hepatocellular carcinoma; SEER, Surveillance, Epidemiology, and End Results; CCS, cause-specific survival.
DISCUSSION
Compared with those who have never married, separated, widowed, or divorced, married patients have longer overall survival and lower mortality for many major causes of death [9–11]. Using the SEER database to investigate the relationship between marital status and survival, the present study showed that in the context of HCC, widowed patients had significantly poorer HCSS than did their married counterparts. Furthermore, in multivariable analyses, the risk for widowed patients persisted even after adjusting for age, race, year of diagnosis, pathology grade, TNM stage, tumor size, and SEER Stage.
One hypothesis for the poor prognosis in unmarried individuals is delayed diagnosis with advanced tumor stage; however, as seen in Table 1, in our study group, however, the percentages of patients with localized and regional tumors or distant metastasis were comparable among the four subgroups. Moreover, widowed patients had the highest percentage of SEER localized stage disease. Widowed patients had worse 5-year HCSS compared with all other groups (all P < 0.001). Furthermore, among the patients at each SEER stage, the widowed group had worse 2-year HCSS compared with all other groups. Notably, at SEER distant stage, there was no significant difference in HCSS between the groups—this may have been the result of the smaller sample size, for which effects are more difficult to detect and/or to quantify (Table 4).
Our data also revealed that unmarried patients had a survival disadvantage that persisted for each different tumor size. In particular, widowed patients suffered from the poorest 5-year HCSS.
In attempting to explain the relationship between marital status and survival, psychosocial factors may provide a reasonable answer. Unmarried and especially, widowed patients may experience a lack of emotional and social support (otherwise provided by a spouse), and may display more distress, depression, and anxiety than do their married counterparts [12]. Additionally, marital status may affect the level of adherence to the treatment plan. Compared with their unmarried counterparts, married patients were shown to be more likely to comply with treatment, to seek treatment at more highly recognized centers, and to accept more aggressive treatment, all of which may make for better cancer control [13].
There is evidence that physiological changes accompanying stress and depression may worsen cancer outcome via different mechanisms. Decreased psychosocial support and increased psychological stress have been shown weaken immune function and in this way, may contribute to tumor progression and mortality [14–16]. Further, perceived lack of social support has been shown to decrease the activity of natural killer cells [17]. As well, chronic stress may cause a prolonged secretion of cortisol [18], which triggers a counterregulatory response in white blood cells, by downregulating their cortisol receptors. This downregulation also degrades the cellular response to anti-inflammatory signals and stimulates an increase in cytokine-mediated inflammatory processes [19], which, in colorectal cancer, has been confirmed to be a poor prognostic factor [20, 21]. Additionally, several other neuroendocrine mediators and cytokines present in depression and stress have been linked with an increased cancer metastasis [16]. Finally, depression and quality of life have been associated with an increased production of vascular endothelial growth factor, which may contribute to endothelial cell migration, proliferation, and proteolytic activity [22]. Accordingly, two meta-analyses confirmed that depression increased cancer mortality by 19% and 39%, respectively [23, 24].
This study analyzed data from the SEER database to add to current knowledge about the relationship between marital status and the postoperative prognosis of HCC; however, the study had several potential limitations. First, the SEER database only provided data on marital status at diagnosis. Whether the marital status varied during therapeutic process is unknown, but any variation would have affected the results. Second, some marital status data may have been inaccurate, for example, some patients classified as married may have actually separated, while other patients classified as never married may have been cohabitating. Third, the quality of the marriage including the “inner correlations” of marital status can also influence the survival of HCC patients—marital distress has also been linked with long-term immune consequences and has been associated with an increased risk of a variety of health problems [25]. Fourth, the SEER HCC database lacks quality data on adjuvant therapy, comorbidities, and recurrence. Finally, we hypothesized that psychosocial factors may have been important factors in the poor survival of unmarried patients, but we could not perform psychological tests to confirm our hypothesis.
Despite these potential limitations, our study results reconfirmed that unmarried patients are at greater risk for cancer-specific mortality. Furthermore, we showed that the unmarried patient groups were heterogeneous and that widowed patients were always at highest risk for death of cancer. Psychosocial factors may be the primary reasons for poor survival in unmarried patients. Therefore, physicians should include consideration of social supports during their care of unmarried patients with HCC and especially, of widowed patients, to help improve postoperative survival.
MATERIALS AND METHODS
Patient selection in the SEER database
The SEER program of the National Cancer Institute is an authoritative source of information on cancer incidence and survival in the United States. The SEER program registries routinely collect data on patient demographics, primary tumor site, the tumor morphology and stage at diagnosis, first course of treatment, and the follow up for survival—the SEER program is the only source of population-based information in the United States that includes the stage of cancer at the time of diagnosis as well as patient survival data.
Seer data contain no identifiers and have been widely used for studies of the relationship between marital status and survival outcome in patients with cancer [4, 5, 26–29]. We used SEER*Stat 8.1.5 software to identify patients with a histopathologic diagnosis of LC between 1998 and 2013. The morphology codes were limited to HCC (8170, 8171, 8172, 8173, 8174, 8175).
We excluded patients who were less than 18 years at diagnosis; did not undergo surgical resection for LC; had multiple primary cancers, of which the LC was not the first; and who had an unknown cause of death or unknown survival length.
According to the SEER staging system, tumors that remained in situ or confined to the organ of origin were considered to be localized; tumors that invaded locally or metastasized to regional lymph nodes were regarded as regional, while those that traveled to distant organs were considered to be distant.
Statistical analysis
We analyzed sex, age, race, primary tumor site, pathology grade, histologic type, TNM stage, tumor size, SEER stage, and marital status at the time of diagnosis. The TNM stage was established according to the criteria described in the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (7th edition). We classified patients as “married,” “never married,” “widowed,” or “separated/divorced.”
The primary endpoint of this study was HCSS, which was derived from the date of diagnosis and the date of cancer-specific death. Deaths attributed to HCC were treated as events, and deaths from other causes were treated as censored observations.
The baseline patient demographics and tumor characteristics were compared using the chi-square test. The HCC death rate was compared between groups using the Kaplan–Meier method. Risk factors for survival outcome were analyzed using multivariate Cox regression models. All statistical analyses were performed using the statistical software package SPSS for Windows, version 22 (IBM Corp, Armonk, NJ, USA). Statistical significance was set at two-sided P < 0.05.
Acknowledgments
This work was supported by six kinds of talents of jiangsu province (No. WSW–087).
Footnotes
CONFLICTS OF INTEREST
The authors made no disclosures.
REFERENCES
- 1.Tinkle CL, Haas-Kogan D. Hepatocellular carcinoma: natural history, current management, and emerging tools. Biologics. 2012;6:207–219. doi: 10.2147/BTT.S23907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liver EAFTSOT EASL–EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Denberg TD, Beaty BL, Kim FJ, Steiner JF. Marriage and ethnicity predict treatment in localized prostate carcinoma. Cancer. 2005;103:1819–1825. doi: 10.1002/cncr.20982. [DOI] [PubMed] [Google Scholar]
- 5.Nelles JL, Joseph SA, Konety BR. The impact of marriage on bladder cancer mortality. Urologic oncology. 2009;27:263–267. doi: 10.1016/j.urolonc.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Li Q, Gan L, Liang L, Li X, Cai S. The influence of marital status on stage at diagnosis and survival of patients with colorectal cancer. Oncotarget. 2015;6:7339–7347. doi: 10.18632/oncotarget.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang XD, Qian JJ, Bai DS, Li ZN, Jiang GQ, Yao J. Marital status independently predicts pancreatic cancer survival in patients treated with surgical resection: an analysis of the SEER database. Oncotarget. 2016;7:24880–24887. doi: 10.18632/oncotarget.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He XK, Lin ZH, Qian Y, Xia D, Jin P, Sun LM. Marital status and survival in patients with primary liver cancer. Oncotarget. 2016 Aug 5; doi: 10.18632/oncotarget.11066. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan RM, Kronick RG. Marital status and longevity in the United States population. Journal of epidemiology and community health. 2006;60:760–765. doi: 10.1136/jech.2005.037606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu YR, Goldman N. Mortality differentials by marital status: an international comparison. Demography. 1990;27:233–250. [PubMed] [Google Scholar]
- 11.Ikeda A, Iso H, Toyoshima H, Fujino Y, Mizoue T, Yoshimura T, Inaba Y, Tamakoshi A, JACC Study Group Marital status and mortality among Japanese men and women: the Japan Collaborative Cohort Study. BMC public health. 2007;7:73. doi: 10.1186/1471-2458-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldzweig G, Andritsch E, Hubert A, Brenner B, Walach N, Perry S, Baider L. Psychological distress among male patients and male spouses: what do oncologists need to know? Ann Oncol. 2010;21:877–883. doi: 10.1093/annonc/mdp398. [DOI] [PubMed] [Google Scholar]
- 13.Iwashyna TJ, Christakis NA. Marriage, widowhood, and health-care use. Soc Sci Med. 2003;57:2137–2147. doi: 10.1016/s0277-9536(02)00546-4. [DOI] [PubMed] [Google Scholar]
- 14.Garssen B, Goodkin K. On the role of immunological factors as mediators between psychosocial factors and cancer progression. Psychiatry research. 1999;85:51–61. doi: 10.1016/s0165-1781(99)00008-6. [DOI] [PubMed] [Google Scholar]
- 15.Sklar LS, Anisman H. Stress and coping factors influence tumor growth. Science. 1979;205:513–515. doi: 10.1126/science.109924. [DOI] [PubMed] [Google Scholar]
- 16.Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future oncology. 2010;6:1863–1881. doi: 10.2217/fon.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy SM, Herberman RB, Whiteside T, Sanzo K, Lee J, Kirkwood J. Perceived social support and tumor estrogen/progesterone receptor status as predictors of natural killer cell activity in breast cancer patients. Psychosomatic medicine. 1990;52:73–85. doi: 10.1097/00006842-199001000-00006. [DOI] [PubMed] [Google Scholar]
- 18.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 19.Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health psychology. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- 20.Formica V, Luccchetti J, Cunningham D, Smyth EC, Ferroni P, Nardecchia A, Tesauro M, Cereda V, Guadagni F, Roselli M. Systemic inflammation, as measured by the neutrophil/lymphocyte ratio, may have differential prognostic impact before and during treatment with fluorouracil, irinotecan and bevacizumab in metastatic colorectal cancer patients. Med Oncol. 2014;31:166. doi: 10.1007/s12032-014-0166-6. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton TD, Leugner D, Kopciuk K, Dixon E, Sutherland FR, Bathe OF. Identification of prognostic inflammatory factors in colorectal liver metastases. BMC Cancer. 2014;14:542. doi: 10.1186/1471-2407-14-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocrine reviews. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 23.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 24.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychological medicine. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaremka LM, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Marital distress prospectively predicts poorer cellular immune function. Psychoneuroendocrinology. 2013;38:2713–2719. doi: 10.1016/j.psyneuen.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, Graham PL, Choueiri TK, Hoffman KE, Martin NE, Hu JC, Nguyen PL. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31:3869–3876. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Wilson SE, Stewart DB, Hollenbeak CS. Marital status and colon cancer outcomes in US Surveillance, Epidemiology and End Results registries: does marriage affect cancer survival by gender and stage? Cancer epidemiology. 2011;35:417–422. doi: 10.1016/j.canep.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Rippentrop JM, Joslyn SA, Konety BR. Squamous cell carcinoma of the penis: evaluation of data from the surveillance, epidemiology, and end results program. Cancer. 2004;101:1357–1363. doi: 10.1002/cncr.20519. [DOI] [PubMed] [Google Scholar]
- 29.Kravdal H, Syse A. Changes over time in the effect of maritalstatus on cancer survival. BMC public health. 2011;11:804. doi: 10.1186/1471-2458-11-804. [DOI] [PMC free article] [PubMed] [Google Scholar]


