Summary
Background
Salivary cortisol measurement is a non-invasive method suitable for use in neonatal research. Mother-infant separation after birth represents stress and skin-to-skin contact (SSC) has numerous benefits. The aim of the study was to measure salivary cortisol in mothers and newborns before and after SSC in order to assess the effect of SSC on mothers’ and infants’ stress and to estimate the efficacy of collecting small saliva samples in newborns.
Methods
Salivary cortisol was measured in 35 mother-infant pairs before and after the first and the fifth SSC in small saliva samples (50 μL) using the high sensitivity Quantitative ELISA-Kit (0.0828 nmol/L) for low cortisol levels detection. Samples were collected with eye sponge during 3 to 5 minutes.
Results
Cortisol level in mothers decreased after SSC: the highest levels were measured before and the lowest after SSC and the differences in values were significant during both the first (p<0.001) and the fifth SSC (p<0.001). During the first SSC the cortisol level decrease was detected in 14 (40%) and an increase in 21 (60%) newborns, and during the fifth SSC a decrease was detected in 16 (45.7%) and an increase in 19 (54.3%) newborns, without confirmed significance of the difference. Saliva sampling efficacy using eye sponge was 75%.
Conclusions
Cortisol level decrease in mothers proves the stress reduction during SSC, while variable cortisol levels in infants do not indicate stress reduction and imply the need for further research. The used sampling method appeared to be one of the most optimal considering the sample volume, sampling time and efficacy.
Keywords: low birth weight, salivary cortisol, skin-to-skin care
Kratak sadržaj
Uvod
Određivanje salivarnog kortizola je, kao neinvazivna metoda, pogodno za primenu u neonatologiji. Separacija majke i novorođenčeta predstavlja stres, a metoda »kontakt koža na kožu« (KKK) ima brojne pozitivne efekte. Cilj studije je da se određivanjem nivoa salivarnog kortizola pre i nakon primene KKK proceni efekat na stres majke i novorođenčeta i da se proceni uspešnost uzimanja malih uzoraka salive kod novorođenčeta.
Metode
Kortizol je određivan kod 35 parova majki i novorođenčadi pre i nakon 1. i pre i nakon 5. KKK, iz malog uzorka salive, 50 μL, kvantitativnim ELISA testom visoke osetljivosti (0,0828 nmol/L), za merenje niskih koncentracija kortizola. Uzimanje uzoraka je vršeno pomoću eye sponge, tokom 3 do 5 minuta.
Rezultati
Kortizol kod majki opada nakon KKK: najviše vrednosti su izmerene pre, a najniže posle primene KKK metode, i razlike u vrednostima su značajne i tokom 1. (p<0,001) i tokom 5. KKK (p<0,001). Kod novorođenčadi je tokom 1. KKK detektovan pad kortizola kod 14 (40%), a porast kod 21 novorođenčeta (60%), a tokom 5. KKK pad kod 16 (45,7%), a porast kod 19 novorođenčadi (54,3%), bez potvrđene značajnosti razlike. Sakupljanje uzoraka salive pomoću eye sponge je pokazalo uspešnost od 75%.
Zaključak
Pad vrednosti kortizola dokazuje redukciju stresa kod majki pri primeni KKK, dok varijabilne vrednosti kortizola kod novorođenčadi ne upućuju na redukciju stresa i ukazuju na potrebu za daljim istraživanjima. Primenjena metoda uzorkovanja salive se pokazala jednom od najoptimalnijih u odnosu na veličinu uzorka, trajanje uzimanja uzorka i uspešnost.
Introduction
Preterm birth is the most common cause of mother-infant separation due to the necessary medical treatment of the newborn in the neonatal intensive care unit. Mothers of preterm infants are exposed to higher amounts of stress and experimental research has proven that prolonged separation causes an increase in the cortisol level of the young and hyperactivity of the hypothalamic-pituitary-adrenal axis with long-term consequences (1–3). Due to repeated exposure to stress and painful procedures during their treatment, extremely premature infants can have altered patterns of cortisol response even at 8 months corrected age (4–6). Considering the longterm side effects of high cortisol levels, it is necessary to use stress reducing methods (5–7). High quality of mother care, high sensitivity and responsiveness to infant signals can reduce stress and therefore also the cortisol reactivity in neonates (7, 8). SSC method recommended for low birth weight infants (LBW), among many other benefits, can also minimize the negative effects of stress and mother-infant separation (9, 10). Correlation of salivary cortisol with plasma free cortisol fraction allowed the non-invasive research of cortisol level in neonates, with saliva sampling and measuring low cortisol levels in small saliva samples representing a special challenge (11–14). The aim of this study was to assess the influence of the late type of SSC on stress level in mothers and their infants by determining the level of salivary cortisol before and after the first and before and after the fifth SSC session. As a secondary outcome, the efficacy of collecting small volume saliva samples in LBW neonates was also estimated.
Materials and Methods
The prospective study was conducted at the Institute of Neonatology in Belgrade during the one year period from 2013 to 2014. Recruitment was consecutive and in order of admission. Inclusion criteria were birth weight under 2500 g, mothers’ presence and their agreement to participate in the study. Exclusion criteria were treatment with corticosteroids, anticonvulsants or sedatives, congenital anomalies, severe forms of intracranial hemorrhage, consciousness disorders. The study was approved by the Ethics Committee of the Institute of Neonatology and it was conducted with prior written notice and parental informed consent. SSC was initiated once the infant was in stable clinical condition and it was performed daily for five consecutive days, during two hours, each time from 10 to 12 a.m., due to the diurnal rhythm of maternal cortisol. Salivary cortisol level in mothers and newborns was determined as basal and reactive on 4 occasions: before and after the first, and before and after the fifth SSC.
The cortisol level was determined using the Quantitative ELISA Kit (Salimetrics, LCC, State College, PA, USA), which is intended for research purposes only, with high sensitivity (0.0828 nmol/L) that allows measurement of low concentrations of cortisol. The measurement was done in duplicate using very small saliva samples of 25 μL (50 μL in total). The samples from the same person before and after the SSC were determined in the same series. Saliva was collected using ′eye sponge′ by Alcon, a hydrocellulose micro sponge with plastic grip, which has great absorption power and ability to re-release saliva (90–95%). Saliva samples were collected without the usage of any salivation stimulating agents, always by the same trained nurse. One hour before the sampling newborns were not fed and mothers did not consume any food, caffeine or nicotine. Eye sponge was held under the tongue during 3 to 5 minutes, until the swelling of the sponge became noticeable. It was then placed in a 5 mL polypropylene cuvette with the lid on and centrifuged at 6000 rpm for 15 minutes. The acquired saliva sample was collected using a pipette and frozen at –20 °C until analyzed.
Statistical analysis was performed using IBM SPSS statistical software (SPSS for Windows, release 21.0, SPSS, Chicago, IL). Data is expressed as mean values with standard deviations or medians with minimum and maximum values for continuous variables. Categorical data is presented as absolute numbers with percentages. Changes in salivary cortisol values in mothers and infants before and after SSC were determined by the Wilcoxon two related samples test. p value < 0.05 was considered to be statistically significant.
Results
Salivary cortisol level was determined in 35 mother-infant pairs whose basic demographic characteristics were – median (range): gestational age 31 (25–37) weeks, birth weight 1545 (745–2450) g, Apgar score at 1 min 7 (1–9), Apgar score at 5 min 8 (6–9), age at the beginning of SSC 27 (13–62) days, 15 male (42.9%), 20 female (57.1%) newborns. It was determined that cortisol level in mothers decreases after SSC. The highest levels of maternal cortisol were measured before the first and the lowest after the first SSC session and the difference was highly significant (p<0.001). Cortisol level in mothers also decreased during the fifth SSC session and the difference in these values was also significant (p<0.001) (Figure 1). The percentage change in mothers’ salivary cortisol level was also analyzed: during the first SSC the mean value of percentage change in cortisol was 57.0 (18.5), while during the fifth SSC the mean percentage change value is lower at 52.7 (22.7). By analyzing the basal cortisol level in mothers before the first and before the fifth SSC, we established that basal cortisol level during the fifth SSC was lower but the difference did not reach statistical significance (p=0.437). During SSC the salivary cortisol level in newborns either increases or decreases. During the first SSC a decrease in cortisol level was detected in 14 (40%) and an increase in 21 (60%) newborns, and during the fifth SSC a decrease was detected in 16 (45.7%) and an increase in 19 (54.3%) newborns (Figure 2). By analyzing the difference in cortisol level change in newborns, the significance of this difference was not confirmed, either during the first (p=0.831) or during the fifth (p=0.961) SSC session. Unlike the basal cortisol levels in mothers, the measured basal cortisol levels in newborns were higher during the fifth than during the first SSC; however, this difference was not significant (p=0.554).
Figure 1.
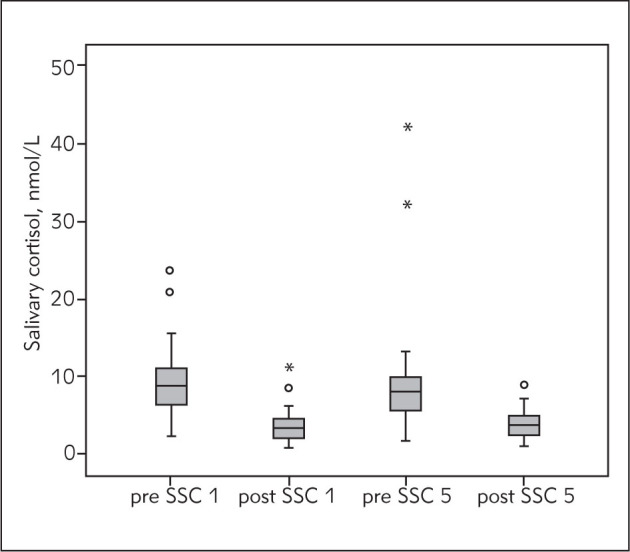
Salivary cortisol changes in mothers during SSC. SSC 1 – first SSC, SSC 5 – fifth SSC, o – outliers, * – extreme values
Figure 2.
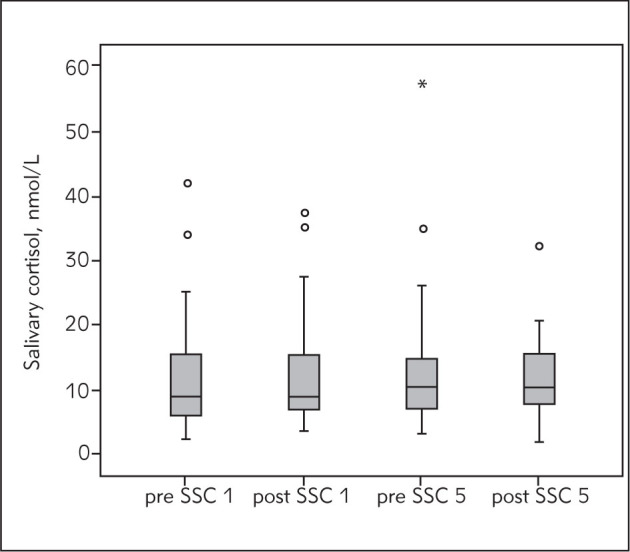
Salivary cortisol changes in infants during SSC. SSC 1 – first SSC, SSC 5 – fifth SSC, o – outliers, * – extreme values
During saliva sampling in newborns, it was necessary to collect 4 samples from each of the 35 neonates, a total of 140 samples. A total of 187 samples was collected, out of which 140 had a volume of 70 mL, with sampling time from 3 to 5 minutes. The sampling method described has shown an efficacy of 75%.
Discussion
Despite numerous evidence of the positive effects of SSC, a number of studies estimating the salivary cortisol level in LBW infants during SSC of late intermittent type is moderate (10, 15). While estimating the stress level by measuring salivary cortisol in term infants, Takahashi et al. have proved that groups with early SSC, immediately after delivery, with duration from 60 to 120 minutes, had significantly lower cortisol levels. Sampling was conducted using the original Sorbettes, sample volume was not listed and cortisol level was determined using a commercial high sensitivity EIA-Kit (Salimetrics, LLC, Pennsylvania, USA) (16). In our study, after SSC the salivary cortisol level in infants either increased or decreased: what made the difference was the population examined, with cortisol being measured in LBW infants with gestational age up to 37 weeks and with SSC being introduced at the average age of 27 days. When comparing the results of both studies, the immaturity of hypothalamic-pituitary-adrenal axis and insufficiently developed circadian rhythm of cortisol in preterm infants should be considered (5, 17, 18). Gitau et al. measured salivary cortisol in 11 preterm infants during SSC and massage and they proved a significant and consistent decrease in cortisol level after only one 20 minute SSC session, with the effects of massage being variable (19). In our study, however, neither the first nor the following SSC sessions, each lasting significantly longer, caused a significant and consistent cortisol level decrease. The question whether the study group being three times bigger (35 compared to 11 infants) has allowed more precise insight in salivary cortisol change, along with the longer session time, remains open. Morelius et al. (20) have compared the influence of Family Centered Care (constant presence of parents) in relation to standard care in 289 preterm infants by measuring the salivary cortisol before and after nappy change as a stressful intervention. Significant differences regarding cortisol reactivity were not proven; however, the correlation between the basal and reactive cortisol levels in mothers and newborns in the Family Centered Group compared to the standard care group was confirmed (20). These results emphasize the importance of parental presence in achieving the mother-infant synchronization and stress reduction and this study certainly is important considering the size of the test group. The same group of authors has compared the effects of continuous SSC to standard care in 37 mother-infant pairs by determining salivary cortisol during nappy change as a stressful intervention. In infants from the SSC group, at the age of one month, cortisol reactivity was lower, whereas at the age of four months the cortisol level in mothers and infants were correlated, which indicates the stress reductive effect of SSC (21). Castral et al. (22) have compared the salivary cortisol level in mothers and infants before and after heel prick, during heel lancing procedure and during SSC and they found no increase in the cortisol level of mothers and infants, which indicates the stress regulatory effect of SSC. Results of the clinical study in which the cortisol level was examined in 17 mothers and their premature born babies during SSC document that the reactive cortisol level in mothers was 32% lower after the first and 22% lower after the fourth SSC session, while the cortisol level in infants was either increasing or decreasing (23). Results of our study were similar to those stated above, with the decrease of salivary cortisol level in mothers being more prominent, which can be connected to the longer use of SSC and longer duration of sessions, while the cortisol level in infants has shown inconsistent changes also in our study. In these studies, Morelius et al. have used their own original modification of the radioimmunoassay (RIA) method for determining salivary cortisol level, in order to achieve a lower detection limit and smaller saliva sample volume up to 10 μL. Saliva sample was collected using two joined cotton swabs with a plastic grip, sampling time was not listed and the sampling efficacy has varied during different phases of the study in the range from 70.6% to 88% (21, 23, 24). This is the smallest saliva sample referred to in the literature so far. The same group of authors also documented the difference in sampling using cotton swabs with plastic and with wooden grip, with the recommendation to avoid the ones with a wooden grip, unless the samples are being centrifuged immediately, since the obtained cortisol levels were otherwise lower (25). The study that compared saliva sampling using cotton swabs and eye sponge in adults and newborns indicated the advantage of using eye sponge in the sense of collecting an adequate sample volume, comfort and simplicity (26). Saliva sampling in preterm newborns represents a challenge because of deficient salivation in comparison to the necessary sample volume and sampling is considered to be successful if a sample volume, sufficient enough to determine the cortisol level, is collected in the shortest time possible. The highest sampling efficacy of 99% was published when using Visipear™ Sorbettes (Salimetrics LCC) during 20 minutes and the lowest efficacy of 46% when using cotton applicators during 5 to 8 minutes. Even in term newborns and with the prior use of salivation stimulating agents, when sampling with the dental cotton gauze, the sampling efficacy achieved was 66%. Therefore, according to the results of sampling analysis in six studies, the use of Sorbettes during 20 minutes is recommended while sampling (27). In the present study we used the eye sponge, sampling time was short and the optimal sample volume of 70 μL was achieved, along with relatively high sampling efficacy compared to the data published in the literature. Only Morelius et al. (25) have cited a smaller sample volume, however, the sampling time was unknown, sampling efficacy was lower and the method used was a modified RIA method.
Conclusion
Cortisol level decrease during SSC proves the stress reduction in mothers, and with repeated SSC sessions the effect is even more prominent. Variable cortisol levels in LBW infants do not indicate stress reduction during SSC, which implies the need for further research. The saliva sampling method used in this study was proven to be one of the most optimal considering the sample volume, sampling time and efficacy and it can be considered a method with significant advantages.
Glossary
Abbreviations
- LBW
low birth weight
- SSC
skin-to-skin care.
Conflict of interest statement
The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Wormald F, Tapia JL, Torres G, Canepa P, Gonzales MA, Rodrigez D. et al. Stress in parents of very low birth weight preterm infants hospitalized in neonatal intensive care units. A multicenter study. Arch Argent Pediatr. 2015;113(4):303–9. doi: 10.5546/aap.2015.eng.303. 1. [DOI] [PubMed] [Google Scholar]
- 2.Shaw RJ, Bernard RS, Deblois T, Ikuta LM, Ginzburg K, Koopman C. The relationship between acute stress disorder and posttraumatic stress disorder in the neonatal intensive care unit. Psychosomatics. 2009;50(2):131–7. doi: 10.1176/appi.psy.50.2.131. [DOI] [PubMed] [Google Scholar]
- 3.Vetulani J. Early maternal separation: a rodent model of depression and a prevailing human condition. Pharmacol Rep. 2013;65(6):1451–61. doi: 10.1016/s1734-1140(13)71505-6. [DOI] [PubMed] [Google Scholar]
- 4.Vinall J, Grunau RE. Impact of repeated procedural pain – related stress in infants born very preterm. Pediatr Res. 2014;75(5):584–7. doi: 10.1038/pr.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthews SG, Owen D, Banjanin S, Andrews MH. Glucocorticoids, hypothalamo-pituitary-adrenal (HPA) development, and life after birth. Endocr Res. 2002;28:709–18. doi: 10.1081/erc-120016991. [DOI] [PubMed] [Google Scholar]
- 6.Grunau RE, Weinberg J, Whitfield MF. Neonatal procedural pain and preterm infants cortisol responses to novelty at 8 months. Pediatrics. 2004;114(1):e77–e84. doi: 10.1542/peds.114.1.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albers EM, Riksen-Walraven JM, Sweep FC, de Weerth C. Maternal behaviour predicts infants cortisol recovery from a mild everyday stressor. J Child Psychol Psychiatry. 2008;49(1):97–103. doi: 10.1111/j.1469-7610.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 8.Bosquet Enlow M, King L, Schreier HM, Howard JM, Rosenfield D, Ritz T. et al. Maternal sensitivity and infant autonomic and endocrine stress responses. Early Hum Dev. 2014;90(7):377–85. doi: 10.1016/j.earlhumdev.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston C, Campbell-Yeo M, Fernandes A, Inglis D, Streiner D, Zee R. Skin-to-skin care for procedural pain in neonates. Cochrane Database of Systematic Reviews. 2014;(Issue 1) doi: 10.1002/14651858.CD008435.pub2. Art. No.: CD008435. [DOI] [PubMed] [Google Scholar]
- 10.Conde-Agudelo A, Díaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birth weight infants. Cochrane Database of Systematic Reviews. 2014;(Issue 4) doi: 10.1002/14651858.CD002771.pub3. Art. No.: CD002771. [DOI] [PubMed] [Google Scholar]
- 11.Calixto C, Martinez FE, Jorge SM, Moreira AC, Martinelli CE Jr. Correlation between plasma and salivary cortisol levels in preterm infants. J Pediatr. 2002;140(2):116–8. doi: 10.1067/mpd.2002.120765. [DOI] [PubMed] [Google Scholar]
- 12.Hellhammer DH, Wüst S, Kudielka BM. Salivary cotisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34:163–71. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Tryphonopoulos PD, Letourneau N, Azar R. Approaches to salivary cortisol collection and analysis in infants. Biol Res Nurs. 2014;16(4):398–408. doi: 10.1177/1099800413507128. [DOI] [PubMed] [Google Scholar]
- 14.Braga F, Infusino I, Panteghini M. Role and responsibilities of laboratory medicine specialists in the verification of metrological traceability of in vitro medical diagnostics. J Med Biochem. 2015;34:282–7. doi: 10.1515/jomb-2015-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz WB. et al. Integration of salivary bio-markers into developmental behaviorally-oriented research: Problems and solutions for collecting specimens. Psychology and Behavior. 2007;92(4):583–90. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi Y, Tamakoshi H, Matsushima M, Kawabe T. Comparison of salivary sortisol, heart rate and oxygen saturation between early skin-to-skin contact with different initiation and duration times in healthy, full term infants. Early Hum Dev. 2011;87(3):151–7. doi: 10.1016/j.earlhumdev.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Castro M, Elias PC, Martinelli CE Jr, Antonini SR, Santiago L, Moreira AC. Salivary cortisol as a tool for physiological studies and diagnostic strategies. Braz J Med Biol Res. 2000;33(10):1171–5. doi: 10.1590/s0100-879x2000001000006. [DOI] [PubMed] [Google Scholar]
- 18.Antonini SR, Jorge SM, Moreira AC. The emergence of salivary cortisol circadian rhytm and its relationship to sleep activity in preterm infants. Clin Endocrinol. 2000;52(4):423–6. [PubMed] [Google Scholar]
- 19.Gitau R, Modi N, Gianakoulopoulos X, Bond C, Glover V, Stevenson J. Acute effects of maternal skin-to-skin contact and massage on saliva cortisol in preterm infants. J Reproduct Infant Psychol. 2002;20(2):83–8. [Google Scholar]
- 20.Mörelius E, Brostrom EB, Westrup B, Sarman I, Ortenstrand A. The Stockholm Neonatal Family-Centred Care Study: Effects on salivary cortisol in infants and their mothers. Early Hum Dev. 2012;88:575–81. doi: 10.1016/j.earlhumdev.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 21.Morelius E, Ortenstrand A, Theodorsson E, Frostell A. A randomised trial of continuous skin-to-skin contact after preterm bitrh and the effects on salivary cortisol, parental stress, depression, and breastfeeding. Early Hum Dev. 2015;91(1):60–70. doi: 10.1016/j.earlhumdev.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Castral TC, Warnock F, Dos Santos CB, Dare MF, Moreira AC, Antonini SR. et al. Maternal mood and concordant maternal and infant salivary cortisol during heel lance while in kangaroo care. Eur J Pain. 2015;19(3):429–38. doi: 10.1002/ejp.566. [DOI] [PubMed] [Google Scholar]
- 23.Mörelius E, Theodorosson E, Nelson N. Salivary Cortisol and Mood and Pain Profiles During Skin-to-Skin Care for an Unselected Group of Mothers and Infants in Neonatal Intensive Care. Pediatrics. 2005;116(5):1105–13. doi: 10.1542/peds.2004-2440. [DOI] [PubMed] [Google Scholar]
- 24.Morelius E, Nelson N, Theodorosson E. Salivary cortisol and administration of concentrated oral glucose in newborn infants: improved detection limit and smaller sample volumes without glucose interference. Scand J Clin Lab Invest. 2004;64(2):113–8. doi: 10.1080/00365510410004452. [DOI] [PubMed] [Google Scholar]
- 25.Morelius E, Nelson N, Theodorosson E. Saliva collection using cotton buds with wooden sticks: a note of caution. Scand J Clin Lab Invest. 2006;66(1):15–8. doi: 10.1080/00365510500402166. [DOI] [PubMed] [Google Scholar]
- 26.de Weerth, Jansen J, Vos MH, Maitimu I, Lentjes EG. A new device for collecting saliva for cortisol determination. Psychoneuroendocrinology. 2007;32(8–10):1144–8. doi: 10.1016/j.psyneuen.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell AJ, Chang J, Yates C, Hall R. Challenges, Guidelines and Systematic Review of Salivary Cortisol Research in Preterm Infants. e-Journal of Neonatology Research. 2012;2(1):44–51. [Google Scholar]


