Summary
Working in laboratories of clinical chemistry, we risk feeling that our personal contribution to quality is small and that statistical models and manufacturers play the major roles. It is seldom sufficiently acknowledged that personal knowledge, skills and common sense are crucial for quality assurance in the interest of patients. The employees, environment and procedures inherent to the laboratory including its interactions with the clients are crucial for the overall result of the total testing chain. As the measurement systems, reagents and procedures are gradually improved, work on the preanalytical, postanalytical and clinical phases is likely to pay the most substantial dividends in accomplishing further quality improvements. This means changing attitudes and behaviour, especially of the users of the laboratory. It requires understanding people and how to engage them in joint improvement processes. We need to use our knowledge and common sense expanded with new skills e.g. from the humanities, management, business and change sciences in order to bring this about together with the users of the laboratory.
Keywords: quality assurance, quality control, metrology, preanalytical error, postanalytical error
Kratak sadržaj
Radeći u kliničko-hemijskoj laboratoriji, može nam se učiniti da je naš lični doprinos kvalitetu mali i da glavne uloge pripadaju statističkim modelima i proizvođačima. Retko se u dovoljnoj meri odaje priznanje ličnom znanju, veštinama i zdravoj logici, koji su od presudne važnosti za osiguranje kvaliteta u interesu pacijenata. Zaposleni, okolina i procedure koje se obavljaju u laboratoriji, uključujući njenu interakciju sa klijentima, ključne su za konačni rezultat u ukupnom lancu testiranja. Kako se sistemi merenja, reagensi i procedure postepeno unapređuju, rad na preanalitičkoj, postanalitičkoj i kliničkoj fazi verovatno će se najznačajnije isplatiti u vidu postizanja daljeg napretka u kvalitetu. Ovo znači da treba promeniti stavove i ponašanje, pre svega korisnika laboratorije. Potrebno je razumeti ljude i to na koji način se oni mogu uključiti u zajedničke procese poboljšanja. Moramo upotrebiti svoje znanje i zdravu logiku upotpunjene novim veštinama npr. iz oblasti društvenih nauka, upravljanja, poslovanja i menjati nauke kako bismo ovo postigli zajedno sa korisnicima laboratorije.
Quality control, quality assurance and total quality management in Clinical chemistry
According to ISO 9000:2005, Clause 3.2.11, quality assurance is a part of quality management, providing confidence that quality requirements will be fulfilled. Quality control is monitoring to indicate needed corrective responses.
Quality control in clinical chemistry has its roots in precision mass production of telephone exchangers at the Western Electric Company in the 1920es which fostered many of the pioneers of quality assurance including Shewhart, Deming and Juran (1–3). Levey and Jennings (4), Henry and Segalove (5) introduced quality control methods including control charts in clinical laboratories in the 1950es focusing on singleton measurement of control samples – which is routine today.
Westgard, deVerdier, Groth and Aronsson (6, 7) addressed the important problem of false rejections of measurements introducing the use of multiple control rules. They naturally used then prevailing batch – oriented measurement systems and methods as paradigms also when estimating the power functions of the control rules and of combinations of them (8, 9). Singleton measurements of control samples became common practice in proficiency testing/ external quality control during this time period, also in programs established for regulatory purposes including CLIA 88 (10) in the U.S.A. and RiliBÄK (11–14) in Germany.
Laessig and Ehrmeyer (15) have shown that CLIA 88 regulatory requirements have resulted in improvements in laboratory medicine, but thoughtfully pose the question »Do fewer PT failures, fewer inspection deficiencies, and a very limited number of government sanctions mean that the quality of patient test results has improved?«. It is similarly not yet established whether accreditation standards and similar schemes including ISO 15189 (16) have improved the diagnostic value of results from accredited laboratories (17). Importantly the introduction of ISO 15189 (16) broadened the scope of accreditation from the measurement process itself to the interaction of the laboratory with its clients and to the total testing chain, including the pre-and postanalytical processes. This is in tune with the widely practiced and well-established approaches of total quality management systems. This development together with the responsibility of the manufacturers for the measurement systems and reagents (18) creates the environment for re-orientation of laboratories of clinical chemistry to closer co-operation with their users.
Total quality management (TQM) in clinical chemistry consists of efforts to establish and maintain a climate of continued improvements in the laboratory in order to deliver high-quality services to health care. Total quality management systems come in numerous variants forwarded by different organizations but are united by the following major cornerstones: 1) customer needs define quality, 2) continuous monitoring (19), systematic analysis and improvement of crucial work processes are needed, 3) the top leadership of the laboratory is responsible for the quality and quality improvements.
The well-established principles of total quality management come in handy when optimizing the total testing chain.
Quality assurance must be implemented, managed and maintained by the leadership of the laboratory.
Procedures, processes and systems and not people represent the major obstacles to optimal quality.
Avoid treating the symptoms. Treat the »disease« instead, with the intention to cure
Every employee is responsible for hers/his part in the overall quality of the laboratory
If a quality problem is discovered in the services of a laboratory the search for root causes should start at the top of the staircase of responsibility. When physical staircases need cleaning, an appropriate cleaning process should start at the top since dust gravitates downwards.
The initial efforts made by the laboratories to acquire accreditation are commonly the most rewarding as they engage all of the employees and the whole organisation in a goal-directed and concerted effort for improvements. As the years pass by, accreditation usually becomes the primary activity of a handful of persons in the laboratory who create a bureaucracy for the purpose. Standards and accreditation are important for quality assurance but in their basic nature they strive for status quo rather than for dynamic development with the inherent risks that changes invite.
The avoidance of the risks of organisational changes is fundamentally not a property of the standards themselves. However, the involved people – both laboratory personnel, and in the accreditation authorities – are afraid that the process of change decreases quality. Consolidating laboratories of e.g. microbiology, virology, immunology, pharmacology, genetics and clinical chemistry laboratories in order to use common automation- and information technologies involves inevitable risks of professional and other inter-personal tensions, which e.g. endanger the demand of the standard for clarity in the »the specified responsibilities, authority, and interrelationships of all personnel« (ISO 15189). Accreditation according to ISO standards, as commonly practiced today, therefore risks becoming not only an obstacle but also a real enemy of the necessary paradigm shift in laboratory medicine made possible by advances in automation and information technologies. Flexible scope of accreditation (17, 20) may represent a partial solution to this challenge, but a more radical scheme of more intensive monitoring by the accreditation authorities during periods of major transitions for accredited laboratories may be needed in order to avoid the need to abandon forma accreditation when performing major restructuring.
The total testing chain is only as strong as its weakest link
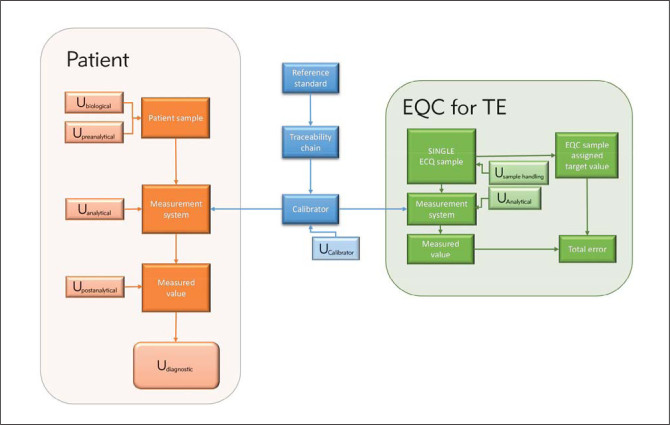
There is an age-old truth in the saying that »a chain is only as strong as its weakest link«. It also holds true for the total testing process in clinical chemistry e.g. as depicted in Figure 1. Errors and uncertainties introduced at any link in the chain influence the clinical value of the laboratory results and in every one of the four phases in the testing process, clinical, pre-clinical, analytic and post-analytic phases (21–27).
Figure 1.

The total testing chain in clinical chemistry involves several professionals and organizations in healthcare from the clinical decision to order a test through the pre-analytical, analytical and post-analytical phases to the value of the test result in the on-going clinical decisions and healthcare process.
Uncertainty of the high-volume measurement methods in clinical chemistry has decreased substantially with the advent of highly automated measurement methods and reference measurement systems. The most substantial improvements have been accomplished in reducing the repeatability and reproducibility. Bias has also been decreased, but not to the same extent (28).
The preanalytical, postanalytical and clinical phases (collectively known as extraanalytical phases) of the testing processes have not been addressed nearly to the same extent as the analytical phase, probably because they involve multiple categories of professionals working in the clinic and are therefore outside of the boundaries of total control of the laboratory. The mode of practice in these fields is also different amongst countries and also within the same country e.g. regarding which personnel category is responsible for preparing the patient/person before blood sampling and for taking the sample. In brief; it depends on whether the taking and handling of samples is under the auspices of the laboratory or not. Research in the fields of pre- and post-analytical factors in laboratory medicine has seen exponential growth during recent years. However, since most of the economy of the laboratory is spent in the analytical phase, it still attracts the main focus of both the diagnostic industry and healthcare.
Another important reason that pre- and postanalytical factors have been studied less than analytical factors is that other research- and administrative paradigms are needed than when studying analytical factors which can and should be addressed by sound principles of e.g. metrology. Optimal pre- and post-analytical procedures are frequently known and agreed on in professional circles. However, it is commonly difficult to convince people – particularly in the clinic – that it is clinically- and cost effective to make the efforts needed to implement them.
The analytical phase of the total testing chain
The quality of the analytical phase of the total testing process has been and is being improved e.g. by the International Standardization Organization (ISO), e.g. through the ISO standard 17511:2003 detailing how the metrological traceability of values assigned to calibrators and control materials is established, The Joint Committee for Traceability in Laboratory Medicine (JCTLM) established in 2002 i.a. by the IFCC and the International Consortium for Harmonization of Clinical Laboratory Results (ICH-CLR) established by The American Association of Clinical Chemistry (AACC) in 2010. The Empower project (29) (http://stt-consulting.com/index.php) is a new promising and energetic newcomer in the field.
Bonini et al. (30) compiled the results of several studies showing that 68–87% of all errors in laboratory medicine occur in the extraanalytical phases (preanalytical, postanalytical and clinical phases) and that correspondingly only 13–32% of errors in the total testing phase can be reduced by diligent work in the analytical phase.
Singleton measurement of control samples for quality control
External quality control procedures in clinical chemistry traditionally focus on singleton- sample methods for quality control, which means that a control sample is measured only once before the result is reported. Total error methods are suitable for evaluating the results in this case since they evaluate the combination of random error and bias (accuracy/total error). Singleton measurements are efficient for regulatory purposes since a minimum number of control samples (one) and measurements (one) are required. The drawback in some situations is that singletons are suboptimal for distinguishing between random error and bias as causes of the (total) error (31) (Figure 2).
Figure 2.
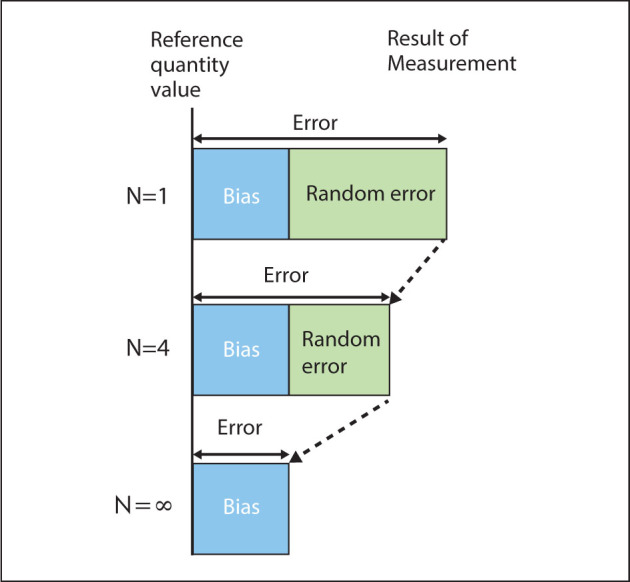
When a mean of a result is reported, the error of the mean is influenced both by bias and random error. The standard error of the mean is inversely related to the square root of the number of replicates and thus decreases quadratically with the number of replicates. As the number of replicates is increased, the contribution of the random error to the measurement error of the mean approaches zero, thereby improving the estimate of the bias.
Bias is commonly estimated by participation in proficiency testing schemes (external quality control), using certified reference materials or by comparisons with reference methods (32, 33). Comparisons are commonly made by stabilized samples which do not necessarily exhibit all the properties of natural patient samples. Natural patient samples are commutable (34) by definition and in practice whereas stabilized control materials may or may not be commutable. If the main purpose of a quality control system is to minimize the overall measurement uncertainty of all measurement systems and methods in an organization or geographical area, the use of fresh split patient samples is more efficient in finding clinically important bias and thereby for minimizing measurement uncertainty, especially when replicate measurements are used for minimizing random error.
Figure 3.

External quality control (ECQ) organizations send out stabilized quality control samples which are analyzed as singletons and evaluated centrally (depicted as dotted arrows). The use of split fresh patient samples (depicted as the solid black ring) including the use of replicate measurements facilitates finding bias and thereby minimization of measurement uncertainty in an organization or a geographical area. A laboratory represented by the yellow circle may preferably serve as a mentor for a certain measurand for the other laboratories in the conglomerate of laboratories serving a certain population (28, 34).
The use of fresh split patient samples for quality control makes common sense for several reasons:
the material has optimal matrix properties (is commutable),
the material is available without cost for all laboratories accepting routine patient samples,
there is general agreement that all measurement systems and reagents should optimally result in identical results when analyzing the same patient samples,
the methods are optimal for identifying the measurement system(s) in the organization that contribute the largest part of the overall measurement uncertainty due to bias.
Split sample methods are laborious in the absence of effective computerized systems, but convenient when properly implemented (34, 35).
Most laboratory organizations that introduce split sample methods prefer to continue their participation in external quality control schemes for the purpose of being able to compare their results more widely.
Traceability
It is comforting when other laboratories measure approximately the same measurement result for the same measurand in the same sample. However, the absence of bias does not, in on its own, constitute a proof of trueness. Absence of bias must be combined with a demonstrated traceability chain – that each measurement result is linked to an independent common »stated reference« through an uninterrupted chain of comparisons to demonstrate traceability. Traceability is »vertical« to the »horizontal« process of establishing the absence of bias. Thus inter-laboratory comparisons by themselves do not provide traceability of the participants’ results. It is the task of the participants’ themselves task to ensure the traceability of their results (36, 37).
Making sure there is traceability of measurement methods of the laboratory takes knowledge, skills and common sense of the engaged persons and makes especially good common sense when the results from the laboratory are to be used in studies involving several countries or when decision limits established in large population studies are implemented.
Harmonization
Only a minor portion of common methods in clinical chemistry are currently traceable. It is, however, possible to harmonize (38) the majority of all measurement methods using commutable sample materials, including patient samples (39, 40). It is not an easy undertaking, but potentially very valuable for the patients. Routine laboratories of clinical chemistry with their abundance of patient samples are in an especially favourable position to participate in harmonization projects which optimally are done in co-operation with reference laboratories and with co-operation of the producers of the relevant measurement systems and reagents (41).
Uncertainty when measuring patient samples vs measurement error in control samples
The purpose of laboratory medicine is to reduce uncertainty when physicians diagnose diseases and monitor treatment effects – diagnostic uncertainty. Clinical chemistry pioneered in establishing the theoretical framework and practical routines for single sample-based external quality control (EQC) and batch-oriented routines for internal quality control. The total error of a measurement system estimated when measuring control samples is frequently the main emphasis of laboratories despite the fact that the total error only represents in the order of 20% of the diagnostic uncertainty related to laboratory medicine (30) (Figure 4).
Figure 4.
The diagnostic uncertainty of a measurement result in a patient sample is a property of the measurement result itself, influenced by several uncertainty components, including biological variation, preanalytical variation, analytical variation (including uncertainty of the calibration) and postanalytical variation. The total error of an external quality control sample, in contrast, is influenced by substantially fewer and smaller uncertainty components and therefore represents a property of the measurement system itself. The total error is commonly used for regulatory or accreditation purposes. EQC = extrenal quality control, TE = Total error.
Measuring the concentration of a measurand in a stabilized control sample in internal quality control or in proficiency testing involves much fewer uncertainty factors than being requested to prepare a patient, take a sample, process the sample, transport the sample, analyse the sample and interpret the results in a clinical context (Figure 4). The uncertainty factors involved when measuring a stabilized control sample are mainly the sample handling and the uncertainty of the measurement system. Therefore, the accuracy/combination of bias and imprecision/ total error of the measurement result represents properties of the measurement system. The total error estimated from singleton measurements of control samples has been found appropriate for regulatory purposes and an extensive theoretical and practical framework has been developed around its use (42, 43). According to a recent definition total analytical error (TAE) defines the interval that contains a specified proportion (usually 95% or 99%) of the distribution of analytical measurement differences between a measurement procedure operating in its stable incontrol state and a comparative measurement procedure that is either a definitive reference method or one that is traceable to one (43). Correspondingly allowable total error (ATE) is an analytical quality requirement that sets a limit for both the imprecision (random error) and bias (systematic error) that are tolerable in a single measurement or single test result.
Regulatory issues are not of primary interest in many countries, certainly in the Nordic countries where the majority of labs are accredited according to ISO 15189. The laboratory organisation that the present writer belongs to caters for all laboratory services for 0,5 million inhabitants including point-of-care measurement methods. All laboratory services (including all specialties) are covered by the same accreditation. The total outcome is king in this environment, e.g. glycaemic control in the diabetic population, glycated haemoglobin and the contribution of the laboratory organization in optimizing treatment. It is a substantial challenge keeping the total CV% for HbA1c below 3% (total CV% for in the order of 100 measurement systems) as demanded by the diabetologists. This means that the performance of a single measurement system in external quality control systems has somewhat lower priority than the contribution of that measurement system to the overall CV% of HbA1c used for the entire population. In an environment of this kind, eliminating the contribution of the poorest performing measurement system (bias and random error) becomes particularly important.
The extraanalytical phases of the total testing chain
Academic organizations and producers of measurement systems and reagents are already heavily involved in improving the measurement part of the total testing process. The extraanalytical phases are also in need of substantial development. Current and future efforts in harmonizing measurement results in clinical chemistry are likely to include extensive cooperation between e.g. clinically active persons, the industry, standardization organizations, professional organizations and individual laboratories. They do also include all aspects of the process from the clinical decision to use the clinical chemistry laboratory in diagnosis through preparing the patient, taking- and transporting the samples (44), measuring the samples and reporting the results and including the interpretation of the results in the clinical (Figure 1).
Preanalytical factors
Statland, Winkel, Guder and other nestors in the field of preanalytics paved the important way for realization of the essence of preanalytics for decreasing diagnostic uncertainty and for standardization in the preanalytical field (45–48). Plebani (49), Lippi (50–53) Lima-Oliveira (54), and Simundic (55) have been particularly influential recently in focusing attention on improved procedures and indicators for improving practices in the preanalytical phase.
Statistical and graphical methods are essential for quality control and for calculating measurement uncertainty in the analytical phase. Statistical methods can also be applied in the preanalytical phase, e.g. for monitoring the occurrences of different kinds of preanalytical errors (56). The information gained in this way should primarily be channeled into practical work in the laboratory and with the users of the laboratory to minimize and – if possible – practically eliminate preanalytical errors. There are limits to the extent which uncertainty in the analytical phase can be reduced. In contrast sources of uncertainty in the preanalytical phase can be practically eliminated by optimizing practices for e.g. patient preparation, phlebotomy and sample transport. Sample transport practices can be improved by investments in e.g. vacuum tube systems or by contracting certified regional transporters of samples, regularly monitoring their performance through sensors regularly sent with the samples.
It is however, even more challenging to change the behavior of nurses, doctors and others responsible for patient preparation, phlebotomy and other preanalytical procedures outside the control of the laboratory. Different circumstances and individuals may also need different means of persuasion and education in order to minimize preanalytical errors. Time is well spent listening to the opinions of the users of the laboratory in different natural situations of co-operation. However, even more structured means of qualitative research (57–59) may be needed for proper understanding of the possible obstacles to change and to elucidate the most promising approaches going forwards. Advanced change management methods may be needed to accomplish the improvements needed. Neither of these technologies are amongst tools that have as yet been widely applied in clinical chemistry. Unfortunately there is no firm evidence as to the best methods to employ for the purpose of changing practices in healthcare (60). Tailored interventions/implementations including the use of guidelines can be effective, but the effect is variable and tends to be small to moderate (60). Studies investigating the components of tailoring (identification of the most important determinants, selecting interventions to address the determinants) are especially lacking. Motivational factors, practice coaches and persistence are amongst the most likely determinants (61–63).
Eliminating preanalytical errors deserve to rank highest on the list of priorities when attempting to continue to reduce diagnostic uncertainty. Structured and persistent work in this area means that personnel from the laboratory need to allocate sufficient time and efforts to this purpose. The fact that laboratories are seldom reimbursed for work in the preanalytical field, commonly means that sufficient emphasis and time is not allocated.
There are several valuable current developments for defining analytical quality specifications (64) and overall diagnostic uncertainty (the combined uncertainty of all uncertainty components involved when using the laboratory to support diagnosis). However, increased emphasis on changing behaviours in the preanalytical field promise to be even more important than developing methods for adding uncertainties arising in the preanalytical phase to the overall diagnostic uncertainty of laboratory results.
Postanalytical factors
Co-operation with clinical disciplines on Health Technology Assessment (HTA), evidence- based medicine (EBM), guidelines etc. is well established both on paper and in practice. Hopefully this and other factors striving for excellence in healthcare can lead to projects aiming for harmonization and improvements of practice especially in the pre-and postanalytical parts of the total testing process.
Important steps can be taken through many channels to improve the clinical use- and value of diagnostic procedures available through clinical chemistry. The laboratory and the clinicians are increasingly making co-operative projects in diagnostic guidelines and in the implementation of these guidelines. I personally believe joint projects of this kind may serve to facilitate other projects in the pre-and postanalytical areas (65).
Motivation, knowledge and common sense
Laboratory medicine performs a highly practical high-volume production, but its cornerstone is intellectual. Motivation is the mother of all intellectual pursuits. All measures that increase the motivation of the employed in the laboratory contribute to the overall quality of the services.
The most important factor for creating and maintaining motivation is the intellectual and organisational environment of the laboratory. Active participation in research projects, organisational and quality improvement projects is motivational. Collaborative projects directly aimed at improving the quality of the services to the patients have especially strong motivational effects when done in collaboration with workers in other areas of healthcare. Research projects in the basic sciences are also important as they bring and maintain knowledge in scientific philosophy and methods, thereby increasing understanding of the meaning and proper interpretation of data.
It is a substantial challenge to maintain motivation throughout extended periods of time especially since demands for the reduction of costs and the number of workers are of regular occurrence. It is therefore important to regularly lift the focus from the mundane challenges of the laboratory and all its employees to the needs of the patients. External inspections of the quality assurance of the laboratory e.g. as part of ISO 15189 accreditation serves an important role in this context as it renews important commitments and focus on purpose.
Common sense is especially important in the extraanalytical phases of the testing chain. Uncertainties in the preanalytical, postanalytical and clinical phases of the testing chain may be partially estimated as type A uncertainties (66) by calculating coefficients of variation. However, the majority of the uncertainty components in the extraanalytical phases need to be quantified as best estimates – type B uncertainties (66). In contrast to imprecision in the analytical phase which cannot be eliminated the goal should be to eliminate uncertainty components in the extraanalytical phases, in order to as much as possible eliminate their contribution to the overall diagnostic uncertainty (Figure 4). This is a lofty but not an unrealistic goal. As a matter of fact, any improvements in phlebotomy practices, sample treatment, sample transport, interpretation of the results in clinical and biologic variation contexts will decrease the contributions of the extraanalytical phases to the overall diagnostic uncertainty. Such crucial improvements will not happen by themselves. They are brought about by the motivation, knowledge and common sense of us – the workers in the laboratories of clinical chemistry- influencing our clinical colleagues for the benefit of the patients.
Conclusion
Clinical chemistry is in the process of paradigm shift from a primary focus on optimizing the measurement methods themselves to more intense collaboration with persons engaged in clinical work in order to reduce preanalytical, postanalytical and clinical uncertainties thereby improving the clinical use of laboratory methods. Manufacturers of measurement systems and reagents now shoulder the main responsibilities for the analytical process leaving time for optimizing preanalytical, postanalytical and clinical processes demanded e.g. by the accreditation standard ISO 15189. In order to shoulder these added responsibilities clinical chemistry needs to use its abundant common sense and learn from the humanities and from management-, business- and change sciences how to proceed in the interest of patients.
Conflict of interest statement
The authors stated that they have no conflicts of interest regarding the publication of this article
References
- 1.Shewhart WA. Economic control of quality of manufactured product. New York: D. Van Nostrand Company; 1931. [Google Scholar]
- 2.Shewhart WA. Statistical method from the viewpoint of quality control. New York: Dover Publications; 1939. [Google Scholar]
- 3.Small BB. Western Electric Company; 1956. http://www.contesolutions.com/Western_Electric_SQC_Handbook.pdf Available from: [Google Scholar]
- 4.Levey S, Jennings ER. The use of control charts in the clinical laboratory. Am J Clin Pathol. 1950;20:1059–66. doi: 10.1093/ajcp/20.11_ts.1059. [DOI] [PubMed] [Google Scholar]
- 5.Henry RJ, Seaglove M. The running of standards in clinical chemistry and the use of the control chart. J Clin Pathol. 1952;5:305–11. doi: 10.1136/jcp.5.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westgard JO, Groth T, Aronsson T, de Verdier CH. Combined Shewhart-cusum control chart for improved quality control in clinical chemistry. Clinical Chemistry. 1977;23(10):1881–7. [PubMed] [Google Scholar]
- 7.Westgard JO, Groth T, Aronsson T, Falk H, de Verdier CH. Performance characteristics of rules for internal quality control: probabilities for false rejection and error detection. Clinical Chemistry. 1977;23(10):1857–67. [PubMed] [Google Scholar]
- 8.Westgard JO, Groth T. Power functions for statistical control rules. Clinical Chemistry. 1979;25(6):863–9. [PubMed] [Google Scholar]
- 9.Westgard JO, Barry PL, Hunt MR, Groth T. A multi-rule Shewhart chart for quality control in clinical chemistry. Clinical Chemistry. 1981;27(3):493–501. [PubMed] [Google Scholar]
- 10.CDC. CLIA 88. Certification of laboratories. http://wwwn.cdc.gov/clia/pdf/PHSA_353.pdf1988 http://wwwn.cdc.gov/clia/pdf/PHSA_353.pdf Available from:
- 11.Bundesärztekammer. Richtlinie der Bundesärztekammer zur Qualitätssicherung laboratoriumsmedizinischer Untersuchungen Gemäβ dem Beschluss des Vorstands der Bundesärztekammer vom 11.04.2014 und 20.06.2014. Deutsches Ärzteblatt. 2014;111(38):A1583–A618. [Google Scholar]
- 12.Vogt W. New Guidelines of the German Medical Association for Quality Assurance of Medical Laboratory Tests. Laboratoriumsmedizin. 2015;39(1):6–7. [Google Scholar]
- 13.Neumaier M. Quality assurance in the medical laboratory: a success story in the health-care system. Laboratoriumsmedizin. 2015;39(1):10. [Google Scholar]
- 14.Neumaier M, Luppa PB, Koschinsky T, Siegel E, Freckmann G, Heinemann L. Updated Requirements for Measurement Quality and Quality Assurance of Point-Of-Care Testing (POCT) – Blood Glucose Measurement Systems with Unit-Use Reagents Suitable for the Initial Diagnosis of Diabetes Manifested inPregnancy or Gestational Diabetes Mellitus (GDM) According to the GDM Guideline of the German Diabetes Association (DDG): Consensus Recommendation by the German United Society for Clinical Chemistry and Laboratory Medicine (DGKL) and the German Diabetes Association 2015. Diabetol Stoffwechs. 2015;10(4):E1–E3. [Google Scholar]
- 15.Ehrmeyer SS, Laessig RH. Has compliance with CLIA requirements really improved quality in US clinical laboratories? Clinica Chimica Acta. 2004;346(1):37–43. doi: 10.1016/j.cccn.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 16.ISO. ISO 15189: 2012 Medical laboratories – Requirements for quality and competence. Geneva: International Standardisation Organisation; 2012. [Google Scholar]
- 17.Plebani M, Sciacovelli L, Chiozza ML, Panteghini M. Once upon a time: a tale of ISO 15189 accreditation. Clinical Chemistry and Laboratory Medicine. 2015;53(8):1127–9. doi: 10.1515/cclm-2015-0355. [DOI] [PubMed] [Google Scholar]
- 18.EU. Eur-Lex. EN: NOT; 1998. Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices.http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:31998L0079 [Google Scholar]
- 19.Kilinc C. Laboratory quality management systems: missions, goals and activities in quality assurance. Clin Bio-chem. 2009;42(4–5):301–2. doi: 10.1016/j.clinbiochem.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Thelen MH, Vanstapel FJ, Kroupis C, Vukasovic I, Boursier G, Barrett E. et al. Flexible scope for ISO 15189 accreditation: a guidance prepared by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) Working Group Accreditation and ISO/CEN standards (WG-A/ISO) Clinical Chemistry and Laboratory Medicine. 2015;53(8):1173–80. doi: 10.1515/cclm-2015-0257. [DOI] [PubMed] [Google Scholar]
- 21.Plebani M, Sciacovelli L, Aita A, Padoan A, Chiozza ML. Quality indicators to detect pre-analytical errors in laboratory testing. Clinica Chimica Acta; 2014;432:44–8. doi: 10.1016/j.cca.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 22.Plebani M. Harmonization in laboratory medicine: the complete picture. Clinical Chemistry and Laboratory Medicine. 2013;51(4):741–51. doi: 10.1515/cclm-2013-0075. [DOI] [PubMed] [Google Scholar]
- 23.Plebani M. Harmonization in laboratory medicine: Requests, samples, measurements and reports. Crit Rev Clin Lab Sci. 2015:1–13. doi: 10.3109/10408363.2015.1116851. [DOI] [PubMed] [Google Scholar]
- 24.Plebani M, Astion ML, Barth JH, Chen W, de Oliveira Galoro CA, Escuer MI. et al. Harmonization of quality indicators in laboratory medicine. A preliminary consensus. Clinical Chemistry and Laboratory Medicine. 2014;52(7):951–8. doi: 10.1515/cclm-2014-0142. [DOI] [PubMed] [Google Scholar]
- 25.Plebani M, Sciacovelli L, Aita A, Padoan A, Chiozza ML. Quality indicators to detect pre-analytical errors in laboratory testing. Clinica Chimica Acta. 2014;432:44–8. doi: 10.1016/j.cca.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 26.Plebani M, Sciacovelli L, Marinova M, Marcuccitti J, Chiozza ML. Quality indicators in laboratory medicine: A fundamental tool for quality and patient safety. Clinical Biochemistry. 2013;46(13–14):1170–4. doi: 10.1016/j.clinbiochem.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Sciacovelli L, O’Kane M, Skaik YA, Caciagli P, Pellegrini C, Da Rin G. et al. Quality Indicators in Laboratory Medicine: from theory to practice. Clinical Chemistry and Laboratory Medicine. 2011;49(5):835–44. doi: 10.1515/CCLM.2011.128. [DOI] [PubMed] [Google Scholar]
- 28.Theodorsson E, Magnusson B, Leito I. Bias in clinical chemistry. Bioanalysis. 2014;6(21):2855–75. doi: 10.4155/bio.14.249. [DOI] [PubMed] [Google Scholar]
- 29.De Grande LA, Goossens K, Van Uytfanghe K, Stockl D, Thienpont LM. The Empower project – a new way of assessing and monitoring test comparability and stability. Clinical Chemistry and Laboratory Medicine. 2015;53(8):1197–204. doi: 10.1515/cclm-2014-0959. [DOI] [PubMed] [Google Scholar]
- 30.Bonini P, Plebani M, Ceriotti F, Rubboli F. Errors in laboratory medicine. Clinical Chemistry. 2002;48(5):691–8. [PubMed] [Google Scholar]
- 31.Kallner A. Is the combination of trueness and precision in one expression meaningful? On the use of total error and uncertainty in clinical chemistry. Clinical Chemistry and Laboratory Medicine. 2016 doi: 10.1515/cclm-2015-0975. January 2016. [DOI] [PubMed] [Google Scholar]
- 32.Büttner J. Reference methods in clinical chemistry. Objectives, trends, problems. Eur J Clin Chem Clin Biochem. 1991;29:221–2. [Google Scholar]
- 33.Thienpont LM. Quality specifications for reference methods. Scand J Clin Lab Invest. 1999;59(7):535–8. doi: 10.1080/00365519950185300. [DOI] [PubMed] [Google Scholar]
- 34.Theodorsson E. Validation and verification of measurement methods in clinical chemistry. Bioanalysis. 2012;4(3):305–20. doi: 10.4155/bio.11.311. http://www.future-science.com/doi/abs/10.4155/bio.11.311?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov [DOI] [PubMed] [Google Scholar]
- 35.Norheim S. Computer Support Simplifying Uncertainty Estimation using Patient Samples. Linkoping: Linkoping University; 2008. http://liu.divaportal.org/smash/record.jsf?pid=diva2:417298 [Google Scholar]
- 36.Braga F, Infusino I, Panteghini M. Role and responsibilities of laboratory medicine specialists in the verification of metrological traceability of in vitro medical diagnostics. J Med Biochem. 2015;34:282–7. doi: 10.1515/jomb-2015-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Bievre P. Do interlaboratory comparisons provide traceability? Accredit Qual Assur. 1999;4(8):342–6. [Google Scholar]
- 38.Greenberg N. Update on current concepts and meanings in laboratory medicine – Standardization, traceability and harmonization. Clinica Chimica Acta. 2014;432:49–54. doi: 10.1016/j.cca.2013.12.045. [DOI] [PubMed] [Google Scholar]
- 39.Greg Miller W, Myers GL, Lou Gantzer M, Kahn SE, Schonbrunner ER, Thienpont LM. et al. Roadmap for harmonization of clinical laboratory measurement procedures. Clinical Chemistry. 2011;57(8):1108–17. doi: 10.1373/clinchem.2011.164012. [DOI] [PubMed] [Google Scholar]
- 40.Thienpont LM, Van Uytfanghe K, De Leenheer AP. Reference measurement systems in clinical chemistry. Clinica Chimica Acta. 2002;323(1–2):73–87. doi: 10.1016/s0009-8981(02)00188-2. [DOI] [PubMed] [Google Scholar]
- 41.Stepman HC, Tiikkainen U, Stockl D, Vesper HW, Edwards SH, Laitinen H. et al. Measurements for 8 common analytes in native sera identify inadequate standardization among 6 routine laboratory assays. Clinical Chemistry. 2014;60(6):855–63. doi: 10.1373/clinchem.2013.220376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Westgard JO, Westgard SA. Quality control review: implementing a scientifically based quality control system. Annals of Clinical Biochemistry. 2016;53(Pt 1):32–50. doi: 10.1177/0004563215597248. [DOI] [PubMed] [Google Scholar]
- 43.Westgard JO. Useful measures and models for analytical quality management in medical laboratories. Clinical Chemistry and Laboratory Medicine. 2016;54:223–33. doi: 10.1515/cclm-2015-0710. [DOI] [PubMed] [Google Scholar]
- 44.Truchaud A, Le Neel T, Brochard H, Malvaux S, Moyon M, Cazaubiel M. New tools for laboratory design and management. Clinical Chemistry. 1997;43(9):1709–15. [PubMed] [Google Scholar]
- 45.Statland BE, Bokelund H, Winkel P. Factors contributing to intra-individual variation of serum constituents. 4. Effects of posture and torniquet application on variation of serum constituents in healthy subjects. Clinical Chemistry. 1974;20:1513–9. [PubMed] [Google Scholar]
- 46.Statland BE, Winkel P. Effects of preanalytical factors on the intraindividual variation of a nalytes in the blood of healthy subjects: consideration of preparation of the subject and time of venipuncture. CRC Crit Rev Clin Lab Sci. 1977;10:105–44. doi: 10.3109/10408367709151694. [DOI] [PubMed] [Google Scholar]
- 47.Guder WG. History of the preanalytical phase: a personal view. Biochem Med (Zagreb) 2014;24(1):25–30. doi: 10.11613/BM.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guder WG. Samples : from the patient to the laboratory. Munich: Wiley VCH; 2003. 3rd, rev. ed. ed. [Google Scholar]
- 49.Plebani M. Quality indicators to detect pre-analytical errors in laboratory testing. Clin Biochem Rev. 2012;33(3):85–8. [PMC free article] [PubMed] [Google Scholar]
- 50.Lippi G, Banfi G, Church S, Cornes M, De Carli G, Grankvist K. et al. Preanalytical quality improvement. In pursuit of harmony, on behalf of European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) Working group for Preanalytical Phase (WG-PRE) Clinical Chemistry and Laboratory Medicine. 2015;53(3):357–70. doi: 10.1515/cclm-2014-1051. [DOI] [PubMed] [Google Scholar]
- 51.Lippi G, Becan-McBride K, Behulova D, Bowen RA, Church S, Delanghe J. et al. Preanalytical quality improvement: in quality we trust. Clinical Chemistry and Laboratory Medicine. 2013;51(1):229–41. doi: 10.1515/cclm-2012-0597. [DOI] [PubMed] [Google Scholar]
- 52.Lippi G, Chance JJ, Church S, Dazzi P, Fontana R, Giavarina D. et al. Preanalytical quality improvement: from dream to reality. Clinical Chemistry and Laboratory Medicine. 2011;49(7):1113–26. doi: 10.1515/CCLM.2011.600. [DOI] [PubMed] [Google Scholar]
- 53.Lippi G, Simundic AM, Plebani M. Phlebotomy, stat testing and laboratory organization: an intriguing relationship. Clinical Chemistry and Laboratory Medicine. 2012;50(12):2065–8. doi: 10.1515/cclm-2012-0374. [DOI] [PubMed] [Google Scholar]
- 54.Lima-Oliveira G, Lippi G, Luca Salvagno G, Picheth G, Cesare Guidi G. Laboratory diagnostics and quality of blood collection. J Med Biochem. 2015;34:288–294. doi: 10.2478/jomb-2014-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simundic AM, Lippi G. Preanalytical phase – a continuous challenge for laboratory professionals. Biochem Med (Zagreb) 2012;22(2):145–9. doi: 10.11613/bm.2012.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plebani M, Sciacovelli L, Aita A, Chiozza ML. Harmonization of pre-analytical quality indicators. Biochem Med (Zagreb) 2014;24(1):105–13. doi: 10.11613/BM.2014.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corbin JM, Strauss AL. Basics of qualitative research: techniques and procedures for developing grounded theory. 3rd ed. Los Angeles, Calif: Sage Publications, Inc; 2008. p. xv. 379 p. p. [Google Scholar]
- 58.Maxwell JA. Qualitative research design : an interactive approach. 3rd ed. Thousand Oaks, Calif: SAGE Publications; 2013. p. xi. 218 p. p. [Google Scholar]
- 59.Denzin NK, Lincoln YS. The Sage handbook of qualitative research. 4th ed. ed. Thousand Oaks: Sage; 2011. [Google Scholar]
- 60.Baker R, Camosso-Stefinovic J, Gillies C, Shaw EJ, Cheater F, Flottorp S. et al. Tailored interventions to address determinants of practice. Cochrane Database Syst Rev. 2015;4:CD005470. doi: 10.1002/14651858.CD005470.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fearing G, Barwick M, Kimber M. Clinical transformation: Manager’s perspectives on implementation of evidence-based practice. Adm Policy Ment Health. 2014;41(4):455–68. doi: 10.1007/s10488-013-0481-9. [DOI] [PubMed] [Google Scholar]
- 62.Bernhardsson S, Larsson ME, Eggertsen R, Olsen MF, Johansson K, Nilsen P. et al. Evaluation of a tailored, multi-component intervention for implementation of evidence-based clinical practice guidelines in primary care physical therapy: a non-randomized controlled trial. BMC Health Services Research. 2014;14:105. doi: 10.1186/1472-6963-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rousseau DM, Gunia BC. Evidence-Based Practice: The Psychology of EBP Implementation. Annu Rev Psychol. 2016;67:667–92. doi: 10.1146/annurev-psych-122414-033336. [DOI] [PubMed] [Google Scholar]
- 64.Sandberg S, Fraser CG, Horvath AR, Jansen R, Jones G, Oosterhuis W. et al. Defining analytical performance specifications: Consensus Statement from the 1st Strategic Conference of the European Federation of Clinical Chemistry and Laboratory Medicine. Clinical Chemistry and Laboratory Medicine. 2015;53:833–835. doi: 10.1515/cclm-2015-0067. [DOI] [PubMed] [Google Scholar]
- 65.Skeie S, Perich C, Ricos C, Araczki A, Horvath AR, Oosterhuis WP. et al. Postanalytical external quality assessment of blood glucose and hemoglobin A1c: an international survey. Clinical Chemistry. 2005;51(7):1145–53. doi: 10.1373/clinchem.2005.048488. [DOI] [PubMed] [Google Scholar]
- 66.JCGM. JCGM 100: 2008, GUM 1995 with minor corrections. Joint Committee for Guides in Metrology; 2008. Evaluation of measurement data — Guide to the expression of uncertainty in measurement.http://www.bipm.org/utils/common/documents/jcgmJCGM_100_2008_E.pdf [Google Scholar]